Introduction
Gastric cancer (GC) and gastroesophageal junction
cancer (GEJC) are the fifth most frequently diagnosed cancer and
the third leading cause of cancer death globally (1,2). GC
and GEJC are frequently asymptomatic in the early stages, and,
often diagnosed at advanced disease stages (3,4). At
present, a combination of a platinum drug (cisplatin or
oxaliplatin) and a fluoropyrimidine (fluorouracil, capecitabine, or
S-1) is regarded as the standard first-line palliative chemotherapy
regimen (5). In total, ~10% of
patients with advanced GC or GEGC will survive for five years,
despite advancements in treatment options (3).
The treatment of advanced cancer has been completely
transformed in recent years by immunotherapy using immune
checkpoint inhibitors. One such checkpoint is the negative
costimulatory receptor known as programmed cell death 1 (PD-1),
which is mostly expressed on peripheral CD4+ and CD8+ T cells,
natural killer T cells, B cells, monocytes and certain dendritic
cell subsets upon their activation (6,7). Tumor
cells frequently use the PD-1 pathway to evade immune surveillance
(6,7). The antitumor immune response is
suppressed when PD-1 binds to its ligands, programmed cell death
ligand 1 (PD-L1) and PD-L2, inhibiting effector T-cell activity
(6,7). PD-L1 is frequently upregulated in GC
and correlated with the depth of tumor invasion, lymph node
metastasis and American Joint Committee on Cancer (AJCC) stage
(8). Pembrolizumab is a humanized,
specific monoclonal antibody against immunoglobulin G4κ that
inhibits the interaction between PD-1 and its ligands, and allows
for the reactivation of the immune response against cancer cells
(9). In other malignancies,
pembrolizumab plus chemotherapy was shown to be effective and
relatively safe (10,11).
Pembrolizumab plus chemotherapy was recently
considered a promising treatment for patients with advanced
GC/GEJC, with positive reported treatment outcomes in patients with
or without surgical treatment (12,13).
Fuchs et al (14) noted that
pembrolizumab monotherapy showed promising activity and a
manageable safety profile in patients with advanced GC/GEJC who had
previously received at least two lines of therapy, and that a
durable response was observed in patients with PD-L1-positive and
PD-L1-negative tumors. Shitara et al (15) also confirmed that pembrolizumab
exhibited a superior safety profile to paclitaxel, despite the fact
that it did not significantly increase overall survival when
administered as a second-line treatment for advanced gastric or
gastroesophageal carcinomas with a PD-L1 combined positive score
(CPS) of 1 or higher. Furthermore, chemotherapeutic drugs such as
5-fluorouracil and cisplatin boost the immunogenicity of cancer
cells and render them more vulnerable to immune-mediated
cytotoxicity (16). However, the
efficacy and side effects of pembrolizumab plus chemotherapy still
lack evidence-based medical evidence to support. Therefore, a
meta-analysis was conducted to evaluate the hot issue.
Materials and methods
Literature search and study
selection
PubMed (https://pubmed.ncbi.nlm.nih.gov/), EMBASE (https://www.embase.com), Cochrane Central Register of
Controlled Trials (https://www.cochranelibrary.com) and Web of Science
(https://clarivate.com) were used for systematic
search. The main search strategy was as follows: (‘esophagogastric
junction carcinoma’ OR ‘EJC’ OR ‘esophagogastric cancer’ OR
‘gastric carcinoma’ OR ‘gastric cancer’ OR ‘GC’) AND
(‘immunotherapy’ OR ‘PD-1’ OR ‘Pembrolizumab’). The last search was
conducted on March 8, 2024. Two authors (JL and XH) independently
reviewed the title and abstract of the citation and obtained the
full text of potentially eligible studies. Disagreements were
resolved by discussion or, if necessary, by a third author (SZ). A
reference list review of all retrieved studies was further screened
for additional eligible studies.
Inclusion and exclusion criteria
Studies that compared the efficacy and side effects
of patients undergoing chemotherapy alone or in combination with
pembrolizumab were included. The studies should meet the following
criteria: i) Randomized clinical trials; ii) advanced GC or GEJC;
and iii) comparable data on overall survival (OS), response rate
(RR) or side effects was available. The main exclusion criteria
included: i) Reviews, letters, case reports and conference
abstracts; ii) animal experiments, in vitro studies and
ongoing studies; iii) studies a with lack of survival data or
adverse effects; and iv) studies were not about advanced
GC/GEJC.
Quality assessment
Two authors (JL and XH) conducted independent
assessments. Disagreements were resolved by discussion or by a
third author (SZ). No studies were excluded on this basis. The risk
of bias in the eligible studies was comprehensively assessed
according to the Cochrane Collaboration's Risk of Bias tool
(17).
Data extraction and synthesis
Two authors (JL and XH) independently extracted the
data from the included studies. The information included the
following outcomes: Name of the trial, publication time and design
of the trial, treatment methods, case characteristics, median
follow-up time, therapeutic efficacy and related side effects.
The meta-analysis was conducted according to the
Cochrane handbook for systematic reviews of interventions. Every
categorical variable in the current investigation was
discontinuous. Forest plots were drawn by Review Manager 5.4
software (Cochrane Institute) automatically. Odds ratio (OR),
P-value and 95% confidence intervals (CI) were used to assess
whether the differences in results were significant. P>0.05 was
considered to indicate a statistically significant difference. The
statistical analysis was deemed to show no substantial
heterogeneity if I2<50%; heterogeneity was deemed to
be present if I2≥50%. The fixed-effects model (FEM) and
random-effects model (REM) were alternated in the calculation mode.
FEM was utilized to handle the data if there was no significant
heterogeneity; REM was employed in other cases. The publication
bias and Egger test were conducted by using STATA 16.0 (Stata Corp
LP).
Results
Study selection
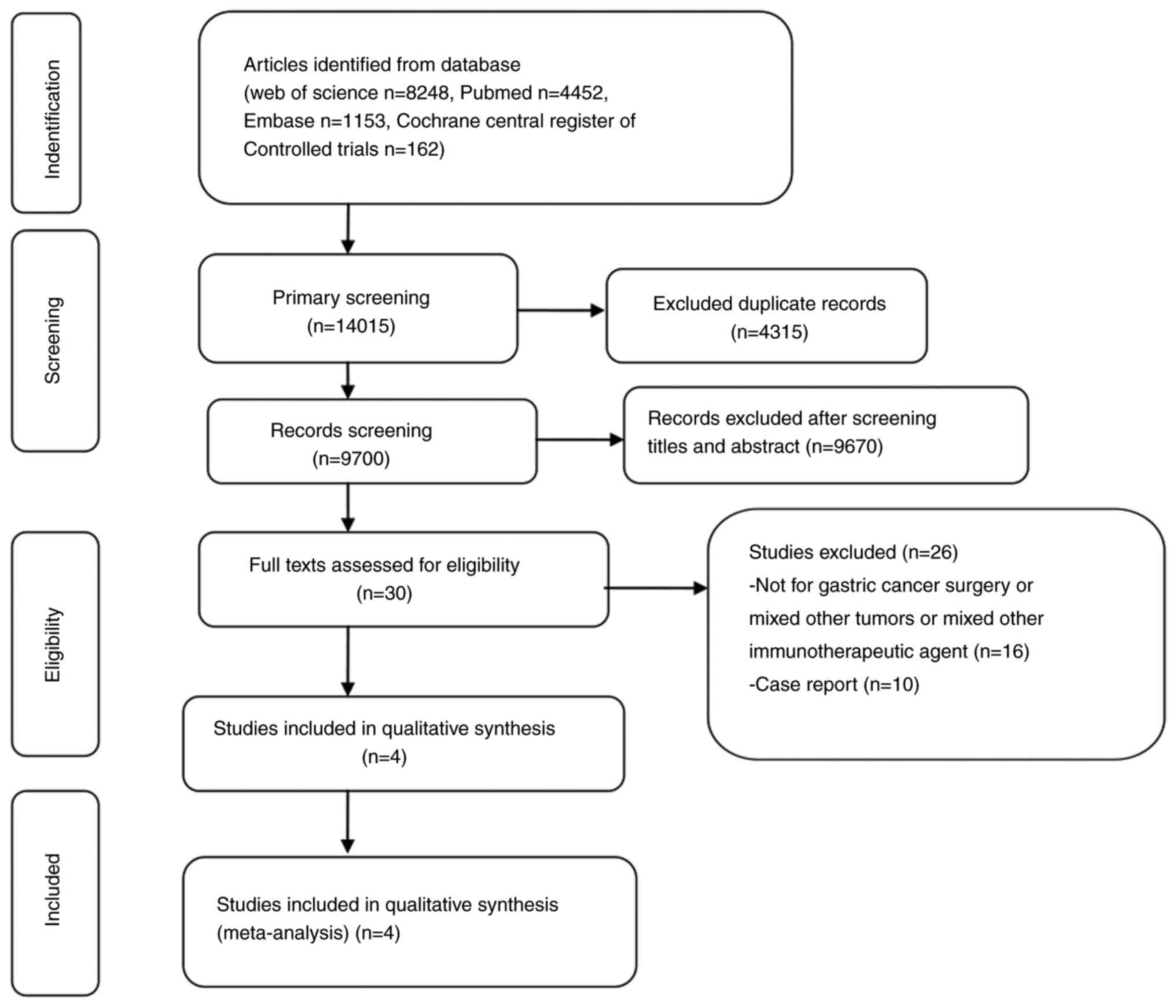
Through a systematic literature search, 14,015
studies were retrieved. After removing duplicate studies,
irrelevant studies were excluded by checking titles and abstracts
one by one. At last, four eligible studies (18–21)
were included. The flow chart of selecting literatures according to
PRISMA guidelines is shown in Fig.
1.
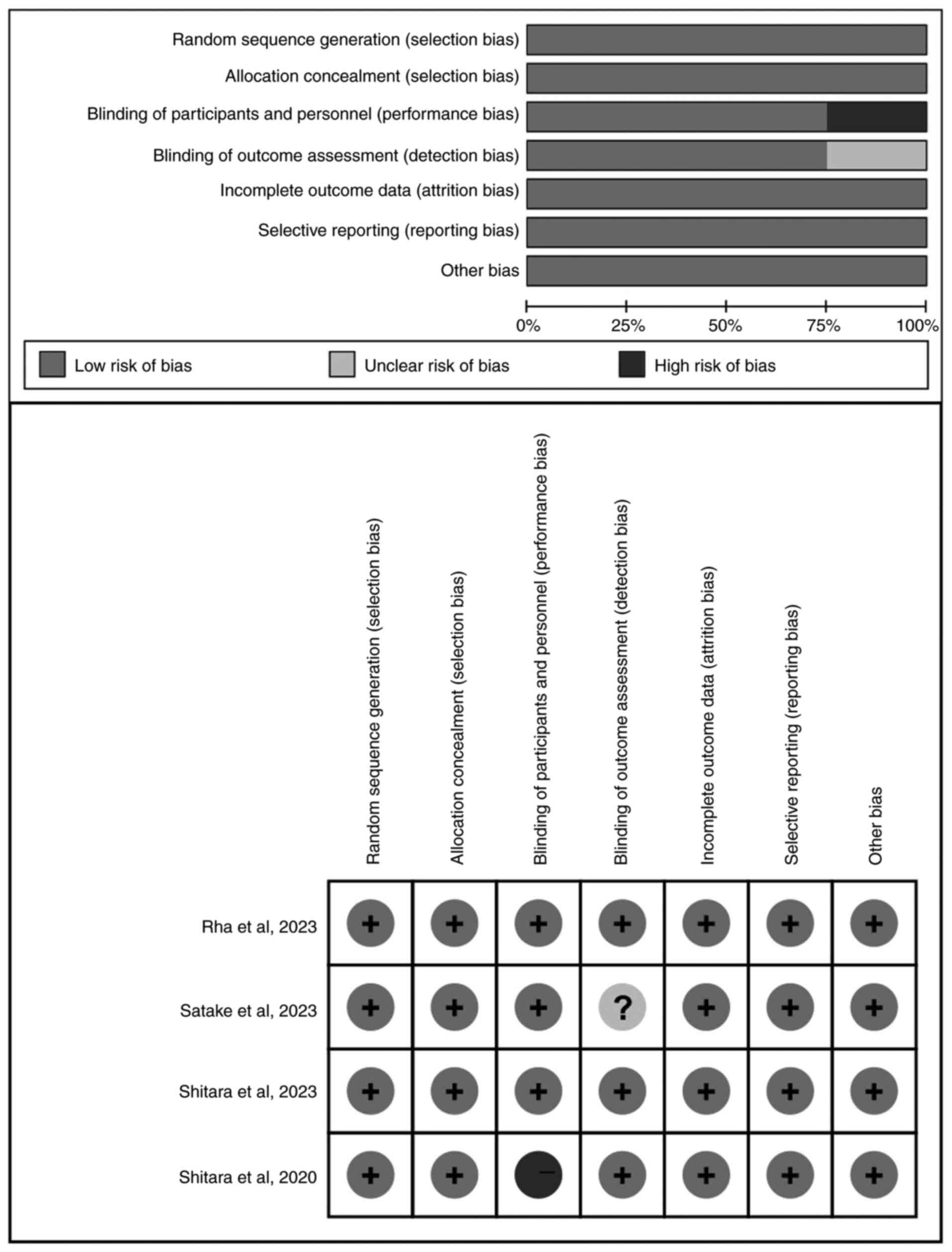
Methodological quality
All included four studies (n=1,821) were multicenter
randomized clinical trials. A total of 3,015 patients were included
in the meta-analysis, of which 1,502 patients received chemotherapy
alone and 1,513 patients received chemotherapy in addition to
pembrolizumab. The baseline characteristics of the included studies
are displayed in Table I. Cochrane
Collaboration's Risk of Bias tool was used to assess the quality of
the included studies. All studies were low risk of bias. The
details of the assessment are shown in Fig. 2.
 | Table I.Study characteristics. |
Table I.
Study characteristics.
| Author, year | Country | Study design | Clinical stage | Therapeutic
regimen | Age, years [median
(range)]. | Number of
patients | Tumor location
G/GEJ | CPS ≥1 | CPS ≥10 | Median follow-up
time, months | Risk of bias | (Refs.) |
|---|
| Shitara et
al, 2020 | Japan | Multicenter | III–IV | i) Pe+C; ii) C | i) 62.0 (22–83); ii) 62.5 (23–87) | i) 257; ii)
250 | i) 170/181; ii)
85/67 | i) 257; ii)
250 | i) 99; ii) 90 | 29.4
(22.0–41.3) | Low | (18) |
| Satake et
al, 2023 | Japan | Multicenter | III–IV | i) Pe+C; ii) C | i) 65.0 (34–83); ii) 67.0 (37–85 | i) 64; ii) 61 | i) 55/9; ii)
57/4 | i) 64; ii) 61 | i) 26; ii) 22 | 24 (19–31) | Low | (19) |
| Shitara et
al, 2023 | Japan | Multicenter | II–IV | i) Pe+C; ii) C | i) 64 (56–70); ii)
63 (55–69) | i) 402; ii)
402 | i) 316/86; ii)
322/79 | i) 293; ii)
307 | i) 104; ii)
116 | 16.9
(0.2–41.0) | Low | (20) |
| Rha et al,
2023 | South Korea | Multicenter | III–IV | i) Pe+C; ii) C | i) 61 (52–67); ii)
62 (52–69) | i) 790; ii)
789 | i) 640/149; ii)
603/185 | i) 618; ii)
617 | i) 279; ii)
272 | 31.0
(23.0–8.3) | Low | (21) |
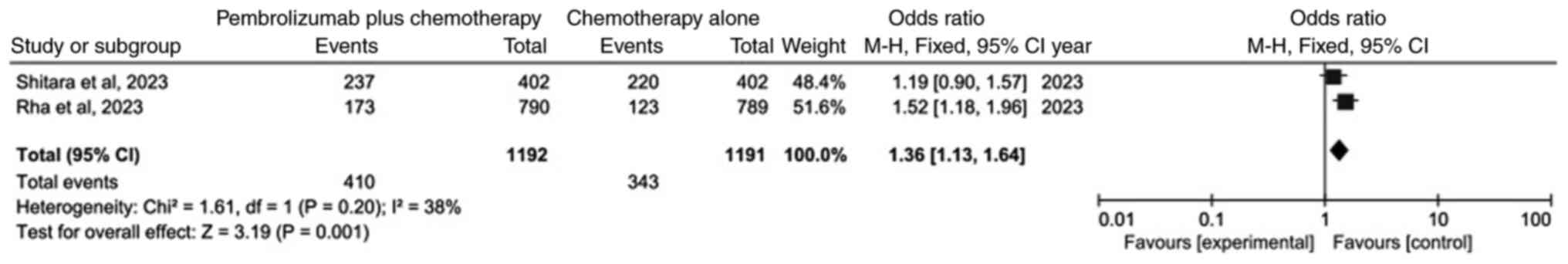
Comparison of OS rate
The meta-analysis of OS following data integration
is demonstrated in Fig. 3. Only two
studies (20,21) were included. The results
demonstrated that, as compared with chemotherapy alone, the OS of
the pembrolizumab plus chemotherapy group was statistically
significant (OR=1.36; 95% CI: 1.13–1.64; P=0.001). This indicated
that OS was higher in the pembrolizumab plus chemotherapy group
than in the chemotherapy alone group.
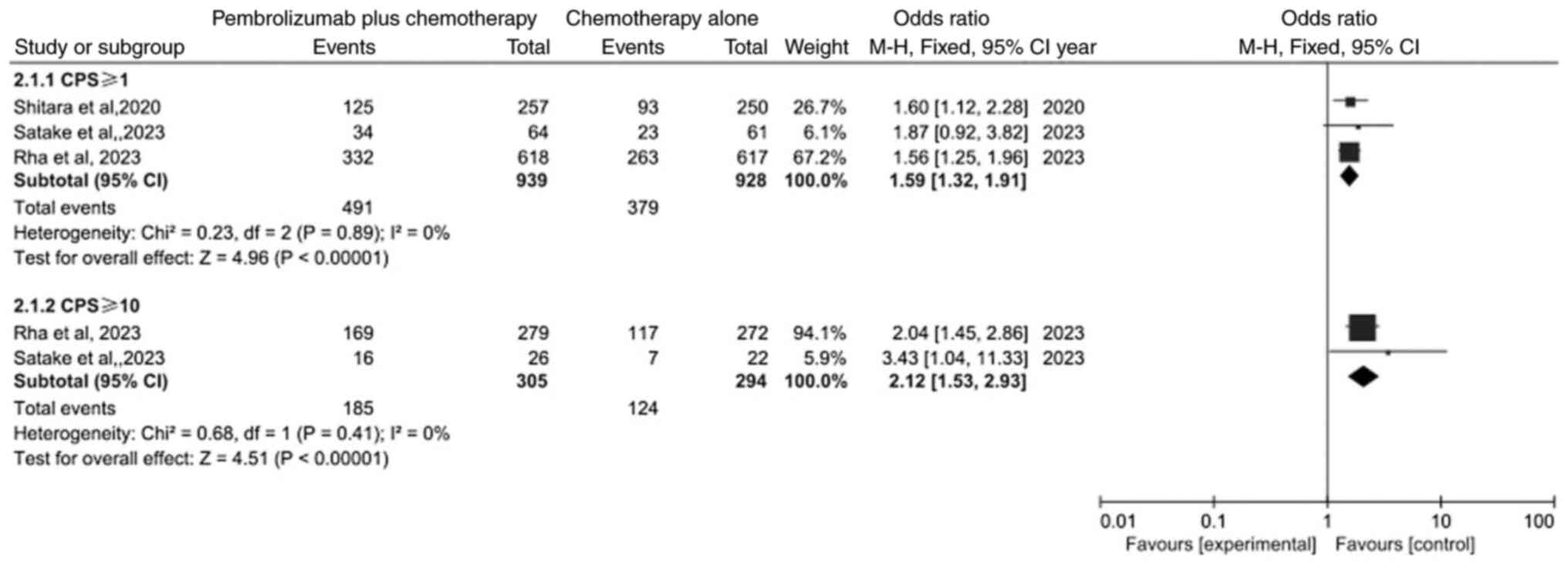
Comparison of response rate (RR)
A subgroup analysis was performed in the
meta-analysis of RR (Fig. 4). The
result identified that the RR both in PD-L1 CPS ≥1 subgroup and
PD-L1 CPS ≥10 subgroup was significantly different between the
pembrolizumab plus chemotherapy group and chemotherapy alone group
(OR=1.59, 95% CI: 1.32–1.91; P<0.00001 and OR=2.12; 95% CI:
1.53–2.93; P<0.00001).
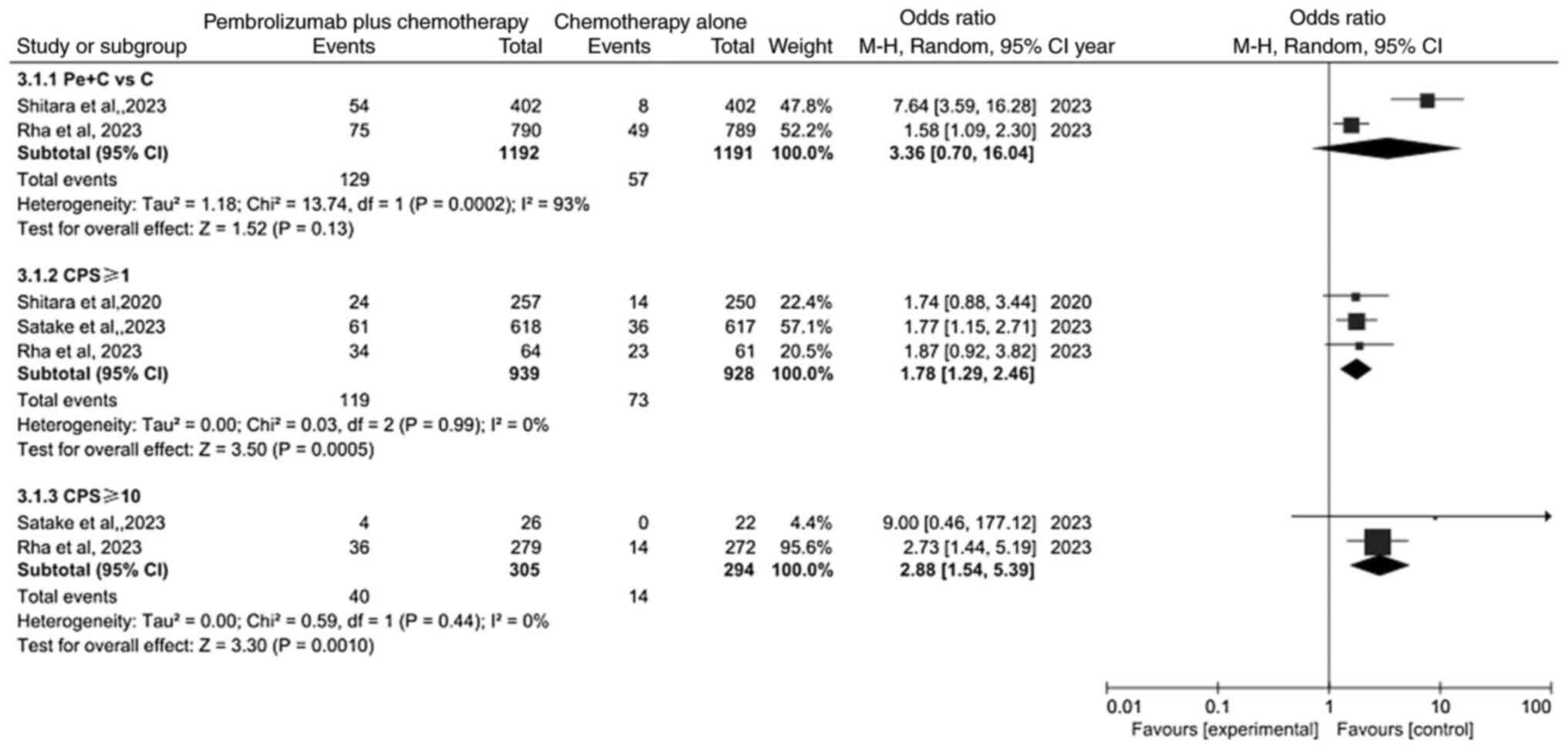
Comparison of complete response (CR)
rate
Meta-analysis of CR is revealed in Fig. 5. Because of heterogeneity (P=0.01,
I2=63%), a random effects model was used for analysis.
The result revealed that the CR rate was not significantly
different between the pembrolizumab plus chemotherapy group and
chemotherapy alone group (OR=3.36; 95% CI: 0.70–16.04; P=0.13). By
contrast, the results showed that the PD-L1 CPS ≥1 subgroup and
PD-L1 CPS ≥10 subgroup were significantly different (OR=1.78; 95%
CI: 1.29–2.46; P=0.0005 and OR=2.88; 95% CI: 1.54–5.39;
P=0.0010).
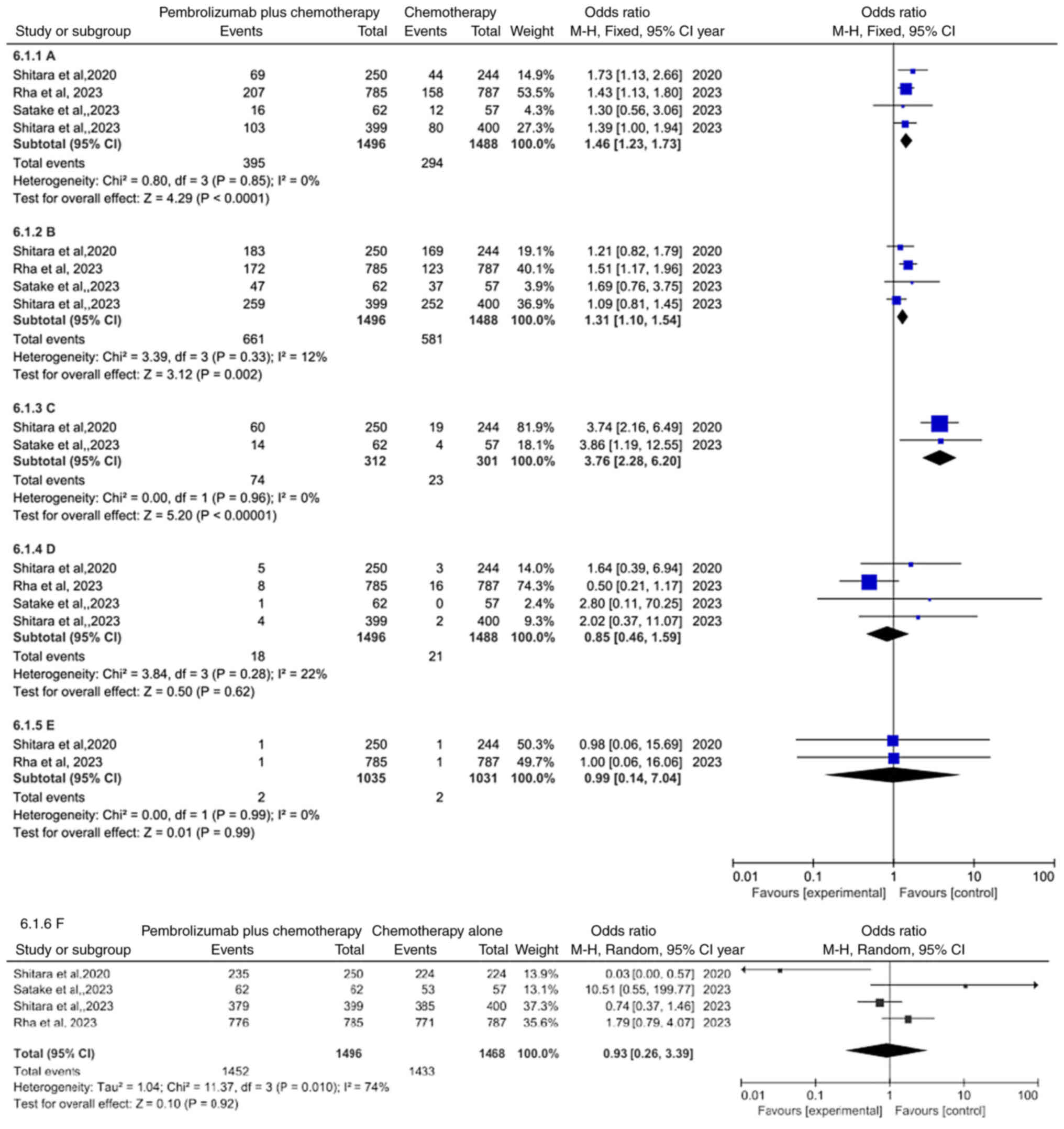
Comparison of safety
Moreover, an analysis was conducted to explore the
side effects of pembrolizumab plus chemotherapy group compared with
chemotherapy alone group (Table
SI). The data integration demonstrated that the occurrence of
treatment-related adverse events leading to discontinuation was
more common in pembrolizumab plus chemotherapy group compared with
chemotherapy alone group (OR=1.46, 95% CI: 1.23–1.73, P<0.0001)
(Fig. 6A), regardless of the 3–5
treatment-related adverse events (OR=1.31; 95% CI: 1.10–1.54;
P=0.002) (Fig. 6B) or
immune-mediated adverse events and infusion reactions (OR=3.76; 95%
CI: 2.28–6.20; P<0.00001) (Fig.
6C). Conversely, compared with chemotherapy alone group,
succumbing to drug-related events (OR=0.85; 95% CI: 0.46–1.59;
P=0.62) (Fig. 6D) was not
statistically significant in pembrolizumab plus chemotherapy group,
regardless of succumbing to immune-mediated events (OR=0.99; 95%
CI: 0.14–7.04; P=0.99) (Fig. 6E) or
the occurrence of treatment-related adverse events (OR=0.93; 95%
CI: 0.26–3.39; P=0.92) (Fig.
6F).
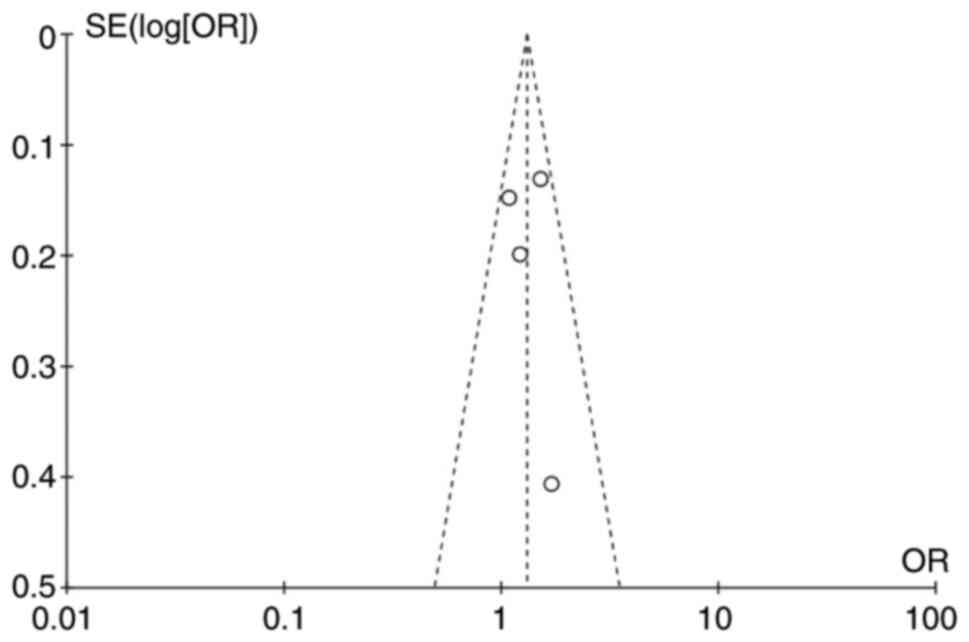
Publication bias
Funnel plots (Fig.
7) were chosen to estimate publication bias in the present
study, and no obvious bias was observed. No significant publication
bias was also identified through the Egger tests (P=0.394).
Discussion
A variety of targeted drugs targeting the programmed
cell death protein 1 (PD-1)/PD-L1 pathway have successfully entered
clinical trials (22–26). Among them, pembrolizumab, as a
representative, has been approved by the FDA for marketing, and has
obtained corresponding indications in melanoma and non-small cell
lung cancer (27–30). As it has been reported, advanced
GC/GEJC obtain a poor prognosis, one of the reasons being the poor
effect of current drugs (31). The
application of pembrolizumab in GC/GEJC opens a new chapter of
prognosis. However, there is an urgent need to investigate the side
effects and effectiveness of pembrolizumab alongside
chemotherapy.
In review of 4 randomized controlled trials
(18–21), the result of the present
meta-analysis revealed that the pembrolizumab plus chemotherapy
group had improved OS than the chemotherapy alone group, but not
discovered in RR and CR. According to the trial by Reck et
al (32), PD-L1 may be a
significant biomarker for predicting pembrolizumab response in
solid tumors. Thus, it suggests that pembrolizumab may have a more
favorable curative outcome for patients with PD-L1 CPS ≥1 and PD-L1
CPS ≥10. The meta-analysis verified that the pembrolizumab plus
chemotherapy group had superior RR and CR than the chemotherapy
alone group in patients with PD-L1 CPS ≥1 and PD-L1 CPS ≥10. The
difference in OS, RR and CR between PD-L1 positive and negative
patients could not be compared. However, the CR was not
significantly different between the pembrolizumab plus chemotherapy
group and chemotherapy alone group.
The most common treatment-related adverse effects
associated with pembrolizumab are fatigue, decreased appetite and
nausea; in most cases they are resolved without or with minimal
treatment (33,34). The results of the present
meta-analysis revealed that compared with chemotherapy alone group,
the occurrence of treatment-related adverse events was not
statistically significant in pembrolizumab plus chemotherapy group,
regardless of succumbing to drug-related events. The most common
grade 3–5 treatment-related adverse events occurring are fatigue,
diarrhea and anemia, which have great influence on patients. The
results of the current meta-analysis revealed that the occurrence
of grade 3–5 treatment-related adverse events was higher in
pembrolizumab plus chemotherapy group than in chemotherapy alone
group; the same result occurred in the occurrence of
treatment-related adverse events leading to discontinuation.
Immune checkpoint inhibitors, which enhance the
capacity of the immune system to eliminate cancer cells, have
revolutionized cancer immunotherapy by focusing on the PD-1
pathway. But because of the way these treatments work, there is a
unique set of difficulties associated with this novel strategy.
Specifically, immune-related adverse events may result from these
therapies. Skin, gastrointestinal tract, liver, endocrine system
and lungs are the most frequently affected organ systems by adverse
events (35). The efficacy of
treatment is influenced by these side effects (36,37).
The meta-analysis revealed that the pembrolizumab plus chemotherapy
group experienced more immune-mediated adverse events and infusion
reactions than the chemotherapy alone group. However, the frequency
of drug-related deaths and immune-mediated deaths did not differ
statistically significantly between the two groups. New side
effects caused by pembrolizumab, such as delayed immune-related
hepatitis (35), aplastic anemia
(38) and severe mucositis
(39), continue to emerge.
Therefore, the side effects of pembrolizumab require continuous
attention. The development of an aptamer against the target can
mitigate these negative effects. Compared with typical antibodies,
aptamers have several benefits, including increased specificity,
decreased immunogenicity and flexible design for fewer side
effects. Aptamers are particularly designed to target and disrupt
receptor-ligand or protein-protein interactions that are involved
in immune checkpoint pathways (40).
The present study also has certain limitations.
First, it is a meta-analysis based on published literature, which
is susceptible to publication bias. Second, the number of included
trials in the present meta-analysis was only four; but all are
high-quality multicenter randomized trials. Third, there are still
insufficient relevant research projects on progression-free
survival, alleviating progression symptoms and enhancing quality of
life. In the future, it is expected that a more in-depth stratified
analysis can be conducted to identify the efficacy and safety of
pembrolizumab plus chemotherapy versus chemotherapy alone for
patients with advanced GC/GEJC. In conclusion, it was revealed that
in treating advanced GC/GEJC, pembrolizumab plus chemotherapy had
improved therapeutic efficacies than chemotherapy alone, as
evidenced by the significantly longer OS. Furthermore, the patients
in PD-L1 CPS ≥1 subgroupand PD-L1 CPS ≥10 subgroup appeared to
benefit from pembrolizumab plus chemotherapy treatment because of
higher RR and CR. However, when compared with the chemotherapy
group, the pembrolizumab plus chemotherapy group experienced a
higher frequency of immune-mediated adverse events, infusion
reactions and treatment-related adverse events that resulted in
treatment discontinuation. These side effects should be given more
consideration.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The data generated in the present study are included
in the figures and/or tables of this article.
Authors' contributions
JL conceptualized and supervised the present study,
and wrote, reviewed and edited the manuscript. JL and XH curated
the data. JL and SZ checked and confirmed the authenticity of the
raw data. JL, SZ and XH conducted formal analysis, developed
methodology, performed software analysis and wrote the original
draft. All authors read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Sung H, Ferlay J, Siegel RL, Laversanne M,
Soerjomataram I, Jemal A and Bray F: Global cancer statistics 2020:
GLOBOCAN estimates of incidence and mortality worldwide for 36
cancers in 185 countries. CA Cancer J Clin. 71:209–249. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Bray F, Ferlay J, Soerjomataram I, Siegel
RL, Torre LA and Jemal A: Global cancer statistics 2018: GLOBOCAN
estimates of incidence and mortality worldwide for 36 cancers in
185 countries. CA Cancer J Clin. 68:394–424. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Li C, Chen D and Yang H: Trends in
incidence, survival and mortality of gastric cancer in the United
States: A population-based study, 2001–2015. Asian Pac J Cancer
Prev. 24:2011–2020. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Li Y, Feng A, Zheng S, Chen C and Lyu J:
Recent estimates and predictions of 5-year survival in patients
with gastric cancer: A model-based period analysis. Cancer Control.
29:107327482210992272022. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Lordick F, Carneiro F, Cascinu S, Fleitas
T, Haustermans K, Piessen G, Vogel A and Smyth EC; ESMO Guidelines
Committee. Electronic address, : simpleclinicalguidelines@esmo.org:
Gastric cancer: ESMO clinical practice guideline for diagnosis,
treatment and follow-up. Ann Oncol. 33:1005–1020. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Francisco LM, Sage PT and Sharpe AH: The
PD-1 pathway in tolerance and autoimmunity. Immunol Rev.
236:219–242. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Pardoll DM: Immunology beats cancer: A
blueprint for successful translation. Nat Immunol. 13:1129–1132.
2012. View
Article : Google Scholar : PubMed/NCBI
|
|
8
|
Geng Y, Wang H, Lu C, Li Q, Xu B, Jiang J
and Wu C: Expression of costimulatory molecules B7-H1, B7-H4 and
Foxp3+ Tregs in gastric cancer and its clinical significance. Int J
Clin Oncol. 20:273–281. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Russo CD, Gagliardi D, Ramlogan R and
Navarra P: Optimizing patient selection to maximize drug efficacy:
The expanding role of pharmacogenomics in the clinical development
of pembrolizumab for the treatment of non-small cell lung cancer.
Clin Ther. 41:982–991. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Langer CJ, Gadgeel SM, Borghaei H,
Papadimitrakopoulou VA, Patnaik A, Powell SF, Gentzler RD, Martins
RG, Stevenson JP, Jalal SI, et al: Carboplatin and pemetrexed with
or without pembrolizumab for advanced, non-squamous non-small-cell
lung cancer: A randomised, phase 2 cohort of the open-label
KEYNOTE-021 study. Lancet Oncol. 17:1497–1508. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Cescon DW, Schmid P, Rugo HS, Im SA, Yusof
MM, Gallardo C, Lipatov O, Barrios CH, Perez-Garcia J, Iwata H, et
al: Health-related quality of life with pembrolizumab plus
chemotherapy vs placebo plus chemotherapy for advanced
triple-negative breast cancer: KEYNOTE-355. J Natl Cancer Inst.
116:717–727. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wakasugi A, Sasaki A, Okamoto R and
Motomura Y: Eldest gastric cancer patient with high microsatellite
instability responding to pembrolizumab. Int Cancer Conf J.
12:59–62. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lin CY, Mehta P, Waters KM, Chang E,
Hendifar A, Osipov A, Burch M, Lin DC, Gangi A, Cho M and Gong J:
Complete response to neoadjuvant pembrolizumab and capecitabine in
microsatellite stable, Epstein-Barr virus-positive, locally
advanced gastric adenocarcinoma: Case report. AME Case Rep.
5:302021. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Fuchs CS, Doi T, Jang RW, Muro K, Satoh T,
Machado M, Sun W, Jalal SI, Shah MA, Metges JP, et al: Safety and
efficacy of pembrolizumab monotherapy in patients with previously
treated advanced gastric and gastroesophageal junction cancer:
Phase 2 clinical KEYNOTE-059 trial. JAMA Oncol. 4:e1800132018.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Shitara K, Özgüroğlu M, Bang YJ, Di
Bartolomeo M, Mandalà M, Ryu MH, Fornaro L, Olesiński T, Caglevic
C, Chung HC, et al: Pembrolizumab versus paclitaxel for previously
treated, advanced gastric or gastro-oesophageal junction cancer
(KEYNOTE-061): A randomised, open-label, controlled, phase 3 trial.
Lancet. 392:123–133. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zitvogel L, Galluzzi L, Smyth MJ and
Kroemer G: Mechanism of action of conventional and targeted
anticancer therapies: Reinstating immunosurveillance. Immunity.
39:74–88. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Higgins JP, Altman DG, Gøtzsche PC, Jüni
P, Moher D, Oxman AD, Savovic J, Schulz KF, Weeks L, Sterne JA, et
al: The cochrane collaboration's tool for assessing risk of bias in
randomised trials. BMJ. 343:d59282011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Shitara K, Van Cutsem E, Bang YJ, Fuchs C,
Wyrwicz L, Lee KW, Kudaba I, Garrido M, Chung HC, Lee J, et al:
Efficacy and safety of pembrolizumab or pembrolizumab plus
chemotherapy vs chemotherapy alone for patients with first-line,
advanced gastric cancer: The KEYNOTE-062 phase 3 randomized
clinical trial. JAMA Oncol. 6:1571–1580. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Satake H, Lee KW, Chung HC, Lee J,
Yamaguchi K, Chen JS, Yoshikawa T, Amagai K, Yeh KH, Goto M, et al:
Pembrolizumab or pembrolizumab plus chemotherapy versus standard of
care chemotherapy in patients with advanced gastric or
gastroesophageal junction adenocarcinoma: Asian subgroup analysis
of KEYNOTE-062. Jpn J Clin Oncol. 53:221–229. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Shitara K, Rha SY, Wyrwicz LS, Oshima T,
Karaseva N, Osipov M, Yasui H, Yabusaki H, Afanasyev S, Park YK, et
al: Neoadjuvant and adjuvant pembrolizumab plus chemotherapy in
locally advanced gastric or gastro-oesophageal cancer
(KEYNOTE-585): An interim analysis of the multicentre,
double-blind, randomised phase 3 study. Lancet Oncol. 25:212–224.
2024. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Rha SY, Oh DY, Yañez P, Bai Y, Ryu MH, Lee
J, Rivera F, Alves GV, Garrido M, Shiu KK, et al: Pembrolizumab
plus chemotherapy versus placebo plus chemotherapy for
HER2-negative advanced gastric cancer (KEYNOTE-859): A multicentre,
randomised, double-blind, phase 3 trial. Lancet Oncol.
24:1181–1195. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Gong J, Chehrazi-Raffle A, Reddi S and
Salgia R: Development of PD-1 and PD-L1 inhibitors as a form of
cancer immunotherapy: A comprehensive review of registration trials
and future considerations. J Immunother Cancer. 6:82018. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Lim SH, Lee KW, Kim JJ, Im HS, Kim IH, Han
HS, Koo DH, Cho JH, Maeng CH, Lee MY, et al: Real-world outcomes of
third-line immune checkpoint inhibitors versus irinotecan-based
chemotherapy in patients with advanced gastric cancer: A Korean,
multicenter study (KCSG ST22-06). BMC Cancer. 24:2522024.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Lévêque I and Spitzer E: Pembrolizumab
with trastuzumab and chemotherapy in advanced or metastatic gastric
or gastroesophageal junction adenocarcinomas with surexpression of
HER2 and CPS ≥1. Bull Cancer. 111:130–132. 2024.(In French).
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Li S, Bao J, Li X, Yang Q, Xu J, Chen S,
Feng G, Gao C, Feng L, Lu B, et al: Multicenter phase I dose
escalation and expansion study of pyrotinib in combination with
camrelizumab and chemotherapy as first-line treatment for
HER2-positive advanced gastric and gastroesophageal junction
adenocarcinoma. EClinicalMedicine. 66:1023142023. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zhu Y, Liu K, Zhu H and Wu H: Immune
checkpoint inhibitors plus chemotherapy for HER2-negative advanced
gastric/gastroesophageal junction cancer: A cost-effectiveness
analysis. Therap Adv Gastroenterol. 16:175628482312072002023.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Robert C, Long GV, Brady B, Dutriaux C,
Maio M, Mortier L, Hassel JC, Rutkowski P, McNeil C,
Kalinka-Warzocha E, et al: Nivolumab in previously untreated
melanoma without BRAF mutation. N Engl J Med. 372:320–330. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Hellmann MD, Paz-Ares L, Caro RB, Zurawski
B, Kim SW, Costa EC, Park K, Alexandru A, Lupinacci L, de la Mora
Jimenez E, et al: Nivolumab plus ipilimumab in advanced
non-small-cell lung cancer. N Engl J Med. 381:2020–2031. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Gong S, Li Q, Yu X and Yang S: Efficacy
and safety of different immunotherapies combined with chemotherapy
as first-line therapy in patients with small cell lung cancer: A
network meta-analysis. Front Immunol. 15:13625372024. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Li Y, Liang X, Li H and Chen X: Efficacy
and safety of immune checkpoint inhibitors for advanced non-small
cell lung cancer with or without PD-L1 selection: A systematic
review and network meta-analysis. Chin Med J (Engl). 136:2156–2165.
2023. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Abderhalden LA, Wu P, Amonkar MM, Lang BM,
Shah S, Jin F, Frederickson AM and Mojebi A: Clinical outcomes for
previously treated patients with advanced gastric or
gastroesophageal junction cancer: A systematic literature review
and meta-analysis. J Gastrointest Cancer. 54:1031–1045. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Reck M, Rodríguez-Abreu D, Robinson AG,
Hui R, Csőszi T, Fülöp A, Gottfried M, Peled N, Tafreshi A, Cuffe
S, et al: Pembrolizumab versus chemotherapy for PD-L1-positive
non-small-cell lung cancer. N Engl J Med. 375:1823–1833. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Fuchs CS, Özgüroğlu M, Bang YJ, Di
Bartolomeo M, Mandala M, Ryu MH, Fornaro L, Olesinski T, Caglevic
C, Chung HC, et al: Pembrolizumab versus paclitaxel for previously
treated PD-L1-positive advanced gastric or gastroesophageal
junction cancer: 2-year update of the randomized phase 3
KEYNOTE-061 trial. Gastric Cancer. 25:197–206. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Bang YJ, Kang YK, Catenacci DV, Muro K,
Fuchs CS, Geva R, Hara H, Golan T, Garrido M, Jalal SI, et al:
Pembrolizumab alone or in combination with chemotherapy as
first-line therapy for patients with advanced gastric or
gastroesophageal junction adenocarcinoma: Results from the phase II
nonrandomized KEYNOTE-059 study. Gastric Cancer. 22:828–837. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
González FS, Palacios CA, Gordón AM,
Gallego JM, Díaz A and Ferrer GM: Delayed immune-related hepatitis
after 24 months of pembrolizumab treatment: A case report and
literature review. Anticancer Drugs. 35:284–287. 2024. View Article : Google Scholar
|
|
36
|
Aggarwal S: Adverse effects of
immuno-oncology drugs-Awareness, diagnosis, and management: A
literature review of immune-mediated adverse events. Indian J
Cancer. 56 (Suppl):S10–S22. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Urwyler P, Earnshaw I, Bermudez M, Perucha
E, Wu W, Ryan S, Mcdonald L, Karagiannis SN, Taams LS, Powell N, et
al: Mechanisms of checkpoint inhibition-induced adverse events.
Clin Exp Immunol. 200:141–154. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Yeung C, Relke N, Good D, Satkunam N and
Mates M: Antithymocyte globulin for aplastic anemia secondary to
pembrolizumab: A case report and review of literature.
Immunotherapy. 15:323–333. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Huntley RE, DeNiro K, Yousef J, Sheedy M
and Dillon JK: Severe mucositis secondary to pembrolizumab: Reports
of two cases, review of the literature, and an algorithm for
management. J Oral Maxillofac Surg. 79:1262–1269. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Kejamurthy P and Devi KTR: Immune
checkpoint inhibitors and cancer immunotherapy by aptamers: An
overview. Med Oncol. 41:402023. View Article : Google Scholar : PubMed/NCBI
|