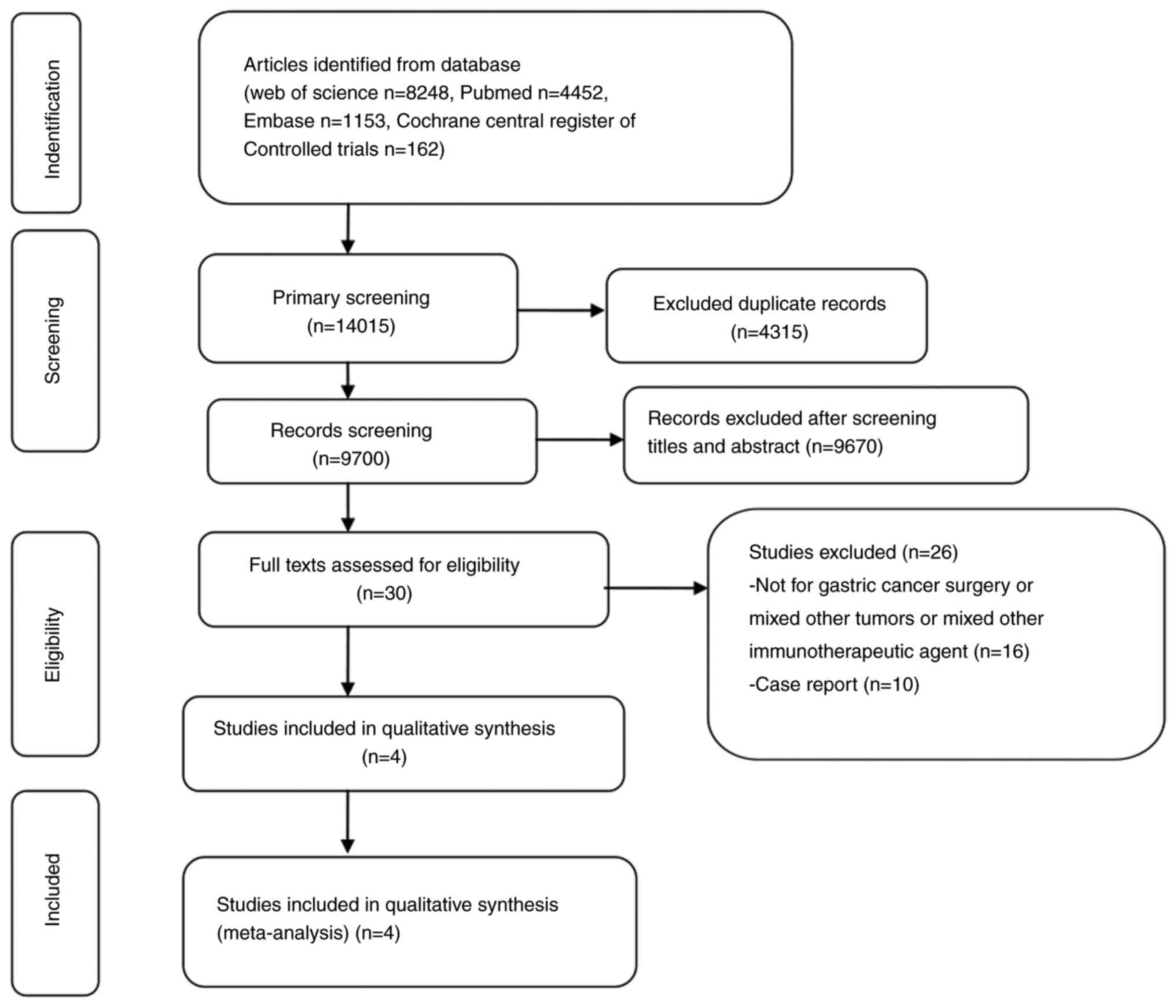
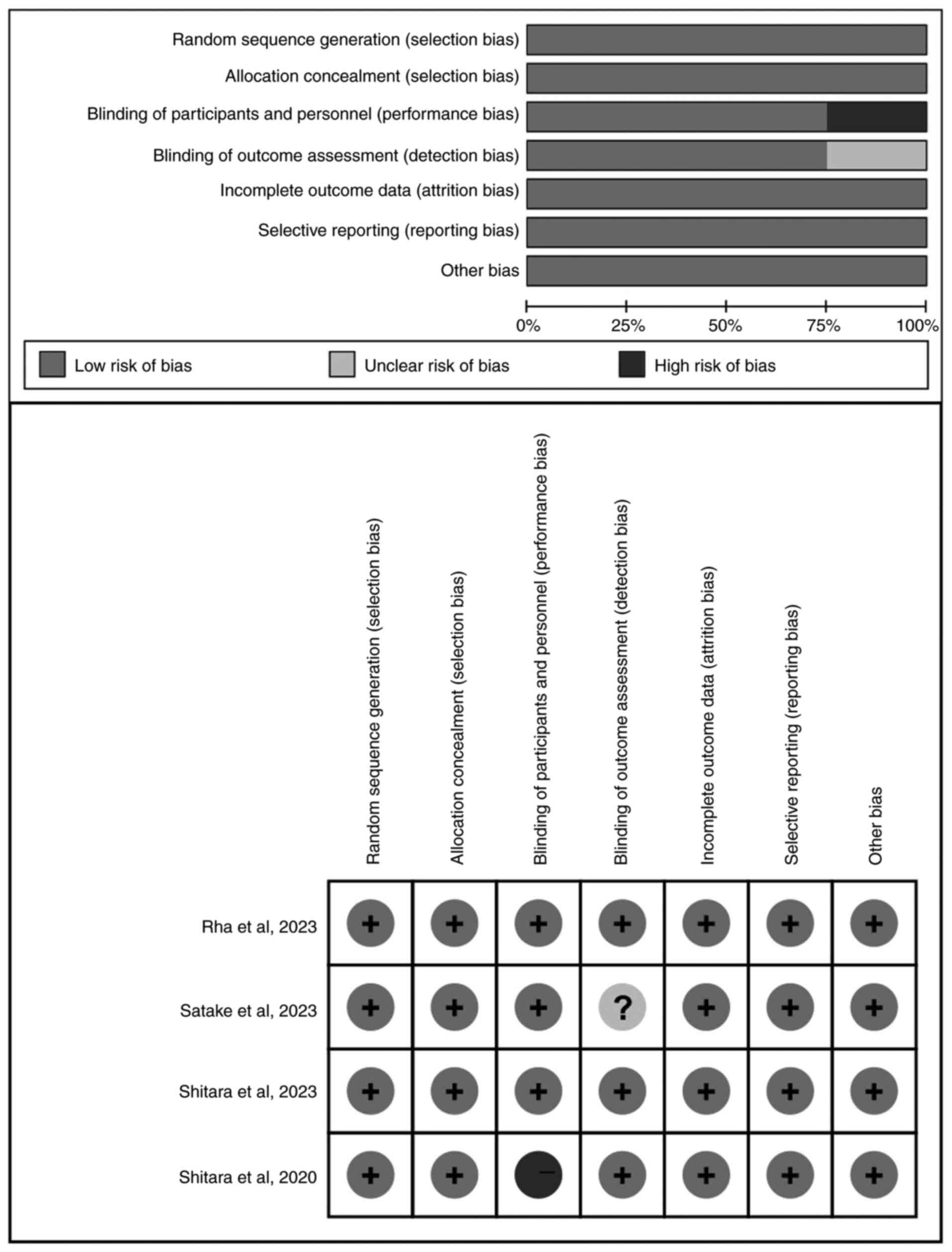
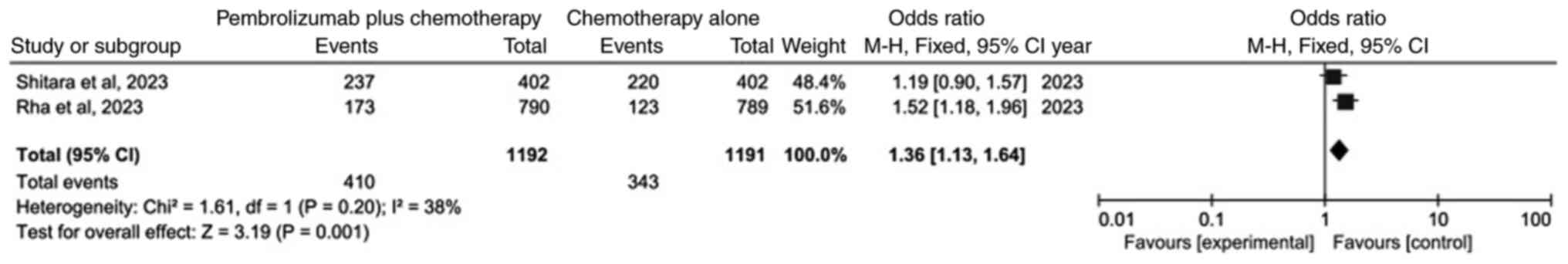
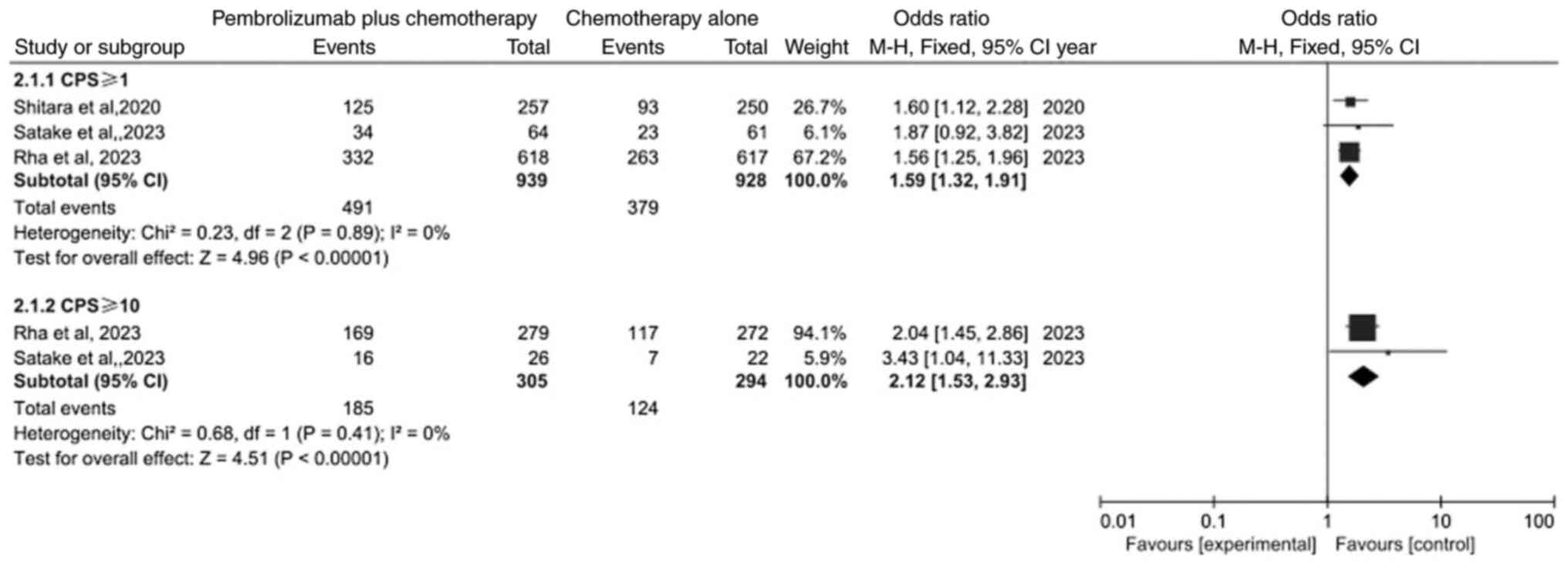
|
1
|
Sung H, Ferlay J, Siegel RL, Laversanne M,
Soerjomataram I, Jemal A and Bray F: Global cancer statistics 2020:
GLOBOCAN estimates of incidence and mortality worldwide for 36
cancers in 185 countries. CA Cancer J Clin. 71:209–249. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Bray F, Ferlay J, Soerjomataram I, Siegel
RL, Torre LA and Jemal A: Global cancer statistics 2018: GLOBOCAN
estimates of incidence and mortality worldwide for 36 cancers in
185 countries. CA Cancer J Clin. 68:394–424. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Li C, Chen D and Yang H: Trends in
incidence, survival and mortality of gastric cancer in the United
States: A population-based study, 2001–2015. Asian Pac J Cancer
Prev. 24:2011–2020. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Li Y, Feng A, Zheng S, Chen C and Lyu J:
Recent estimates and predictions of 5-year survival in patients
with gastric cancer: A model-based period analysis. Cancer Control.
29:107327482210992272022. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Lordick F, Carneiro F, Cascinu S, Fleitas
T, Haustermans K, Piessen G, Vogel A and Smyth EC; ESMO Guidelines
Committee. Electronic address, : simpleclinicalguidelines@esmo.org:
Gastric cancer: ESMO clinical practice guideline for diagnosis,
treatment and follow-up. Ann Oncol. 33:1005–1020. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Francisco LM, Sage PT and Sharpe AH: The
PD-1 pathway in tolerance and autoimmunity. Immunol Rev.
236:219–242. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Pardoll DM: Immunology beats cancer: A
blueprint for successful translation. Nat Immunol. 13:1129–1132.
2012. View
Article : Google Scholar : PubMed/NCBI
|
|
8
|
Geng Y, Wang H, Lu C, Li Q, Xu B, Jiang J
and Wu C: Expression of costimulatory molecules B7-H1, B7-H4 and
Foxp3+ Tregs in gastric cancer and its clinical significance. Int J
Clin Oncol. 20:273–281. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Russo CD, Gagliardi D, Ramlogan R and
Navarra P: Optimizing patient selection to maximize drug efficacy:
The expanding role of pharmacogenomics in the clinical development
of pembrolizumab for the treatment of non-small cell lung cancer.
Clin Ther. 41:982–991. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Langer CJ, Gadgeel SM, Borghaei H,
Papadimitrakopoulou VA, Patnaik A, Powell SF, Gentzler RD, Martins
RG, Stevenson JP, Jalal SI, et al: Carboplatin and pemetrexed with
or without pembrolizumab for advanced, non-squamous non-small-cell
lung cancer: A randomised, phase 2 cohort of the open-label
KEYNOTE-021 study. Lancet Oncol. 17:1497–1508. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Cescon DW, Schmid P, Rugo HS, Im SA, Yusof
MM, Gallardo C, Lipatov O, Barrios CH, Perez-Garcia J, Iwata H, et
al: Health-related quality of life with pembrolizumab plus
chemotherapy vs placebo plus chemotherapy for advanced
triple-negative breast cancer: KEYNOTE-355. J Natl Cancer Inst.
116:717–727. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wakasugi A, Sasaki A, Okamoto R and
Motomura Y: Eldest gastric cancer patient with high microsatellite
instability responding to pembrolizumab. Int Cancer Conf J.
12:59–62. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lin CY, Mehta P, Waters KM, Chang E,
Hendifar A, Osipov A, Burch M, Lin DC, Gangi A, Cho M and Gong J:
Complete response to neoadjuvant pembrolizumab and capecitabine in
microsatellite stable, Epstein-Barr virus-positive, locally
advanced gastric adenocarcinoma: Case report. AME Case Rep.
5:302021. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Fuchs CS, Doi T, Jang RW, Muro K, Satoh T,
Machado M, Sun W, Jalal SI, Shah MA, Metges JP, et al: Safety and
efficacy of pembrolizumab monotherapy in patients with previously
treated advanced gastric and gastroesophageal junction cancer:
Phase 2 clinical KEYNOTE-059 trial. JAMA Oncol. 4:e1800132018.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Shitara K, Özgüroğlu M, Bang YJ, Di
Bartolomeo M, Mandalà M, Ryu MH, Fornaro L, Olesiński T, Caglevic
C, Chung HC, et al: Pembrolizumab versus paclitaxel for previously
treated, advanced gastric or gastro-oesophageal junction cancer
(KEYNOTE-061): A randomised, open-label, controlled, phase 3 trial.
Lancet. 392:123–133. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zitvogel L, Galluzzi L, Smyth MJ and
Kroemer G: Mechanism of action of conventional and targeted
anticancer therapies: Reinstating immunosurveillance. Immunity.
39:74–88. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Higgins JP, Altman DG, Gøtzsche PC, Jüni
P, Moher D, Oxman AD, Savovic J, Schulz KF, Weeks L, Sterne JA, et
al: The cochrane collaboration's tool for assessing risk of bias in
randomised trials. BMJ. 343:d59282011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Shitara K, Van Cutsem E, Bang YJ, Fuchs C,
Wyrwicz L, Lee KW, Kudaba I, Garrido M, Chung HC, Lee J, et al:
Efficacy and safety of pembrolizumab or pembrolizumab plus
chemotherapy vs chemotherapy alone for patients with first-line,
advanced gastric cancer: The KEYNOTE-062 phase 3 randomized
clinical trial. JAMA Oncol. 6:1571–1580. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Satake H, Lee KW, Chung HC, Lee J,
Yamaguchi K, Chen JS, Yoshikawa T, Amagai K, Yeh KH, Goto M, et al:
Pembrolizumab or pembrolizumab plus chemotherapy versus standard of
care chemotherapy in patients with advanced gastric or
gastroesophageal junction adenocarcinoma: Asian subgroup analysis
of KEYNOTE-062. Jpn J Clin Oncol. 53:221–229. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Shitara K, Rha SY, Wyrwicz LS, Oshima T,
Karaseva N, Osipov M, Yasui H, Yabusaki H, Afanasyev S, Park YK, et
al: Neoadjuvant and adjuvant pembrolizumab plus chemotherapy in
locally advanced gastric or gastro-oesophageal cancer
(KEYNOTE-585): An interim analysis of the multicentre,
double-blind, randomised phase 3 study. Lancet Oncol. 25:212–224.
2024. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Rha SY, Oh DY, Yañez P, Bai Y, Ryu MH, Lee
J, Rivera F, Alves GV, Garrido M, Shiu KK, et al: Pembrolizumab
plus chemotherapy versus placebo plus chemotherapy for
HER2-negative advanced gastric cancer (KEYNOTE-859): A multicentre,
randomised, double-blind, phase 3 trial. Lancet Oncol.
24:1181–1195. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Gong J, Chehrazi-Raffle A, Reddi S and
Salgia R: Development of PD-1 and PD-L1 inhibitors as a form of
cancer immunotherapy: A comprehensive review of registration trials
and future considerations. J Immunother Cancer. 6:82018. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Lim SH, Lee KW, Kim JJ, Im HS, Kim IH, Han
HS, Koo DH, Cho JH, Maeng CH, Lee MY, et al: Real-world outcomes of
third-line immune checkpoint inhibitors versus irinotecan-based
chemotherapy in patients with advanced gastric cancer: A Korean,
multicenter study (KCSG ST22-06). BMC Cancer. 24:2522024.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Lévêque I and Spitzer E: Pembrolizumab
with trastuzumab and chemotherapy in advanced or metastatic gastric
or gastroesophageal junction adenocarcinomas with surexpression of
HER2 and CPS ≥1. Bull Cancer. 111:130–132. 2024.(In French).
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Li S, Bao J, Li X, Yang Q, Xu J, Chen S,
Feng G, Gao C, Feng L, Lu B, et al: Multicenter phase I dose
escalation and expansion study of pyrotinib in combination with
camrelizumab and chemotherapy as first-line treatment for
HER2-positive advanced gastric and gastroesophageal junction
adenocarcinoma. EClinicalMedicine. 66:1023142023. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zhu Y, Liu K, Zhu H and Wu H: Immune
checkpoint inhibitors plus chemotherapy for HER2-negative advanced
gastric/gastroesophageal junction cancer: A cost-effectiveness
analysis. Therap Adv Gastroenterol. 16:175628482312072002023.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Robert C, Long GV, Brady B, Dutriaux C,
Maio M, Mortier L, Hassel JC, Rutkowski P, McNeil C,
Kalinka-Warzocha E, et al: Nivolumab in previously untreated
melanoma without BRAF mutation. N Engl J Med. 372:320–330. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Hellmann MD, Paz-Ares L, Caro RB, Zurawski
B, Kim SW, Costa EC, Park K, Alexandru A, Lupinacci L, de la Mora
Jimenez E, et al: Nivolumab plus ipilimumab in advanced
non-small-cell lung cancer. N Engl J Med. 381:2020–2031. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Gong S, Li Q, Yu X and Yang S: Efficacy
and safety of different immunotherapies combined with chemotherapy
as first-line therapy in patients with small cell lung cancer: A
network meta-analysis. Front Immunol. 15:13625372024. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Li Y, Liang X, Li H and Chen X: Efficacy
and safety of immune checkpoint inhibitors for advanced non-small
cell lung cancer with or without PD-L1 selection: A systematic
review and network meta-analysis. Chin Med J (Engl). 136:2156–2165.
2023. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Abderhalden LA, Wu P, Amonkar MM, Lang BM,
Shah S, Jin F, Frederickson AM and Mojebi A: Clinical outcomes for
previously treated patients with advanced gastric or
gastroesophageal junction cancer: A systematic literature review
and meta-analysis. J Gastrointest Cancer. 54:1031–1045. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Reck M, Rodríguez-Abreu D, Robinson AG,
Hui R, Csőszi T, Fülöp A, Gottfried M, Peled N, Tafreshi A, Cuffe
S, et al: Pembrolizumab versus chemotherapy for PD-L1-positive
non-small-cell lung cancer. N Engl J Med. 375:1823–1833. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Fuchs CS, Özgüroğlu M, Bang YJ, Di
Bartolomeo M, Mandala M, Ryu MH, Fornaro L, Olesinski T, Caglevic
C, Chung HC, et al: Pembrolizumab versus paclitaxel for previously
treated PD-L1-positive advanced gastric or gastroesophageal
junction cancer: 2-year update of the randomized phase 3
KEYNOTE-061 trial. Gastric Cancer. 25:197–206. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Bang YJ, Kang YK, Catenacci DV, Muro K,
Fuchs CS, Geva R, Hara H, Golan T, Garrido M, Jalal SI, et al:
Pembrolizumab alone or in combination with chemotherapy as
first-line therapy for patients with advanced gastric or
gastroesophageal junction adenocarcinoma: Results from the phase II
nonrandomized KEYNOTE-059 study. Gastric Cancer. 22:828–837. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
González FS, Palacios CA, Gordón AM,
Gallego JM, Díaz A and Ferrer GM: Delayed immune-related hepatitis
after 24 months of pembrolizumab treatment: A case report and
literature review. Anticancer Drugs. 35:284–287. 2024. View Article : Google Scholar
|
|
36
|
Aggarwal S: Adverse effects of
immuno-oncology drugs-Awareness, diagnosis, and management: A
literature review of immune-mediated adverse events. Indian J
Cancer. 56 (Suppl):S10–S22. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Urwyler P, Earnshaw I, Bermudez M, Perucha
E, Wu W, Ryan S, Mcdonald L, Karagiannis SN, Taams LS, Powell N, et
al: Mechanisms of checkpoint inhibition-induced adverse events.
Clin Exp Immunol. 200:141–154. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Yeung C, Relke N, Good D, Satkunam N and
Mates M: Antithymocyte globulin for aplastic anemia secondary to
pembrolizumab: A case report and review of literature.
Immunotherapy. 15:323–333. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Huntley RE, DeNiro K, Yousef J, Sheedy M
and Dillon JK: Severe mucositis secondary to pembrolizumab: Reports
of two cases, review of the literature, and an algorithm for
management. J Oral Maxillofac Surg. 79:1262–1269. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Kejamurthy P and Devi KTR: Immune
checkpoint inhibitors and cancer immunotherapy by aptamers: An
overview. Med Oncol. 41:402023. View Article : Google Scholar : PubMed/NCBI
|