Introduction
Osteosarcoma (OS) is one of the most common primary
bone malignancies; in 2003–2007, for most countries, OS represented
20–40% of all bone cancers) (1). OS
is characterized by its aggressiveness and high metastatic
potential, coupled with rapid rates of progression and
chemoresistance (2). Due to
developments in novel resection surgical techniques and multidrug
adjuvant chemotherapeutic methods, the 5-year survival rates have
improved to 70–80% by the mid-1980s (3). However, the 5-year survival rate of
patients showing chemoresistance is markedly lower at <20%;
patients with OS may demonstrate superior outcomes with additional
therapies, including small molecule targeted agents (such as
endothelin-1) (4), but these
strategies are not making breakthroughs in clinical trials because
of the complex biology of OS (5,6).
Therefore, novel therapeutic approaches for OS, especially those
with complex gene regulatory networks, are needed.
Over the past decades, new classes of non-coding
RNAs (ncRNAs) have been discovered, including circular RNAs
(circRNAs), microRNAs (miRNAs) and long non-coding RNAs (lncRNAs)
(3–5). During this time period, different
regulatory functions associated with ncRNAs have been revealed. In
particular, ncRNAs have been reported to effectively regulate
essential protein effectors of cellular function that are important
for the development of OS (6–9).
Knockout of MALAT1 can reduce the expression of RhoA, cell nuclear
antigen and vascular growth factor (angiomotin), thereby inhibiting
the proliferation and invasion of human OS cells and inhibiting
their metastasis (10). Amongst the
various known ncRNA families, circRNAs are conserved types of
special RNAs with covalent closed-loop properties, rendering them
highly stable and more resistant to degradation by endonucleases
(11). CircRNAs are widely found in
various types of tissue and organs such as circRNA ZKSCAN1 in
non-small cell lung cancer, circRNA CAMSAP1 in colorectal cancer,
circRNA FBXW4 in trophoblast cell and circRNA MAN1A2 in esophageal
squamous cell carcinoma (12).
Circular RNAs may function similarly to regulate the activity of
other miRNAs. circular RNAs may bind and sequester RNA-binding
proteins or even base pair with RNAs besides microRNAs, resulting
in the formation of large RNA-protein complexes (13). Previous studies have reported that
circRNAs participate widely in disease development such as type2
diabetes, hypertension, spondyloarthropathies, osteoarthritis and
serve important roles in cancer, such as thyroid and cervical
cancer (14,15). In addition, circRNAs have been
reported contribute to the onset and development of OS by
controlling proliferation, invasive and metastatic ability, in
addition to apoptosis, tumor cell metabolism and drug resistance
(16–19). Therefore, understanding the function
of circRNAs is of importance for elucidating the molecular
mechanism underlying the development of OS whilst overcoming its
chemoresistance potential.
In the present study, the differential expression of
hsa_circ_0064636 in OS was assessed and its regulatory mechanism
was evaluated using bioinformatic methods to determine its
expression profile and prognostic significance. The aim was to
provide a novel experimental basis and direction for future studies
on molecular markers of OS.
Materials and methods
Materials and equipment
Human normal osteoblast (hFOB1.19) and OS (HOS,
SJSA-1 and MG63) cell lines were purchased from Shenzhen Haodi
Huatuo Biotechnology Co., Ltd. TRIzol reagent was purchased from
Invitrogen; Thermo Fisher Scientific, Inc. PrimeScript™ RT reagent
Kit with gDNA Eraser and TB Green® Fast qPCR Mix were
purchased from Takara Bio, Inc. A LightCycler® 96
real-time quantitative PCR instrument was purchased from Roche
Diagnostics.
Cell culture
hFOB1.19 cells were grown in DMEM/F12 supplemented
with 10% FBS with the addition of geneticin (0.3 mg/ml; all Thermo
Fisher Scientific, Inc.), penicillin (100 U/ml) and streptomycin
(100 U/ml). By contrast, the OS cell lines were grown in DMEM/F12
medium supplemented with 10% FBS, streptomycin (100 U/ml) and
penicillin (100 U/ml). All cells were incubated at 37°C in a
humidified atmosphere with 5% CO2. Medium was replaced
with fresh complete medium every 2–3 days for further incubation
and when the cell density reached >90%, before the cells were
passaged at a ratio of 1:3.
RNA extraction and reverse
transcription-quantitative (RT-q)PCR
Total cellular RNA was extracted using TRIzol and
cDNA was synthesized using the miRNA First Strand cDNA Synthesis
Kit (Sangon Biotech Co., Ltd.) at 25°C for 5 min, 42°C for 30 min,
85°C for 5 min. qPCR was performed using the SYBR green PCR mix (TB
Green® Fast qPCR Mix) and divergent primers from
hsa_circ_0064636 were used with reaction conditions as follows:
Pre-denaturation at 95°C for 10 min, followed by denaturation at
95°C for 15 sec and annealing at 60°C for 30 sec for 45 cycles. The
expression levels of GAPDH (circRNA) and U6 (miRNA) were used as
internal controls. The relative expression of the screened genes
was calculated using the 2−ΔΔCq method (20). The primers used in the present study
are presented in Table I; primer
sequence for U6 is not available.
 | Table I.Primer sequences used for reverse
transcription-quantitative PCR. |
Table I.
Primer sequences used for reverse
transcription-quantitative PCR.
| Gene | Sequence
(5′-3′) |
|---|
|
Hsa_circ_0064636 | F:
GCTTCCCCTGTCTCCACATA |
|
| R:
ATGTCCAAAGGGTTTCAGCA |
| GAPDH | F:
CCACTCCTCCACCTTTGAC |
|
| R:
ACCCTGTTGCTGTAGCCA |
| Ubiquitination | F:
TCCAGAGAACCTGCTACCCT |
| factor E4A | R:
AGTTACATCTTCAAAATGGG |
|
| CTCC |
| Voltage
dependent | F:
GGAAGGCAGAAGATGGCTGT |
| anion channel
1 | R:
GTCCACGTGCAAGCTGATCT |
Construction of the circRNA/miRNA/mRNA
network
miRNAs interacting with hsa_circ_0064636 were
predicted using the circRNA target miRNA prediction tools RNAhybrid
(21), TargetScan (22) and miRanda (23) before being subjected to intersection
analysis to screen out miRNAs that were commonly identified by the
three prediction tools. For miRNA targeting, gene prediction tools,
including RNAhybrid, TargetScan, miRanda, miRWalk (24), miRMap (25) and miRNAMap (26) were used to predict target genes for
the intersecting miRNAs. These were compared with the screened
miRNAs, before seven (six database prediction ensembles and a
variance analysis) intersects from the differentially expressed
miRNAs were finally identified. The selected circRNA-miRNAs and
miRNA-mRNAs were then used to construct and visualize the
regulatory network of circRNA/miRNA/mRNA using Cytoscape 3.8.0
(cytoscape.org/). Venn diagrams of predicted miRNAs or target genes
were plotted using the R (version 4.2.3) package ‘Venn’ (version
1.11).
Analysis of the differential
expression of miRNAs
The GSE65071 (9 normal samples from healthy
individuals, 14 OS samples) and GSE16088 (9 normal samples, 14 OS
samples) expression datasets and RNA-seq ere downloaded from the
Gene Expression Omnibus (GEO; http://www.ncbi.nlm.nih.gov/geo/) database and the
‘affy’ (to read gene expression microarray data from affymetix)
package (version 3.18) was used. The ‘Limma’ (version
3.54.2).package was used to identify differentially expressed genes
between normal and tumor samples with log2[fold change
(FC)]>1 and adjusted P<0.05 set as the significance criteria.
The differentially expressed genes were plotted using the ‘ggplot2’
(version 3.4.3) package and a volcano plot was produced.
Survival analysis
PROGgeneV2 (progtools.net/gene/filter.php) (27) was used along with aforementioned GEO
data to investigate the prognostic significance of genes. The
target genes obtained from the aforementioned intersection) were
input into a PROGgeneV2 before the dataset for OS was selected and
the overall survival map was constructed based on the median
expression of a given gene for classification into high and low
expression groups for plotting Kaplan-Meier curves with PROGgeneV2.
‘PROGeneV2’ was used for hypothesis testing using the ‘Suvival’
(version 3.5.3) package in R (version 4.2.3). Statistical analysis
was performed using the log-rank test and the threshold for a
meaningful survival prognosis was set as P<0.05. Based on the
survival analysis, a new circRNA/miRNA/mRNA visual regulatory
sub-network was obtained using Cytoscape (cytoscape.org/)
3.8.0.
Statistical analysis
GraphPad Prism 8 (Dotmatics) software was used to
statistically analyze the experimental data. The test data were
normally distributed. Data are presented as the means ± SD of three
independent experiments. P<0.05 was considered to indicate a
statistically significant difference. Statistical comparison
between groups was evaluated using one-way ANOVAwith the Dunnett's
post hoc test.
Results
Analysis of differentially expressed
miRNAs and mRNAs in OS
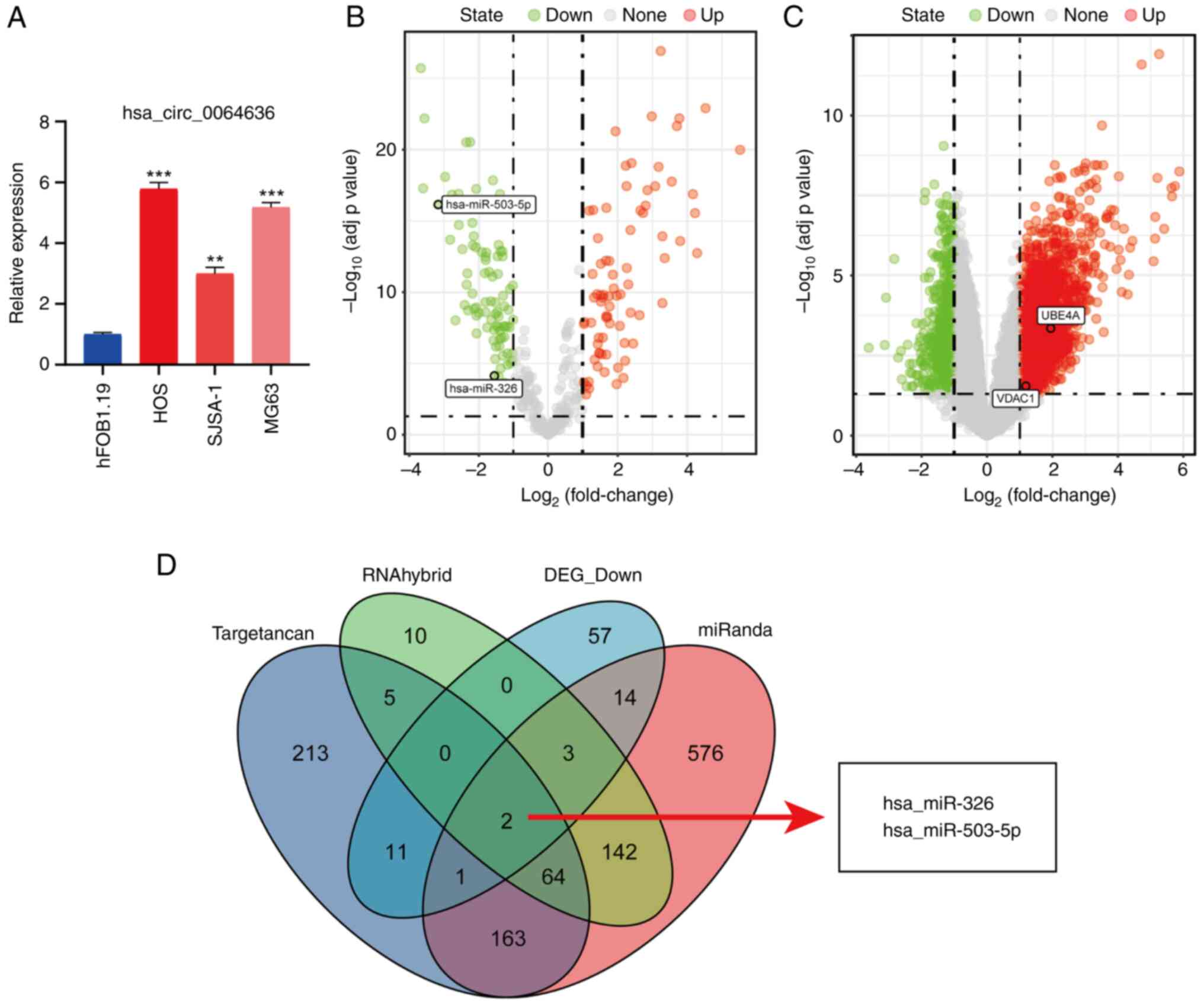
The expression of hsa_circ_0064636 was found to be
significantly higher in the three human OS cell lines compared with
that in the hFOB1.19 osteoblasts (Fig.
1A). A total of 114 miRNAs were shown to be upregulated, whilst
117 were downregulated in OS (compared with normal group) based on
the miRNA (GSE65071) expression profile data. Differentially
expressed miRNAs were visualized using a volcano plot (Fig. 1B). Differential analysis of the mRNA
expression (GSE16088) spectrum data identified 716 mRNAs that were
downregulated and 1,924 mRNAs that were upregulated(OS versus
norma). Differentially expressed mRNAs were visualized using a
volcano plot (Fig. 1C).
Hsa_circ_006463 targets the predicted
miRNA
To assess the regulatory mechanism of
hsa_circ_006463 in OS, miRNAs targeted by hsa_circ_006463 were
predicted using three databases. TargetScan, miRanda and RNAhybrid
predicted 498, 965 and 226 target miRNAs, respectively.
Differential expression analysis yielded 88 target (downregulated)
miRNAs. Intersecting miRNAs targeted by hsa_circ_006463 according
to all four different databases used were found to be miR-326 and
miR-503-5p (Fig. 1D).
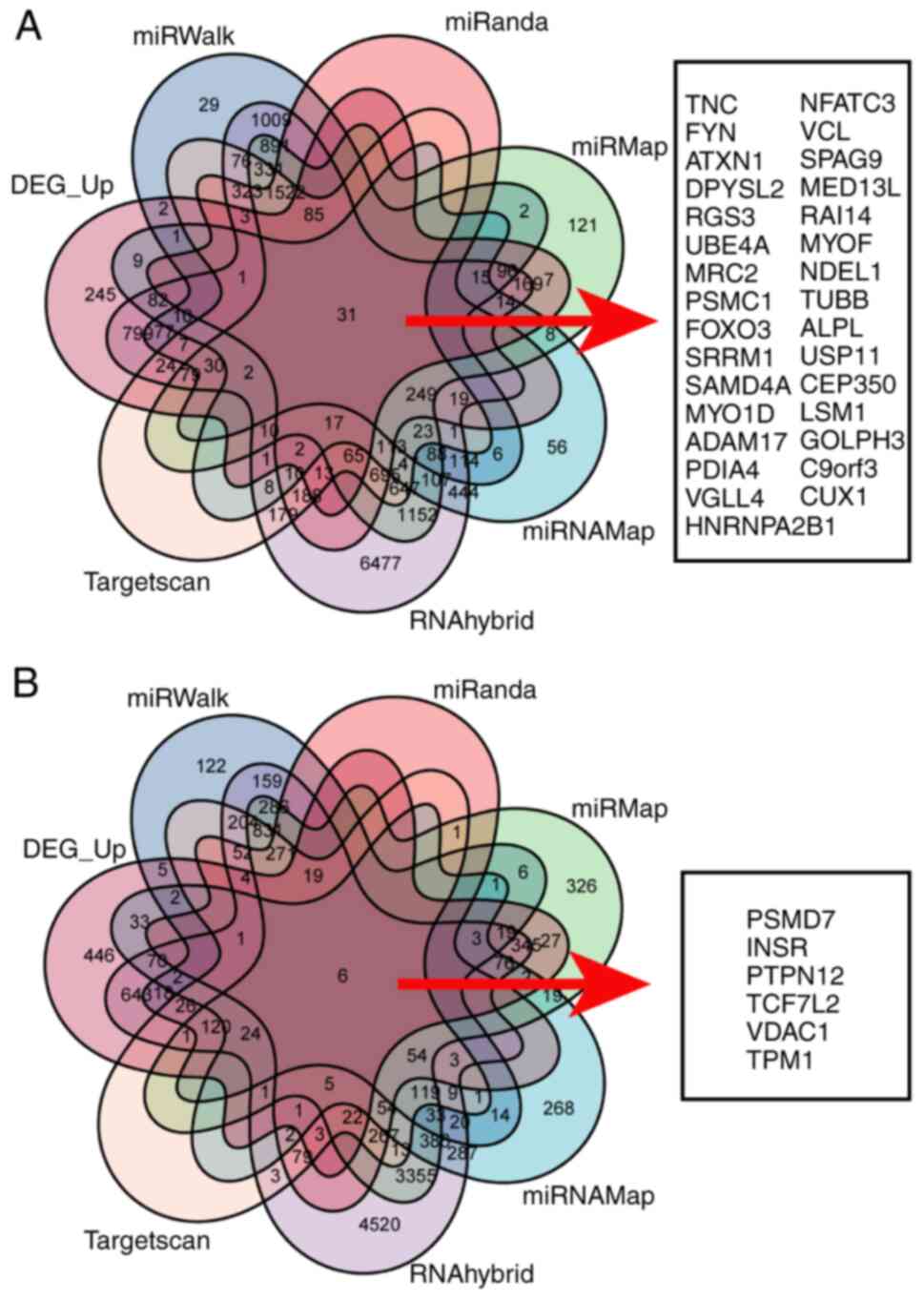
Prediction and analysis of miR-326 and
miR-503-5p targets
The regulatory action of hsa-miR-326 and miR-503-5p
in OS was then assessed based on the possible binding of target
mRNAs. miR-326 target genes were predicted using the miRWalk,
miRanda, miRMap, miRNAMap, RNAhybrid and TargetScan databases, with
5,071, 3,364, 6,616, 6,716, 16,373 and 4,830 target genes
identified, respectively. Intersection with 1,924 upregulated
differential genes identified 31 target genes using Venn diagram
analysis (Fig. 2A). Target genes of
miR-503-5p were also analyzed using the miRWalk, miRanda, miRMap,
miRNAMap, RNAhybrid and TargetScan, with 2,034, 844, 6,692, 1,496,
12,416 and 2,196 target genes identified, respectively. These were
subjected to intersection analysis with 1,924 differentially
upregulated genes and 31 target genes were identified (Fig. 2B).
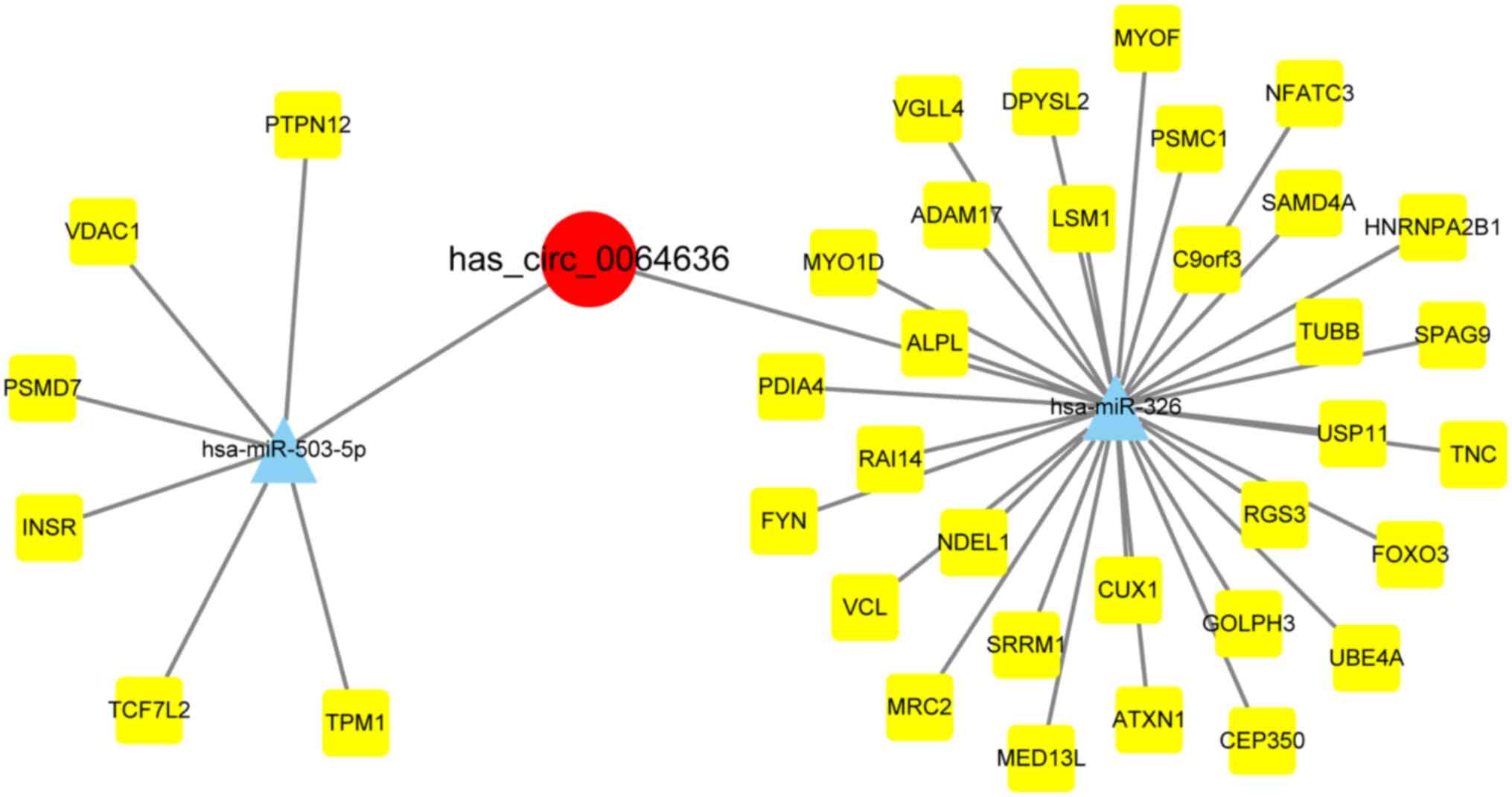
Construction of the circRNA/miRNA/mRNA
network
In total, six databases were used to predict binding
targets of miR-326 and miR-503-5p, yielded 31 and 6 target gene
relationships, respectively. These were used to construct
relationship networks (Fig. 3).
Hsa_circ_0064636 was therefore predicted to target miR-326 and
miR-503-5p. The potential target genes of miR-326 included TNC,
HNRNPA2B1, ATXN1, DPYSL2, RGS3, ubiquitination factor E4A (UBE4A),
MRC2, PSMC1, FOXO3, SRRM1, SAMD4A, MYO1D, ADAM17, PDIA4, VGLL4,
FYN, NFATC3, VCL, SPAG9, MED13L, RAI14, MYOF, NDEL1, TUBB, ALPL,
USP11, CEP350, LSM1, GOLPH3, C9orf3 and CUX1 (31 target genes). By
contrast, miR-503-5p target genes were PSMD7, INSR, PTPN12, TCF7L2,
voltage dependent anion channel 1 (VDAC1) and TPM1 (6 target
genes).
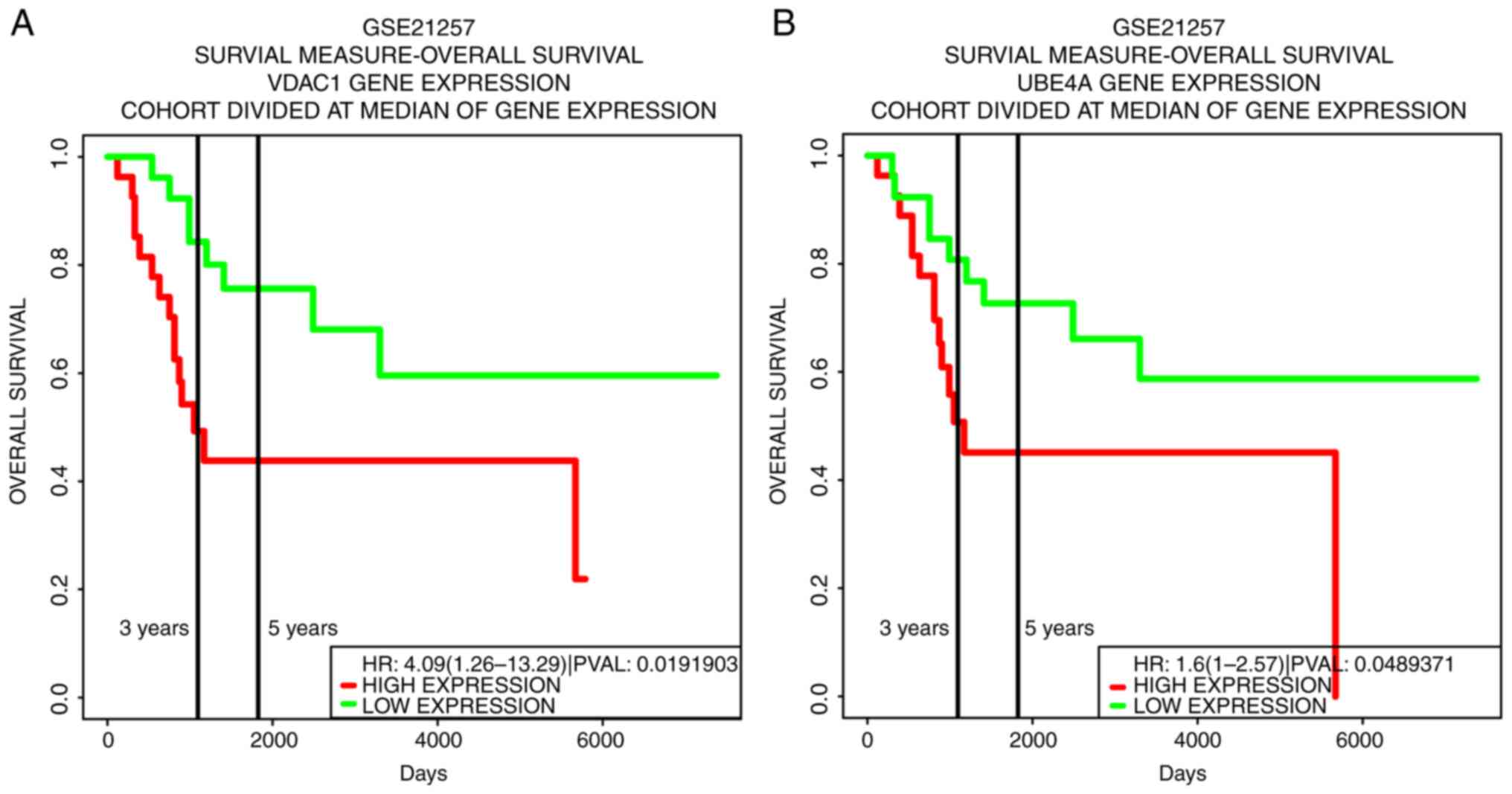
Survival analysis
Survival curves for overall survival were plotted
for 31 of the 37 target genes, as shown in Table II. For survival analysis of the
target genes, only two differences were found significant, namely
UBE4A and VDAC1 (Fig. 4). This
implies that patients with osteosarcoma have poorer prognostic
survival when UBE4A and VDAC1 are highly expressed. According to
Fig. 2, UBE4A was a target gene of
miR-326 whereas VDAC1 was a target gene of miR-503-5p.
 | Table II.Target gene survival analysis. |
Table II.
Target gene survival analysis.
| Gene | miRNA | Hazard ratio | LCI (95%) | UCI (95%) | P-value |
|---|
| TNC | hsa-miR-326 | 0.80 | 0.59 | 1.09 | 0.16087 |
| ATXN1 | hsa-miR-326 | 1.06 | 0.45 | 2.51 | 0.89614 |
| DPYSL2 | hsa-miR-326 | 0.72 | 0.31 | 1.66 | 0.44617 |
| RGS3 | hsa-miR-326 | 0.59 | 0.21 | 1.61 | 0.30120 |
| UBE4A | hsa-miR-326 | 1.60 | 1.00 | 2.57 | 0.04894 |
| MRC2 | hsa-miR-326 | 0.81 | 0.38 | 1.73 | 0.59453 |
| PSMC1 | hsa-miR-326 | 0.38 | 0.10 | 1.40 | 0.14712 |
| SRRM1 | hsa-miR-326 | 1.11 | 0.35 | 3.51 | 0.85323 |
| SAMD4A | hsa-miR-326 | 0.75 | 0.25 | 2.28 | 0.61097 |
| MYO1D | hsa-miR-326 | 0.76 | 0.4 | 1.45 | 0.40852 |
| ADAM17 | hsa-miR-326 | 0.4 | 0.06 | 2.86 | 0.36201 |
| PDIA4 | hsa-miR-326 | 0.81 | 0.38 | 1.74 | 0.58828 |
| VGLL4 | hsa-miR-326 | 1.03 | 0.46 | 2.27 | 0.95088 |
| FYN | hsa-miR-326 | 1.66 | 0.7 | 3.95 | 0.25158 |
| NFATC3 | hsa-miR-326 | 4.94 | 0.76 | 32.03 | 0.09380 |
| VCL | hsa-miR-326 | 0.60 | 0.29 | 1.24 | 0.16888 |
| SPAG9 | hsa-miR-326 | 0.45 | 0.18 | 1.17 | 0.10087 |
| RAI14 | hsa-miR-326 | 0.33 | 0.16 | 0.68 | 0.00261 |
| NDEL1 | hsa-miR-326 | 0.81 | 0.35 | 1.9 | 0.62756 |
| TUBB | hsa-miR-326 | 1.59 | 0.92 | 2.78 | 0.09927 |
| ALPL | hsa-miR-326 | 1.07 | 0.83 | 1.39 | 0.59196 |
| USP11 | hsa-miR-326 | 0.5 | 0.18 | 1.38 | 0.18324 |
| CEP350 | hsa-miR-326 | 0.88 | 0.35 | 2.21 | 0.78379 |
| LSM1 | hsa-miR-326 | 1.58 | 0.78 | 3.22 | 0.20742 |
| GOLPH3 | hsa-miR-326 | 1.54 | 0.94 | 2.5 | 0.08360 |
| PSMD7 | hsa-miR-503-5p | 0.70 | 0.32 | 1.54 | 0.37880 |
| INSR | hsa-miR-503-5p | 0.13 | 0.01 | 1.79 | 0.12555 |
| PTPN12 | hsa-miR-503-5p | 1.62 | 0.89 | 2.94 | 0.11362 |
| TCF7L2 | hsa-miR-503-5p | 3.99 | 0.79 | 20.11 | 0.09396 |
| VDAC1 | hsa-miR-503-5p | 4.09 | 1.26 | 13.29 | 0.01919 |
| TPM1 | hsa-miR-503-5p | 0.73 | 0.47 | 1.16 | 0.185767 |
| HNRNPA2B1 | hsa-miR-326 | - | - | - | - |
| FOXO3 | hsa-miR-326 | - | - | - | - |
| MYOF | hsa-miR-326 | - | - | - | - |
| C9orf3 | hsa-miR-326 | - | - | - | - |
| CUX1 | hsa-miR-326 | - | - | - | - |
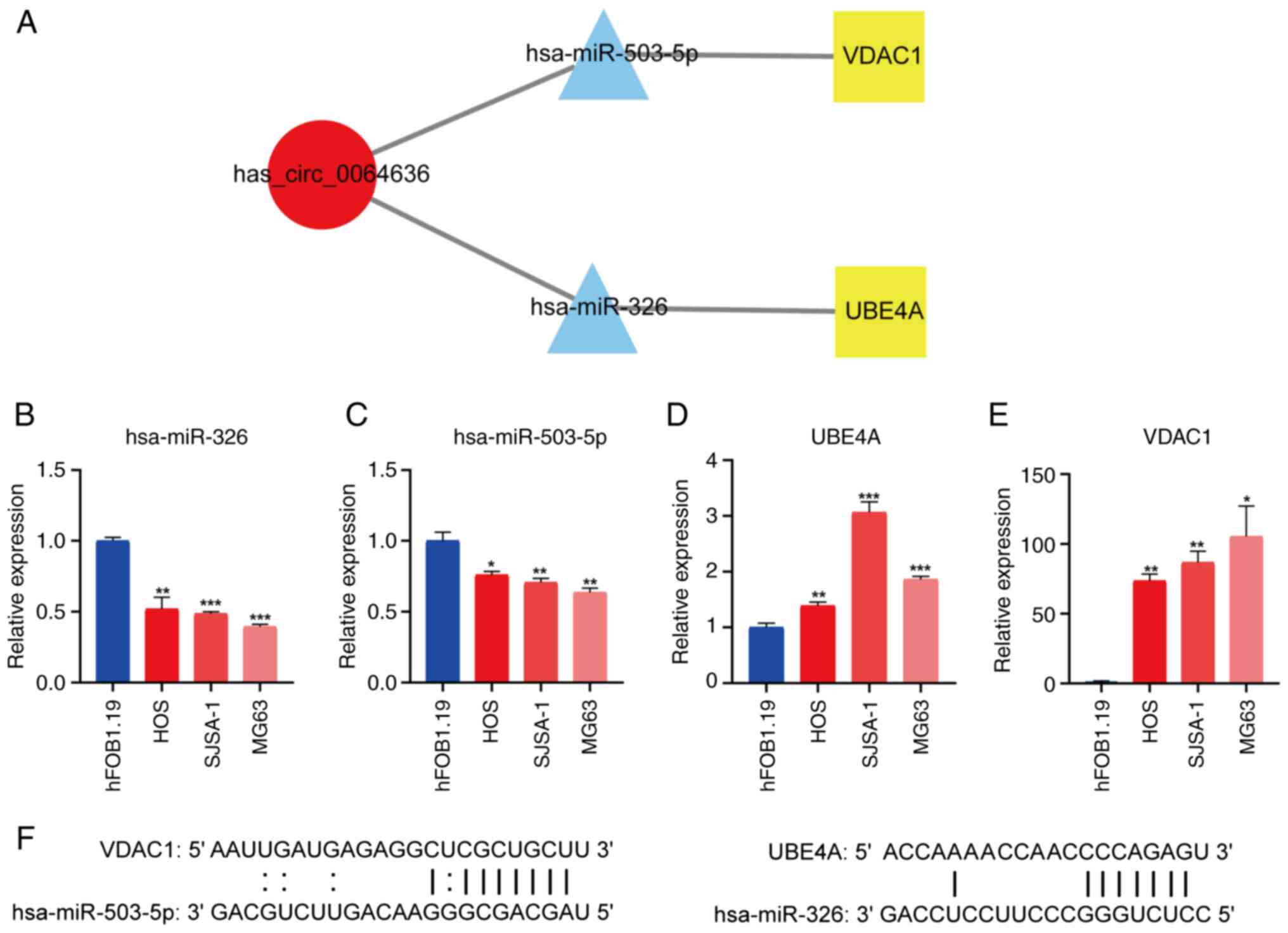
CircRNA/miRNA/mRNA network has
prognostic significance in OS
Based on the original circRNA/miRNA/mRNA competing
endogenous RNA interaction network, though RAI14 was significant in
the survival analysis, RAI14 is highly expressed in osteosarcoma
compared with normal group, but survival analysis of RAI14 low
expression group has better survival prognosis). Thus only two
target genes with prognostic significance for survival (UBE4A and
VDAC1) were retained before a new sub-network with prognostic
implications was created (Fig.
5A).
Validation of differential miRNA and
mRNA expression in OS cell lines
Differential expression of hsa_circ_0064636 was next
assessed using RT-qPCR in human (HOS, SJSA-1, MG63) OS cell lines
and the human osteoblast (hFOB1.19) cell line. The expression of
hsa_circ_0064636 was found to be significantly higher in the human
OS cell line compared with that in the osteoblast cell line
(Fig. 1A). The expression levels of
hsa-miR-326 (Fig. 5B) and
hsa-miR-503 (Fig. 5C) were
significantly lower in OS cell lines compared with those in the
osteoblast cell line. UBE4A (Fig.
5D) and VDAC1 (Fig. 5E)
expression were found to be significantly increased in the OS cell
lines compared with that in the human osteoblast cell line. A
schematic representation of the target gene and corresponding miRNA
binding site are presented in Fig.
5F.
Discussion
OS is one of the most common malignancies in the
bone, Treatment of OS remains a major challenge. Existing treatment
options for OS include as surgery and chemotherapy, but the
prognosis of patients remains unsatisfactory (28). Although four genes [BRCA1, mutS
homolog 2, CCND1 (cyclin D1) and ITGA5 (integrin subunit alpha 5)]
have been documented to associate with the development and
progression of OS, the focus has been only on protein-coding genes
or miRNAs (29). In addition,
molecular mechanisms underlying the development and progression of
OS remain poorly understood. A number of ncRNAs have been reported
to be important in the development of OS (30–33).
The lncRNA TMPO-AS1 was previously found to decreases
miR-199a-5p/WNT7B(elevated) axis, thereby functioning as a ceRNA
that promotes tumorigenesis in OS (34). In addition, miR-1236-3p
overexpression inhibits cell proliferation and induces apoptosis by
targeting Krueppel-like factor 8 (35).
Unlike other ncRNAs, including lncRNAs and miRNAs,
circRNAs are highly conserved and stable in mammalian cells. These
properties render them potentially ideal biomarkers and therapeutic
targets (36). Previous studies
have reported the important role of circRNAs in different
processes, including tumorigenesis, development and metastasis, of
tumors such as gastric (37),
colorectal (38), lung (39) and cervical (40) cancer. Circ-transcriptional Adaptor
2A was previously found to be differentially expressed between OS
cell lines (HOS, 143B,U2OS,SJSA-1,MG63) and corresponding
non-cancer cell lines (HEK-293 and hFOB1.19), with higher
expression in OS (41). In the
present study, the significantly upregulated expression of
hsa_circ_0064636 in OS cell lines compared with normal tissue cell
lines was found before a regulatory network of its miRNA targets
and downstream mRNA targets was constructed.
In the present study, miR-326 and miR-503-5p were
predicted to be the target miRNAs of hsa_circ_0064636 using
multiple databases, yielding circRNA/miRNA interactions. miR-326
and miR-503-5 were identified from the GSE65071 dataset and were
downregulated in OS samples compared with normal samples, before
their expression in OS samples was verified. Here, hsa_circ_0064636
was found to be significantly upregulated in OS, miR-326 to promote
cervical cancer progression through up-regulation of ELK1 (42). The overexpression of miR-326 can
lead to the inhibition of proliferation and invasion, whilst
inducing apoptosis and autophagy in cervical cancer cells (42). In addition, miR-326 expression was
previously found to be significantly downregulated in prostate
cancer (43), which associated with
the prognoses of these patients. By contrast, miR-503-5p has been
reported to inhibit tumorigenesis, angiogenesis and
lymphangiogenesis in colon cancer through the direct inhbition of
VEGF-A expression (44). In
hepatocellular carcinoma, miR-503-5p was documented to regulate
epithelial-to-mesenchymal transition, metastasis and prognosis
through the inhibition of WEE1 (45). miR-326 and miR-503-5p are key for
the suppression of numerous tumors such as prostatic carcinoma and
colon cancer (44,46), where their expression is low in
certain cancers such as miR-326 is low expressed in lung
adenocarcinoma and miR-503-5p is low expressed in colon cancer.
Therefore, hsa_circ_0064636 may promote OS development by
suppressing the expression of miR-326 and miR-503-5p. To the best
of our knowledge, studies on miR-326 and miR-503-5p in OS are
lacking.
The present study performed miRNA prediction using
miRWalk, miRanda, miRMap, miRNAMap, RNAhybrid and TargetScan.
Significantly differentially expressed genes in OS were used to
screen for potential target genes of miR-326 and miR-503-5p to
obtain a circRNA/miRNA/mRNA ceRNA interaction network.
Subsequently, mRNAs with prognostic significance were also screened
to obtain a circRNA/miRNA/mRNA regulatory sub-network. UBE4A was
identified to be a potential direct target of miR-326 whereas VDAC1
was found to be a potential direct target of miR-503-5p. A
significant difference in prognosis between UBE4A and VDAC1 was
confirmed in a survival analysis of OS patients.. The results of
survival analysis showed that the prognosis of patients was
significantly worse in the high UBE4A and VDAC1 expression group
compared with that in the low expression group.
UBE4A belongs to the U-box ubiquitin ligase class of
enzymes. The encoded protein is involved in polyubiquitin chain
assembly and regulates chromosome condensation and
polyubiquitination segregation through securing (47). Auto-antibodies against the encoded
protein (recombinant C-terminal UBE4A Protein) are potential
markers for scleroderma and Crohn's disease (47,48).
UBE4A was found to be significantly upregulated in ovarian
plasmatic cystic carcinoma compared with the adjacent normal
tissues. The VDAC1 channel forms a major component of the outer
mitochondrial membrane (49). VDAC1
is expressed in all compartments, including mitochondria, plasma
membrane. This protein regulates major metabolic and energetic
functions in the cell, including Ca2+ homeostasis,
oxidative stress and mitochondria-mediated apoptosis (50,51).
VDAC1 may be associated with the destruction of nerve cells, where
its overexpression triggers cell death (52). Furthermore, VDAC1 was found to be a
tumor promoter in cervical cancer, where its knockdown in cervical
cancer cell line S12 increased the rate of apoptosis in a manner
that was partially reversed by the overexpression of VDAC1
(53).
In the present study, RT-qPCR analysis demonstrated
that hsa_circ_00063636, VDAC1 and UBE4A were highly expressed in OS
cell lines, whereas miR-326 and miR-503-5p expression was
significantly lower in OS cell lines. These results were consistent
with those of the bioinformatics analysis. A limitation of the
present study is that the regulatory network was not validated
experimentally and further investigations are required such as
experimentally verifying miRNA binding to mRNAs. In the present
study, bioinformatic methods are used to predict potential
regulatory networks, with emphasis on discovering new targets and
potential networks. However, further verification of the regulation
between miRNA and mRNA is required.
In conclusion, a sub-regulatory network of
hsa_circ_0064636-miR-326/miR-503-5p-UBE4A/VDAC1 was identified with
significance for survival prognosis. RT-qPCR experiments
demonstrated that hsa_circ_0064636, UBE4A and VDAC1 were
significantly and differentially overexpressed in OS cell lines,
whilst miR-326 and miR-503-5p were downregulated. It was
hypothesized that hsa_circ_0064636 may be involved in the
development of OS by acting as a sponge, thereby suppressing
miR-326 and miR-503-5p to facilitate the upregulation of VDAC1 and
UBE4A.
Acknowledgements
Not applicable.
Funding
The present study received financial support from the National
Natural Science Foundation of China (grant no. 81960400).
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
GHY and NCH confirm the authenticity of all the raw
data. GHY and NCH designed the study and drafted the manuscript.
CTC, JWC and HJH interpreted data. JWC and HJH revised the
manuscript. All authors have read and approved the final version of
the manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
GEO
|
Gene Expression Omnibus
|
|
miRNA
|
microRNA
|
|
OS
|
osteosarcoma
|
|
circRNAs
|
circular RNAs
|
|
HR
|
hazard ratio
|
|
lncRNA
|
long non-coding RNA
|
|
RT-qPCR
|
reverse transcription-quantitative
PCR
|
|
VDAC1
|
voltage dependent anion channel 1
|
|
UBE4A
|
ubiquitination factor E4A
|
References
|
1
|
Valery PC, Laversanne M and Bray F: Bone
cancer incidence by morphological subtype: A global assessment.
Cancer Causes Control. 26:1127–1139. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Saraf AJ, Fenger JM and Roberts RD:
Osteosarcoma: Accelerating progress makes for a hopeful future.
Front Oncol. 8:42018. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Chou AJ and Gorlick R: Chemotherapy
resistance in osteosarcoma: Current challenges and future
directions. Expert Rev Anticancer Ther. 6:1075–1085. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Fizazi K, Yang J, Peleg S, Sikes CR,
Kreimann EL, Daliani D, Olive M, Raymond KA, Janus TJ, Logothetis
CJ, et al: Prostate cancer cells-osteoblast interaction shifts
expression of growth/survival-related genes in prostate cancer and
reduces expression of osteoprotegerin in osteoblasts. Clin Cancer
Res. 9:2587–2597. 2003.PubMed/NCBI
|
|
5
|
Otoukesh B, Boddouhi B, Moghtadaei M,
Kaghazian P and Kaghazian M: Novel molecular insights and new
therapeutic strategies in osteosarcoma. Cancer Cell Int.
18:1582018. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Bishop MW, Janeway KA and Gorlick R:
Future directions in the treatment of osteosarcoma. Curr Opin
Pediatr. 28:26–33. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Nemeth K, Bayraktar R, Ferracin M and
Calin GA: Non-coding RNAs in disease: From mechanisms to
therapeutics. Nat Rev Genet. 25:211–232. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wang K, Jiang W, Cheng C, Li Y and Tu M:
Pathological and therapeutic aspects of long noncoding RNAs in
osteosarcoma. Anticancer Agents Med Chem.
13:10.2174/1871520617666170213122442. 2017.
|
|
9
|
Jones KB, Salah Z, Del Mare S, Galasso M,
Gaudio E, Nuovo GJ, Lovat F, LeBlanc K, Palatini J, Randall RL, et
al: miRNA signatures associate with pathogenesis and progression of
osteosarcoma. Cancer Res. 72:1865–1877. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Cai X, Liu Y, Yang W, Xia Y, Yang C, Yang
S and Liu X: Long noncoding RNA MALAT1 as a potential therapeutic
target in osteosarcoma. J Orthop Res. 34:932–941. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Liu CX, Li X, Nan F, Jiang S, Gao X, Guo
SK, Xue W, Cui Y, Dong K, Ding H, et al: Structure and degradation
of circular RNAs regulate PKR activation in innate immunity. Cell.
177:865–880.e21. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Memczak S, Jens M, Elefsinioti A, Torti F,
Krueger J, Rybak A, Maier L, Mackowiak SD, Gregersen LH, Munschauer
M, et al: Circular RNAs are a large class of animal RNAs with
regulatory potency. Nature. 495:333–338. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Das A, Sinha T, Shyamal S and Panda AC:
Emerging role of circular RNA-protein interactions. Noncoding RNA.
7:482021.PubMed/NCBI
|
|
14
|
Wilusz JE and Sharp PA: Molecular biology.
A circuitous route to noncoding RNA. Science. 340:440–441. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Yang F, Chen Y, Xue Z, Lv Y, Shen L, Li K,
Zheng P, Pan P, Feng T, Jin L and Yao Y: High-throughput sequencing
and exploration of the lncRNA-circRNA-miRNA-mRNA network in type 2
diabetes mellitus. Biomed Res Int. 2020:81625242020.PubMed/NCBI
|
|
16
|
Trang NTN, Lai CY, Tsai HC, Huang YL, Liu
SC, Tsai CH, Fong YC, Tzeng HE and Tang CH: Apelin promotes
osteosarcoma metastasis by upregulating PLOD2 expression via the
Hippo signaling pathway and hsa_circ_0000004/miR-1303 axis. Int J
Biol Sci. 19:412–425. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yu L, Zhu H, Wang Z, Huang J, Zhu Y, Fan
G, Wang Y, Chen X and Zhou G: Circular RNA circFIRRE drives
osteosarcoma progression and metastasis through
tumorigenic-angiogenic coupling. Mol Cancer. 21:1672022. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zhang M, Yu GY, Liu G and Liu WD: Circular
RNA circ_0002137 regulated the progression of osteosarcoma through
regulating miR-433-3p/IGF1R axis. J Cell Mol Med. 26:1806–1816.
2022. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Liu Y, Qiu G, Luo Y, Li S, Xu Y, Zhang Y,
Hu J, Li P, Pan H and Wang Y: Circular RNA ROCK1, a novel circRNA,
suppresses osteosarcoma proliferation and migration via altering
the miR-532-5p/PTEN axis. Exp Mol Med. 54:1024–1037. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Rehmsmeier M, Steffen P, Höchsmann M and
Giegerich R: Fast and effective prediction of microRNA/target
duplexes. RNA. 10:1507–1517. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Agarwal V, Bell GW, Nam JW and Bartel DP:
Predicting effective microRNA target sites in mammalian mRNAs.
Elife. 4:e050052015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Riffo-Campos ÁL, Riquelme I and
Brebi-Mieville P: Tools for sequence-based miRNA target prediction:
What to choose? Int J Mol Sci. 17:19872016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Dweep H, Sticht C, Pandey P and Gretz N:
miRWalk-database: Prediction of possible miRNA binding sites by
‘walking’ the genes of three genomes. J Biomed Inform. 44:839–847.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Vejnar CE, Blum M and Zdobnov EM: miRmap
web: Comprehensive microRNA target prediction online. Nucleic Acids
Res. 41:W165–W168. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Hsu SD, Chu CH, Tsou AP, Chen SJ, Chen HC,
Hsu PW, Wong YH, Chen YH, Chen GH and Huang HD: miRNAMap 2.0:
Genomic maps of microRNAs in metazoan genomes. Nucleic Acids Res.
36:D165–D169. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Goswami CP and Nakshatri H: PROGgeneV2:
Enhancements on the existing database. BMC Cancer. 14:9702014.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Gillespie EF, Yang JC, Mathis NJ, Marine
CB, White C, Zhang Z, Barker CA, Kotecha R, McIntosh A, Vaynrub M,
et al: Prophylactic radiation therapy versus standard of care for
patients with high-risk asymptomatic bone metastases: A
multicenter, randomized phase II clinical trial. J Clin Oncol.
42:38–46. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Yang J and Wang N: Analysis of the
molecular mechanism of osteosarcoma using a bioinformatics
approach. Oncol Lett. 12:3075–3080. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Wang J, Liu S, Shi J, Li J, Wang S, Liu H,
Zhao S, Duan K, Pan X and Yi Z: The role of miRNA in the diagnosis,
prognosis, and treatment of osteosarcoma. Cancer Biother
Radiopharm. 34:605–613. 2019.PubMed/NCBI
|
|
31
|
Wang JY, Yang Y, Ma Y, Wang F, Xue A, Zhu
J, Yang H, Chen Q, Chen M, Ye L, et al: Potential regulatory role
of lncRNA-miRNA-mRNA axis in osteosarcoma. Biomed Pharmacother.
121:1096272020. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Hei R, Chen J, Zhang X, Bian H, Chen C, Wu
X, Han T, Zhang Y, Gu J, Lu Y and Zheng Q: CircNRIP1 acts as a
sponge of miR-1200 to suppress osteosarcoma progression via
upregulation of MIA2. Am J Cancer Res. 12:2833–2849.
2022.PubMed/NCBI
|
|
33
|
Qin S, Wang Y, Ma C and Lv Q: Competitive
endogenous network of circRNA, lncRNA, and miRNA in osteosarcoma
chemoresistance. Eur J Med Res. 28:3542023. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Cui H and Zhao J: LncRNA TMPO-AS1 serves
as a ceRNA to promote osteosarcoma tumorigenesis by regulating
miR-199a-5p/WNT7B axis. J Cell Biochem. 121:2284–2293. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Sun Y, Cao L, Lin JT, Yuan Y, Cao ZL and
Jia JD: Upregulated miRNA-1236-3p in osteosarcoma inhibits cell
proliferation and induces apoptosis via targeting KLF8. Eur Rev Med
Pharmacol Sci. 23:6053–6061. 2019.PubMed/NCBI
|
|
36
|
Jeck WR, Sorrentino JA, Wang K, Slevin MK,
Burd CE, Liu J, Marzluff WF and Sharpless NE: Circular RNAs are
abundant, conserved, and associated with ALU repeats. RNA.
19:141–157. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Li R, Jiang J, Shi H, Qian H, Zhang X and
Xu W: CircRNA: A rising star in gastric cancer. Cell Mol Life Sci.
77:1661–1680. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Ju HQ, Zhao Q, Wang F, Lan P, Wang Z, Zuo
ZX, Wu QN, Fan XJ, Mo HY, Chen L, et al: A circRNA signature
predicts postoperative recurrence in stage II/III colon cancer.
EMBO Mol Med. 11:e101682019. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Zong L, Sun Q, Zhang H, Chen Z, Deng Y, Li
D and Zhang L: Increased expression of circRNA_102231 in lung
cancer and its clinical significance. Biomed Pharmacother.
102:639–644. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Song T, Xu A, Zhang Z, Gao F, Zhao L, Chen
X, Gao J and Kong X: CircRNA hsa_circRNA_101996 increases cervical
cancer proliferation and invasion through activating TPX2
expression by restraining miR-8075. J Cell Physiol.
234:14296–14305. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Wu Y, Xie Z, Chen J, Chen J, Ni W, Ma Y,
Huang K, Wang G, Wang J, Ma J, et al: Circular RNA circTADA2A
promotes osteosarcoma progression and metastasis by sponging
miR-203a-3p and regulating CREB3 expression. Mol Cancer. 18:732019.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Tang Q, Chen Z, Zhao L and Xu H: Circular
RNA hsa_circ_0000515 acts as a miR-326 sponge to promote cervical
cancer progression through up-regulation of ELK1. Aging (Albany
NY). 11:9982–9999. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Moya L, Meijer J, Schubert S, Matin F and
Batra J: Assessment of miR-98-5p, miR-152-3p, miR-326 and miR-4289
expression as biomarker for prostate cancer diagnosis. Int J Mol
Sci. 20:11542019. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Wei L, Sun C, Zhang Y, Han N and Sun S:
miR-503-5p inhibits colon cancer tumorigenesis, angiogenesis, and
lymphangiogenesis by directly downregulating VEGF-A. Gene Ther.
29:28–40. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Jiang SP and Li ZR: MiR-503-5p regulates
cell epithelial-to-mesenchymal transition, metastasis and prognosis
of hepatocellular carcinoma through inhibiting WEE1. Eur Rev Med
Pharmacol Sci. 23:2028–2037. 2019.PubMed/NCBI
|
|
46
|
Liang X, Li Z, Men Q, Li Y, Li H and Chong
T: miR-326 functions as a tumor suppressor in human prostatic
carcinoma by targeting Mucin1. Biomed Pharmacother. 108:574–583.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Contino G, Amati F, Pucci S, Pontieri E,
Pichiorri F, Novelli A, Botta A, Mango R, Nardone AM, Sangiuolo FC,
et al: Expression analysis of the gene encoding for the U-box-type
ubiquitin ligase UBE4A in human tissues. Gene. 328:69–74. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Sakiyama T, Fujita H and Tsubouchi H:
Autoantibodies against ubiquitination factor E4A (UBE4A) are
associated with severity of Crohn's disease. Inflamm Bowel Dis.
14:310–317. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Yi X, Xiao F, Zhong X, Duan Y, Liu K and
Zhong C: A Ca2+ chelator ameliorates chromium (VI)-induced
hepatocyte L-02 injury via down-regulation of voltage-Dependent
anion channel 1 (VDAC1) expression. Environ Toxicol Pharmacol.
49:27–33. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Liu C, Li H, Duan W, Duan Y, Yu Q, Zhang
T, Sun YP, Li YY, Liu YS and Xu SC: MCU upregulation overactivates
mitophagy by promoting VDAC1 dimerization and ubiquitination in the
hepatotoxicity of cadmium. Adv Sci (Weinh). 10:22038692023.
View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Shoshan-Barmatz V, De Pinto V,
Zweckstetter M, Raviv Z, Keinan N and Arbel N: VDAC, a
multi-functional mitochondrial protein regulating cell life and
death. Mol Aspects Med. 31:227–285. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Shoshan-Barmatz V, Nahon-Crystal E,
Shteinfer-Kuzmine A and Gupta R: VDAC1, mitochondrial dysfunction,
and Alzheimer's disease. Pharmacol Res. 131:87–101. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Zhang C, Ding W, Liu Y, Hu Z, Zhu D, Wang
X, Yu L, Wang L, Shen H, Zhang W, et al: Proteomics-based
identification of VDAC1 as a tumor promoter in cervical carcinoma.
Oncotarget. 7:52317–52328. 2016. View Article : Google Scholar : PubMed/NCBI
|