Introduction
Liver cancer is the fourth leading cause of death
worldwide, with approximately one-half of the cases occurring in
China. Approximately 466,000 new cases of hepatocellular carcinoma
are reported annually in China, constituting 55.4% of the global
incidence. Concurrently, ~422,000 associated deaths are recorded
per annum, representing 53.9% of worldwide mortality from this
disease (1,2). Abdominal surgery, liver
transplantation and local thermal ablation are commonly used
methods for treating liver cancer (3,4).
Thermal ablation utilizes energy such as radiofrequency, microwave
or laser energy to generate high temperatures and destroy tumor
tissues for therapeutic purposes (5). Compared to radiofrequency and laser
ablation, microwave ablation (MWA) can heat tissue more rapidly,
reaching higher temperatures, and thus more quickly destroy tumor
cells. MWA has a smaller thermal deposition effect on blood vessels
and bile ducts, which means it is safer when treating tumors close
to these structures, reducing damage to surrounding tissues.
Additionally, microwaves can penetrate tissues more effectively and
deliver energy to greater depths, making it more effective in
treating deeper-seated tumors (6,7).
However, adjacent to the deep diaphragm muscle, some
lesions may be difficult to visualize due to the influence of gas
in the lungs, posing challenges for ablation (8). Furthermore, the heat generated during
ablation can affect the diaphragm muscle and potentially cause
damage (9). Therefore, when
performing MWA for liver cancer adjacent to the deep diaphragm
muscle under ultrasound guidance, issues such as the quality of the
ultrasound images and diaphragm muscle protection need to be
addressed.
To protect the diaphragm muscle from thermal injury,
artificial ascites injected into the perihepatic peritoneal space
can be utilized as a heat barrier to separate the ablation zone
from the diaphragm (10,11). The present study aimed to
retrospectively analyze the safety and effectiveness of artificial
ascites-assisted MWA for liver cancer adjacent to the deep
diaphragm muscle, as well as perioperative nursing measures to
ensure the safety of the procedure and reduce complications.
Materials and methods
Study design
The study has been approved by the Ethics Committee
of Hangzhou Xixi Hospital (Hangzhou, China) and strictly adheres to
the ethical guidelines outlined in the Helsinki Declaration.
Patients with liver cancer who underwent artificial
ascites-assisted MWA treatment near the diaphragm at Hangzhou Xixi
Hospital between January 2016 and December 2022 were included as
the study subjects, with all patients or their guardians providing
signed informed consent forms.
Inclusion criteria
The inclusion criteria were as follows: i) A
clinical diagnosis of liver cancer that met the Milan criteria
(6) or histopathological
confirmation through preoperative puncture biopsy. Milan criteria
specific contents include: For a solitary tumor, the diameter
should not exceed 5 cm. For multiple tumors, the total number of
tumors should be ≤3, and the maximum diameter of each individual
tumor should not exceed 3 cm. There should be no evidence of
macrovascular invasion, lymph node metastasis or extrahepatic
metastasis; ii) a tumor located within 1 cm of the diaphragm, with
a single lesion diameter not exceeding 5 cm, or multiple lesions
(≤3) with each individual lesion diameter not exceeding 3 cm; and
iii) Child-Pugh class A or B (6).
Child-Pugh class A indicates relatively preserved liver function,
characterized by low total bilirubin levels, high serum albumin
levels, mild prolongation of prothrombin time, minimal or no
ascites, and only mild symptoms of hepatic encephalopathy. By
contrast, patients with Child-Pugh class B, representing moderate
to severe impairment of liver function, exhibit worsening in one or
more of these parameters, including elevated bilirubin levels,
decreased albumin level and significantly prolonged prothrombin
time. They may also present with moderate ascites or some degree of
hepatic encephalopathy.
Exclusion criteria
The exclusion criteria were as follows: i) The
presence of major vascular invasion and/or distant metastasis; and
ii) a lack of regular follow-up after surgery or patients who were
lost to follow-up.
Artificial ascites technique
The tumor location was initially assessed and
visualized clearly using ultrasound. Subsequently, a single cavity
central venous catheter set was utilized to draw 5 ml of 0.9%
sodium chloride solution and pre-place the guide wire into the
empty needle tube. With ultrasound guidance, the needle tip was
carefully inserted parallel to the liver surface, targeting the
puncture point near the gall bladder at the lower margin of the
right anterior lobe of the liver. Upon encountering a sudden
decrease in resistance, the 0.9% sodium chloride solution was
injected, and if no resistance was encountered, the wire was
advanced further. In the absence of resistance, the wire was
expeditiously introduced into the abdominal cavity, followed by the
withdrawal of the needle and placement of the drainage tube along
the guide wire. Throughout the procedure, the patient maintained a
Trendelenburg position and continuous instillation of 0.9% sodium
chloride solution at 40°C into the abdominal cavity was performed
until the separation distance between the liver and the diaphragm
reached a minimum of 0.5 cm.
Ablation procedure
MyLab™ X90 (Esaote SPA) color doppler
ultrasound machine was used, equipped with contrast-enhanced
ultrasound function and abdominal convex array probes CA431 and
CA541, with a frequency range from 1 to 8 MHz. MWA was performed
using a KY-2000 MWA instrument (Nanjing Kangyou Co., Ltd.). After
the establishment of artificial ascites, ultrasound-guided MWA was
performed. All patients underwent general anesthesia and the
procedure was performed according to the ablation plan determined
preoperatively: The ablation power was set at 60 W, the size of the
ablation area was ~3.5×2.5 cm and the ablation time at each point
was 6 min. The microwave ablation needle has a temperature sensor
that displays the internal temperature in real time. Tumors with a
diameter of <2 cm were ablated with a single needle and a single
point, while tumors with a diameter of 2–5 cm were ablated with a
single needle and multiple points, or double needles and multiple
points overlapping. Ablation was required to extend 0.5 cm beyond
the tumor margin to ensure a safe margin. Immediately after
ablation, contrast-enhanced ultrasound assessment was performed to
determine whether the ablation zone reached the safe boundary. If
the safe boundary was reached, the ablation was terminated;
otherwise, additional ablation was performed immediately until the
safe boundary was reached.
Tumor image quality score
The study evaluated the visualization of liver
cancer through the assessment of ultrasound images before and after
the introduction of artificial ascites. The images were scored by
three experienced radiologists independently, with each image
receiving a score that was then averaged to determine the final
score. The scoring criteria were as follows: 1 point, the lesion
was affected by gas in the lung and could not be visualized; 2
points, the visualized region represented <1/3 of the lesion; 3
points, the visualized region represented between 1/3 and 2/3 of
the lesion; 4 points, the visualized lesion represented >2/3 of
the lesion; and 5 points, complete and clear visualization of the
lesion. This scoring system can help physicians evaluate the
quality and visualization of ultrasound images of liver cancer to
guide subsequent treatment and decision-making.
Perioperative nursing
Preoperative nursing involved the nurses explaining
the purpose, methods, advantages, surgical process, precautions and
possible complications of MWA to the patients and their families in
detail, so as to eliminate the anxiety and tension of patients.
These patients received Situation, Background, Assessment and
Recommendations communication mode of care, in addition to the
routine preoperative care provided (12).
During the operation, other nursing duties were
required. Firstly, venous access is established to replenish and
rescue fluid circulation. The position is then assisted in choosing
the supine or lateral position according to the location of the
lesion. The operation of all equipment was tested, checking whether
the connecting instrument pipeline was smooth. The doctor was then
assisted to operate the MWA instrument, closely observing the ECG
monitor, and watching for bleeding, changes in heart rate, dyspnea
and other reactions.
Postoperative nursing care included monitoring the
vital signs and clinical symptoms of the patients for the first 72
h after ablation to detect any early complications such as fever,
swelling, pain and bleeding. In particular, severe complications,
which may result in significant morbidity and disability, would
require an increased level of care, hospitalization or a
significantly prolonged hospital stay.
The aforementioned nursing measures for MWA before,
during and after operation were designed to ensure the smooth
operation and promote the recovery of patients. Nurses aimed to
closely observe any changes to the condition of the patients during
the whole process and communicate with doctors in time to ensure
the safety and comfort of the patients.
Complications
Pain and fever are common mild complications after
ablation, and even pleural effusion may occur. Burns and
perforations of the diaphragm, gastrointestinal injuries, severe
bleeding and stress ulcers require further treatment. In
particular, severe complications that may lead to significant
morbidity and disability require increased levels of care,
hospitalization or significantly prolonged hospital stays.
Follow-up
Patients with complications were managed according
to the complication, while those without serious complications were
discharged 3–7 days after surgery. Contrast-enhanced magnetic
resonance imaging (MRI) or contrast-enhanced computed tomography
(CT) was performed at 1 month after ablation to evaluate the
short-term efficacy of MWA. Complete ablation was defined as
ablation completely covering the lesion without abnormal
enhancement. The ablation was defined as incomplete ablation if it
did not completely cover the lesion and there was neoplastic
enhancement. If residual lesions were present, repeat thermal
ablation or surgical resection may be required. Subsequently,
contrast-enhanced MRI or contrast-enhanced CT was performed every
3–6 months to assess local tumor progression (LTP) in comparison
with the previous images, and technical efficacy and LTP were
recorded.
Statistical analysis
Data analysis and mapping were performed using the R
software (version 3.5.3; R Core Team). Patient age, tumor size and
sonographic quality score were used as measurement data and
expressed as mean ± SD. The paired t-test was used for comparison
of image score before and after the induction of artificial
ascites. To evaluate different radiologists in ultrasonic image
visual grading consistency, Cohen's κ coefficient predominate
analysis method was applied. The number of cases of adverse
reactions and complications, the number of cases of tumor complete
ablation and the number of cases of LTP were included as the count
data and expressed as n (%). P<0.05 was considered to indicate a
statistically significant difference.
Results
Baseline characteristics
A total of 62 lesions in 54 patients were included
in this study, and all patients underwent percutaneous MWA. The
cohort consisted of 44 men and 10 women, with ages ranging from 35
to 82 years and a mean age of 55.64±10.33 years. Among the
patients, 46 had a single lesion, while 8 had two lesions. A total
of 39 patients were receiving treatment for the first time, while
15 had recurrent lesions (7 with prior surgical resection and 8
with prior thermal ablation). The distribution of the tumors was
predominantly concentrated in liver segments I (1; 1.6%), IV
(6.5%), VII (23; 37.1%) and VIII (34; 54.8%). The size of the
tumors ranged from 14 to 49 mm, with a mean size of 31.64±8.37 mm
(Table I).
 | Table I.Clinical information of patients
(n=54) and characteristics of liver cancer. |
Table I.
Clinical information of patients
(n=54) and characteristics of liver cancer.
| Characteristic | Value | P-value |
|---|
| Age, years | 55.64±10.33 |
|
| Sex, n (%) |
|
|
| Male | 44 (81.5) |
|
|
Female | 10 (18.5) |
|
| Number of lesions, n
(%) |
|
|
|
Single | 46 (85.2) |
|
|
Multiple | 8 (14.8) |
|
| Liver segment
distribution, n (%)a |
|
|
| I | 1 (1.6) |
|
| IV | 4 (6.5) |
|
| VII | 23 (37.1) |
|
| VIII | 34 (54.8) |
|
| Treatment mode, n
(%) |
|
|
|
First | 39 (72.2) |
|
| Second or
more | 15 (27.8) |
|
| Tumor size, mm | 31.64±8.37 |
|
| Image score |
| <0.05 |
|
Before | 3.57±0.79 |
|
|
After | 4.89±0.33 |
|
| Artificial ascites
dosage, ml |
|
|
|
Minimum | 500 |
|
| Max | 2,000 |
|
| Mean ±
SD | 1,021±544 |
|
| Microwave ablation, n
(%)a |
|
|
| Single
needle and point | 45 (72.6) |
|
| Multiple
points | 17 (27.4) |
|
| Complications, n
(%) |
|
|
|
Diaphragmatic injury | 0 (0.0) |
|
|
Hematoma | 0 (0.0) |
|
| Pleural
effusion | 1 (1.9) |
|
|
Fever | 11 (20.4) |
|
| Pain | 12 (22.2) |
|
| Efficacy, n (%) |
|
|
| Complete
ablation | 51 (94.4) |
|
|
Recurrence | 3 (5.6) |
|
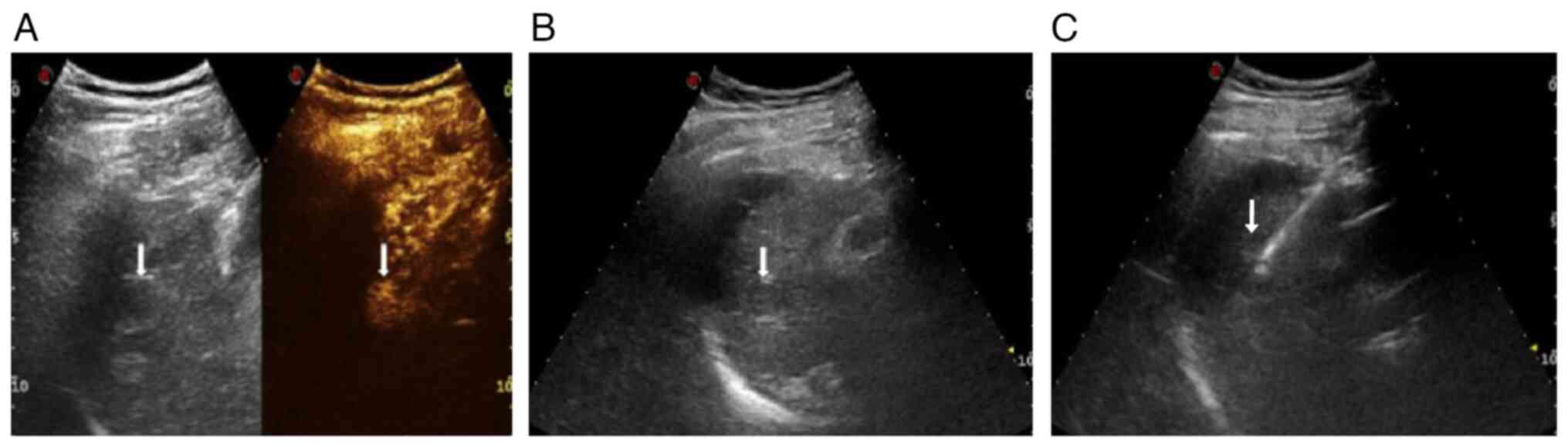
Ultrasonic image evaluation
The κ-value among the three radiologists was 0.878,
indicating a high degree of agreement among the three radiologists
in the visual evaluation of ultrasound images. The score for
ultrasonographic image quality before artificial ascites was
3.57±0.79, while after artificial ascites it increased
significantly to 4.89±0.33 (t=16.324; P<0.05), indicating a
marked improvement in lesion image quality following artificial
ascites treatment (Fig. 1).
Amount of artificial ascites
Artificial ascites successfully separated the
diaphragm from the liver at a distance of ≥0.5 cm in all 54
patients. The mean dosage of 0.9% sodium chloride solution was
1,021±544 ml (range, 500-2,000 ml) (Table I).
Microwave ablation
Among the 62 lesions, 45 lesions were ablated with
single needle and single point, and 17 lesions were ablated with
single needle and multiple points or double needle and multiple
points overlapping. In 13 cases, the ablation range was
insufficient, and additional ablation was performed immediately
until the safe boundary was reached.
Complications
There were no treatment-related deaths or
cardiopulmonary complications due to fluid overload. No diuretics
or punctures were required to manage any of the patients infused
with artificial ascites. The ascites was completely absorbed before
the patient was discharged. There was no injury to the diaphragm,
no burn to the skin at the puncture site and no abdominal
hemorrhage. A single patient developed right-sided pleural
effusion, which was not drained, and on the seventh postoperative
day, the pleural effusion had disappeared on re-examination. The
most common minor complications after ablation were pain and fever.
A total of 11 patients developed fever after treatment and were
treated with antipyretics (Table
I). A total of 12 patients required analgesics after
treatment.
Efficacy
Enhanced MRI or CT examination was conducted within
1 month of ablation, resulting in a complete ablation rate of 94.4%
(51/54). Only 3 patients experienced recurrence and subsequently
underwent further ultrasound-guided MWA treatment. The patients
were followed up for 12 to 48 months post-surgery, with a median
follow-up period of 20 months. According to the enhanced MRI or CT
results, the LTP rate was found to be 5.6% (3/54).
Discussion
MWA has been shown to effectively control and
eliminate liver cancer lesions (13); it preserves the patient's normal
liver tissue to the greatest extent and reduces the impact on liver
function (14). The artificial
ascites technique can separate the diaphragm from the liver,
improving the image quality of liver cancer ablation near the
diaphragm and reducing thermal damage caused by microwave energy.
Perioperative high-quality nursing can enhance the safety and
therapeutic effect of MWA. Therefore, safe and effective treatment
methods, along with systematic nursing measures, are crucial for
ensuring rapid patient recovery and surgical success.
Artificial ascites improves visualization of
adjacent deep diaphragmatic muscle lesions by increasing the
distance between the lesion and lung, thereby reducing interference
from gas in the lung and enhancing sonographic image quality
(15,16). Quality scores of tumor
ultrasonographic images before and after artificial ascites were
established in the present study. The results showed a significant
improvement in ultrasonographic image quality after artificial
ascites, leading to improved visualization and integrity of tumors
adjacent to the top of the diaphragm. This ultimately enhances
puncture accuracy, ablation effectiveness and operator confidence.
The thermal barrier formed by artificial ascites reduces thermal
damage to the diaphragm during ablation (17), as evidenced by no instances of
diaphragmatic injury among patients in the present study. Huang
et al (15) conducted a
study on patients with previous history of abdominal surgery to
evaluate the feasibility and effectiveness of artificial
ascites-assisted thermal ablation in the treatment of liver cancer
near the gastrointestinal tract. Artificial ascites was
successfully utilized in 38 out of 40 operations, resulting in a
success rate of 95%, and effectively protecting the surrounding
organs. However, the use of artificial ascites may be limited by
abdominal adhesions, which can hinder the separation of fluid from
the patient's gastrointestinal tissue, particularly in patients
with a history of previous abdominal surgery. However, in the
present study, none of the cases exhibited any radiographic
evidence of diaphragmatic injury during postoperative review, and
no abnormalities such as perforation were detected during
follow-up, indicating that artificial ascites played a protective
isolating role.
In the present study, only 1 patient developed a
small pleural effusion that was not treated with drainage. In this
patient, the lesion was large and tightly connected to the
diaphragm, along with a history of surgery and the presence of
visceral adhesions. Injecting too much ascites may lead to the
occurrence of pleural effusion, and may also be related to indirect
stimulation of the diaphragm and lung after heating of the ascites
(18). During ablation, it is
important to closely monitor bubble coverage resulting from thermal
ablation and adjust the power emission of the ablation needle to
effectively prevent burns.
Mild adverse effects, such as a low fever and
epigastric pain, are commonly observed after liver cancer ablation.
These symptoms generally do not require special treatment and will
resolve on their own (19). In
fact, artificial ascites infusion can even reduce the pain during
subcapsular radiofrequency ablation of hepatocellular carcinoma
(20). In the present study, no
stress response were triggered by the infusion of artificial
ascites. The management of a perioperative stress response is very
difficult, and it will even affect the operation and the evaluation
of the curative effect (21).
Although the present study did not observe any cases of stress
response, it does not rule out its occurrence in future cases, and
adequate monitoring during the perioperative period is
necessary.
Studies have indicated that failure to achieve a
safe boundary during liver cancer ablation is a significant factor
in postoperative LTP (22). In the
present study, contrast-enhanced ultrasonography was utilized
during the ablation process to assess immediate efficacy. For
patients with inadequate ablation, additional procedures were
performed until a safe margin was reached. At 1 month
post-operation, the complete ablation rate was 94.4% (51/54), which
aligns with a previous report on thermal ablation for liver cancer
adjacent to the diaphragm (16).
Perioperative nursing primarily involves
comprehensive care of the psychological, physiological and social
well-being of the patients throughout the entire operation process
to ensure they receive satisfactory treatment outcomes (23). Strengthening preoperative education
can alleviate patient anxiety and improve surgical compliance.
Nursing staff should be knowledgeable about and proficient in the
treatment procedures related to ablation technology, while also
developing relevant nursing process plans. Postoperative care is
equally important for ensuring rehabilitation and timely management
of unexpected events. Close observation of the patient's condition
after surgery is essential, along with implementing relevant
treatment measures in collaboration with physicians to ensure
smooth progress across all aspects of the operation (24). Safe and effective treatment methods
alongside systematic nursing measures are crucial for promoting
rapid patient recovery and increasing surgical success rates
(25).
The present study has several limitations. Firstly,
it is retrospective with a small sample size; therefore,
prospective large-sample studies are necessary to validate the
safety and efficacy of artificial ascites in liver cancer ablation.
Additionally, the tumor sonographic quality score used in this
study was subjective and dependent on operator experience.
In conclusion, artificial ascites not only enhances
lesion sonography visualization but also helps prevent thermal
ablation-induced diaphragm burns to reduce complications.
Perioperative treatment and rehabilitation of the patients with
high quality nursing is beneficial.
Acknowledgements
The authors would like to thank Professor Liping
Zheng (Hangzhou Xixi Hospital, Hangzhou, China) for providing
suggestions and support.
Funding
This study was supported by funding from the Zhejiang Province
Medical and Health Science and Technology Program Project (grant
nos. 2022KY1019 and 2021KY937), and the Hangzhou Science and
Technology Plan Guidance Project (grant no. 20201231Y033).
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
QA, YP and DL contributed to the data curation,
analysis, investigation and writing of the manuscript. FL, ZK and
DL performed and analyzed the ultrasonography examinations and
operation of the patients. XZ designed and supervised the project,
acquired funding and revised the manuscript. QA reviewed and edited
the writing, and validated the whole analysis process with regard
to study design and expectations. All authors read and approved the
final manuscript. QA and DL confirm the authenticity of all the raw
data.
Ethics approval and consent to
participate
This study was conducted according to the
Declaration of Helsinki. Ethics approval was obtained from the
Medical Ethics Committee of Hangzhou Xixi Hospital (Hangzhou,
China; approval no. 2021KY937) and written informed consent was
obtained from all patients or their guardians.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no conflict of
interest.
References
|
1
|
Fujita M, Yamaguchi R, Hasegawa T, Shimada
S, Arihiro K, Hayashi S, Maejima K, Nakano K, Fujimoto A, Ono A, et
al: Classification of primary liver cancer with immunosuppression
mechanisms and correlation with genomic alterations. Ebiomedicine.
53:1026592020. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Wang SJ, Chao D, Wei W, Nan G, Li JY, Liu
FL, Li L, Jiang JL, Cui HY and Chen ZN: CD147 promotes collective
invasion through cathepsin B in hepatocellular carcinoma. J Exp
Clin Cancer Res. 39:1452020. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Zhang Q, Chen WW, Sun X, Qian D, Tang DD,
Zhang LL, Li MY, Wang LY, Wu CJ and Peng W: The versatile emodin: A
natural easily acquired anthraquinone possesses promising
anticancer properties against a variety of cancers. Int J Biol Sci.
18:3498–3527. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Zhou Z, Li Y, Hao H, Wang Y, Zhou Z, Wang
Z and Chu X: Screening hub genes as prognostic biomarkers of
hepatocellular carcinoma by bioinformatics analysis. Cell
Transplant. 28 (1 Suppl):76S–86S. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Meng L, Wei Y and Xiao Y:
Chemo-immunoablation of solid tumors: A new concept in tumor
ablation. Front Immunol. 13:10575352023. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Yu J, Cheng ZG, Han ZY, Liu FY, Zheng RQ,
Cheng W, Wei Q, Yu SY, Li QY, He GZ, et al: Period-dependent
survival benefit of percutaneous microwave ablation for
hepatocellular carcinoma: A 12-year real-world, multicentric
experience. Liver Cancer. 11:341–353. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ozen M and Raissi D: Editorial comment: A
review on radiofrequency, microwave and high-intensity focused
ultrasound ablations for hepatocellular carcinoma with cirrhosis.
Hepatobiliary Surg Nutr. 11:453–456. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
van de Berg NJ, Meeuwsen FC, Doukas M,
Kronreif G, Moelker A and van den Dobbelsteen JJ: Steerable needles
for radio-frequency ablation in cirrhotic livers. Sci Rep.
11:3092021. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Tay BWR, Huang DQ, Mark M, Thong NW, Guan
HL, Gee LS, Cheng LH, Mei LY, Thurairajah P, Chen LJ, et al:
Comparable outcomes in early hepatocellular carcinomas treated with
trans-arterial chemoembolization and radiofrequency ablation.
Biomedicines. 10:23612022. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Li B, Liu C, Xu XX, Li Y, Du Y, Zhang C,
Zheng HJ and Yang HF: Clinical application of artificial ascites in
assisting CT-guided percutaneous cryoablation of hepatic tumors
adjacent to the gastrointestinal tract. Sci Rep. 7:166892017.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Chu MO, Shen CH, Chang TS, Xu HW, Yen CW,
Lu SN and Hung CH: Pretreatment inflammation-based markers predict
survival outcomes in patients with early stage hepatocellular
carcinoma after radiofrequency ablation. Sci Rep. 8:166112018.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Yun J, Lee YJ, Kang K and Park J:
Effectiveness of SBAR-based simulation programs for nursing
students: A systematic review. BMC Med Educ. 23:5072023. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Fan Z, Zhou P, Jin B, Li G, Feng L, Zhuang
C and Wang S: Recent therapeutics in hepatocellular carcinoma. Am J
Cancer Res. 13:261–275. 2023.PubMed/NCBI
|
|
14
|
Adnan A, Muñoz NM, Prakash P, Habibollahi
P, Cressman ENK and Sheth RA: Hyperthermia and tumor immunity.
Cancers (Basel). 13:25072021. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Huang Q, Li J, Zeng Q, Tan L, Zheng R, He
X and Li K: Value of artificial ascites to assist thermal ablation
of liver cancer adjacent to the gastrointestinal tract in patients
with previous abdominal surgery. BMC Cancer. 20:7632020. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wang CC and Kao JH: Artificial ascites is
feasible and effective for difficult-to-ablate hepatocellular
carcinoma. Hepatol Int. 9:514–519. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Nishimura M, Nouso K, Kariyama K, Wakuta
A, Kishida M, Wada N, Higashi T and Yamamoto K: Safety and efficacy
of radiofrequency ablation with artificial ascites for
hepatocellular carcinoma. Acta Med Okayama. 66:279–284.
2012.PubMed/NCBI
|
|
18
|
Song SG, Hur YH, Cho JY, Shin MH, Yoon EJ
and Kim JW: Pleural effusion after hepatic radiofrequency ablation
with artificial ascites: Clinical spectrum and significance. J Vasc
Interv Radiol. 31:1636–1644.e1. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhang Y, Wang J, Li H, Zheng T, Jiang H,
Li M and Song B: Performance of LI-RADS version 2018 CT treatment
response algorithm in tumor response evaluation and survival
prediction of patients with single hepatocellular carcinoma after
radiofrequency ablation. Ann Transl Med. 8:3882020. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Park SJ, Lee DH and Han JK: Reducing pain
by artificial ascites infusion during radiofrequency ablation for
subcapsular hepatocellular carcinoma. Cardiovasc Intervent Radiol.
44:565–573. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Berghi ON, Dumitru M, Vrinceanu D, Cergan
R, Jeican II, Giurcaneanu C and Miron A: Current approach to
medico-legal aspects of allergic reactions. Rom J Leg Med.
29:328–331. 2021. View Article : Google Scholar
|
|
22
|
Jiang C, Liu B, Chen S, Peng Z, Xie X and
Kuang M: Safety margin after radiofrequency ablation of
hepatocellular carcinoma: precise assessment with a
three-dimensional reconstruction technique using CT imaging. Int J
Hyperthermia. 34:1135–1141. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Yuan Y, Li Y, Yang G, Zhang L and Ye J:
Effect of Comprehensive nursing approach in perioperative stage of
patients with hepatocellular carcinoma interventional therapy. Evid
Based Complement Alternat Med. 2022:68624632022. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Melliza DM and Woodall M: Radiofrequency
ablation of liver tumors: The complementary roles of the clinic and
research nurse. Gastroenterol Nurs. 23:210–214. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zhang S, Yang Y, Liu W, Yuan B, Tao C and
Dou G: Effects of nursing care using a fast-track surgery approach
in 49 patients with early-stage hepatocellular carcinoma undergoing
first-line treatment with radiofrequency ablation: A retrospective
study. Med Sci Monit. 29:e9390442023. View Article : Google Scholar : PubMed/NCBI
|