Introduction
Clinical studies to establish novel effective drug
therapy for thymic epithelial tumors (TETs), which are tumors
located in the anterior mediastinum, are difficult to plan, due to
the lack of a large number of cases. The European Society for
Medical Oncology guidelines recommend cisplatin + doxorubicin +
cyclophosphamide and platinum-based anticancer therapies, such as
carboplatin + paclitaxel, for thymic carcinoma (TC) (1).
The multi-vascular endothelial growth factor (VEGF)
receptor (VEGFR) inhibitor lenvatinib has been shown to be
effective for TC, based on the results of the REMOMA trial, and can
now be used in clinical practice (2). Lenvatinib, a molecularly targeted drug
against tyrosine kinases, is effective in patients with TC, but it
also carries the risk of several side effects, such as proteinuria
and hypothyroidism (3). We
hypothesize that more effective treatment plans for TC can be
formulated if the efficacy can be predicted before prescription. By
doing so, the prognosis of patients with TET could be improved.
VEGFR-1 and −2 are mainly involved in tumor
angiogenesis; however, VEGFR-1 also has a main role in modulating
the inflammatory response (4). It
has also been reported that VEGFR-2 is primarily involved in
activating signals for tumor angiogenesis (5). The present study first assessed
VEGFR-2 to identify a group of patients who would respond to
lenvatinib in TET. In a previous study, VEGF expression was
evaluated in 17 thymic specimens, with VEGFR-1 and −2 expression
reported in the normal thymic region and in the lesion area, and
VEGF expression was reported in the tumor epithelial cells of
thymomas, in the vascular endothelium and in the stroma close to
the tumor (6). Predicting the
response to lenvatinib prior to the administration of
pharmacotherapy may help to optimize the postoperative management
of advanced TC and the treatment of recurrent disease. In the
present study, immunohistochemistry (IHC) was used to assess the
expression of VEGFR-2 protein, as potentially relevant to the
efficacy of VEGF inhibitors in patients with TETs, including TC.
The present study also evaluated thymomas that were completely
resected by surgery to assess the possibility of expanding the
therapeutic indications.
Patients and methods
Patients
The present study analyzed completely resected tumor
tissues from 144 TETs from patients aged 25–87 years who were
treated at Nagoya City University Hospital (Nagoya, Japan) between
April 2004 and March 2021. Exclusion criteria were as follows: i)
Cases in which postoperative pathology did not diagnose TETs; ii)
cases in which surgery did not result in specimen removal; and iii)
cases in which the specimen block was no longer available. There
were no missing data, except for 7 cases in which outpatient
follow-up was stopped midway through. Patients who were lost to
follow-up early were not excluded from prognostic studies or other
analyses.
The present study was performed in accordance with
the Declaration of Helsinki and was approved by the institutional
review board of Nagoya City University Graduate School of Medical
Sciences in 2020 (approval no. 70-19-0016). The requirement for
individual patient consent was waived due to the retrospective
nature of this study and since individuals were not identified. The
original hematoxylin and eosin-stained slides were reviewed by a
pathologist. The 2021 edition of the World health Organization
classification was used for histopathology (7) and the 8th edition of the Union for
International Cancer Control classification was used for typing and
staging of TETs (8).
IHC staining
VEGFR-2 protein expression was assessed using IHC,
with hematoxylin and eosin-stained sections (5 µM-thick) evaluated
using microscopy. Immunostaining was performed as follows:
Deparaffinization was performed by immersion in xylene, followed by
immersion in 100, 90, 80 and 70% ethanol for 5 min each. After
washing in running water, antigen activation was performed by heat
treatment (120°C for 15 min) in 10 mM citrate buffer (pH 6.0),
washing in phosphate-buffered saline (PBS), and soaking in methanol
with 0.3% hydrogen peroxide for 30 min to prevent endogenous
peroxidase activity. After another wash in PBS, the slides were
covered with Block Ace (undiluted at room temperature for 10 min)
(Megmilk Snow Brand Co., Ltd.), a normal animal serum, to block
non-specific reactions, and allowed to react for 10 min in a wet
box. The slides were then coated with 200-fold diluted mouse
anti-VEGFR-2 antibodies (cat. no. sc-393163; Santa Cruz
Biotechnology, Inc.) and kept in a 4°C, dark place in a wet box
overnight. They were then washed with PBS and coated with mouse
horseradish peroxidase-conjugated EnVision+ Single Reagent
(undiluted; room temperature for 45 min) (cat. no. K400111-2;
Agilent Technologies, Inc.) and incubated for 45 min in a wet box.
Subsequently, they were washed with PBS and incubated for 10 min in
DAB/hydrogen peroxide (Sigma-Aldrich; Merck KGaA) reaction solution
after checking for staining. The slides were then washed with
running water. Nuclei were stained with hematoxylin (room
temperature for 1 min), dehydrated in 100% ethanol and xylene, and
permeated and sealed.
Evaluation methods
VEGFR-2 staining was evaluated under an optical
microscope at ×400 magnification. The plasma membrane of the tumor
cells (epithelial cells) was evaluated for staining intensity and
the proportion of staining. Lymphocytes were excluded from the
evaluation.
As there is no established method for evaluating
immunostaining for VEGFR-2, the method for determining human
epidermal growth factor (EGF) receptor 2, a membrane protein like
VEGFR, was referred to. The evaluation of immunostaining was
prepared according to the method for determining immunostaining in
the American Society of Clinical Oncology/College of American
Pathologists guidelines (9). No
staining of the plasma membrane was scored as 0. Patchy staining of
the plasma membrane was scored as 1, and a specimen with >50% of
the plasma membrane stained was scored as 2. Complete
circumferential staining of all the cell membranes was scored as 3.
The % stained cells within the tumor cells was also assessed. In
the case of thymoma type AB and tumors with mixed parts with
multiple scores, the part with the highest score was targeted for
evaluation.
Statistical analysis
All statistical analyses were performed using EZR
(version 1.61; Division of Hematology, Saitama Medical Center,
Jichi Medical University) (10).
Pearson's correlation coefficient was used to
determine correlation coefficients. Welch's test was use for
comparisons between two groups when the variance was not considered
equal. The Kruskal-Wallis test was used to compare the other
groups, followed by the Steel-Dwass multiple comparisons test.
P<0.05 was considered to indicate a statistically significant
difference. The log-rank test was used to compare overall survival
(OS) and disease-free survival (DFS) between groups using
Kaplan-Meier curves. Univariate and multivariate analyses were
performed using Cox proportional hazard regression models.
Results
VEGFR-2 staining score in 144 patients
with TETs
The expression of VEGFR-2 was assessed in 144
patients who underwent surgical resection of a TET (Table I). The mean and median ages of the
patients at the time of surgery were 59.0 and 58 years,
respectively. The histological types of thymomas were as follows:
Type A (n=15), type AB (n=30), type B1 (n=39), type B2 (n=34), type
B3 (n=13) and TC (n=13) (5). The
pathological stages of thymoma were as follows: Stage 1 (n=31),
stage 2 (n=73), stage 3 (n=15) and stage 4 (n=12). The pathological
stages of TC were as follows: Stage 1 (n=8), stage 2 (n=1), stage 3
(n=2) and stage 4 (n=2). The following treatments were
administered: Preoperative treatment (n=18), steroid pulse therapy
(n=13), carboplatin + paclitaxel (n=2), radiotherapy (n=1),
cisplatin + etoposide (n=1) and doxorubicin + cisplatin +
vincristine + cyclophosphamide therapy (n=1) (data not shown).
 | Table I.Patient characteristics (n=144). |
Table I.
Patient characteristics (n=144).
| Characteristic | Value |
|---|
| Age, n (%) |
|
| <60
years | 74 (51.4) |
| ≥60
years | 70 (48.6) |
| Sex, n (%) |
|
| Male | 70 (48.6) |
|
Female | 74 (51.4) |
| Pathology, n (%) |
|
| Thymoma
type A | 15 (10.4) |
| Thymoma
type AB | 30 (20.8) |
| Thymoma
type B1 | 39 (27.1) |
| Thymoma
type B2 | 34 (23.6) |
| Thymoma
type B3 | 13 (9.0) |
| Thymic
carcinoma | 13 (9.0) |
| Tumor
size, mma | 78.5 (50) |
| Thymoma UICC stage, n
(%) |
|
| 1 | 31 (21.5) |
| 2 | 73 (50.7) |
| 3 | 15 (10.4) |
| 4 | 12 (8.3) |
| Thymic carcinoma UICC
stage, n (%) |
|
| 1 | 8 (5.6) |
| 2 | 1 (0.7) |
| 3 | 2 (1.4) |
| 4 | 2 (1.4) |
Table II presents
the VEGFR-2 staining scores for all cases according to the
pathological type of TETs. The immunohistochemical staining score
for VEGFR-2 protein was 0 in 50 cases, 1 in 66 cases, 2 in 22
cases, and 3 in 6 cases.
 | Table II.Number of VEGFR-2 stained cases per
pathological type of thymus epithelial tumor. |
Table II.
Number of VEGFR-2 stained cases per
pathological type of thymus epithelial tumor.
|
| VEGFR-2 score |
|
|---|
|
|
|
|
|---|
| Pathology | 0 | 1 | 2 | 3 | Total |
|---|
| Thymoma type A | 2 | 12 | 1 | 0 | 15 |
| Thymoma type
AB | 9 | 20 | 1 | 0 | 30 |
| Thymoma type
B1 | 30 | 6 | 3 | 0 | 39 |
| Thymoma type
B2 | 6 | 19 | 8 | 1 | 34 |
| Thymoma type
B3 | 2 | 4 | 4 | 3 | 13 |
| Thymic
carcinoma | 1 | 5 | 5 | 2 | 13 |
| Total | 50 | 66 | 22 | 6 | 144 |
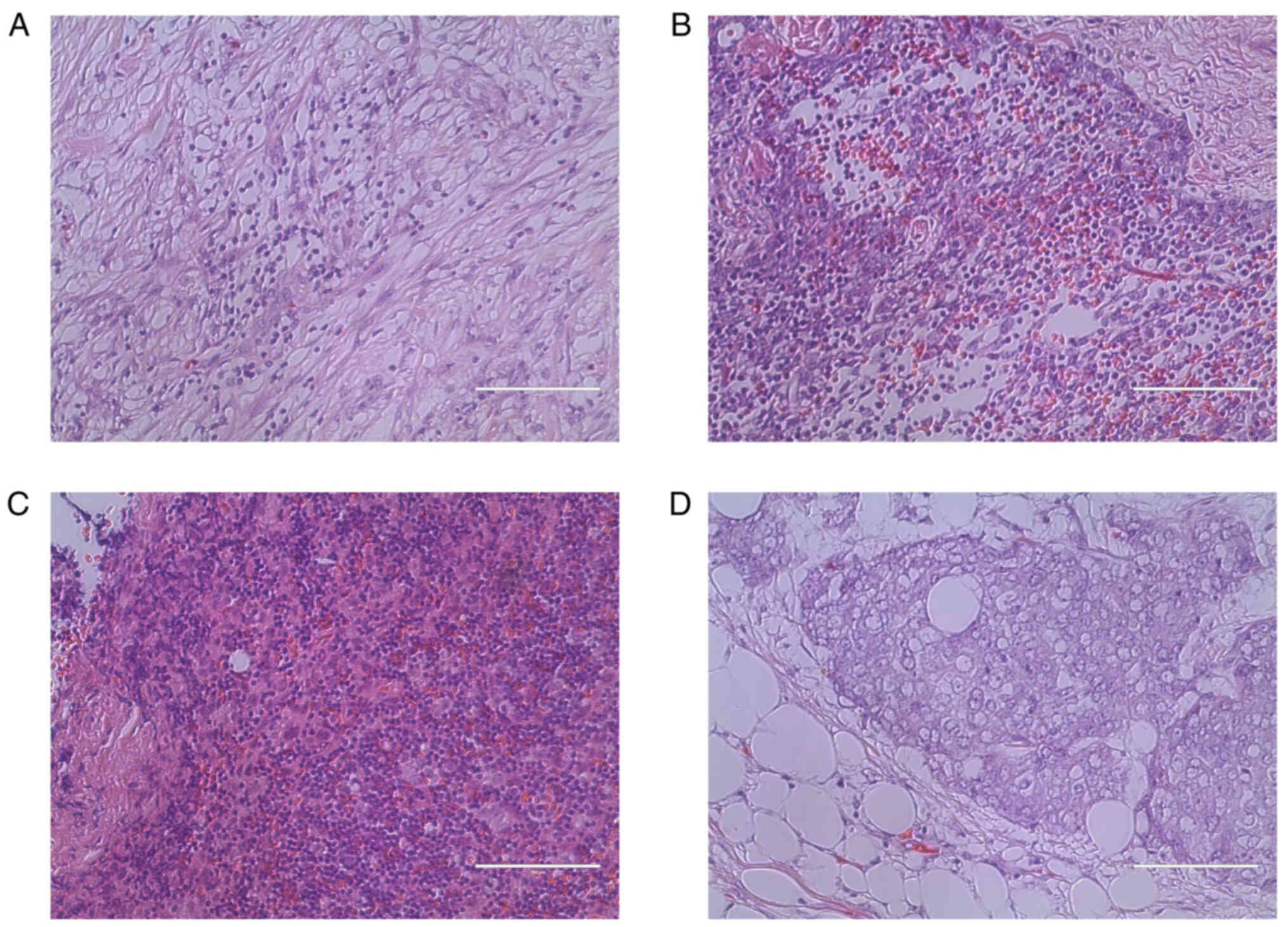
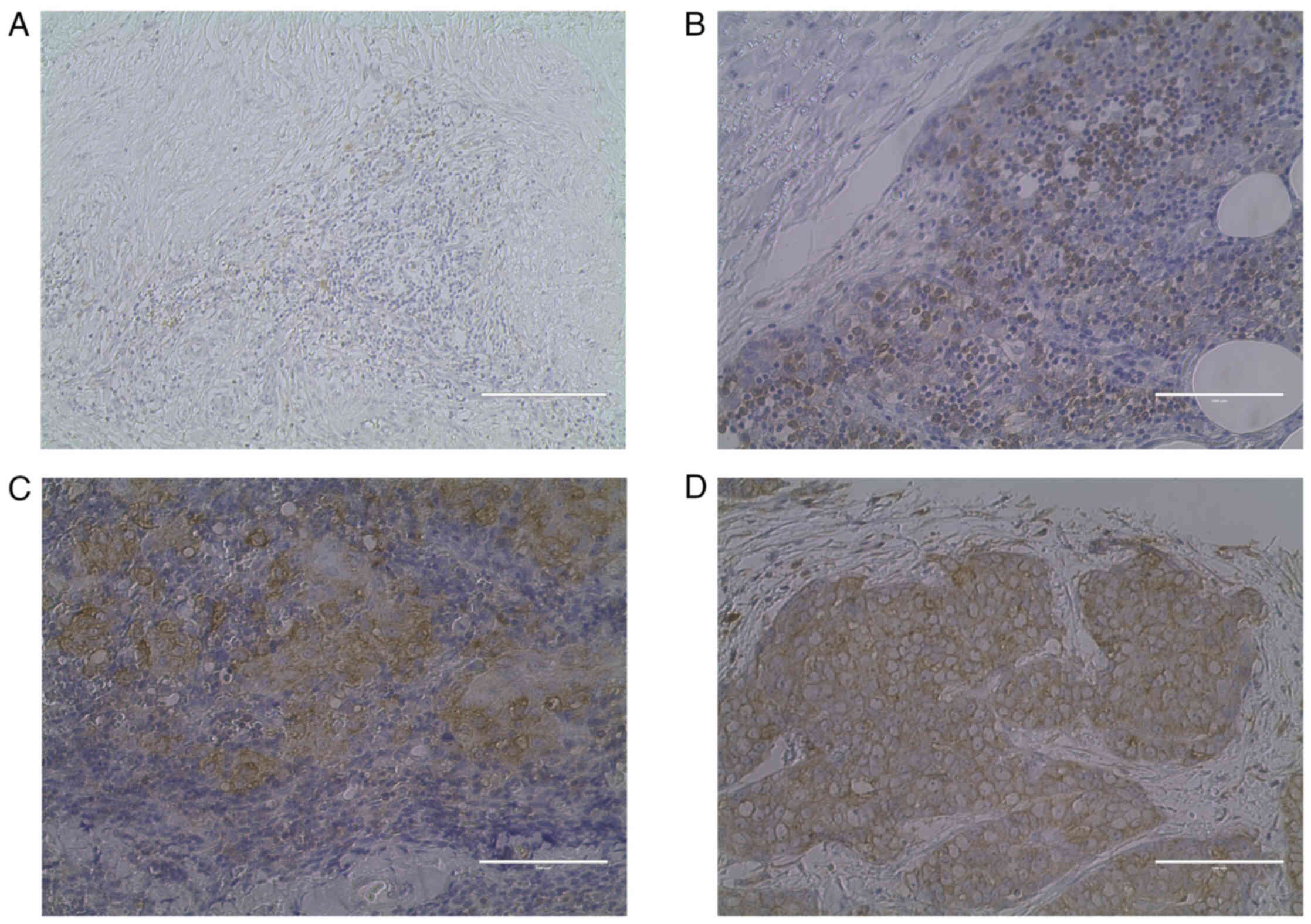
Representative images of hematoxylin and eosin
staining and IHC are presented in Figs.
1 and 2. A case of type A
thymoma is shown in Figs. 1A and
2A (VEGFR-2 staining intensity
score, 0; 0%); a case of type B1 thymoma is shown in Figs. 1B and 2B (VEGFR-2 staining intensity score, 1;
40%); a case of type B3 thymoma is shown in Figs. 1C and 2C (VEGFR-2 staining intensity score, 2;
65%); and a case of TC is shown in Figs. 1D and 2D (VEGFR-2 staining intensity score, 3;
95%).
The relationship between the staining score and the
pathological stage is presented in Table III. There was a significant
positive correlation between the VEGFR-2 staining score and the
pathological stage (correlation coefficient, 0.167; P=0.04). A
significant positive correlation was also demonstrated between the
tumor diameter and VEGFR-2 staining score (correlation coefficient,
0.339; P=0.00003). There was no correlation with any other
clinicopathological factor (data not shown).
 | Table III.Number of VEGFR-2 stained cases per
pathological stage of thymus epithelial tumor. |
Table III.
Number of VEGFR-2 stained cases per
pathological stage of thymus epithelial tumor.
|
| VEGFR-2 score |
|
|---|
|
|
|
|
|---|
| Pathological
stage | 0 | 1 | 2 | 3 | Total |
|---|
| I | 15 | 14 | 2 | 0 | 31 |
| II | 26 | 43 | 10 | 3 | 82 |
| III | 4 | 6 | 6 | 2 | 18 |
| IV | 5 | 3 | 4 | 1 | 13 |
| Total | 50 | 66 | 22 | 6 | 144 |
Association between the VEGFR-2
staining score value and clinicopathological factors
When the product of the VEGFR-2 staining score and
the % stained cells (defined as the VEGFR-2 staining score value)
was evaluated, the mean value was 18.5, and the median value was 5.
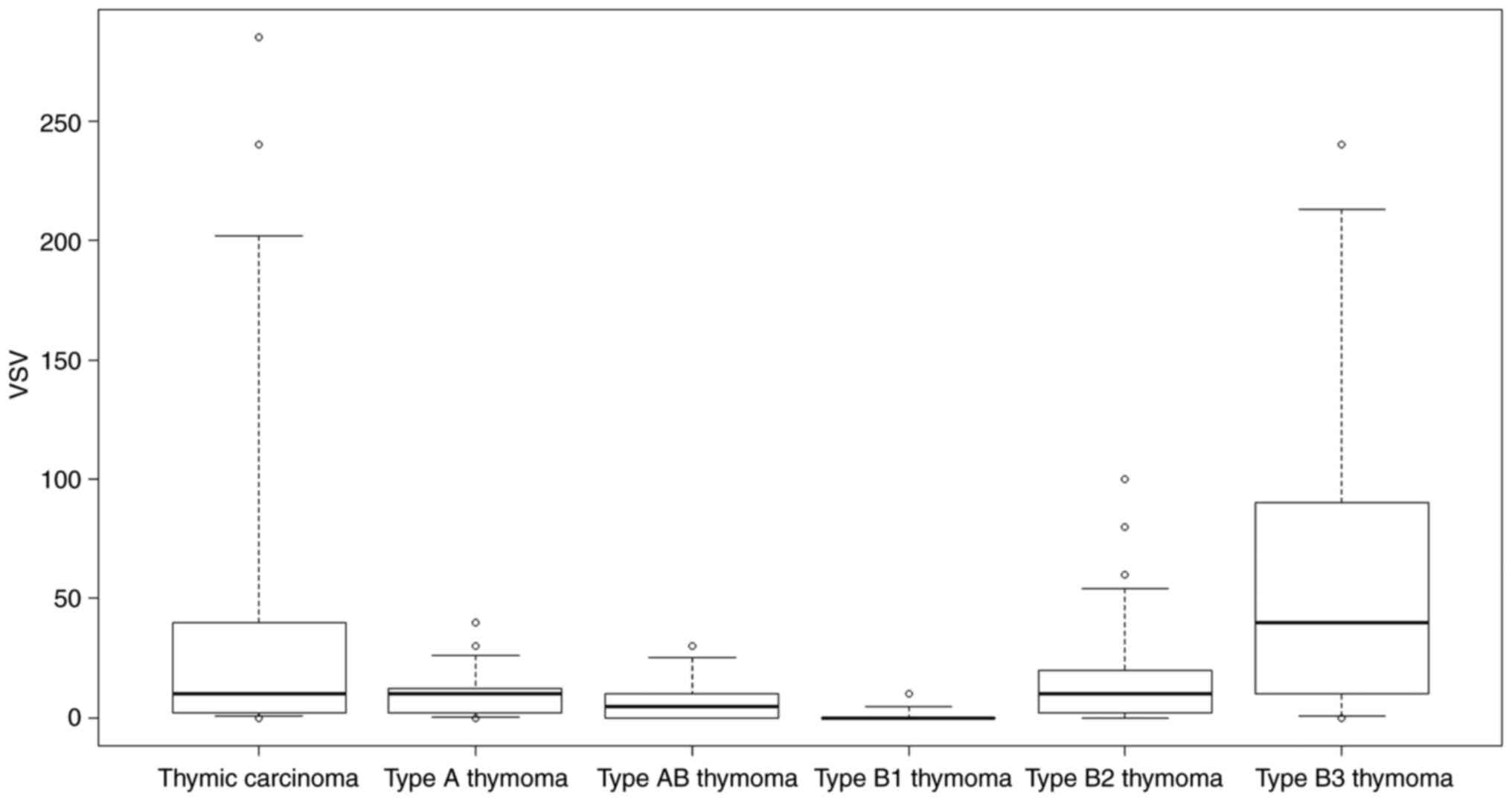
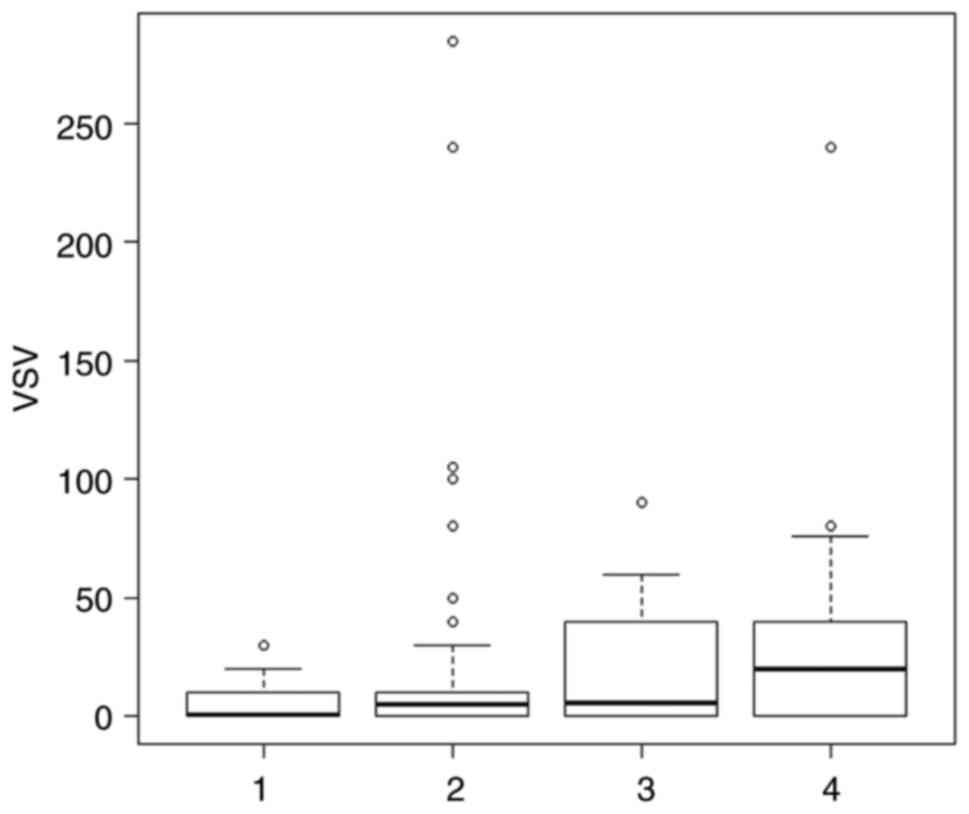
Fig. 3 demonstrates the range of
VEGFR-2 staining scores for each type of TET. Type B1 thymoma had
the lowest staining score.
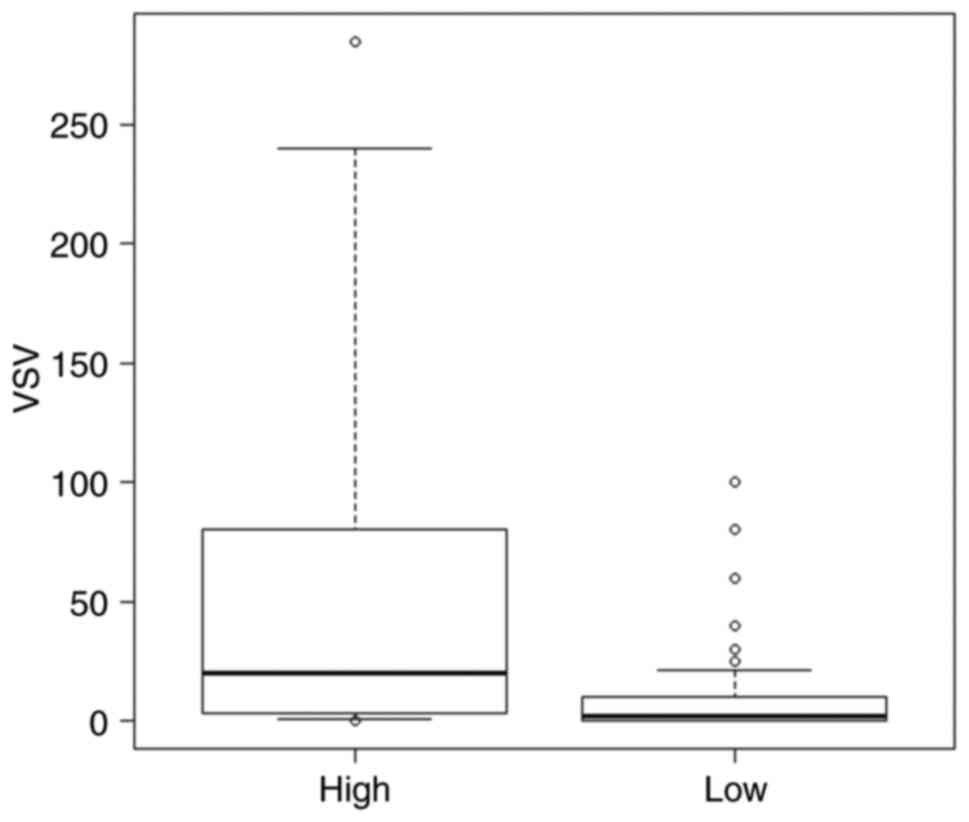
In the present study, Type A, AB, B1 and B2 thymomas
were categorized as low-grade TETs, whilst type B3 thymomas and TCs
were categorized as high-grade TETs. The mean ± standard deviation
of the VEGFR-2 staining score value was 9.11±88.05 for low-grade
TET, compared with 61.03±88.06 for high-grade TET. The values were
significantly higher in the high-grade TET group compared with in
the low-grade TET group (P=0.0063; Fig.
4).
Preoperative treatment
The association between preoperative steroid pulse
therapy and the VEGFR-2 staining score value was analyzed and the
results demonstrated that the VEGFR-2 staining score values of
patients who received steroid pulse therapy did not differ from
those of patients who did not receive steroid pulse therapy (data
not shown).
No significant differences were identified between
the groups categorized according to pathological stage (P=0.226;
Fig. 5). Advanced-stage TET (stages
3–4) had a higher VEGFR-2 staining score in comparison with
early-stage TET (stage 1–2); however, the difference between the
groups was not statistically significant (P=0.24).
Association between the VEGFR-2
staining score value and the prognosis
The 5-year OS and DFS rates for the overall
population were 53.1 and 50.8%, respectively. The 5-year OS and DFS
rates in the low-grade TET group (OS, 53.4%; DFS, 54.3%) were
notably higher than those in the high-grade TET group (OS, 36.6%;
DFS, 32.2%); however, the differences were not significant (OS,
P=0.48; DFS, P=0.408). Furthermore, the 5-year OS and DFS rates of
patients with negative VEGFR-2 staining score values (OS, 66.5%;
DFS, 65.5%) were higher than those of patients with positive
VEGFR-2 staining score values (OS, 42.5%; DFS, 45.4%), and the
differences were found to be significant (OS, P=0.000078; DFS,
P=0.00051).
Table IV shows
univariate and multivariate analysis of TETs prognosis. Univariate
analyses identified preoperative therapy (P=0.00869), VEGFR-2
staining score value >1 (P=0.0438) and advanced pathological
stage (P=0.000253) as significant risk factors for OS. Age
(P=0.5661), sex (P=0.06429), histological grade (P=0.1139), smoking
history (P=0.9193), and tumor size (P=0.6264) were not associated
with OS. Advanced pathological stage was the only factor that
showed a significant association with OS in the multivariate
analysis (P=0.008076).
 | Table IV.Univariate and multivariate analysis
of thymic epithelial tumor prognosis. |
Table IV.
Univariate and multivariate analysis
of thymic epithelial tumor prognosis.
|
| Univariate
analysis | Multivariate
analysis |
|---|
|
|
|
|
|---|
| Variable | HR (95% CI) | P-value | HR (95% CI) | P-value |
|---|
| Age | 1.01
(0.975–1.047) | 0.5661 |
|
|
| Sex |
|
|
|
|
|
Male | 2.58
(0.95–7.02) | 0.0643 |
|
|
|
Female | 1 |
|
|
|
| Preoperative
therapy |
|
|
|
|
|
Yes | 4.70
(1.48–14.92) | 0.0087 | 1.67
(0.47–5.99) | 0.4302 |
| No | 1 |
|
|
|
| Histological
grade |
|
|
|
|
|
Low | 1 |
|
|
|
|
High | 2.33
(0.82–6.65) | 0.1139 |
|
|
| VEGFR score |
|
|
|
|
|
Positive | 3.25
(1.03–10.22) | 0.0438 | 2.23
(0.71–70.34) | 0.1714 |
|
Negative | 1 |
|
|
|
| Smoking |
|
|
|
|
|
Never | 1 |
|
|
|
|
Smoker | 1.05
(0.40–2.77) | 0.9193 |
|
|
| Pathological
stage |
|
|
|
|
| 1 and
2 | 1 |
|
|
|
| 3 and
4 | 2.45
(1.52–3.95) | 0.0003 | 2.08
(1.21–3.58) | 0.0080 |
| Tumor size |
|
|
|
|
| ≤5
cm | 1 |
|
|
|
| >5
cm | 1.27
(0.49–3.30) | 0.6264 |
|
|
Of the 13 deaths in the current study, 7 were
tumor-related deaths and 6 were deaths from other diseases.
Discussion
TET is a rare tumor, and the elucidation of improved
treatment for advanced and relapsed cases, especially for TC and
type B3 thymoma, is needed. Overcoming the difficulties of planning
a clinical study to establish novel effective drug therapies, a
previous study reported that a daily dose of 24 mg lenvatinib
demonstrated a 38.1% response rate, and achieved a marked reduction
in tumor size for recurrent or unresectable TC (2). Regarding VEGFR molecular-targeted
therapeutic drug inhibitor for TC and B3 thymomas, the RELEVENT
phase II trial reported that carboplatin + paclitaxel +
ramucirumab, a monoclonal antibody with antiangiogenic activity
that specifically targets the extracellular domain of VEGFR-2, was
effective for untreated metastatic TC and B3 thymoma (11).
To date, no genetic driver mutations have been
reported in thymomas and TC that could serve as therapeutic targets
for effective therapy, to the best of our knowledge. A previous
study reported that no obvious driver mutations can be detected in
thymomas and TC (12). According to
the results of a biomarker analysis in a phase 2 trial of
lenvatinib for advanced medullary thyroid cancer, several factors,
including high serum VEGF, soluble VEGFR-3 and platelet-derived
growth factor-α, and low baseline levels of soluble Tie-2, were
reported as positive predictive markers for the efficacy of
lenvatinib (13). Circulating
fibroblast growth factor (FGF)19 and FGF23 were also indicated as
positive predictive markers for the efficacy of lenvatinib in
hepatocellular and thyroid carcinoma, respectively (14,15).
For the growth of tumors, blood vessels are needed
to carry nutrients and oxygen along with waste products and
metabolites. The limit of diffusion in the interstitial fluid is
~200 µm, and as long as tumors are small, metabolic maintenance is
possible without blood vessels entering the tumor. However, for
tumors >1-2 mm, angiogenesis into the tumor is required.
Angiogenesis is associated with tumor growth and distant
metastasis, and angiogenic factors include VEGF, basic fibroblast
growth factor, angiopoietin, hepatocyte growth factor, EGF and
placental growth factor (16). The
findings of the present study suggest that low-grade TETs,
specifically thymoma types A and B1, express minimal VEGFR-2
expression levels. Conversely, high-grade TETs, including TC and
thymoma type B3, demonstrated elevated VEGFR-2 expression levels.
Larger tumors and more advanced-stage lesions were also
demonstrated to express VEGFR-2. To the best of our knowledge, no
other studies have evaluated tumor size and the expression of
VEGFR-2. The expression of VEGF mRNA has been reported to be
increased in many solid tumors, including brain, gastric, renal,
colon and ovarian tumors (17–19).
Since the formation of blood vessels is necessary for solid tumors
to grow beyond a certain size, it is conceivable that the
expression of VEGF, which is strictly specific to vascular
endothelial cells, increases during tumor growth (20).
Factors known to adversely affect the recurrence and
life expectancy in patients with TETs include tumor size,
incomplete resection and pathological staging; however, age, sex,
and myasthenia gravis are not considered to have a prognostic
impact (21,22). Furthermore, colorectal cancer
(23), gastric cancer (24), cervical cancer (25) and papillary thyroid cancer (26,27)
have been reported to have an adverse effect on the prognosis in
VEGF- and VEGFR-positive case groups. However, to the best of our
knowledge, there are no previous studies assessing the relationship
between VEGF or VEGFR and prognosis in thymoma and TC. In the
univariate analysis in the present study, a high expression of
VEGFR-2 was associated with a worse prognosis; however, the
expression of VEGFR-2 showed no prognostic impact in the
multivariate analysis. The present study investigated the PFS and
OS according to differences in VEGFR-2 expression; however, as the
numbers of cases with thymic cancer and type B3 thymoma, and stage
III and IV disease are small, no significant differences were
observed in terms of PFS or OS.
If the expression of VEGFR-2 is confirmed to predict
the efficacy of lenvatinib in future studies, there may be
benefits, such as increased complete resection rates, if lenvatinib
can be used as a preoperative therapy for TETs with surrounding
organ invasion. To this end, future research should evaluate
VEGFR-2 in biopsy specimens and surgical specimens from patients
treated with lenvatinib for the postoperative recurrence of TET and
patients who are diagnosed with unresectable TET, as well as the
antitumor efficacy of lenvatinib and the subsequent recurrence
rate. Alternatively, tumors that require concomitant resection
prior to treatment may no longer require concomitant resection
after treatment. Furthermore, future research should use cell lines
to perform a functional analysis of VEGFR-2.
The present study had several limitations, including
the inclusion of cases in which tissue sections were stored for
long periods, the retrospective case-count design, the single
center setting, and the fact that immunostaining of the tissues was
performed by hand, which may have resulted in uneven staining in
comparison with machine staining. Moreover, the selection bias was
not ignored. In particular, the number of the cases with thymic
cancer and type B3 thymoma, and stage III and IV disease was low,
making it difficult to fully examine them. Despite these
limitations, there are no reports assessing VEGFR-2 expression in
TETs, to the best of our knowledge, and it is hoped that the
present study is a useful report suggesting that VEGFR may be a
novel target for the treatment of TETs. Finally, of the 13 deaths
in the current study, seven were tumor-related deaths and six were
deaths from other diseases. As a high proportion of the deaths from
other diseases, the influence of this factor could not be
excluded.
In conclusion, among TETs, the expression of VEGFR-2
was notably high in TETs that were of a higher grade, larger tumors
and advanced stages. Future research should evaluate the effects of
modulating VEGFR-2 expression on other thymic cancer cells using
cell lines for VEGFR-2 and other receptors in TET.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
KC and KO conceived and designed the study. KC, TM
and SY performed the experiments, acquired data and assessed the
authenticity of all raw data to ensure its legitimacy. KC, KY, TsT,
RO, RN and TaT analyzed the data. KC drafted the manuscript. KO and
KC confirm the authenticity of all the raw data. All authors have
read and approved the final manuscript.
Ethics approval and consent to
participate
The present study was performed in accordance with
the Declaration of Helsinki and approved by the Institutional
Ethics Committee of Nagoya City University Graduate School of
Medical Sciences (Nagoya, Japan; approval no. 70-19-0016). The
requirement for individual patient consent was waived due to the
retrospective nature of the present study and since individuals
were not identified.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
TET
|
thymus epithelial tumor
|
|
TC
|
thymic carcinoma
|
|
VEGF
|
vascular endothelial growth factor
|
|
IHC
|
immunohistochemistry
|
|
VEGFR
|
VEGF receptor
|
|
PBS
|
phosphate-buffered saline
|
|
EGF
|
epidermal growth factor
|
References
|
1
|
Girard N, Ruffini E, Marx A, Faivre-Finn C
and Peters S; ESMO Guidelines Committee, : Thymic epithelial
tumours: ESMO clinical practice guidelines for diagnosis, treatment
and follow-up. Ann Oncol. 26 (Suppl 5):v40–v55. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Sato J, Satouchi M, Itoh S, Okuma Y, Niho
S, Mizugaki H, Murakami H, Fujisaka Y, Kozuki T, Nakamura K, et al:
Lenvatinib in patients with advanced or metastatic thymic carcinoma
(REMORA): A multicentre, phase 2 trial. Lancet Oncol. 21:843–850.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Zhu C, Ma X, Hu Y, Guo L, Chen B, Shen K
and Xiao Y: Safety and efficacy profile of lenvatinib in cancer
therapy: A systematic review and meta-analysis. Oncotarget.
7:44545–44557. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Luttun A, Tjwa M, Moons L, Wu Y,
Angelillo-Scherrer A, Liao F, Nagy JA, Hooper A, Priller J, De
Klerck B, et al: Revascularization of ischemic tissues by PlGF
treatment, and inhibition of tumor angiogenesis, arthritis and
atherosclerosis by anti-Flt1. Nat Med. 8:831–840. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ellis LM and Hicklin DJ: VEGF-targeted
therapy: Mechanisms of anti-tumour activity. Nat Rev Cancer.
8:579–591. 2008. View
Article : Google Scholar : PubMed/NCBI
|
|
6
|
Cimpean AM, Marius R, Svetlana E, Cornea R
and Viorica B: Immunohistochemical expression of vascular
endothelial growth factor A (VEGF) and its receptors (VEGFR1, 2) in
normal and pathologic conditions of human thymus. Ann Anat.
190:238–245. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
WHO classification of tumours editorial
board, . International agency for research on cancer: Thoracic
Tumours. 5th Edition. Lyon; France: 2021
|
|
8
|
Brierley JD, Gospodarowicz MK and
Wittekind C: The TNM classification of malignant tumors. 8th
Edition. Wiley Blackwell; Oxford: 2017
|
|
9
|
Wolff AC, Hammond MEH, Allison KH, Harvey
BE, Mangu PB, Bartlett JMS, Bilous M, Ellis IO, Fitzgibbons P,
Hanna W, et al: Human epidermal growth factor receptor 2 testing in
breast cancer: American society of clinical oncology/college of
American pathologists clinical practice guideline focused update.
Arch Pathol Lab Med. 142:1364–1382. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kanda Y: Investigation of the freely
available easy-to-use software ‘EZR’ for medical statistics. Bone
Marrow Transplant. 48:452–458. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Imbimbo M, Vitali M, Fabbri A, Ottaviano
M, Pasello G, Petrini I, Palmieri G, Berardi R, Zucali P,
Ganzinelli M, et al: RELEVENT trial: Phase II trial of ramucirumab,
carboplatin, and paclitaxel in previously untreated thymic
carcinoma/B3 thymoma with area of carcinoma. Clin Lung Cancer.
19:e811–e814. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Petrini I, Meltzer PS, Kim IK, Lucchi M,
Park KS, Fontanini G, Gao J, Zucali PA, Calabrese F, Favaretto A,
et al: A specific missense mutation in GTF2I occurs at high
frequency in thymic epithelial tumors. Nat Genet. 46:844–849. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Schlumberger M, Jarzab B, Cabanillas ME,
Robinson B, Pacini F, Ball DW, McCaffrey J, Newbold K, Allison R,
Martins RG, et al: A phase II trial of the multitargeted tyrosine
kinase inhibitor lenvatinib (E7080) in advanced medullary thyroid
cancer. Clin Cancer Res. 22:44–53. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Chuma M, Uojima H, Numata K, Hidaka H,
Toyoda H, Hiraoka A, Tada T, Hirose S, Atsukawa M, Itokawa N, et
al: Early changes in circulating FGF19 and ang-2 levels as possible
predictive biomarkers of clinical response to lenvatinib therapy in
hepatocellular carcinoma. Cancers (Basel). 12:2932020. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Tahara M, Schlumberger M, Elisei R, Habra
MA, Kiyota N, Paschke R, Dutcus CE, Hihara T, McGrath S, Matijevic
M, et al: Exploratory analysis of biomarkers associated with
clinical outcomes from the study of lenvatinib in differentiated
cancer of the thyroid. Eur J Cancer. 75:213–221. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Bergers G and Benjamin LE: Tumorigenesis
and the angiogenic switch. Nat Rev Cancer. 3:401–410. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Dvorak HF, Brown LF, Detmar M and Dovrak
AM: Vascular permeability factor/vascular endothelial growth
factor, microvascular hyperpermeability, and angiogenesis. Am J
Pathol. 146:1029–1039. 1995.PubMed/NCBI
|
|
18
|
Mustonen T and Alitalo K: Endothelial
receptor tyrosine kinases involved in angiogenesis. J Cell Biol.
129:895–898. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ferrara N, Houck K, Jakeman L and Leung
DW: Molecular and biological properties of the vascular endothelial
growth factor family of proteins. Endocr Rev. 13:18–32. 1992.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Luo JC, Yamaguchi S, Shinkai A, Shitara K
and Shibuya M: Significant expression of vascular endothelial
growth factor/vascular permeability factor in mouse ascites tumors.
Cancer Res. 58:2652–2660. 1998.PubMed/NCBI
|
|
21
|
Detterbeck F, Youssef S, Ruffini E and
Okumura M: A review of prognostic factors in thymic malignancies. J
Thorac Oncol. 6 (7 Suppl 3):S1698–S1704. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Asamura H, Nakagawa K, Matsuno Y, Suzuki
K, Watanabe S and Tsuchiya R: Thymoma needs a new staging system.
Interact Cardiovasc Thorac Surg. 3:163–167. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ishigami SI, Arii S, Furutani M, Niwano M,
Harada T, Mizumoto M, Mori A, Onodera H and Imamura M: Predictive
value of vascular endothelial growth factor (VEGF) in metastasis
and prognosis of human colorectal cancer. Br J Cancer.
78:1379–1384. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ichikura T, Tomimatsu S, Ohkura E and
Mochizuki H: Prognostic significance of the expression of vascular
endothelial growth factor (VEGF) and VEGF-C in gastric carcinoma. J
Surg Oncol. 78:132–137. 2001. View
Article : Google Scholar : PubMed/NCBI
|
|
25
|
Loncaster JA, Cooper RA, Logue JP,
Davidson SE, Hunter RD and West CM: Vascular endothelial growth
factor (VEGF) expression is a prognostic factor for radiotherapy
outcome in advanced carcinoma of the cervix. Br J Cancer.
83:620–625. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Klein M, Vignaud JM, Hennequin V,
Toussaint B, Bresler L, Plénat F, Leclère J, Duprez A and Weryha G:
Increased expression of the vascular endothelial growth factor is a
pejorative prognosis marker in papillary thyroid carcinoma. J Clin
Endocrinol Metab. 86:656–658. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Hsueh C, Lin JD, Wu IC, Chao TC, Yu JS,
Liou MJ and Yeh CJ: Vascular endothelial growth factors and
angiopoietins in presentations and prognosis of papillary thyroid
carcinoma. J Surg Oncol. 103:395–399. 2011. View Article : Google Scholar : PubMed/NCBI
|