Introduction
According to the World Health Organization
classification of tumors of the digestive system (fifth edition)
published in 2019, malignant tumors of the digestive system include
colorectal, gastric, esophageal, pancreatic and liver cancer,
cholangiocarcinoma, lymphoma and mesenchymal tumors (1). The results of a survey of cancer cases
and deaths in China in 2022 conducted by the National Cancer Center
of the Chinese Academy of Medical Sciences and Peking Union Medical
College indicated that lung, colorectal, stomach, liver and breast
cancer were the top five causes of cancer-associated mortality
among Chinese residents (2). The
incidence and mortality rates of digestive system tumors remains
high, with an estimated 4.8 million new cases and 3.4 million
related deaths worldwide in 2019, accounting for 26% of the global
cancer incidence and 35% of all cancer-related deaths. Therefore,
digestive system tumors still pose a major challenge to global
public health (3). Most malignant
tumors of the digestive system still lack effective targeted drugs,
and conventional radiotherapy and chemotherapy have limited
therapeutic effects (4). Therefore,
early detection and radical surgery remain the main routes to
reduce distant metastases and disease mortality. At present, the
mainstream diagnostic methods are abdominal high-resolution CT
(5), abdominal B ultrasound
(6), fibrogastroscopy and
fibrocolonoscopy (7). Therefore,
biomarkers with high sensitivity and specificity are required for
early disease detection and intervention. In addition, serological
tumor markers, a non-invasive diagnostic method, are widely
applicable and relatively safe, and have an important role in the
screening of malignant tumors of the digestive system (8).
Cysteine proteases can regulate physiological
processes by controlling the hydrolysis of target proteins. The
activity of cysteine proteases is strictly regulated at various
levels, including genetic and epigenetic factors that control gene
expression and protein biosynthesis, post-translational
modifications that affect protein transport and proenzyme
activation (9). If the
protease-inhibitor-substrate balance is disrupted, it may lead to
changes in protease signaling and result in the occurrence and
development of diseases, such as various inflammatory conditions,
neurodegenerative and cardiovascular diseases, viral infections,
atherosclerosis and osteoporosis (10). The development of cancer is a
multi-step process, where different procedures are reflected by
distinct genetic changes, which gradually transform normal cells
into highly malignant cells. For example, mutations in the
catalytic subunit of the PI3-kinase subtype within tumor cells can
excessively activate the PI3-kinase signal transduction pathway,
while carcinogenic mutations in Ras genes can impair Ras GTPase
activity, thereby compromising the negative feedback mechanism of
signal transduction. The mutation of different genes can further
exacerbate the malignant proliferation of cells. These processes
mainly include maintaining proliferation signals, escaping growth
inhibitors, resisting cell apoptosis, inducing angiogenesis and
migration and activating invasion and metastasis pathways (11). Cysteine proteases, as pro-invasive
enzymes, interfere with cytokine/chemokine signaling, regulate cell
adhesion, migration and endocytosis, participate in the antitumor
immune response and cell apoptosis and promote cell invasion,
angiogenesis and metastasis through their effects on extracellular
matrix (12). Cysteine protease
inhibitors are reversible or irreversible inhibitors that widely
exist in living organisms, and can limit the excessive activity of
cysteine proteases, which can indirectly reflect the equilibrium
state between the two molecules and influence the proliferation of
tumor cells in patients (13).
Cystatin is a protease inhibitor that is found in a
number of human cells and tissues. There are 12 different types of
cystatin, which are divided into types I, II and III based on the
differences in their in vivo distribution (14). The distribution of type I is
intracellular, while type II is extracellular, and type III
primarily consists of intravascular inhibitors (14). Cystatin type II is composed of
non-glycosylated proteins, including cystatin C, D, E/M, F, G, S,
SN and SA (14). Cystatin-S (CST4),
a member of the cystatin superfamily, is also known as cystatin
SA-III. CST4 can specifically bind to cysteine protease to regulate
its activity (15). It has been
shown that CST4 enhances the invasiveness of gastric cancer and
promotes its progression by regulating extracellular leucine rich
repeat and fibronectin type III domain containing 2 signaling
(16). Furthermore, it has been
demonstrated that high CST4 expression in ovarian cancer is closely
related to a poor prognosis (17).
A recent study has also shown that both serum CST4 and DR-70 have
diagnostic value in patients with early colorectal cancer, and the
combined detection of CST4 and DR-70 is used to further improve the
early diagnosis of this disease (18). However, the clinical value of CST4
combined with related tumor markers in the diagnosis of digestive
system malignancies has not been elucidated in the current
literature. In addition, Yang et al (19) found through cancer gene mapping that
CST4 was highly expressed in esophageal squamous cell carcinoma,
and survival analysis showed that patients with high CST4
expression had a poor overall survival. A previous study has also
shown that CST4 can promote the occurrence of bone metastasis in
combination with two plasminogen activators (the tissue-type and
urokinase-type plasminogen activators) in vivo (20). In conclusion, CST4 still shows
promise as a general tumor indicator, but clinical data from more
tumor types are required.
α-Fetoprotein (AFP), an indicator used early in the
detection of malignant digestive system tumors, is now mostly used
in the early screening of liver cancer. Although the diagnostic
efficacy of AFP alone is poor, AFP can have better accuracy when
combined with other indicators (21). Carcinoembryonic antigen (CEA), an
indicator used early in the screening of malignant gastrointestinal
tumors, is not only widely used in the diagnosis and prognosis
management of colorectal cancer (22,23),
but it also has certain application prospects in the monitoring of
postoperative recurrence of gastric cancer when combined with
related serological indicators (24). Carbohydrate antigen (CA)199, CA125,
CA153 and CA724 are tumor markers widely used in the clinical
diagnosis of malignant digestive system tumors and have good
applications in the early diagnosis and prognosis evaluation of
colorectal (25), gastric (26) and pancreatic (27) cancer. Although the mainstream
auxiliary diagnostic markers of digestive system malignancies, such
as CEA, AFP and serum oncology markers, are still the commonly used
indicators of digestive malignant disease, a novel type of oncology
indicator with higher sensitivity and specificity is still
required.
Therefore, the aim of the present study was to
evaluate the serum CST4 level and its positivity rate in patients
with malignant and benign digestive system diseases, to analyze the
sensitivity and specificity of CST4 in the diagnosis of malignant
digestive system tumors and examine whether there are differences
in the diagnostic efficacy of CST4 compared with other tumor
markers.
Materials and methods
Patients
A combined total of 200 in-patients and out-patients
who visited the Department of Gastrointestinal Surgery,
Gastroenterology and Oncology at the affiliated Chaohu Hospital of
Anhui Medical University (ChaoHu, China) between June 2022 and
March 2023 were included in the present retrospective study. These
200 patients included 100 patients with malignant digestive system
tumors (the observation group) and 100 patients with benign
diseases (the control group). In the observation group, 26 patients
underwent chemotherapy, 2 patients received immunotherapy, 1
patient received targeted therapy, and 1 patient underwent
radiation therapy. Additionally, a total of 32 patients underwent
two or more combination therapies. Furthermore, it should be noted
that adjuvant therapy was not administered to a total of 38
patients. The control group consisted of patients who were selected
from the ward or outpatient department and all of them had
digestive diseases, excluding individuals without any underlying
health conditions. The inclusion criteria for patients in the
observation group were as follows: i) Patients are 18 years of age
or older; ii) patients have complete medical records at the
hospital; and iii) patients with a clinical, imaging and
pathological diagnosis of a digestive system malignant tumor. The
exclusion criteria for the observation group were as follows: i)
Patients are <18 years old or have incomplete medical records;
ii) confirmation of non-digestive system malignant tumor through
clinical diagnosis and pathology, with exclusion of patients with
metastasis of non-digestive system malignant tumors to the
digestive system; and iii) confirmation of double primary
malignancies through clinical diagnosis, imaging study and
pathology, but inclusion if all sources of malignancy are from the
digestive system and exclusion if the sources are different. The
inclusion criteria for the control group were as follows: i)
Patients are 18 years of age or older; ii) patients have complete
medical records at the hospital; and iii) patients with a clinical,
imaging and pathological diagnosis of a benign condition. The
exclusion criteria for the control group were as follows: i)
Patients are <18 years old or have incomplete medical records;
and ii) patients with a history of malignancy. Ethics approval was
granted by the Ethics Committee of The Affiliated Chaohu Hospital
of Anhui Medical University (approval no. KYXM-202201-058; Chaohu,
China).
Data collection
The relevant information was retrieved from the
electronic database and medical record system of The Affiliated
Chaohu Hospital of Anhui Medical University. The patient
information collected includes: i) Records of admission or
outpatient visits; ii) records of disease course; iii) records of
laboratory examinations; iv) records of imaging examinations; v)
reports of pathology findings; and vi) discharge summaries. The key
variables included in the analysis encompassed age, sex, history of
hypertension and diabetes, diagnosis, pathology, distant metastasis
and levels of CST4, AFP, CEA, CA199, CA125, CA153 and CA724.
Detection method
The kits and analytical methods were utilized in
accordance with the instructions provided by the manufacturer. CST4
levels were detected using a human CST4 ELISA kit (cat. no.
20173403280; Shanghai Liangrun Biomedical Technology Co., Ltd.).
The cut-off value used to indicate positive cancer results was
101.0 U/ml. The tumor markers AFP, CEA, CA199, CA125, CA153 and
CA724 were detected through electrochemiluminescence immunoassays
using the Cobas® e801 analytical unit (Roche
Diagnostics). AFP levels were detected using Elecsys AFP kit (cat.
no. 07026706190); CEA levels were detected using Elecsys CEA kit
(cat. no. 07027079190); CA199 levels were detected using Elecsys
CA199 kit (cat. no. 07027028190); CA125 levels were detected using
Elecsys CA125 II kit (cat. no. 07026986190); CA153 levels were
detected using Elecsys CA125 II kit (cat. no. 07027001190); and
CA724 levels were detected using Elecsys CA724 kit (cat. no.
07324910190) (all from Roche Diagnostics). The cut-off values used
for AFP, CEA, CA199, CA125, CA153 and CA724 to indicate positive
cancer results were 10.0 ng/ml, 5.0 ng/ml, 39.0 U/ml, 35.0 U/ml,
31.3 U/ml and 8.2 U/ml, respectively.
Statistical analysis
All data in the present study were analyzed using
SPSS 29.0 (IBM Corp.), JMP 16.2.0 (SAS Institute, Inc.) and
GraphPad Prism 8.0 (Dotmatics). Data normality was assessed using
the Kolmogorov-Smirnov and Shapiro-Wilk tests. Data conforming to a
normal distribution are presented as the mean ± standard deviation.
Data that did not follow a normal distribution are presented as the
median and interquartile range (IQR). Given the presence of
multiple groups of tumor indicators, their specific distributions
are provided in Table SI.
Mann-Whitney U test was employed to compare two groups of data. For
multiple datasets, Kruskal-Wallis followed by the Steel-Dwass post
hoc test was used. The McNemar test followed by Bonferroni
correction was used to compare the rates of diagnostic methods. The
McNemar test requires the addition of 0.5 to each cell count if
both cells have a value of 0, ensuring compatibility with the SPSS
29.0 software package for accurate result output. The χ2
test and Fisher's exact test were used to compare qualitative data.
The comprehensive diagnostic efficiency was examined using the area
under the receiver operating characteristic (ROC) curve, and the
Delong test followed by Bonferroni correction was performed to
compare the areas under the curve (AUCs). The probability value of
combined diagnosis was fitted using binary logistic regression and
then the ROC curves were plotted. After counting the number of true
positives (TP), false positives (FP), true negatives (TN) and false
negatives (FN) of each indicator, the following formula was used to
calculate the relevant indicators: Sensitivity=TP/(TP + FN);
Specificity=TN/(FP + TN); Positive predictive value=TP/(TP + FP);
Negative predictive value=TN/(FN + TN);
+LR=Sensitivity/(1-Specificity); -LR=(1-Sensitivity)/Specificity;
Accuracy=(TP + TN)/(TP + FP + TN + FN). All statistical analyses
were two-tailed, and P<0.05 was considered to indicate a
statistically significant difference.
Results
Patient clinical data
A total of 100 patients with digestive malignancies
and 100 patients with benign diseases were included in the present
study. Patients in the observation group were aged 43–86 years
[median (IQR), 70.00 (60.00, 75.75) years] and included 71 men and
29 women. The observation group included 15 patients with
esophageal malignancies, 40 patients with stomach malignancies, 12
patients with pancreatic malignancies, 16 patients with colon
malignancies and 17 patients with rectal malignancies. Patients in
the control group were aged 18–101 years (mean ± standard
deviation, 63.00±14.61 years) and included 58 men and 42 women. The
control group consisted of 45 patients with gastrointestinal polyps
and 55 patients with non-gastrointestinal polyps, presenting with
gastritis, gastric ulcer, constipation, diarrhea, colitis, abnormal
appetite and other related conditions. The clinical data of these
patients are presented in Table I.
There was no significant difference in sex distribution between the
observation and control groups (P>0.05), but there was a
significant difference in the age of the two groups. The number of
elderly patients (aged ≥60 years) in the observation group was
higher than that in the control group (P<0.01), indicating that
the risk of digestive malignancies increased with age. There was no
significant difference in the number of patients with hypertension
and diabetes between the observation and control groups
(P>0.05), indicating that these conditions were not associated
with digestive malignancies.
 | Table I.Clinical data of patients with
digestive system malignant tumors and benign diseases. |
Table I.
Clinical data of patients with
digestive system malignant tumors and benign diseases.
| Characteristic | Digestive malignant
tumors, n (%) | Digestive benign
diseases, n (%) | χ2 | P-value | OR (95% CI) |
|---|
| Sex |
|
|
|
|
|
|
Male | 71 (71.00) | 58 (58.00) | 3.690 | 0.055 | 1.224
(0.994–1.508) |
|
Female | 29 (29.00) | 42 (42.00) |
|
|
|
| Age, years |
|
|
|
|
|
|
≥60 | 76 (76.00) | 56 (56.00) | 8.913 | 0.003 | 1.357
(1.105–1.667) |
|
<60 | 24 (24.00) | 44 (44.00) |
|
|
|
| Hypertension |
|
|
|
|
|
|
Yes | 26 (26.00) | 28 (28.00) | 0.101 | 0.750 | 0.929
(0.588–1.465) |
| No | 74 (74.00) | 72 (72.00) |
|
|
|
| Diabetes |
|
|
|
|
|
|
Yes | 11 (11.00) | 11 (11.00) | 0.000 | 1.000 | 1.000
(0.455–2.200) |
| No | 89 (89.00) | 89 (89.00) |
|
|
|
Comparison of the serum levels of CST4
and related tumor markers
To facilitate a more comprehensive evaluation of the
serum distribution of seven indicators, the control group was
further divided into two subgroups, one comprising patients with
gastrointestinal polyps and the other consisting of patients with
non-gastrointestinal polyps. The results demonstrated that there
were no significant differences in the serum levels of CST4, CEA,
CA199, CA125, CA153 and CA724 between patients with
gastrointestinal polyps and patients with non-gastrointestinal
polyps (P>0.05; Fig. 1A and
C-G). The levels of AFP were significantly different between
patients with gastrointestinal polyps and patients with
non-gastrointestinal polyps (P<0.05; Fig. 1B). The serological levels of CST4,
CEA, AFP, CA199, CA125, CA153 and CA724 exhibited a statistically
significant increase in patients with digestive system malignancies
compared to those with non-gastrointestinal polyps (P<0.05;
Fig. 1A-G). The serological levels
of CST4, CEA, CA199 and CA125 exhibited a statistically significant
increase in patients with digestive system malignancies compared to
those with gastrointestinal polyps (P<0.05; Fig. 1A and C-E).
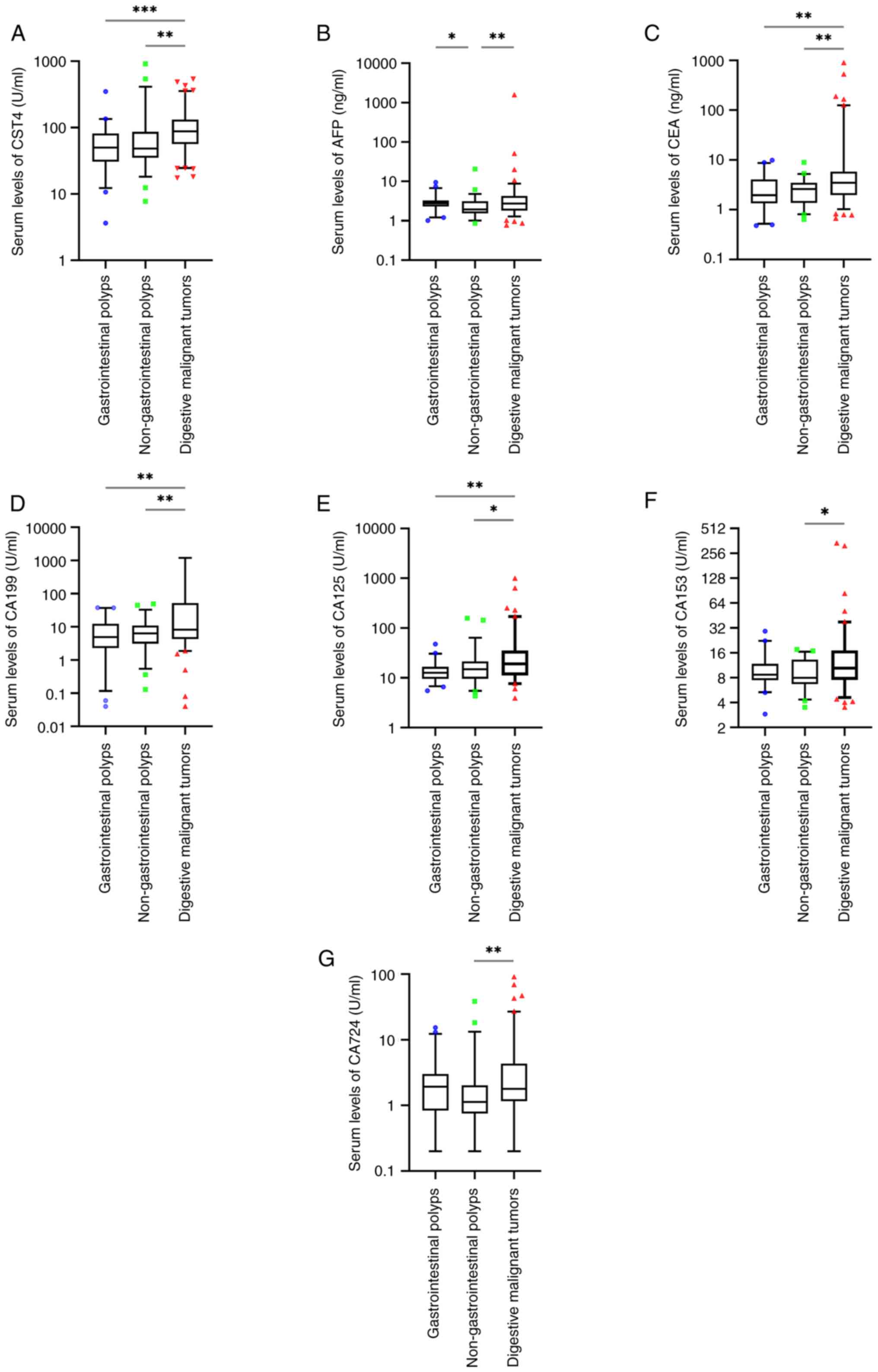 | Figure 1.Serum levels of human (A) CST4, (B)
AFP, (C) CEA, (D) CA199, (E) CA125, (F) CA153 and (G) CA724 in
patients with digestive malignant tumors, gastrointestinal polyps
and non-gastrointestinal polyps. *P<0.05; **P<0.01;
***P<0.001. CST4, cystatin-S; AFP, α-fetoprotein; CEA,
carcinoembryonic antigen; CA, carbohydrate antigen. |
Application of CST4 and related tumor
markers in the clinical diagnosis of malignant digestive
tumors
Through the analyses of relevant data, it was found
that the positive rates of CST4, CEA, CA199, CA125 and CA724 in the
observation group were significantly higher than those in the
control group (P<0.01), while the positive rates of AFP and
CA153 were not significantly different between the two groups
(P>0.05) (Table II). The ROC
curves were evaluated for the aforementioned tumor markers
(including CST4, CEA, AFP, CA199, CA125, CA153 and CA724) to
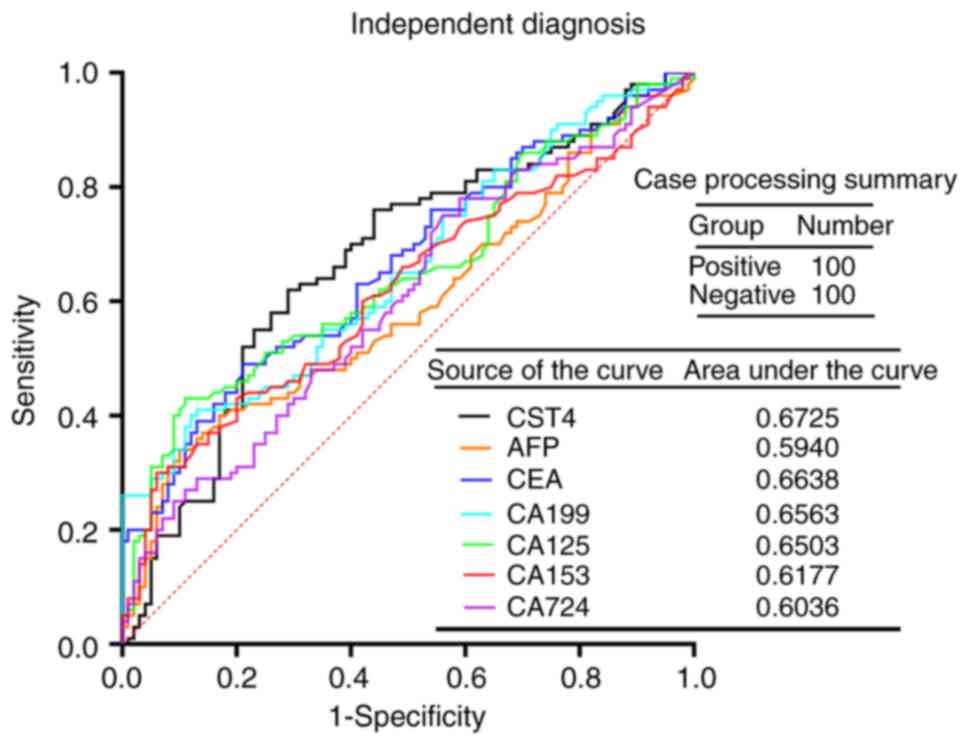
examine the diagnostic value of these markers in cancer (Fig. 2). The results demonstrated that the
AUC of CST4 was 0.6725, which was followed by CEA (AUC, 0.6638).
Since the difference among the AUCs of each index was small, paired
AUC comparisons were made to examine whether there were any
differences. The results indicated that there were no significant
differences in the paired comparisons between the AUC values of
CST4, AFP, CEA, CA199, CA125, CA153 and CA724 (P>0.05; Table III).
 | Table II.Comparison of related tumor markers
in the diagnosis of digestive malignant tumors. |
Table II.
Comparison of related tumor markers
in the diagnosis of digestive malignant tumors.
|
| Group |
|
|
|---|
|
|
|
|
|
|---|
| Biomarker | Digestive malignant
tumors, n (%) | Digestive benign
diseases, n (%) | χ2 | P-value |
|---|
| CST4 |
|
|
|
|
|
Positive | 38 (38.00) | 17 (17.00) | 11.060 | 0.001 |
|
Negative | 62 (62.00) | 83 (83.00) |
|
|
| AFP |
|
|
|
|
|
Positive | 5 (5.00) | 1 (1.00) | N/A | 0.212 |
|
Negative | 95 (95.00) | 99 (99.00) |
|
|
| CEA |
|
|
|
|
|
Positive | 31 (31.00) | 2 (2.00) | 30.521 | <0.001 |
|
Negative | 69 (69.00) | 98 (98.00) |
|
|
| CA199 |
|
|
|
|
|
Positive | 26 (26.00) | 2 (2.00) | 23.920 | <0.001 |
|
Negative | 74 (74.00) | 98 (98.00) |
|
|
| CA125 |
|
|
|
|
|
Positive | 25 (25.00) | 5 (5.00) | 15.686 | <0.001 |
|
Negative | 75 (75.00) | 95 (95.00) |
|
|
| CA153 |
|
|
|
|
|
Positive | 5 (5.00) | 0 (0.00) | N/A | 0.059 |
|
Negative | 95 (95.00) | 100 (100.0) |
|
|
| CA724 |
|
|
|
|
|
Positive | 18 (18.00) | 6 (6.00) | 6.818 | 0.009 |
|
Negative | 82 (82.00) | 94 (94.00) |
|
|
 | Table III.Comparison of AUCs of relevant
indicators. |
Table III.
Comparison of AUCs of relevant
indicators.
| Groups
compared | Z-value | P-value | AUC variance |
|---|
| CST4-AFP | 1.390 | 1.000 | 0.079 |
| CST4-CEA | 0.161 | 1.000 | 0.009 |
| CST4-CA199 | 0.332 | 1.000 | 0.016 |
| CST4-CA125 | 0.451 | 1.000 | 0.022 |
| CST4-CA153 | 1.032 | 1.000 | 0.055 |
| CST4-CA724 | 1.229 | 1.000 | 0.069 |
| AFP-CEA | −1.298 | 1.000 | −0.070 |
| AFP-CA199 | −1.118 | 1.000 | −0.062 |
| AFP-CA125 | −1.002 | 1.000 | −0.056 |
| AFP-CA153 | −0.436 | 1.000 | −0.024 |
| AFP-CA724 | −0.178 | 1.000 | −0.010 |
| CEA-CA199 | 0.160 | 1.000 | 0.008 |
| CEA-CA125 | 0.260 | 1.000 | 0.014 |
| CEA-CA153 | 0.913 | 1.000 | 0.046 |
| CEA-CA724 | 1.161 | 1.000 | 0.060 |
| CA199-CA125 | 0.119 | 1.000 | 0.006 |
| CA199-CA153 | 0.789 | 1.000 | 0.039 |
| CA199-CA724 | 0.956 | 1.000 | 0.053 |
| CA125-CA153 | 0.635 | 1.000 | 0.033 |
| CA125-CA724 | 0.816 | 1.000 | 0.047 |
| CA153-CA724 | 0.249 | 1.000 | 0.014 |
Application of CST4 combined with
related tumor markers in the clinical diagnosis of malignant
digestive system tumors
In clinical practice, to improve the identification
of patients with gastrointestinal tumors at an early stage,
multiple tumor indicators are often combined in parallel for
diagnosis (28,29). Before the introduction of CST4 as a
routine diagnostic indicator at The Affiliated Chaohu Hospital of
Anhui Medical University, there were three diagnostic groups
(traditional groups) available for the diagnosis of malignant
tumors of the digestive system: i) Group A, CEA + AFP; ii) Group B,
CA199 + CA125 + CA153 + CA724; and iii) Group C, AFP + CEA + CA199
+ CA125 + CA153 + CA724. After incorporating CST4 as a routine
diagnostic indicator, it was introduced into the traditional groups
with the aim to improve the diagnostic effect. The new groups
involving CST4 are as follows: i) group D, CST4 + CEA + AFP; ii)
group E, CST4 + CA199 + CA125 + CA153 + CA724; and iii) group F,
CST4 + AFP + CEA + CA199 + CA125 + CA153 + CA724. The results of
the data analysis demonstrated that the positive rate of these six
groups in the observation group was significantly higher than that
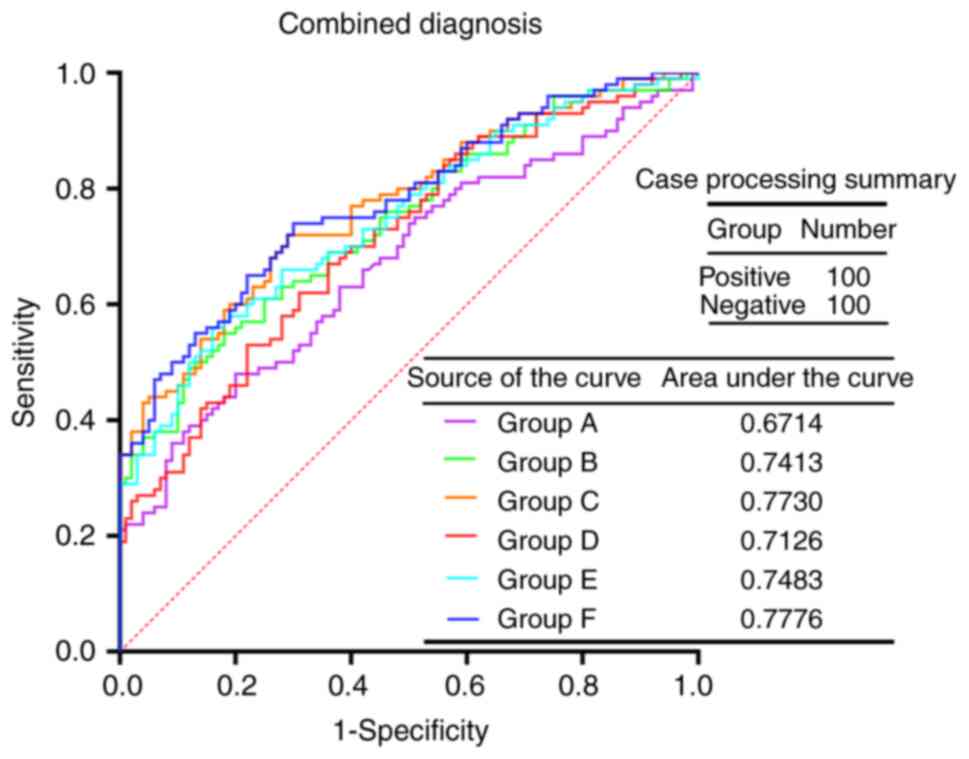
in the control group (P<0.001; Table IV). The ROC curves were evaluated
for the aforementioned six diagnostic groups to examine their
diagnostic value for cancer (Fig.
3). The results demonstrated that group F had the highest AUC
(0.7776), which was followed by group C (AUC, 0.7730). Since the
difference among the AUCs of each index was small, paired AUC
comparisons were made to examine whether there were any
differences. There were no significant differences in the paired
comparisons between the AUC values of groups A, B, C, D, E and F
(P>0.05; Table V).
 | Table IV.Comparison of combined diagnostic
groups in the diagnosis of malignant tumors of the digestive
system. |
Table IV.
Comparison of combined diagnostic
groups in the diagnosis of malignant tumors of the digestive
system.
|
| Group |
|
|
|---|
|
|
|
|
|
|---|
| Diagnostic
group | Digestive malignant
tumors, n (%) | Digestive malignant
tumors, n (%) | χ2 | P-value |
|---|
| A |
|
|
|
|
|
Positive | 32 (32.00) | 11 (11.00) | 13.065 | <0.001 |
|
Negative | 68 (68.00) | 89 (89.00) |
|
|
| B |
|
|
|
|
|
Positive | 50 (50.00) | 13 (13.00) | 31.723 | <0.001 |
|
Negative | 50 (95.00) | 87 (99.00) |
|
|
| C |
|
|
|
|
|
Positive | 62 (62.00) | 23 (23.00) | 31.120 | <0.001 |
|
Negative | 38 (38.00) | 77 (77.00) |
|
|
| D |
|
|
|
|
|
Positive | 56 (56.00) | 27 (27.00) | 17.321 | <0.001 |
|
Negative | 44 (44.00) | 73 (73.00) |
|
|
| E |
|
|
|
|
|
Positive | 63 (63.00) | 26 (26.00) | 27.715 | <0.001 |
|
Negative | 37 (37.00) | 74 (74.00) |
|
|
| F |
|
|
|
|
|
Positive | 71 (71.00) | 35 (35.00) | 26.014 | <0.001 |
|
Negative | 29 (29.00) | 65 (65.00) |
|
|
 | Table V.Comparison of AUCs for relevant joint
indicators. |
Table V.
Comparison of AUCs for relevant joint
indicators.
| Groups
compared | Z-value | P-value | AUC variance |
|---|
| Group A-Group
B | −1.634 | 1.000 | −0.070 |
| Group A-Group
C | −2.716 | 0.099 | −0.102 |
| Group A-Group
D | −1.013 | 1.000 | −0.041 |
| Group A-Group
E | −1.723 | 1.000 | −0.077 |
| Group A-Group
F | −2.778 | 0.082 | −0.106 |
| Group B-Group
C | −1.402 | 1.000 | −0.032 |
| Group B-Group
D | 0.733 | 1.000 | 0.029 |
| Group B-Group
D | −0.312 | 1.000 | −0.007 |
| Group B-Group
F | −1.401 | 1.000 | −0.036 |
| Group C-Group
D | 1.900 | 0.862 | 0.060 |
| Group C-Group
D | 1.094 | 1.000 | 0.025 |
| Group C-Group
F | −0.228 | 1.000 | −0.005 |
| Group D-Group
D | −0.904 | 1.000 | −0.036 |
| Group D-Group
F | −2.250 | 0.366 | −0.065 |
| Group D-Group
F | −1.160 | 1.000 | −0.029 |
Diagnostic efficacy of related tumor
markers in the diagnosis of digestive system malignancies
In a single-index diagnosis, CST4 (38.00%) had the
highest sensitivity, which was followed by CEA (31.00%); however,
CA153 (100.00%) had the highest specificity, which was followed by
AFP (99.00%). In addition, CEA (64.50%) had the highest accuracy in
a single-index diagnosis, which was followed by CA199 (62.00%).
Compared with the traditional groups of tumor markers (including
AFP, CEA, CA199, CA125, CA153 and CA724), the introduction of the
CST4 index improved the sensitivity (Table VI). The sensitivity of Group D
increased by 24% compared to Group A, while Group E experienced a
13% increase in sensitivity compared to Group B Additionally, Group
F observed a 9% increase in sensitivity when compared to Group C
(Table VI).
 | Table VI.Evaluation of the diagnostic efficacy
of related tumor markers in digestive malignant tumors. |
Table VI.
Evaluation of the diagnostic efficacy
of related tumor markers in digestive malignant tumors.
| Tumor marker | Sensitivity
(%) | Specificity
(%) | PPV (%) | NPV (%) | Accuracy (%) | LR+ | LR- |
|---|
| CST4 | 38.00 | 83.00 | 69.09 | 57.24 | 60.50 | 2.24 | 0.75 |
| AFP | 5.00 | 99.00 | 83.33 | 51.03 | 52.00 | 5.00 | 0.96 |
| CEA | 31.00 | 91.00 | 93.94 | 58.68 | 64.50 | 15.5 | 0.70 |
| CA199 | 26.00 | 98.00 | 92.86 | 56.98 | 62.00 | 13.00 | 0.76 |
| CA125 | 25.00 | 95.00 | 83.33 | 55.88 | 60.00 | 5.00 | 0.79 |
| CA153 | 5.00 | 100.00 | 100.00 | 51.28 | 52.50 | N/A | 0.95 |
| CA724 | 18.00 | 94.00 | 75.00 | 53.41 | 56.00 | 3.00 | 0.87 |
| Group A | 32.00 | 89.00 | 74.42 | 56.69 | 61.46 | 2.91 | 0.76 |
| Group B | 50.00 | 87.00 | 79.37 | 63.50 | 68.50 | 3.85 | 0.57 |
| Group C | 62.00 | 65.00 | 63.91 | 63.11 | 63.50 | 1.77 | 0.58 |
| Group D | 56.00 | 73.00 | 67.47 | 62.39 | 64.50 | 2.07 | 0.60 |
| Group E | 63.00 | 74.00 | 70.79 | 66.67 | 68.50 | 2.42 | 0.50 |
| Group F | 71.00 | 65.00 | 66.98 | 69.15 | 68.00 | 2.03 | 0.45 |
Comparison of the sensitivity and
specificity of CST4 and related tumor markers in digestive system
cancer diagnosis
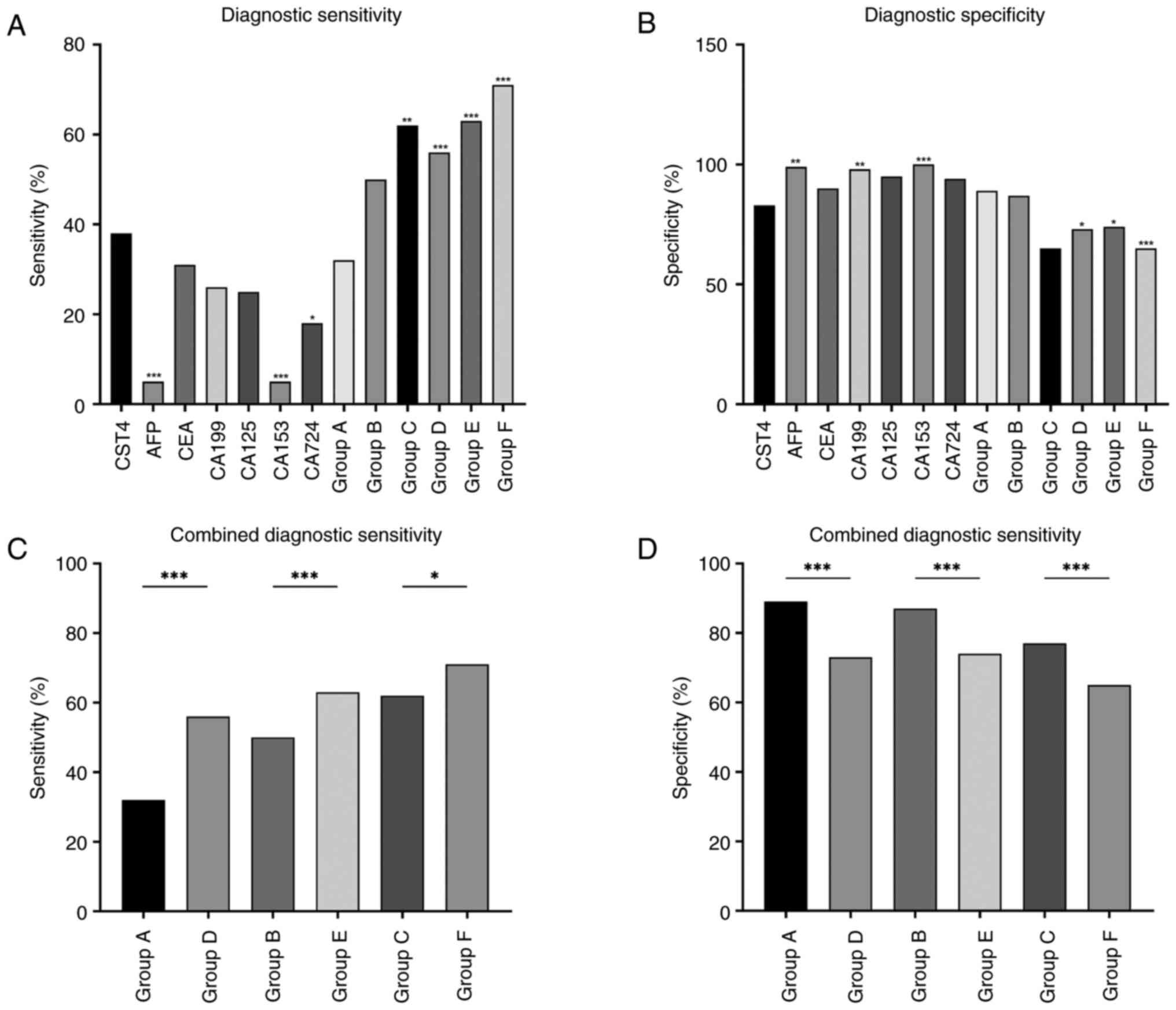
The sensitivity of AFP and CA153 in the
observational group was significantly lower compared with that of
CST4 (P<0.001; Fig. 4A), and the
sensitivity of CA724 was also significantly lower compared with
that of CST4 (P<0.05; Fig. 4A).
The sensitivity of group C (including AFP, CEA, CA199, CA125, CA153
and CA724) was significantly higher than that of CST4 alone
(P<0.01; Fig. 4A). In addition,
the sensitivity of CST4 combined with the other related tumor
markers was significantly higher than that of CST4 alone
(P<0.001; Fig. 4A). The
specificity of AFP, CA199 and CA153 was significantly higher
compared with that of CST4 (P<0.01; Fig. 4B), and there was no significant
difference between the specificity of CST4, CA125 and CA724 in the
observation group (P>0.05; Fig.
4B). The specificity of CST4 combined with the other tumor
markers was significantly lower than that of CST4 alone (P<0.05;
Fig. 4B). In order to evaluate the
sensitivity and specificity of diagnosis after the introduction of
CST4 in the traditional groups, the sensitivity and specificity of
group A vs. group D, group B vs. group E and group C vs. group F
were compared, as shown in Fig. 4C and
D. It was found that the sensitivity of the traditional marker
groups was significantly improved by the addition of CST4
(P<0.05; Fig. 4C); however, the
specificity was significantly decreased (P<0.001; Fig. 4D). Detailed information on group
distributions and comparisons is presented in Table SII, Table SIII, Table SIV, Table SV, Table SVI, Table SVII, Table SVIII, Table SIX.
Association between CST4 and the
clinical features of patients with malignant digestive tumors and
benign diseases
The association between the serum CST4 level and the
relevant clinical features of the observation and control groups
was analyzed, to further explore the role of CST4 in malignant
digestive tumors (Table VII). In
patients with malignant digestive tumors and benign diseases, the
positive rate of CST4 was independent of sex, age, diabetes and
hypertension (P>0.05).
 | Table VII.Association between the positive rate
of cystatin-S and clinical data characteristics of patients with
digestive malignant tumors and benign diseases. |
Table VII.
Association between the positive rate
of cystatin-S and clinical data characteristics of patients with
digestive malignant tumors and benign diseases.
|
| Digestive malignant
tumors | Digestive benign
diseases |
|---|
|
|
|
|
|---|
| Characteristic | n | Positive | Negative | χ2 | P-value | n | Positive | Negative | χ2 | P-value |
|---|
| Sex |
|
|
|
|
|
|
|
|
|
|
|
Male | 71 | 27 | 44 | <0.001 | 0.993 | 58 | 12 | 46 | 1.332 | 0.248 |
|
Female | 29 | 11 | 18 |
|
| 42 | 5 | 37 |
|
|
| Age, years |
|
|
|
|
|
|
|
|
|
|
|
<60 | 24 | 9 | 15 | 0.003 | 0.954 | 44 | 5 | 39 | 1.769 | 0.184 |
|
≥60 | 76 | 29 | 47 |
|
| 56 | 12 | 44 |
|
|
| Hypertension |
|
|
|
|
|
|
|
|
|
|
|
Yes | 26 | 10 | 16 | 0.003 | 0.955 | 28 | 3 | 25 | - | 0.383 |
| No | 74 | 28 | 46 |
|
| 72 | 14 | 58 |
|
|
| Diabetes |
|
|
|
|
|
|
|
|
|
|
|
Yes | 11 | 5 | 6 | - | 0.744 | 11 | 2 | 9 | - | 1.000 |
| No | 89 | 33 | 56 |
|
| 89 | 15 | 74 |
|
|
Association between clinical features
and distant metastasis of malignant digestive tumors
In the observation group, 40 out of 100 patients
developed distant metastases. As shown in Table VIII, distant tumor metastasis in
the observation group was not associated with sex, age,
hypertension or diabetes (P>0.05).
 | Table VIII.Association between distant
metastasis and clinical data characteristics of patients. |
Table VIII.
Association between distant
metastasis and clinical data characteristics of patients.
| Characteristic | n | Patients with
metastasis | Patients without
metastasis | χ2 | P-value |
|---|
| Sex |
|
|
|
|
|
|
Male | 71 | 26 | 45 | 1.166 | 0.280 |
|
Female | 29 | 14 | 15 |
|
|
| Age, years |
|
|
|
|
|
|
<60 | 24 | 11 | 13 | 0.448 | 0.503 |
|
≥60 | 76 | 29 | 47 |
|
|
| Hypertension |
|
|
|
|
|
|
Yes | 26 | 7 | 19 | 2.503 | 0.114 |
| No | 74 | 33 | 41 |
|
|
| Diabetes |
|
|
|
|
|
|
Yes | 11 | 3 | 8 | - | 0.518 |
| No | 89 | 37 | 52 |
|
|
Association between the serum levels
of tumor markers and distant metastasis of malignant digestive
tumors
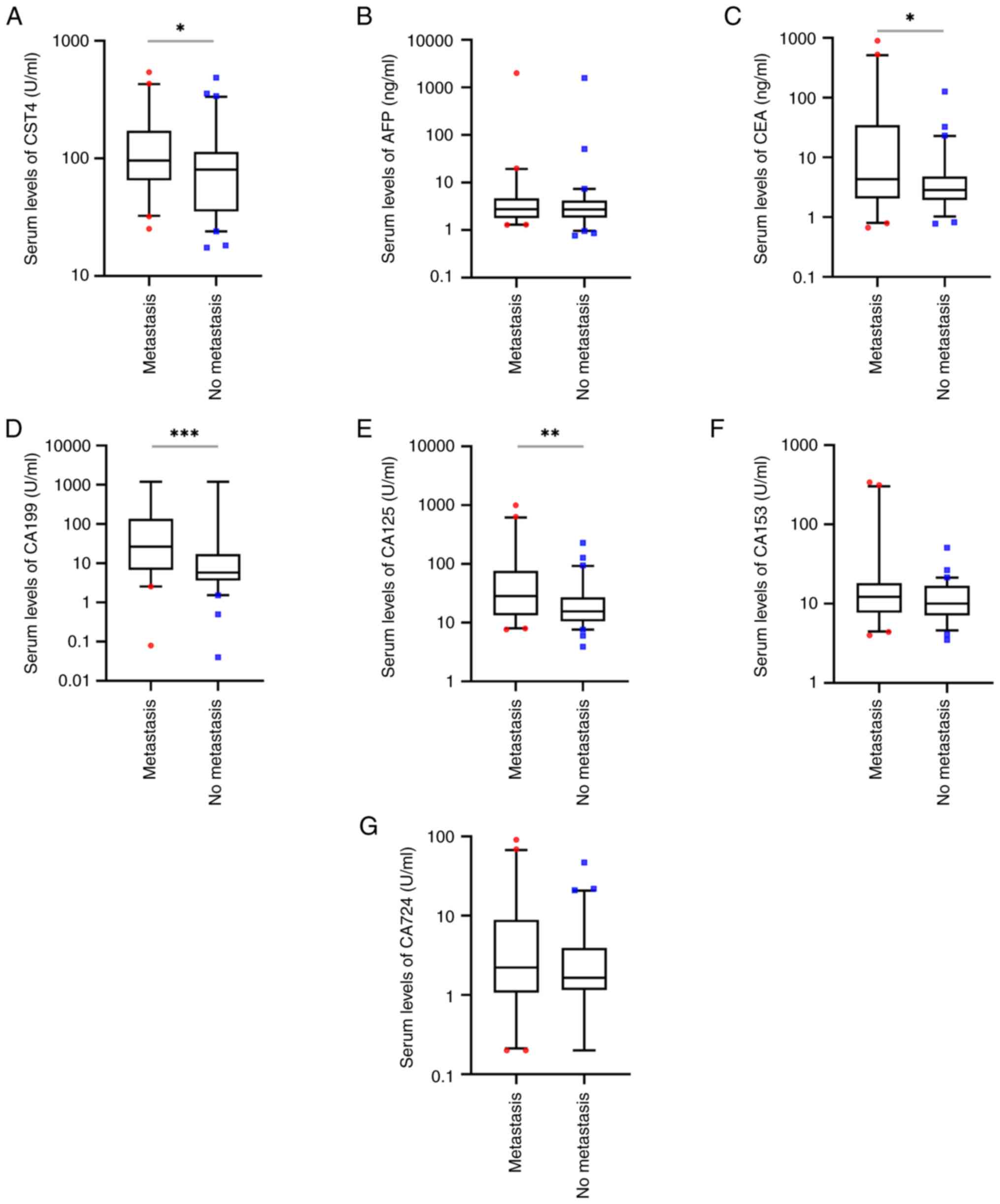
In the present study, the serum levels of AFP, CA153
and CA724 were not significantly different in the 40 patients with
distant metastasis compared with the 60 patients without metastasis
(P>0.05; Fig. 5B, F and G);
however, the serum levels of CST4, CEA, CA199 and CA125 were
significantly higher in patients with distant metastasis compared
with those without metastasis (P<0.05; Fig. 5A and C-E). The positive rates of
CST4, AFP, CA153 and CA724 in patients with distant metastasis were
not significantly different from those in patients without distant
metastasis (P>0.05; Table IX);
however, the positive rates of CEA, CA199 and CA125 were
significantly higher in patient with distant metastasis compared
with those in patients without distant metastasis (P<0.01;
Table IX).
 | Table IX.Comparison of positive rates of
related tumor markers in patients with and without metastasis. |
Table IX.
Comparison of positive rates of
related tumor markers in patients with and without metastasis.
|
| Metastasis, n
(%) |
|
|
|---|
|
|
|
|
|
|---|
| Biomarker | Yes | No | χ2 | P-value |
|---|
| CST4 |
|
|
|
|
|
Positive | 19 (47.50) | 19 (31.67) | 2.554 | 0.110 |
|
Negative | 21 (52.50) | 41 (68.34) |
|
|
| AFP |
|
|
|
|
|
Positive | 3 (7.50) | 2 (3.33) | - | 0.386 |
|
Negative | 37 (92.50) | 58 (96.67) |
|
|
| CEA |
|
|
|
|
|
Positive | 19 (47.50) | 12 (20.00) | 8.485 | 0.004 |
|
Negative | 21 (52.50) | 48 (80.00) |
|
|
| CA199 |
|
|
|
|
|
Positive | 17 (42.50) | 9 (15.00) | 9.433 | 0.002 |
|
Negative | 23 (57.50) | 51 (85.00) |
|
|
| CA125 |
|
|
|
|
|
Positive | 16 (40.00) | 9 (15.00) | 8.000 | 0.005 |
|
Negative | 24 (60.00) | 51 (85.00) |
|
|
| CA153 |
|
|
|
|
|
Positive | 4 (10.00) | 1 (1.67) | - | 0.154 |
|
Negative | 36 (90.00) | 59 (98.33) |
|
|
| CA724 |
|
|
|
|
|
Positive | 10 (25.00) | 8 (13.33) | 2.213 | 0.185 |
|
Negative | 30 (75.00) | 52 (86.67) |
|
|
Association between the serum levels
of tumor markers and tumor types
The results demonstrated significant differences in
the serologic levels of CST4, CA199 and CA153 between pancreatic
cancer and gastrointestinal or non-gastrointestinal polyps
(P<0.05; Fig. 6A, D and F). The
serologic level of CA199 also exhibited a significant difference
between pancreatic cancer and esophageal, stomach and rectal cancer
(P<0.05; Fig. 6D). The serologic
level of CA153 also exhibited a significant difference between
pancreatic cancer and esophageal and stomach cancer (P<0.05;
Fig. 6F). Significant differences
were observed in the serologic levels of CA125 between patients
with esophageal cancer and those with gastrointestinal polyps
(P<0.05; Fig. 6E). There was a
significant difference in serum CA724 levels between patients with
esophageal cancer or stomach cancer, and those with
non-gastrointestinal polyps (P<0.05; Fig. 6G).
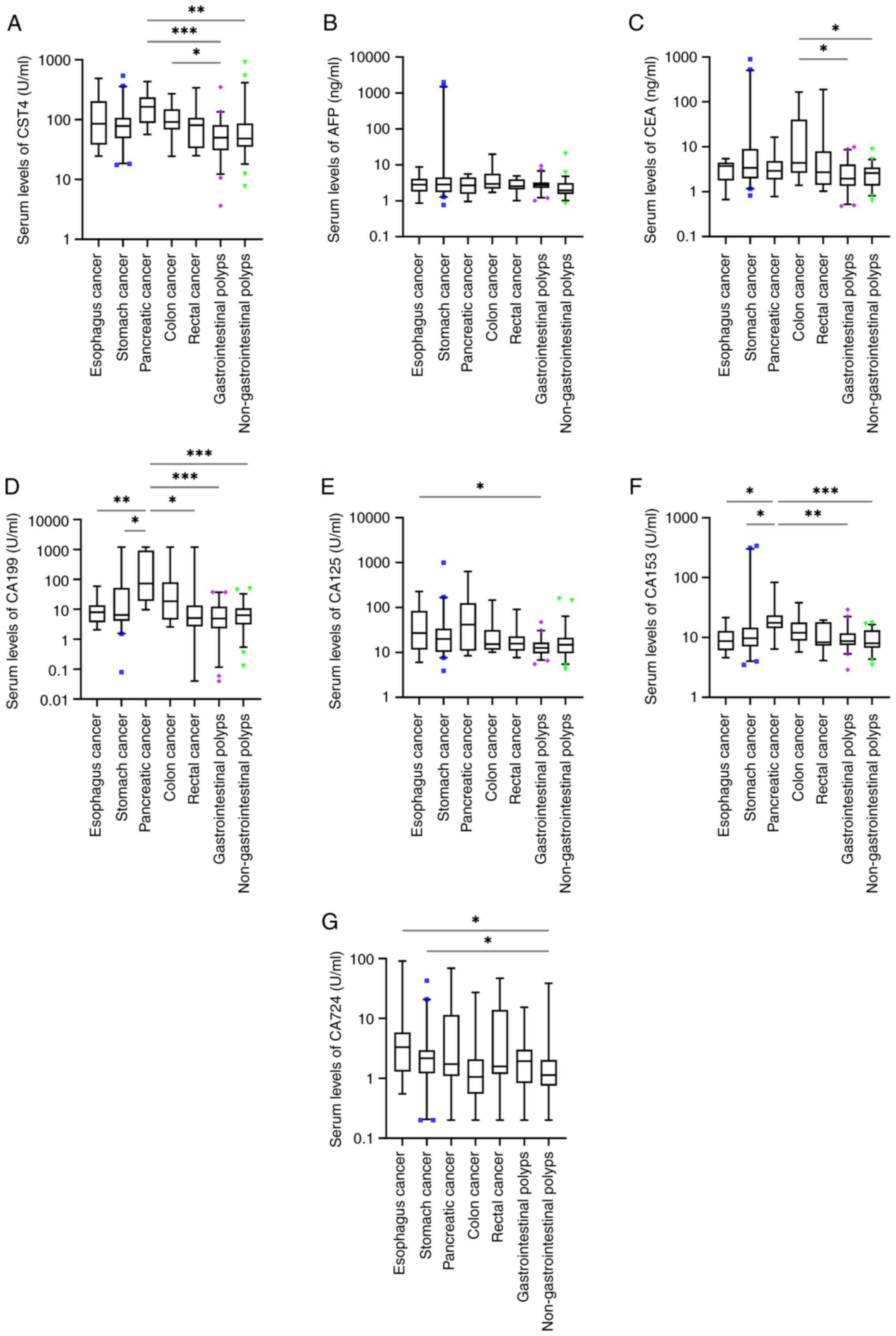 | Figure 6.Serum levels of (A) CST4, (B) AFP,
(C) CEA, (D) CA199, (E) CA125, (F) CA153 and (G) CA724 in patients
with esophageal, stomach, pancreatic, colon and rectal cancer,
gastrointestinal polyps and non-gastrointestinal polyps.
*P<0.05; **P<0.01; ***P<0.001. CST4, cystatin-S; AFP,
α-fetoprotein; CEA, carcinoembryonic antigen; CA, carbohydrate
antigen. |
Discussion
Due to the crucial role of cysteine protease in
tumor regulation (10), cysteine
protease inhibitors can serve as a reliable indicator of tumor
progression. CST4, functioning as a potent cysteine protease
inhibitor, holds great promise as a novel diagnostic marker for
digestive system malignancies. By utilizing retrospective data
analysis, the present study compared CST4 with other tumor markers
to objectively evaluate its diagnostic efficacy and distribution
among different patient populations. In the present study, the
serum levels of CST4, CEA, AFP, CA199, CA125, CA153 and CA724
exhibited significant differences between patients without
gastrointestinal polyps and those with gastrointestinal
malignancies. Moreover, there were also significant differences in
the levels of CST4, CEA, CA199 and CA125 between patients with
gastrointestinal polyps and those diagnosed with gastrointestinal
malignancies. The current findings are consistent with the results
of previous studies (15,18), suggesting an important role of CST4
in the screening of gastrointestinal malignancies, as well as the
ability to differentiate between gastrointestinal polyps and
malignant tumors. In the present study, the serum levels of AFP and
CA724 in patients without gastrointestinal polyps were
significantly different from those in patients with digestive
system malignancies, but there were no significant differences in
these two markers between patients with digestive system
malignancies and gastrointestinal polyps. AFP is widely used in the
screening and diagnosis of hepatocellular malignancies (30), but its application in
gastrointestinal polyps has been little studied; therefore, the
reason for the aforementioned result is unclear. A previous study
has shown that the expression level of CA199 in colon polyps is
higher than that in normal colon mucosa (31), and another study has demonstrated
that CA199 is closely related to the recurrence of colorectal
polyps (32).
The positive rates of CST4, CEA, CA199, CA125 and
CA724 in patients with malignant digestive tumors were
significantly higher than those in patients with benign diseases,
while the positive rates of AFP and CA153 were not significantly
different between the two groups. AFP is mainly used in the
diagnosis of hepatocellular and pancreatic malignancies. However,
the patients selected in the present study did not have
hepatocellular malignancies, and the proportion of patients with
pancreatic cancer was relatively low, which may be the reason for
the low AFP positive rate. CA153 has been widely used in the
diagnosis and evaluation of breast cancer and has also been applied
in the diagnosis of ovarian, pancreatic, gastric and lung cancer
(33). However, malignant tumors
are not the only cause of increases in serum CA153; benign
diseases, such as chronic active hepatitis, cirrhosis, sarcoidosis
and megaloblastic anemia may also lead to changes in the CA153
level. Therefore, the false negative and false positive rates of
CA153 as a biomarker can both be high, which may be the reason for
the low CA153 positive rate in the current study (33). Although in the present study the
positive rate of CA724 was significantly different between the two
patient groups, it has been reported that the CA724 level also
increases in individuals with gout arthritis and benign diseases,
and may also be affected by drugs (34). CEA is mainly used as a diagnostic
indicator for colorectal cancer, but it is also elevated in other
cancer types and diseases, such as pulmonary fibrosis and
Alzheimer's disease (35). CA199 is
widely distributed in normal human tissues and organs, and its
distribution is closely related to genotype. CA199 biosynthesis
depends on the enzymatic activity of fucosyltransferase-2 (FUT2)
and fucosyltransferase-3 (FUT3) (36). The activity of both enzymes is
determined by the FUT2 and FUT3 genotype. The presence of mutations
in genotypes results in alterations in the activity of FUT2 and
FUT3. The T59G mutation of the FUT3 gene has been shown to
significantly affect patient serum CA199 levels (37). In addition to malignant tumors,
other diseases, such as pancreatitis, hepatitis, cirrhosis and
pulmonary fibrosis, also result in an increase or decrease in the
CA199 serum level (38,39). In addition to being a traditional
tumor marker, CA125 has also been used as an evaluation indicator
of heart failure (40,41).
In the present study, the sensitivity of CST4 alone
was the highest (38.00%), followed by CEA (31.00%), CA199 (26.00%)
and CA125 (25.00%). The sensitivity of CST4 in patients with a
digestive system malignancy was not significantly different from
that of CEA, CA199 and CA125, but it was significantly higher than
that of AFP, CA153 and CA724. The specificity of CA153 and AFP was
the highest in patients with benign diseases of the digestive
system (100.00 and 99.00%, respectively). However, when the ROC
curve was constructed to comprehensively evaluate the diagnostic
efficiency of the aforementioned indicators, it was found that
there were no notable differences in the comprehensive performance
of each diagnostic indicator. In addition, although the AUC values
were different, statistically significant differences between were
not observed. There are few published reference values for CST4,
but the sensitivity of CST4 in detecting digestive system tumors in
the present study was lower than that noted in the studies by Dou
et al (15) and Cai et
al (18) in patients with
colorectal or gastric cancer, with no marked difference in
specificity. In the study of Dou et al the ELISA detection
system for CST4 showed significantly better sensitivities of 69.0
and 69.0%, and specificities of 85.6 and 83.6%, for gastric cancer
and colorectal cancer, respectively. Additionally, the study
conducted by Cai et al demonstrated that the AUC of serum
CST4 in patients with early colorectal cancer was 0.927, exhibiting
a sensitivity of 57.8% and a specificity of 95.3%. When compared
with cystatin-SN (CST1), the specificity and sensitivity of CST4
was similar (33). In the study of
Wang et al (42),the
diagnostic sensitivity of CST1 for early esophageal squamous cell
carcinoma was 31.25% (specificity 92.64%, AUC 0.654). When
considering that the cases in the studies by Dou et al
(15) and Cai et al
(18) were mainly patients with
colorectal and gastric malignancies, while other malignant tumors
of the digestive system in addition to colorectal malignancies were
included in the present study, the positive rate of detection may
be decreased. In addition, we hypothesize that, as some patients
included in the present study had undergone associated surgical and
chemoradiotherapy treatment at the time of examination and the
growth of tumor cells in the body was inhibited, this resulted in
reduced cysteine protease secretion and thus indirectly reduced
CST4 secretion.
Since multiple indicators are often combined in the
diagnosis of digestive system tumors, six diagnostic groups were
established in the present study (43,44).
Among them, diagnostic group A is the traditional group used in
previous large-scale screening, diagnostic group B is the glycogen
marker group that has been commonly used in the past (45,46),
diagnostic group C includes the six markers used in digestive
system tumor screening at The Affiliated Chaohu Hospital of Anhui
Medical University, and the diagnostic groups D, E and F correspond
to groups A, B and C combined with CST4, respectively. In the
sensitivity and specificity analyses of the diagnostic groups, it
was found that the sensitivity of the diagnostic groups was
significantly increased following the inclusion of CST4, but the
specificity was significantly decreased. Therefore, the
introduction of CST4 may help to screen positive patients, but
caution should be taken regarding the false positive results that
may be produced. When evaluating the diagnostic efficacy of group
E, it was observed that the inclusion of CST4 resulted in a
sensitivity of 63.00%, specificity of 74%, accuracy of 68.50%, and
LR+ of 2.42. In comparison to group B, there was a significant
increase in sensitivity by 13%; however, specificity decreased by
13%. The accuracy remained unchanged while the LR+ decreased by
1.43. In addition, CST4 combined with group C (group F) had a
sensitivity of 71.00%, an accuracy of 68.00% and an LR+ of 2.03,
but the specificity was 18% lower compared with than of CST4 alone.
ROC curves were constructed to analyze the diagnostic efficiency of
the aforementioned groups, and the results demonstrated that there
were no notable differences in the comprehensive performance
between the diagnostic groups. Therefore, there was little
difference in the diagnostic efficiency among the diagnostic
groups, and the addition of CST4 exhibited no notable
advantages.
The association between CST4 expression and the
clinical characteristics of malignant digestive tumors and benign
diseases were also evaluated in the present study. The results
demonstrated that CST4 was not associated with age, sex,
hypertension or diabetes. Since a considerable number of patients
with malignant digestive tumors in the present study had distant
metastases, the clinical features, serum levels and positive rates
of tumor markers in these patients were further examined. The
results demonstrated that distant metastasis was not associated
with sex, age, hypertension or diabetes in the observation group.
The serum levels of CST4, CEA, CA199 and CA125 in the patients with
distant metastasis were significantly higher than those in the
patients without distant metastasis, while the serum levels of AFP,
CA153 and CA724 were not significantly different. Subsequent
investigations demonstrated that the positive rates of CEA, CA199
and CA125 in the patients with distant metastasis were
significantly higher than that in the patients without distant
metastasis, while there was no difference in the positive rates of
CST4, AFP, CA153 and CA724 between the two groups. As most patients
with distant metastasis are in the advanced stage of the disease
and have low nutritional status and protein synthesis (47), this may lead to a decrease in CST4
secretion and thus a decrease of the positive detection rate.
The serum levels of various tumor markers in
different tumor types were also analyzed in the present study, and
it was found that there were no significant differences in the
serum levels of CST4, AFP, CEA, CA125 and CA724 among the different
types of digestive tract tumors assessed. The serum levels of CA199
were significantly higher in pancreatic cancer compared with
esophageal, stomach and rectal cancer, and the serum levels of
CA153 in pancreatic cancer were significantly higher than those in
esophageal and stomach cancer.
The presence of advanced-stage disease, along with
evident spread and metastasis at the time of diagnosis in some
patients within the observation group, may contribute to a certain
degree of elevation in the CST4 index among individuals with
malignant tumors of the digestive system compared with the
gastrointestinal and non-gastrointestinal polyps groups. However,
it cannot be ruled out that malignant pancreatic tumor cells
themselves may promote the upregulation of CST4. The high
expression of CA199 and CA153 in pancreatic cancer observed in the
present study was also consistent with the results of previous
studies (48,49). The results of the present study also
demonstrated that the CST4 level in patients with malignant
pancreatic tumor was significantly increased compared with that in
the gastrointestinal and non-gastrointestinal polyps groups. In
addition, the levels of CST4 were significantly increased in the
colon cancer group compared with in the gastrointestinal polyp
group. A previous study on the early diagnosis of patients with
colorectal malignant tumors has demonstrated that CST4 has good
diagnostic efficacy (18). As few
patients with rectal tumors were included in the present study,
there was no significant difference in the CST4 levels between
these patients and the gastrointestinal or non-gastrointestinal
polyps patients groups. A previous study on CST1 in esophageal
malignancies (42) demonstrated a
significant elevation of CST1 levels in the group with esophageal
malignancies compared to the group with esophageal benign lesions.
However, there was no significant difference in the CST4 level
between patients with esophageal cancer and the gastrointestinal or
non-gastrointestinal polyps groups in the present study. Although
CST1 and CST4 are tumor markers of the same type, their distinct
characteristics may lead to different sensitivities in different
tumor types. The inclusion of a larger cohort of patients with
esophageal malignancies in future studies is warranted to further
substantiate any potential disparities in the distribution patterns
of CST1 and CST4 among these patients. The limitation of the
present preliminary study is that the number of included patients
with digestive system malignancies was small (100 patients), and
that gallbladder and hepatocellular malignancies were not included,
which may be the reason for the low positive rate of AFP. Since
most patients had undergone a certain course of chemoradiotherapy
or targeted therapy, it was not possible to further stratify the
patients and analyze the expression of CST4 in patients at each
stage. In addition, as certain patients were not at the affiliated
Chaohu Hospital of Anhui Medical University for long-term
treatment, and certain patients may refuse further treatment
resulting in a loss of follow-up, association analyses of the
treatment response, prognosis and survival rate could not be
conducted for these patients.
The findings of the present study demonstrated a
significant upregulation in the expression level of CST4 among
patients diagnosed with malignant digestive tumors, as compared to
those with gastrointestinal polyps and non-gastrointestinal polyps.
The expression level of CST4 was not affected by age, sex,
hypertension or diabetes, nor by gastrointestinal benign
proliferative diseases during screening. Although CST4 was more
sensitive than the other tumor markers when used alone, its
specificity and accuracy exhibited no specific advantages.
Therefore, it is suggested that CST4 could be combined with other
tumor markers to establish more effective diagnostic tools, and to
improve the accuracy of the diagnosis of malignant digestive system
tumors. It is also necessary to establish an effective multi-index
and multi-parameter combined detection model to improve the
accuracy of cancer diagnosis.
In conclusion, the findings of the present study
suggested that serum CST4 testing may be a promising and convenient
diagnostic tool, but CST4 needs to be combined with other tumor
markers to further improve its diagnostic efficacy. In addition,
further large-scale, extensive, prospective, multi-center studies
are required to confirm the clinical significance of serum CST4
testing in the diagnosis of digestive malignant tumors.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
The present study was funded by the Scientific Research Project
of the Health Commission of Anhui Province (grant no.
AHWJ2021a013), the Major Project of Humanities and Social Sciences
Research in Anhui (grant no. SK2021ZD0032) and Anhui Medical
University Clinical and Early Discipline Co-construction
Project.
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
MZ and DZ conceived of the study; MZ, XF and DZ
participated in the design of the study; DZ, XF, SX, ML, LG and RZ
participated in data collection; XF and SX analyzed and interpreted
the data; SX and ML drafted the manuscript; MZ, XF, DZ, SX, ML, LG
and RZ revised and edited the manuscript. MZ and DZ confirm the
authenticity of all the raw data. All authors read and approved the
final manuscript.
Ethics approval and consent to
participate
Ethics approval was granted by The Ethics Committee
of The Affiliated Chaohu Hospital of Anhui Medical University
(approval no. KYXM-202201-058; Chaohu, China). The data utilized in
the present study were obtained from the hospital's electronic
medical record system; therefore, informed consent for
participation was waived by the ethics committee.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
WHO Classification of Tumours Editorial
Board. WHO Classification of Tumors, . Digestive System Tumours.
5th edition. International Agency for Research on Cancer; Lyon:
2019
|
|
2
|
Xia C, Dong X, Li H, Cao M, Sun D, He S,
Yang F, Yan X, Zhang S, Li N and Chen W: Cancer statistics in China
and United States, 2022: Profiles, trends, and determinants. Chin
Med J (Engl). 135:584–590. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Arnold M, Abnet CC, Neale RE, Vignat J,
Giovannucci EL, McGlynn KA and Bray F: Global burden of 5 major
types of gastrointestinal cancer. Gastroenterology.
159:335–349.e15. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Zhang Y, Sun J, Song Y, Gao P, Wang X,
Chen M, Li Y and Wu Z: Roles of fusion genes in digestive system
cancers: Dawn for cancer precision therapy. Crit Rev Oncol Hematol.
171:1036222022. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Bentley-Hibbert S and Schwartz L: Use of
imaging for GI cancers. J Clin Oncol. 33:1729–1736. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Foley KG, Pearson B, Riddell Z and Taylor
SA: Opportunities in cancer imaging: A review of oesophageal,
gastric and colorectal malignancies. Clin Radiol. 76:748–762. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Torab FC, Bokobza B and Branicki F:
Laparoscopy in gastrointestinal malignancies. Ann NY Acad Sci.
1138:155–161. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Nowak KM and Chetty R: Predictive and
prognostic biomarkers in gastrointestinal tract tumours. Pathology.
56:205–213. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Turk B, Turk D and Turk V: Protease
signalling: The cutting edge. EMBO J. 31:1630–1643. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Breznik B, Motaln H and Lah Turnšek T:
Proteases and cytokines as mediators of interactions between cancer
and stromal cells in tumours. Biol Chem. 398:709–719. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Hanahan D and Weinberg RA: Hallmarks of
cancer: The next generation. Cell. 144:646–674. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Mitrović A, Pečar Fonović U and Kos J:
Cysteine cathepsins B and X promote epithelial-mesenchymal
transition of tumor cells. Eur J Cell Biol. 96:622–631. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Turk B, Turk D and Salvesen GS: Regulating
cysteine protease activity: Essential role of protease inhibitors
as guardians and regulators. Curr Pharm Des. 8:1623–1637. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Liu Y and Yao J: Research progress of
cystatin SN in cancer. Onco Targets Ther. 12:3411–3419. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Dou Y, Lv Y, Zhou X, He L, Liu L, Li P,
Sun Y, Wang M, Gao M and Wang C: Antibody-sandwich ELISA analysis
of a novel blood biomarker of CST4 in gastrointestinal cancers.
Onco Targets Ther. 11:1743–1756. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhang YQ, Zhang JJ, Song HJ and Li DW:
Overexpression of CST4 promotes gastric cancer aggressiveness by
activating the ELFN2 signaling pathway. Am J Cancer Res.
7:2290–2304. 2017.PubMed/NCBI
|
|
17
|
Wang S, Wang C, Liu O, Hu Y, Li X and Lin
B: Prognostic value of immune-related cells and genes in the tumor
microenvironment of ovarian cancer, especially CST4. Life Sci.
277:1194612021. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Cai L, Tu M, Yin X, Zhang S, Zhuang W, Xia
Y, Zhang Y, Zhang L, Yu L, Chi L and Huang Y: Combination of serum
CST4 and DR-70 contributes to early diagnosis of colorectal cancer.
Clin Chim Acta. 531:318–324. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Yang G, Zhang Y, Lin H, Liu J, Huang S,
Zhong W, Peng C and Du L: CircRNA circ_0023984 promotes the
progression of esophageal squamous cell carcinoma via regulating
miR-134-5p/cystatin-s axis. Bioengineered. 13:10578–10593. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Blanco MA, LeRoy G, Khan Z, Alečković M,
Zee BM, Garcia BA and Kang Y: Global secretome analysis identifies
novel mediators of bone metastasis. Cell Res. 22:1339–1355. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Tian S, Chen Y, Zhang Y and Xu X: Clinical
value of serum AFP and PIVKA-II for diagnosis, treatment and
prognosis of hepatocellular carcinoma. J Clin Lab Anal.
37:e248232023. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Hori Y, Seo S, Yoh T, Ueno K, Morino K,
Toda R, Nishio T, Koyama Y, Fukumitsu K, Ishii T, et al: Impact of
preoperative CEA uptrend on survival outcomes in patients with
colorectal liver metastasis after hepatectomy. Ann Surg Oncol.
29:6745–6754. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Rao H, Wu H, Huang Q, Yu Z and Zhong Z:
Clinical value of serum CEA, CA24-2 and CA19-9 in patients with
colorectal cancer. Clin Lab. April 1–2021.(Epub ahead of print).
doi: 10.7754/Clin.Lab.2020.200828. View Article : Google Scholar
|
|
24
|
Shibata C, Nakano T, Yasumoto A, Mitamura
A, Sawada K, Ogawa H, Miura T, Ise I, Takami K, Yamamoto K and
Katayose Y: Comparison of CEA and CA19-9 as a predictive factor for
recurrence after curative gastrectomy in gastric cancer. BMC Surg.
22:2132022. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Gao Y, Wang J, Zhou Y, Sheng S, Qian SY
and Huo X: Evaluation of Serum CEA, CA19-9, CA72-4, CA125 and
ferritin as diagnostic markers and factors of clinical parameters
for colorectal cancer. Sci Rep. 8:27322018. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Li X, Li S, Zhang Z and Huang D:
Association of multiple tumor markers with newly diagnosed gastric
cancer patients: A retrospective study. PeerJ. 10:e134882022.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Fahrmann JF, Schmidt CM, Mao X, Irajizad
E, Loftus M, Zhang J, Patel N, Vykoukal J, Dennison JB, Long JP, et
al: Lead-time trajectory of CA19-9 as an anchor marker for
pancreatic cancer early detection. Gastroenterology.
160:1373–1383.e6. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Cordero OJ, De Chiara L, Lemos-González Y,
Páez de la Cadena M and Rodríguez-Berrocal FJ: How the measurements
of a few serum markers can be combined to enhance their clinical
values in the management of cancer. Anticancer Res. 28:2333–2341.
2008.PubMed/NCBI
|
|
29
|
Wilhelmsen M, Christensen IJ, Rasmussen L,
Jørgensen LN, Madsen MR, Vilandt J, Hillig T, Klaerke M, Nielsen
KT, Laurberg S, et al: Detection of colorectal neoplasia:
Combination of eight blood-based, cancer-associated protein
biomarkers. Int J Cancer. 140:1436–1446. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Sauzay C, Petit A, Bourgeois AM, Barbare
JC, Chauffert B, Galmiche A and Houessinon A: Alpha-foetoprotein
(AFP): A multi-purpose marker in hepatocellular carcinoma. Clin
Chim Acta. 463:39–44. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Imamura Y, Yasutake K, Yoshimura Y, Oya M,
Matsushita K, Tokisue M and Sashikata T: Contents of tissue CEA and
CA19-9 in colonic polyp and colorectal cancer, and their clinical
significance. Gastroenterol Jpn. 25:186–192. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Tong J, Wang Y, Chang B, Zhang D and Wang
B: Associations between tumor markers and the risk of colorectal
polyp recurrence in Chinese people. Int J Clin Exp Med.
8:6397–6405. 2015.PubMed/NCBI
|
|
33
|
Li X, Xu Y and Zhang L: Serum CA153 as
biomarker for cancer and noncancer diseases. Prog Mol Biol Transl
Sci. 162:265–276. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Zhang Y, Zhang M, Bai X, Li C and Zhang L:
Increased serum CA724 levels in patients suffering gout vs cancers.
Prog Mol Biol Transl Sci. 162:177–186. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Hao C, Zhang G and Zhang L: Serum CEA
levels in 49 different types of cancer and noncancer diseases. Prog
Mol Biol Transl Sci. 162:213–227. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Narimatsu H, Iwasaki H, Nakayama F,
Ikehara Y, Kudo T, Nishihara S, Sugano K, Okura H, Fujita S and
Hirohashi S: Lewis and secretor gene dosages affect CA19-9 and
DU-PAN-2 serum levels in normal individuals and colorectal cancer
patients. Cancer Res. 58:512–518. 1998.PubMed/NCBI
|
|
37
|
Wannhoff A, Werner S, Tao S, Brenner H and
Gotthardt DN: Validation of a genotype-based algorithm that
identifies individuals with low, intermediate, and high serum CA199
levels in cancer-free individuals and in patients with colorectal
cancer. J Gastrointest Oncol. 13:1711–1721. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Teng D, Wu K, Sun Y, Zhang M, Wang D, Wu
J, Yin T, Gong W, Ding Y, Xiao W, et al: Significant increased
CA199 levels in acute pancreatitis patients predicts the presence
of pancreatic cancer. Oncotarget. 9:12745–12753. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Zeng P, Li H, Chen Y, Pei H and Zhang L:
Serum CA199 levels are significantly increased in patients
suffering from liver, lung, and other diseases. Prog Mol Biol
Transl Sci. 162:253–264. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Llàcer P, Núñez J, Manzano L, Cepeda
Rodrigo JM, Salamanca Bautista P, Guzmán García M, Trullás Vila JC,
Quirós López R, López Reboiro ML and Montero-Pérez-Barquero M; en
representación de los investigadores del registro Rica, :
Carbohydrate antigen 125 (CA125) as a prognostic marker in the
elderly with acute heart failure and preserved ejection fraction.
Med Clin (Barc). 159:164–170. 2022.(In English, Spanish).
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Núñez J, Bayés-Genís A, Revuelta-López E,
Ter Maaten JM, Miñana G, Barallat J, Cserkóová A, Bodi V,
Fernández-Cisnal A, Núñez E, et al: Clinical role of CA125 in
worsening heart failure: A BIOSTAT-CHF study subanalysis. JACC
Heart Fail. 8:386–397. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Wang J, Yu L, Sun Y, Zhang L, Tu M, Cai L,
Yin X, Pan X, Wang T and Huang Y: Development and evaluation of
serum CST1 detection for early diagnosis of esophageal squamous
cell carcinoma. Cancer Manag Res. 13:8341–8352. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Edoo MIA, Chutturghoon VK, Wusu-Ansah GK,
Zhu H, Zhen TY, Xie HY and Zheng SS: Serum biomarkers AFP, CEA and
CA19-9 combined detection for early diagnosis of hepatocellular
carcinoma. Iran J Public Health. 48:314–322. 2019.PubMed/NCBI
|
|
44
|
Wojtalewicz N, Vierbaum L, Kaufmann A,
Schellenberg I and Holdenrieder S: Longitudinal evaluation of AFP
and CEA external proficiency testing reveals need for method
harmonization. Diagnostics (Basel). 13:20192023. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Tang Y, Cui Y, Zhang S and Zhang L: The
sensitivity and specificity of serum glycan-based biomarkers for
cancer detection. Prog Mol Biol Transl Sci. 162:121–140. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Chen C, Chen Q, Zhao Q, Liu M and Guo J:
Value of combined detection of serum CEA, CA72-4, CA19-9, CA15-3
and CA12-5 in the diagnosis of gastric cancer. Ann Clin Lab Sci.
47:260–263. 2017.PubMed/NCBI
|
|
47
|
Marcolini EG, Putnam AT and Aydin A:
History and perspectives on nutrition and hydration at the end of
life. Yale J Biol Med. 91:173–176. 2018.PubMed/NCBI
|
|
48
|
Ho JJ, Chung YS, Yuan M, Henslee JG and
Kim YS: Differences in expression of SPan-1 and CA15-3 antigens in
blood and tissues. Int J Cancer. 52:693–700. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Liu L, Xu HX, Wang WQ, Wu CT, Xiang JF,
Liu C, Long J, Xu J, Fu de L, Ni QX, et al: Serum CA125 is a novel
predictive marker for pancreatic cancer metastasis and correlates
with the metastasis-associated burden. Oncotarget. 7:5943–5956.
2016. View Article : Google Scholar : PubMed/NCBI
|