Introduction
Hepatocellular carcinoma (HCC) is the most common
primary liver cancer and a major cause of cancer-related mortality
(1,2). In 2008, Llovet et al (3) showed that sorafenib increased overall
survival (OS) compared to placebo, thus introducing an effective
systemic therapy for advanced HCC.
In 2018, Kudo et al (4) showed that lenvatinib was not inferior
to sorafenib in the treatment of advanced HCC, whereafter the
former was introduced as a first-line chemotherapy option. Since
the introduction of multitarget tyrosine kinase inhibitors (TKIs),
such as sorafenib and lenvatinib, markers that may help predict
their therapeutic efficacy have been actively explored. Such an
attempt was made by Marisi et al (5), who did not identify factors predicting
sorafenib response. Following the advent of TKIs, a new era of
combination therapies has emerged, including combination treatments
with TKIs and immunotherapy, such as immune checkpoint inhibitors
(ICIs). In 2020, Finn et al (6) showed that atezolizumab plus
bevacizumab combination therapy resulted in superior overall
survival (OS) and progression-free survival (PFS) compared to
sorafenib, thereby changing the first-line treatment of patients
with unresectable HCC. Markers predicting the efficacy of the
atezolizumab plus bevacizumab combination, including programmed
cell death ligand 1 (PD-L1), are the subject of active research
(7). Both TKIs and ICIs exert
immunomodulatory effects on the tumor microenvironment (TME)
(8). In 2013, Sprinzl et al
(9) showed that sorafenib enhances
anti-tumor immune responses by regulating macrophages, in addition
to its direct effect on tumor cells. In 2019, Kato et al
(10) demonstrated that lenvatinib
reduced tumor-associated macrophage (TAM) infiltration, thereby
enhancing anti-tumor immunity.
The liver TME is defined as the sum of stromal and
tumor cells within the extracellular matrix, along with their
secretome. Chronic insults from various etiologies, including
hepatitis B, hepatitis C, alcoholic and non-alcoholic
steatohepatitis, which are characterized by sequelae of
inflammation and oxidative DNA damage, promote tumorigenesis
through the accumulation of mutations and epigenetic rewiring
(11). TKIs interact with tyrosine
kinase receptors, inhibiting the autophosphorylation of their
cytoplasmic domains to exert their anti-angiogenic effects
(12). Sorafenib regulates TAMs and
enhances T-cell responses, thereby enhancing anti-tumor immunity
(9,13). Lenvatinib was shown to target
fibroblast growth factor receptors, leading to greater efficacy of
anti-programmed cell death 1 (PD-1) therapy (14). A recent meta-analysis concluded that
PD-L1 expression was associated with a superior objective response
rate in patients with advanced HCC treated with PD-1 or PD-L1
inhibitors (15).
In addition to such systemic treatments, Tischfield
et al (16) demonstrated
that locoregional therapies (LRTs), such as transarterial
embolization, also induce changes in the TME. Hepatic artery
infusion chemotherapy (HAIC) is a popular LRT option in Eastern
Asia, particularly in South Korea and Japan. Considering the
immunomodulatory effects of LRTs reported in multiple studies and
reviews (8), the present study set
out to determine whether the expression of factors related to the
anti-tumor immune response, particularly PD-L1 expression, can
predict the efficacy of HAIC in HCC.
Materials and methods
Study design and population
A total of 40 patients diagnosed with HCC who had
undergone HAIC and a liver biopsy between January 2020 and May 2023
at Seoul St. Mary's Hospital (Seoul, Korea) were retrospectively
enrolled. These patients were diagnosed based on radiological and
histological findings, including multiphasic computed tomography
and magnetic resonance imaging (17). The patients' hospital records were
reviewed and their tumor response, PFS, disease control rate (DCR),
objective response rate (ORR) and OS were evaluated. The DCR was
defined as the proportion of patients who showed complete response,
partial response or stable disease after therapy. The ORR was
defined as the proportion of patients that responded either
partially or fully to therapy: partial response or complete
response. Patients were diagnosed with HCC based on the imaging
criteria of the American Association for the Study of Liver
Disease, the 2022 Korean Liver Cancer Association and the National
Cancer Center Korea practice guidelines (17,18).
Biopsy samples were immunohistochemically assessed for PD-L1
positivity using combined positivity scores (CPSs) (19). The study protocol was approved by
the Institutional Review Board of Seoul St. Mary's Hospital (Seoul,
Korea; approval no. KC23RISI0656). The study conformed to the
ethical guidelines of the Declaration of Helsinki.
Immunohistochemistry
Immunohistochemistry was performed on core-needle
liver biopsy samples. A 4-µm-thick cross-section of a
paraffin-embedded block from the biopsy sample was placed on a
glass slide. Deparaffinization, rehydration and antigen retrieval
were performed using CC1 antigen retrieval solution (Ventana
Medical Systems) and an automated slide stainer (Ventana Medical
Systems) for 64 min. The sample was incubated with antibodies
against PD-L1 (1:50 dilution; cat. no. M3653; Dako) for 32 min at
37°C and washed. Finally, the slides were counterstained with
hematoxylin I and bluing reagent (Ventana Medical Systems) for 4
min at room temperature. The CPS for PD-L1 expression were
determined (19). In the present
study, slides with ≥1% PD-L1-positive cells were considered
PD-L1-positive samples. Sangro et al (20) also used the 1% threshold when
determining PD-L1 positivity in their study on the association of
inflammatory biomarkers with prognosis in nivolumab-treated
patients with HCC.
Response evaluation
Response was evaluated using the modified Response
Evaluation Criteria in Solid Tumors (21). All CT and MRI scans of the patients
were examined by more than one doctor from The Department of
Gastroenterology and Hepatology, and one doctor from The Department
of Radiology. Accordingly, tumors with no arterial enhancement were
defined as those showing a complete response (CR). Tumors with the
sum of the diameters of viable lesions reduced by >30% were
defined as showing a partial response (PR). Tumors with viable
lesion diameters that had increased by >20% were defined as
progressive disease (PD). Tumors that did not meet the criteria for
PR or PD were defined as having stable disease (SD).
Statistical analysis
SPSS version 26 software (IBM Corp.) was used for
statistical analyses. Categorical variables were analyzed using
Fisher's extract test or the Freeman-Halton extension for Fisher's
extract test in the case of multiple groups, and continuous
variables were analyzed using an independent t-test. Patient
survival was analyzed using the Kaplan-Meier method and survival
curves were analyzed using the log-rank test. Cox proportional
hazards regression analysis was used to analyze factors associated
with survival. P<0.05 was considered to indicate statistical
significance.
Results
Baseline characteristics
Table I presents the
baseline characteristics of the 40 enrolled patients. A total of 36
(90%) of the patients were men and 4 (10%) were women. The mean age
was 61.23±14.51 (range, 26–89) years. Hepatitis B infection was the
most common cause of HCC [23 (57.6%) patients]. A total of 7
(17.5%) patients had a history of excessive alcohol consumption.
Another 10 (25%) patients had no known risk factors for fatty liver
disease. The mean tumor size was 9.53±4.43 cm. A total of 5 (12.5%)
patients had a single HCC lesion, while 35 (87.5%) had multiple
lesions. Furthermore, 31 (77.5%) patients had portal vein invasion,
18 (45%) had extrahepatic metastasis, 32 (80%) had Child-Pugh class
A liver function, 8 (20%) had Child-Pugh class B liver function, 18
(45%) had a history of treatment, 6 (15%) had Barcelona Clinic
Liver Cancer (BCLC) stage B disease and 34 (85%) had BCLC stage C
disease. Table II shows the
baseline characteristics of patients with and without PD-L1
positivity. There was a significant difference in enrolled patients
with and without PD-L1 positivity in BCLC stage (Table II; P=0.026).
 | Table I.Baseline characteristics of enrolled
patients (n=40). |
Table I.
Baseline characteristics of enrolled
patients (n=40).
| Item | Value |
|---|
| Sex
(male/female) | 36 (90)/4 (10) |
| Age, years | 61.23±14.51 |
| Etiology |
|
|
HBV | 23 (57.5) |
|
HCV | 0 (0) |
| Alcohol
abuse | 7 (17.5) |
|
Unknown | 10 (25) |
| Tumor size, cm | 9.53±4.43 |
| Tumor number |
|
|
Single | 5 (12.5) |
|
Multiple | 35 (87.5) |
| Portal vein
invasion |
|
|
Yes/No | 31 (77.5)/9
(22.5) |
| Extrahepatic
metastasis | 18 (45.0) |
| Child-Pugh
score |
|
| A | 32 (80.0) |
| B | 8 (20.0) |
| C | 0 (0.0) |
| Previous treatment
history | 18 (45.00) |
| BCLC stage |
|
| B | 6 (15.0) |
| C | 34 (85.0) |
 | Table II.Baseline characteristics of subgroups
according to PD-L1 positivity. |
Table II.
Baseline characteristics of subgroups
according to PD-L1 positivity.
| Baseline
characteristics | PD-L1-positive
cells ≥1% (n=30) | PD-L1-positivity
<1% (n=10) | P-value |
|---|
| Sex
(male/female) | 26/4 | 10/0 | 0.556 |
| Age, years | 59.6±16.20 | 66.10±5.59 | 0.067 |
| Etiology |
|
| 0.122 |
|
HBV | 20 | 3 |
|
|
HCV | 0 | 0 |
|
| Alcohol
abuse | 4 | 3 |
|
|
Others | 6 | 4 |
|
| Tumor size, cm | 9.75±4.49 | 8.90±4.42 | 0.747 |
| Tumor number |
|
| 0.584 |
|
Single | 3 | 2 |
|
|
Multiple | 27 | 8 |
|
| Portal vein
invasion |
|
| 0.190 |
|
Yes/No | 25/6 | 5/4 |
|
| Extrahepatic
metastasis | 15 | 3 | 0.190 |
| Child-Pugh
score |
|
| 0.165 |
| A | 22 | 10 |
|
| B | 8 | 0 |
|
| C | 0 | 0 |
|
| Previous treatment
history |
|
| 0.231 |
|
Yes/No | 12/18 | 6/4 |
|
| BCLC stage |
|
| 0.026 |
| B | 2 | 4 |
|
| C | 28 | 6 |
|
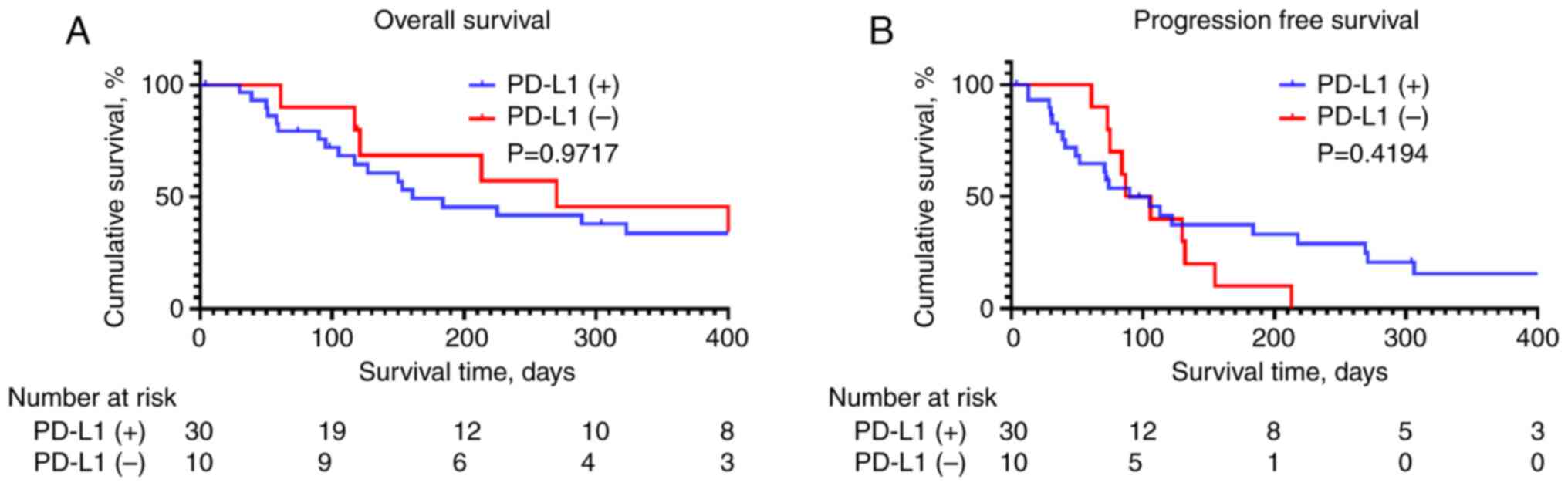
OS and PFS according to PD-L1
expression
OS and PFS did not significantly differ between
patients with and without PD-L1 expression (Fig. 1; P=0.9717 and 0.4194, respectively).
In addition, no significant differences were noted in the objective
response rate (ORR) and disease control rate (DCR) (Table III; P=0.633 and 0.508,
respectively). HAIC response also did not differ based on PD-L1
expression (Table III, P=0.595).
Specifically, among patients with ≥1% PD-L1-positive cells, 1
(3.3%) showed a CR, 3 (10%) showed a PR, 15 (50%) showed SD and 11
(36.7%) showed PD. Among those with <1% PD-L1-positive cells, 1
patient (10%) showed a CR, 6 patients (60%) showed SD and 3
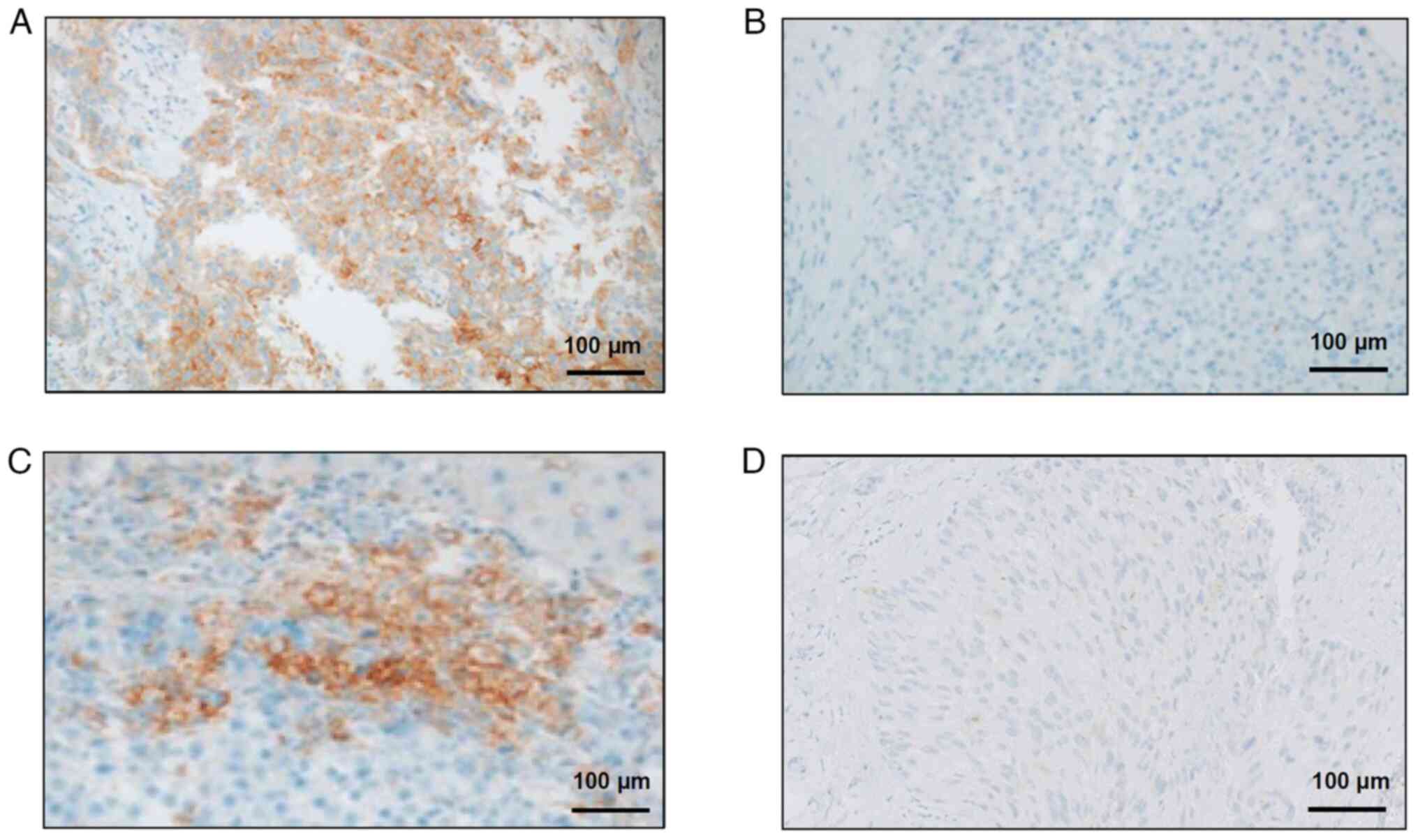
patients (30%) showed PD. Fig. 2
displays representative immunohistochemical findings for the
enrolled patients, including samples with or without PD-L1
expression from patients whose disease did or did not progress
(Fig. 2A-D).
 | Table III.Treatment response of enrolled
patients according to PD-L1 positivity. |
Table III.
Treatment response of enrolled
patients according to PD-L1 positivity.
| Parameter | PD-L1-positive
cells ≥1% (n=30) | PD-L1-positive
cells <1% (n=10) | P-value |
|---|
| Treatment
response |
|
| 0.595 |
|
Complete response | 1 (3.3) | 1 (10) |
|
| Partial
response | 3 (10) | 0 |
|
| Stable
disease | 15 (50) | 6 (60) |
|
|
Progressive disease | 11 (36.7) | 3 (30) |
|
| Objective response
rate | 4/30 | 1/10 | 0.633 |
| Disease control
rate | 19/30 | 7/10 | 0.508 |
Factors associated with prognosis
Table IV shows the
results of the Cox regression analysis performed to identify the
factors associated with OS and PFS. With regard to OS, the Eastern
Cooperative Oncology Group (ECOG) performance status and liver
function, represented by the Child-Pugh class, were significantly
associated with a better prognosis (P<0.001 and P=0.02,
respectively). Multivariate Cox regression analysis revealed a
significant association between ECOG performance status and a
better prognosis [hazard ratio=4.000 (95% CI: 1.937–8.262),
P<0.001]. None of the factors analyzed was significantly
associated with PFS.
 | Table IV.Univariate and multivariate analyses
of factors associated with overall and progression-free survival of
enrolled patients. |
Table IV.
Univariate and multivariate analyses
of factors associated with overall and progression-free survival of
enrolled patients.
|
| Overall
survival | Progression-free
survival |
|---|
|
|
|
|
|---|
| Variable | Univariate
P-value | Multivariate
P-value | HR (95% CI) | Univariate
P-value | Multivariate
P-value | HR (95% CI) |
|---|
| PD-L1
positivity | 0.706 | - |
| 0.622 |
|
|
| Sex (male vs.
female) | 0.363 | - |
| 0.602 |
|
|
| Agea | 0.353 | - |
| 0.480 |
|
|
|
Etiologyb | 0.099 | - |
| 0.186 |
|
|
| Tumor
sizea | 0.366 | - |
| 0.827 |
|
|
| Multiple tumor
lesions | 0.435 | - |
| 0.413 |
|
|
| Portal vein
invasion | 0.806 | - |
| 0.790 |
|
|
| Distant
metastasis | 0.525 | - |
| 0.447 |
|
|
| ECOG performance
statusa | <0.001 | <0.001 | 4.000
(1.937–8.262) | 0.226 |
|
|
| Child-Pugh class
A | 0.020 | 0.062 | 2.670
(0.953–7.479) | 0.510 |
|
|
| Previous
treatment | 0.625 |
|
| 0.567 |
|
|
Discussion
In the case of patients with advanced HCC for whom
surgical treatment, such as resection, transplantation or LRT,
including transarterial catheter embolization, is not an option,
systemic therapy with atezolizumab plus bevacizumab is the first
line of treatment (22). In Eastern
Asia, HAIC is considered when systemic chemotherapy is not
effective, particularly in HCC with portal vein invasion (23). The theoretical rationale is that,
unlike normal hepatocytes, which receive most of their perfusion
from the portal vein, HCC cells receive most of their perfusion
from the hepatic artery (24).
Transarterial chemoembolization (TACE) is another popular LRT that
may damage and impair liver function (25). HAIC is significantly less toxic than
TACE (26). In 2015, Song et
al (27) reported comparable OS
and time to progression between sorafenib and HAIC in patients with
HCC with portal vein invasion. A study from 2019 suggested that
HAIC is effective in patients regardless of portal vein invasion or
extrahepatic metastasis (28).
Comparable OS and PFS between lenvatinib and HAIC have also been
reported by Lee et al (29).
It is worth noting that lenvatinib has been reported to have
similar efficacy to first-line atezolizumab/bevacizumab,
particularly in specific cases, such as patients with autoimmune
disease or other patients receiving immunosuppressants (30,31).
Comparable OS and PFS were also previously reported between
atezolizumab/bevacizumab and HAIC (32). Recently, Iwamoto et al
(33) proposed a new era of
multidisciplinary therapeutic strategies encompassing LRT, with an
emphasis on the importance of HAIC.
In the current era of personalized medicine,
patients are increasingly administered various combination
regimens, which include ICIs and LRTs. As ever more treatment
modalities become available, identifying biomarkers that predict
their efficacy is essential, with extensive research focusing on
the TME in this regard. Tischfield et al (16) demonstrated that transarterial
embolization induces dynamic alterations in the TME. Cell death
induced by LRTs results in the release of tumor antigens, which
stimulate antigen-presenting cells, triggering an anti-tumor immune
response (34). As LRTs may also
exert immunomodulatory effects, the present study focused on the
association between PD-L1 expression and HAIC efficacy.
In one study, the presence of PD-L1 in patients with
HCC treated with ICI was associated with superior outcomes. The
KEYNOTE-224 open-label phase II trial using pembrolizumab analyzed
the association of PD-L1 with ORR and PFS, reporting a better
prognosis in patients with PD-L1-positive tumors (35). In the phase III IMbrave150 trial,
PD-L1 expression was associated with superior outcomes of
atezolizumab plus bevacizumab combination therapy in terms of PFS
and ORR (36). By contrast, in the
CheckMate040 randomized clinical trial, PD-L1 expression was not
associated with better treatment outcomes (37). PD-L1 is expressed in tumor cells,
normal hepatocytes, sinusoidal cells and Kupffer cells (38). PD-L1 expression by neoplastic cells
is associated with poor prognosis and characteristics include
macrovascular invasion and poor differentiation (39). PD-L1 is known to be expressed on
TAMs as well as on tumor cells in HCC. Furthermore,
PD-L1-expressing TAMs are associated with tumor immunogenicity
(40). A previous study by our
group demonstrated that PD-L1 is highly expressed in TAMs and
cancer-associated fibroblasts in the TME of HCC (41).
In the current study, patients diagnosed with
advanced HCC who were treated with HAIC showed similar outcomes in
terms of OS, PFS, ORR and DCR, regardless of PD-L1 positivity.
Considering the compelling evidence that PD-L1 positivity elicits
significantly superior outcomes in patients treated with
atezolizumab plus bevacizumab combination chemotherapy, HAIC should
be acknowledged as a favorable treatment option for patients
diagnosed with advanced HCC, particularly those with portal vein
invasion without PD-L1 positivity.
The present study had certain limitations, owing to
the retrospective nature of its design, which included selection
bias. The small number of cases was also a limitation, considering
its effect on the statistical power of the results. In addition,
there was a significant difference in BCLC stage between patients
with and without PD-L1 expression, which was ignored due to the
small sample size of the current study. Furthermore, the lack of a
longer follow-up duration represents an additional limitation to
the present study. Finally, biopsy samples were obtained at a
single timepoint per patient, thus not recapitulating the
heterogeneity and dynamics of PD-L1 expression in tumors. Ideally,
a prospective study with a larger patient pool would yield more
meaningful results.
In conclusion, OS and PFS did not differ based on
PD-L1 expression between patients with advanced HCC, suggesting
that HAIC shows consistent efficacy, irrespective of PD-L1 status.
A multidisciplinary approach including various systemic and
locoregional treatment options is globally employed for the
treatment of advanced HCC, in parallel to an emphasis on
monotherapy. There is considerable interest in uncovering positive
and negative factors predicting treatment response. The current
findings support the use of HAIC in patients with advanced HCC with
portal vein tumor thrombosis whose tumors lack PD-L1
expression.
Acknowledgements
Not applicable.
Funding
The present study was supported by The Basic Science Research
Program of the National Research Foundation of Korea funded by the
Korean government (grant no. 2021R1C1C1005844 to PSS), the 2022
Leader Research Fund of Seoul St. Mary's Hospital of the Catholic
University of Korea (grant no. 2022-001 to PSS) and The Korean
Liver Foundation (grant no. 2022-1 to PSS).
Availability or data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
JHK, YHK, SL and PSS contributed to the conception
and design of the study, data interpretation, writing the first
draft of the paper and critical revision of the manuscript. JWH,
HCN, SK, CK and JWJ contributed to the study design and data
analysis. JSY, JSO, HJC, JYC and SKY contributed to the data
interpretation and critical revision of the manuscript. PSS and SHL
conceived the idea of this study and contributed to the study
conception, study design, data interpretation and critical revision
of the manuscript. JK and PSS checked and confirm the authenticity
of the raw data. All authors contributed to the manuscript and have
read and approved the submitted version.
Ethics approval and consent to
participate
The study was conducted in accordance with the
Declaration of Helsinki and approved by the Institutional Review
Board of Seoul St. Mary's Hospital (Seoul, Korea; approval no.
KC23RISI0656). Patient consent was waived owing to the
retrospective nature of the study and the analysis used anonymous
clinical data.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Villanueva A: Hepatocellular carcinoma. N
Engl J Med. 380:1450–1462. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Korean Liver Cancer Association (KLCA) and
National Cancer Center (NCC) Korea, . 2022 KLCA-NCC Korea practice
guidelines for the management of hepatocellular carcinoma. J Liver
Cancer. 23:1–120. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Llovet JM, Ricci S, Mazzaferro V, Hilgard
P, Gane E, Blanc JF, de Oliveira AC, Santoro A, Raoul JL, Forner A,
et al: Sorafenib in advanced hepatocellular carcinoma. N Engl J
Med. 359:378–390. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kudo M, Finn RS, Qin S, Han KH, Ikeda K,
Piscaglia F, Baron A, Park JW, Han G, Jassem J, et al: Lenvatinib
versus sorafenib in first-line treatment of patients with
unresectable hepatocellular carcinoma: A randomised phase 3
non-inferiority trial. Lancet. 391:1163–1173. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Marisi G, Cucchetti A, Ulivi P, Canale M,
Cabibbo G, Solaini L, Foschi FG, De Matteis S, Ercolani G,
Valgiusti M, et al: Ten years of sorafenib in hepatocellular
carcinoma: Are there any predictive and/or prognostic markers?
World J Gastroenterol. 24:4152–4163. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Finn RS, Qin S, Ikeda M, Galle PR, Ducreux
M, Kim TY, Kudo M, Breder V, Merle P, Kaseb AO, et al: Atezolizumab
plus bevacizumab in unresectable hepatocellular carcinoma. N Engl J
Med. 382:1894–1905. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Han JW and Jang JW: Predicting outcomes of
atezolizumab and bevacizumab treatment in patients with
hepatocellular carcinoma. Int J Mol Sci. 24:117992023. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Han JW and Yoon SK: Immune responses
following locoregional treatment for hepatocellular carcinoma:
Possible roles of adjuvant immunotherapy. Pharmaceutics.
13:13872021. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Sprinzl MF, Reisinger F, Puschnik A,
Ringelhan M, Ackermann K, Hartmann D, Schiemann M, Weinmann A,
Galle PR, Schuchmann M, et al: Sorafenib perpetuates cellular
anticancer effector functions by modulating the crosstalk between
macrophages and natural killer cells. Hepatology. 57:2358–2368.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kato Y, Tabata K, Kimura T,
Yachie-Kinoshita A, Ozawa Y, Yamada K, Ito J, Tachino S, Hori Y,
Matsuki M, et al: Lenvatinib plus anti-PD-1 antibody combination
treatment activates CD8+ T cells through reduction of
tumor-associated macrophage and activation of the interferon
pathway. PLoS One. 14:e02125132019. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Hernandez-Gea V, Toffanin S, Friedman SL
and Llovet JM: Role of the microenvironment in the pathogenesis and
treatment of hepatocellular carcinoma. Gastroenterology.
144:512–527. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Chen R, Li Q, Xu S, Ye C, Tian T, Jiang Q,
Shan J and Ruan J: Modulation of the tumour microenvironment in
hepatocellular carcinoma by tyrosine kinase inhibitors: From
modulation to combination therapy targeting the microenvironment.
Cancer Cell Int. 22:732022. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Sunay MME, Foote JB, Leatherman JM,
Edwards JP, Armstrong TD, Nirschl CJ, Hicks J and Emens LA:
Sorafenib combined with HER-2 targeted vaccination can promote
effective T cell immunity in vivo. Int Immunopharmacol. 46:112–123.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Yi C, Chen L, Lin Z, Liu L, Shao W, Zhang
R, Lin J, Zhang J, Zhu W, Jia H, et al: Lenvatinib targets FGF
receptor 4 to enhance antitumor immune response of anti-programmed
cell death-1 in HCC. Hepatology. 74:2544–2560. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Yang Y, Chen D, Zhao B, Ren L, Huang R,
Feng B and Chen H: The predictive value of PD-L1 expression in
patients with advanced hepatocellular carcinoma treated with
PD-1/PD-L1 inhibitors: A systematic review and meta-analysis.
Cancer Med. 12:9282–9292. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Tischfield DJ, Gurevich A, Johnson O,
Gatmaytan I, Nadolski GJ, Soulen MC, Kaplan DE, Furth E, Hunt SJ
and Gade TPF: Transarterial embolization modulates the immune
response within target and nontarget hepatocellular carcinomas in a
rat model. Radiology. 303:215–225. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Marrero JA, Kulik LM, Sirlin CB, Zhu AX,
Finn RS, Abecassis MM, Roberts LR and Heimbach JK: Diagnosis,
staging, and management of hepatocellular carcinoma: 2018 Practice
guidance by the American association for the study of liver
diseases. Hepatology. 68:723–750. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Korean Liver Cancer Association (KLCA) and
National Cancer Center (NCC) Korea, . 2022 KLCA-NCC Korea practice
guidelines for the management of hepatocellular carcinoma. Clin Mol
Hepatol. 28:583–705. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Paver EC, Cooper WA, Colebatch AJ,
Ferguson PM, Hill SK, Lum T, Shin JS, O'Toole S, Anderson L,
Scolyer RA and Gupta R: Programmed death ligand-1 (PD-L1) as a
predictive marker for immunotherapy in solid tumours: A guide to
immunohistochemistry implementation and interpretation. Pathology.
53:141–156. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Sangro B, Melero I, Wadhawan S, Finn RS,
Abou-Alfa GK, Cheng AL, Yau T, Furuse J, Park JW and Boyd Z:
Association of inflammatory biomarkers with clinical outcomes in
nivolumab-treated patients with advanced hepatocellular carcinoma.
J Hepatol. 73:1460–1469. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Llovet JM and Lencioni R: mRECIST for HCC:
Performance and novel refinements. J Hepatol. 72:288–306. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Reig M, Forner A, Rimola J, Ferrer-Fàbrega
J, Burrel M, Garcia-Criado Á, Kelley RK, Galle PR, Mazzaferro V,
Salem R, et al: BCLC strategy for prognosis prediction and
treatment recommendation: The 2022 update. J Hepatol. 76:681–693.
2022. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Song MJ: Hepatic artery infusion
chemotherapy for advanced hepatocellular carcinoma. World J
Gastroenterol. 21:3843–3849. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Breedis C and Young G: The blood supply of
neoplasms in the liver. Am J Pathol. 30:969–977. 1954.PubMed/NCBI
|
|
25
|
Torimura T and Iwamoto H: Optimizing the
management of intermediate-stage hepatocellular carcinoma: Current
trends and prospects. Clin Mol Hepatol. 27:236–245. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
He MK, Le Y, Li QJ, Yu ZS, Li SH, Wei W,
Guo RP and Shi M: Hepatic artery infusion chemotherapy using
mFOLFOX versus transarterial chemoembolization for massive
unresectable hepatocellular carcinoma: A prospective non-randomized
study. Chin J Cancer. 36:832017. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Song DS, Song MJ, Bae SH, Chung WJ, Jang
JY, Kim YS, Lee SH, Park JY, Yim HJ, Cho SB, et al: A comparative
study between sorafenib and hepatic arterial infusion chemotherapy
for advanced hepatocellular carcinoma with portal vein tumor
thrombosis. J Gastroenterol. 50:445–454. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Sung PS, Yang K, Bae SH, Oh JS, Chun HJ,
Nam HC, Jang JW, Choi JY and Yoon SK: Reduction of intrahepatic
tumour by hepatic arterial infusion chemotherapy prolongs survival
in hepatocellular carcinoma. Anticancer Res. 39:3909–3916. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Lee J, Han JW, Sung PS, Lee SK, Yang H,
Nam HC, Yoo SH, Lee HL, Kim HY, Lee SW, et al: Comparative analysis
of lenvatinib and hepatic arterial infusion chemotherapy in
unresectable hepatocellular carcinoma: A multi-center, propensity
score study. J Clin Med. 10:40452021. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Chan LL and Chan SL: The evolving role of
lenvatinib at the new era of first-line hepatocellular carcinoma
treatment. Clin Mol Hepatol. 29:909–923. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Lee MMP, Chan LL and Chan SL: The role of
lenvatinib in the era of immunotherapy of hepatocellular carcinoma.
J Liver Cancer. 23:262–271. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Kim JH, Nam HC, Kim CW, Cho HS, Yoo JS,
Han JW, Jang JW, Choi JY, Yoon SK, Yang H, et al: Comparative
analysis of atezolizumab plus bevacizumab and hepatic artery
infusion chemotherapy in unresectable hepatocellular carcinoma: A
multicenter, propensity score study. Cancers (Basel). 15:42332023.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Iwamoto H, Shimose S, Shirono T, Niizeki T
and Kawaguchi T: Hepatic arterial infusion chemotherapy for
advanced hepatocellular carcinoma in the era of chemo-diversity.
Clin Mol Hepatol. 29:593–604. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Greten TF, Mauda-Havakuk M, Heinrich B,
Korangy F and Wood BJ: Combined locoregional-immunotherapy for
liver cancer. J Hepatol. 70:999–1007. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Zhu AX, Finn RS, Edeline J, Cattan S,
Ogasawara S, Palmer D, Verslype C, Zagonel V, Fartoux L, Vogel A,
et al: Pembrolizumab in patients with advanced hepatocellular
carcinoma previously treated with sorafenib (KEYNOTE-224): A
non-randomised, open-label phase 2 trial. Lancet Oncol. 19:940–952.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Cheng AL, Qin S, Ikeda M, Galle PR,
Ducreux M, Kim TY, Lim HY, Kudo M, Breder V, Merle P, et al:
Updated efficacy and safety data from IMbrave150: Atezolizumab plus
bevacizumab vs sorafenib for unresectable hepatocellular carcinoma.
J Hepatol. 76:862–873. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Yau T, Kang YK, Kim TY, El-Khoueiry AB,
Santoro A, Sangro B, Melero I, Kudo M, Hou MM, Matilla A, et al:
Efficacy and safety of nivolumab plus ipilimumab in patients with
advanced hepatocellular carcinoma previously treated with
sorafenib: The CheckMate 040 randomized clinical trial. JAMA Oncol.
6:e2045642020. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Yamazaki T, Akiba H, Iwai H, Matsuda H,
Aoki M, Tanno Y, Shin T, Tsuchiya H, Pardoll DM, Okumura K, et al:
Expression of programmed death 1 ligands by murine T cells and APC.
J Immunol. 169:5538–5545. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Calderaro J, Rousseau B, Amaddeo G, Mercey
M, Charpy C, Costentin C, Luciani A, Zafrani ES, Laurent A, Azoulay
D, et al: Programmed death ligand 1 expression in hepatocellular
carcinoma: Relationship With clinical and pathological features.
Hepatology. 64:2038–2046. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Sung PS: Crosstalk between
tumor-associated macrophages and neighboring cells in
hepatocellular carcinoma. Clin Mol Hepatol. 28:333–350. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Park JG, Roh PR, Kang MW, Cho SW, Hwangbo
S, Jung HD, Kim HU, Kim JH, Yoo JS, Han JW, et al: Intrahepatic IgA
complex induces polarization of cancer-associated fibroblasts to
matrix phenotypes in the tumor microenvironment of HCC. Hepatology.
Feb 15–2024.(Epub ahead of print). View Article : Google Scholar
|