Introduction
With the ever-increasing incidence of breast cancer
(BC) and improved diagnosis and treatment methods, BC survival
rates are increasing, making it more likely that a woman will
develop a second BC in the contralateral breast after completion of
the initial treatment (1). Among
patients with BC, contralateral (C)BC is the most common secondary
cancer event, with an incidence of 0.5–1.0% per year (2,3).
Several risk factors for CBC have been identified, including young
age (2,4,5) and a
family history of BC (5).
Conversely, treatments, such as systemic adjuvant chemotherapy
(6–8) and hormonal therapies, including
tamoxifen or aromatase inhibitors, are known to reduce the risk of
CBC (8,9).
Based on this understanding, the contralateral
breast can be categorized into synchronous and metachronous (M)CBC.
This classification stems from the report by Kilgore (10) on ‘synchronous carcinoma’ in 1921,
which was later expanded by Haagensen and Rosato (11), who introduced temporal variance in
bilateral BC. The distinction between synchronous and MCBC has been
a subject of ongoing research, with time intervals for
classification varying widely, such as 1 month (12,13), 3
months (14), 6 months (15) and 12 months (16–18).
Most existing studies on the risk of metachronous CBC have relied
predominantly on data from single institutions and notably,
large-scale studies on Asian populations are scarce (14,17,18).
MCBC is a distinct pathological entity characterized
by the development of a novel primary carcinoma in the opposite
breast following the initial diagnosis. Contrary to synchronous
tumors that are present in temporal proximity to the primary
neoplasm, metachronous growths emerge after a lapse of time,
marking them as separate oncogenic occurrences rather than as
expansions or metastatic sequelae of the original cancer (19). The critical delineation between
metachronous BC and metastatic spread is a profound consequence of
clinical decision-making, with implications for treatment
modalities and prognostic deliberations (20). Despite the challenges of confirming
their etiological independence, MCBCs are widely acknowledged as
de novo primary cancers, separate from the first occurrence.
This understanding has a definitive bearing on surveillance,
therapeutic stratification and prognostication in the continuum of
care for survivors of BC (21).
Considering the bilateral nature of the breast, extensive research
has been performed on the laterality of BC, with studies
demonstrating a higher incidence (22) and severity (23) of left-sided BC; however, studies
extensively assessing the impact of the laterality of the initial
ipsilateral BC as a risk factor for the development of MCBC are
limited.
The present study used prospectively maintained
clinical data from the Korean Breast Cancer Registry and aimed to
assess the risk factors associated with the occurrence of MCBC. In
this context, mortality is considered a competing factor in
mitigating biases arising from deaths occurring before the
development of MCBC (21).
Furthermore, as MCBC allows for a clear delineation of primary and
secondary cancers in a chronological sequence (22,23),
the present study also aimed to evaluate whether the laterality of
the initial ipsilateral (I)BC is a risk factor for the development
of MCBC.
Materials and methods
Data source and study population
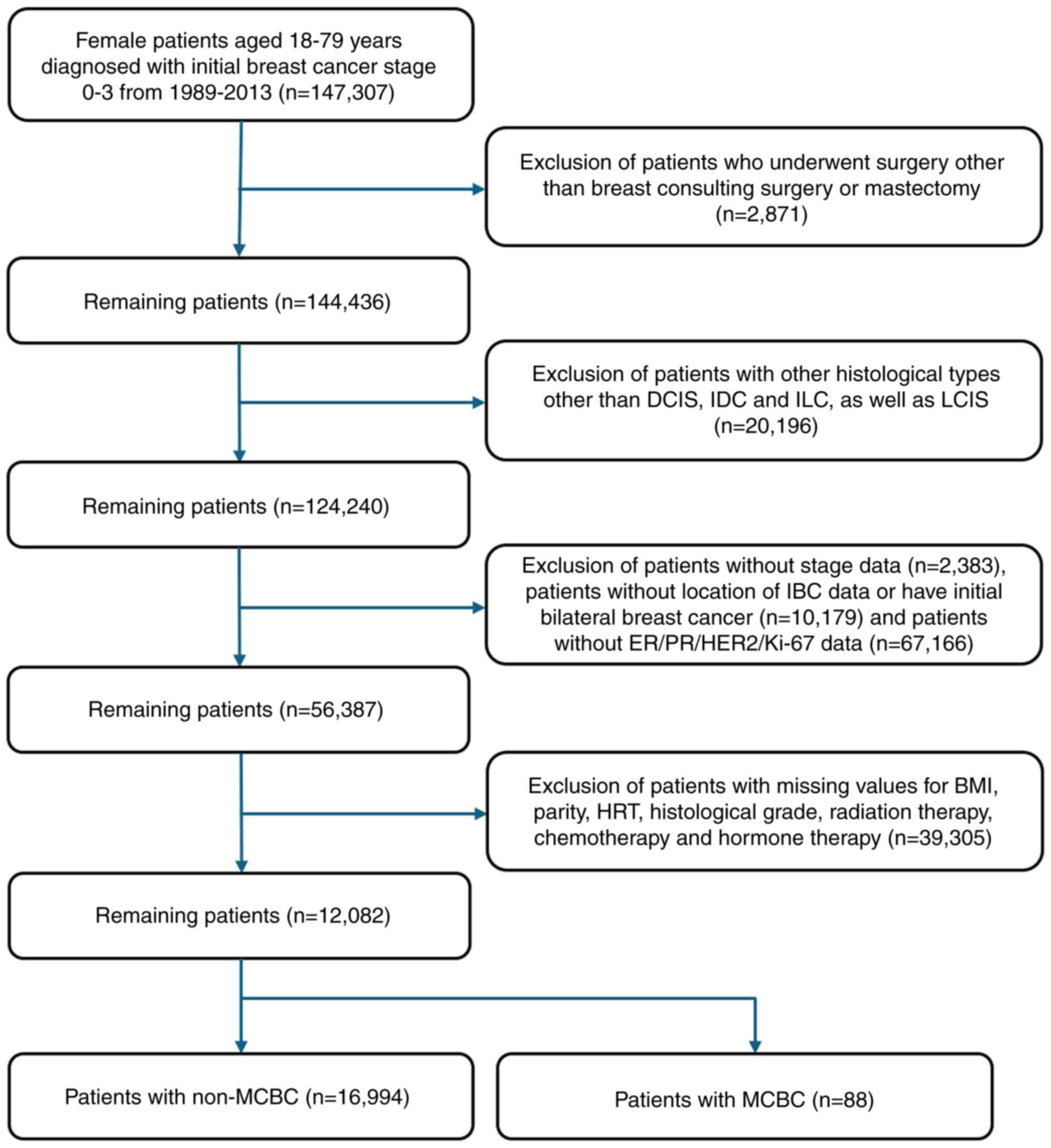
The present retrospective study was based on
prospectively collected and maintained databases of patients who
underwent BC surgery at 102 general hospitals. Female patients aged
18–79 years who were diagnosed with stage 0–3 BC by American Joint
Committee on Cancer Tumor-Node-Metastasis staging (24) and underwent breast-conserving
surgery or mastectomy as the primary breast surgery with curative
intent between 1989–2013 were included in the present study.
Patients who developed distant metastasis after the diagnosis of
IBC but before the diagnosis of MCBC were excluded from the first
step in determining eligibility. This is because metastatic BC,
which may be indistinguishable from metachronous BC, was suspected
in these circumstances. Patients without laterality data for
initial IBC or MCBC, estrogen receptor (ER), progesterone receptor
(PR), human epidermal growth factor receptor 2 (HER2) and Ki-67
results, which are necessary immunohistochemistry data for
molecular subtype classification, and those without stage results
were excluded (Fig. 1).
Assessment
Patients who met the criteria were divided into MCBC
and non-MCBC groups based on the development of MCBC. MCBC was
defined as secondary contralateral BC diagnosed >12 months after
the diagnosis of primary BC. The clinical characteristics of the
patients included the following: Age at the time of primary cancer
diagnosis; operation type (breast-conserving surgery or
mastectomy); laterality of primary IBC; tumor-node-metastasis (TNM)
stage; body mass index (BMI); parity; hormone replacement therapy
(HRT) experience; histologic grade; nuclear grade; histological
type; subtype; expression status of ER, PR and HER2; Ki-67
proliferation index; and history of chemotherapy, radiation therapy
and hormone therapy.
The seventh edition criteria of the AJCC was used to
classify the TNM staging (24). ER,
PR and HER2 statuses were determined using immunohistochemistry as
previously described (25). HER2
overexpression was defined as negative for immunohistochemistry
grades 0–2+, whereas grade 3+ was considered positive. Patients
with a grade of 1+ or 2+ underwent additional fluorescence in
situ hybridization assessments as previously described
(25). Based on ER, PR, HER2 and
Ki-67 markers, tumors were grouped into five subtypes: Luminal A
(ER+ and/or PR+, HER2− and Ki-67
<14.0%), Luminal B (positive for hormone receptors,
HER2− and Ki-67 ≥14.0%), Luminal HER2 (positive for
hormone receptors and HER2+), HER2 amplified
(ER− and PR−, but HER2+), and
triple-negative (TN)BC (ER−, PR− and
HER2−).
Statistical analysis
The clinical characteristics of the MCBC and
non-MCBC groups were compared using the χ2 and Fisher's
exact tests. Kaplan-Meier analysis was used to compare the overall
survival (OS) and BC-specific survival (BCSS) between the two
groups, and the significance of the differences was assessed using
the log-rank test. In addition, the Fine-Gray subdistributional
hazard model was used to evaluate the risk factors for MCBC,
considering patient mortality as a competing risk. In this context,
‘time’ was defined in a multifaceted manner to account for diverse
clinical outcomes, representing the period from the date of IBC
diagnosis to that of the occurrence of MCBC. For patients who died,
‘time’ spanned from their IBC diagnosis to the date of death. Where
neither MCBC nor death occurred, ‘time’ was considered up to the
last date on which the status of patient mortality and CBC
occurrence was confirmed. This comprehensive approach allowed for a
more delicate and precise assessment of MCBC risk over time,
incorporating the critical aspects of patient mortality. This model
included the following: Age; operation type; laterality; stage;
BMI; delivery status; HRT; histological grade; nuclear grade;
histological type; ER, PR, HER2 and Ki-67 status; and a history of
radiotherapy, chemotherapy and hormone therapy. The model's
assumption of proportional hazards was verified, and the fit of the
model was assessed by calculating the P-values of the residuals.
Statistical analyses were performed using the Statistical Package
for Social Science version 29.0.1.0 (IBM Corp.) and R software
version 4.3.0 (R Foundation for Statistical Computing). P<0.05
was considered to indicate a statistically significant
difference.
Compliance with ethical standards
The study protocol was reviewed and approved by the
Institutional Review Board of the Catholic University of Korea
(Suwon, Republic of Korea; approval no. VC24ZISI0020) in accordance
with the ethical guidelines of the Institutional and/or National
Research Committee and the tenets of the 1964 Declaration of
Helsinki and its later amendments. All the patient data were
collected and maintained by the Korean Breast Cancer Society. All
the patients provided written informed consent for the storage and
use of their information for research purposes.
Results
Baseline characteristics
When comparing the proportions between the non-MCBC
and MCBC groups using the χ2 and Fisher's exact tests
for baseline characteristics, the proportion of individuals <40
years of age was significantly higher in the MCBC group than in the
non-MCBC group (14.9% vs. 31.8%; P<0.001). Significant
differences were also observed in histological and nuclear grades,
particularly in grade 3, where there was a significant difference
in proportions between groups (38.4% vs. 52.3%; P=0.021 and 42.0%
vs. 53.4%; P=0.016, respectively). Subtype distribution varied
significantly between the non-MCBC and MCBC groups. Specifically,
the Luminal A subtype occurred less frequently in the MCBC group
compared with the non-MCBC group, and a greater proportion of TNBC
was observed in the MCBC group relative to the non-MCBC group
(34.4% vs. 20.5%; P=0.006 and 16.6% vs. 29.5%; P=0.006,
respectively). Moreover, patients negative for both ER and PR were
significantly more common in the MCBC group than in the non-MCBC
group (30.8% vs. 47.7%; P=0.001 and 41.2% vs. 58.0%; P=0.001,
respectively). Furthermore, patients with Ki-67 scores of ≥14% were
significantly more prevalent in the MCBC group, with 56.0% in the
non-MCBC group compared with 67.0% in the MCBC group (P=0.038).
Finally, a significantly higher proportion of patients did not
receive hormonal therapy in the MCBC group compared with the
non-MCBC group (30.1% vs. 42.0%; P=0.014; Table I).
 | Table I.Patient characteristics. |
Table I.
Patient characteristics.
| Characteristic | Non-metachronous BC
(n=16,994) | Metachronous BC
(n=88) | P-value |
|---|
| Age, years |
|
| <0.001 |
|
≤40 | 2,531 (14.9) | 28 (31.8) |
|
|
>40 | 14,463 (85.1) | 60 (68.2) |
|
| Location of initial
breast cancer (laterality) |
|
| 0.301 |
|
Left | 8,717 (51.3) | 50 (56.8) |
|
|
Right | 8,277 (48.7) | 38 (43.2) |
|
| Operation |
|
| 0.352 |
|
BCS | 10,478 (61.7) | 50 (56.8) |
|
|
Mastectomy | 6,516 (38.3) | 38 (43.2) |
|
| Stage |
|
| 0.273 |
| 0 | 139 (0.8) | 1 (1.1) |
|
| 1 | 7,708 (45.4) | 46 (52.3) |
|
| 2 | 6,866 (40.4) | 35 (39.8) |
|
| 3 | 2,281 (13.4) | 6 (6.8) |
|
| NAC |
|
| 0.815 |
| No | 15,433 (90.8) | 83 (94.3) |
|
|
Yes | 1,561 (9.2) | 5 (5.7) |
|
| BMI,
kg/m2 |
|
| 0.249 |
|
<25 | 11,777 (69.3) | 62 (70.5) |
|
|
≥25 | 5,217 (30.7) | 26 (29.5) |
|
| Parity |
|
| 0.091 |
| No | 608 (3.6) | 5 (5.7) |
|
|
Yes | 16,386 (96.4) | 83 (94.7) |
|
| Oral
contraceptive |
|
| 0.091 |
| No | 14,566 (87.5) | 70 (81.4) |
|
|
Yes | 2,089 (12.5) | 16 (18.6) |
|
| HRT |
|
| 0.954 |
| No | 15,419 (90.7) | 80 (90.9) |
|
|
Yes | 1,575 (9.3) | 8 (9.1) |
|
| Histological
grade |
|
| 0.021 |
| 1 | 2,402 (14.1) | 12 (13.6) |
|
| 2 | 8,062 (47.4) | 30 (34.1) |
|
| 3 | 6,530 (38.4) | 46 (52.3) |
|
| Nuclear grade |
|
| 0.016 |
| 1 | 1,305 (7.7) | 10 (11.4) |
|
| 2 | 8,550 (50.3) | 31 (35.2) |
|
| 3 | 7,139 (42.0) | 47 (53.4) |
|
| Histological
type |
|
| 0.749 |
|
Ductal | 16,485 (97.0) | 85 (96.6) |
|
|
Lobular | 509 (3.0) | 3 (3.4) |
|
| Lymphovascular
invasion |
|
| 0.116 |
| No | 9,347 (61.4) | 52 (70.3) |
|
|
Yes | 5,888 (38.6) | 22 (29.7) |
|
| Subtype |
|
| 0.006 |
| Luminal
A | 5,848 (34.4) | 18 (20.5) |
|
| Luminal
B | 4,271 (25.1) | 20 (22.7) |
|
| Luminal
HER2 | 2,038 (12.0) | 11 (12.5) |
|
| HER2
amplified | 2,018 (11.9) | 13 (14.8) |
|
|
TNBC | 1,819 (16.6) | 26 (29.5) |
|
| Estrogen
receptor |
|
| 0.001 |
|
Negative | 5,229 (30.8) | 42 (47.7) |
|
|
Positive | 11,765 (69.2) | 46 (52.3) |
|
| Progesterone
receptor |
|
| 0.001 |
|
Negative | 7,002 (41.2) | 51 (58.0) |
|
|
Positive | 9,992 (58.8) | 37 (42.0) |
|
| HER2 |
|
| 0.455 |
|
Negative | 12,938 (76.1) | 64 (72.7) |
|
|
Positive | 4,056 (23.9) | 24 (27.3) |
|
| Ki-67 |
|
| 0.038 |
|
<14% | 7,472 (44.0) | 29 (33.0) |
|
|
≥14% | 9,522 (56.0) | 59 (67.0) |
|
| Radiation
therapy |
|
| 0.275 |
| No | 5,078 (29.9) | 31 (35.2) |
|
|
Yes | 11,916 (70.1) | 57 (64.8) |
|
| Chemotherapy |
|
| 0.190 |
| No | 4,944 (29.1) | 20 (22.7) |
|
|
Yes | 12,050 (70.9) | 68 (77.3) |
|
| Hormonal
therapy |
|
| 0.014 |
| No | 5,107 (30.1) | 37 (42.0) |
|
|
Yes | 11,887 (69.9) | 51 (58.0) |
|
Cumulative incidence
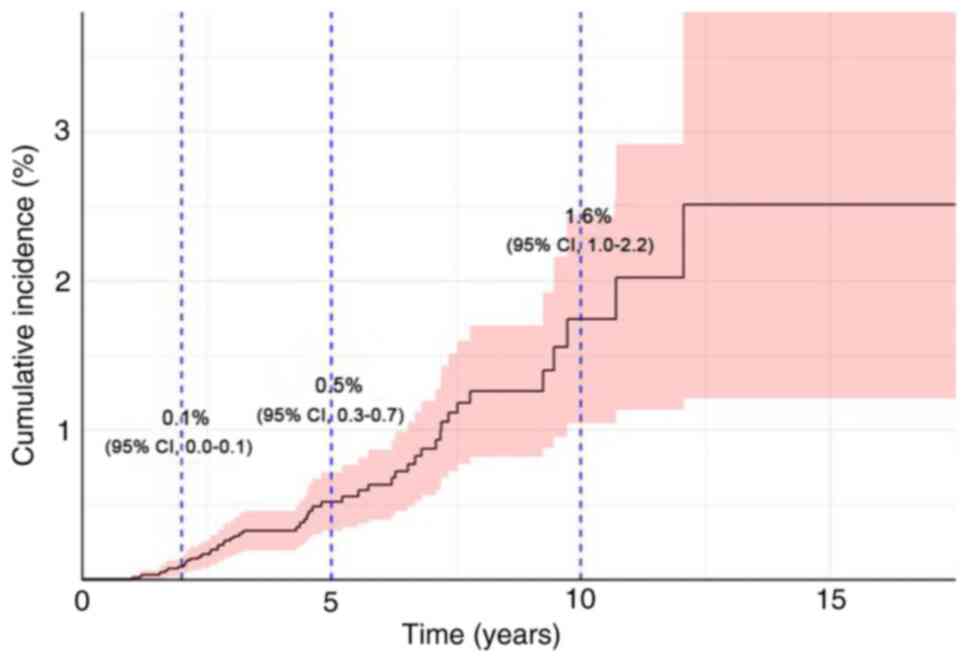
In the present study, the overall cumulative
incidence of MCBC was assessed. The data indicated an incidence of
0.1% in the first year, which increased to 0.5% by the fifth year
and further increased to 1.6% by the tenth year. These findings
demonstrated a progressive increase in the risk of MCBC over time.
The confidence intervals (CIs) at these time points were 0.0–0.1
for year 1, 0.3–0.7 for year 5, and 1.0–2.2 for year 10, which
reflect the statistical variability of the estimates (Fig. 2).
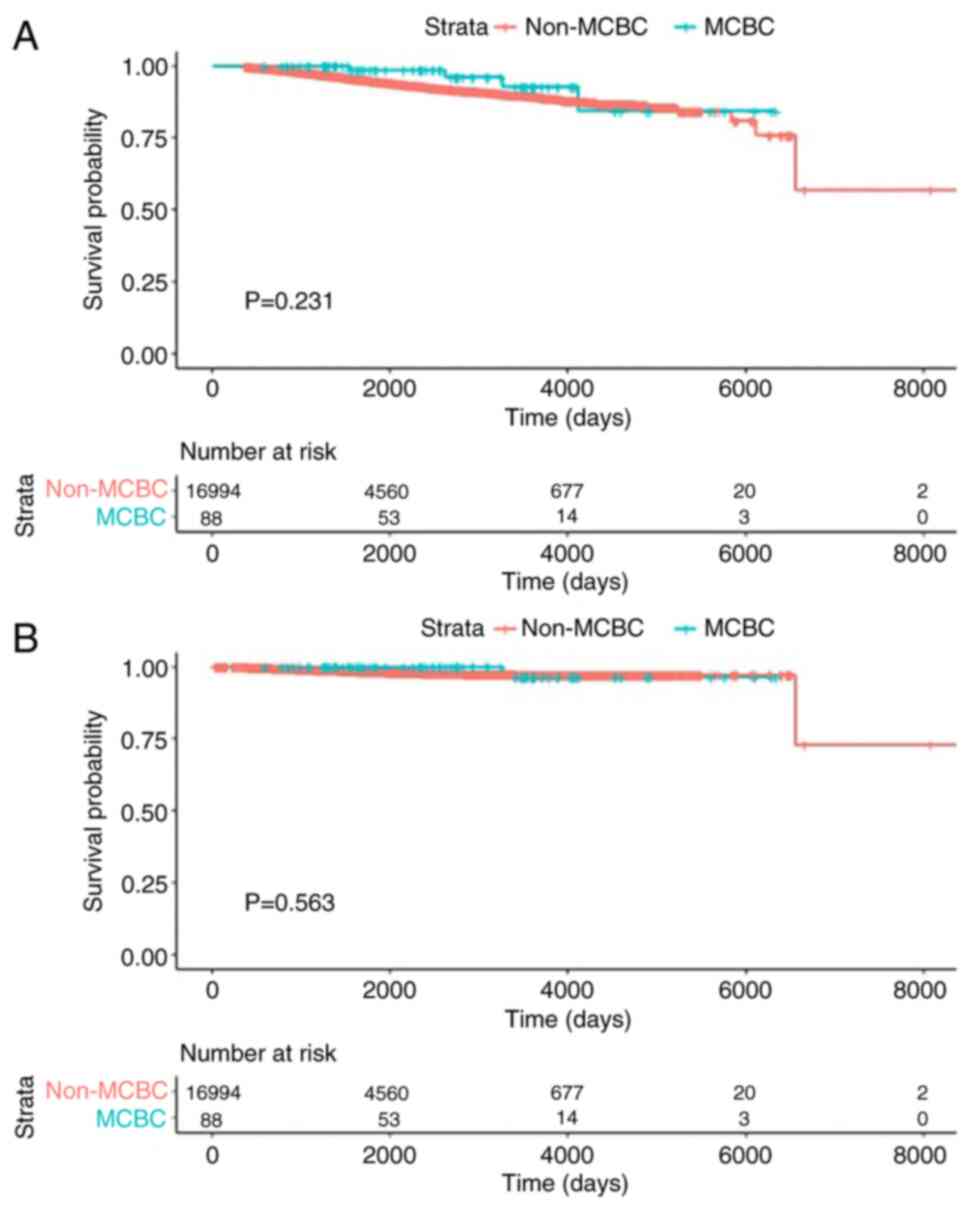
Survival analysis
Survival outcomes were assessed using the
Kaplan-Meier method, with the log-rank test used to evaluate the
statistical significance between survival curves. At a median
follow-up of 3.63 years, the log-rank test revealed no significant
differences in OS or BCSS between the non-MCBC and MCBC cohorts
(OS, P=0.23 and BCSS, P=0.56). The estimated 5-year OS rate was
94.7% in the non-MCBC group compared with 98.5% in the MCBC group,
and the 10-year OS rates were 89.3 and 92.7%, respectively. For
BCSS, the rates at 5 years were 98.3% in patients without MCBC and
100% in those with MCBC; at 10 years, the rates were 97.3 and
96.4%, respectively (Fig. 3).
Subdistributional Cox regression
To evaluate the determinants of the incidence of
MCBC with death as a competing risk factor, a competing risk
regression analysis was performed. The analysis indicated that
patients aged ≤40 years had a significantly higher risk of
developing MCBC compared with those who were >40 years. This was
evidenced by a subdistributional hazard ratio (SHR) of 0.428 (95%
CI, 0.223–0.819; P=0.010). Furthermore, a Ki-67 index of ≥14%
significantly increased the risk of MCBC, with an SHR of 1.966 (95%
CI, 1.053–3.668; P=0.034), indicating a pronounced susceptibility
for MCBC in patients with elevated Ki-67 levels. Conversely,
PR+ was associated with a decreased risk of MCBC, with
an SHR of 0.441 (95% CI, 0.203–0.956; P=0.038), suggesting a
protective effect against MCBC development. Variables such as
laterality, BMI and hormonal treatment of the initial BC were not
significantly associated with the risk of MCBC. Moreover, the
cancer stage at diagnosis did not significantly alter the risk
profile of MCBC in this analysis. Treatment modalities, including
radiation therapy, chemotherapy and hormone therapy, as well as
histological and nuclear grades, ER status and HER2 status, did not
demonstrate significant associations with MCBC risk (Table II).
 | Table II.Subdistributional Cox regression
analysis. |
Table II.
Subdistributional Cox regression
analysis.
| Factor | HR | 95% CI | P-value |
|---|
| Age, years |
|
|
|
|
≤40 | - | - | - |
|
>40 | 0.428 | 0.223–0.819 | 0.010 |
| Location of initial
breast cancer (laterality) |
|
|
|
|
Left | - | - | - |
|
Right | 1.020 | 0.590–1.759 | 0.936 |
| Stage |
|
|
|
| 0 | - | - | - |
| 1 | 0.361 | 0.040–3.179 | 0.359 |
| 2 | 0.269 | 0.029–2.432 | 0.241 |
| 3 | 0.249 | 0.021–2.939 | 0.274 |
| BMI,
kg/m2 |
|
|
|
|
<25 | - | - | - |
|
≥25 | 1.097 | 0.588–2.042 | 0.772 |
| Parity |
|
|
|
| No | - | - | - |
|
Yes | 0.524 | 0.170–1.609 | 0.258 |
| HRT |
|
|
|
| No | - | - | - |
|
Yes | 1.793 | 0.761–4.221 | 0.180 |
| Histological
grade |
|
|
|
| 1 | - | - | - |
| 2 | 0.735 | 0.212–2.538 | 0.626 |
| 3 | 1.170 | 0.318–4.296 | 0.805 |
| Nuclear grade |
|
|
|
| 1 | - | - | - |
| 2 | 0.515 | 0.154–1.712 | 0.279 |
| 3 | 0.507 | 0.129–1.983 | 0.334 |
| Histological
type |
|
|
|
|
Ductal | - | - | - |
|
Lobular | 2.002 | 0.257–15.540 | 0.510 |
| Estrogen
receptor |
|
|
|
|
Negative | - | - | - |
|
Positive | 0.709 | 0.305–1.646 | 0.421 |
| Progesterone
receptor |
|
|
|
|
Negative | - | - | - |
|
Positive | 0.441 | 0.203–0.956 | 0.038 |
| HER2 |
|
|
|
|
Negative | - | - | - |
|
Positive | 0.681 | 0.303–1.527 | 0.353 |
| Ki-67 |
|
|
|
|
<14% | - | - | - |
|
≥14% | 1.966 | 1.053–3.668 | 0.034 |
| Radiation
therapy |
|
|
|
| No | - | - | - |
|
Yes | 1.257 | 0.352–4.477 | 0.718 |
| Chemotherapy |
|
|
|
| No | - | - | - |
|
Yes | 1.136 | 0.508–2.536 | 0.763 |
| Hormonal
therapy |
|
|
|
| No | - | - | - |
|
Yes | 1.904 | 0.848–4.270 | 0.118 |
Discussion
There is no universally established definition for
metachronous BC. If a patient is diagnosed with IBC and
subsequently develops contralateral BC, the criteria to determine
whether it should be considered metachronous or metastatic BC are
not clearly defined. When local recurrence or distant metastasis
follows IBC, contralateral BC may be more likely to be classified
as metastatic; however, in the absence of locoregional recurrence
or distant metastasis, it is often regarded as metachronous BC
(20,26). In cases where it is difficult to
distinguish between metachronous BC and metachronous
recurrence/occurrence, a comprehensive genetic analysis, such as
next-generation sequencing using DNA extracted from primary and
secondary BCs, is theoretically required. Nevertheless, as
metachronous BC is considered to be increasing in frequency, but
not absolutely, and as only longitudinal data from multiple
institutions enable in-depth studies, tumor registry data, as used
in the present research, remains the primary source, and extensive
genetic analysis faces practical challenges. Encouragingly, recent
research suggests that most bilateral BCs, including metachronous
types, may not be genetically related in terms of clonal
relationships, although this does not apply to all instances
(20,26).
The present retrospective study aimed to elucidate
risk factors associated with MCBC using data from the Korean Breast
Cancer Registry. Upon analyses of baseline characteristics, it was
observed that patients aged <40 years, with histological and
nuclear grade 3 tumors, and the TNBC subtype, as well as patients
negative for hormonal receptors (ER and PR), a Ki-67 index of ≥14%,
and those who had not received adjuvant hormonal therapy, were
present at a significantly higher proportion in the MCBC group than
in the non-MCBC group. Conversely, variables such as the type of
surgery performed, administration of systemic chemotherapy, stage
of IBC and the status of HER2 did not demonstrate a significant
difference between the MCBC and non-MCBC groups.
Furthermore, in the present study, IBC laterality
demonstrated a preference for the left breast in both cohorts, with
a higher, albeit not statistically significant, prevalence in the
MCBC group compared with the non-MCBC group (51.3 vs. 56.8%;
P=0.301; Table I). This finding
aligns with that in the established literature suggesting a higher
incidence of left-sided BC (22,27).
A detailed analysis of the cumulative incidence of
MCBC was performed. The findings revealed a cumulative incidence of
0.1% at the end of the first year. This incidence gradually
escalated, reaching 0.5% by the fifth year and further rising to
1.6% by the tenth year. These results not only delineate a
progressive increase in the risk of MCBC over time but also
indicate the importance of prolonged and vigilant monitoring. The
confidence intervals, registering at 0.0–0.1 for the first year,
0.3–0.7 for the fifth year, and 1.0–2.2 for the tenth year,
indicate the statistical variability inherent in the findings. This
upward trajectory in incidence emphasizes the necessity for
continuous surveillance of BC survivors, extending well beyond the
initial decade post-diagnosis.
Contrary to previous research suggesting that
patients with bilateral BC have a worse prognosis than those with
unilateral disease (28), the
analysis in the present study demonstrated no significant
differences in the survival rates between patients with MCBC and
those with non-MCBC. However, this is consistent with another
Korean study (29), which also
reported no notable survival differences between these groups,
suggesting that improvements in treatment protocols, the importance
of early detection, and possibly unique genetic or environmental
factors within the Korean population may contribute to diminishing
the traditional survival gap between unilateral and bilateral BC
cases. Nevertheless, it is not yet clear whether the findings of
the present study are unique to the Korean population or whether
they reflect global trends. To better understand these nuances,
further research involving a broader international sample and the
examination of additional variables, such as genetic markers,
lifestyle impacts and health system differences, is required. Such
research could reveal the extent to which these outcomes are
influenced by regional characteristics and help to tailor BC
treatment and survivorship planning on a global scale.
Moreover, the Fine-Gray subdistribution hazard model
was applied to assess the risk factors for MCBC, considering
mortality as a competing risk. This method was selected due to its
ability to account for competing risks, thereby offering a more
precise estimation of the incidence and impact of covariates over
time (30,31). The results revealed that an age of
≤40 years at the time of IBC diagnosis, having a PR−
status, and having a Ki-67 score of ≥14% were significant risk
factors for the onset of MCBC; however, the other variables
assessed were not significant risk factors for MCBC. The current
results align with the that of the existing literature that has
identified a younger age at initial BC diagnosis as a risk factor
for CBC (2,4,5) and
highlights the significance of PR status and high Ki-67 scores in
the context of MCBC risk (27,28).
However, whilst in previous studies, chemotherapy and hormone
therapy have been associated with a decrease in the incidence of
CBC, the findings of the present study did not demonstrate
significant evidence supporting this perspective (6–9).
Moreover, during the data collection phase of the present study,
ductal carcinoma in situ was classified as a ductal
histological type, and lobular carcinoma in situ (LCIS) was
not included as a lobular histological type. This decision reflects
the current understanding that LCIS is not a form of cancer but is
a risk factor for the development of BC. Consequently, patients
with LCIS were excluded from the analysis. The results of the
present study indicated that the histological type was not a
significant risk factor for the development of MCBC, which is
consistent with other studies that have also reported that the
histological type does not constitute a risk factor for the
occurrence of CBC (32,33).
Furthermore, whilst a PR− status was
identified as a significant risk factor for the development of
MCBC, ER status did not emerge as a significant risk factor in the
Fine-Gray subdistribution hazard model. This distinction is
noteworthy because it deviates from the common understanding that
both hormone receptors typically serve a role in BC prognosis
(27–29). Thus, the findings of the present
study provide a new perspective on the differential impacts of
hormone receptor status on the risk of MCBC development. Potential
reasons for the lack of significance of ER status in this context
warrant further investigation and could help refine risk
stratification and management in patients with IBC.
Family history is recognized as a risk factor for
CBC (5); however, in the present
study, it was not used as a variable due to the large number of
missing values. For instance, of the 88 individuals in the MCBC
group, only 11 had a known family history. Family histories of the
remaining 77 patients were undocumented. Therefore, an association
between family history and the occurrence of MCBC could not be
ascertained. Future research may further explore this relationship,
including an assessment of MCBC occurrence in patients with
hereditary BC with BC gene (BRCA)1 and BRCA2 mutations.
The present study, which considered mortality as a
competing factor, revealed the possible risk factors for MCBC. This
methodology has enabled an in-depth understanding of the factors
influencing the occurrence of MCBC, particularly the identification
of PR− status and high Ki-67 scores as new risk
indicators. These findings offer a revised perspective on the
management and monitoring of patients with BC.
The multivariate analysis indicated that the
laterality of IBC is not a significant risk factor for MCBC;
however, this finding does not diminish the relevance of laterality
as a variable for future studies. It is conceivable that in
subsequent studies involving diverse ethnic patient cohorts or
using different research methodologies, consideration of IBC
laterality may yield valuable insights. Therefore, laterality is
worthy of careful consideration in future BC research.
Despite the insights provided by the present study,
there are inherent limitations in its retrospective design. This
approach is susceptible to missing data across parameters, accuracy
challenges, and potential selection and information bias. Reliance
on clinical records may hinder data standardization and introduce
the risk of unaccounted-for confounding factors. Additionally, the
ethnic and geographical homogeneity of the cohort constrains the
broader applicability of the findings. Therefore, future research
should endeavor to use prospective designs, encompass diverse
populations, and implement rigorous standardization to enhance the
robustness and generalizability of the outcomes.
Nonetheless, the present study offers significant
insights into the risk factors for MCBC; however, these results
should be approached with caution and regarded as the foundation
for subsequent prospective investigations. Future studies should
include broader demographics by incorporating patients from diverse
ethnicities and regions, thereby expanding the sample range and
enhancing the generalizability of the findings. Such studies,
ideally encompassing larger and more varied cohorts and using
prospective methodologies, are crucial to affirm the findings of
the present study and extend their relevance and applicability
across different clinical contexts and populations. The inclusion
of varied ethnic and regional backgrounds in future research would
help determine whether the identified risk factors are universally
applicable or whether they exhibit variation among different
groups. This expansion is vital not only for the validation of
results but also for tailoring preventive strategies and
interventions to address the specific needs of distinct
populations.
In conclusion, the present comprehensive study on
MCBC within the Korean population identified age, PR status and
Ki-67 scores as significant risk factors but did not substantiate
certain traditional views regarding histological types and therapy
implications. Additionally, despite the limitations inherent to
retrospective analyses, the findings suggest that bilateral BC does
not inherently confer a worse prognosis than unilateral BC does in
this demographic, indicating a potential shift in understanding the
dynamics of BC progression and outcomes. Future research should
focus on expanding these findings through prospective studies and
broader international collaborations to confirm these observations
and explore the impact of genetic, lifestyle and healthcare system
factors on BC prognosis, ultimately contributing to targeted and
effective patient care across diverse populations.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The data generated in the present study may be
requested from the Korea Breast Cancer Society due to restrictions
on the availability of these data, which were used under license
for the current study, and so are not publicly available.
Authors' contributions
YJS designed and supervised the study and revised
the manuscript. BKP analyzed the data from the Korean Breast Cancer
Registry, and conceived and modified the manuscript. JS helped
conceive the study, and drafted and modified the original
manuscript. YJS and BKP confirm the authenticity of all the raw
data. All authors have read and approved the final manuscript.
Ethics approval and consent to
participate
The present research received approval from the
Institutional Review Board of the Catholic University of Korea
(approval no. VC24ZISI0020). Written informed consent was obtained
from all patients for the use and storage of their data in the
present research.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
BC
|
breast cancer
|
|
BCSS
|
BC-specific survival
|
|
BMI
|
body mass index
|
|
CBC
|
contralateral BC
|
|
CI
|
confidence interval
|
|
ER
|
estrogen receptor
|
|
HER2
|
human epidermal growth factor receptor
2
|
|
HRT
|
hormone replacement therapy
|
|
IBC
|
ipsilateral BC
|
|
LCIS
|
lobular carcinoma in situ
|
|
MCBC
|
metachronous contralateral BC
|
|
TNBC
|
triple-negative BC
|
|
OS
|
overall survival
|
|
PR
|
progesterone receptor
|
|
SHR
|
subdistributional hazard ratio
|
|
TNM
|
tumor-node-metastasis
|
References
|
1
|
Hartman M, Czene K, Reilly M, Adolfsson J,
Bergh J, Adami HO, Dickman PW and Hall P: Incidence and prognosis
of synchronous and metachronous bilateral breast cancer. J Clin
Oncol. 25:4210–4216. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Nichols HB, Berrington de González A,
Lacey JV Jr, Rosenberg PS and Anderson WF: Declining incidence of
contralateral breast cancer in the United States from 1975 to 2006.
J Clin Oncol. 29:1564–1569. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Rosen PP, Groshen S, Kinne DW and Hellman
S: Contralateral breast carcinoma: An assessment of risk and
prognosis in stage I (T1N0M0) and stage II (T1N1M0) patients with
20-year follow-up. Surgery. 106:904–910. 1989.PubMed/NCBI
|
|
4
|
Hankey BF, Curtis RE, Naughton MD, Boice
JD Jr and Flannery JT: A retrospective cohort analysis of second
breast cancer risk for primary breast cancer patients with an
assessment of the effect of radiation therapy. J Natl Cancer Inst.
70:797–804. 1983.PubMed/NCBI
|
|
5
|
Reiner AS, John EM, Brooks JD, Lynch CF,
Bernstein L, Mellemkjær L, Malone KE, Knight JA, Capanu M, Teraoka
SN, et al: Risk of asynchronous contralateral breast cancer in
noncarriers of BRCA1 and BRCA2 mutations with a family history of
breast cancer: A report from the Women's environmental cancer and
radiation epidemiology study. J Clin Oncol. 31:433–439. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Bernstein JL, Thompson WD, Risch N and
Holford TR: Risk factors predicting the incidence of second primary
breast cancer among women diagnosed with a first primary breast
cancer. Am J Epidemiol. 136:925–936. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Bertelsen L, Bernstein L, Olsen JH,
Mellemkjaer L, Haile RW, Lynch CF, Malone KE, Anton-Culver H,
Christensen J, Langholz B, et al: Effect of systemic adjuvant
treatment on risk for contralateral breast cancer in the Women's
Environment, Cancer and Radiation Epidemiology Study. J Natl Cancer
Inst. 100:32–40. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Early Breast Cancer Trialists'
Collaborative Group (EBCTCG), : Effects of chemotherapy and
hormonal therapy for early breast cancer on recurrence and 15-year
survival: An overview of the randomised trials. Lancet.
365:1687–1717. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Davies C, Pan H, Godwin J, Gray R,
Arriagada R, Raina V, Abraham M, Medeiros Alencar VH, Badran A,
Bonfill X, et al: Long-term effects of continuing adjuvant
tamoxifen to 10 years versus stopping at 5 years after diagnosis of
oestrogen receptor-positive breast cancer: ATLAS, a randomised
trial. Lancet. 381:805–816. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kilgore AR: The incidence of cancer in the
second breast: After radical removal of one breast for cancer. J Am
Med Assoc. 77:454–457. 1921. View Article : Google Scholar
|
|
11
|
Haagensen CD and Rosato FE: Diseases of
the breast. Ann Surg. 176:6901972. View Article : Google Scholar
|
|
12
|
Prior P and Waterhouse JA: Incidence of
bilateral tumours in a population-based series of breast-cancer
patients. I. Two approaches to an epidemiological analysis. Br J
Cancer. 37:620–634. 1978. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Healey EA, Cook EF, Orav EJ, Schnitt SJ,
Connolly JL and Harris JR: Contralateral breast cancer: Clinical
characteristics and impact on prognosis. J Clin Oncol.
11:1545–1552. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Intra M, Rotmensz N, Viale G, Mariani L,
Bonanni B, Mastropasqua MG, Galimberti V, Gennari R, Veronesi P,
Colleoni M, et al: Clinicopathologic characteristics of 143
patients with synchronous bilateral invasive breast carcinomas
treated in a single institution. Cancer. 101:905–912. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
de la Rochefordiere A, Asselain B, Scholl
S, Campana F, Ucla L, Vilcoq JR, Durand JC, Pouillart P and
Fourquet A: Simultaneous bilateral breast carcinomas: A
retrospective review of 149 cases. Int J Radiat Oncol Biol Phys.
30:35–41. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Al-Jurf AS, Jochimsen PR, Urdaneta LF and
Scott DH: Factors influencing survival in bilateral breast cancer.
J Surg Oncol. 16:343–348. 1981. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Heron DE, Komarnicky LT, Hyslop T,
Schwartz GF and Mansfield CM: Bilateral breast carcinoma: Risk
factors and outcomes for patients with synchronous and metachronous
disease. Cancer. 88:2739–2750. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Quan G, Pommier SJ and Pommier RF:
Incidence and outcomes of contralateral breast cancers. Am J Surg.
195:645–650; discussion 650. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Londero AP, Bernardi S, Bertozzi S,
Angione V, Gentile G, Dri C, Minucci A, Caponnetto F and Petri R:
Synchronous and metachronous breast malignancies: A cross-sectional
retrospective study and review of the literature. Biomed Res Int.
2014:2507272014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Begg CB, Ostrovnaya I, Geyer FC,
Papanastasiou AD, Ng CKY, Sakr RA, Bernstein JL, Burke KA, King TA,
Piscuoglio S, et al: Contralateral breast cancers: Independent
cancers or metastases. Int J Cancer. 142:347–356. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Hamy AS, Abécassis J, Driouch K, Darrigues
L, Vandenbogaert M, Laurent C, Zaccarini F, Sadacca B, Delomenie M,
Laas E, et al: Evolution of synchronous female bilateral breast
cancers and response to treatment. Nat Med. 29:646–655. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Al Saad S, Al Shenawi H, Almarabheh A, Al
Shenawi N, Mohamed AI and Yaghan R: Is laterality in breast Cancer
still worth studying? Local experience in Bahrain. BMC Cancer.
22:9682022. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Abdou Y, Gupta M, Asaoka M, Attwood K,
Mateusz O, Gandhi S and Takabe K: Left sided breast cancer is
associated with aggressive biology and worse outcomes than right
sided breast cancer. Sci Rep. 12:133772022. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Edge SB and Compton CC: The American Joint
Committee on Cancer: The 7th edition of the AJCC cancer staging
manual and the future of TNM. Ann Surg Oncol. 17:1471–1474. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wolff AC, Somerfield MR, Dowsett M,
Hammond MEH, Hayes DF, McShane LM, Saphner TJ, Spears PA and
Allison KH: Human epidermal growth factor receptor 2 testing in
breast cancer: ASCO-college of american pathologists guideline
update. J Clin Oncol. 41:3867–3872. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Song F, Li X, Song F, Zhao Y, Li H, Zheng
H, Gao Z, Wang J, Zhang W and Chen K: Comparative genomic analysis
reveals bilateral breast cancers are genetically independent.
Oncotarget. 6:31820–31829. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kim BK, Choi JE, Youn HJ, Park HS, Kim D,
Oh SJ, Lee HJ, Lee J and Sun WY; Korean Breast Cancer Society, :
Clinicopathological features and prognosis associated with breast
cancer laterality: A nationwide study from the Korean Breast Cancer
Society. Ann Surg Treat Res. 103:119–128. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Pan B, Xu Y, Zhou YD, Yao R, Wu HW, Zhu
QL, Wang CJ, Mao F, Lin Y, Shen SJ and Sun Q: The prognostic
comparison among unilateral, bilateral, synchronous bilateral, and
metachronous bilateral breast cancer: A meta-analysis of studies
from recent decade (2008–2018). Cancer Med. 8:2908–2918. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kim H, Yoon TI, Kim S, Lee SB, Kim J,
Chung IY, Ko BS, Lee JW, Son BH, Gwark S, et al: Survival after
development of contralateral breast cancer in Korean patients with
breast cancer. JAMA Netw Open. 6:e23335572023. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Austin PC and Fine JP: Practical
recommendations for reporting Fine-Gray model analyses for
competing risk data. Stat Med. 36:4391–4400. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Zhang Z: Survival analysis in the presence
of competing risks. Ann Transl Med. 5:472017. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Broët P, de la Rochefordière A, Scholl SM,
Fourquet A, Mosseri V, Durand JC, Pouillart P and Asselain B:
Contralateral breast cancer: Annual incidence and risk parameters.
J Clin Oncol. 13:1578–1583. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Gao X, Fisher SG and Emami B: Risk of
second primary cancer in the contralateral breast in women treated
for early-stage breast cancer: A population-based study. Int J
Radiat Oncol Biol Phys. 56:1038–1045. 2003. View Article : Google Scholar : PubMed/NCBI
|