Introduction
Anal squamous cell carcinoma (SCC) presents a unique
challenge in cancer care, with most cases diagnosed as
local-regional disease (1). While
treatment approaches have undergone limited changes over the past
40 years and favorable outcomes have been observed, a challenging
subgroup with a poor prognosis necessitates careful treatment
evaluation (2–4). The European Society for Medical
Oncology, European Society of Surgical Oncology, and European
Society for Therapeutic Radiology and Oncology guidelines
prioritize only clinical digital rectal examinations until 26 weeks
after the start of treatment (5).
Currently, no test or marker apart from clinical examination is
able to predict the response to treatment. Incomplete responses
adversely affect survival (6–8), and
limited options in such cases create issues for clinicians and
patients. This underscores the imperative for the early recognition
and tailored treatment of this challenging subgroup.
In head and neck cancers, human papilloma virüs
(HPV) positivity is associated with improved treatment responses
and survival outcomes (9,10). The positive association between HPV
status and p16 expression, particularly in head and neck tumors,
has been thoroughly investigated. As a result, p16 expression is
now integrated into the staging process for head and neck cancers
as a reliable indicator of HPV infection (11,12).
Further investigations into the relationship between HPV status and
p16 expression have led to their inclusion as prognostic factors in
the National Comprehensive Cancer Network Guidelines for anal SCC
(13,14). However, their clinical utility and
role in treatment require further clarification. The implications
of p53 status in anal cancer are also unclear (15). The different p53 mutations and
functions in HPV-positive and -negative tumors are further
complicating factors (16). In anal
SCC, the expression of p53, which is often described as a negative
prognostic factor in numerous types of cancer (17), has not been fully evaluated.
Immunotherapy has emerged as a promising means of
treating local-regional and metastatic anal cancer, as it is for
numerous other types of cancer. Programmed death ligand 1 (PD-L1)
is a key component of the immune checkpoint pathway during the late
effector phase of the immune response; therefore, the investigation
of its expression is of interest. Previous data have indicated
uncertainty regarding the associations between PD-1 expression on
tumor-infiltrating lymphocytes and PD-L1/2 expression on tumor
cells with poor prognosis (18),
and discrepancies in these associations also exist for anal cancer
(19–21).
The influence of HPV status and p16, p53 and PD-L1
expression on treatment response, recurrence patterns and overall
survival (OS) in anal SCC were investigated in the current study
with the aim of gaining prognostic insights. Another aim was to
elucidate the immunological landscape of anal SCC, facilitating the
exploration of targeted therapies involving immune checkpoint
inhibitors.
Materials and methods
Patients
A retrospective evaluation was conducted on 42
patients diagnosed with anal SCC and treated with definitive
radiotherapy (RT)/chemoradiotherapy (CRT) at the Department of
Radiation Oncology of Ege University (Izmir, Türkiye) between
January 2006 and January 2022. Exclusion criteria comprised age
<18 years, metastatic disease, a history of other cancers within
the last 3 years, anal carcinoma in situ or anal
intraepithelial neoplasm, prior RT, human immunodeficiency virus
(HIV) positivity, unavailable current status data, and lack of
diagnostic biopsy material. The study was approved by the Medical
Research Ethics Committee at Ege University (Izmir, Türkiye;
reference: 21-3.1T/63). Written informed consent was obtained from
all patients.
Treatment
The staging elements of tumor (T), node (N) and
metastasis (M) were evaluated according to version 9 of the
American Joint Committee on Cancer staging system before treatment
(22).
The sequential boost technique (23) was used for the treatment of the
high- and low-risk clinical target volumes (CTVs). After delivering
the initial dose to the low-risk CTV, treatment focuses on a
smaller, high-risk target volume. This involves administering an
additional, intensified dose of radiation, commonly known as the
‘boost’. High-risk CTV included the gross disease CTV, mesorectum,
presacral nodes, and bilateral internal and external iliac nodes
below the sacroiliac joint. Additional inguinal nodes were included
in the high-risk CTV if gross inguinal nodal involvement was
present. Low-risk CTV encompassed the high-risk CTV, and presacral,
bilateral internal and external iliac nodes above the sacroiliac
joint to the L5/S1 vertebral body junction. Bilateral inguinal
nodes were included in the low-risk CTV if there was no evident
involvement of these nodes. A dose comprising 45 Gy in 1.8-Gy
fractions was applied to the low-risk CTV followed by a 5.4–9-Gy
boost in 1.8-Gy fractions to the high-risk CTV. Use of the
simultaneous integrated boost technique (24) delivers stage-based doses to
different target volumes with the same number of fractions,
simplifying planning but reducing the biological dose to elective
nodal areas. The single CTV included the gross disease CTV,
bilateral inguinal nodes, mesorectum, presacral nodes, and
bilateral internal and external iliac nodes above the sacroiliac
joint to the L5/S1 vertebral body junction. A dose of 45–54 Gy was
targeted at the single CTV, with a specific dose within of 54–59.4
Gy for the gross disease CTV.
Chemotherapy received by the patients included
mitomycin-C (MMC; 10 mg/m2) administered intravenously
on days 1 and 29, together with 5-fluorouracil (5-FU; 1,000
mg/m2) administered by continuous infusion for 24 h on
days 1–4 and 29–32. No cases received induction chemotherapy.
Post-treatment evaluation
At 3 months after the completion of treatment,
patients underwent an initial evaluation of local response using
standard anoscopy and digital rectal examination to identify
indications of lesion progression. At 6 months post-treatment, a
comprehensive examination was conducted, including digital rectal
examination, endoscopic examination, computed tomography and pelvic
magnetic resonance imaging. Fluorodeoxyglucose positron emission
tomography was also performed when clinically indicated,
encompassing the assessment of suspicious pelvic and inguinal
nodes, as well as distant metastasis. If suspicion arose regarding
a residual lesion, a biopsy was performed for histopathological
confirmation. Those patients with confirmed residual lesions based
on biopsy findings were referred for surgical intervention. The
response status was assessed following the Response Evaluation
Criteria in Solid Tumors (RECIST 1.1) (25).
A complete response was defined as the absence of
disease in the primary tumor site and regional lymph nodes within 6
months after the completion of RT/CRT. A non-complete response was
categorized as stable disease, partial response or progression.
Recurrence definitions
Local-regional recurrence (LRR) refers to
progression independent of time after definitive RT/RCT, such that
it was not possible to perform salvage surgery, or the reappearance
of cancer in the primary tumor and pelvic area ≥6 months
post-treatment in patients with a complete response. Distant
recurrence (DR) indicates the presence of metastasis outside the
pelvic or inguinal lymph node areas, regardless of local-regional
status.
Follow-up and monitoring
Patients underwent regular follow-up after their
treatment, which involved digital rectal examinations every 3
months for the first 2 years, biannually from year 3 to 5, and
yearly thereafter. Females underwent gynecological examinations,
and imaging was conducted as necessary. The assessment included the
detection of LRR and DR, with time intervals measured from the end
of RT.
HPV-DNA analysis and typing
First, tissue microarrays were prepared from
Formalin-fixed paraffin-embedded (FFPE) blocks for each patient
with a thickness of 2 µm. From these, two FFPE sections were cut,
each labeled with biopsy number, block name, and date, and barcode.
FFPE sections were processed with 300 µl deparaffinization solution
(Qiagen, Inc.) in 1.5- or 2-ml microcentrifuge tubes, followed by
10 sec of vigorous vortexing at room temperature. After
centrifugation at 15,000 × g for 10 sec at 25°C to bring the sample
to the bottom of the tube, the samples were incubated at 56°C for 3
min and then cooled to room temperature. HPV DNA amplification was
performed using the QIASCREEN HPV PCR kit (Qiagen, Inc.).
Deparaffinized samples were treated with 25 µl Buffer FTB, 55 µl
RNase-free water, and 20 µl proteinase K, with controlled
temperature incubations. Following removal of the upper phase, the
aqueous lysate was subjected to RNase A treatment and a final
incubation with proteinase K. DNA was then extracted using Buffer
AL and ethanol, with purification using QIAamp UCP MinElute columns
during centrifugation at 15,000 × g for 1 min at 25°C, followed by
elution using Buffer ATE. This produced high-quality DNA for
downstream molecular analyses. Quality control was performed using
spectrophotometry and gel electrophoresis, with optional
enhancements for small elution volumes. Small elution volumes are
the final liquid volumes used to collect DNA from purification
columns. They are employed when working with limited sample sizes
or to achieve higher DNA concentrations, using less elution buffer
to concentrate the DNA. The sequences of the primers in the PCR kit
are proprietary and were not revealed by the company.
The study targeted 15 high-risk HPV types, namely
HPV 16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, 66, 67 and 68,
which were grouped into HPV 16, HPV 18 and other high-risk types.
In the analysis phase, patients were categorized as HPV positive or
HPV negative.
Expression analysis and scoring of
p16, p53 and PD-L1
First, tissue microarrays were prepared from FFPE
blocks for each patient with a thickness of 2 µm. From these, five
FFPE sections were cut, each labeled with biopsy number, block
name, date and barcode. The sections were incubated at 60°C in an
oven for at least 2 h for deparaffinization. Following this initial
preparation step, the process continued with the p16, p53 and PD-L1
immunohistochemical (IHC) staining using the Benchmark XT device
(Roche Tissue Diagnostics). Treatment steps included antigen
retrieval, reagent application, and the use of CINtec P16
Histology, CONFIRM anti-p53 (DO-7) Primary Antibody, and
VENTANA® PD-L1 (SP263) Assay, all from Roche Tissue
Diagnostics.
Viable tumor tissue without necrosis was examined.
Positive p16 expression was defined as intense cytoplasmic and
nuclear staining in >5% of tumor cells (26,27).
For p53 expression, positivity was defined as intense cytoplasmic
and nuclear staining in >5% of tumor cells (26,27).
The tumor proportion score (TPS) was calculated as the percentage
of PD-L1-positive tumor cells among all viable tumor cells: TPS
(%)=[(PD-L1-positive tumor cells/total viable cells) ×100]. The
combined positive score (CPS) was determined by dividing the sum of
PD-L1-positive tumor cells, lymphocytes and macrophages by the
total number of viable tumor cells: CPS (%)=[(PD-L1-positive tumor
cells + lymphocytes + macrophages/total viable cells) ×100]
(28). The analysis was conducted
blindly.
Statistical analysis
The associations among HPV-DNA status, p16, p53,
PD-L1 expression, complete response and clinical factors were
assessed using Pearson's Chi-square or Fisher's exact tests.
Univariate and multivariate Cox regression analyses were performed
to identify independent prognostic factors for OS and disease-free
survival (DFS), including factors achieving P<0.01 from the
univariate analysis in the multivariate analysis. Survival analysis
was performed by the generation of Kaplan-Meier curves, and the
log-rank test was used for this analysis. IBM SPSS Statistics
version 25 (IBM Corp.) was utilized for statistical analysis.
P<0.05 was considered to indicate a statistically significant
result.
Results
Patient characteristics
A total of 42 patients with anal SCC participated in
the study, with a median age of 61 years (age range, 35–86 years).
Among them, 25 (59.5%) were female and 17 (40.5%) were male. The
distribution of stages was as follows: 2 (4.8%) cases in stage I, 6
(14.3%) in stage IIA, 7 (16.7%) in stage IIB, 4 (9.5%) in stage
IIIA, 3 (7.1%) in stage IIIB and 20 (47.6%) in stage IIIC.
Concurrent chemotherapy (5-FU/MMC) with RT was administered in 36
cases (85.7%). This treatment approach took into account the
patients' comorbidities, including severe hypersensitivity or
allergy, myelosuppression, renal or hepatic impairment, and
cardiopulmonary disease. The characteristics of the patients and
the therapies received are presented in Table I.
 | Table I.Patient and clinical
characteristics. |
Table I.
Patient and clinical
characteristics.
| Characteristic | Value |
|---|
| Sex, n (%) |
|
|
Female | 25 (59.5) |
|
Male | 17 (40.5) |
| Median age (range),
years | 61 (35–86) |
| T stage, n (%) |
|
| T1 | 2 (4.8) |
| T2 | 11 (26.2) |
| T3 | 18 (42.8) |
| T4 | 11 (26.2) |
| N stage, n (%) |
|
| N0 | 18 (42.8) |
|
N1a | 16 (38.1) |
|
N1b | 1 (2.4) |
|
N1c | 7 (16.7) |
| Stage, n (%) |
|
| I | 2 (4.8) |
|
IIA | 6 (14.3) |
|
IIB | 7 (16.7) |
|
IIIA | 4 (9.5) |
|
IIIB | 3 (7.1) |
|
IIIC | 20 (47.6) |
| Median total
radiotherpay dose (range), Gy | 59.4
(50.4–59.4) |
| Concurrent
chemotherapy, n (%) |
|
|
Yes | 36 (85.7) |
| No | 6 (14.3) |
In terms of treatment response, 30 cases (71.4%)
achieved a complete response, 5 (11.9%) had a partial response, 4
(9.5%) had a stable response and 3 (7.2%) experienced progression.
Among the 12 patients without a complete response, 8 (19%)
underwent abdominoperineal resection surgery, 1 (2.4%) underwent
wide local excision, 2 (4.8%) were referred for systemic treatment
due to the detection of distant metastasis and 1 (2.4%) experienced
progression, leading to early death due to acute abdominal
perforation. Of the six patients who did not receive chemotherapy,
only one did not exhibit a complete response.
During the follow-up period (median length, 64
months), the 24 surviving cases comprised 20 (47.6%) patients who
remained disease-free and 4 (9.5%) who had ongoing disease.
Recurrence was observed in 16 cases (38.1%), with 3 (7.1%) having
only LRR, 6 (14.3%) having only DR and 7 (16.7%) experiencing both
LRR and DR. The median recurrence time was 10 months (range, 1–72
months). The median local recurrence-free time was 16.5 months
(range, 3–72 months) and the median DR-free time was 11 months
(range, 1–96 months). The most common metastatic sites were the
lungs, followed by the liver. In addition, no statistical
difference was observed in recurrence (P=0.510), LRR (P=0.614) and
DR (P=0.433) between RT with or without chemotherapy.
HPV status and p16 expression
The 42 patients comprised 30 (71.4%) cases who were
HPV positive and 12 (28.6%) who were HPV negative. Within the
HPV-positive subgroup, 22 (73.3%) were solely positive for HPV 16,
which was the most prevalent HPV type. Additionally, 5 (16.6%)
cases were positive for other high-risk HPV types, 2 (6.7%) had a
combination of HPV 16 with other HPV types, and 1 (3.4%) had a
combination of HPV 16, HPV 18 and other HPV types. The total study
cohort included 31 (73.8%) p16-positive cases and 11 (26.2%)
p16-negative cases. A robust association was observed between
HPV-positive and p16-positive tumors (P<0.001).
HPV-negative and p16-negative tumors were found to
be associated with male sex (P=0.001 and P<0.001, respectively).
No significant associations were found with other patient or
clinical factors for both HPV status and p16 expression.
Recurrence was observed in 9 (30%) of 30 cases with
HPV-positive status and in 7 (58.3%) of 12 cases with HPV-negative
status, although this difference lacked statistical significance
(P=0.088). Also without reaching statistical significance,
HPV-negative tumors exhibited a trend for higher LRR (16.7% in HPV
positive vs. 41.7% in HPV negative; P=0.086) and DR (26.7% in HPV
positive vs. 41.7% in HPV negative; P=0.277). Patients with
HPV-positive tumors demonstrated a statistically significant
improved outcome (P=0.008); 21 (70%) patients with HPV-positive
tumors survived, compared with only 43 (25%) patients with
HPV-negative tumors. The clinical, recurrence and IHC
characteristics of HPV-positive and -negative tumors are presented
in Table II.
 | Table II.Clinical and immunohistochemical
characteristics of HPV-positive and -negative anal squamous cell
carcinoma. |
Table II.
Clinical and immunohistochemical
characteristics of HPV-positive and -negative anal squamous cell
carcinoma.
|
Characteristics | HPV-positive, n
(%) | HPV-negative, n
(%) | P-value |
|---|
| Sex |
|
| 0.001 |
|
Female | 23 (76.7) | 2 (16.7) |
|
|
Male | 7 (23.3) | 10 (83.3) |
|
| Age, years |
|
| 0.406 |
|
<65 | 17 (56.7) | 8 (66.7) |
|
|
≥65 | 13 (43.3) | 4 (33.3) |
|
| T stage |
|
| 0.446 |
|
T1-T2 | 10 (33.3) | 3 (25) |
|
|
T3-T4 | 20 (66.7) | 9 (75) |
|
| N status |
|
| 0.600 |
| N- | 13 (43.3) | 5 (41.7) |
|
| N+ | 17 (53.7) | 7 (58.3) |
|
| Clinical stage |
|
| 0.292 |
|
I–II | 2 (40) | 3 (25) |
|
|
III | 18 (60) | 9 (75) |
|
| Recurrence |
|
| 0.088 |
|
Yes | 9 (30) | 7 (58.3) |
|
| No | 21 (70) | 5 (41.7) |
|
| Locoregional
recurrence |
|
| 0.086 |
|
Yes | 5 (16.7) | 5 (41.7) |
|
| No | 25 (83.3) | 7 (58.3) |
|
| Distant
recurrence |
|
| 0.277 |
|
Yes | 8 (26.7) | 5 (41.7) |
|
| No | 22 (73.3) | 7 (58.3) |
|
| Survival
status |
|
| 0.008 |
|
Alive | 21 (70) | 3 (25) |
|
|
Deceased | 9 (30) | 9 (75) |
|
| p16 expression |
|
| <0.001 |
|
Positive | 29 (96.7) | 2 (16.7) |
|
|
Negative | 1 (3.3) | 10 (83.3) |
|
| p53 expression |
|
| 0.222 |
|
Positive | 9 (30) | 6 (50) |
|
|
Negative | 21 (70) | 6 (50) |
|
p53 expression
The 42 cases included 15 (35.7%) patients who were
p53 positive. No significant associations were identified between
p53 expression and p16 expression (P=0.333), HPV status (P=0.193)
or other patient characteristics (Table III).
 | Table III.Clinical and immunohistochemical
characteristics of p53-positive and -negative anal squamous cell
carcinoma. |
Table III.
Clinical and immunohistochemical
characteristics of p53-positive and -negative anal squamous cell
carcinoma.
|
Characteristics | p53-positive, n
(%) | p53-negative, n
(%) | P-value |
|---|
| Sex |
|
| 0.613 |
|
Female | 9 (60) | 16 (59.3) |
|
|
Male | 6 (40) | 11 (40.7) |
|
| Age, years |
|
| 0.055 |
|
<65 | 6 (40) | 19 (70.4) |
|
|
≥65 | 9 (60) | 8 (29.6) |
|
| T stage |
|
| 0.534 |
|
T1-T2 | 5 (33.3) | 8 (29.6) |
|
|
T3-T4 | 10 (66.7) | 19 (70.4) |
|
| N status |
|
| 0.094 |
| N- | 9 (60) | 9 (33.3) |
|
| N+ | 6 (40) | 18 (66.7) |
|
| Clinical stage |
|
| 0.220 |
|
I–II | 7 (46.7) | 8 (29.6) |
|
|
III | 8 (53.3) | 19 (70.4) |
|
| Recurrence |
|
| 0.006 |
|
Yes | 10 (66.7) | 6 (22.2) |
|
| No | 5 (33.3) | 21 (77.8) |
|
| Locoregional
recurrence |
|
| 0.014 |
|
Yes | 7 (46.7) | 3 (11.1) |
|
| No | 8 (53.3) | 24 (88.9) |
|
| Distant
recurrence |
|
| 0.101 |
|
Yes | 7 (46.7) | 6 (22.2) |
|
| No | 8 (53.3) | 21 (77.8) |
|
| Survival
status |
|
| 0.307 |
|
Alive | 7 (46.7) | 17 (63) |
|
|
Deceased | 8 (53.3) | 10 (37) |
|
| HPV status |
|
| 0.193 |
|
Positive | 9 (60) | 21 (77.8) |
|
|
Negative | 6 (40) | 6 (22.2) |
|
| p16 expression |
|
| 0.333 |
|
Positive | 10 (66.7) | 21 (77.8) |
|
|
Negative | 5 (33.3) | 6 (22.2) |
|
Recurrence was observed in 10 (66.7%) patients with
p53-positive tumors, but only 6 (22.2%) patients with p53-negative
tumors; a significant association was established between
recurrence and p53-positive tumors (P=0.006). Similarly, p53
positivity was associated with an increased occurrence of LRR, with
7 of the 10 cases of LRR being p53 positive (P=0.014; Table III).
In the 30 cases with HPV-positive tumors, 5 (55.6%)
of the 9 patients with p53-positive tumors and 4 (19%) of the 21
patients with p53-negative tumors exhibited recurrence. p53
expression was found to be associated with increased recurrence in
the HPV-positive subgroup (P=0.046). However, a similar predictive
effect was not observed for survival status (alive vs. ex) within
the HPV-positive subgroup.
In the 12 cases with HPV-negative tumors, an equal
distribution of p53-positive and -negative patients was noted (6 of
each). The analysis did not reveal a significant impact of p53
expression on the recurrence and survival status (alive vs.
deceased) in HPV-negative cases (P=0.079 and P=0.505,
respectively).
PD-L1 expression
The median TPS was 1% (range, 0–100%), with a mean
value of 8%, while the median CPS was 3% (range, 0–100%), with a
mean value of 10%. PD-L1 expression was higher in tumor cells
compared with immune cells.
Exploring the number of cases with TPS <1 or ≥1%,
as well as CPS <1% and ≥1%, revealed that 19 cases (45.2%) had a
TPS <1% and 23 cases (54.8%) had a TPS ≥1%. In addition, 11
cases (26.2%) had a CPS <1% and 31 cases (73.8%) had a CPS ≥1%.
The distribution of TPS and CPS cases exhibited a statistically
significant difference (P<0.001).
A significant association of female sex with TPS
≥1%-positive (P=0.038) and CPS ≥1%-positive (P=0.011) tumors was
observed. Statistical significance was established for the
associations of CPS positivity with HPV-positive (P=0.026) and
p16-positive tumors (P=0.013). Although no statistically
significant association was noted in terms of recurrence (P=0.109),
out of the 16 cases with recurrence, 14 were found to have a CPS
≥1%. In addition, all 10 cases with LRR were positive for PD-L1
(P=0.031) (Table IV).
 | Table IV.Clinical and immunohistochemical
characteristics associated with TPS (<1 vs. ≥1%) and CPS (<1
vs. ≥1%). |
Table IV.
Clinical and immunohistochemical
characteristics associated with TPS (<1 vs. ≥1%) and CPS (<1
vs. ≥1%).
|
Characteristics | TPS <1%, n
(%) | TPS ≥1%, n (%) | P-value | CPS <1%, n
(%) | CPS ≥1%, n (%) | P-value |
|---|
| Sex |
|
| 0.038 |
|
| 0.011 |
|
Female | 8 (42.1) | 17 (73.9) |
| 3 (27.3) | 22 (71) |
|
|
Male | 11 (57.9) | 6 (26.1) |
| 8 (72.7) | 9 (29) |
|
| Age, years |
|
| 0.453 |
|
| 0.299 |
|
<65 | 12 (63.2) | 13 (56.5) |
| 8 (72.7) | 17 (54.8) |
|
|
≥65 | 7 (36.8) | 10 (43.5) |
| 3 (27.3) | 14 (45.2) |
|
| T stage |
|
| 0.401 |
|
| 0.759 |
|
T1-T2 | 5 (26.3) | 8 (34.8) |
| 3 (27.3) | 10 (32.3) |
|
|
T3-T4 | 14 (73.7) | 15 (65.2) |
| 8 (72.7) | 21 (67.7) |
|
| N status |
|
| 0.344 |
|
| 0.224 |
| N- | 7 (36.8) | 11 (47.8) |
| 3 (27.3) | 15 (48.4) |
|
| N+ | 12 (63.2) | 12 (52.2) |
| 8 (72.7) | 16 (51.6) |
|
| Clinical stage |
|
| 0.428 |
|
| 0.496 |
|
I–II | 6 (31.6) | 9 (39.1) |
| 3 (27.3) | 12 (38.7) |
|
|
III | 13 (68.4) | 14 (60.9) |
| 8 (72.7) | 19 (61.3) |
|
| Complete
response |
|
| 0.371 |
|
| 0.149 |
|
Yes | 4 (21.1) | 7 (30.4) |
| 6 (54.5) | 24 (77.5) |
|
| No | 15 (78.9) | 16 (69.6) |
| 5 (45.5) | 7 (22.6) |
|
| Recurrence |
|
| 0.320 |
|
| 0.109 |
|
Yes | 6 (31.6) | 10 (43.5) |
| 2 (18.2) | 14 (45.2) |
|
| No | 13 (68.4) | 13 (56.5) |
| 9 (81.8) | 17 (54.8) |
|
| Locoregional
recurrence |
|
| 0.230 |
|
| 0.031 |
|
Yes | 3 (15.8) | 7 (30.4) |
| 0 (0) | 10 (32.3) |
|
| No | 16 (84.2) | 16 (69.6) |
| 11 (100) | 21 (67.7) |
|
| Distant
recurrence |
|
| 0.599 |
|
| 0.286 |
|
Yes | 6 (31.6) | 7 (30.4) |
| 2 (18.2) | 11 (35.5) |
|
| No | 13 (68.4) | 16 (69.6) |
| 9 (81.8) | 20 (64.5) |
|
| Survival
status |
|
| 0.474 |
|
| 0.695 |
|
Alive | 12 (63.2) | 12 (52.2) |
| 6 (54.5) | 18 (58.1) |
|
|
Deceased | 7 (36.8) | 11 (47.8) |
| 5 (45.5) | 13 (41.9) |
|
| HPV status |
|
| 0.078 |
|
| 0.026 |
|
Positive | 11 (57.9) | 19 (82.6) |
| 5 (45.5) | 25 (80.6) |
|
|
Negative | 8 (42.1) | 4 (17.4) |
| 6 (54.5) | 6 (19.4) |
|
| p16 expression |
|
| 0.141 |
|
| 0.013 |
|
Positive | 12 (63.2) | 19 (82.6) |
| 5 (45.5) | 26 (83.9) |
|
|
Negative | 7 (36.8) | 4 (17.4) |
| 6 (54.5) | 5 (16.1) |
|
| p53 expression |
|
| 0.203 |
|
| 0.158 |
|
Positive | 5 (26.3) | 10 (43.5) |
| 2 (18.2) | 13 (41.9) |
|
|
Negative | 14 (73.7) | 13 (56.5) |
| 9 (81.8) | 18 (58.1) |
|
The distribution of TPS and CPS values for PD-L1
expression in cases according to HPV status, p16 and p53 expression
is shown in Table V.
 | Table V.Distribution of TPS and CPS
values. |
Table V.
Distribution of TPS and CPS
values.
| A, TPS, n (%) |
|---|
|
|---|
|
Characteristics | <1% | ≥1 to <5% | ≥5 to <10% | ≥10 to <25% | ≥25 to <50% | ≥50% |
|---|
| HPV status |
|
|
|
|
|
|
|
Positive | 11 (26.2) | 8 (19) | 4 (9.5) | 2 (4.8) | 4 (9.5) | 1 (2.4) |
|
Negative | 8 (19) | 2 (4.8) | 0 (0) | 1 (2.4) | 0 (0) | 1 (2.4) |
| p16 expression |
|
|
|
|
|
|
|
Positive | 12 (28.6) | 8 (19) | 4 (9.5) | 2 (4.8) | 4 (9.5) | 1 (2.4) |
|
Negative | 7 (16.7) | 2 (4.8) | 0 (0) | 1 (2.4) | 0 (0) | 1 (2.4) |
| p53 expression |
|
|
|
|
|
|
|
Positive | 5 (11.9) | 4 (9.5) | 1 (2.4) | 2 (4.8) | 3 (7.1) | 0 (0) |
|
Negative | 14 (33.3) | 6 (14.3) | 3 (7.1) | 1 (2.1) | 1 (2.4) | 2 (4.8) |
| Total | 19 (45.2) | 10 (23.8) | 4 (9.5) | 3 (7.1) | 4 (9.5) | 2 (4.8) |
|
| B, CPS, n
(%) |
|
|
Characteristics | <1% | ≥1 to
<5% | ≥5 to
<10% | ≥10 to
<25% | ≥25 to
<50% | ≥50% |
|
| HPV status |
|
|
|
|
|
|
|
Positive | 5 (11.9) | 11 (26.2) | 7 (16.7) | 2 (4.8) | 4 (9.5) | 1 (2.4) |
|
Negative | 6 (14.3) | 4 (9.5) | 0 (0) | 1 (2.4) | 0 (0) | 1 (2.4) |
| p16 expression |
|
|
|
|
|
|
|
Positive | 5 (11.9) | 12 (28.6) | 7 (16.7) | 2 (4.8) | 4 (9.5) | 1 (2.4) |
|
Negative | 6 (14.3) | 3 (7.1) | 0 (0) | 1 (2.4) | 0 (0) | 1 (2.4) |
| p53 expression |
|
|
|
|
|
|
|
Positive | 2 (4.8) | 7 (16.7) | 1 (2.4) | 2 (4.8) | 3 (7.1) | 0 (0) |
|
Negative | 9 (21.4) | 8 (19) | 6 (14.3) | 1 (2.4) | 1 (2.4) | 2 (4.8) |
| Total | 11 (26.2) | 15 (35.7) | 7 (16.7) | 3 (7.1) | 4 (9.5) | 2 (4.8) |
Factors and outcomes associated with
treatment response
Both HPV-negative and p16-negative tumors were found
to be strongly associated with the absence of a complete response
to definitive RT/CRT (both P<0.001). The presence of HPV and p16
positivity was identified as a significant predictor for achieving
a complete response. The absence of a complete response was also
associated with an increased recurrence rate (P=0.016), elevated DR
rate (P=0.015) and unfavorable survival status (alive vs. deceased;
P=0.049) (Table VI).
 | Table VI.Clinical and immunohistochemical
characteristics associated with response status. |
Table VI.
Clinical and immunohistochemical
characteristics associated with response status.
|
Characteristics | Complete response
(+), n (%) | Complete response
(−), n (%) | P-value |
|---|
| Sex |
|
| 0.127 |
|
Female | 20 (66.7) | 5 (58.3) |
|
|
Male | 10 (33.3) | 7 (41.7) |
|
| Age, years |
|
| 0.921 |
|
<65 | 18 (60) | 7 (41.7) |
|
|
≥65 | 12 (40) | 5 (58.3) |
|
| T stage |
|
| 0.554 |
|
T1-T2 | 9 (30) | 4 (33.3) |
|
|
T3-T4 | 21 (70) | 8 (66.7) |
|
| N status |
|
| 0.400 |
| N- | 12 (40) | 6 (50) |
|
| N+ | 18 (60) | 6 (50) |
|
| Clinical stage |
|
| 0.566 |
|
I–II | 11 (36.7) | 4 (33.3) |
|
|
III | 19 (63.3) | 8 (66.7) |
|
| Recurrence |
|
| 0.016 |
|
Yes | 8 (26.7) | 8 (66.7) |
|
| No | 22 (73.3) | 4 (33.3) |
|
| Locoregional
recurrence |
|
| 0.296 |
|
Yes | 6 (20) | 4 (33.3) |
|
| No | 24 (80) | 8 (66.7) |
|
| Distant
recurrence |
|
| 0.015 |
|
Yes | 6 (20) | 7 (41.7) |
|
| No | 24 (80) | 5 (58.3) |
|
| Survival
status |
|
| 0.049 |
|
Alive | 20 (66.7) | 4 (33.3) |
|
|
Deceased | 10 (33.3) | 8 (66.7) |
|
| HPV status |
|
| <0.001 |
|
Positive | 27 (90) | 3 (25) |
|
|
Negative | 3 (10) | 9 (75) |
|
| p16 expression |
|
| <0.001 |
|
Positive | 27 (90) | 4 (33.3) |
|
|
Negative | 3 (10) | 8 (66.7) |
|
| p53 expression |
|
| 0.193 |
|
Positive | 9 (30) | 6 (50) |
|
|
Negative | 21 (70) | 6 (50) |
|
| PD-L1 expression,
TPS % |
|
| 0.371 |
|
<1 | 4 (21.1) | 15 (78.9) |
|
| ≥1 | 7 (30.4) | 16 (69.6) |
|
| PD-L1 expression,
CPS % |
|
| 0.149 |
|
<1 | 6 (54.5) | 5 (45.5) |
|
| ≥1 | 24 (77.5) | 7 (22.6) |
|
Survival outcomes
The 3-year and 5-year OS rates of the study cohort
were 78.4 and 66.7%, respectively, while the corresponding DFS
rates were 72 and 65.5%.
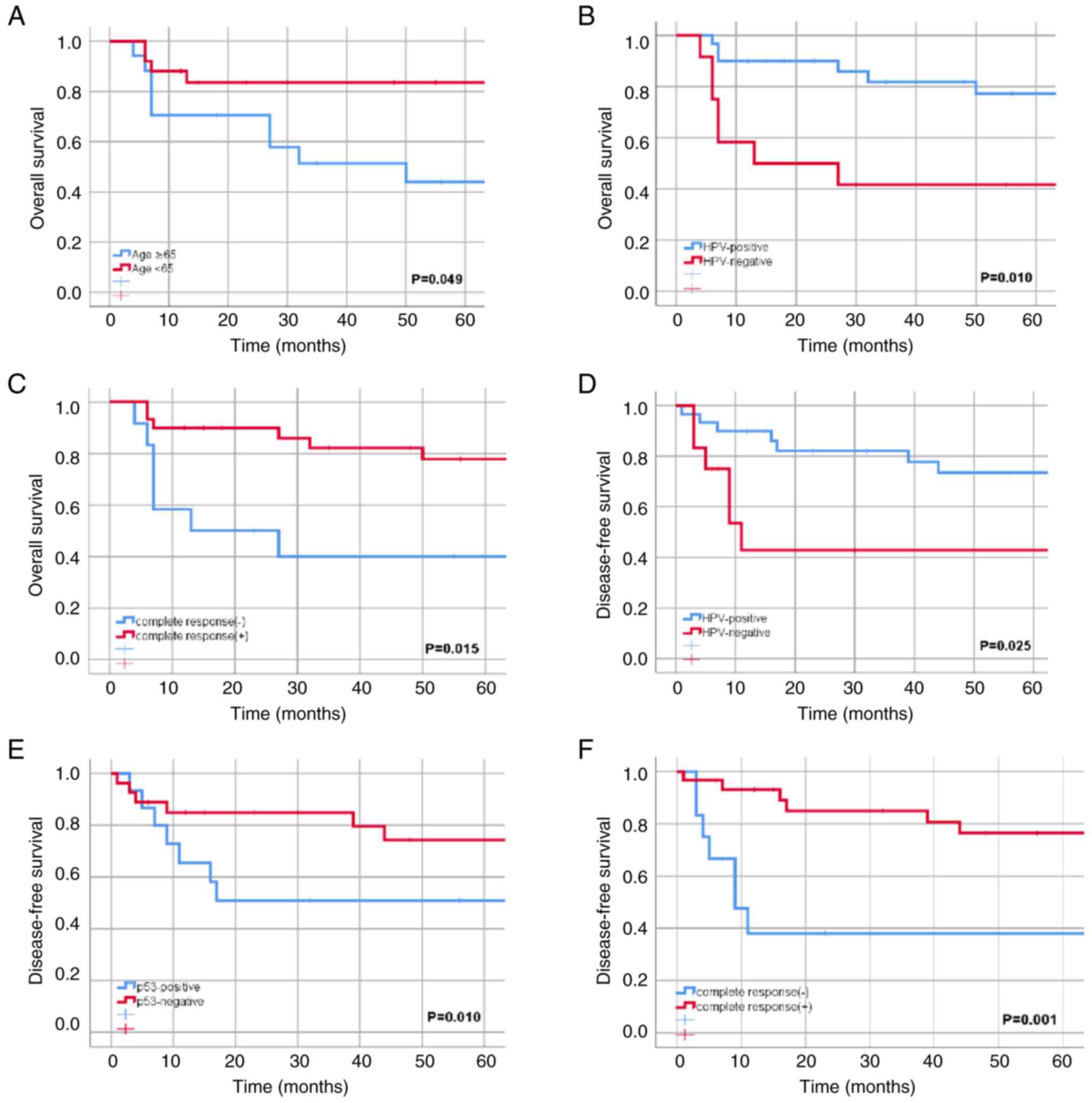
In univariate analyses, age (<65 vs. ≥65 years)
was found to have a significant association with improved 5-year OS
(P=0.049; Fig. 1A). No significant
associations were found for other clinical factors (T stage, N
status and clinical stage) or patient characteristics with
survival. HPV-positive tumors exhibited higher rates for 5-year OS
(77.3 vs. 41.7%; P=0.010; Fig. 1B)
and DFS (73.4 vs. 42.9%; P=0.025; Fig.
1D). Although p53 expression was not found to have a
significant association with 5-year OS (94.6 vs. 72.2%; P=0.642),
it was strongly associated with a decreased 5-year DFS (50.9 vs.
74.2%; P=0.010; Fig. 1E). A
complete response was also significantly associated with an
improved 5-year OS (77.8 vs. 40%; P=0.015; Fig. 1C) and DFS (76.5 vs. 38.1%; P=0.001;
Fig. 1F). Conversely, no
significant impacts of p16 expression on 5-year OS (P=0.183) and
DFS (P=0.160), or of PD-L1 expression on 5-year OS (P=0.963) and
DFS (P=0.179) were observed.
In the multivariate analysis, age (<65 vs. ≥65
years; P=0.010) and HPV positivity (P=0.002) emerged as independent
prognostic factors for 5-year OS, while a complete response
(P=0.007) and p53 expression (P=0.038) were identified as
independent prognostic factors for 5-year DFS.
Discussion
In early studies of concurrent CRT, a complete
response rate of 80–90% was reported (2,29,30),
while Ajani et al (31)
observed a 73% complete response rate. In the present study, the
complete response rate was 71.4%, which is lower compared with that
in previous studies. However, the present study did not identify
any factors influencing the treatment-associated complete response
rate. A complete response was found to be associated with
recurrence and survival as an independent prognostic factor in the
present series, aligning with previous reports (6–8). It is
important to highlight that the factors found to be influencing a
complete response in the previous studies were limited to clinical
data and treatment strategy, and sufficient information for a
complete response evaluation regarding the identification of
guiding HPV status and molecular characteristics were lacking. The
inclusion of these factors is an notable aspect of the present
study. In that context, HPV negativity was found to be strongly
associated with resistance to definitive CRT, consistent with the
findings of Soares et al (32). The lower complete response rate in
the present study compared with previous studies may be explained
by there being a higher proportion of HPV-negative tumors (28.6%)
in the present study (33–35). The robust association of HPV
positivity with a complete response and the significant impact of a
complete response on DR in the present data indirectly suggest that
HPV status should be considered when assessing the risk of DR.
Indeed, the present study was consistent with previous studies
(36,37) in finding significantly increased
5-year OS and DFS rates in HPV-positive patients. HPV status was
identified to be a stage-independent prognostic factor.
Several studies have emphasized that p16 expression
alone serves as a favorable prognostic factor for anal SCC and is
linked to a higher likelihood of achieving a complete response
(26,38,39),
while the study by Ajani et al did not find this association
(31). In the present study cohort,
p16 expression demonstrated a significant association with an
increased complete response rate. Regarding survival, the
association of p16 positivity with OS is currently uncertain, but
it may be linked with increased DFS (13,26,37).
In the present study cohort, despite the strong association between
HPV positivity and p16 expression, the favorable survival outcomes
observed in the HPV-positive subgroup were not mirrored in p16
expression. Given the discordant survival outcomes, prioritizing
the use of HPV in prognostic evaluations for anal SCC cases appears
consistent and rational.
In HPV-assocated cancers, the association between
p53 mutations and prognosis is not conclusive due to differences in
mutation and function. p53 mutations can be examined using IHC and
mutational analyses, including sequencing or PCR-single-strand
conformation polymorphism. IHC expression analysis of p53 using a
p53 antibody, which was also employed in the current study for the
detection of p53 mutations, is frequently used due to its
simplicity and accessibility compared with mutational analysis
(26). The rationale for using IHC
analysis in the detection of p53 is the rapid degradation of the
non-mutant wild-type p53 under normal cellular conditions, which
makes it challenging to detect. By contrast, mutant p53 has a
longer half-life, leading to its accumulation within cells and
increased IHC staining (40).
HPV-negative cancers generally exhibit more mutations and
functional losses in the p53 gene compared with HPV-positive
cancers (41). Consequently, in
HPV-positive tumors, low p53 expression is expected
immunohistochemically. However, the HPV-induced degradation of p53
may mask increased p53 expression immunohistochemically in
HPV-positive tumors with p53 mutations, causing potential confusion
in the analysis. In SCCs of the head and neck, the correspondence
between both tests has been investigated, and IHC and mutation
analysis were reported to be consistent with each other for the
detection of p53 mutations; it is noteworthy that the majority of
the cases were HPV negative (64%) (42). By contrast, Meulendijks et al
(43) noted a low concordance
between p53 gene mutation and abnormal p53 expression (>70 and
0%, respectively) in their analysis of anal SCC cases, of which 87%
were HPV positive.
A variable range of 34–100% has been reported for
p53 mutations in anal SCC (15). In
the present study, p53 mutations were detected in 35.7% of the
patients. Previous studies have suggested that HPV-negative tumors
may be more resistant to definitive CRT due to an association
between the p53 mutation and HPV-negative status (43–46).
Soares et al (32) detected
p53 gene mutations in all HPV-negative cases and identified a
significant association between poor treatment response at 6 months
and the p53 mutation. Contrary to this, the present data did not
reveal any association of p53 expression with either HPV-negative
status or a complete response.
Meulendijks et al (43) noted that the presence of p53
mutations was associated with HPV- and p16-negative tumors, but did
not significantly affect survival or serve as a prognostic factor
in the HPV-negative subgroup. Similarly, in the present study, p53
expression exhibited no impact on recurrence or survival in the
HPV-negative subgroup. However, a notable finding was the
first-time identification of a significant association between p53
expression and increased recurrence in the HPV-positive subgroup.
Consistent with the present study, Gilbert et al (26) found that high p53 expression
(>5%) was associated with increased recurrence and, similarly,
Bruyere et al (47) found
that abnormal p53 expression (0 or >50%) had an association with
increased recurrence. Meulendijks et al (43) found that abnormal P53 expression and
the presence of a P53 gene mutation exhibited an independent
association with poor local-regional control. The present study
demonstrated that LRR was associated with >5% p53 expression. In
terms of survival outcomes linked to p53 expression, previous
studies have presented ambiguous results (31,32,48–50).
However, the present study is consistent with the aforementioned
research, with the exception of the study of Zhu et al
(49), in indicating a reduction in
DFS.
In non-metastatic anal SCC, PD-L1 expression rates
have been reported in previous studies by Armstrong et al
(50), who found >5% PD-L1
expression in 40.5% of cases; Iseas et al (51), who observed a CPS >1% in 57% of
cases; and Chan et al (52),
who found a TPS >1% in 71.4% of cases. In the present study, the
prevalance of PD-L1 expression with TPS ≥1 was 54.8%, and with CPS
≥1 was 73.8%. Consistent with the findings of Iseas et al
(51), higher PD-L1 expression was
observed in tumor cells than in immune cells in the present study,
which has an impact on treatment decisions. Therefore, the use of
PD-1 or PD-L1 inhibitors may be effective for tumor regression. The
quantity and quality of lymphocytes in the tumor microenvironment,
regulated by various types of T cells, are highly important. It has
been shown that the number, location and quality of CD8+
T cells positively correlate with the prognosis of a number of
malignant tumors. However, regulatory T cells can inhibit the
immune response against tumor cells, which is related to the
failure of immunotherapy (53).
Ongoing studies are aiming to enhance the immune response, and
include the use of adaptive T-cell therapies such as CAR-T cells
and tumor-infiltrating lymphocytes. RT is a significant stimulator
and enhancer of lymphocytes, which counters the immunosuppressive
effect of PD-L1 (53).
The existing evidence on the relationships between
HPV status, p16, p53 expression and PD-L1 in non-metastatic cases
of anal SCC is contradictory (19,52).
However, PD-L1 positivity has been shown to be associated with HPV
negativity in anogenital tumors (54). In the present study, ≥1% PD-L1
positivity was significantly associated with HPV and p16
positivity, highlighting their potential for guiding immunotherapy
decisions in patients with anal SCC.
Iseas et al (51) reported higher complete response
rates and improved OS in PD-L1-positive cases with CPS >1% after
definitive CRT. In addition, Chan et al (52) found an improved 10-year OS in cases
with ≥5% PD-L1 positivity, and Wessely et al (19) reported an improved OS for patients
who were PD-L1 positive with TPS >1%. By contrast, Zhao et
al (21) observed a tendency
for PD-L1 positivity to be associated with worse DFS and OS. In the
present study, PD-L1 positivity (CPS >1%) was identified as a
factor increasing the LRR, although no statistically significant
association was found between PD-L1 (CPS >1%) status and
complete response, 5-year OS or DFS. The present study presents a
well-suited population for the low-incidence anal SCC, as the
exclusion of HIV-positive patients ensured a homogeneous group with
a consistent treatment approach. Despite being retrospective, all
data were obtained and cases with missing information were
excluded. Treatment response, HPV status and IHC analysis were
meticulously evaluated by an experienced team. However, the limited
number of patients (14.3%) who did not receive chemotherapy in the
present study could be considered a limitation, despite no
statistical significance being observed between the patients who
received chemotherapy and those who did not in terms of treatment
response and survival. As a useful suggestion, p53 mutation
analysis alongside p53 expression should be evaluated in future
studies.
The present study also highlights the crucial role
of HPV vaccination. Anal cancer prevention strategies mirror those
for cervical cancer prevention, focusing on both primary and
secondary prevention methods. Providing the 9-valent HPV vaccine to
girls and boys before the onset of sexual activity could
effectively prevent nearly all anal cancers. There is evidence to
suggest that vaccination may also reduce the risk of recurrent
precancerous lesions and potentially prevent the progression to
anal cancer, particularly in high-risk individuals (55). However, addressing the needs of
individuals with persistent HPV infection requires a different
approach. Therapeutic vaccination aims to stimulate cellular
immunity against existing HPV infections and lesions, potentially
preventing cancer progression. Multiple therapeutic vaccines are
currently in clinical development, utilizing various platforms
(56).
Despite the high efficacy of HPV vaccines in
preventing infection, several challenges persist. Guidelines
advocate for the cancer screening of vaccinated individuals,
underscoring the ongoing importance of preventive measures.
Disparities in global HPV vaccination rates highlight that targeted
interventions are necessary to ensure equitable access to
vaccination. In addition, there is a concerning lack of awareness
among adolescents, including medical students, regarding HPV and
its vaccines. Greater efforts, potentially including mandatory
measures, are required to increase awareness, particularly among
males and young people (57).
Accordingly, a risk-adaptive approach is necessary for patients
with anal SCC. Further randomized controlled studies comparing
patients with and without HPV based on risk stratification are
essential. The findings of the present study suggest that
additional agents tailored to the treatment for both subgroups are
necessary.
Concerning the HPV-positive subgroup, a strong
association with complete response and significantly improved
overall and DFS were observed. Although p16 expression was found to
be associated with an improved treatment response, survival
outcomes were more consistently associated with HPV positivity. A
number of recommendations for the HPV-positive subgroup may be
made. Firstly, in the entire cohort, p53 exhibited an association
with increased recurrence and LRR. However, it may be beneficial to
assess the impact of p53 mutation within HPV-positive and -negative
subgroups separately due to the p53 status in HPV-negative tumors
having no significant effect on survival or prognosis, contrasting
with HPV-positive tumors, where p53 expression or mutation
exhibited an association with heightened recurrence. Addressing
mutated p53 in HPV-positive tumors may involve directly targeting
the aberrant protein to restore the wild-type conformation and
transcriptional activity. Agents for targeting p53 include COTI-2
and Ad-p53, which are being studied in head and neck cancers
(58). Furthermore, treatments
targeting HPV-positive cancer cells, even without mutated p53,
could focus on viral enzymes E6/E7, responsible for p53
degradation. Notable examples are Ad-E6/E7-As and bortezomib
(58). Further studies are
warranted to uncover p53 reactivators that are more specific, safe
and efficient, in order to provide an enhanced treatment of anal
SCC. Secondly, in the present study PD-L1 expression was found to
be associated with HPV and p16 positivity, suggesting a potential
role in guiding immunotherapy decisions for this subgroup of
patients. The integration of HPV status with p16, p53 and PD-L1
expression analysis may provide a comprehensive understanding of
the immunological landscape, and aid in risk stratification and
personalized treatment approaches for HPV-positive anal SCC.
Thirdly, additional genetic alterations may influence the survival
in patients with HPV-positive tumors, including somatic PIK3CA exon
9/20 and KMT2C pathogenic variants. These mutations may play a key
role in tumor biology and the response to treatment, further
highlighting the complexity of the molecular mechanisms involved in
HPV-positive tumors (59). Finally,
while efforts to further increase survival outcomes in the
HPV-positive subgroup are necessary, the reduction of potential
side effects is also important. For example, the potential for dose
de-escalation and the substitution of chemotherapy with alternative
therapeutic agents may be considered.
The HPV-negative subgroup is currently overlooked
due to its relatively lower incidence, and there is currently no
randomized controlled trial focusing on this subgroup. Based on the
key insights gained from the present study, further research
focusing on the HPV-negative subgroup of patients with anal SCC
would be beneficial. Recommendations for the HPV-negative subgroup
include investigating the hypothesis that RT dose escalation based
on HPV status could be successful for the HPV-negative resistant
subgroup. In addition, mutational profiles that notably differ
between HPV-positive and HPV-negative patients may be explored, as
they could suggest multiple avenues for the investigation of
targeted therapies in anal SCC (59). Also, hyperthermia could be
considered as a potential means of increasing the sensitivity of
cancer cells to therapeutic agents, inducing direct cytotoxicity,
triggering anticancer immune responses and improving drug delivery,
as supported by previous studies (60,61).
Hypoxia-sensitizing methods may also be explored, particularly in
radioresistant HPV-negative or p16-negative subgroups, as in the
DAHANCA 5 trials (46,62). Furthermore, the potential of
metformin in the prevention of multidrug resistance and the
resensitization of cancer cells to standard chemotherapeutic
agents, as well as enhancing cancer cell sensitivity to RT, may be
considered (63). Notable PD-L1
positivity in the HPV-negative subgroup suggests that immunotherapy
may be considered as an aggressive option, including concurrent RT.
While the expression of p53 in HPV-negative tumors appears to have
no significant impact on survival or prognosis, additional larger
population-based studies are required to confirm these findings
within this subgroup. These approaches all have the potential to be
further optimized with nanoparticle-based treatments, highlighting
the advancement of radiosensitizer nanoparticles. However, despite
progress, challenges remain in the translation of
nanoparticle-enhanced RT from the laboratory to clinical practice,
including concerns about biosafety, nanoparticle clearance and the
optimization of nanoparticle properties for effective interaction
with radiation and biological systems (64).
Acknowledgements
Not applicable.
Funding
The study was supported by the Office of Scientific Research
Projects at Ege University under project number 22981.
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
BBT conceived and designed the analysis, collected
data, contributed data or analysis tools, performed analyses, wrote
the paper and supervised the study. FS, MSe and MSo collected data
and contributed data or analysis tools. DY contributed data or
analysis tools and performed analyses. SO conceived and designed
the analysis, collected data and supervised the study. BBT, FS, DY
and SO confirm the authenticity of all the raw data. All authors
read and approved the final manuscript.
Ethics approval and consent to
participate
The present study was approved by the Medical
Research Ethics Committee at Ege University (Izmir, Türkiye;
reference: 21-3.1T/63). Written informed consent was obtained from
all patients.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
CPS
|
combined positive score
|
|
CRT
|
chemoradiotherapy
|
|
CTV
|
clinical target volume
|
|
DFS
|
disease-free survival
|
|
DR
|
distant recurrence
|
|
FFPE
|
formalin-fixed, paraffin-embedded
|
|
HIV
|
human immunodeficiency virus
|
|
HPV
|
human papillomavirus
|
|
IHC
|
immunohistochemical
|
|
LRR
|
local-regional recurrence
|
|
MMC
|
mitomycin-C
|
|
OS
|
overall survival
|
|
RT
|
radiotherapy
|
|
SCC
|
squamous cell carcinoma
|
|
TPS
|
tumor proportion score
|
|
5-FU
|
5-fluorouracil
|
References
|
1
|
National Cancer Institute, . SEER cancer
statistics factsheets: Anal Cancer. Available from:. http://seer.cancer.gov/statfacts/html/anus.htmlJun
7–2021
|
|
2
|
Bartelink H, Roelofsen F, Eschwege F,
Rougier P, Bosset JF, Gonzalez DG, Peiffert D, van Glabbeke M and
Pierart M: Concomitant radiotherapy and chemotherapy is superior to
radiotherapy alone in the treatment of locally advanced anal
cancer: Results of a phase III randomized trial of the European
organization for research and treatment of cancer radiotherapy and
gastrointestinal cooperative groups. J Clin Oncol. 15:2040–2049.
1997. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Oliveira SC, Moniz CM, Riechelmann R, Alex
AK, Braghirolli MI, Bariani G, Nahas C and Hoff PM: Phase II study
of capecitabine in substitution of 5-FU in the chemoradiotherapy
regimen for patients with localized squamous cell carcinoma of the
anal canal. J Gastrointest Cancer. 47:75–81. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Peiffert D, Tournier-Rangeard L, Gérard
JP, Lemanski C, François E, Giovannini M, Cvitkovic F, Mirabel X,
Bouché O, Luporsi E, et al: Induction chemotherapy and dose
intensification of the radiation boost in locally advanced anal
canal carcinoma: Final analysis of the randomized UNICANCER ACCORD
03 trial. J Clin Oncol. 30:1941–1948. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Rao S, Guren MG, Khan K, Brown G, Renehan
AG, Steigen SE, Deutsch E, Martinelli E and Arnold D; ESMO
Guidelines Committee. Electronic address, : simpleclinicalguidelines@esmo.org:
Anal cancer: ESMO clinical practice guidelines for diagnosis,
treatment and follow-up*. Ann Oncol. 32:1087–1100. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Glynne-Jones R, Sebag-Montefiore D,
Meadows HM, Cunningham D, Begum R, Adab F, Benstead K, Harte RJ,
Stewart J, Beare S, et al: Best time to assess complete clinical
response after chemoradiotherapy in squamous cell carcinoma of the
anus (ACT II): A post-hoc analysis of randomised controlled phase 3
trial. Lancet Oncol. 18:347–356. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Chapet O, Gerard JP, Riche B, Alessio A,
Mornex F and Romestaing P: Prognostic value of tumor regression
evaluated after first course of radiotherapy for anal canal cancer.
Int J Radiat Oncol Biol Phys. 63:1316–1324. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Gerard JP, Ayzac L, Hun D, Romestaing P,
Coquard R, Ardiet JM and Mornex F: Treatment of anal canal
carcinoma with high dose radiation therapy and concomitant
fluorouracil-cisplatinum. Long-term results in 95 patients.
Radiother Oncol. 46:249–256. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ang KK, Harris J, Wheeler R, Weber R,
Rosenthal DI, Nguyen-Tân PF, Westra WH, Chung CH, Jordan RC, Lu C,
et al: Human papillomavirus and survival of patients with
oropharyngeal cancer. N Engl J Med. 363:24–35. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ragin CC and Taioli E: Survival of
squamous cell carcinoma of the head and neck in relation to human
papillomavirus infection: Review and meta-analysis. Int J Cancer.
121:1813–1820. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Rischin D, Young RJ, Fisher R, Fox SB, Le
QT, Peters LJ, Solomon B, Choi J, O'Sullivan B, Kenny LM and
McArthur GA: Prognostic significance of p16INK4A and human
papillomavirus in patients with oropharyngeal cancer treated on
TROG 02.02 phase III trial. J Clin Oncol. 28:4142–4148. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Lewis JS Jr: p16 Immunohistochemistry as a
standalone test for risk stratification in oropharyngeal squamous
cell carcinoma. Head Neck Pathol. 6 (Suppl 1):S75–S82. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Urgoiti GB, Gustafson K, Klimowicz AC,
Petrillo SK, Magliocco AM and Doll CM: The prognostic value of HPV
status and p16 expression in patients with carcinoma of the anal
canal. PLoS One. 9:e1087902014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
NCCN, . NCCN Guidelines for Anal Cancer
Version 1.2024. https://www.nccn.org/professionals/physician_gls/pdf/anal_blocks.pdfMarch
4–2024
|
|
15
|
Lampejo T, Kavanagh D, Clark J, Goldin R,
Osborn M, Ziprin P and Cleator S: Prognostic biomarkers in squamous
cell carcinoma of the anus: A systematic review. Br J Cancer.
103:1858–1869. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Gillison ML, Chaturvedi AK, Anderson WF
and Fakhry C: Epidemiology of human papillomavirus-positive head
and neck squamous cell carcinoma. J Clin Oncol. 33:3235–3242. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Balachandran S and Narendran A: The
developmental origins of cancer: A review of the genes expressed in
embryonic cells with ımplications for tumorigenesis. Genes (Basel).
14:6042023. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Doroshow DB, Bhalla S, Beasley MB, Sholl
LM, Kerr KM, Gnjatic S, Wistuba II, Rimm DL, Tsao MS and Hirsch FR:
PD-L1 as a biomarker of response to immune-checkpoint inhibitors.
Nat Rev Clin Oncol. 18:345–362. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wessely A, Heppt MV, Kammerbauer C, Steeb
T, Kirchner T, Flaig MJ, French LE, Berking C, Schmoeckel E and
Reinholz M: Evaluation of PD-L1 expression and HPV genotyping in
anal squamous cell carcinoma. Cancers (Basel). 12:25162020.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Balermpas P, Martin D, Wieland U,
Rave-Fränk M, Strebhardt K, Rödel C, Fokas E and Rödel F: Human
papilloma virus load and PD-1/PD-L1, CD8+ and FOXP3 in anal cancer
patients treated with chemoradiotherapy: Rationale for
immunotherapy. Oncoimmunology. 6:e12883312017. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhao YJ, Sun WP, Peng JH, Deng YX, Fang
YJ, Huang J, Zhang HZ, Wan DS, Lin JZ and Pan ZZ: Programmed
death-ligand 1 expression correlates with diminished CD8+ T cell
infiltration and predicts poor prognosis in anal squamous cell
carcinoma patients. Cancer Manag Res. 10:1–11. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Goodman KA, Gollub M, Eng C, Brierley J,
Palefsky J, Gress D, Williams A and Goldberg R: Anus: AJCC Cancer
Staging Manual. 9th edition. Washington MK: American College of
Surgeons; 2022
|
|
23
|
Glynne-Jones R, Nilsson PJ, Aschele C, Goh
V, Peiffert D, Cervantes A and Arnold D; ESMO; ESSO; ESTRO, : Anal
cancer: ESMO-ESSO-ESTRO clinical practice guidelines for diagnosis,
treatment and follow-up. Radiother Oncol. 111:330–339. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kachnic LA, Winter K, Myerson RJ, Goodyear
MD, Willins J, Esthappan J, Haddock MG, Rotman M, Parikh PJ, Safran
H and Willett CG: RTOG 0529: A phase 2 evaluation of dose-painted
intensity modulated radiation therapy in combination with
5-fluorouracil and mitomycin-C for the reduction of acute morbidity
in carcinoma of the anal canal. Int J Radiat Oncol Biol Phys.
86:27–33. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Eisenhauer EA, Therasse P, Bogaerts J,
Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S,
Mooney M, et al: New response evaluation criteria in solid tumours:
Revised RECIST guideline (version 1.1). Eur J Cancer. 45:228–247.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Gilbert DC, Williams A, Allan K, Stokoe J,
Jackson T, Linsdall S, Bailey CM and Summers J: p16INK4A, p53, EGFR
expression and KRAS mutation status in squamous cell cancers of the
anus: Correlation with outcomes following chemo-radiotherapy.
Radiother Oncol. 109:146–151. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Yang Y, Forslund A, Remotti H, Lönnroth C,
Andersson M, Brevinge H, Svanberg E, Lindnér P, Hafström L, Naredi
P and Lundholm K: P53 mutations in primary tumors and subsequent
liver metastases are related to survival in patients with
colorectal carcinoma who undergo liver resection. Cancer.
91:727–736. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
de Ruiter EJ, Mulder FJ, Koomen BM, Speel
EJ, van den Hout MFCM, de Roest RH, Bloemena E, Devriese LA and
Willems SM: Comparison of three PD-L1 immunohistochemical assays in
head and neck squamous cell carcinoma (HNSCC). Mod Pathol.
34:1125–1132. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Northover J, Glynne-Jones R,
Sebag-Montefiore D, James R, Meadows H, Wan S, Jitlal M and
Ledermann J: Chemoradiation for the treatment of epidermoid anal
cancer: 13-year follow-up of the first randomised UKCCCR anal
cancer trial (ACT I). Br J Cancer. 102:1123–1128. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
James RD, Glynne-Jones R, Meadows HM,
Cunningham D, Myint AS, Saunders MP, Maughan T, McDonald A, Essapen
S, Leslie M, et al: Mitomycin or cisplatin chemoradiation with or
without maintenance chemotherapy for treatment of squamous-cell
carcinoma of the anus (ACT II): A randomised, phase 3, open-label,
2×2 factorial trial. Lancet Oncol. 14:516–524. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Ajani JA, Wang X, Izzo JG, Crane CH, Eng
C, Skibber JM, Das P and Rashid A: Molecular biomarkers correlate
with disease-free survival in patients with anal canal carcinoma
treated with chemoradiation. Dig Dis Sci. 55:1098–1105. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Soares PC, Abdelhay ES, Thuler LCS, Soares
BM, Demachki S, Ferro GVR, Assumpção PP, Lamarão LM, Pinto LF and
Burbano RMR: HPV positive, wild type TP53, and p16 overexpression
correlate with the absence of residual tumors after
chemoradiotherapy in anal squamous cell carcinoma. BMC
Gastroenterol. 18:302018. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Lu DW, El-Mofty SK and Wang HL: Expression
of p16, Rb, and p53 proteins in squamous cell carcinomas of the
anorectal region harboring human papillomavirus DNA. Mod Pathol.
16:692–699. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Mai S, Welzel G, Ottstadt M, Lohr F,
Severa S, Prigge ES, Wentzensen N, Trunk MJ, Wenz F, von
Knebel-Doeberitz M and Reuschenbach M: Prognostic relevance of HPV
ınfection and p16 overexpression in squamous cell anal cancer. Int
J Radiat Oncol Biol Phys. 93:819–827. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Foster CC, Lee AY, Furtado LV, Hart J,
Alpert L, Xiao SY, Hyman NH, Sharma MR and Liauw SL: Treatment
outcomes and HPV characteristics for an institutional cohort of
patients with anal cancer receiving concurrent chemotherapy and
intensity-modulated radiation therapy. PLoS One. 13:e01942342018.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Baricevic I, He X, Chakrabarty B, Oliver
AW, Bailey C, Summers J, Hampson L, Hampson I, Gilbert DC and
Renehan AG: High-sensitivity human papilloma virus genotyping
reveals near universal positivity in anal squamous cell carcinoma:
Different implications for vaccine prevention and prognosis. Eur J
Cancer. 51:776–785. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Yhim HY, Lee NR, Song EK, Kwak JY, Lee ST,
Kim JH, Kim JS, Park HS, Chung IJ, Shim HJ, et al: The prognostic
significance of tumor human papillomavirus status for patients with
anal squamous cell carcinoma treated with combined
chemoradiotherapy. Int J Cancer. 129:1752–1760. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Rödel F, Wieland U, Fraunholz I, Kitz J,
Rave-Fränk M, Wolff HA, Weiss C, Wirtz R, Balermpas P, Fokas E and
Rödel C: Human papillomavirus DNA load and p16INK4a expression
predict for local control in patients with anal squamous cell
carcinoma treated with chemoradiotherapy. Int J Cancer.
136:278–288. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Serup-Hansen E, Linnemann D,
Skovrider-Ruminski W, Høgdall E, Geertsen PF and Havsteen H: Human
papillomavirus genotyping and p16 expression as prognostic factors
for patients with American Joint Committee on cancer stages I to
III carcinoma of the anal canal. J Clin Oncol. 32:1812–1817. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Neal CP, Garcea G, Doucas H, Manson MM,
Sutton CD, Dennison AR and Berry DP: Molecular prognostic markers
in resectable colorectal liver metastases: A systematic review. Eur
J Cancer. 42:1728–1743. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Sun Y, Wang Z, Qiu S and Wang R:
Therapeutic strategies of different HPV status in head and neck
squamous cell carcinoma. Int J Biol Sci. 17:1104–1118. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Ganly I, Soutar DS, Brown R and Kaye SB:
p53 alterations in recurrent squamous cell cancer of the head and
neck refractory to radiotherapy. Br J Cancer. 82:392–398. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Meulendijks D, Tomasoa NB, Dewit L, Smits
PH, Bakker R, van Velthuysen ML, Rosenberg EH, Beijnen JH,
Schellens JH and Cats A: HPV-negative squamous cell carcinoma of
the anal canal is unresponsive to standard treatment and frequently
carries disruptive mutations in TP53. Br J Cancer. 112:1358–1366.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Cacheux W, Rouleau E, Briaux A, Tsantoulis
P, Mariani P, Richard-Molard M, Buecher B, Dangles-Marie V, Richon
S, Lazartigues J, et al: Mutational analysis of anal cancers
demonstrates frequent PIK3CA mutations associated with poor outcome
after salvage abdominoperineal resection. Br J Cancer.
114:1387–1394. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Mireștean CC, Iancu RI and Iancu DPT: p53
modulates radiosensitivity in head and neck cancers-from classic to
future horizons. Diagnostics (Basel). 12:30522022. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Liu C, Mann D, Sinha UK and Kokot NC: The
molecular mechanisms of increased radiosensitivity of HPV-positive
oropharyngeal squamous cell carcinoma (OPSCC): An extensive review.
J Otolaryngol Head Neck Surg. 47:592018. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Bruyere D, Monnien F, Colpart P, Roncarati
P, Vuitton L, Hendrick E, Lepinoy A, Luquain A, Pilard C, Lerho T,
et al: Treatment algorithm and prognostic factors for patients with
stage I–III carcinoma of the anal canal: A 20-year multicenter
study. Mod Pathol. 34:116–130. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Allal AS, Waelchli L and Bründler MA:
Prognostic value of apoptosis-regulating protein expression in anal
squamous cell carcinoma. Clin Cancer Res. 9:6489–6496.
2003.PubMed/NCBI
|
|
49
|
Zhu X, Jamshed S, Zou J, Azar A, Meng X,
Bathini V, Dresser K, Strock C, Yalamarti B, Yang M, et al:
Molecular and immunophenotypic characterization of anal squamous
cell carcinoma reveals distinct clinicopathologic groups associated
with HPV and TP53 mutation status. Mod Pathol. 34:1017–1030. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Armstrong SA, Malley R, Wang H, Lenz HJ,
Arguello D, El-Deiry WS, Xiu J, Gatalica Z, Hwang JJ, Philip PA, et
al: Molecular characterization of squamous cell carcinoma of the
anal canal. J Gastrointest Oncol. 12:2423–2437. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Iseas S, Golubicki M, Robbio J, Ruiz G,
Guerra F, Mariani J, Salanova R, Cabanne A, Eleta M, Gonzalez JV,
et al: A clinical and molecular portrait of non-metastatic anal
squamous cell carcinoma. Transl Oncol. 14:1010842021. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Chan AM, Urgoiti GR, Jiang W, Lee S,
Kornaga E, Mathen P, Yeung R, Enwere EK, Box A, Konno M, et al: The
prognostic impact of PD-L1 and CD8 expression in anal cancer
patients treated with chemoradiotherapy. Front Oncol.
12:10002632022. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Chen C, Liu Y and Cui B: Effect of
radiotherapy on T cell and PD-1/PD-L1 blocking therapy in tumor
microenvironment. Hum Vaccin Immunother. 17:1555–1567. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Qin Y, Luan J, Zhou X and Li Y: PD-L1
expression in anogenital and oropharyngeal squamous cell carcinomas
associated with different clinicopathological features, HPV status
and prognosis: A meta-analysis. Biosci Rep. 41:BSR202036692021.
View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Stier EA, Chigurupati NL and Fung L:
Prophylactic HPV vaccination and anal cancer. Hum Vaccin
Immunother. 12:1348–1351. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Skolnik JM and Morrow MP: Vaccines for
HPV-associated diseases. Mol Aspects Med. 94:1012242023. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Garolla A, Graziani A, Grande G, Ortolani
C and Ferlin A: HPV-related diseases in male patients: An
underestimated conundrum. J Endocrinol Invest. 47:261–274. 2024.
View Article : Google Scholar : PubMed/NCBI
|
|
58
|
de Bakker T, Journe F, Descamps G, Saussez
S, Dragan T, Ghanem G, Krayem M and Van Gestel D: Restoring p53
function in head and neck squamous cell carcinoma to ımprove
treatments. Front Oncol. 11:7999932022. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Hamza A, Masliah-Planchon J, Neuzillet C,
Lefèvre JH, Svrcek M, Vacher S, Bourneix C, Delaye M, Goéré D,
Dartigues P, et al: Pathogenic alterations in PIK3CA and KMT2C are
frequent and independent prognostic factors in anal squamous cell
carcinoma treated with salvage abdominoperineal resection. Int J
Cancer. 154:504–515. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Mao W, Li W and Hu X: Tumor hyperthermia
research progress and application prospect in tumoroids (Review).
Mol Clin Oncol. 20:312024. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Ott OJ, Schmidt M, Semrau S, Strnad V,
Matzel KE, Schneider I, Raptis D, Uter W, Grützmann R and Fietkau
R: Chemoradiotherapy with and without deep regional hyperthermia
for squamous cell carcinoma of the anus. Strahlenther Onkol.
195:607–614. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Telarovic I, Wenger RH and Pruschy M:
Interfering with tumor hypoxia for radiotherapy optimization. J Exp
Clin Cancer Res. 40:1972021. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Ugwueze CV, Ogamba OJ, Young EE, Onyenekwe
BM and Ezeokpo BC: Metformin: A possible option in cancer
chemotherapy. Anal Cell Pathol (Amst). 27:71809232020.PubMed/NCBI
|
|
64
|
Liu J, Wu J, Chen T, Yang B, Liu X, Xi J,
Zhang Z, Gao Y and Li Z: Enhancing X-Ray sensitization with
multifunctional nanoparticles. Small. 26:e24009542024. View Article : Google Scholar : PubMed/NCBI
|