Introduction
Endometrial carcinoma (EC) is one of the most
prevalent female genital tract malignancies worldwide (1–3). In
2019, the incidence rate of EC was 6.39 per 100,000 people in China
and this is increasing (4). The
standard treatment strategy for patients with EC is surgery, after
which the decision to receive adjuvant radiotherapy or chemotherapy
is made based on the patient's disease condition (5–8).
Currently, some advances have been made in establishing a consensus
on risk classification for EC, which is beneficial for
best-tailored management to improve the prognosis of patients with
EC (9,10). However, as a clinically
heterogeneous disease, there are still certain patients with EC
whose survival is unsatisfactory (11–13).
Thus, identifying potential biomarkers for the stratified
management of EC is vital.
Kinesin family protein 2A (KIF2A) is a member of the
kinesin-13 family that functions by restraining the process of
microtubule polymerization and is considered to participate in the
progression of a variety of tumors, including gynecological cancers
(14–17). For example, one study showed that
KIF2A promotes the migration and invasion of cervical cancer cells
(14). Additionally, another study
suggested that the overactivation of KIF2A induces the
depolymerization of microtubules, thereby promoting the progression
of epithelial ovarian cancer (15).
Clinically, a previous study indicated that KIF2A expression was
elevated in tumor tissues compared with non-cancerous tissues, and
high KIF2A expression was associated with unsatisfactory overall
survival (OS) in patients with epithelial ovarian cancer (18). Additionally, another study indicated
that KIF2A serves as a potential biomarker for disease monitoring
and prognostication in patients with cervical cancer (19). However, to the best of our
knowledge, the clinical role of KIF2A as a potential biomarker in
patients with EC has not been previously investigated.
Therefore, the present study aimed to evaluate KIF2A
expression as well as its correlation with prognosis in patients
with EC.
Materials and methods
Patients
A total of 230 patients with EC who underwent
surgical resection in Handan Central Hospital (Handan, China)
between January 2015 and December 2019 were retrospectively
screened. The inclusion criteria were as follows: i) EC diagnosis
by histopathological examination; ii) ≥18 years of age; iii)
underwent surgical resection; iv) accessible and available tumor
formalin-fixed paraffin-embedded (FFPE) specimens; and v) adequate
clinical characteristics data and information for at least one
follow-up. Patients who had other malignancies, were pregnant or
were lactating were excluded. The present study was approved by the
Ethics Committee of Handan Central Hospital (approval no.
2023020615; Handan, China), and informed consent (written or oral)
was obtained from every patient or their family member.
Sample collection
Data on demographics, disease, treatment and
follow-up were obtained from the patients with EC. Disease-free
survival (DFS) and OS were calculated. The last follow-up was in
January 2023. Additionally, 230 FFPE specimens of tumor tissue and
50 FFPE specimens of non-tumor tissue were obtained from the
hospital for examination of KIF2A expression.
Immunohistochemical (IHC)
staining
KIF2A expression was assessed using IHC staining.
Stored specimens were cut into 4-µm thick sections, deparaffinized
with xylene, and rehydrated using a descending ethanol series.
Microwave heating (100°C for 7 min) was used for antigen retrieval.
Endogenous peroxidase activity was quenched using 3%
H2O2. Sections were then blocked using 5%
goat serum (cat. no. ab7481; Abcam) at room temperature for 10 min.
In preliminary experiments, the sections were incubated with rabbit
polyclonal anti-KIF2A antibodies (cat. no. ab197988; Abcam) at
three different concentrations (1:100, 1:200 and 1:300) overnight
at 4°C. In the subsequent experiments, the slides were incubated
overnight with rabbit polyclonal anti-KIF2A antibodies (1:200) at
4°C. After incubation, the slides were washed three times with TBST
(containing 0.1% Tween-20). The slides were then incubated with
goat anti-rabbit IgG H&L (HRP) secondary antibodies (1:1,000;
cat. no. ab6721; Abcam) at room temperature for one hour. After
incubation, the slides were washed three times with TBST. The
slides were then stained using a DAB Peroxidase Substrate Kit
[Absin (Shanghai) Biotechnology, Co., Ltd.) and hematoxylin at room
temperature for 30 min. Sections were then sealed with Glycerol
Jelly Mounting Medium and imaged. A section in which the primary
antibody was omitted served as a negative control. IHC scoring was
performed by two investigators separately using a light microscopy
and Image-Pro Plus 6.0 (Media Cybernetics Inc.), which was used to
assess KIF2A density and intensity scores. Briefly, staining
intensity was scored as 0 (negative), 1 (weak), 2 (moderate) or 3
(strong), and staining density was scored as 0 (0%), 1 (1–25%), 2
(26–50%), 3 (51–75%) or 4 (76–100%). The mean of the KIF2A density
and intensity scores obtained by the two investigators was taken.
The final IHC score (ranging from 0–12) was calculated by
multiplying staining intensity and staining density. For subsequent
analysis, tumor KIF2A expression was divided into high (IHC score
>3) and low (IHC score ≤3) (20–22).
The present study ensured consistency in personnel, experimental
conditions, instrumentation and reagents to control for possible
batch effects.
Database verification
To further verify the correlation between KIF2A
expression and OS, information from 541 patients with EC was
gathered from The Human Protein Atlas database (https://www.proteinatlas.org), in which the KIF2A
expression was classified as low or high with a cut-off value of
5.2.
Statistical analysis
SPSS v24.0 (IBM Corp.) was used for statistical
analysis, and GraphPad Prism v7.01 (Dotmatics) was utilized for
graphing and statistical analysis. Comparisons were performed using
an unpaired t-test or ANOVA with the Tukey's post hoc test.
Correlations were analyzed using Spearman's rank correlation test.
Kaplan-Meier curves with the log-rank test were used for DFS and OS
assessment. Time-dependent receiver-operating characteristic
analyses for relapse and death risk were performed and the area
under the curve (AUC) was calculated (AUC >0.7 was considered to
indicate good predictive utility). Independent factors related to
DFS or OS were screened using forward-step multivariate Cox's
proportional hazard regression analyses. P<0.05 was considered
to indicate a statistically significant difference.
Results
Clinical features of patients with
EC
There were 230 patients with EC with a mean age of
58.1±8.0 years. There were 46 (20.0%) and 184 (80.0%) premenopausal
and postmenopausal patients with EC, respectively. Regarding the
histological subtype, there were 163 (70.9%) patients with
endometrioid carcinoma G1/G2, 17 (7.4%) patients with endometrioid
carcinoma G3, 34 (14.8%) patients with serous endometrial carcinoma
and 16 (7.0%) patients with clear cell endometrial carcinoma.
Additionally, 66 (28.7%) patients demonstrated lymphovascular
invasion. There were 137 (59.6%) patients with the International
Federation of Gynecology and Obstetrics (FIGO) stage I, 27 (11.7%)
patients with FIGO stage II, 45 (19.6%) patients with FIGO stage
III and 21 (9.1%) patients with FIGO stage IV (FIGO 2009 revision)
(23). Additional information on
patients with EC was presented in Table
I.
 | Table I.Clinical characteristics of patients
with endometrial carcinoma (n=230). |
Table I.
Clinical characteristics of patients
with endometrial carcinoma (n=230).
| Clinical
characteristic | Value |
|---|
| Mean age ± SD,
years | 58.1±8.0 |
| Menopausal status, n
(%) |
|
|
Pre-menopause | 46 (20.0) |
|
Post-menopause | 184 (80.0) |
| Diabetes, n (%) |
|
| No | 176 (76.5) |
| Yes | 54 (23.5) |
| Hypertension, n
(%) |
|
| No | 140 (60.9) |
| Yes | 90 (39.1) |
| Histological subtype,
n (%) |
|
|
Endometrioid carcinoma
G1/G2 | 163 (70.9) |
|
Endometrioid carcinoma G3 | 17 (7.4) |
| Serous
endometrial carcinoma | 34 (14.8) |
| Clear
cell endometrial carcinoma | 16 (7.0) |
| Myometrial invasion
≥50%, n (%) |
|
| No | 139 (60.4) |
|
Yes | 91 (39.6) |
| Cervical invasion,
n (%) |
|
| None or
epithelial | 175 (76.1) |
|
Stromal | 55 (23.9) |
| Lymphovascular
invasion, n (%) |
|
| No | 164 (71.3) |
|
Yes | 66 (28.7) |
| FIGO stage, n
(%) |
|
| I | 137 (59.6) |
| II | 27 (11.7) |
|
III | 45 (19.6) |
| IV | 21 (9.1) |
| Adjuvant
radiotherapy, n (%) |
|
| No | 61 (26.5) |
|
Yes | 169 (73.5) |
| Adjuvant
chemotherapy, n (%) |
|
| No | 151 (65.7) |
|
Yes | 79 (34.3) |
Comparison of KIF2A expression in
tumor and non-tumor tissues in patients with EC
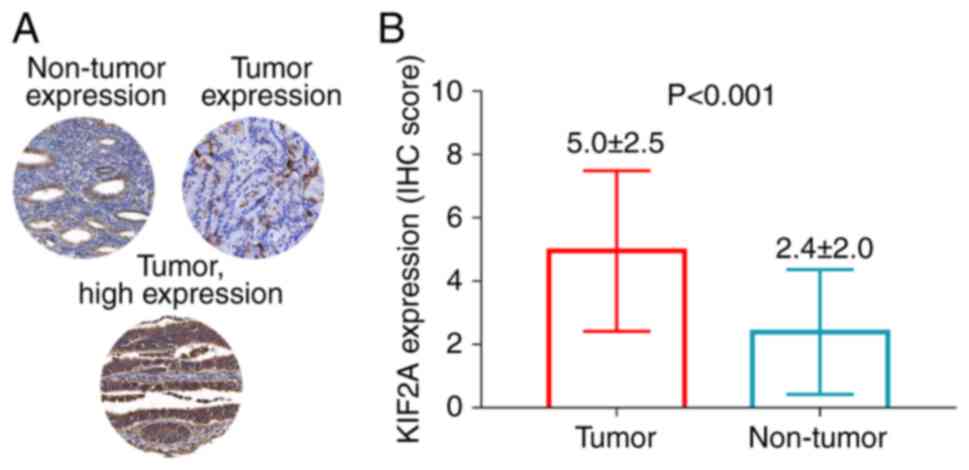
In non-tumor tissue, KIF2A was expressed in
glandular cells but not in endometrial stroma cells. Meanwhile, in
tumor tissue, KIF2A was expressed in the cytoplasm, membranes and
nucleus (Fig. 1A). An unpaired
t-test analysis showed that KIF2A expression was significantly
higher in tumor tissue compared with non-tumor tissue in patients
with EC (P<0.001; Fig. 1B).
Correlation of tumor KIF2A expression
with clinicopathologic features in patients with EC
KIF2A expression in tumor tissue was significantly
related to lymphovascular invasion (P=0.004) and higher FIGO stage
(P=0.001) in patients with EC. However, there was no linkage of
KIF2A expression in tumor tissue with other clinicopathologic
features in patients with EC, including menopausal status,
diabetes, hypertension, histological subtype and cervical invasion
(all P>0.05; Table II).
Furthermore, the results for the comparison by Tukey's post hoc
test regarding histological subtype and FIGO stage data are shown
in Table SI.
 | Table II.Correlation of tumor KIF2A expression
with clinical characteristics. |
Table II.
Correlation of tumor KIF2A expression
with clinical characteristics.
| Clinical
characteristic | KIF2A
expression | P-value |
|---|
| Age, years |
| 0.834 |
|
<60 | 4.9±2.3 |
|
|
≥60 | 5.0±2.8 |
|
| Menopausal
status |
| 0.573 |
|
Pre-menopause | 4.8±2.5 |
|
|
Post-menopause | 5.0±2.6 |
|
| Diabetes |
| 0.917 |
| No | 4.9±2.5 |
|
|
Yes | 5.0±2.8 |
|
| Hypertension |
| 0.490 |
| No | 4.9±2.5 |
|
|
Yes | 5.1±2.6 |
|
| Histological
subtype |
| 0.229 |
|
Endometrioid carcinoma
G1/G2 | 4.8±2.3 |
|
|
Endometrioid carcinoma G3 | 5.4±2.7 |
|
| Serous
endometrial carcinoma | 5.2±3.1 |
|
| Clear
cell endometrial carcinoma | 5.9±3.0 |
|
| Myometrial invasion
≥50% |
| 0.159 |
| No | 4.8±2.5 |
|
|
Yes | 5.2±2.6 |
|
| Cervical
invasion |
| 0.091 |
| None or
epithelial | 4.8±2.4 |
|
|
Stromal | 5.5±3.0 |
|
| Lymphovascular
invasion |
| 0.004 |
| No | 4.6±2.5 |
|
|
Yes | 5.7±2.6 |
|
| FIGO stage |
| 0.001 |
| I | 4.6±2.4 |
|
| II | 4.9±2.7 |
|
|
III | 5.2±2.3 |
|
| IV | 6.7±3.0 |
|
| Adjuvant
radiotherapy |
| 0.253 |
| No | 4.6±2.6 |
|
|
Yes | 5.1±2.5 |
|
| Adjuvant
chemotherapy |
| 0.063 |
| No | 4.7±2.5 |
|
|
Yes | 5.4±2.7 |
|
Correlation of tumor KIF2A expression
with accumulating DFS and OS rates in patients with EC
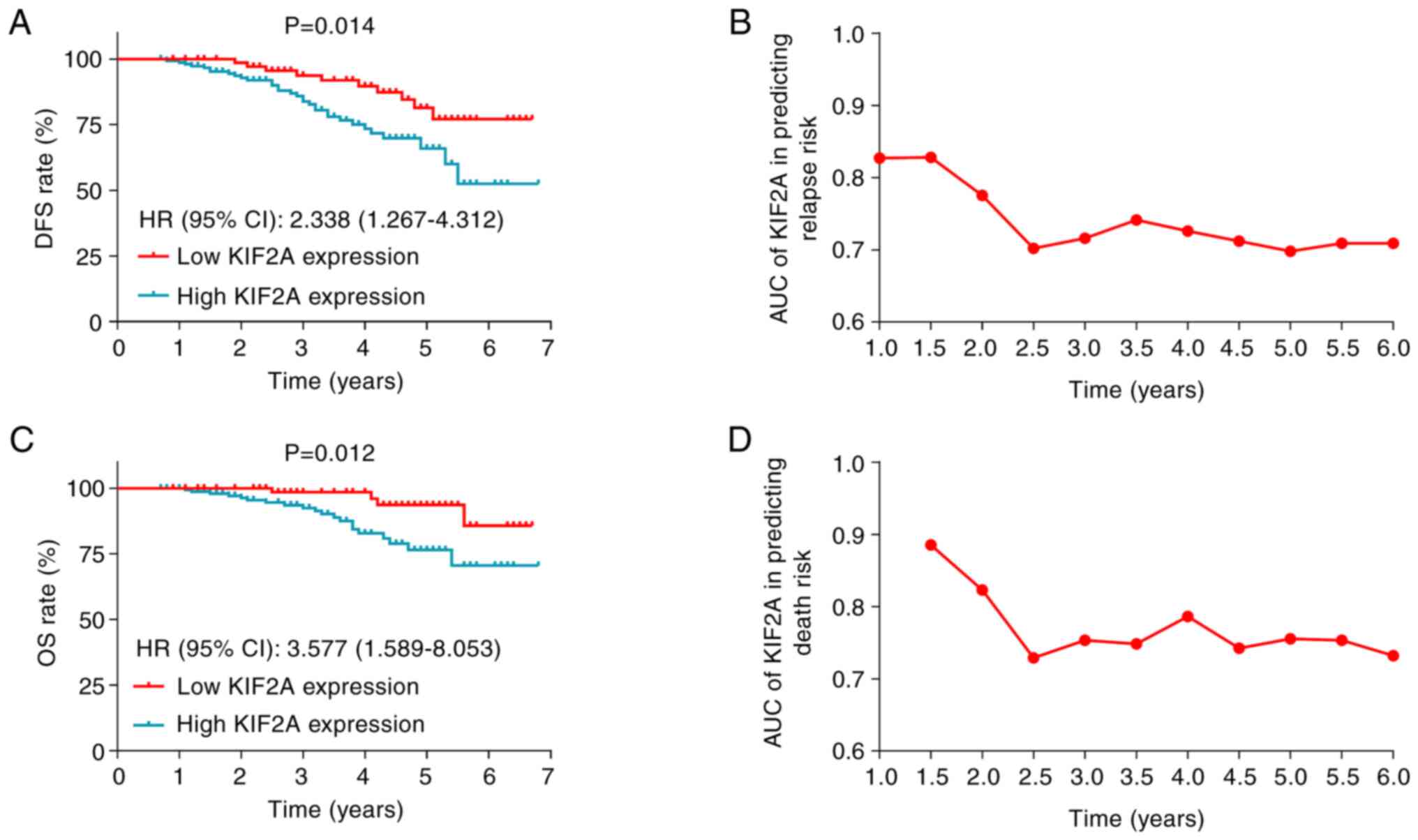
High tumor KIF2A expression was associated with
significantly lower DFS in patients with EC [P=0.014; hazard ratio
(HR)=2.338; Fig. 2A]. Moreover, the
AUC of KIF2A expression in tumor tissues, for predicting relapse
risk over 6 years remained stable at ≥0.7 (Fig. 2B). High KIF2A expression in tumor
tissues was also correlated with significantly reduced OS in
patients with EC (P=0.012; HR=3.577; Fig. 2C). Furthermore, the AUC of KIF2A
expression in tumor tissues, for predicting death risk over 6 years
also maintained stable at ≥0.7 (Fig.
2D).
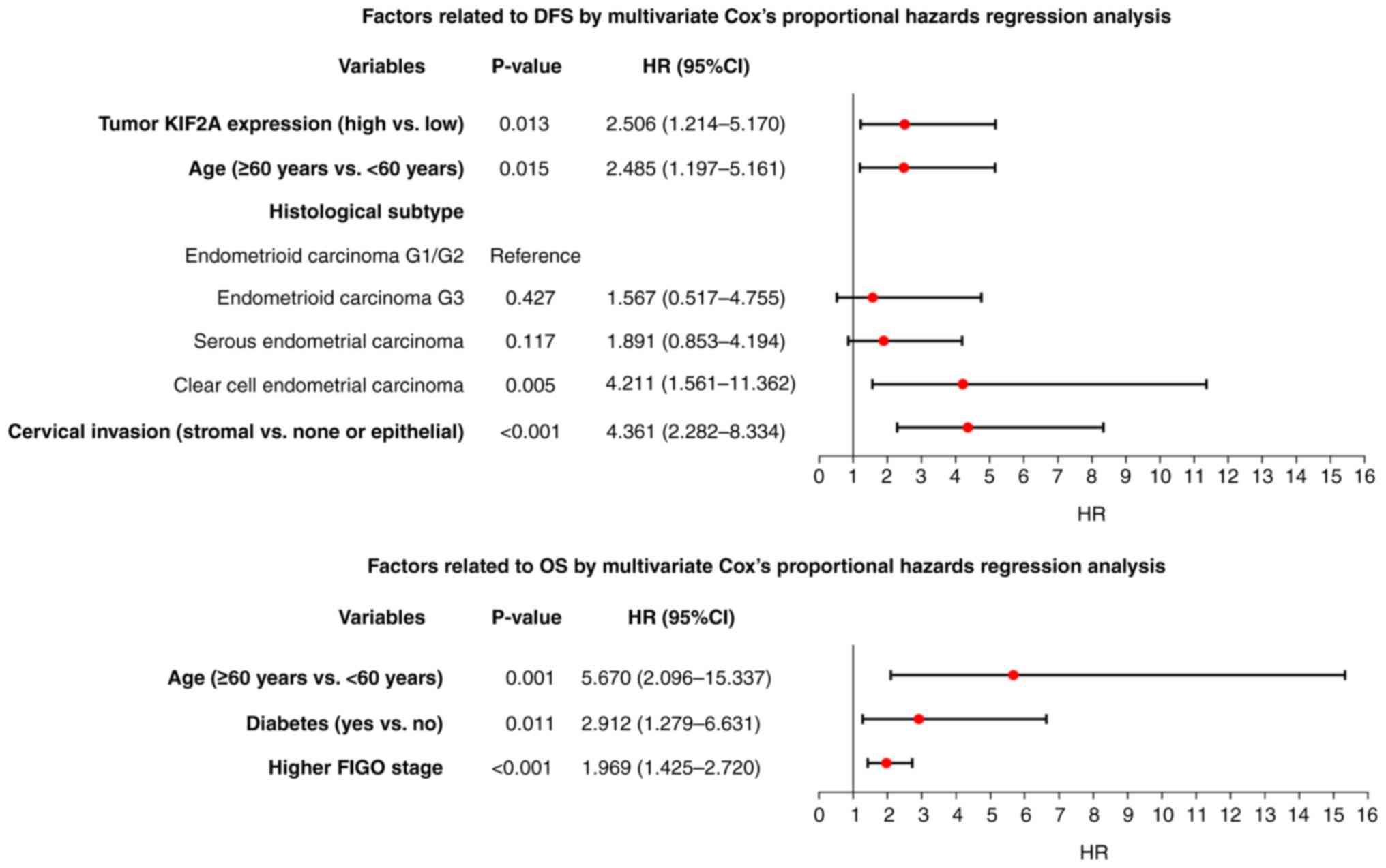
Independent factors linked with DFS
and OS in patients with EC
KIF2A expression in tumor tissues (high vs. low;
HR=2.506; P=0.013), age (≥60 years vs. <60 years; HR=2.485;
P=0.015), histological subtype (clear cell endometrial carcinoma
vs. endometrioid carcinoma G1/G2; HR=4.211; P=0.005) and cervical
invasion (stromal vs. none or epithelial; HR=4.361; P<0.001)
independently estimated shorter DFS in patients with EC (Fig. 3A). KIF2A expression in tumor tissues
was not independently associated with OS. However, age (≥60 years
vs. <60 years; HR=5.670; P=0.001), diabetes (yes vs. no;
HR=2.912; P=0.011) and higher FIGO stage (HR=1.969; P<0.001)
were independently linked with shorter OS in patients with EC
(Fig. 3B).
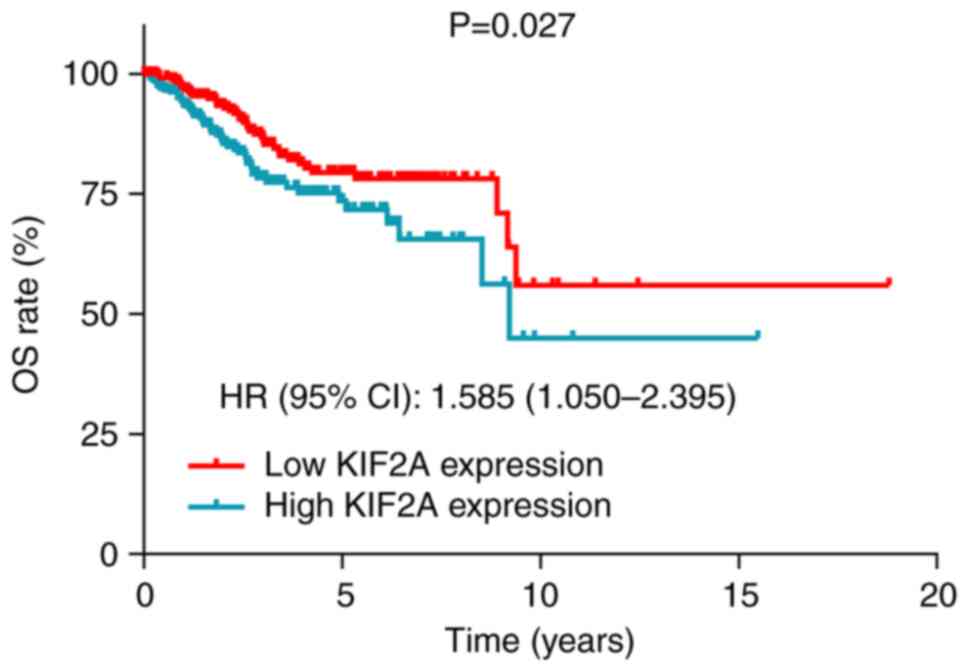
Data from the Human Protein Atlas database indicated
that high tumor KIF2A expression was significantly associated with
decreased OS in patients with EC (P=0.027; HR=1.585; Fig. 4).
Discussion
KIF2A is an oncogene that has been reported to be
abnormally expressed in certain cancers, such as ovarian cancer,
cervical carcinoma and breast cancer (24–27).
These results demonstrated similarity to the present study, which
revealed that KIF2A expression was significantly higher in tumor
tissue compared with non-tumor tissue in patients with EC. This may
be since the increase in KIF2A could reflect the increase in cell
proliferation ability. Furthermore, the proliferative ability of EC
cells in tumor tissues has been previously reported to be faster
than that of cells in non-tumor tissues (28).
Clinically, high KIF2A expression is associated with
worse tumor features in certain cancers (19,27).
For example, one study reported that there was a positive
correlation of KIF2A expression with lymph node metastasis and FIGO
stage in patients with cervical cancer (19). Furthermore, another study reported
that higher KIF2A expression was related to higher tumor stages in
patients with breast cancer (27).
The present study indicated that KIF2A was associated with
lymphovascular invasion and higher FIGO stage in patients with EC.
This could be because KIF2A might increase the migration and
invasion of EC cells through the membrane type 1-matrix
metalloproteinase and phosphatidylinositol-3-kinase/protein kinase
B pathway signaling pathways (16,17),
indicating a possible link with lymphovascular invasion. KIF2A
could also increase the malignant behavior of EC cells by
regulating microtubule dynamics, thus stimulating the progression
of EC (29–31), indicating a possible link with FIGO
stage (32,33).
Notably, previous studies have reported that KIF2A
expression was correlated with unfavorable survival in many cancer
patients (24,34). One study reported that KIF2A
expression in tumor tissues was linked with unsatisfactory DFS in
basal-like breast cancer patients (24). Furthermore, another study reported
that KIF2A expression in tumor tissues was related to shortened OS
in hepatocellular carcinoma patients (34). Similarly, the present study
indicated that tumor KIF2A expression was related to shorter DFS
and OS, and independently forecast unsatisfactory DFS in patients
with EC. Moreover, data from the Human Protein Atlas database
showed that patients with high KIF2A expression had shortened OS in
patients with EC. The probable causes could be that, based on the
content described above, KIF2A expression was related to
unfavorable disease features and thus lead to poor survival in
patients with EC. A possible cause could also be that KIF2A
promoted EC progression through a series of pathways, including
promoting membrane type 1-matrix metalloproteinase, facilitating
phosphatidylinositol-3-kinase/protein kinase B pathway signaling
pathways and regulating microtubule dynamics, thus negatively
affecting the prognosis of patients with EC (16,17,29,30).
Moreover, KIF2A might affect the chemosensitivity of EC cells to
platinum-based regimens, thus reducing the survival rate of
patients with EC (28).
The present study had several limitations. Firstly,
this was a retrospective study, which could cause a certain degree
of selection bias. Secondly, the present study only detected the
KIF2A expression in tissues and further studies should consider
exploring its expression in circulating samples. Thirdly, the
detailed mechanism through which KIF2A participates in the
progression of EC was not elucidated and requires further
investigation in future studies.
In conclusion, KIF2A is highly expressed in tumor
tissue and is associated with lymphovascular invasion and advanced
FIGO stage, as well as undesirable DFS and OS in patients with
EC.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
YS and WZ contributed to study conception and
design. Material preparation, data collection and analysis were
performed by YS, LY, YH and JW. The first draft of the manuscript
was written by YS, JW and WZ. LY, YH and WZ revised the manuscript.
YS and WZ confirm the authenticity of all the raw data. All authors
read and approved the final manuscript.
Ethics approval and consent to
participate
This study obtained the approval from the Ethics
Committee of Handan Central Hospital (approval no. 2023020615;
Handan, China), and informed consent (written or oral) was obtained
from each patient or their family member.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Use of artificial intelligence tools
During the preparation of this work, AI tools were
used to improve the readability and language of the manuscript, and
subsequently, the authors revised and edited the content produced
by the AI tools as necessary, taking full responsibility for the
ultimate content of the present manuscript.
References
|
1
|
Koskas M, Amant F, Mirza MR and Creutzberg
CL: Cancer of the corpus uteri: 2021 update. Int J Gynaecol Obstet.
155 (Suppl 1):S45–S60. 2021. View Article : Google Scholar
|
|
2
|
Concin N, Matias-Guiu X, Vergote I, Cibula
D, Mirza MR, Marnitz S, Ledermann J, Bosse T, Chargari C, Fagotti
A, et al: ESGO/ESTRO/ESP guidelines for the management of patients
with endometrial carcinoma. Int J Gynecol Cancer. 31:12–39. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Yang Z, Yang X, Liu X, Ma K, Meng YT, Yin
HF, Wen J, Yang JH, Zhen Z, Feng ZH and Liao QP: Clinical
characteristics and prognostic characterization of endometrial
carcinoma: A comparative analysis of molecular typing protocols.
BMC Cancer. 23:2432023. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Zhang SZ, Zhang L and Xie L: Cancer Burden
in China during 1990–2019: Analysis of the global burden of
disease. Biomed Res Int. 2022:39180452022.PubMed/NCBI
|
|
5
|
Kovacevic N: Surgical treatment and
fertility perservation in endometrial cancer. Radiol Oncol.
55:144–149. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
van den Heerik ASVM, Horeweg N, de Boer
SM, Bosse T and Creutzberg CL: Adjuvant therapy for endometrial
cancer in the era of molecular classification: Radiotherapy,
chemoradiation and novel targets for therapy. Int J Gynecol Cancer.
31:594–604. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Tung HJ, Huang HJ and Lai CH: Adjuvant and
post-surgical treatment in endometrial cancer. Best Pract Res Clin
Obstet Gynaecol. 78:52–63. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Crosbie EJ, Kitson SJ, McAlpine JN,
Mukhopadhyay A, Powell ME and Singh N: Endometrial cancer. Lancet.
399:1412–1428. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Lee YC, Lheureux S and Oza AM: Treatment
strategies for endometrial cancer: Current practice and
perspective. Curr Opin Obstet Gynecol. 29:47–58. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kasius JC, Pijnenborg JMA, Lindemann K,
Forsse D, van Zwol J, Kristensen GB, Krakstad C, Werner HMJ and
Amant F: Risk stratification of endometrial cancer patients: FIGO
stage, biomarkers and molecular classification. Cancers (Basel).
13:58482021. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
McAlpine J, Leon-Castillo A and Bosse T:
The rise of a novel classification system for endometrial
carcinoma; integration of molecular subclasses. J Pathol.
244:538–549. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wang N, Zhang J, Fan X and Ma J, He J,
Kang S, Cheng J and Ma J: Identification of risk factors for the
prognosis of Chinese patients with endometrial carcinoma. Medicine
(Baltimore). 100:e273052021. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Liang S and Zhang Y: Clinical pathological
characteristics and survival of high-grade endometrioid carcinoma.
J Obstet Gynaecol Res. 47:3644–3651. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wang Y, Wang J, Zhao A, Huang X and Zhang
X: HPV16 E6E7 up-regulates KIF2A expression by activating JNK/c-Jun
signal, is beneficial to migration and invasion of cervical cancer
cells. Open Med (Wars). 17:1780–1787. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Xu Y, Wang W, Chen J, Mao H, Liu Y, Gu S,
Liu Q, Xi Q and Shi W: High neuropilin and tolloid-like 1
expression associated with metastasis and poor survival in
epithelial ovarian cancer via regulation of actin cytoskeleton. J
Cell Mol Med. 24:9114–9124. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhao P, Lan F, Zhang H, Zeng G and Liu D:
Down-regulation of KIF2A inhibits gastric cancer cell invasion via
suppressing MT1-MMP. Clin Exp Pharmacol Physiol. 45:1010–1018.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wang K, Lin C, Wang C, Shao Q, Gao W, Song
B, Wang L, Song X, Qu X and Wei F: Silencing Kif2a induces
apoptosis in squamous cell carcinoma of the oral tongue through
inhibition of the PI3K/Akt signaling pathway. Mol Med Rep.
9:273–278. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wang D, Zhu H, Ye Q, Wang C and Xu Y:
Prognostic Value of KIF2A and HER2-Neu overexpression in patients
with epithelial ovarian cancer. Medicine (Baltimore). 95:e28032016.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Lei G, Xin X and Hu X: Clinical
significance of kinesin family member 2A as a facilitating
biomarker of disease surveillance and prognostication in cervical
cancer patients. Ir J Med Sci. 191:665–670. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Tian Y, Zhao K, Yuan L, Li J, Feng S, Feng
Y, Fang Z, Li H and Deng R: EIF3B correlates with advanced disease
stages and poor prognosis, and it promotes proliferation and
inhibits apoptosis in non-small cell lung cancer. Cancer Biomark.
23:291–300. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wei DC, Yeh YC, Hung JJ, Chou TY, Wu YC,
Lu PJ, Cheng HC, Hsu YL, Kuo YL, Chen KY, et al: Overexpression of
T-LAK cell-originated protein kinase predicts poor prognosis in
patients with stage I lung adenocarcinoma. Cancer Sci. 103:731–738.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Neven P, Van Calster B, Van den Bempt I,
Van Huffel S, Van Belle V, Hendrickx W, Decock J, Wildiers H,
Paridaens R, Amant F, et al: Age interacts with the expression of
steroid and HER-2 receptors in operable invasive breast cancer.
Breast Cancer Res Treat. 110:153–159. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Amant F, Mirza MR, Koskas M and Creutzberg
CL: Cancer of the corpus uteri. Int J Gynaecol Obstet. 143 (Suppl
2):S37–S50. 2018. View Article : Google Scholar
|
|
24
|
Yang H and Liu Y: Kinesin family member 2A
serves as a potential biomarker reflecting more frequent lymph node
metastasis and tumor recurrence risk in basal-like breast cancer
patients. Front Surg. 9:8892942022. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Sheng N, Xu YZ, Xi QH, Jiang HY, Wang CY,
Zhang Y and Ye Q: Overexpression of KIF2A is Suppressed by miR-206
and associated with poor prognosis in ovarian cancer. Cell Physiol
Biochem. 50:810–822. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Wang ZX, Ren SC, Chang ZS and Ren J:
Identification of kinesin family member 2A (KIF2A) as a promising
therapeutic target for osteosarcoma. Biomed Res Int.
2020:71027572020.PubMed/NCBI
|
|
27
|
Wang C, Xie X, Li W and Jiang D:
Expression of KIF2A, NDC80, CDK1, and CCNB1 in breast cancer
patients: Their interaction and linkage with tumor features and
prognosis. J Clin Lab Anal. 36:e246472022. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Bai F, He Z, Zhou H and Gan W: Kinesin
family member 2A links with advanced tumor stage, reduced
chemosensitivity and worse prognosis in gastric cancer. J Clin Lab
Anal. 36:e243132022. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zaganjor E, Osborne JK, Weil LM,
Diaz-Martinez LA, Gonzales JX, Singel SM, Larsen JE, Girard L,
Minna JD and Cobb MH: Ras regulates kinesin 13 family members to
control cell migration pathways in transformed human bronchial
epithelial cells. Oncogene. 33:5457–5466. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Schimizzi GV, Currie JD and Rogers SL:
Expression levels of a kinesin-13 microtubule depolymerase
modulates the effectiveness of anti-microtubule agents. PLoS One.
5:e113812010. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Xu L, Zhang X, Wang Z, Zhao X, Zhao L and
Hu Y: Kinesin family member 2A promotes cancer cell viability,
mobility, stemness, and chemoresistance to cisplatin by activating
the PI3K/AKT/VEGF signaling pathway in non-small cell lung cancer.
Am J Transl Res. 13:2060–2076. 2021.PubMed/NCBI
|
|
32
|
Gou R, Zheng M, Hu Y, Gao L, Wang S, Liu
O, Li X, Zhu L, Liu J and Lin B: Identification and clinical
validation of NUSAP1 as a novel prognostic biomarker in ovarian
cancer. BMC Cancer. 22:6902022. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Chang H, Wang J, Tian Y, Xu J, Gou X and
Cheng J: The TPX2 gene is a promising diagnostic and therapeutic
target for cervical cancer. Oncol Rep. 27:1353–1359.
2012.PubMed/NCBI
|
|
34
|
Liu W, Xu C, Meng Q and Kang P: The
clinical value of kinesin superfamily protein 2A in hepatocellular
carcinoma. Clin Res Hepatol Gastroenterol. 45:1015272021.
View Article : Google Scholar : PubMed/NCBI
|