Introduction
Glycoalkaloids are secondary metabolites from plants
of the solanaceae family, which include widely consumed staple
foods such as eggplant, tomato, and potato. There, they exert a
natural protective function against fungi, bacteria, and predators
(1). In recent years, there has
been increasing interest in the possible antitumor activities of
steroidal glycoalkaloids (2).
Several publications can be found on the glycoalkaloid α-solanine
(Molecular formula: C45H73NO15),
which is found mainly in potatoes, where it accounts for up to 95%
of the total glycoalkaloid content (3). Due to its known acute toxicity in
mammals and thus also in humans, the Food and Agriculture
Organization (FAO) of the United Nations (UN) and the World Health
Organization (WHO) recommend a maximum daily dose of 200 mg kgG-1
(3). An additional concern is
possible teratogenic effects, especially neural tube defects
(NTDs). These have been demonstrated in various animal experiments,
although the effect did not occur in a study with non-human
primates (4). Regarding the
antitumor effects of glycoalkaloids, especially α-solanine, a
growing number of publications in recent years investigated these
mechanisms in various tumor entities, including breast, lung,
pancreas, liver, prostate, and skin (5,6). In
terms of toxicity threshold and mechanisms of action, results and
the conclusions drawn of them in literature showed a high variance.
Activation of apoptosis via different pathways is often cited as a
cause of tumor toxicity. α-solanine stimulated apoptosis-activating
proteins in the liver carcinoma cell line HepG2 (ASK1: apoptosis
signal-regulating kinase 1, TBP-2: tetrahymena piggyBac transposase
2) (7), in the colorectal carcinoma
cell line RKO (Caspase-3) (8) and
in the pancreatic carcinoma cell lines SW1990 and Panc-1 (each
caspase-3) (9). In addition,
influences on cell cycle regulation were investigated in the liver
carcinoma cell line HepG2 (10).
Despite these studies raising hope for its potential
usefulness as a tumor therapeutic agent, to our knowledge, there
are no studies on the effect of α-solanine on human head and neck
squamous cell carcinoma (HNSCC). HNSCC are a heterogeneous tumor
group with more than 890.000 new cases and more than 450.000 deaths
in the year 2018 alone (11). The
exploration of potential new therapies is an urgent topic, as
drug-based tumor therapies gain increasingly higher
significance.
The aim of this study was to investigate the effect
of α-solanine on HNSCC cells for the first time. Furthermore, the
cytotoxicity as well as potential genotoxicity and functional
impairment of a non-malignant cell line of Human Umbilical Vein
Endothelial Cells (HUVECs) was evaluated at subtoxic doses.
Materials and methods
Characterization of reagents and cell
cultures
α-solanine (from potato sprouts, >95%) was
purchased from Merck (Darmstadt, Germany). Two HNSCC derived cell
lines were used. The first, established from a hypopharyngeal
carcinoma (FaDu) (12), was
cultured in MEM Eagle (Sigma) with penicillin/streptomycin (Sigma),
FBS 10% (Anprotec) and Glutamin 1% (Sigma). The tongue carcinoma
cell line (CAL-33; Leibniz Institute DSMZ-German Collection of
Microorganisms and Cell Cultures Gmbh) was cultured in DMEM (Gibco)
with FBS 10% (Anprotec) and penicillin/streptomycin (Sigma). Cells
were incubated at 37°C with 5% CO2 in 75 cm2
flasks (Greiner). The medium was replaced every second day. Cells
were passaged by trypsinization (0.25% trypsin-EDTA (Gibco) (1×);
Invitrogen (Gibco), Life Technologies, Karlsruhe, Germany) before
reaching 80% of confluence. Then the cells were washed (1×
centrifugation, 1,000 rpm, 5 min) and either seeded in treatment
wells or in new 75 cm2 flasks. Non-malignant HUVECs
(pooled donors, C12203, PromoCell GmbH, Heidelberg, Germany) were
treated according to the procedures described in a previous study
by our group (13). Briefly, the
cells were cultured in an endothelial cell growth medium with
supplements (ECGM; Provitro GmbH, Berlin, Germany) and in 1%
penicillin/streptomycin. Mycoplasma testing has been carried out
for the cell lines used.
Cytotoxicity analysis in the MTT
assay
Before the application of α-solanine, the number of
FaDu, CAL-33, and HUVECs was determined using an electronic cell
counter (Casy Technology, Innovatis AG, Reutlingen, Germany) and
1×104 cells per well were seeded in a 96-well plate
(Greiner, 655180, flat bottom). After 24 h incubation, the medium
was replaced with rpmI 1640 medium supplemented with α-solanine at
concentrations of 3–30 µM for FaDu, 1–30 µM for CAL-33 and 1–18 µM
for HUVECs. Previously, an adequate concentration range was
determined from a wide panel of doses. Untreated negative controls
were cultivated in rpmI 1640 only on the same cell culture
plate.
After subsequent incubation for 24 h, cell viability
was assessed with
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT;
Sigma Aldrich) colorimetric staining method (14). The expansion medium was removed and
100 µl of MTT solution (1 mg/ml dissolved in medium) was added to
each well. This was followed by a 4 h incubation at 37°C with 5%
CO2. MTT was then replaced with isopropanol, which
dissolves the formed formazan crystals within 30 min at room
temperature. Cells were kept dark. To remove all particles from the
samples and avoid interference during measurements, the contents of
each well were transferred to an Eppendorf tube, centrifuged (1,000
rpm, 5 min) and then transferred to a new 96-well plate without
resuspending the pellet. Finally, the color conversion of the
purple formazan dye was measured by an enzyme-linked immunosorbent
assay reader at a wavelength of 570 nm. 1 mM tert-butyl
hydroperoxide (t-BHP; Sigma-Aldrich, St. Louis, MO, USA) served as
a positive control of metabolically inactive cells and medium
without α-solanine was used as a negative control. All measurements
were carried out in technical triplicates for FaDu, CAL-33 and
HUVECs.
Cell cycle analysis
For cell cycle analysis, FaDu were incubated with
α-solanine at concentrations of 12, 15, 18 and 21 µM. After 24 h,
the cells were fixed in the dark in 70% ethanol at 4°C for 2 h
followed by centrifugation at 500 g for 5 min at 4°C. Then, 500 ml
PI/RNase Staining Buffer (Becton-Dickinson Bioscience) was added
followed by an incubation in the dark at 4°C for 15 min.
Subsequently, flow cytometer (FACScanto, Becton-Dickinson)
Measurements were performed.
Flow cytometry
Time- and dose-dependent apoptosis and necrosis of
tumor cells were measured by flow cytometry using an Annexin V
propidium iodide (PI) kit (Becton-Dickinson Bioscience, Heidelberg,
Germany) according to the manufacturer's protocol. Here, a
distinction was made between viable cells, apoptotic cells (Annexin
V), and necrotic or late-apoptotic cells (propidium iodide (PI)).
FaDu and CAL-33 (2×10^5 (200,000)/well) were seeded in 6-well
plates and incubated with α-solanine for 24 h. The experimental
concentration range included concentrations of 12, 15, 18, 21, 24,
27 and 30 µM. After exposure, adherent cells were harvested by
trypsinization as described above and added to the preserved
medium. After two washing steps with centrifugation (500 g, 5 min,
4°C) and addition of phosphate buffer saline (PBS, Roche), the cell
pellet was resuspended with 100 µl binding buffer (BD Pharmingen).
For staining, 5 µl of Annexin V-APC and 5 µl of PI were added.
After 15 min of incubation in the dark, the fluorescence of
1×104 cells per sample was measured by flow
cytometry.
Genotoxicity evaluation with the comet
assay
For the detection of DNA strand breaks and alkali
labile as well as incomplete excision repair sites in single cells,
the comet-assay (alkaline version of the single-cell microgel
electrophoresis) was used. Subcytotoxic doses of α-solanine in
HUVECs were evaluated in the MTT assay as previously described.
Doses of 1, 2, 4 and 6 µM were applied in the comet assay and a
negative control was performed using culture medium. Additionally,
200 µM direct alkylating methyl methanesulfonate (MMS,
Sigma-Aldrich) was applied for 24 h as a positive control. The test
concentrations were applied for 24 h. The comet assay was performed
as described before by our group (15). For each concentration, 50 cells on 2
assay (slide) replicates were evaluated, resulting in 100 cells
evaluated per concentration. Cell nucleoli were analyzed
semi-automatically with a DMLB fluorescence microscope (Leica
Microsystems, Wetzlar, Germany). The image analysis software COMET
5.5 (Kinetic Imaging, Liverpool, UK) was used to measure the comet
and identify heads and tails. The percentage of DNA in the tail
(TD), the tail length (TL), and the product of the percent of DNA
in the tail and the mean migration distance (olive tail moment
(OTM)), were evaluated (Kinetic Imaging Limited, KOMET, Singe Cell
Gel Elektrophoresis Analysis, Version 4). For statistical analysis,
OTM values were evaluated.
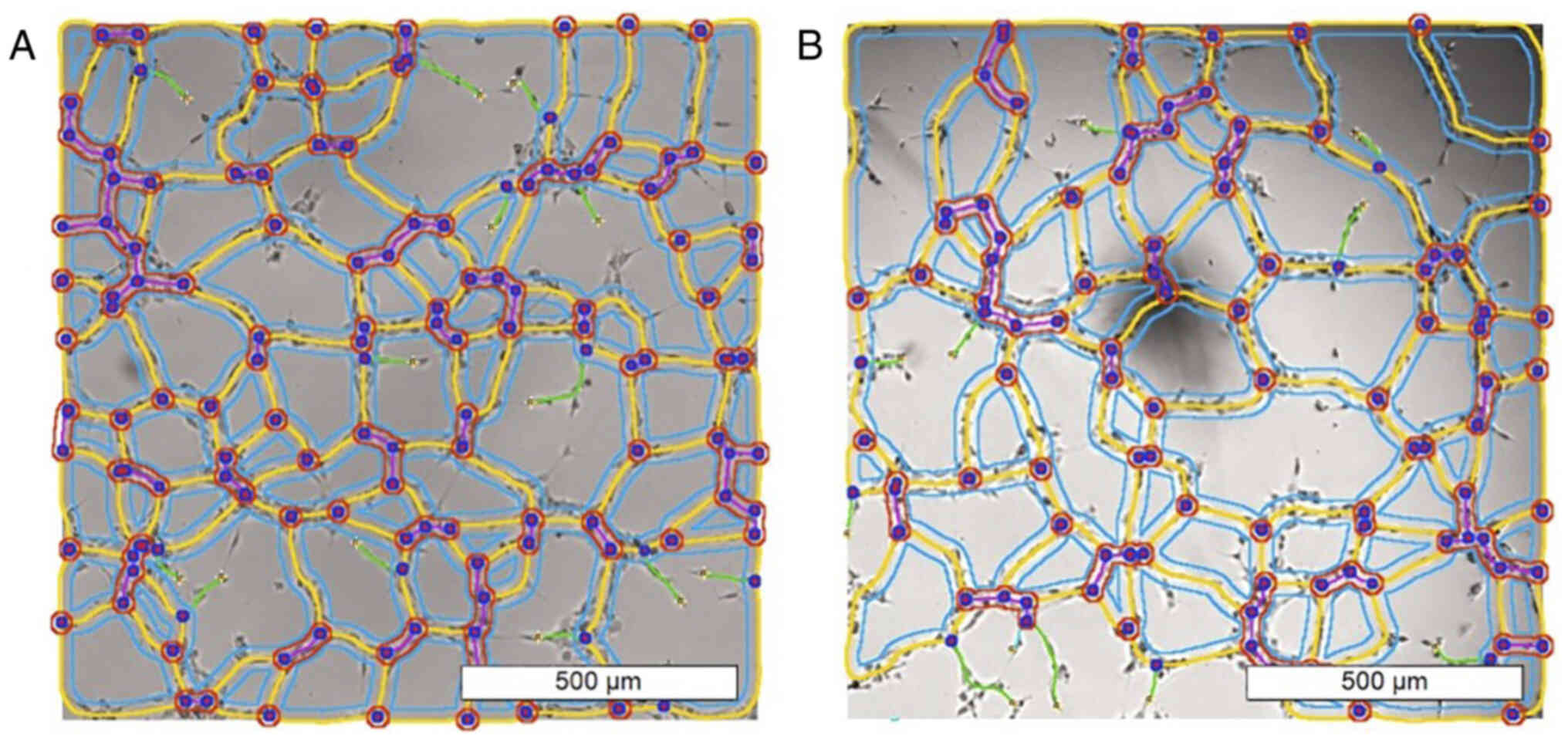
Functionality evaluation in the
capillary tube formation assay
To evaluate the effects of α-solanine on vascular
endothelial cells' proliferation and tube formation as a model of
neoangiogenesis and tissue repair, the capillary tube formation
assay was used (16).
10 µl of Matrigel (Sigma-Aldrich, St. Louis, MO,
USA) was transferred for µ-slide angiogenesis (µ-Slide Angiogenesis
plate, ibidi GmbH, Martinsried, Germany). The plates were placed in
an incubator under standard conditions (humidity chamber, 37°C) for
30–60 min. Afterwards, 50 µl of the test cell suspension
(containing 1×104 cells each) were transferred into each
well. The plate was incubated and analyzed after 6 and 24 h. Images
were acquired using an inverted phase contrast microscope (Leica
Microsystems, Wetzlar, Germany).
All test concentrations were performed three times.
The image sections with the best capillary tube formation were
carefully selected and used for automatic image analysis, i.e.,
three images per test concentration were analyzed. For this
purpose, NIH ImageJ software (ImageJ 1.53v) with the Angiogenesis
Analyzer plugin (CARPENTIER, 2012) was used as suggested by
DeCicco-Skinner et al (16).
Statistical analysis
GraphPad Prism software 9 (GraphPad Software, Inc.,
La Jolla, CA, USA) was used for statistical analyses. One-way ANOVA
and Dunnett's tests were used to test for statistical differences
between the cell viability (MTT assay) of treated samples in
comparison with the negative control. The mean values of the OTM of
treatment group and control groups and between cells with or
without fpg-treatment (comet assay) were also compared with one-way
ANOVA and Dunnett's tests. The results of flow cytometry (viable,
apoptotic and necrotic cells) in comparison to the negative control
and also the results of the capillary tube formation assay, each in
comparison to the negative controls, were also analyzed using
one-way ANOVA and Dunnett's tests. The P-value for statistical
significance was set at P<0.05 and marked with asterisks in
plots.
Results
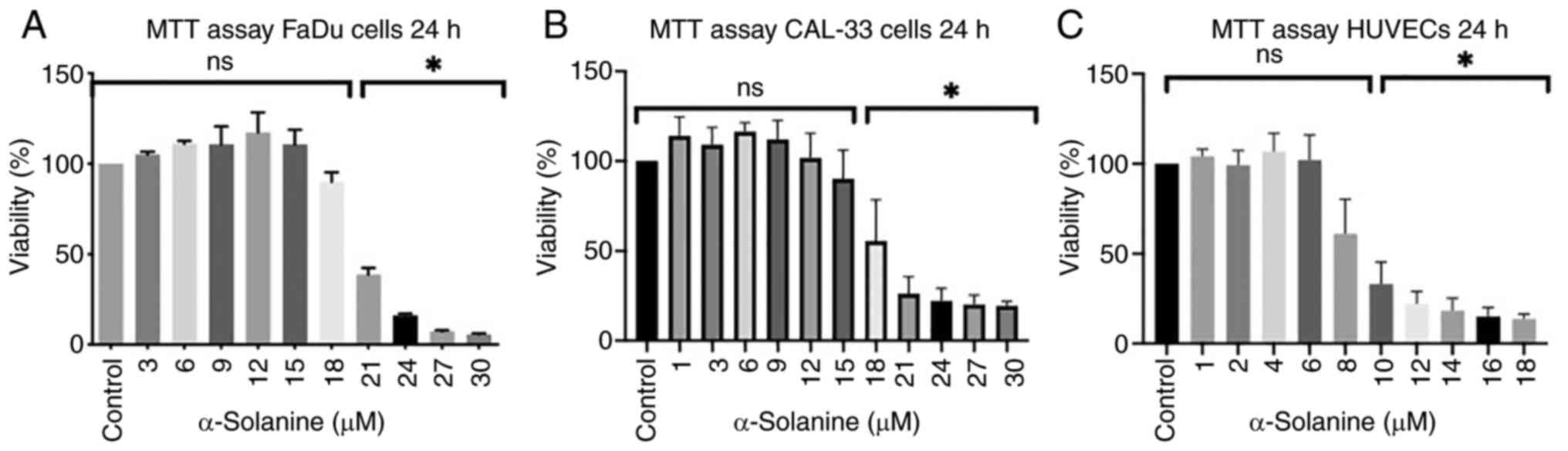
Cytotoxicity analysis
The MTT assay was conducted to estimate the
viability of cells immediately after 24 h of exposure to
α-solanine. Fig. 1, Fig. 2, Fig.
3 show the results for the different cell lines expressed as
the percentage of viable cells compared to the untreated control
groups which were defined as 100%. In the MTT assay, a
dose-dependent decrease in cell viability was observed in FaDu,
CAL33, and HUVECs, respectively (Fig.
1). Significant cytotoxicity of α-solanine was reached at 21 µM
in FaDu, at 21 µM in CAL-33 and at 10 µM in HUVECs.
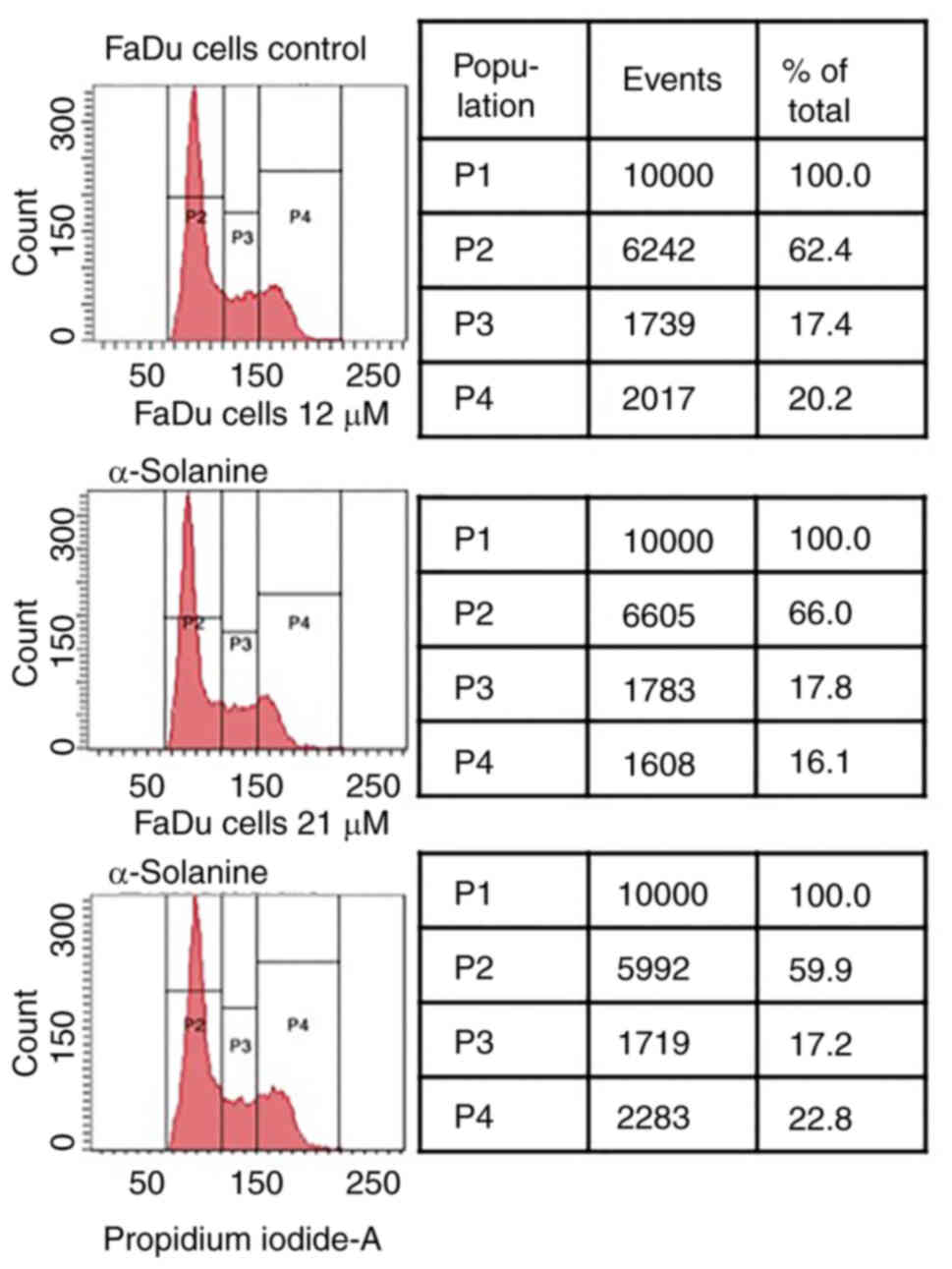
Cell cycle analysis
No dose-dependent changes in the phases of the cell
cycle were observed after exposure of FaDu to α-solanine doses of
12 to 21 µM compared to the control group (Fig. 2).
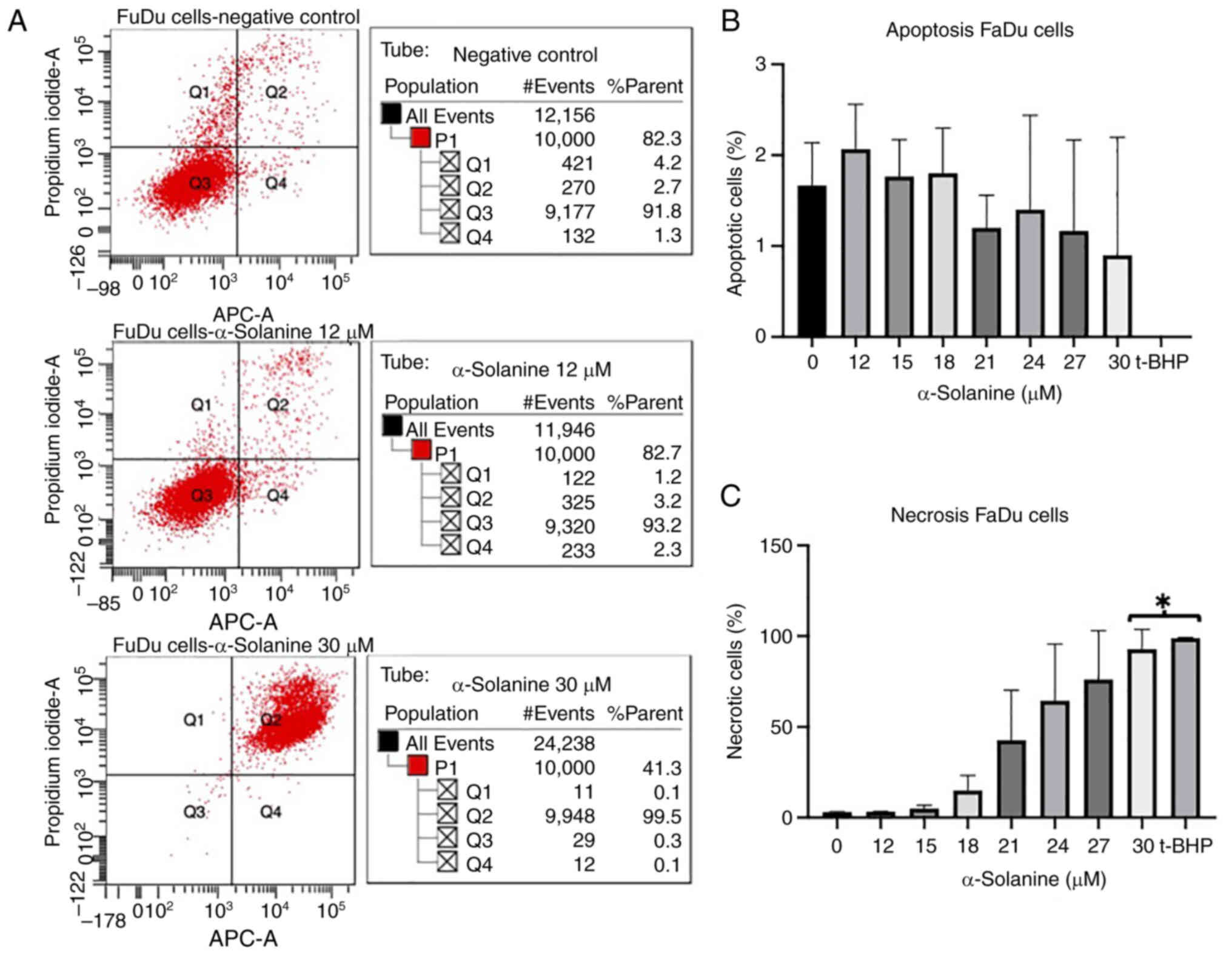
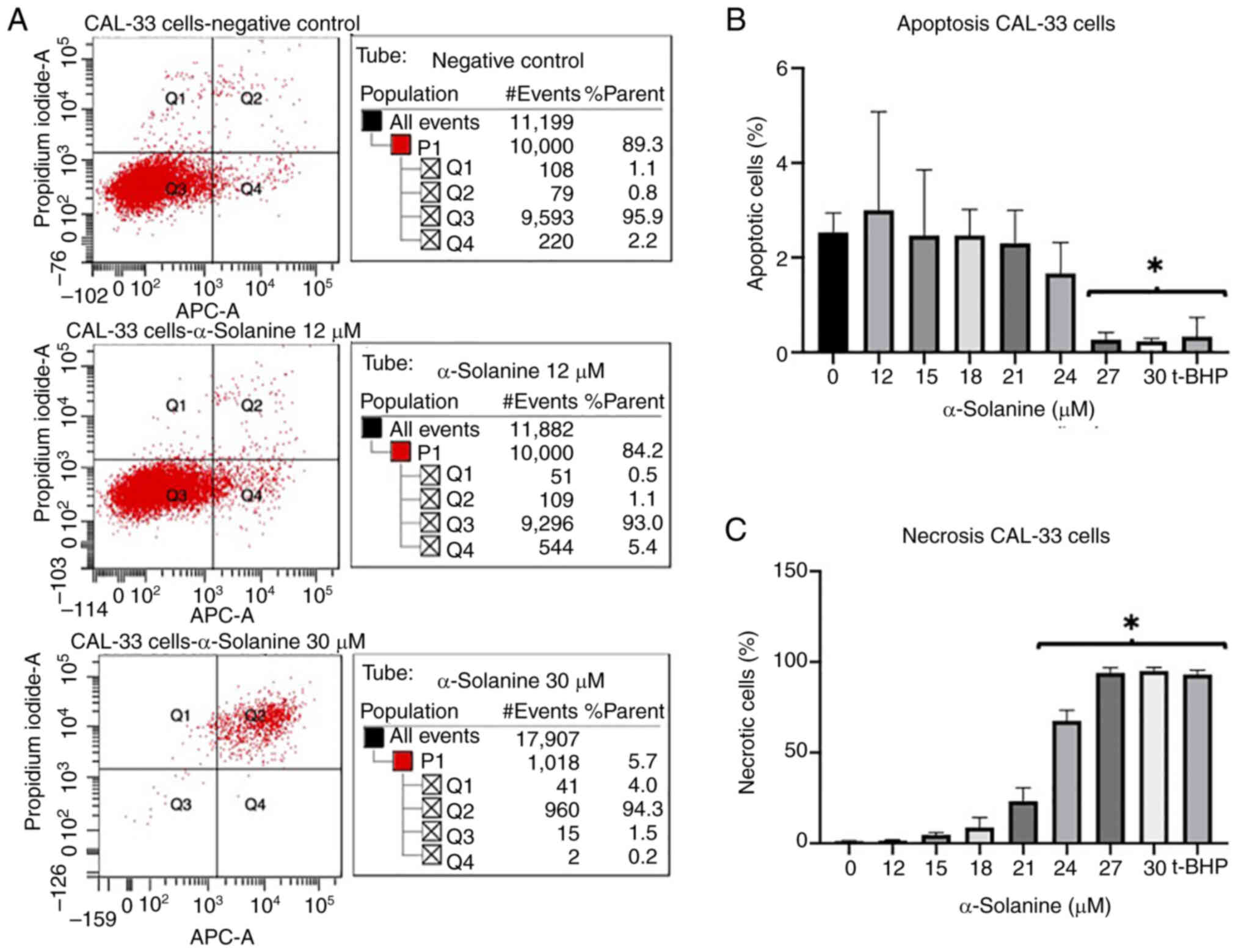
Annexin V-propidium iodide FACS
At an α-solanine concentration of 12 µM and above,
FaDu and CAL-33 showed a slight increase in the rate of apoptosis
and necrosis. At an α-solanine concentration of 15 µM, the
apoptosis rate of cells did not change while the number of necrotic
cells increased. At an α-solanine concentration of 30 µM and above,
only necrotic cells were detected (Figs. 3 and 4). 1 µM t-BHP as a positive control
induced necrosis in all cells. Control groups with untreated cells
showed viability of 91.8% for FaDu and 95.9% for CAL-33,
respectively. The flow cytometry plots for all groups quantified in
the diagrams are shown in Figs. S1
and S2.
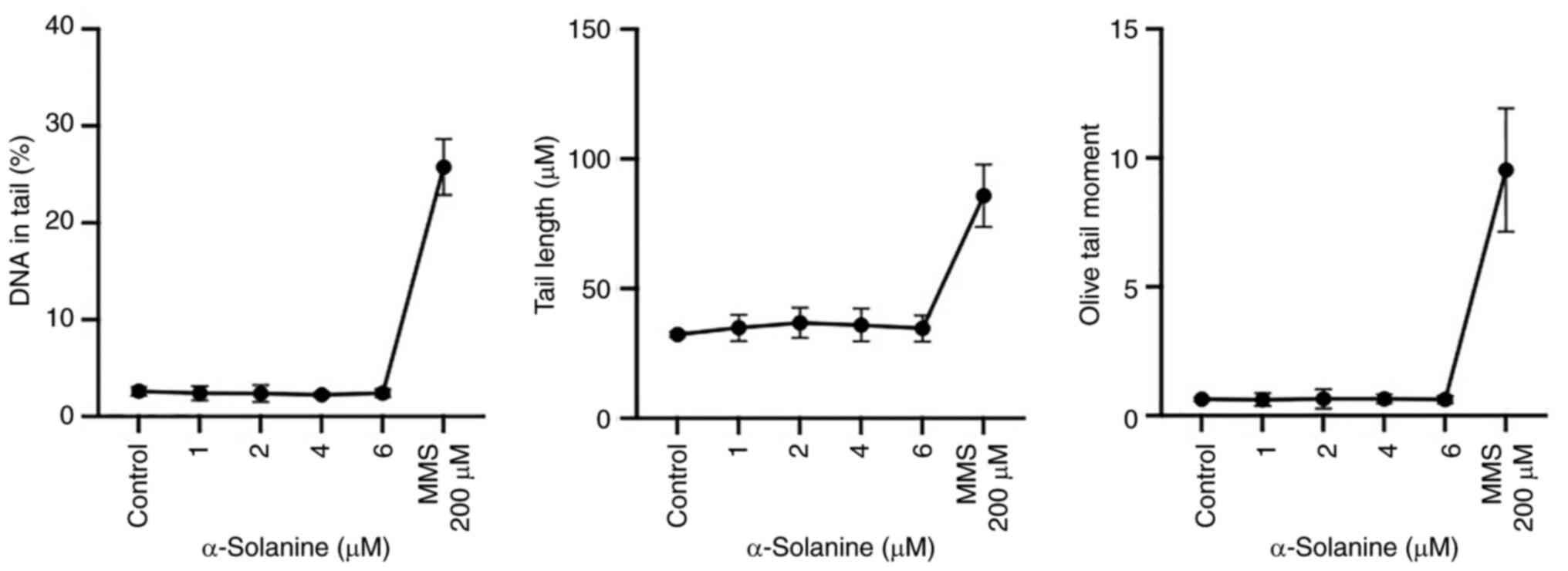
Genotoxicity
HUVECs were exposed to subcytotoxic α-solanine
concentrations of 1, 2, 4, and 6 µM for 24 h to evaluate DNA
damage.
200 µM mMS for 24 h served as a positive control for
genotoxicity. The negative control was the mean OTM of untreated
HUVECs. No DNA damage was detected at the doses used, whereas
significant damage was induced by mMS (positive control) (Figs. 5 and 6).
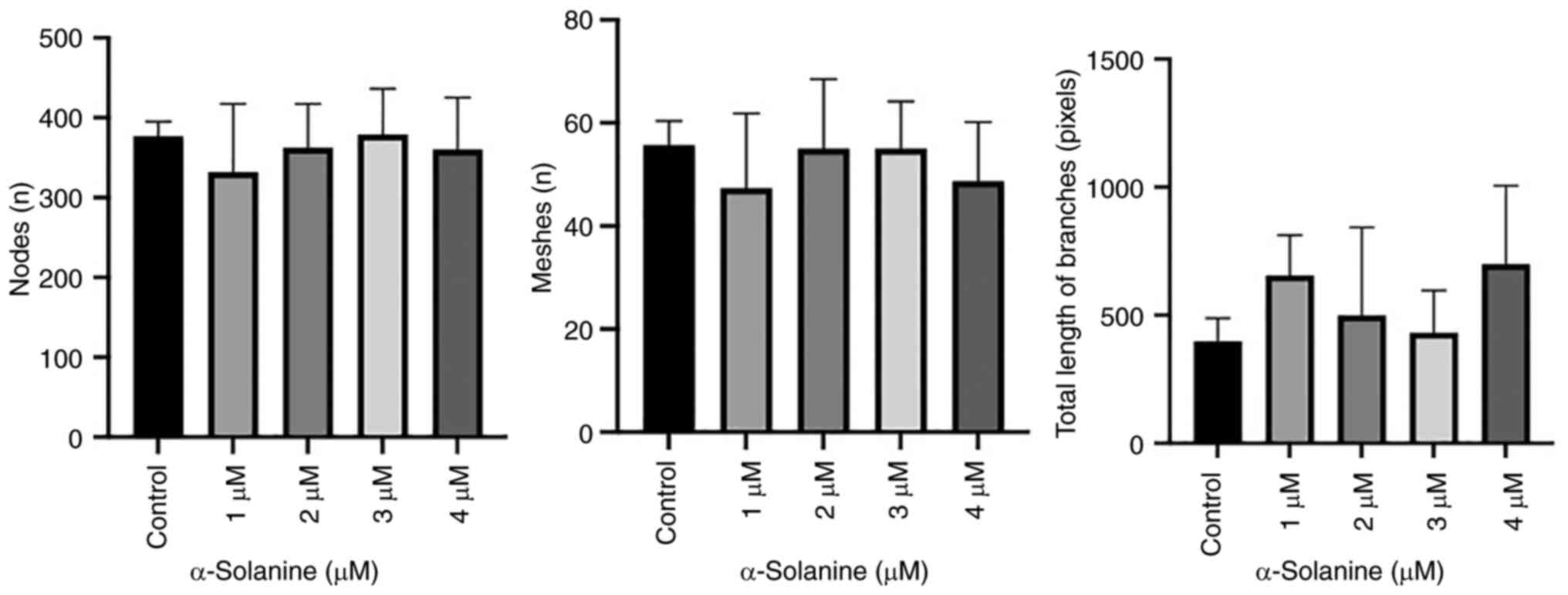
Functional impairment
At subcytotoxic doses, the capillary tube formation
assay on HUVECs indicated no significant decrease in the number of
meshes and nodes or the total length of the branches (Figs. 7 and 8). The images of all analyzed groups are
shown in Fig. S3.
Discussion
The aim of the current study was to evaluate the
tumortoxic effect of α-solanine on two HNSCC cell lines in
vitro. In addition, the toxicity of α-solanine as well as
possible effects on DNA integrity and cellular function with regard
to angiogenesis in non-malignant cells, in this case HUVECs, was
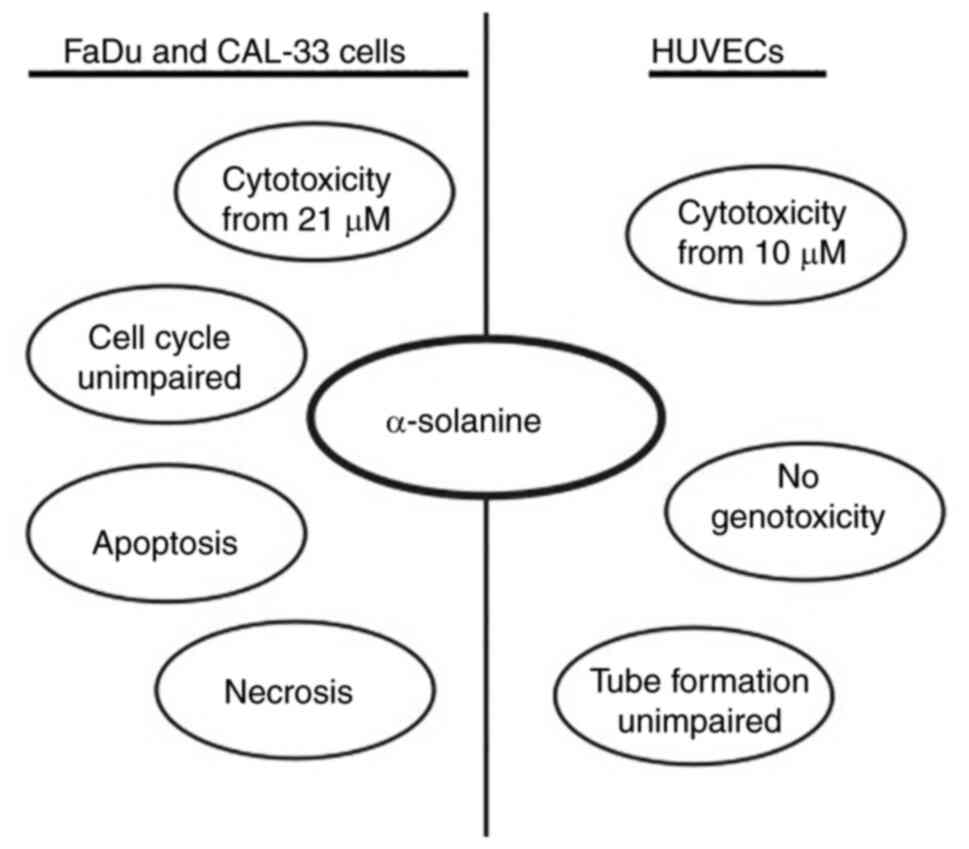
investigated (Summarized in Fig.
9).
Previous studies have so far yielded promising
results regarding a possible benefit of glycoalkaloids in the
therapy of human malignant tumors. This has brought a lot of
attention to this group of substances in recent years. In
particular, α-solanine has been shown to have a strong antitumor
effect and, being a globally available resource due to the wide
distribution of solanaceae plant family, has attracted a lot of
interest.
HNSCC is the sixth most common cancer in humans and
the most common head and neck tumor entity (11). Standard therapies include surgery
with or without adjuvant radiotherapy. Alternatively, irradiation
or chemoradiation are adequate primary treatment options.
Chemotherapy alone is administered only in the palliative setting.
The introduction of immune checkpoint inhibitors (ICI) has
significantly changed the treatment approach of HNSCC, swiftly
becoming the new standard of care as first line therapy in
individuals with recurrent and/or metastatic disease. For example,
overall survival of patients with metastatic or recurrent HNSCC was
prolonged by several months with the PD1 blocker pembrolizumab
compared to the control groups with conventional therapies
(17). Despite the tremendous
progress that the introduction of ICI has resulted in, the median
overall survival of many patients remains unsatisfactory and the
response rates are still low, even after specific patient selection
via PD-L1 expression in the tumor and the immune infiltrate
(18). In addition, there is a
significant cost to the health care system. Therefore, the
discovery of new approaches to drug tumor therapy for HNSCC is
essential. Approaches with the least possible side effects on
non-malignant cells are of greatest interest.
Glycoalkaloids such as α-solanine inhibit tumor cell
growth. However, to date, no study has addressed the effects of
α-solanine on HNSCC. The present study is the first investigation
of the effects of α-solanine on HNSCC cell lines in
vitro.
Toxicity analysis in tumor cell lines FaDu and CAL33
showed a dose-dependent decrease of cell viability after 24 h at
α-solanine concentrations of 21 and 21 µM, respectively. In
non-malignant HUVECs also treated for 24 h, relevant cytotoxicity
was already observed at a concentration of 10 µM α-solanine.
There are several studies on the tumor toxicity of
α-solanine in other types of cancer. In prostate carcinoma cells,
for example, a significant reduction in cell viability was
demonstrated from a concentration of 16 µM α-solanine for 24 and 48
h respectively (19). The toxicity
threshold in endometrial carcinoma cells after treatment for 24 h
was 30 µM, twice as high (20). In
another study, melanoma cells showed a significant and
dose-dependent decrease in cell viability starting at a
concentration of 23 µM α-solanine.
The selective effect of therapeutics on tumor cells
without affecting non-malignant cells is essential. One research
group investigated the toxicity of α-solanine on non-malignant
human keratinocytes and human fibroblasts. In this study an
α-solanine concentration above 23 µM after treatment for 24 h
revealed dose-dependent toxicity in both non-malignant cells
(21). However, the non-malignant
control cells selected here are known to be quite resilient and
grow well under a wide range of conditions. Ideally, a potential
new anticancer drug should also be tolerable for less resistant
cells that are exposed to the drug.
In HUVECs, α-solanine induced toxicity starting at a
concentration of 10 µM. This concentration is far below the
toxicity threshold of α-solanine on keratinocytes and fibroblasts.
Thus, the threshold for α-solanine toxicity could vary in a
cell-type-specific manner. This greatly complicates the application
of the compound in the clinical setting.
To determine whether cell death is caused by
apoptosis or necrosis, the Annexin V assay was performed in the
present work. Although administration of low doses of α-solanine
initially showed an increase in apoptosis and necrosis in tumor
cells, there was a marked dose-dependent increase in tumor cell
necrosis without an alteration in the rate of apoptosis. This
observation suggests that apoptosis-independent cell death
mechanisms may also be important in α-solanine's mode of action at
higher concentrations. However, this does not provide any
information as to whether the non-apoptotic effect was achieved,
for example, by necroptosis, autophagy or ferroptosis.
In previous studies on non-HNSCC cell lines, authors
have discussed various cell death mechanisms induced by α-solanine,
many of which depend on apoptosis.
In the liver carcinoma cell line HepG2, the
formation of reactive oxygen species (ROS), stimulation of
apoptosis-inducing proteins ASK1 (apoptosis signal-regulating
kinase 1) and TBP-2 (tetrahymena piggyBac transposase 2), and
inhibited expression of proliferation-associated proteins, such as
HDAC1, has been demonstrated (7).
Another study using HepG2 cells also supports the mode of action
through apoptosis-dependent cell death, as decreased Bcl-2
expression was demonstrated (22).
In the colon carcinoma cell line HT-29, a mechanism for apoptosis
induction by the α-solanine-like glycoalkaloid α-chaconine was
demonstrated. This was mainly induced by activation of the
pro-apoptotic caspase-3 pathway and inhibition of phosphorylation
of ERK1 and ERK2 (extracellular signal-regulated protein kinases 1
and 2) (23). The effect of ERK1
and ERK2 extends to different cell functions, such as cell cycle
progression, migration, survival, differentiation, metabolism,
proliferation and transcription (3). The pro-apoptotic effect via the
caspase-3 pathway was also demonstrated for α-solanine in the
SW1990 and Panc-1 pancreatic carcinoma cell lines (9). In the prostate cancer cell line DU145,
α-solanine showed apoptotic effects mediated by synergistic cyclin
suppression, induction of ROS and activation of P38 (24).
Apoptosis-independent modes of action for α-solanine
also were reported, for example through increased autophagy rate,
inhibition of angiogenesis, and regulation of the cell cycle. In
tumor cell lines of various origins, α-solanine induced autophagy
through endoplasmic reticulum stress and suppression of the
Akt/mTOR pathway, which plays a role in tumor cell proliferation
(25). A study by Lv et al
(26) suggested an effect of
α-solanine by suppressing proliferation, angiogenesis and
metastasis. Among other things, they showed that α-solanine
significantly reduced the expression of vascular endothelial growth
factor (VEGF) and that the tube formation of endothelial cells was
altered after treatment with α-solanine. The authors considered
this to have potential benefits in the treatment of pancreatic
cancer. In melanoma cell lines, α-solanine at subtoxic doses
suppressed cell migration and invasion by decreasing the activity
of mMP-2 and mMP-9 and causing suppression of phosphorylation of
JNK, PI3K, and Akt. In contrast to other studies, the authors found
no effects on the phosphorylation of ERK (22).
In the present study, no significant changes in cell
cycle phases were observed after 24 h exposure of FaDu to
α-solanine doses ranging from 12 to 21 µM. In the literature, a
connection between the apoptotic effect of α-solanine and other
glycoalkaloids, e.g., with MAP kinase P38 or ERK1/ERK2 acting on
the cell cycle, has been observed (24,25).
Cell cycle observations of HepG2 cells after treatment with
α-solanine showed that cells in the G(2)/M phases disappeared, while cells in the
S phase increased (23).
Various effects of α-solanine in humans have been
described in literature, ranging from toxicity to teratogenicity.
After evaluating the cytotoxicity threshold in non-malignant cells,
we found no effect of α-solanine on functionality with regard to
angiogenesis and proliferation in HUVECs, nor were there any
genotoxic effects. Unfortunately, the toxicity threshold was higher
in both malignant cell lines than in benign cells. One way to
address this problem is to use a co-drug to prevent damage to the
human cells without compromising tumor toxicity. For example,
previous studies have shown that increased intake of folic acids is
an effective method of preventing NTDs, but not cytotoxicity in
general (3). However, this fact
cannot be transferred to the in vivo situation. Further
possibilities would be to evaluate the optimization of exposure
times or recovery times and also the use of lower doses of
α-solanine as a co-drug with other potent drugs. Here, further
studies are needed.
In summary, the lack of functional effects and
genotoxicity on non-malignant cells in the subtoxic regime and the
toxic effects of α-solanine on tumor cells are promising. However,
the exact mechanisms of action of α-solanine require further
investigation. Application as a tumor therapy is currently not
probable due to the high toxicity in non-malignant cells. A
reduction or prevention of toxicity in non-malignant cells without
a simultaneous weakening of the tumor-toxic effects might be a
long-term goal. A large number of non-malignant cells are available
for safety testing. The usage of primary human cells, which are
potentially exposed to the drug during treatment of HNSCC, would be
relevant especially.
Supplementary Material
Supporting Data
Acknowledgements
The authors would like to thank Mr. Michael Kessler
(Research Assistant, Department of Otorhinolaryngology, Plastic,
Aesthetic and Reconstructive Head and Neck Surgery, University
Hospital Wuerzburg, Wuerzburg, Germany) for their technical
assistance.
Funding
Funding: Not applicable.
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
AVF and AS designed the study. AVF performed the
experiments. AVF, AS and TG confirm the authenticity of all raw
data. AVF and TEK analysed and interpreted the data. AVF wrote the
manuscript. SH, CW, TM, TG and RH contributed to data collection
and interpretation, and critically reviewed the manuscript. All
authors read and approved the final version of the manuscript.
Ethics approval and consent to
participate
The Medical Ethics Committee at the
Julius-Maximilians-University (Würzburg, Germany) had no objections
regarding the use of anonymous commercial cell lines in this
project and confirmed that the requirement for ethics approval can
be waived for the study.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Friedman M: Potato glycoalkaloids and
metabolites: Roles in the plant and in the diet. J Agric Food Chem.
54:8655–8681. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Friedman M: Chemistry and anticarcinogenic
mechanisms of glycoalkaloids produced by eggplants, potatoes, and
tomatoes. J Agric Food Chem. 63:3323–3337. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ordóñez Vásquez A, Aguirre-Arzola V,
Garza-Ramos M, Urrutia-Baca V and Suárez-Obando F: Toxicity,
teratogenicity and anti-cancer activity of α-solanine: A
perspective on anti-cancer potential. Int J Pharmacol. 15:301–310.
2019. View Article : Google Scholar
|
|
4
|
Allen JR, Marlar RJ, Chesney CF, Helgeson
JP, Kelman A, Weckel G, Traisman E and White JW Jr: Teratogenicity
studies on late blighted potatoes in nonhuman primates (Macaca
mulatta and Saguinus labiatus). Teratology. 15:17–23. 1977.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hassan SH, Gul S, Zahra HS, Maryam A,
Shakir HA, Khan M and Irfan M: Alpha solanine: A novel natural
bioactive molecule with anticancer effects in multiple human
malignancies. Nutr Cancer. 73:1541–1552. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Luo S, Tian GJ, Yu FX and Wen ZD: A
narrative review of the antitumor studies of solanine. Transl
Cancer Res. 10:1578–1582. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Meng XQ, Zhang W, Zhang F, Yin SY, Xie HY,
Zhou L and Zheng SS: Solanine-induced reactive oxygen species
inhibit the growth of human hepatocellular carcinoma HepG2 cells.
Oncol Lett. 11:2145–2151. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Yan X, Li M, Chen L, Peng X, Que ZJ, An
HM, Shen KP and Hu B: α-Solanine inhibits growth and metastatic
potential of human colorectal cancer cells. Oncol Rep.
43:1387–1396. 2020.PubMed/NCBI
|
|
9
|
Sun H, Lv C, Yang L, Wang Y, Zhang Q, Yu
S, Kong H, Wang M, Xie J, Zhang C and Zhou M: Solanine induces
mitochondria-mediated apoptosis in human pancreatic cancer cells.
Biomed Res Int. 2014:8059262014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
El-Daly SM, Gouhar SA, Gamal-Eldeen AM,
Abdel Hamid FF, Ashour MN and Hassan NS: Synergistic effect of
α-solanine and cisplatin induces apoptosis and enhances cell cycle
arrest in human hepatocellular carcinoma cells. Anticancer Agents
Med Chem. 19:2197–2210. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Johnson DE, Burtness B, Leemans CR, Lui
VWY, Bauman JE and Grandis JR: Head and neck squamous cell
carcinoma. Nat Rev Dis Primers. 6:922020. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Rangan SR: A new human cell line (FaDu)
from a hypopharyngeal carcinoma. Cancer. 29:117–121. 1972.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Scherzed A, Hackenberg S, Froelich K, Rak
K, Schendzielorz P, Gehrke T, Hagen R and Kleinsasser N: The
differentiation of hMSCs counteracts their migration capability and
pro-angiogenic effects in vitro. Oncol Rep. 35:219–226.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Mosmann T: Rapid colorimetric assay for
cellular growth and survival: Application to proliferation and
cytotoxicity assays. J Immunol Methods. 65:55–63. 1983. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Scherzad A, Hackenberg S, Schramm C,
Froelich K, Ginzkey C, Hagen R and Kleinsasser N: Geno- and
cytotoxicity of salinomycin in human nasal mucosa and peripheral
blood lymphocytes. Toxicol In Vitro. 29:813–818. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
DeCicco-Skinner KL, Henry GH, Cataisson C,
Tabib T, Gwilliam JC, Watson NJ, Bullwinkle EM, Falkenburg L,
O'Neill RC, Morin A and Wiest JS: Endothelial cell tube formation
assay for the in vitro study of angiogenesis. J Vis Exp.
e513122014.PubMed/NCBI
|
|
17
|
Burtness B, Harrington KJ, Greil R,
Soulières D, Tahara M, de Castro G Jr, Psyrri A, Basté N, Neupane
P, Bratland Å, et al: Pembrolizumab alone or with chemotherapy
versus cetuximab with chemotherapy for recurrent or metastatic
squamous cell carcinoma of the head and neck (KEYNOTE-048): A
randomised, open-label, phase 3 study. Lancet. 394:1915–1928. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Szturz P and Vermorken JB: Translating
KEYNOTE-048 into practice recommendations for head and neck cancer.
Ann Transl Med. 8:9752020. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Shen KH, Liao AC, Hung JH, Lee WJ, Hu KC,
Lin PT, Liao RF and Chen PS: α-Solanine inhibits invasion of human
prostate cancer cell by suppressing epithelial-mesenchymal
transition and mMPs expression. Molecules. 19:11896–11914. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Karaboga Arslan AK and Yerer MB:
α-Chaconine and α-Solanine inhibit RL95-2 endometrium cancer cell
proliferation by reducing expression of Akt (Ser473) and ERα
(Ser167). Nutrients. 10:6722018. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Lu MK, Shih YW, Chang Chien TT, Fang LH,
Huang HC and Chen PS: α-Solanine inhibits human melanoma cell
migration and invasion by reducing matrix metalloproteinase-2/9
activities. Biol Pharm Bull. 33:1685–1691. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ji YB, Gao SY, Ji CF and Zou X: Induction
of apoptosis in HepG2 cells by solanine and Bcl-2 protein. J
Ethnopharmacol. 115:194–202. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Yang SA, Paek SH, Kozukue N, Lee KR and
Kim JA: Alpha-chaconine, a potato glycoalkaloid, induces apoptosis
of HT-29 human colon cancer cells through caspase-3 activation and
inhibition of ERK 1/2 phosphorylation. Food Chem Toxicol.
44:839–846. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Pan B, Zhong W, Deng Z, Lai C, Chu J, Jiao
G, Liu J and Zhou Q: Inhibition of prostate cancer growth by
solanine requires the suppression of cell cycle proteins and the
activation of ROS/P38 signaling pathway. Cancer Med. 5:3214–3222.
2016. View
Article : Google Scholar : PubMed/NCBI
|
|
25
|
Hasanain M, Bhattacharjee A, Pandey P,
Ashraf R, Singh N, Sharma S, Vishwakarma AL, Datta D, Mitra K and
Sarkar J: α-Solanine induces ROS-mediated autophagy through
activation of endoplasmic reticulum stress and inhibition of
Akt/mTOR pathway. Cell Death Dis. 6:e18602015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Lv C, Kong H, Dong G, Liu L, Tong K, Sun
H, Chen B, Zhang C and Zhou M: Antitumor efficacy of α-solanine
against pancreatic cancer in vitro and in vivo. PLoS One.
9:e878682014. View Article : Google Scholar : PubMed/NCBI
|