Introduction
Gastric granular cell tumors (GCTs) are exceedingly
rare. GCTs in general represent an uncommon subset of soft-tissue
neoplasms. The origin of the tissue remains controversial, although
an accumulating number of studies have suggested that it may have
originated via Schwann cell differentiation (1–3). In a
previous retrospective analysis of 410,000 surgical specimens
collected over a 32-year period from the National Naval Medical and
Dental Centers (Bethesda, USA) and the Georgetown University
Hospital (Washington, USA) Lack et al (4) reported that the overall incidence of
GCTs was 0.03%. GCTs can occur in various parts of the body,
although they most commonly arise in the skin and subcutaneous
tissues of the head, neck, trunk, limbs and vulva. Gastrointestinal
involvement accounts for only 4–6% of all GCTs (5). However, this involvement primarily
affects the esophagus and colorectum, with gastric occurrences
being particularly scarce. Mobarki et al (6) previously reported 42 GCTs cases,
including resections and biopsies identified in electronic medical
records of the Pathology Department in the University Hospital of
Saint Etienne (Saint Etienne, France) over a 21-year period. Only 8
cases (7 esophageal and 1 right colonic) in the gastrointestinal
tract were found. In another study, An et al (7) reported 98 cases of GCTs in the
gastrointestinal tract, comprising 73 esophageal (75%), 21
colorectal (21%) and 4 gastric (4%) cases.
The detection of gastric GCTs has been facilitated
by the use of endoscopy and endoscopic ultrasonography (EUS). In
endoscopy, GCTs are typically sessile, small in size,
yellowish-white and covered by a normal mucous membrane (8). The histology of GCTs is characterized
by abundant eosinophilic granules in the cytoplasm and
immunohistochemistry staining yielding positivity for S-100, CD68
and transcription factor SOX-10 (SOX-10) (9). Similar to the majority of
subepithelial lesions (SELs), GCTs are difficult to obtain
sufficient quantities of tumor tissues from using traditional
gastroscopy biopsy techniques, as the tumor surface is covered with
normal mucosa, making disease diagnosis difficult (10). EUS can be used to adequately observe
the characteristics of SELs, including location, size, echo and
boundary, facilitating the diagnosis and treatment of such
submucosal lesions (11). The
majority of gastric GCTs are benign and have favorable prognosis,
but occasionally they may exhibit aggressive characteristics, such
as local recurrence or distant metastasis (12,13).
The treatment method of gastric GCTs remains unclear, with
possibilities including endoscopic resection and traditional
surgical resection. Due to its malignant potential, a review of
such gastric GCT cases is necessary (14).
The present study documents the case of a
52-year-old man with a gastric body GCT that was completely excised
by endoscopic submucosal dissection (ESD) and showed
immunohistochemical positivity for S100, with CD34 expression
surrounding the gastric GCT cells. The patient recovered well
postoperatively. The aim of the present case was to contribute to
the diagnostic and therapeutic understanding of gastric GCTs.
Case report
In May 2023, a 52-year-old man visited the
Gastroenterology Clinic of the People's Hospital of Putuo District
(Zhoushan, China) due to upper abdominal fullness over the last
week. No significant abdominal pain, nausea, vomiting, diarrhea,
constipation, hematemesis, melena, or cessation of flatus or stool
passage was reported. Gastroscopy indicated a mucosal elevation in
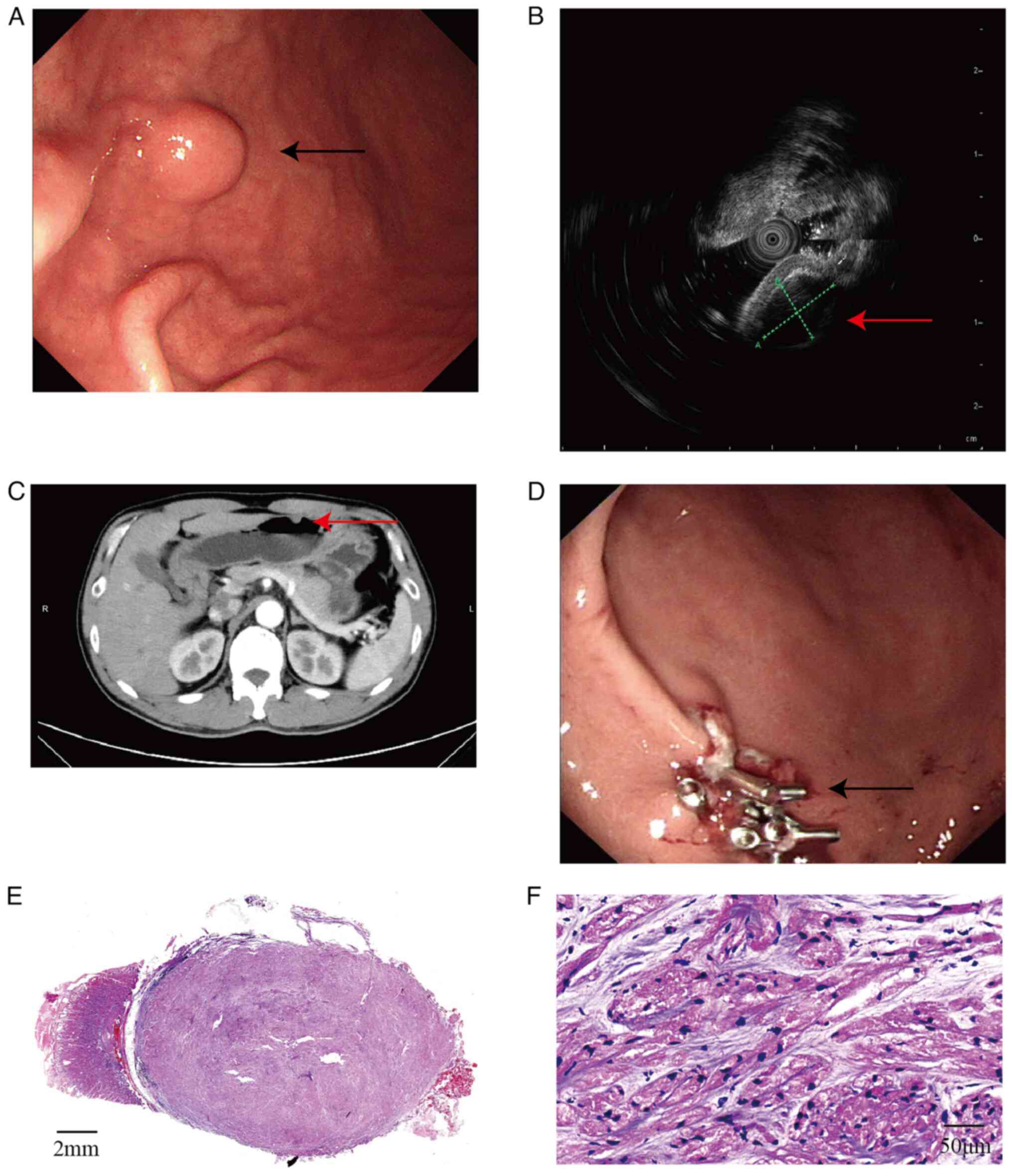
the anterior wall of the lower part of the gastric body (Fig. 1A). A 20-MHz EUS probe (model no.
IM-02M-01; InnerMedical, Co., Ltd.; Table I) revealed a hypoechoic mass in the
third layer (submucosal layer), measuring 10.34×7.06 mm, with
uniform internal echoes and distinct boundaries. The remaining
layers displayed clear and intact echogenicity (Fig. 1B). Under gastroscopy, the surface
mucosa appeared normal, without features of low-grade dysplasia,
high-grade dysplasia or early carcinoma, such as red discoloration
of the mucosal surface, depressed lesions or mucosal ulcers. The
lesion was preliminarily diagnosed as a submucosal tumor, such as a
leiomyoma, gastrointestinal stromal tumor (GIST), lipoma,
schwannoma or neuroendocrine tumor. Abdominal computed tomography
(Aquilion 16; Canon Medical Systems Corporation; Table I) showed a localized nodular
elevation on the greater curvature of the stomach body that was ~8
mm in diameter, with mild continuous enhancement post-contrast
(Fig. 1C). No enlarged lymph nodes
were observed around the stomach. Laboratory investigations
revealed that this patient presented with positive fecal occult
blood. Other tests, including complete blood count, coagulation
profile, renal function and tumor markers (such as chromogranin A,
carcinoembryonic antigen and carbohydrate antigen 19–9), were all
found to be within the normal limits. There was also no history of
esophageal cancer, gastric cancer, intestinal cancer or
gastrointestinal granulosa cell tumors in family members.
 | Table I.Imaging parameters of computed
tomography and US. |
Table I.
Imaging parameters of computed
tomography and US.
| Imaging method | Machine model | Supplier | Imaging
parameters |
|---|
| Computed
tomography | Aquilion 16 | Canon medical
Systems Corporation | Fastest scan time,
0.5 sec/360°; scan layers, 16; scan thickness, 4 mm; image
reconstruction speed, 10 frames/sec; image matrix, 512×512; maximum
continuous scanning time of a single spiral, 100 sec |
| US | IM-02M-01 | InnerMedical, Co.,
Ltd. | Scan angle, 360°;
frequency-scanning, 20 Hz; gain, 1–16; contrast, 1–5; grayscale
mapping, 1–16; monitor resolution, 1,920×1,080 |
The patient was therefore admitted to the People's
Hospital of Putuo District in June 2023 for further diagnosis and
treatment, with a provisional diagnosis of a gastric
space-occupying lesion. Subsequently, 2 days later, ESD was
performed. After verifying patient information and successful
intubation under general anesthesia, a polypoid elevation was
observed in the stomach body. The surface mucosa was slightly
congested. After circumferential marking, indigo carmine and saline
were injected submucosally for elevation. The lesion was well
lifted and the surrounding mucosa was incised using a Dual knife
(KD-650L; Olympus Corp.). Submucosal dissection was progressively
performed using Dual and insulation-tipped diathermic knife
(KD-611L; Olympus Corp.). The lesion was completely excised using a
snare. The operative site showed no perforation. Visible vessels on
the wound surface were coagulated with hemostatic forceps, before
the local surface was closed using titanium clips (Fig. 1D). Post-operation, to prevent
complications and promote wound healing, the patient was
administered 1.5 g cefuroxime sodium twice daily for 2 days as a
preventive measure against infection. Additionally, a single dose
of 80 mg carbazochrome sodium sulfonate was administered on the day
of surgery to facilitate hemostasis, and 60 mg omeprazole was
prescribed twice daily for two days to suppress gastric acid
production. The patient recovered well and was discharged 3 days
after surgery. The last time the patient visited the
Gastroenterology Outpatient Clinic for a follow-up was 1 month
later in July 2023, and there was no abdominal pain or distention.
A follow-up visit was recommended 3–6 months after discharge;
however, the patient has not yet scheduled this appointment. During
the telephone follow-up conversation after 6 and 10 months of the
operation, the patient reported that the symptoms had been
relieved.
In terms of the pathology, the tumor was located in
the submucosal layer and was nodular, with a gray-yellow cut
surface and clear boundaries. Microscopically, tumor cells were
arranged in bundles, nests and sheets, where the stroma was
predominantly clear, with a loose matrix containing basophilic
fibrous material and minimal blood vessel proliferation. The tumor
cells were oval or polygonal with abundant eosinophilic granular
cytoplasm. The nuclei were small, hyperchromatic, oval, short
spindle-shaped and occasionally angular, with inconspicuous
nucleoli (Fig. 1E and F).
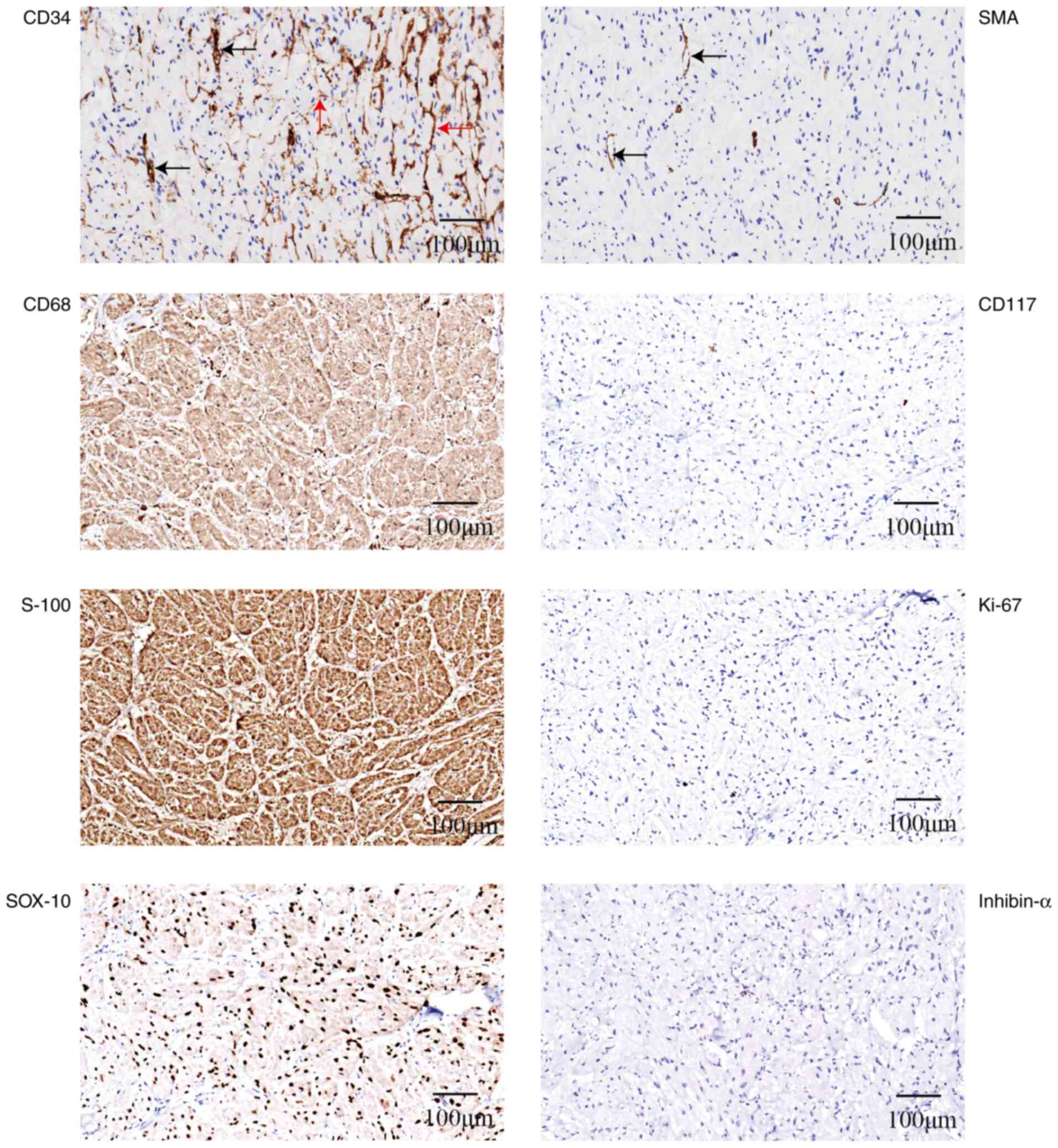
Immunohistochemistry revealed the following staining results:
CD117(−), Ki-67 (low expression, <1%), smooth muscle actin
(SMA)(−), S-100(+), SOX-10(+), CD68(+) and inhibin-α(−). CD34
staining was negative in the tumor cells but positive in the cells
surrounding the tumor cell nests (Fig.
2). Tumor cells arranged in sheets and nests contained a large
number of eosinophilic granules in the cytoplasm and expressed
S-100 and CD68. These features closely resembled the GCTs commonly
found in the skin, distinguishing them from neuroendocrine
neoplasms. The negative expression of SMA and CD117 effectively
ruled out the possibility of a gastric leiomyoma or GIST.
Furthermore, the cells lacked characteristics such as a spindle
shape, nuclear pleomorphism, prominent nucleoli, a high
nuclear-to-cytoplasmic ratio, necrosis and significant mitoses. As
the characteristics aligned with the internationally recommended
Fanburg-Smith criteria (15) for
diagnosing benign and malignant GCTs, the final diagnosis was of a
benign gastric GCT. In the follow-ups until May 2024, the patient's
symptoms have improved; however, due to work commitments, a visit
to the hospital for a CT scan and gastroscopy review is temporarily
not possible.
Hematoxylin and eosin staining
(H&E), and immunohistochemistry
For H&E staining, the tissues were first fixed
in a 10% formaldehyde solution for a period of 24 h at room
temperature. After that, they were subjected to a dehydration
process and subsequently embedded in paraffin. Next, they were cut
into 4-µm thick sections. These sections underwent a dewaxing
procedure utilizing xylene and ethanol before being thoroughly
rinsed with water. The prepared sections were then sequentially
stained with hematoxylin for 8 min and eosin dyes for 1 min at room
temperature. Subsequently, the slices were observed under a light
microscope.
S100 (cat. no. CSM-0101), CD34 (cat. no. CCM-0550),
CD68 (cat. no. CCM-0701), SOX-10 (cat. no. CSR-0180), SMA (cat. no.
CAM-0191), Ki-67 (cat. no. CKM-0032) and α-inhibin (cat. no.
CIM-0151) antibodies were purchased from Celnotve Biotechnology,
Co., Ltd. CD117 (cat. no. YR145) was purchased from MXB
Biotechnology; Beijing Strong Biotechnologies. Paraffin sections of
tissues were cut into 4-µm thick sections and mounted onto slides.
After deparaffinization, rehydration and blocking endogenous
peroxidase activity with 3% hydrogen peroxide for 10 min at room
temperature, the slides were incubated with the appropriate
aforementioned primary antibody (1:100) for 12 h at 4°C in a
humidified chamber. Subsequently, the slides were incubated with
the secondary antibody (cat. no. SD3003; Celnotve Biotechnology,
Co., Ltd.) for 30 min at room temperature, and then incubated with
the DAB chromogenic solution (cat. no. SD3004; Celnotve
Biotechnology, Co., Ltd.) for 5 min. Finally, counterstaining was
achieved through a 3-min incubation with hematoxylin at room
temperature. After the staining was completed, a light microscope
was used to observe the slides.
Literature review
For the literature review, the key words ‘gastric
granulosa cell tumor’, ‘gastrointestinal granulosa cell tumor’,
‘digestive tract granulosa cell tumor’ and ‘granular cell tumor’
were searched for in PubMed data (https://pubmed.ncbi.nlm.nih.gov/) recorded between
1970 and 2024, and the English full-text study was obtained.
Inclusion criteria were as follows: Case reports of gastric GCTs.
Exclusion criteria were as follows: Articles for which full text
could not be obtained. The literature was then reviewed, and the
clinical and pathological characteristics of 42 gastric GCT cases
were summarized, including country, sex, age, symptoms, location,
size, immunohistochemistry results, treatment methods, follow-up
information and outcome (Table II)
(16–48).
 | Table II.Literature review results of 42 cases
of gastric GCT. |
Table II.
Literature review results of 42 cases
of gastric GCT.
| Authors, year |
Country/ethnicity | Age, years | Sex | Maximum diameter,
mm | Site | Others | Treatment | Follow-up time,
months | Outcome | (Refs.) |
|---|
| Naidech et
al, 1971 | US/black | 32 | F | 40 | Muscular layer | - | Partial
gastrectomy | - | - | (16) |
| Naidech et
al, 1971 | US/black | 31 | F | 10 | Submucosa | - | Excision of the
tumor | - | - | (16) |
| Goodman and Cooper,
1972 | US/black | 32 | F | 15 | Submucosa | Appendicitis | Gastric wedge
resection | - | - | (17) |
| Miranda, 1976 | US/black | 51 | M | 15 | Submucosa and
muscular layer | Esophageal squamous
cell carcinoma |
Esophagogastrectomy | - | - | (18) |
| Miranda, 1976 | US/black | 51 | M | 8 | Submucosa | - | Autopsy | - | - | (18) |
| Ross, 1977 | US/white | 32 | M | 20 | Submucosa and
muscular layer | - | Gastrotomy | 8 | No recurrence | (19) |
| Chen, 1981 | US | 76 | F | - | - | - | Partial
gastrectomy | 8 | No recurrence | (20) |
| Abdelwahab and
Klein, 1983 | US/black | 39 | F | 20 | Submucosa | Chronic
pancreatitis | Gastric wedge
resection | - | - | (21) |
| Seo et al,
1984 | US/black | 53 | F | i) 18; ii) 10 | i) Submucosa and
deeply into the serosa; ii) Submucosa | Ovarian fibroma and
vulva GCTs | Gastric wedge
resection | - | - | (22) |
| Seo et al,
1984 | US/black | 35 | F | 10 | Submucosa | - | Billroth I
operation | - | - | (22) |
| Shah and Mazza,
1986 | US/white | 62 | M | 10 | - | - | Endoscopic
resection | - | - | (23) |
| Yamaguchi et
al, 1989 | Japan | 64 | M | 15 | Submucosa | Adenocarcinoma | Partial
gastrectomy | 84 | Asymptomatic | (24) |
| Joshi et al,
1992 | US/black | 52 | F | 35 | Submucosa, extend
to the serosa | Esophageal, colon
GCTs and esophageal squamous cell carcinoma | Partial
gastrectomy | 24 | Died of esophageal.
cancer | (25) |
| Biikel et
al, 1994 | Israel | 52 | - | 10 | Submucosa | Inflammatory
fibroid polyp | Gastrostomy | - | - | (26) |
| White et al,
1994 | US/black | 43 | M | 25 | Submucosa | - | Partial
gastrectomy | - | - | (27) |
| Yasuda et
al, 1995 | Japan | 52 | F | 8 | Submucosa | - | Endoscopic
resection | - | - | (28) |
| Matsumoto et
al, 1996 | Japan | 64 | F | 70 | Submucosa | - | Partial
gastrectomy | 27 | Recurred after 21
months | (13) |
| David et al,
1999 | US/black | 45 | F | 15 | Submucosa | Esophageal
GCTs |
Esophagogastrectomy | 24 | Asymptomatic | (29) |
| Eguchi et
al, 2002 | Japan | 64 | M | - | - | Gastric lymphoma
and adenocarcinoma | Total
gastrectomy | 8 | Asymptomatic | (30) |
| Maekawa et
al, 2003 | Japan | 53 | M | 15,7,5 | Submucosa | Esophageal
GCTs | Total
gastrectomy | 14 | Asymptomatic | (31) |
| Mitomi et
al, 2004 | Japan | 50 | F | 19,19 | Submucosa | Esophageal and
colon GCTs | Local
resection | 36 | No recurrence | (32) |
| Patti et al,
2006 | Italy | 49 | F | 20 | Muscular layer | - | Gastric wedge
resection | 24 | No recurrence | (33) |
| Lowe et al,
2007 | US/white | 54 | M | 30 | - | - | Gastric wedge
resection | - | No recurrence | (34) |
| John et al,
2009 | US/black | 30 | F | - | Submucosa and
muscular layer | Esophageal
GCTs | Transhiatal
esophago gastrectomy | - | - | (35) |
| Rekha and Srinivas,
2010 | Indian | 30 | M | 20 | - | - | Unknown | - | - | (36) |
| Pertile et
al, 2010 | Italy | 45 | M | 20 | - | - | Gastric wedge
resection | - | - | (37) |
| Monahan et
al, 2009 | Britain | 49 | M | 8 | Submucosa | - | Endoscopic
resection | - | - | (38) |
| Gilg et al,
2012 | France | 61 | F | 20 | - | - | Gastric wedge
resection | - | - | (39) |
| Krishnan, 2013 | Nigerian | 54 | F | 12 | Submucosa | - | Endoscopic
resection | 12 | Asymptomatic | (40) |
| Min et al,
2013 | Korea | 49 | M | 10 | Submucosa | - | Endoscopic
resection | - | No recurrence | (41) |
| Lipkin-Moore et
al, 2014 | US | 55 | M | 10 | Submucosa | Esophageal and
colon GCTs | Gastric wedge
resection | 24 | No recurrence | (42) |
| Takaya et
al, 2017 | Japan | 16 | M | 15 | Submucosa | - | Endoscopic
resection | 12 | No recurrence | (43) |
| Hnach et al,
2017 | Morocco | 20 | - | 25 | - | - | Endoscopic
resection | - | - | (44) |
| Jain et al,
2018 | Indian | 28 | F | 7 | Submucosa | - | Gastric wedge
resection | 6 | Asymptomatic | (45) |
| Watanabe et
al, 2018 | Japan | 71 | M | 30,18 | Submucosa | Adenocarcinoma and
cecum GCTs | Total
gastrectomy | 20 | No recurrence | (46) |
| Santos et
al, 2019 | Portugal | 52 | F | 24 | Submucosa, muscular
layer and subserosa | - | Atypical gastric
resection | 18 | No recurrence | (3) |
| Yasuda et
al, 2020 | Japan | 46 | F | 15 | Submucosa and
muscular layer | Hepatic
hemangioma | Local
resection | 16 | No recurrence | (5) |
| Kawai et al,
2021 | Japan | 61 | M | 5 | - | - | Follow-up | 6 | No recurrence | (47) |
| Taban et al,
2021 | Romania | 52 | F | 6 | Submucosa | - | Endoscopic
resection | 5 | No recurrence | (9) |
| Nitta et al,
2021 | Japan | 75 | F | 5 | Submucosa | Adenocarcinoma | Partial
gastrectomy | 24 | No recurrence | (14) |
| Kent and Saulino,
2024 | Japan | 35 | F | 8,6,4 | Submucosa and
muscular layer | - | Gastric sleeve
operation | - | - | (48) |
| Present study | China | 52 | M | 10 | Submucosa | - | Endoscopic
resection | 10 | No recurrence | - |
Discussion
The histopathophysiology of GCTs remains unclear.
Initially identified in 1926 by Abrikossoff (49) in five cases of tongue tumors, the
tumor cells were found to be strikingly similar to skeletal muscle
cells, rendering them being described as ‘granular cell
myoblastomas’ at the time. In subsequent studies, the presence of
S100 positivity, coupled with the complex granular and lysosomal
ultrastructure observed under electron microscopy, suggested a
potential differentiation towards Schwann cells (1,2).
However, previous genetic studies on GCT cells did not demonstrate
monosomy or deletions in the long arm of chromosome 22, a
characteristic differing from that of schwannomas (15). Additionally, reports of
S100-negative granular cell tumors have emerged (50–52).
Therefore, the histopathophysiology of GCTs remains to be fully
elucidated, necessitating further research. In the present gastric
GCT case, CD34 expression was observed, but not in the tumor cells
themselves; immunohistochemistry showed CD34-positive cells or
matrix surrounding the tumor cells or nests, with SMA highlighting
only a small vascular component within the tumor tissue. Previous
studies have confirmed the specificity of glucose transporter
protein 1 and epithelial membrane antigen for perineurial cells,
whereas CD34 appeared to be immunoreactive to endoneurial
fibroblasts (53). Díaz-Flores
et al (54) previously
observed CD34-positive interstitial cells around S100-positive
Schwann cells. The present findings corroborate this observation,
potentially suggesting a Schwann cell origin for GCTs. However,
whether the CD34-positive cells are reactive or are part of the
tumor, akin to the bidirectional differentiation that is commonly
observed in breast fibroadenomas and salivary gland tumors,
warrants further investigation.
From the present literature review, it was found
that GCTs are mostly solitary, but can also present as multiple
tumors in the same body (22,31,32,46,48) or
involve multiple organs (25,29,31,32,35,42,46).
Gastric GCTs typically involve the esophageal or colorectal
regions. The site of gastric GCT occurrence shows no predilection
and can occur in the cardia, body and antrum. The age of onset
varies widely, with the mean age of patients with GCTs being 48
years (median, 50 years; range, 16–76 years). There is also no
significant sex predisposition (18 males and 22 females). Among the
42 cases of gastric GCT recorded, there were 12 African and 12
Japanese individuals, which may suggest a higher prevalence of GCTs
among these individuals. According to Patti et al (33), gastric GCTs are typically diagnosed
in individuals aged between 40 and 60 years, with no specific sex
predisposition. However, do seem to be ethnic considerations, since
the majority of instances were observed in the Japanese population.
Gastric GCTs lack a specifically defined set of clinical symptoms
and laboratory findings. Patients with gastric GCTs will typically
present with abdominal fullness, discomfort, abdominal pain or
indigestion. Gastric GCTs are frequently discovered incidentally
during imaging, gastroscopy or other surgeries. In the present
case, the patient was a 52-year-old man who presented with
abdominal fullness, and whose nodule was subsequently discovered
during endoscopy. When ulcerated, the tumors may present with
gastrointestinal bleeding. In addition, gastric GCTs can coexist
with other diseases, such as gastric adenocarcinoma, esophageal
cancer and gastric ulcers, manifesting symptoms of the concurrent
diseases.
Endoscopically, gastric GCTs are typically covered
with yellow, white or normal mucosa, presenting as polypoid or
hemispherical protrusions into the stomach, and are mostly sessile.
Macroscopically, they appear as well-demarcated, firm submucosal
nodules, occasionally involving the muscularis propria or serosa. A
number of patients do present with multiple nodules, with a total
of 48 gastric GCTs studied in 42 patients. The location of 39
nodules was clearly defined, 27 of which were submucosal and 12 of
which involved the serosa or muscle layer. The typical size is
small, with 79% being <2 cm in size and the largest reaching 7
cm (13). The application of
magnifying endoscopy or chromoendoscopy in SELs may be limited due
to the normal mucosal covering (55). However, EUS is effective in
characterizing the features of SELs (such as location, size, echo
pattern and boundaries), aiding in narrowing the differential
diagnoses and assessing the feasibility of endoscopic resection
(11). Gastric GCTs on EUS
typically present as uniform, hypoechoic lesions originating from
the second or third layer (mucosal or submucosal) and occasionally
from the fourth layer (if involving the muscularis propria).
According to a retrospective study on SELs by Kida et al
(56), besides GCTs, neuroendocrine
tumors, lipomas and GISTs can also originate from the third layer.
Apart from lipomas, which typically show hyperechoic features,
GISTs, neuroendocrine tumors, leiomyomas and schwannomas can also
present as hypoechoic. Abdominal computed tomography can be used to
image well-demarcated intramural nodules with insignificant or mild
enhancement, in addition to evaluating the overall condition of
surrounding organs and lymph nodes. The EUS examination of the
present case revealed a distinct, hypoechoic nodule positioned in
the third layer, indicating the feasibility of resection via
ESD.
However, distinguishing gastric GCTs from other
submucosal tumors is challenging using imaging techniques alone. A
definitive diagnosis typically requires pathological confirmation.
Unlike epithelial tumors, due to the coverage of a normal mucosa,
traditional endoscopic forceps biopsies, even with multiple
attempts at the same site, will frequently fail to provide an
accurate diagnosis of a gastric GCT. A EUS-guided fine-needle
aspiration biopsy can yield satisfactory tumor tissue samples for
diagnosis (10). In the present
case, the gastric GCT was located in the submucosa, with normal
mucosa on the surface, similar to most gastric GCTs.
Histologically, gastric GCTs are comprised of large
polygonal or oval cells laden with abundant eosinophilic granules.
The tumor margins can be well defined or infiltrative. Pareja et
al (57) previously found that
loss-of-function mutations in the ATPase H+ transporting
accessory protein 1 (ATP6AP)1 and ATP6AP2 genes may
be the driving factors of GCT through whole-exome sequencing and
targeted sequencing of GCT. This finding suggested that
intracellular vesicles found in GCT are acidic cytoplasmic granules
that have accumulated due to vesicle acidification impairments.
Immunohistochemically, apart from being consistently S100-positive
(100%) and exhibiting mild Ki-67 positivity (<10%; 100%), they
can also express CD68, SOX10, CD56, Nestin and inhibin (7,9). The
tumors are typically negative for CD34, CD117, SMA and human
melanoma black 45. Na et al (58) previously studied the
immunohistochemical expression profile of 30 cases of colorectal
GCT, before finding that the tumors were positive for S-100 (100%),
CD68 (100%), neuron-specific enalase (100%), Nestin (100%), SOX-10
(100%), Ki-67 <1% (100%), CD56 (93%), synaptophysin (93%),
calretinin (53%), CD163 (23%), CD57 (21%), p53 (32%) and inhibin-α
(17%). In another previous study, An et al (7) found that the gastric GCTs were
positive for S-100 (100%), CD56 (100%), SOX10 (100%), CD68 (67%)
and inhibin-α (33%). However, in the present case, S-100, CD68 and
SOX10 staining was positive, whereas inhibin-α staining was
negative. Inhibin, a polypeptide hormone, is secreted by the
granulosa cells of the ovary and the Sertoli cells of the testis
(59). Inhibin negatively regulates
the synthesis of follicle-stimulating hormone and the secretion of
anterior pituitary, regulating gonad function (60), and its expression is closely
associated with the differentiation of sex cells and steroid cells
(61). While numerous studies have
documented the presence of positive expression of inhibin-α in GCTs
located in various parts of the body (62–64),
other two studies revealed that only 52 or 17% of GCTs,
respectively, exhibited positive inhibin-α expression (7,58).
Currently, the precise role of inhibin-α expression in cell
differentiation and the pathogenesis of GCTs remains enigmatic.
Taban et al (9) also
previously noted CD34 expression within the tumor, which appeared
to be more pronounced at the periphery. Special periodic
acid-Schiff staining can be used to reveal cytoplasmic granules
(43). Gastric GCTs, due to their
rich cytoplasm and granules, can be misdiagnosed as perivascular
epithelioid cell tumors, which express both muscular and
melanocytic markers (65). In
addition, gastric GCTs typically present as non-neoplastic lesions,
such as histiocytic aggregates. Diagnostic differentiation is also
required from S100-expressing schwannomas, which exhibit distinct
Antoni A and B areas, and are frequently accompanied by a
lymphocyte cuff that is lacking abundant cytoplasmic granules
(66). The most common submucosal
tumors in the stomach, GISTs, are predominantly located in the
muscularis layer and express CD117, GIST1 and CD34 (67). However, the pattern of CD34
expression surrounding tumor cell nests in GCTs distinguishes them
from the expression CD34 characteristics observed in GISTs.
Although the majority of gastric GCTs (98%) are
benign, there have been reports of malignant gastric GCTs (13). A previously reported 64-year-old
female patient with a gastric tumor exhibiting S100-positive
eosinophilic granular cells, atypical giant nuclei and mitotic
figures was found to relapse 2 years later. Although an elevated
Ki-67 index, necrosis and/or mitotic activity are frequently
associated with malignant behavior, metastasis remains the only
definitive sign of malignancy. Fanburg-Smith et al (15) previously studied 73 GCT cases and
proposed the following six diagnostic criteria for differentiating
benign GCTs from malignant GCTs: i) Necrosis; ii) spindle cell
morphology; iii) nuclear pleomorphism; iv) prominent nucleoli; v)
high nuclear-cytoplasmic ratio; and vi) increased mitotic activity
(>2 mitoses per 10 high-power fields at ×200 magnification).
Tumors meeting three or more of the aforementioned criteria are
classified to be histologically malignant GCTs, whilst those
meeting only one or two of the criteria would be considered
atypical GCTs. However, there have been reports of histologically
benign GCTs with metastases (2).
Machado et al (68)
suggested abandoning the distinction between benign and atypical
GCTs, since they rarely metastasize and those that do, typically
occur as a result of local recurrence due to incomplete resection.
Instead, GCTs with various unfavorable histological features should
be labeled as ‘GCTs with increased risk of metastasis’, rather than
‘malignant GCTs’. The tumor cells in the present case had pycnotic
nuclei, inconspicuous nucleoli, a low nucleus-to-cytoplasm ratio,
no nuclear division, no pleomorphism or fusiform cells, no necrosis
and Ki-67 <1%. Abdominal computed tomography showed no abnormal
lymph nodes around the stomach. The patient's symptoms have been
significantly relieved, as assessed at 10 months post-surgery.
These characteristics are in line with the diagnosis of a benign
GCT.
A consensus on the treatment of gastric GCTs has not
yet been fully established. In the present literature review, it
was found that surgical excision was performed in 30 patients,
including partial gastrectomy, wedge resection and local excision.
In total, 3 patients underwent a total gastrectomy due to suspected
lymph node metastasis, multiple gastric GCTs or concurrent
adenocarcinoma. Endoscopic resections were performed in 9 patients,
where, except for the malignant GCT that recurred after 2 years,
there were no recurrences or metastases during the follow-up
period. According to the 2022 European Society of Gastrointestinal
Endoscopy (ESGE) guidelines (69),
asymptomatic gastric GCTs with a clear diagnosis should not be
monitored. If the diagnosis is unclear, endoscopic monitoring
should be performed at 3–6 months, then every 2–3 years for lesions
<10 mm in diameter and every 1–2 years for lesions 10–20 mm in
diameter. For lesions >20 mm in diameter, ESGE recommends
monitoring with endoscopy and EUS at 6 months, then every 6–12
months thereafter. However, challenges remain in diagnosing and
monitoring compliance, since <2% of cases show potentially
malignant biological behavior in gastric GCTs (70). Ryu et al (71) previously conducted a retrospective
analysis of 35 cases of esophageal GCT that underwent endoscopic
resection, which revealed that diagnostic endoscopic resection of
submucosal tumors not only aids in a clear diagnosis, but can also
serve certain therapeutic effects. Additionally, it was noted that
various methods of endoscopic resections conferred no significant
difference on therapeutic outcomes. Endoscopic mucosal resection
(EMR) was originally introduced clinically for gastric lesions in
1984 by Tada et al (72) as
a ‘strip biopsy’, which evolved into ESD, a variant of EMR
(73). The majority of the 42 cases
of gastric GCT in the present literature review were benign, where
77% were ≤2 cm in size. There was no recurrence in the 9 cases of
endoscopic resection. Kahng et al (74) previously examined 25 patients with
gastrointestinal GCT, totaling 27 gastrointestinal GCT tumors.
Specifically, 20 were diagnosed in the esophagus, 5 in the stomach
and 2 in the colon. All GCTs were resected endoscopically, with a
median size of 10 mm. The mean follow-up period was 15 months,
during which there were no recurrences. Endoscopic resection was
therefore considered a safe and effective treatment for this
condition. Another study by Yasuda et al (5) reviewed 12 cases of 34 gastric GCTs
that underwent endoscopic resection. In total, 75.6% of tumors
showed a diameter of ≤2 cm and no local recurrence was observed. It
was therefore concluded that endoscopic resection is a viable
treatment option. Of the 42 patients included in the present
review, 9 underwent an endoscopic resection. Except for 1 patient
whose tumor size was 2.5 cm, the other 8 patients all had tumors ≤2
cm in diameter. In addition, with the exception of 2 cases in which
the location of the tumor was not disclosed, the tumors in the
other 7 patients were located in the submucosa and did not invade
the muscle layer. There was no recurrence after surgery. Therefore,
it could be suggested that endoscopic resection is a feasible
treatment option for gastric GCTs ≤2 cm, provided they do not
involve the muscularis propria or have normal submucosal lifting
during surgery. Otherwise, a combination of tumor removal and
partial gastric wedge resection may be considered, including ≥1 cm
of the normal tissue (33). Due to
the rarity of the malignant gastric GCT cases, a complete tumor
resection with clear margins is necessary, although evidence for
lymph node dissection remains inconclusive.
In conclusion, GCTs are rare. The presence of
CD34-positive interstitial cells surrounding S100-positive tumor
cells in GCTs indicate that these cells are part of the tumor,
providing evidence for the Schwann cell origin of gastric GCTs. The
combination of EUS and endoscopic needle biopsy can enhance the
diagnostic accuracy for gastric GCTs. The majority of gastric GCTs
are benign and <2 cm in size, making endoscopic resection (such
as EMR and ESD) a viable treatment option.
Acknowledgements
Not applicable.
Funding
The present case was supported by a grant from the Project of
the Science and Technology Bureau of Putuo District of Zhoushan
City (grant no. 2021GY304).
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
HXL diagnosed this case and managed the article
design. MZ completed the immunohistochemistry. HXL and MZ collected
data and obtained medical images. HZ analyzed the data and wrote
the paper. YYZ analyzed the data. HXL and HZ reviewed, verified and
confirm the authenticity of all the raw data. All authors have read
and approved the manuscript.
Ethics approval and consent to
participate
Ethics approval (approval no. 2024002KYLW) was
obtained from the Ethics Committee of People's Hospital of Putuo
District (Zhoushan, China) and an informed consent statement for
participation was obtained from the patient.
Patient consent for publication
Written informed consent for publication was
obtained from the patient.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Stefansson K and Wollmann RL: S-100
protein in granular cell tumors (granular cell myoblastomas).
Cancer. 49:1834–1838. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ordonez NG and Mackay B: Granular cell
tumor: A review of the pathology and histogenesis. Ultrastruct
Pathol. 23:207–222. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Santos C, Araújo AV, Contente H and Branco
C: Gastric granular cell tumour, a rare entity. BMJ Case Rep.
12:e2275102019. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lack EE, Worsham RGF, Callihan MD,
Crawford BE, Klappenbach S, Rowden G and Chun B: Granular cell
tumor: A clinicopathologic study of 110 patients. J Surg Oncol.
13:301–316. 2006. View Article : Google Scholar
|
|
5
|
Yasuda A, Yasuda T, Imamoto H, Hiraki Y,
Momose K, Kato H, Iwama M, Shiraishi O, Shinkai M, Imano M and
Kimura Y: A case of a gastric granular cell tumor preoperatively
diagnosed and successfully treated by single-incision laparoscopic
surgery. Surg Case Rep. 6:442020. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Mobarki M, Dumollard JM, Col PD, Camy F,
Peoc'h M and Karpathiou G: Granular cell tumor a study of 42 cases
and systemic review of the literature. Pathol Res Pract.
216:1528652020. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
An S, Jang J, Min K, Kim MS, Park H, Park
YS, Kim J, Lee JH, Song HJ, Kim KJ, et al: Granular cell tumor of
the gastrointestinal tract: Histologic and immunohistochemical
analysis of 98 cases. Hum Pathol. 46:813–819. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Radaelli F and Minoli G: Granular cell
tumors of the gastrointestinal tract: Questions and answers.
Gastroenterol Hepatol (N Y). 5:798–800. 2009.PubMed/NCBI
|
|
9
|
Taban SM, Barna RA, Dema AL, Ratiu IM,
Popa O and Plopeanu AD: Unexpected diagnosis for a gastric polyp:
Granular cell tumor: Case report and review of the literature. Exp
Ther Med. 21:5362021. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Sekine M, Asano T and Mashima H: The
diagnosis of small gastrointestinal subepithelial lesions by
endoscopic ultrasound-guided fine needle aspiration and biopsy.
Diagnostics (Basel). 12:8102022. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Gong EJ and Kim DH: Endoscopic
ultrasonography in the diagnosis of gastric subepithelial lesions.
Clin Endosc. 49:425–433. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Nasser H, Ahmed Y, Szpunar SM and Kowalski
PJ: Malignant granular cell tumor: A look into the diagnostic
criteria. Pathol Res Pract. 207:164–168. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Matsumoto H, Kojima Y, Inoue T, Takegawa
S, Tsuda H, Kobayashi A and Watanabe K: A malignant granular cell
tumor of the stomach: Report of a case. Surg Today. 26:119–122.
1996. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Nitta T, Ohta M, Kataoka J, Ishii M, Ueda
Y, Senpuku S, Takeshita A and Ishibashi T: Granular cell tumor
coexisting with adenocarcinoma in the stomach: Report of a rare
case. Ann Med Surg (Lond). 65:1022712021.PubMed/NCBI
|
|
15
|
Fanburg-Smith JC, Meis-Kindblom JM, Fante
R and Kindblom LG: Malignant granular cell tumor of soft tissue:
Diagnostic criteria and clinicopathologic correlation. Am J Surg
Pathol. 22:779–794. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Naidech HJ, Axelrod RS and Seliger G:
Granular cell tumor (myoblastoma) of the stomach. Am J Roentgenol
Radium Ther Nucl Med. 113:245–247. 1971. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Goodman MD and Cooper PH: Granular cell
tumor (myoblastoma) of the stomach. A case report with
ultrastructural findings and review of the literature. Am J Dig
Dis. 17:1117–1126. 1972. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Miranda D: Benign granular cell tumor
(‘myoblastoma’) of the stomach. Am J Gastroenterol. 65:344–348.
1976.PubMed/NCBI
|
|
19
|
Ross JS: Massive upper gastrointestinal
hemorrhage from a granular cell tumor of the stomach. Am J
Gastroenterol. 68:595–598. 1977.PubMed/NCBI
|
|
20
|
Chen TK: Multifocal benign granular cell
tumor of the stomach. J Clin Gastroenterol. 3:65–67. 1981.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Abdelwahab IF and Klein MJ: Granular cell
tumor of the stomach: A case report and review of the literature.
Am J Gastroenterol. 78:71–76. 1983.PubMed/NCBI
|
|
22
|
Seo IS, Azzarelli B, Warner TF, Goheen MP
and Senteney GE: Multiple visceral and cutaneous granular cell
tumors ultrastructural and lmmunocytochemical evidence of schwann
cell origin. Cancer. 53:2104–2110. 1984. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Shah AN and Mazza BM: Endoscopic removal
of a granular cell tumor of the stomach. Gastrointest Endosc.
32:541986. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Yamaguchi K, Maeda S and Kitamura K:
Granular cell tumor of the stomach coincident with two early
gastric carcinomas. Am J Gastroenterol. 84:656–659. 1989.PubMed/NCBI
|
|
25
|
Joshi A, Chandrasoma P and Kiyabu M:
Multiple granular cell tumors of the gastrointestinal tract with
subsequent development of esophageal squamous carcinoma. Dig Dis
Sci. 37:1612–1618. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Bickel A, Szvalb S, Eitan A and Cohen I:
The coexistence of inflammatory fibroid polyp and cranular cell
tumor in the same gastric lesion. Am J Gastroenterol. 89:2090–2091.
1994.PubMed/NCBI
|
|
27
|
White JG, eI-Newihi HM and Hauser CJ:
Granular cell tumor of the stomach presenting as gastric outlet
obstruction. Am J Gastroenterol. 89:2259–2260. 1994.PubMed/NCBI
|
|
28
|
Yasuda I, Tomita E, Nagura K, Nishigaki Y,
Yamada O and Kachi H: Endoscopic removal of granular cell tumors.
Gastrointest Endosc. 41:163–167. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
David O and Jakate S: Multifocal granular
cell tumor of the esophagus and proximal stomach with infiltrative
pattern: A case report and review of the literature. Arch Pathol
Lab Med. 123:967–973. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Eguchi S, Matsuo S, Hidaka M, Azuma T,
Yamaguchi S, Hayashi T, Eguchi S and Kanematsu T: Concomitant
triple lesions of adenocarcinoma, malignant lymphoma, and granular
cell tumor of the stomach. J Clin Gastroenterol. 35:107–109. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Maekawa H, Maekawa T, Yabuki K, Sato K,
Tamazaki Y, Kudo K, Wada R and Matsumoto M: Multiple
esophagogastric granular cell tumors. J Gastroenterol. 38:776–780.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Mitomi H, Matsumoto Y, Mori A, Arai N,
Ishii K, Tanabe S, Kobayashi K, Sada M and Mieno H: Multifocal
granular cell tumors of the gastrointestinal tract:
Immunohistochemical findings compared with those of solitary
tumors. Pathol Int. 54:47–51. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Patti R, Almasio PL and Vita GD: Granular
cell tumor of stomach: A case report and review of literature.
World J Gastroenterol. 12:3442–3445. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Lowe DL, Chaudhary AJ, Lee JR, Chamberlain
SM, Schade RR and Cuartas-Hoyos U: Four cases of patients with
gastrointestinal granular cell tumors. South Med J. 100:298–300.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
John BK, Dang NC, Hussain SA, Yang GC,
Cham MD, Yantiss R, Joseph AS, Giashuddin SM, Lee PC, Fleming R and
Somnay K: Multifocal granular cell tumor presenting as an
esophageal stricture. J Gastrointest Cancer. 39:107–113. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Rekha K and Srinivas CN: Granular cell
tumor of gastric mucosa. Indian J Pathol Microbiol. 53:578–579.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Pertile D, Scabini S, Romairone E,
Scordamaglia R, Rimini E and Ferrando V: Gastric Abrikosoff tumor
(granular cell tumor): Case report. G Chir. 31:433–434.
2010.PubMed/NCBI
|
|
38
|
Monahan KJ, Pelling M, Goldin R and Hoare
J: Endoscopic removal of a granular cell tumor from the stomach
using the Duette Multiband Mucosectomy kit. Dig Dis Sci.
55:2688–2690. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Gilg MM, Mrak K, Vieth M and Langner C:
Granular cell tumor of the stomach. Pathologe. 33:61–64. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Krishnan A, Ramakrishnan R and Menon M:
Endoscopic removal of granular cell tumors of stomach: Case report
and review of literature. Gastroenterology Res. 6:240–243.
2013.PubMed/NCBI
|
|
41
|
Min KW, Lee KG, Han H, Jang SM and Paik
SS: Gastric granular cell tumour clinically mimicking carcinoid
tumour treated by endoscopic submucosal dissection. ANZ J Surg.
84:985–986. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Lipkin-Moore Z, Thomas RM and Rothstein
RD: Multifocal synchronous granular cell tumors of the
gastrointestinal tract. ACG Case Rep J. 1:193–195. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Takaya H, Kawaratani H, Kaneko M, Takeda
S, Sawada Y, Kitade M, Moriya K, Namisaki T, Sawai M, Mitoro A, et
al: Gastric granular cell tumor in a youth excised by endoscopic
submucosal dissection: A case report and literature review. Acta
Gastroenterol Belg. 80:317–319. 2017.PubMed/NCBI
|
|
44
|
Hnach Y, Allaoui M and Oukabli M: Gastric
Abrikossoff tumor: about a new case. The Pan African Medical
Journal. 28:2202017.(In French). PubMed/NCBI
|
|
45
|
Jain A, Karegar M, Joshi A and Rojekar A:
Granular cell tumour in stomach: A case report. Indian J Surg
Oncol. 9:598–600. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Watanabe Y, Watanabe M, Suehara N,
Ishikawa N, Shinkawa T, Hosokawa T, Akiho H, Mine M, Tamiya S,
Nishihara K and Nakano T: Early gastric cancer with diffuse
heterotopic gastric glands and granular cell tumors mimicking
advanced gastric cancer. Int J Surg Case Rep. 46:41–46. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Kawai M, Goda N, Nikaido M and Fukuda A: A
case of gastric granular cell tumor. JGH Open. 5:966–967. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Kent T and Saulino D: Incidental
multifocal granular cell tumor in the setting of chronic gastritis
discovered following gastric sleeve operation: A case report and
brief review of the literature. J Surg Case Rep. 2024:rjad7002024.
View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Abrikossoff A: Concerning myomas starting
from the striated voluntary musculature. Virchows Arch Pathol Anat.
215–233. 1926.(In German). View Article : Google Scholar
|
|
50
|
Chen SY, Sadanand A, Dillon PA, He M,
Dehner LP and Leonard DS: Non-Neural (S-100 Negative) bronchial
granular cell tumor causing acute respiratory failure. Fetal
Pediatr Pathol. 39:85–89. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Schoedel KE, Bastacky S and Silverman A:
An S100 negative granular cell tumor with malignant potential:
Report of a case. J Am Acad Dermatol. 39:894–898. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Mejía H, Rubiano MFO, Osorio VLD and
González MI: S100 negative granular cell tumor of the oral cavity:
Dermoscopy and surgical approach. An Bras Dermatol. 94:79–81. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Hirose T, Tani T, Shimada T, Ishizawa K,
Shimada S and Sano T: Immunohistochemical demonstration of
EMA/Glut1-positive perineurial cells and CD34-positive fibroblastic
cells in peripheral nerve sheath tumors. Mod Pathol. 16:293–298.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Díaz-Flores L, Gutiérrez R, García MP,
Gayoso S, Gutiérrez E, Díaz-Flores L Jr and Carrasco JL: Telocytes
in the normal and pathological peripheral nervous system. Int J Mol
Sci. 21:43202020. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Goto O, Kaise M and Iwakiri K:
Advancements in the diagnosis of gastric subepithelial tumors. Gut
Liver. 16:321–330. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Kida M, Kawaguchi Y, Miyata E, Hasegawa R,
Kaneko T, Yamauchi H, Koizumi S, Okuwaki K, Miyazawa S, Iwai T, et
al: Endoscopic ultrasonography diagnosis of subepithelial lesions.
Dig Endosc. 29:431–443. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Pareja F, Brandes AH, Basili T, Selenica
P, Geyer FC, Fan D, Da Cruz Paula A, Kumar R, Brown DN,
Gularte-Mérida R, et al: Loss-of-function mutations in ATP6AP1 and
ATP6AP2 in granular cell tumors. Nat Commun. 9:35332018. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Na JI, Kim HJ, Jung JJ, Kim Y, Kim SS, Lee
JH, Lee KH and Park JT: Granular cell tumours of the colorectum:
Histopathological and immunohistochemical evaluation of 30 cases.
Histopathology. 65:764–774. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Murakata LA and Ishak KG: Expression of
inhibin-alpha by granular cell tumors of the gallbladder and
extrahepatic bile ducts. Am J Surg Pathol. 25:1200–1203. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Stenvers KL and Findlay JK: Inhibins: From
reproductive hormones to tumor suppressors. Trends Endocrinol
Metab. 21:174–180. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Weidemann S, Noori NA, Lennartz M,
Lennartz M, Reiswich V, Dum D, Menz A, Chirico V, Hube-Magg C,
Fraune C, et al: Inhibin alpha expression in human tumors: A tissue
microarray study on 12,212 tumors. Biomedicines. 10:25072022.
View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Ortiz-Hidalgo C and Frías-Soria CL:
Análisis histopatológico e inmunohistoquímico del tumor de células
granulares. Estudios de 12 casos con una breve nota histórica. Rev
Esp Patol. 52:11–19. 2019.(In Spanish). PubMed/NCBI
|
|
63
|
Le BH, Boyer PJ, Lewis JE and Kapadia SB:
Granular cell tumor: Immunohistochemical assessment of
inhibin-alpha, protein gene product 9.5, S100 protein, CD68, and
Ki-67 proliferative index with clinical correlation. Arch Pathol
Lab Med. 128:771–775. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Fine SW and Li M: Expression of calretinin
and the alpha-subunit of inhibin in granular cell tumors. Am J Clin
Pathol. 119:259–264. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Doyle LA, Hornick JL and Fletcher CDM:
PEComa of the gastrointestinal tract clinicopathologic study of 35
cases with evaluation of prognostic parameters. Am J Surg Pathol.
37:1769–1782. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Lauricella S, Valeri S, Mascianà G, Gallo
IF, Mazzotta E, Pagnoni C, Costanza S, Falcone L, Benvenuto D,
Caricato M and Capolupo GT: What about gastric Schwannoma? A review
article. J Gastrointest Cancer. 52:57–67. 2020. View Article : Google Scholar
|
|
67
|
Papke DJ and Hornick JL: Recent
developments in gastroesophageal mesenchymal tumours.
Histopathology. 78:171–186. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Machado I, Cruz J, Lavernia J and
Llombart-Bosch A: Solitary, multiple, benign, atypical, or
malignant: The ‘Granular Cell Tumor’ puzzle. Virchows Arch.
468:527–538. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Deprez PH, Moons LMG, O'Toole D, Gincul R,
Seicean A, Pimentel-Nunes P, Fernández-Esparrach G, Polkowski M,
Vieth M, Borbath I, et al: Endoscopic management of subepithelial
lesions including neuroendocrine neoplasms: European society of
gastrointestinal endoscopy (ESGE) guideline. Endoscopy. 54:412–429.
2022. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Barakata M, Kar AA, Pourshahid S, Ainechi
S, Lee HJ, Othman M and Tadros M: Gastrointestinal and biliary
granular cell tumor: Diagnosis and management. Ann Gastroenterol.
31:439–447. 2018.PubMed/NCBI
|
|
71
|
Ryu DG, Choi CW, Kim SJ, Hwang CS, Kang
DH, Kim HW, Park SB and Son BS: Clinical outcomes of esophageal
granular cell tumors with different endoscopic resection methods.
Sci Rep. 13:107382023. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Tada M, Karita M, Yanai H and Takemoto T:
Endoscopic therapy of early gastric cancer by strip biopsy. Gan To
Kagaku Ryoho. 15:1460–1465. 1988.(In Japanese). PubMed/NCBI
|
|
73
|
Maple JT, Dayyeh BK, Chauhan SS, Hwang JH,
Komanduri S, Manfredi M, Konda V, Murad FM, Siddiqui UD and
Banerjee S: Endoscopic submucosal dissection. Gastrointest Endosc.
81:1311–1325. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Kahng DH, Kim GH, Park DY, Jeon MS, Yi JW,
Choi YY and Song GA: Endoscopic resection of granular cell tumors
in the gastrointestinal tract: A single center experience. Surg
Endosc. 27:3228–3236. 2013. View Article : Google Scholar : PubMed/NCBI
|