Introduction
Lung cancer is the most common malignancy worldwide,
and non-small cell lung cancer (NSCLC) accounts for 85–90% of all
lung cancer cases. The histological subtypes of NSCLC are
adenocarcinoma, squamous cell carcinoma and large cell carcinoma
(1). It has been reported that ~50%
of patients with newly diagnosed NSCLC present with metastasis, and
the prognosis of these patients is poor, with a 5-year survival
rate of <5% (2,3).
For advanced NSCLC without driver gene alterations,
such as those in EGFR, KRAS, ALK, ROS1 and BRAF, platinum-based
doublet chemotherapy is considered to be the most common standard
first-line treatment approach (4).
In patients with advanced non-squamous carcinoma NSCLC, the
treatment can be combined with bevacizumab and maintained with
pemetrexed or bevacizumab following standard therapy (5–7). For
patients with driver gene alterations, targeted therapy is often
the first treatment approach, since the relevant agents can improve
overall survival (OS) and the overall response rate (ORR). However,
several patients cannot benefit from target therapy due to the
occurrence of therapeutic resistance (8).
With increasing awareness of tumor immune escape
mechanisms, immunotherapy has become an effective treatment
approach for patients with NSCLC (9). To avoid elimination by immune cells,
the programmed cell death protein-1 (PD-1)/programmed cell death 1
ligand 1 (D-L1) pathway serves a notable role in tumor cells. PD-L1
is expressed by tumor cells and binds to PD-1, which is expressed
by CD4+/CD8+ T cells, which suppresses T cell
proliferation and cytokine secretion. Therefore, the immune system
cannot recognize and target the cancer cells for clearance
(10,11). Over the last decade, immune
checkpoint inhibitors (ICIs) targeting PD-1/PD-L1 have been widely
used to treat cancer worldwide. ICI monotherapy or ICIs combined
with chemotherapy have become the novel standard treatment strategy
for advanced NSCLC without driver gene mutations (9). Previous clinical studies, such as
KEYNOTE-024, KEYNOTE-042, IMpower110, ORIENT-11 and OAK, have
demonstrated that ICIs could improve the prognosis of patients with
advanced NSCLC compared with chemotherapy (12–16).
Therefore, immunotherapy is considered to be an effective treatment
strategy for patients with advanced NSCLC.
Despite the promising results that have been
reported for aforementioned clinical trials on immunotherapy, it
should be noted that the participants in these trials, who are
selected based on strict criteria, are commonly in good physical
condition and of a younger median age compared with patients in
real-world clinical settings. Therefore, real-world studies in
clinical settings are required to assess the effectiveness and
safety of immunotherapy. In the present study, a retrospective
analysis of data from patients with advanced NSCLC who were treated
with PD-1/PD-L1 ICIs at Weifang People's Hospital (The First
Affiliated Hospital of Shandong Second Medical University; Weifang,
China) was performed to evaluate the efficacy and response of these
patients to ICIs in clinical practice. The results of the present
study were then compared with previous research data.
Materials and methods
Patient selection and data
collection
Patients who were treated with PD-1/PD-L1 inhibitors
at Weifang People's Hospital (The First Affiliated Hospital of
Shandong Second Medical University; Weifang, China) between January
1, 2019 and December 31, 2022 were retrospectively included in the
present study. The inclusion criteria were as follows: i) Patients
with an age of ≥18 years; ii) histologically diagnosed with
advanced-stage or recurrent NSCLC; iii) treated with PD-1/PD-L1
inhibitors combined with or without chemotherapy; iv) patients with
asymptomatic or stable brain metastasis were included; and v) those
with measurable disease based on the Response Evaluation Criteria
in Solid Tumors (RECIST; version 1.1) (17). Patients who received <2 cycles of
immunotherapy or those with other types of tumors were excluded
from the study. Clinical data, including age, sex, smoking status,
Eastern Cooperative Oncology Group (ECOG) score (18), histology, stage (19), EGFR mutation, PD-L1 status, previous
systemic therapy, radiotherapy status, brain/liver metastases upon
starting ICIs and PD-1/PD-L1 drugs, were collected from electronic
medical records. Due to tissue puncture or patient refusal, the
PD-L1 status was unknown in 22 cases.
Response evaluation and endpoint
Disease responses were assessed using RECIST
(version 1.1) and classified as complete response (CR), partial
response (PR), stable disease (SD) or progressive disease (PD). The
primary endpoint was progression-free survival (PFS) and OS. PFS
was defined as the time from the start of ICI therapy to
radiologically confirmed PD or death due to any cause. Furthermore,
OS was defined as the time from the start of ICI treatment to death
due to any cause. Secondary objectives included the ORR and disease
control rate (DCR). ORR was defined as the percentage of patients
who displayed confirmed CR or PR, while DCR was defined as the
percentage of patients with confirmed CR, PR or SD.
Statistical analysis
All statistical analyses were performed using SPSS
26.0 software (IBM Corp.) and GraphPad Prism 9.0 (Dotmatics). Data
are presented as the median (interquartile range). The survival
curves for PFS and OS were drawn using Kaplan-Meier estimates and
compared using log-rank tests. Significant prognostic factors were
identified using univariate and multivariate Cox regression
analyses. A two-sided P<0.05 was considered to indicate a
statistically significant difference.
Results
Patient characteristics
A total of 133 patients with advanced-stage NSCLC,
who received ICI therapy between January 1, 2019 and December 31,
2022, were included in the present study. The final follow-up date
was August 24, 2023. The baseline demographic and clinical
characteristics of patients are listed in Table I. The median age at the start of ICI
treatment was 63 years (range, 32–83 years). More than half of
patients (n=83; 62.41%) had an ECOG score of 0 and the remaining 50
patients (37.59%) had an ECOG score of ≥1. A total of 104 patients
(78.20%) were men and 91 patients (68.42%) were smokers. The
majority of patients (n=113; 84.96%) had stage IV cancer, with at
least one organ metastasis. The remaining 20 patients had stage III
cancer, with only lymph node metastasis. Among patients who
experienced organ metastasis, 38 (28.57%) and 26 (19.55%) patients
exhibited brain and liver metastasis, respectively. Furthermore, 90
patients (67.67%) were diagnosed with adenocarcinoma, while the
remaining 43 (32.33%) were diagnosed with squamous cell carcinoma.
Among the 109 patients who underwent EGFR testing, 23 patients
(17.29%) tested positive for EGFR mutations. All patients had
previously received at least one first-line chemotherapy and 51
patients (38.35%) had received ≥2 systemic therapy lines. A total
of 47 patients (35.34%) underwent radiotherapy. The PD-L1 status
was assessed as the percentage of tumor cell and immune cell areas
[tumor proportion score (TPS)] showing PD-L1 staining (20). A total of 111 patients (83.46%)
accepted PD-L1 testing in the current study. Among these patients,
13 (9.77%) had a TPS of <1%. In 46 patients (34.59%), the TPS
ranged between 1 and 49%, and 52 patients (39.10%) had a TPS ≥50%.
The majority of patients (n=114; 85.71%) received either
camrelizumab, sintilimab or tislelizumab, which were the three main
ICIs used at Weifang People's Hospital (The First Affiliated
Hospital of Shandong Second Medical University; Weifang,
China).
 | Table I.Patient characteristics (n=133). |
Table I.
Patient characteristics (n=133).
|
Characteristics | Value |
|---|
| Median age, years
(range) | 63 (32–83) |
| Sex, n (%) |
|
|
Male | 104 (78.20) |
|
Female | 29 (21.80) |
| Smoking status, n
(%) |
|
|
Smoker | 91 (68.42) |
|
Non-smoker | 42 (31.58) |
| ECOG PS score, n
(%) |
|
| 0 | 83 (62.41) |
| ≥1 | 50 (37.59) |
| Histology, n
(%) |
|
|
Adenocarcinoma | 90 (67.67) |
|
Squamous cell carcinoma | 43 (32.33) |
| Stage, n (%) |
|
|
III | 20 (15.04) |
| IV | 113 (84.96) |
| EGFR mutation, n
(%) |
|
|
Wild-type | 86 (64.66) |
|
Mutation | 23 (17.29) |
|
Unknown | 24 (18.05) |
| PD-L1 status, n
(%) |
|
|
<1% | 13 (9.77) |
|
1-49% | 46 (34.59) |
|
≥50% | 52 (39.10) |
|
Unknown | 22 (16.54) |
| Previous systemic
therapy, n (%) |
|
|
<2 | 82 (61.65) |
| ≥2 | 51 (38.35) |
| Radiotherapy
status, n (%) |
|
| No | 86 (64.66) |
|
Yes | 47 (35.34) |
| Brain metastasis, n
(%) |
|
| No | 95 (71.43) |
|
Yes | 38 (28.57) |
| Liver metastasis, n
(%) |
|
| No | 107 (80.45) |
|
Yes | 26 (19.55) |
| PD-1/PD-L1 drugs, n
(%) |
|
|
Camrelizumab | 41 (30.83) |
|
Sintilimab | 37 (27.82) |
|
Tislelizumab | 36 (27.07) |
|
Toripalimab | 8 (6.02) |
|
Pembrolizumab | 8 (6.02) |
|
Durvalumab | 2 (1.50) |
|
Nivolumab | 1 (0.75) |
Clinical efficacy
The median follow-up period was 14.1 months (range,
1.2–53.2 months). As shown in Table
II, 2, 54, 60 and 17 patients achieved CR, PR, SD and PD,
respectively, yielding an ORR of 42.1% and DCR of 87.2%. The median
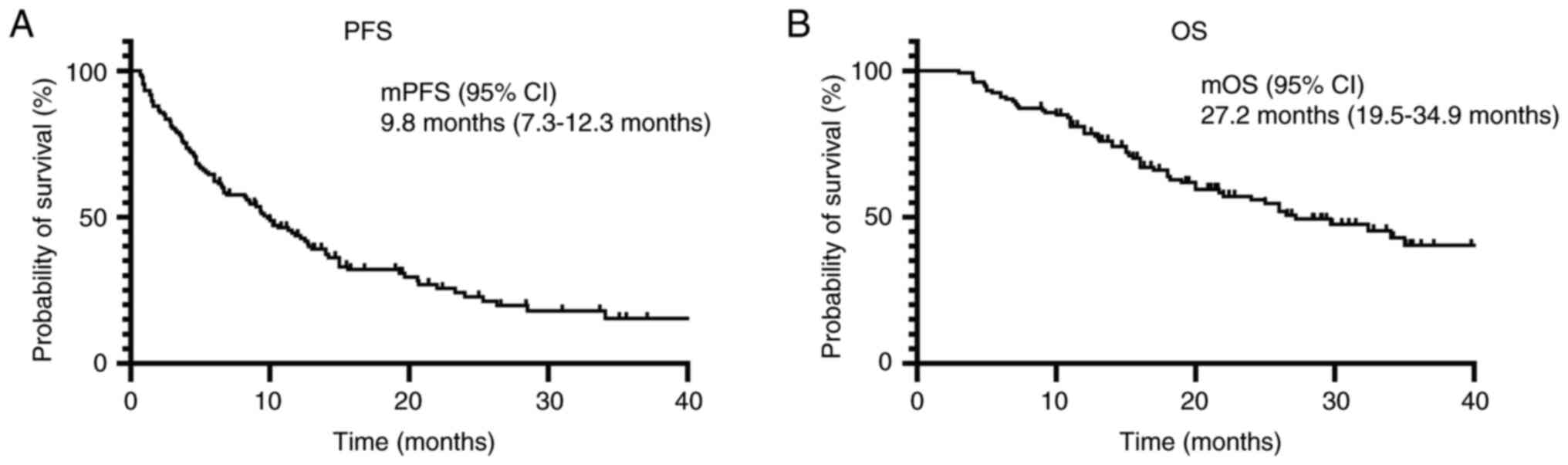
PFS and OS times were 9.8 and 27.2 months, respectively (Fig. 1A and B).
 | Table II.Treatment response. |
Table II.
Treatment response.
| Response | No. (%) |
|---|
| Complete
response | 2 (1.5) |
| Partial
response | 54 (40.6) |
| Stable disease | 60 (45.1) |
| Progressive
disease | 17 (12.8) |
| Overall response
rate | 56 (42.1) |
| Disease control
rate | 116 (87.2) |
Prognostic significance
To assess the association between patient
characteristics and PFS and OS, univariate and multivariate Cox
regression analyses were performed. Univariate Cox regression
analysis demonstrated that sex, clinical stage, PD-L1 status,
previous systemic therapy, and brain and liver metastases were
associated with PFS (Table III).
Furthermore, ECOG status, clinical stage, PD-L1 status and brain
metastasis were associated with OS (Table IV). In multivariate Cox regression
analysis, only PD-L1 status (TPS ≥50%) was considered to be a
significant indicator for both favorable PFS and OS. Furthermore,
an ECOG score of ≥1 was associated with decreased OS. Based on the
Cox regression analysis results, subgroup and Kaplan-Meier analyses
of PFS and OS were performed.
 | Table III.Univariate and multivariate Cox
regression analysis of factors associated with progression-free
survival. |
Table III.
Univariate and multivariate Cox
regression analysis of factors associated with progression-free
survival.
|
| Univariate
analysis |
| Multivariate
analysis |
|
|---|
|
|
|
|
|
|
|---|
|
Characteristics | HR | 95% CI | P-value | HR | 95% CI | P-value |
|---|
| Age (<63 vs. ≥63
years) | 0.783 | 0.523–1.173 | 0.236 |
|
|
|
| Sex (male vs.
female) | 1.796 | 1.128–2.860 | 0.014 | 1.309 | 0.780–2.197 | 0.309 |
| Smoking history
(smoker vs. non-smoker) | 0.748 | 0.487–1.149 | 0.185 |
|
|
|
| ECOG status (0 vs.
≥1) | 1.108 | 0.733–1.677 | 0.626 |
|
|
|
| Stage (III vs.
IV) | 3.781 | 1.743–8.204 | 0.001 | 1.938 | 0.822–4.573 | 0.131 |
| Histology
(adenocarcinoma vs. squamous cell carcinoma) | 1.192 | 0.788–1.803 | 0.406 |
|
|
|
| EGFR status
(wild-type vs. mutation) | 1.626 | 0.973–2.718 | 0.064 |
|
|
|
| PD-L1 status
(<50% vs. ≥50%) | 0.501 | 0.314–0.800 | 0.004 | 0.493 | 0.306–0.795 | 0.004 |
| Previous systemic
therapy (<2 vs. ≥2) | 1.567 | 1.044–2.350 | 0.030 | 1.206 | 0.752–1.933 | 0.437 |
| Radiotherapy status
(no vs. yes) | 1.012 | 0.668–1.533 | 0.955 |
|
|
|
| Brain metastases
(no vs. yes) | 1.816 | 1.185–2.782 | 0.006 | 1.331 | 0.822–2.153 | 0.245 |
| Liver metastases
(no vs. yes) | 1.765 | 1.101–2.831 | 0.018 | 1.406 | 0.814–2.428 | 0.221 |
 | Table IV.Univariate and multivariate Cox
regression analysis of factors associated with overall
survival. |
Table IV.
Univariate and multivariate Cox
regression analysis of factors associated with overall
survival.
|
| Univariate
analysis |
| Multivariate
analysis |
|
|---|
|
|
|
|
|
|
|---|
|
Characteristics | HR | 95% CI | P-value | HR | 95% CI | P-value |
|---|
| Age (<63 vs. ≥63
years) | 1.013 | 0.603–1.703 | 0.960 |
|
|
|
| Sex (male vs.
female) | 1.373 | 0.774–2.435 | 0.279 |
|
|
|
| Smoking history
(smoker vs. non-smoker) | 0.772 | 0.457–1.306 | 0.335 |
|
|
|
| ECOG status (0 vs.
≥1) | 1.685 | 1.002–2.833 | 0.049 | 2.118 | 1.176–3.816 | 0.012 |
| Stage (III vs.
IV) | 4.087 | 1.278–13.069 | 0.018 | 2.654 | 0.630–11.176 | 0.183 |
| Histology
(adenocarcinoma vs. squamous cell carcinoma) | 1.185 | 0.697–2.016 | 0.531 |
|
|
|
| EGFR status
(wild-type vs. mutation) | 1.576 | 0.859–2.893 | 0.142 |
|
|
|
| PD-L1 status
(<50% vs. ≥50%) | 0.465 | 0.260–0.831 | 0.010 | 0.432 | 0.238–0.783 | 0.006 |
| Previous systemic
therapy (<2 vs. ≥2) | 1.372 | 0.824–2.284 | 0.224 |
|
|
|
| Radiotherapy status
(no vs. yes) | 0.879 | 0.517–1.496 | 0.635 |
|
|
|
| Brain metastases
(no vs. yes) | 1.807 | 1.073–3.042 | 0.026 | 1.123 | 0.622–2.026 | 0.700 |
| Liver metastases
(no vs. yes) | 1.735 | 0.976–3.082 | 0.060 |
|
|
|
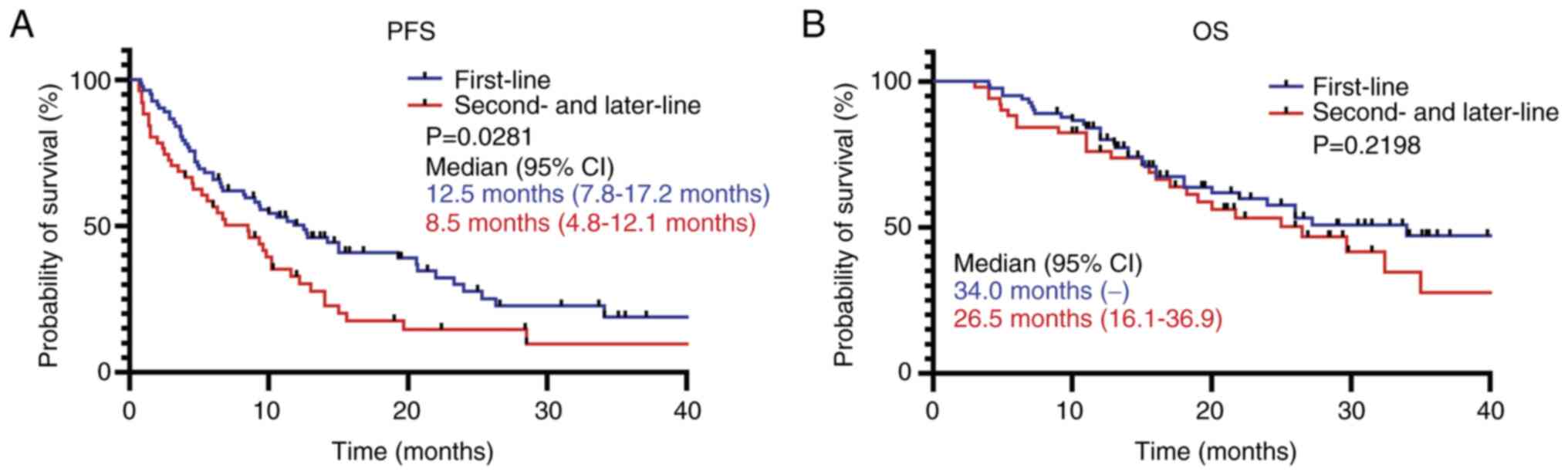
The effect of treatment lines on PFS and OS was
analyzed, and the results demonstrated that patients who were
treated with ICIs as first-line therapy had a significantly longer
PFS compared with patients who were treated with ICIs as a
second-line or later therapy. However, no significant difference in
terms of OS was observed (Fig.
2).
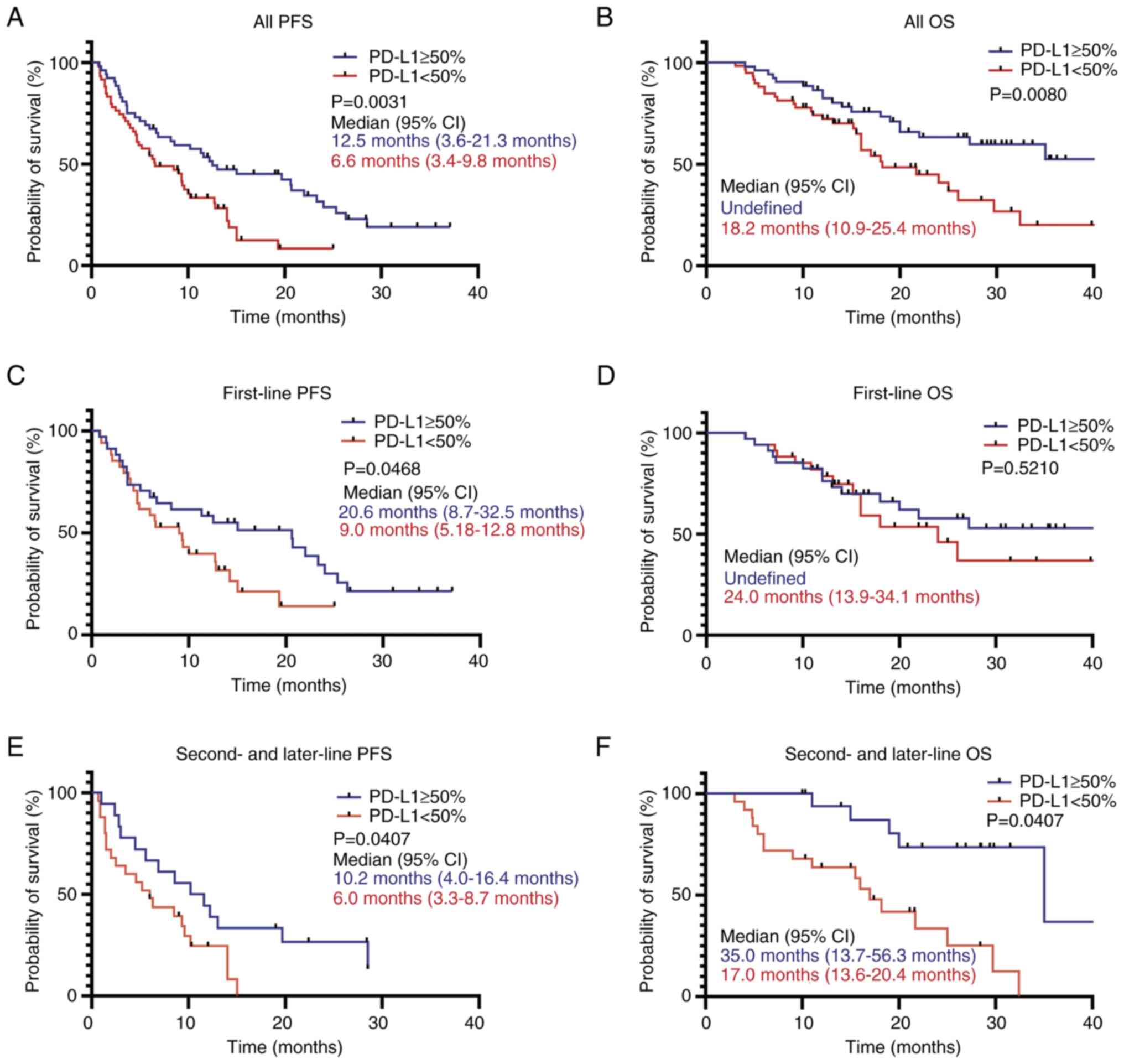
PD-L1 status (TPS <50%) was a poor prognostic
factor associated with PFS and OS (Fig.
3). Patients with a PD-L1 TPS of ≥50% had a significantly
longer PFS, regardless of the previous systemic therapy compared
with patients with a PD-L1 TPS of <50% (Fig. 3A, C and E). For OS, patients with a
PD-L1 TPS of ≥50% had a good prognosis (Fig. 3B), whereas no significant difference
was observed between groups based on PD-L1 status in patients
treated with ICIs as a first-line therapy (Fig. 3D), while patients with a PD-L1 TPS
of ≥50% treated with ICIs as a second-line and subsequent therapies
had a significantly longer OS compared with patients with a PD-L1
TPS of <50% (Fig. 3F).
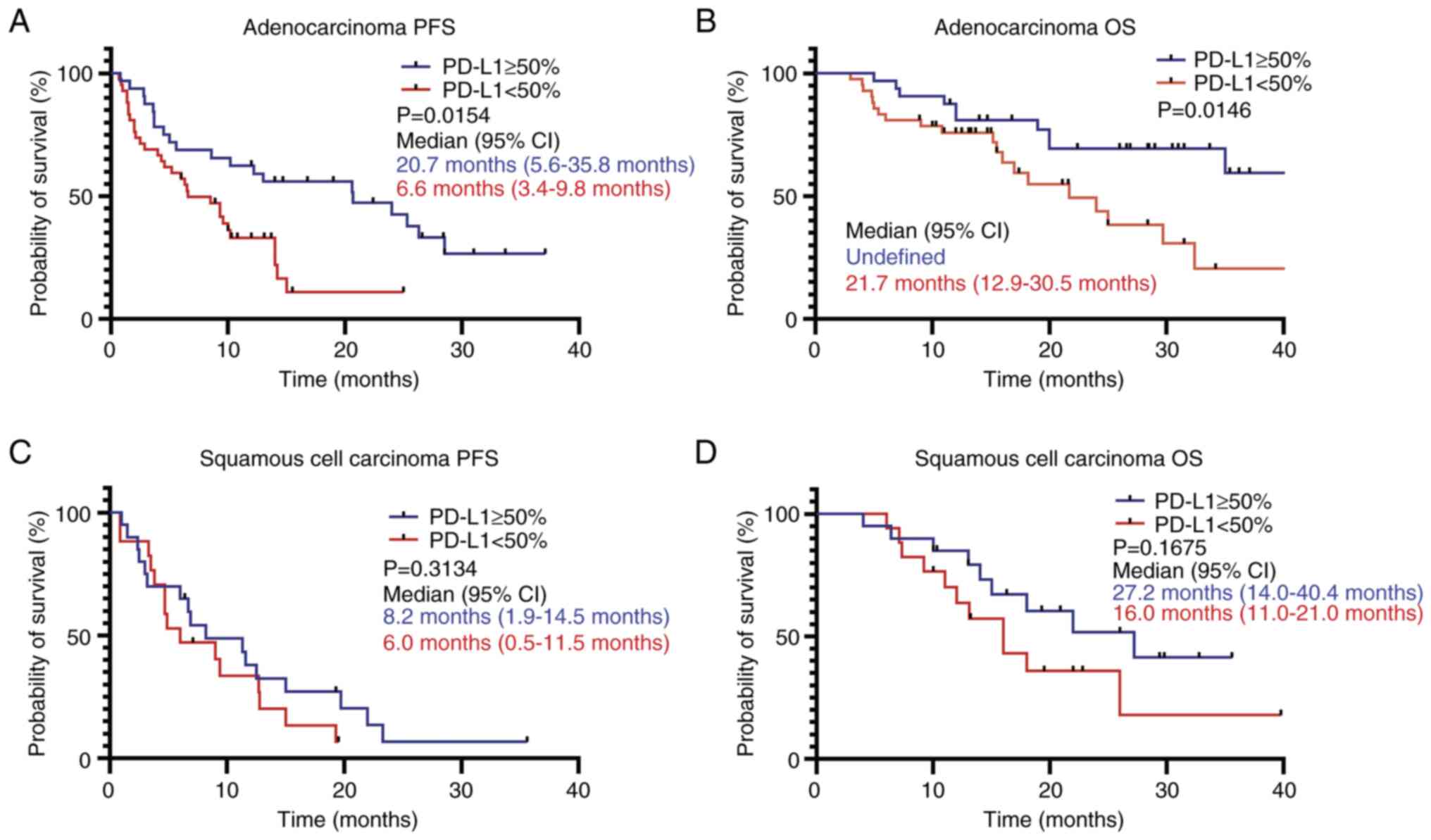
Patients were then further subdivided into two
subgroups based on the histopathological type, and the association
between the PD-L1 TPS and PFS and OS was assessed. The results
demonstrated that in patients with adenocarcinoma, those with a
PD-L1 TPS of ≥50% had significantly longer PFS and OS times
compared with patients with a PD-L1 TPS of <50% (Fig. 4A and B), while the PD-L1 TPS status
had no significant impact on PFS and OS in patients with squamous
cell carcinoma (Fig. 4C and D).
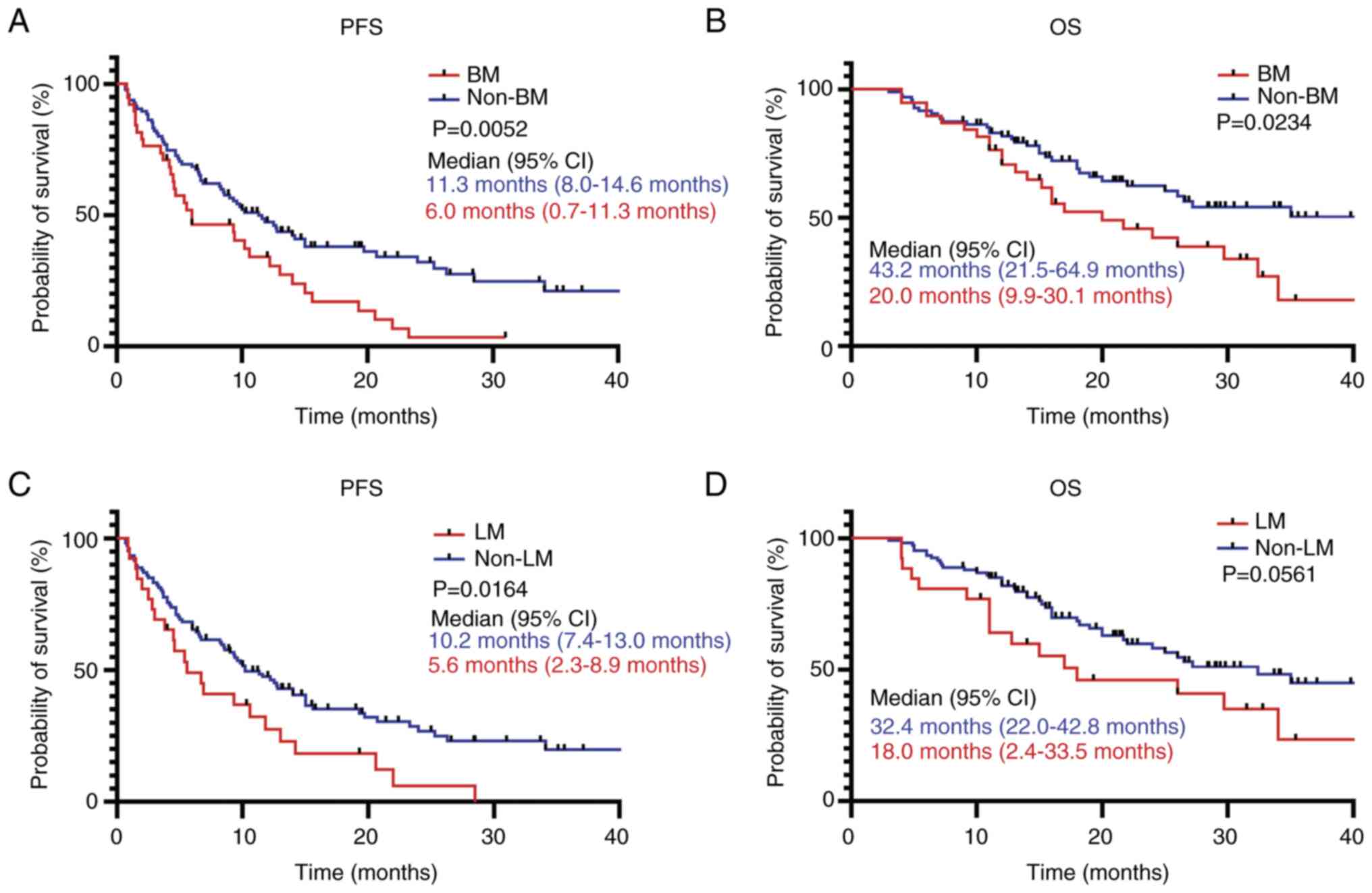
The PFS and OS of patients with brain and liver
metastasis was assessed. It was demonstrated that patients with
brain metastasis had significantly shorter PFS and OS times
compared with patients without brain metastases (Fig. 5A and B). Furthermore, patients with
liver metastasis had a significantly shorter PFS compared with
patients without liver metastases; however, no significant
difference was observed in terms of OS (Fig. 5C and D).
Discussion
The advent of immunotherapy has addressed the
shortcomings in the treatment of patients with advanced NSCLC
without specific driver gene mutations. Numerous large-scale
clinical studies have suggested that ICIs could notably prolong the
survival of patients with advanced NSCLC, thus heralding a novel
era in cancer treatment (12,13,21,22).
However, in the real world, clinicians often encounter a broader
and unselected group of patients with lung cancer, while data on
the real-world use of immunotherapy are limited. Therefore, further
evaluation of the outcomes observed in clinical trials within the
practical context is imperative.
The median age of patients in the present study was
63 years, which was higher compared with the median ages (≤60
years) of patients enrolled in phase III clinical trials, and
allowed for a more accurate depiction of the real-world treatment
conditions by not selecting patients (22,23).
The present results demonstrated that, among 133 patients treated
with ICIs, the median PFS and OS times were 9.8 and 27.2 months,
respectively, while the ORR was 42.1%. Furthermore, among patients
who received ICIs as a first-line therapy, the median PFS and OS
times were 12.5 and 34.0 months, respectively. In the KEYNOTE 189
study (21), patients treated with
pembrolizumab in combination with chemotherapy as a first-line
therapy for metastatic non-squamous NSCLC had a median PFS of 9.0
months, a median OS of 22.0 months and an ORR of 47.6%. Compared
with the KEYNOTE 189 study, the present study included patients
with squamous or non-squamous NSCLC and revealed moderately
improved PFS and OS. Furthermore, among patients treated with ICIs
as second- or later-line therapy, a median PFS time of 8.5 months
and a median OS time of 26.5 months were observed. Based on
long-term follow-up data for ICIs as a second-line therapy, the
phase III clinical trial CheckMate 078 (22) assessed the efficacy and safety of
nivolumab in the treatment of patients with advanced NSCLC, with a
median PFS time of 2.8 months and a median OS time of 12.0 months
reported in patients in the nivolumab group. In the present study,
the follow-up data for second- and later-line treatments
demonstrated improved PFS and OS compared with the existing
clinical research data. Patients with EGFR/ALK alterations were
excluded in the CheckMate 078 trial, while these were included in
the present study. It was demonstrated that the EGFR mutation
status did not affect the efficacy of later-line ICI treatment. In
terms of ICI efficacy as first- or later-line therapies, the
results of the present study demonstrated that patients treated
with first-line ICIs had a longer PFS time compared with those
treated with ICIs as second- or later-line therapy. In summary,
first-line therapy with ICIs exhibited superior efficacy compared
with second-line ICI treatment.
Numerous prior studies have demonstrated that
combining immunotherapy with chemotherapy could extend the survival
of patients with advanced NSCLC compared with chemotherapy alone.
Furthermore, higher PD-L1 expression levels were associated with
more pronounced survival benefits (21,23,24).
In the present study, among all patients who
received ICIs, 83.46% underwent PD-L1 TPS testing. The present
study aimed to assess the association between PD-L1 expression in
tumors and the prognosis of patients with NSCLC treated with
immunotherapy. The results of the present study demonstrated that
patients with a PD-L1 TPS ≥50% had a significantly longer median
PFS and OS time compared with patients with a PD-L1 TPS <50%.
Survival analysis of patients treated with first-line ICI therapy
also demonstrated a longer PFS time in patients with a PD-L1 TPS
≥50%. However, no significant difference was observed in this group
for OS. Furthermore, in terms of later-line treatment, patients
with a PD-L1 TPS ≥50% exhibited a significantly longer median PFS
and OS time. These findings suggested that in second-line and
subsequent immunotherapy treatments, individuals with high PD-L1
expression could notably benefit from ICI treatment compared with
those with low or no PD-L1 expression. Furthermore, multivariate
analysis further verified that PD-L1 TPS was a significant
independent prognostic predictor for both PFS and OS. Furthermore,
the proportion of patients with a PD-L1 TPS ≥50% was ~20% in
patients with NSCLC, as confirmed by immunohistochemistry in a
previous study (25). In the
present study, a number of patients with a PD-L1 TPS <50% did
not receive ICI treatment due to financial reasons, whereas
patients with PD-L1 TPS ≥50% were more willing to use the
immunotherapy; therefore, the proportion of patients with a PD-L1
TPS ≥50% will be higher in the present study compared with the
proportion of patients confirmed by immunohistochemistry in a
previous study (25). This may be
the reason why the PFS and OS were superior in the present study
compared with the results of the clinical trials. In the real-world
setting, the aforementioned findings substantiated the potential
use of high PD-L1 expression in tumors as a biomarker for the
selection of patients with advanced NSCLC who could benefit from
immunotherapy.
In the present study, no association between the
PD-L1 TPS and the effectiveness of ICIs was observed in patients
with squamous cell carcinoma. This finding was consistent with the
outcomes of two prior clinical trials, which compared the
efficiency of nivolumab with docetaxel in patients with advanced
NSCLC (26,27). The CheckMate 017 study, which
focused on patients with squamous cell carcinoma, reported that the
treatment efficacy was not associated with the PD-L1 TPS. By
contrast, the CheckMate 057 study, which enrolled patients with
adenocarcinoma, demonstrated an association between treatment
efficacy and the PD-L1 TPS, thus the present study verified these
previously reported results.
The majority of patients with NSCLC with brain
metastasis were excluded from previous clinical trials (28–30).
Therefore, data on the effectiveness and safety of immunotherapy in
patients with NSCLC exhibiting brain metastasis are limited. Thus,
patients with asymptomatic or stable brain metastasis were
included. In the present study, ~28.57% of the patients exhibited
brain metastasis, which is a higher percentage compared with what
has been commonly recorded in several large-scale clinical trials
(23). The results of the present
study demonstrated that patients with brain metastasis had a
shorter PFS and OS time in the overall population that was treated
with immunotherapy. This aligned with the results of a study by
Waterhouse et al (31),
which reported that brain metastasis was associated with reduced OS
in patients with squamous cell carcinoma NSCLC treated with either
a single-agent immunotherapy or with immunotherapy combined with
chemotherapy. Similar findings were obtained in patients with
non-squamous cell carcinoma treated with immunotherapy combined
with chemotherapy (12,21). The KEYNOTE-189 trial reported that,
in a subgroup of patients with brain and liver metastasis, patients
who received pembrolizumab in combination with chemotherapy
exhibited improved PFS and OS compared with those treated with
placebo combined with chemotherapy (21). However, when the results of two
other studies, namely IMpower130 (26) and IMpower132 (32), were reviewed, the results
demonstrated that patients in the liver metastasis subgroup did not
exhibit survival benefits from immunotherapy combined with
chemotherapy. Notably, in these studies, patients with metastasis
in one or multiple organs were also included, which would affect
the prognosis of patients. Consequently, further clinical research
is needed to verify the response of patients with advanced NSCLC
and liver metastasis to immunotherapy. The results of the present
study also demonstrated that patients without liver metastasis had
a longer PFS time compared with those with liver metastasis.
Consistently, previous studies indicated that liver metastasis was
closely associated with worse survival outcomes in patients
receiving nivolumab, with a noticeably longer median PFS time
observed in patients without liver metastasis compared with those
with liver metastasis (33,34).
The current published immunotherapy data from
real-world patients with lung cancer have some limitations
(35–37). Firstly, the sample sizes were
commonly small (<100 patients), and the studies only included a
particular subgroup of patients, such as elderly patients, those
treated with PD-1/PD-L1 monotherapy or patients experiencing
immune-related adverse events. Although data from several large
studies were extracted from the corresponding databases, the
detailed patient records were not always available (31,38).
In summary, in the present study, the inclusion
criteria resulted in a more comprehensive cohort and relevant
subgroup analyses were also conducted, which could assist
clinicians in assessing the safety and efficacy of ICIs in the
treatment of patients with advanced lung cancer in routine oncology
practice.
However, the present study has some limitations.
This was a small, single-center, retrospective study, which could
be affected by inherent selection bias. Furthermore, seven types of
ICIs were used in present study, which may have resulted in
differences in efficacy among patients who received different
treatments. Furthermore, patients treated with both single-agent
and combination immunotherapy, with varying treatment regimens and
multiple confounding factors, were included, thus possibly
affecting the assessment of immunotherapy efficacy. Therefore,
larger, multicenter, prospective studies are required to further
confirm the results of the present study and provide a more solid
basis for identifying potential benefits of immunotherapy.
In conclusion, the present study demonstrated that
PD-L1 status was an independent predictor for PFS and OS in
patients who were treated with ICIs plus chemotherapy with advanced
adenocarcinoma, but not in those with squamous cell carcinoma. When
the aforementioned regimens were used as first-line therapy, an
increase in PFS but not OS was observed. An increase in PFS was
also demonstrated in patients treated with first-line ICI therapy
with a PD-L1 TPS of ≥50%. Furthermore, when the ICIs were used as
second- or later-line therapy, the PFS and OS were both
significantly increased in patients with a PD-L1 TPS ≥50%. Patients
with brain metastasis had a shorter PFS and OS time compared with
those without metastases. Patients with liver metastasis showed
poor PFS; however, it was not significantly associated with OS.
Finally, although the present study had some limitations, these
results may aid clinicians in decision-making and provide other
options for patients with advanced NSCLC.
Acknowledgements
Not applicable.
Funding
The present study was supported by the Shandong Medical
Association (Grant No. YXH2022ZX02032).
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
ZX, HZ and GM contributed to the response evaluation
and data analysis. ZX, HZ, WM, JD, XW, BY, NW, YD, QZ and NL
performed the data collection and analysis. ZX and ZL wrote and
edited the manuscript. ZX, HZ and ZL confirm the authenticity of
all the raw data. XZ, GY, SL and ZL designed the present study. All
authors have read and approved the final manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of Weifang People's Hospital (The First Affiliated
Hospital of Shandong Second Medical University) (approval no.
KYLL20230112-1; Weifang, China) and was performed according to the
Declaration of Helsinki. Informed consent was not required, since
patient data were de-identified prior to receipt, and the
requirement for informed consent was waived by the Ethics Committee
of Weifang People's Hospital (The First Affiliated Hospital of
Shandong Second Medical University).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Li Y, Yan B and He S: Advances and
challenges in the treatment of lung cancer. Biomed Pharmacother.
169:1158912023. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Gao S, Li N, Wang S, Zhang F, Wei W, Li N,
Bi N, Wang Z and He J: Lung Cancer in People's Republic of China. J
Thorac Oncol. 15:1567–1576. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Sung H, Ferlay J, Siegel RL, Laversanne M,
Soerjomataram I, Jemal A and Bray F: Global cancer statistics 2020:
GLOBOCAN estimates of incidence and mortality worldwide for 36
cancers in 185 countries. CA Cancer J Clin. 71:209–249. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Schiller JH, Harrington D, Belani CP,
Langer C, Sandler A, Krook J, Zhu J and Johnson DH; Eastern
Cooperative Oncology Group, : Comparison of four chemotherapy
regimens for advanced non-small-cell lung cancer. N Engl J Med.
346:92–98. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Cufer T, Ovcaricek T and O'Brien ME:
Systemic therapy of advanced non-small cell lung cancer:
Major-developments of the last 5-years. Eur J Cancer. 49:1216–1225.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Sandler A, Gray R, Perry MC, Brahmer J,
Schiller JH, Dowlati A, Lilenbaum R and Johnson DH:
Paclitaxel-carboplatin alone or with bevacizumab for non-small-cell
lung cancer. N Engl J Med. 355:2542–2550. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kader YA, Le Chevalier T, El-Nahas T and
Sakr A: Comparative study analyzing survival and safety of
bevacizumab/carboplatin/paclitaxel and cisplatin/pemetrexed in
chemotherapy-naive patients with advanced non-squamous bronchogenic
carcinoma not harboring EGFR mutation. Onco Targets Ther.
6:803–809. 2013.PubMed/NCBI
|
|
8
|
Chen P, Liu Y, Wen Y and Zhou C: Non-small
cell lung cancer in China. Cancer Commun (Lond). 42:937–970. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kaur J, Elms J, Munn AL, Good D and Wei
MQ: Immunotherapy for non-small cell lung cancer (NSCLC), as a
stand-alone and in combination therapy. Crit Rev Oncol Hematol.
164:1034172021. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kim H and Chung JH: PD-L1 testing in
non-small cell lung cancer: Past, present, and future. J Pathol
Transl Med. 53:199–206. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ramagopal UA, Liu W, Garrett-Thomson SC,
Bonanno JB, Yan Q, Srinivasan M, Wong SC, Bell A, Mankikar S,
Rangan VS, et al: Structural basis for cancer immunotherapy by the
first-in-class checkpoint inhibitor ipilimumab. Proc Natl Acad Sci
USA. 114:E4223–E4232. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Reck M, Rodriguez-Abreu D, Robinson AG,
Hui R, Csoszi T, Fulop A, Gottfried M, Peled N, Tafreshi A, Cuffe
S, et al: Updated Analysis of KEYNOTE-024: Pembrolizumab versus
platinum-based chemotherapy for advanced non-small-cell lung cancer
with PD-L1 tumor proportion score of 50% or Greater. J Clin Oncol.
37:537–546. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Mok TSK, Wu YL, Kudaba I, Kowalski DM, Cho
BC, Turna HZ, Castro GJ Jr, Srimuninnimit V, Laktionov KK,
Bondarenko I, et al: Pembrolizumab versus chemotherapy for
previously untreated, PD-L1-expressing, locally advanced or
metastatic non-small-cell lung cancer (KEYNOTE-042): A randomised,
open-label, controlled, phase 3 trial. Lancet. 393:1819–1830. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Jassem J, de Marinis F, Giaccone G,
Vergnenegre A, Barrios CH, Morise M, Felip E, Oprean C, Kim YC,
Andric Z, et al: Updated Overall Survival Analysis From IMpower110:
Atezolizumab versus platinum-based chemotherapy in treatment-naive
programmed death-ligand 1-Selected NSCLC. J Thorac Oncol.
16:1872–1882. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Yang Y, Sun J, Wang Z, Fang J, Yu Q, Han
B, Cang S, Chen G, Mei X, Yang Z, et al: Updated overall survival
data and predictive biomarkers of sintilimab plus pemetrexed and
platinum as first-line treatment for locally advanced or metastatic
nonsquamous NSCLC in the Phase 3 ORIENT-11 Study. J Thorac Oncol.
16:2109–2120. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Rittmeyer A, Barlesi F, Waterkamp D, Park
K, Ciardiello F, von Pawel J, Gadgeel SM, Hida T, Kowalski DM, Dols
MC, et al: Atezolizumab versus docetaxel in patients with
previously treated non-small-cell lung cancer (OAK): A phase 3,
open-label, multicentre randomised controlled trial. Lancet.
389:255–265. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Eisenhauer EA, Therasse P, Bogaerts J,
Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S,
Mooney M, et al: New response evaluation criteria in solid tumours:
Revised RECIST guideline (version 1.1). Eur J Cancer. 45:228–247.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Oken MM, Creech RH, Tormey DC, Horton J,
Davis TE, Mcfadden ET and Carbone PP: Toxicity and response
criteria of the Eastern Cooperative Oncology Group. Am J Clin
Oncol. 5:649–655. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Hwang JK, Page BJ, Flynn D, Passmore L,
Mccau E, Brady J, Yang IA, Marshall H, Windsor M, Bowman RV, et al:
Validation of the Eighth Edition TNM Lung Cancer Staging System. J
Thorac Oncol. 15:649–654. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Tsao MS, Kerr KM, Kockx M, Beasley MB,
Borczuk AC, Botling J, Bubendorf L, Chirieac L, Chen G, Chou TY, et
al: PD-L1 Immunohistochemistry comparability study in real-life
clinical samples: Results of blueprint phase 2 project. J Thorac
Oncol. 13:1302–1311. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Gadgeel S, Rodriguez-Abreu D, Speranza G,
Esteban E, Felip E, Domine M, Hui R, Hochmair MJ, Clingan P, Powell
SF, et al: Updated Analysis From KEYNOTE-189: Pembrolizumab or
placebo plus pemetrexed and platinum for previously untreated
metastatic nonsquamous non-small-cell lung cancer. J Clin Oncol.
38:1505–1517. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wu YL, Lu S, Cheng Y, Zhou C, Wang J, Mok
T, Zhang L, Tu HY, Wu L, Feng J, et al: Nivolumab versus docetaxel
in a predominantly chinese patient population with previously
treated advanced NSCLC: CheckMate 078 randomized phase III clinical
trial. J Thorac Oncol. 14:867–875. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zhou C, Chen G, Huang Y, Zhou J, Lin L,
Feng J, Wang Z, Shu Y, Shi J, Hu Y, et al: Camrelizumab plus
carboplatin and pemetrexed versus chemotherapy alone in
chemotherapy-naive patients with advanced non-squamous
non-small-cell lung cancer (CameL): A randomised, open-label,
multicentre, phase 3 trial. Lancet Respir Med. 9:305–314. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Lu S, Wang J, Yu Y, Yu X, Hu Y, Ai X, Ma
Z, Li X, Zhuang W, Liu Y, et al: Tislelizumab plus chemotherapy as
first-line treatment for locally advanced or metastatic nonsquamous
NSCLC (RATIONALE 304): A Randomized phase 3 trial. J Thorac Oncol.
16:1512–1522. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Negrao MV, Skoulidis F, Montesion M,
Schulze K, Bara I, Shen V, Xu H, Hu S, Sui D, Elamin YY, et al:
Oncogene-specific differences in tumor mutational burden, PD-L1
expression, and outcomes from immunotherapy in non-small cell lung
cancer. J Immunother Cancer. 9:e0028912021. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Horn L, Spigel DR, Vokes EE, Holgado E,
Ready N, Steins M, Poddubskaya E, Borghaei H, Felip E, Paz-Ares L,
et al: Nivolumab versus docetaxel in previously treated patients
with advanced non-small-cell lung cancer: Two-Year outcomes from
two Randomized, open-label, phase III trials (CheckMate 017 and
CheckMate 057). J Clin Oncol. 35:3924–3933. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Borghaei H, Gettinger S, Vokes EE, Chow
LQM, Burgio MA, de Castro Carpeno J, Pluzanski A, Arrieta O,
Frontera OA, Chiari R, et al: Five-Year outcomes from the
Randomized, phase III Trials CheckMate 017 and 057: Nivolumab
versus docetaxel in previously treated non–small-cell lung cancer.
J Clin Oncol. 39:723–733. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Borghaei H, Paz-Ares L, Horn L, Spigel DR,
Steins M, Ready NE, Chow LQ, Vokes EE, Felip E, Holgado E, et al:
Nivolumab versus docetaxel in advanced nonsquamous non-small-cell
lung cancer. N Engl J Med. 373:1627–1639. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Gandhi L, Rodriguez-Abreu D, Gadgeel S,
Esteban E, Felip E, De Angelis F, Domine M, Clingan P, Hochmair MJ,
Powell SF, et al: Pembrolizumab plus chemotherapy in metastatic
non-small-cell lung cancer. N Engl J Med. 378:2078–2092. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
West H, Mccleod M, Hussein M, Morabito A,
Rittmeyer A, Conter HJ, Kopp HG, Daniel D, Mccune S, Mekhail T, et
al: Atezolizumab in combination with carboplatin plus
nab-paclitaxel chemotherapy compared with chemotherapy alone as
first-line treatment for metastatic non-squamous non-small-cell
lung cancer (IMpower130): A multicentre, randomised, open-label,
phase 3 trial. Lancet Oncol. 20:924–937. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Waterhouse D, Lam J, Betts KA, Yin L, Gao
S, Yuan Y, Hartman J, Rao S, Lubinga S and Stenehjem D: Real-world
outcomes of immunotherapy-based regimens in first-line advanced
non-small cell lung cancer. Lung Cancer. 156:41–49. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Nishio M, Barlesi F, West H, Ball S,
Bordoni R, Cobo M, Longeras PD, Goldschmidt J Jr, Novello S,
Orlandi F, et al: Atezolizumab plus chemotherapy for first-line
treatment of nonsquamous NSCLC: Results from the Randomized phase 3
IMpower132 Trial. J Thorac Oncol. 16:653–664. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Tournoy KG, Thomeer M, Germonpre P,
Derijcke S, De Pauw R, Galdermans D, Govaert K, Govaerts E,
Schildermans R, Declercq I, et al: Does nivolumab for progressed
metastatic lung cancer fulfill its promises? An efficacy and safety
analysis in 20 general hospitals. Lung Cancer. 115:49–55. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Funazo T, Nomizo T and Kim YH: Liver
metastasis is associated with poor progression-free survival in
patients with non-small cell lung cancer treated with nivolumab. J
Thorac Oncol. 12:e140–e141. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Ikeuchi N, Igata F, Kinoshita E, Kawabata
T, Tan I, Osaki Y, Otsuka R, On R, Ikeda T, Nakao A, et al:
Real-world efficacy and safety of atezolizumab plus bevacizumab,
paclitaxel and carboplatin for first-line treatment of Japanese
patients with metastatic non-squamous non-small cell lung cancer.
Anticancer Res. 43:713–724. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Zheng L, Xiong A, Wang S, Xu J, Shen Y,
Zhong R, Lu J, Chu T, Zhang W, Li Y, et al: Decreased
monocyte-to-lymphocyte ratio was associated with satisfied outcomes
of first-line PD-1 inhibitors plus chemotherapy in stage IIIB-IV
non-small cell lung cancer. Front Immunol. 14:10943782023.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Gu X, Shi Z, Shao L, Zhang Y, Zhang Y,
Song Z, Wang W and Lou G: Efficacy and safety of maintenance immune
checkpoint inhibitors with or without pemetrexed in advanced
non-squamous non-small cell lung cancer: A retrospective study. BMC
Cancer. 22:5762022. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Perol M, Felip E, Dafni U, Polito L, Pal
N, Tsourti Z, Ton TGN, Merritt D, Morris S, Stahel R and Peters S:
Effectiveness of PD-(L)1 inhibitors alone or in combination with
platinum-doublet chemotherapy in first-line (1L) non-squamous
non-small-cell lung cancer (Nsq-NSCLC) with PD-L1-high expression
using real-world data. Ann Oncol. 33:511–521. 2022. View Article : Google Scholar : PubMed/NCBI
|