Introduction
Lung cancer continues to represent the foremost
cause of cancer-associated mortality globally, with an annual death
toll surpassing 1.6 million (1).
Among cases of primary lung cancer, non-small cell lung carcinoma
(NSCLC) accounts for 85%, with lung adenocarcinoma (LADC) being the
predominant subtype, constituting-40% of cases. Patients with stage
IV NSCLC, however, have a dismal prognosis, with a five-year
survival rate ranging from a mere 1–10% (2). Although traditional chemotherapy,
targeted therapy, immunotherapy and local treatments have all been
employed to combat metastatic NSCLC, the emergence of drug
resistance curtails the overall efficacy of chemotherapy, targeted
therapy and immunotherapy interventions (3).
In the Chinese population, mutations of the
epidermal growth factor receptor (EGFR) have been recognized as the
most prevalent driver of oncogenic mutations in patients with
NSCLC, exhibiting a prevalence rate of 47.5%. These mutations are
primarily manifested in exons 18, 19, 20 and 21, with deletions in
exon 19 (19Del) and the exon 21 L858R mutation accounting for
85–90% of the cases (4). Of note,
patients with NSCLC harboring the EGFR 19Del and L858R mutations
showcase favorable responses to EGFR-tyrosine kinase inhibitors
(TKIs) (5). In line with the 2022
National Comprehensive Cancer Network guidelines, osimertinib,
erlotinib and gefitinib are recommended as category 1 treatment
options for patients with confirmed EGFR 19Del or the L858R
mutation (6). A common phenomenon
in patients with NSCLC with EGFR mutations is oligoprogression,
occurring in 30–50% of cases during targeted therapy, and at an
even higher rate in patients receiving a combination of
chemotherapy and immunotherapy (7).
It is considered that local treatment options targeting these
oligoprogressive lesions are able to eradicate insensitive and
drug-resistant clones, potentially restoring treatment sensitivity
(8). Local thermal ablation, a
precise and minimally invasive technique, may be applied in
patients with both early-stage and metastatic NSCLC with tumors ≤3
cm. The advantages of local thermal ablation over open surgery
include shorter hospital stays, reduced risks, minimized blood
loss, manageable or moderate pain levels, limited impact on lung
function and the possibility of repeated treatments (9). Combining microwave ablation (MWA) with
TKI treatment has led to significant improvements in both
progression-free survival (PFS) and overall survival (OS) rates for
patients with oligometastatic NSCLC. Of note, in a study by Wei
et al (10), compared with
the single-agent group, the MWA consolidation group exhibited a
markedly enhanced PFS time (34.8 vs. 16.7 months, respectively) and
OS time (22.7 vs. 12.9 months, respectively). The present case
report describes a patient with LADC featuring EGFR 19Del mutations
who experienced multiple instances of oligoprogression, but
displayed a favorable response to a combination therapy comprising
MWA, chemotherapy and TKI drugs. In addition, the patient had a
history of metachronous colon cancer, along with liver and lung
metastases spanning over 2 years. The treatment regimen included
radical resection of bowel cancer, liver metastasectomy,
chemotherapy for bowel cancer combined with bevacizumab and
ablation for lung metastases. Remarkably, the patient has achieved
a long-term survival duration of 110 months and remained alive
without evaluable lesions on CT imaging.
Case report
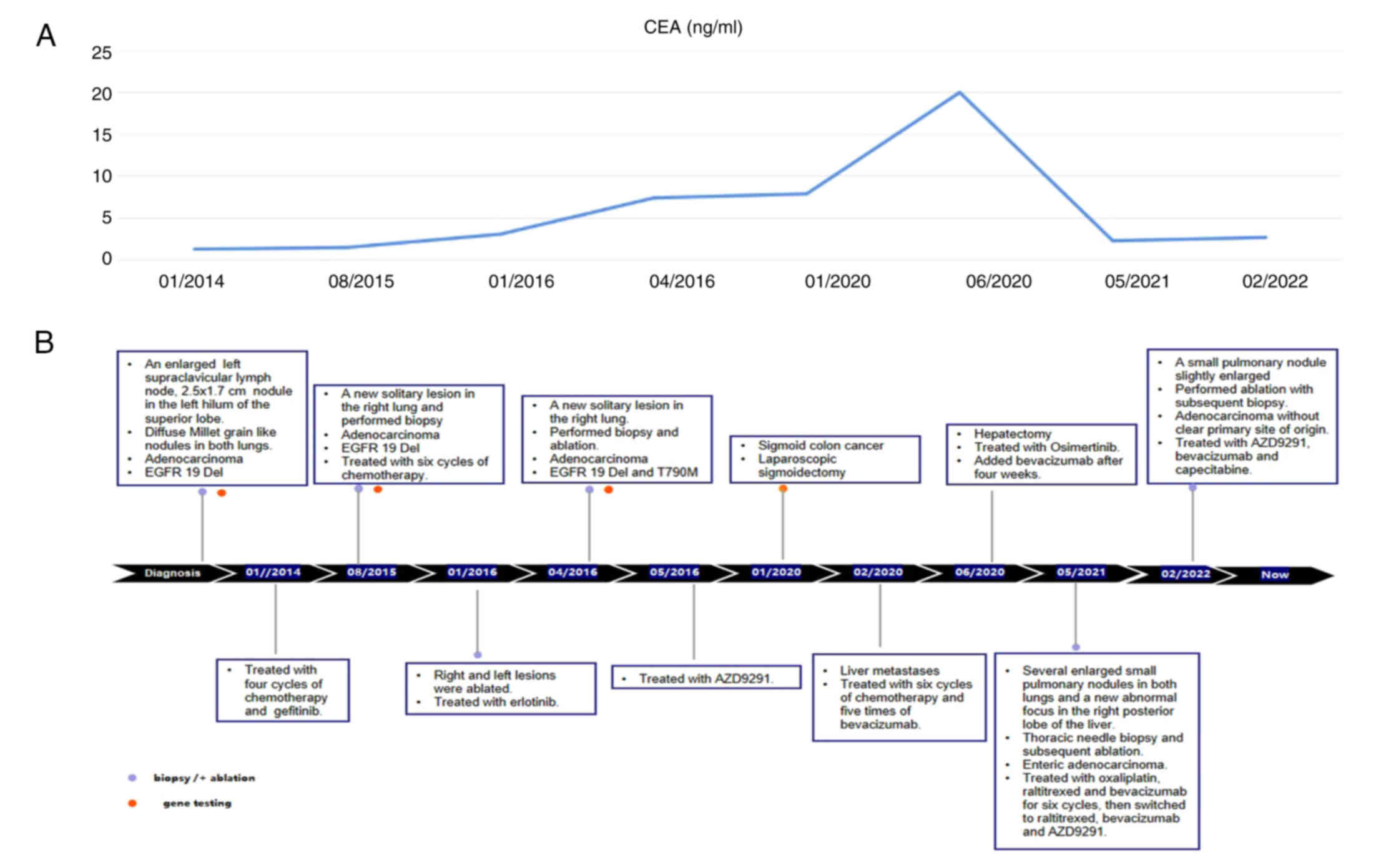
In January 2014, a 50-year-old Chinese female
non-smoker with no family history of cancer presented at Shandong
Provincial Hospital Affiliated to Shandong First Medical University
(Jinan, China) with a persistent cough lasting over 10 days. During
the physical examination, an enlarged, firm lymph node measuring 1
cm in diameter was detected in the left supraclavicular region,
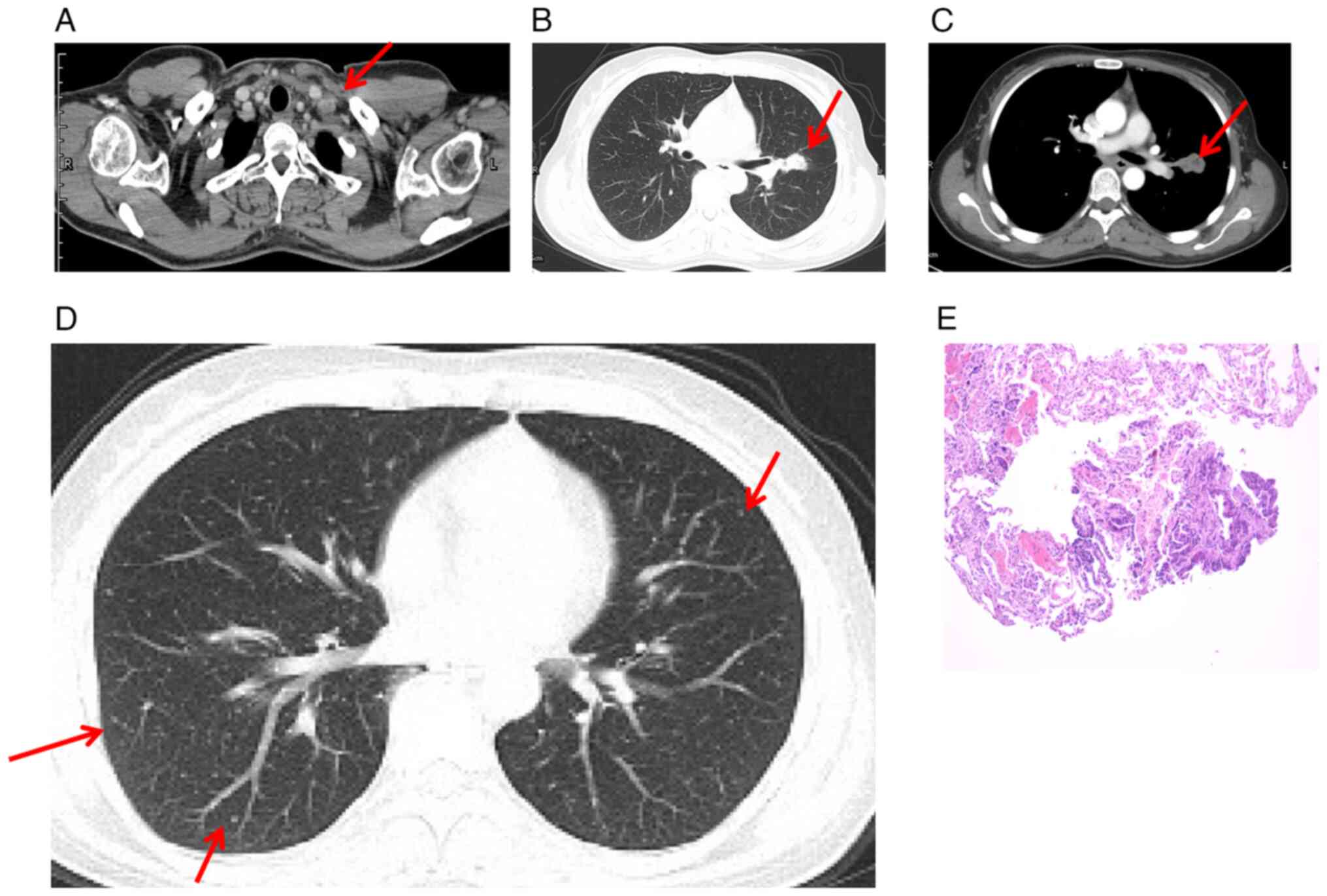
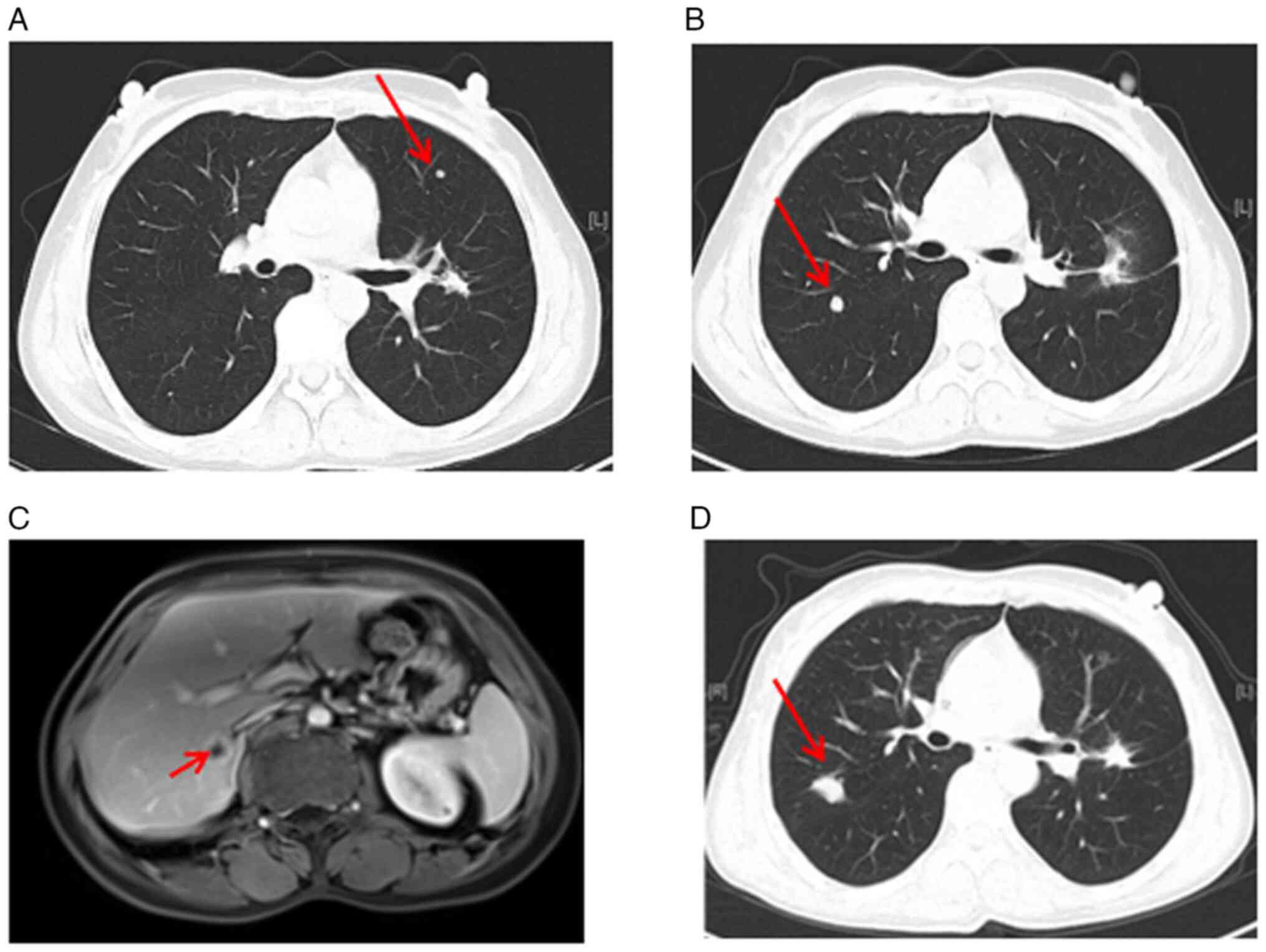
which was confirmed by computed tomography (CT) (Fig. 1A). In addition, CT scan of the chest
revealed a 2.5×1.7 cm pulmonary nodule in the left hilum of the
superior lobe (Fig. 1B and C). Of
note, diffuse Millet grain-like nodules were observed in both
lungs, indicating potential metastasis (Fig. 1D).
Following the biopsy on the lymph node in the left
supraclavicular region, the patient was diagnosed with LADC
(Fig. 1E), specifically classified
as clinical stage cT1N3M1 (cIVB, IASLC 7th edition of the TNM
Classification for Lung Cancer) (11). The subsequent reverse
transcription-quantitative (RT-q)PCR gene testing revealed the
presence of EGFR 19Del. A treatment plan was initiated, starting
with four cycles of chemotherapy (120 mg docetaxel on day 1 and 40
mg cisplatin on days 1, 2 and 3; every three weeks as one cycle).
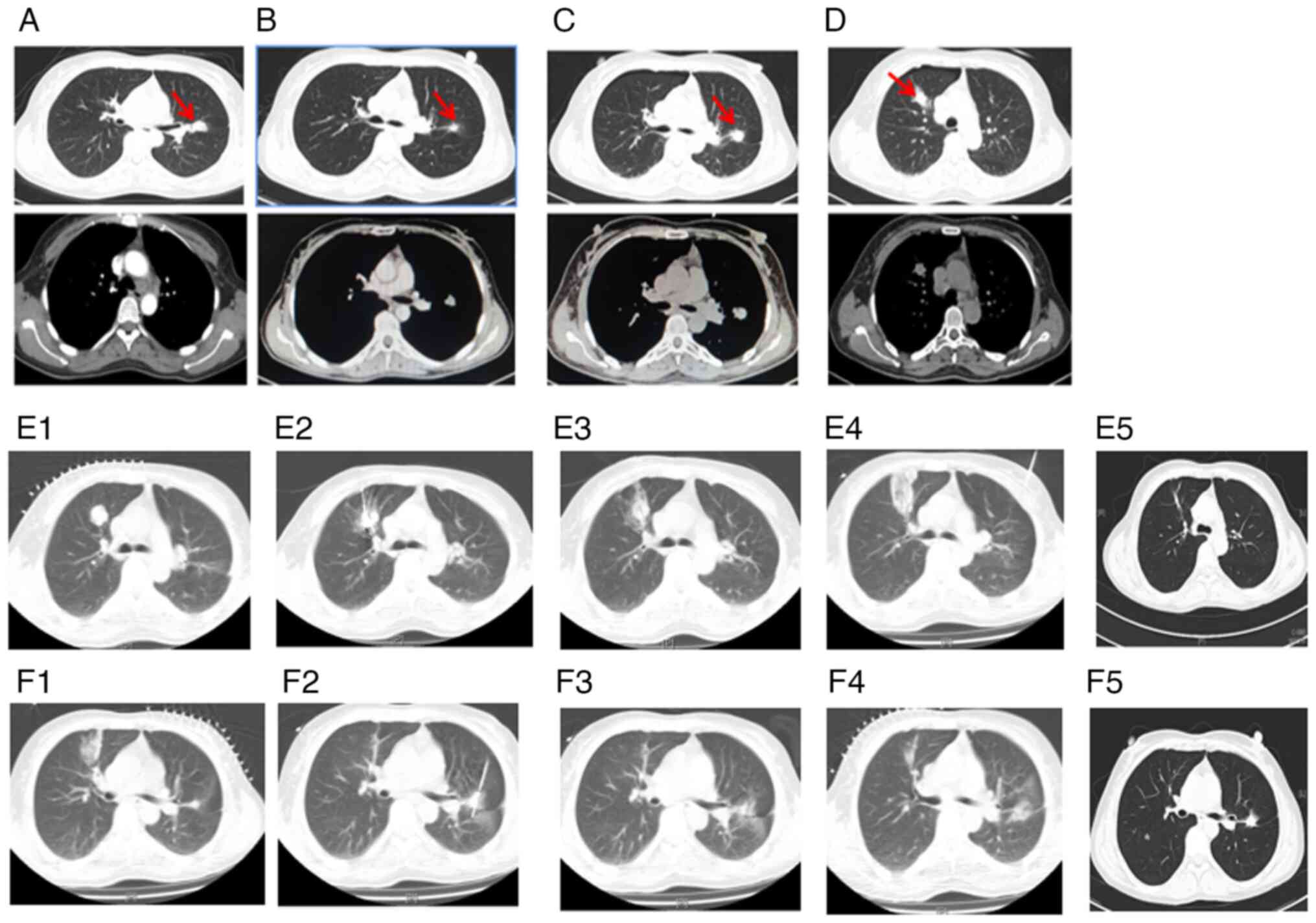
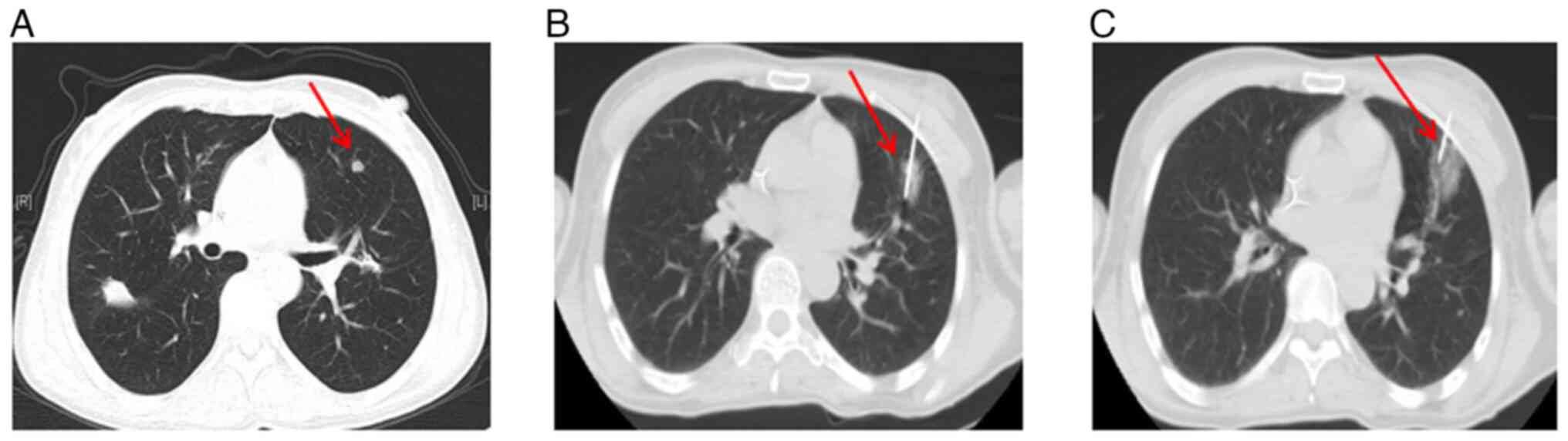
This regimen led to a partial response (Fig. 2A, Table
I), as assessed according to the Response Evaluation Criteria
in Solid Tumors version 1.1 (RECIST 1.1) criteria and the patient
was subsequently treated with gefitinib (Iressa; 250 mg/day), the
patient further improved on gefitinib, maintaining progression-free
survival (PFS) compared to the end of chemotherapy treatment for 16
months (Fig. 2B, Table I).
 | Table I.Timeline of the clinical course. |
Table I.
Timeline of the clinical course.
| Time-point | Tumor location | Testing and
results | Treatment |
|---|
| January 2014 | Left superior
lobe | RT-qPCR analysis:
EGFR exon 19Del. | Four cycles
chemotherapy (120 mg docetaxel on day 1, 40 mg cisplatin on days
1,2,3; every three weeks as one cycle), then gefitinib (Iressa; 250
mg/day) and ablation. |
| August 2015 | A new solitary
lesion right lung | RT-qPCR in August
2015: Adenocarcinoma with EGFR exon 19Del; examination by NGS in
September 2019: EGFR 19Del and EGFR exon 20 T790M. | Six cycles of
chemotherapy (0.8 pemetrexed on day 1 and 70 mg Nedaplatin on days
1 and 2; every three weeks as one cycle), then erlotinib (Tarceva;
150 mg/day) and ablation. |
| April 2016 | Another new nodule
in the right lung | RT-qPCR in January
2016: Adenocarcinoma with EGFR exon 19Del; examination by NGS in
September 2019: EGFR 19Del and EGFR exon 20 T790M. | Ablation in May
2016; osimertinib (AZD9291; 80 mg/day). |
| January 2020 | Colonoscopy
identified multiple polyps in the cyclointestinal neoplasm located
22–26 cm from the anus | Colonoscopy;
pathology confirmed primary colon adenocarcinoma. IHC results
showed positive expression of MLH1, MSH2, MSH6 and
PMS2, whereas PDL1 expression was negative. The NGS
test conducted on this specimen revealed distinct mutations in
KRAS, PIK3CA, PTEN, APC, TP53, TSC1 and AMER1. | In January 2020, a
laparoscopic sigmoidectomy was performed. Following the surgery,
the patient underwent oxaliplatin (200 mg on day 1) and
capecitabine (2.0 g, bid, on days 1–14) chemotherapy, every three
weeks as one cycle, while continuing treatment with
osimertinib. |
| February 2020 | A 1.07×0.95 cm mass
in the right posterior liver | MRI scanning | Five cycles of
chemotherapy (200 mg oxali-platin on day 1 and 2.0 g capecitabine,
bid, on days 1–14) and four cycles of bevacizumab (7.5 mg/kg;)
every three weeks as one cycle before undergoing hepatic
metastasectomy in June 2020. |
| May 2021 | Several newly
enlarged small pulmonary nodules in the bilateral lungs; pelvic MRI
identified a new abnormal focus in the right posterior lobe of the
liver | In May 2021, a
percutaneous thoracic needle biopsy guided by MRI was performed to
obtain tissue specimens from the lung nodule. The biopsy revealed a
small amount of adenocarcinoma tissue. IIHC analysis showed weak
CDX2 expression, negative CK7 expression, positive CK20 expression,
negative napsinA expression, partial SATB2 expression and negative
TTF1 expression. | Oxaliplatin (200 mg
on day 1), raltitrexed (5 mg on day 1) and bevacizumab (7.5 mg/kg;
every three weeks as one cycle) for six cycles. Subsequently, the
treatment regimen was adjusted to include raltitrexed, bevacizumab
and osimertinib. Notably, the liver metastasis disappeared based on
the MRI examination conducted in November 2022. |
| February 2022 | A small pulmonary
nodule showed slight enlargement, prompting an ablation procedure
and a subsequent biopsy | The pathology
report confirmed adenocarcinoma, although the primary site of
origin could not be determined. | Ablation and
treatment with AZD9291, bevaci-zumab and capecitabine up to the
present time. |
However, in August 2015, the primary nodule in the
left lung was found enlarged (Fig.
2C, Table I). and a new
solitary lesion was identified in the right lung, indicating
disease progression (Fig. 2D,
Table I). Despite the progression,
biopsy and RT-qPCR gene testing of this new lesion still confirmed
the presence of LADC with EGFR exon 19Del only. Before 2016,
next-generation sequencing (NGS) was not available in hardly any
hospitals in China, and thus, only PCR was used for tumor gene
testing. The sample collected in August 2015 was confirmed by NGS
testing in September 2019, indicating that the patient harbored
both EGFR exon 19Del and EGFR exon 20 T790M mutation, which
provided an explanation of why the patient showed progression at
this time (Table II). In response,
the patient underwent ablation of both the new right lung lesion
(Fig. 2E) and the primary left lung
lesion in January 2016 (Fig. 2F).
Subsequently, a treatment course consisting of six cycles of
chemotherapy (0.8 g pemetrexed on day 1 and 70 mg Nedaplatin on
days 1 and 2; every three weeks as one cycle) resulted in stable
disease (Fig. 3A). Erlotinib
(Tarceva; 150 mg/day) was administered for 3 months thereafter
(Fig. 3B, Table I). However, a CT scan in April 2016
revealed another new nodule in the right lung and enlargement of
one mediastinal lymph node (Fig.
3C). The subsequent pathology report of the puncture biospy of
the lesion confirmed LADC with EGFR 19Del by PCR at that time and
EGFR 19Del as well as EGFR exon 20 T790M mutations through NGS
testing in 2019 (Table II). The
new right lung lesion was ablated just after biopsy in May 2016
(Table I, Fig. 3D).
 | Table II.NGS genetic testing results. |
Table II.
NGS genetic testing results.
| Time-point samples
were obtained | Tumor position | Gene mutation
item | Mutation
status |
|---|
| August 2015 (the
sample was confirmed by NGS testing in September 2019) | Right lung | EGFR | Exon 19Del
(E746-A750Del) |
|
|
| EGFR | Exon 20 T790M |
|
|
| TP53 | Exon 8 R282W |
|
|
| Tumor mutational
burden | 5.6 muts/Mb |
|
|
| Microsatellite
stable status | Microsatellite
stable |
| April 2016 (the
sample was confirmed by NGS testing in September 2019) | Left lung | EGFR | Exon 19Del
(E746-A750Del) |
|
|
| EGFR | Exon 20 T790M |
|
|
| Tumor mutational
burden | 7.5 muts/Mb |
|
|
| Microsatellite
stable status | Microsatellite
stable |
| January 2020 | Rectal colon | KRAS | G13D1 (exon 2) |
|
|
| PIK3CA | M1043I (exon
21) |
|
|
| PTEN | C.635-1G>A
(splice site changes) |
|
|
| APC | K560* (exon
14) |
|
|
| APC | K1551* (exon
16) |
|
|
| TP53 | R248W (exon 7) |
|
|
| TSC1 | R1097H (exon
23) |
|
|
| AMER1 | R353* (exon2) |
|
|
| Tumor mutational
burden | 5.1 muts/Mb |
|
|
| Microsatellite
stable status | Microsatellite
stable |
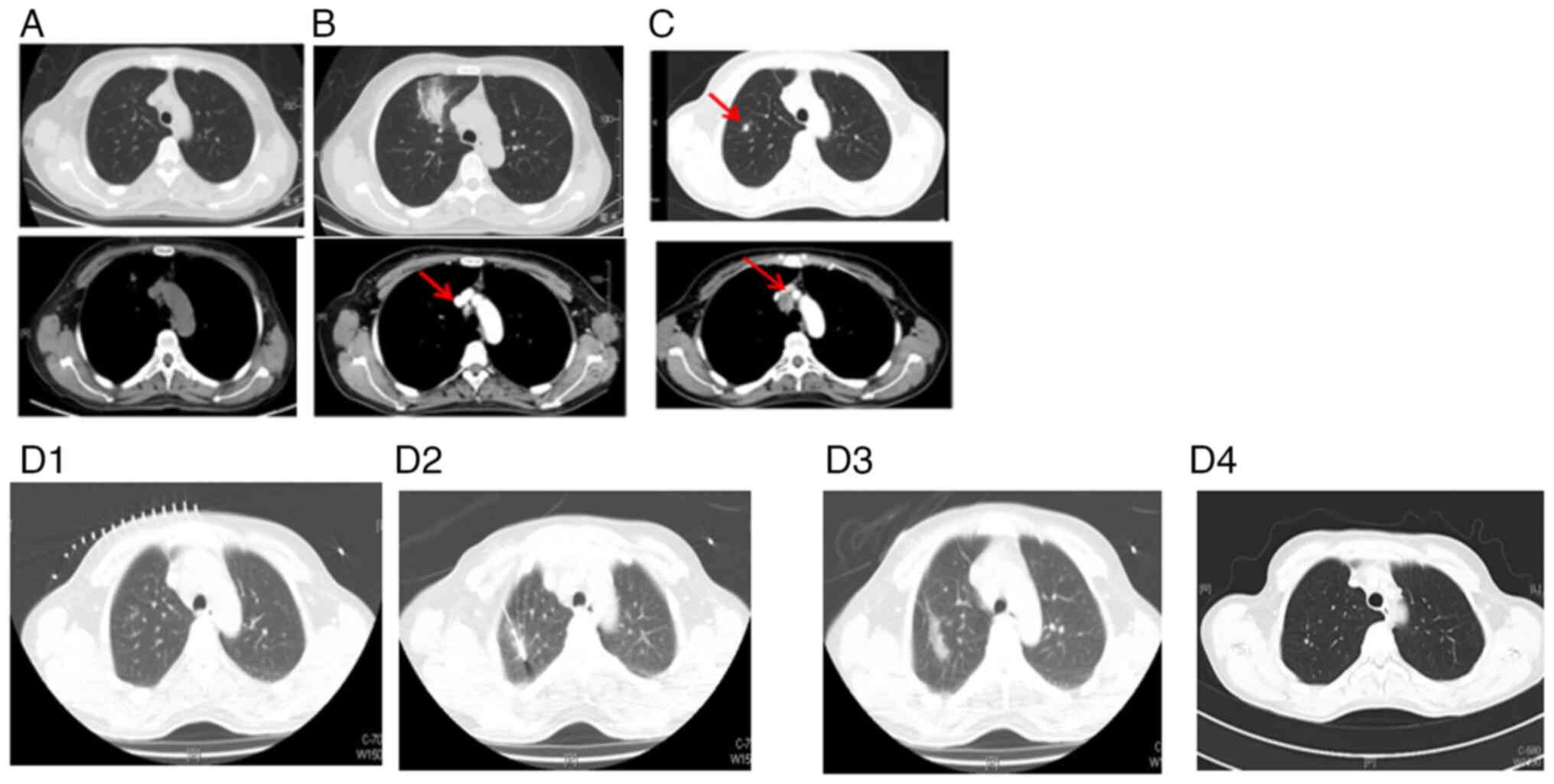
Subsequently, in May 2016, the patient began
treatment with osimertinib (AZD9291; 80 mg/day), resulting in rapid
shrinkage of the mediastinal lymph node after one month. The
treatment proved effective, continuing to provide a benefit for the
patient for 82 months (Fig. 4). Of
note, no evaluable lesions were detected since the patient started
treatment with osimertinib. In September 2017, a blood RT-qPCR test
for EGFR exon 19Del and EGFR exon 20 T790M yielded negative
results. Lung tumor markers are substances that can be measured in
the body to help diagnose and monitor the progression of lung
cancer. In the regular dynamic follow-up for a series of lung tumor
markers [carcinoembryonic antigen (CEA), cancer antigen 125,
squamous cell carcinoma antigen, neuron-specific enolase,
cytokeratin 19 fragment and pro-gastrin-releasing peptide have been
tested numerous times from January 2014 during treatment. However,
in January 2020, the level of the CEA was found to be significantly
increased to 22.2 ng/ml, surpassing the normal range of <5.0
ng/ml (Fig. 5A and B). This
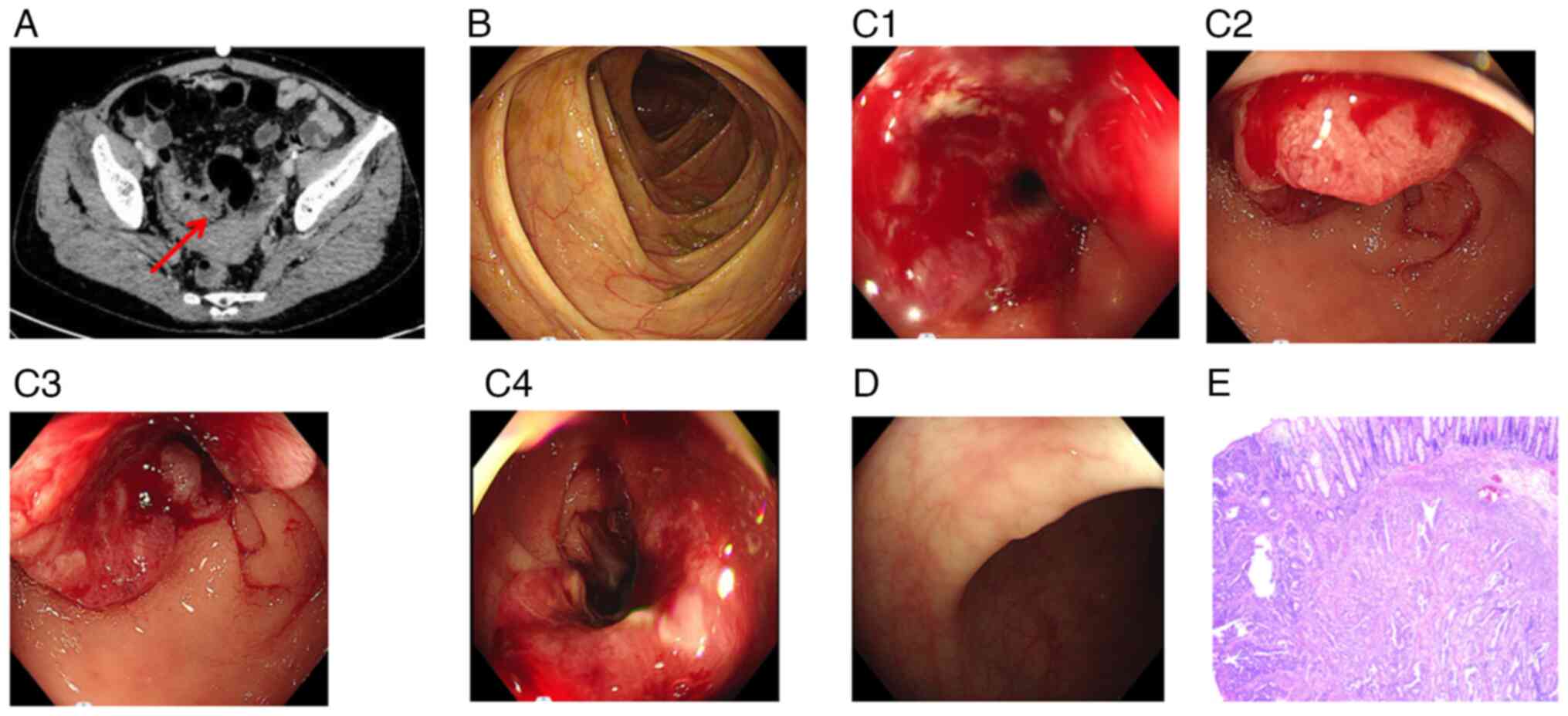
increase prompted a CT scan, which revealed significant
inhomogeneous wall thickening of the sigmoid colon, with the
thickest wall measuring-1.8 cm (Fig.
6A).
The colonoscopy results did not reveal abnormal
signs in the ileocecus area (Fig.
6B). Colonoscopy confirmed the presence of a cyclointestinal
neoplasm located 22–26 cm from the anus. Views with different
angles of this neoplasm are shown in (Fig. 6C1-C4), with the pathology results
confirming primary colon adenocarcinoma. Additionally, multiple
polyps were identified within the colon and rectum (Fig. 6D). In January 2020, a laparoscopic
sigmoidectomy was performed under general anesthesia. Pathological
examination revealed moderately differentiated adenocarcinoma, with
a mass size of 4×0.8 cm, indicating primary colon adenocarcinoma
(Fig. 6E). Malignant cells had
invaded the serosa and metastasized to peri-intestinal lymph nodes
(1/10), along with the formation of a tumor nodule measuring 0.3 cm
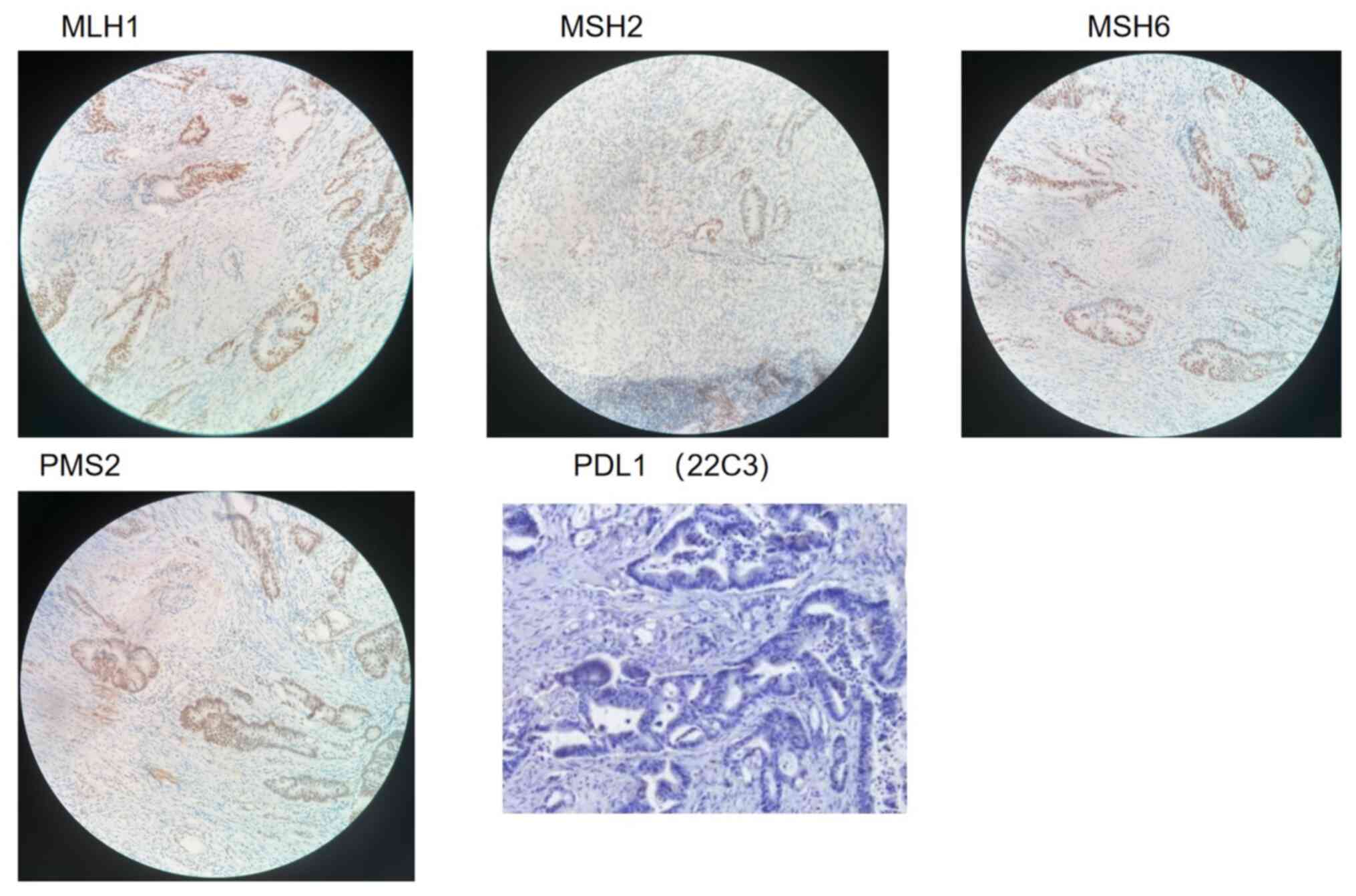
in diameter in the lymph node. Immunohistochemistry (IHC) results
showed positive expression of the DNA mismatch repair protein mutL
homolog 1 (MLH1), MutS homolog 2 (MSH2), MutS homolog 6 (MSH6) and
PMS1 homolog 2 (PMS2), whereas the expression of programmed
death-ligand 1 (PD-L1) was negative (Fig. 7). The NGS test conducted on this
tumor specimen revealed distinct mutations in the genes KRAS,
phosphatidylinositol-4,5-bisphosphate 3-kinase catalytic subunit
alpha, phosphatase and tensin homolog, adenomatous polyposis coli
(APC), tumor protein p53, tuberous sclerosis 1 and APC membrane
recruitment protein 1, differing from those found in the patient's
lung cancer specimens (Table II).
These findings suggested the presence of metachronous lung and
colon adenocarcinoma, with the tumor exhibiting microsatellite
stability and a low tumor mutational burden. Following the surgery,
the patient underwent oxaliplatin (200 mg on day 1) and
capecitabine [2.0 g twice daily (bid), days 1–14, every three weeks
as one cycle] chemotherapy, while continuing treatment with
osimertinib.
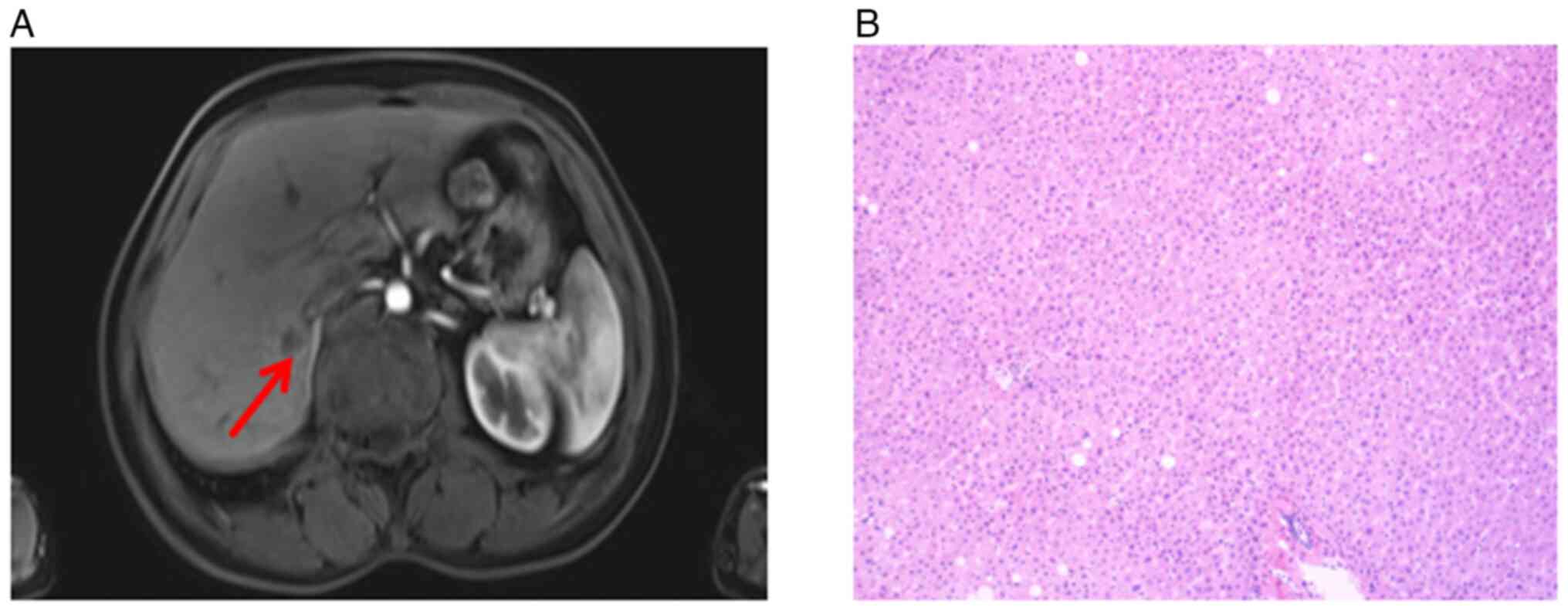
In February 2020, 43 days after the sigmoidectomy, a
follow-up magnetic resonance image (MRI) of the pelvic cavity,
which had never been performed before sigmoidectomy, revealed a
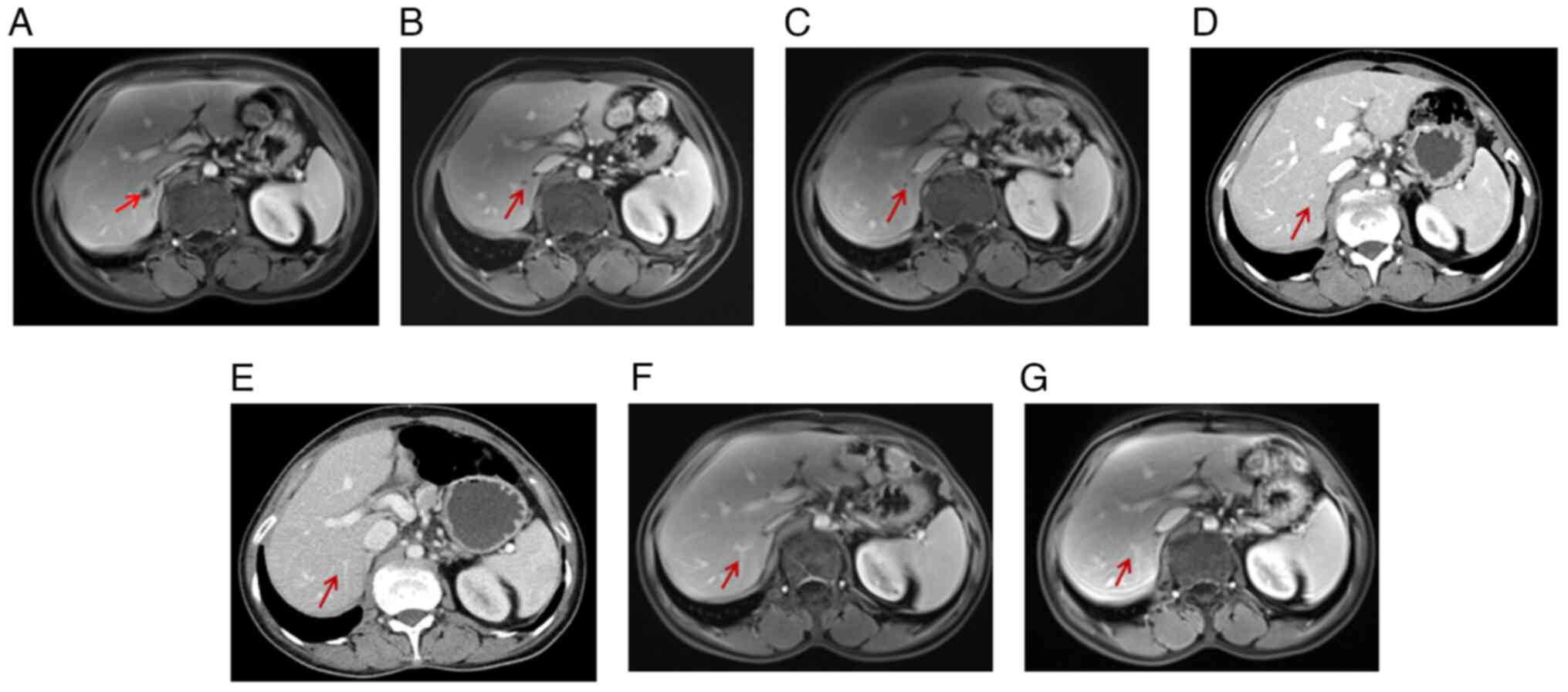
1.07×0.95 cm mass in the right posterior liver (Fig. 8A). Subsequently, the patient
received five cycles of neoadjuvant chemotherapy (200 mg
oxaliplatin on day 1 and 2.0 g capecitabine, bid, days 1–14) and
four cycles of bevacizumab (7.5 mg/kg; day 1), every three weeks as
one cycle, before undergoing hepatic metastasectomy in June 2020.
During the operation, a hepatic tumor lesion was detected by
B-ultrasound, but the pathologist did not find any malignant cells
in the excised specimen following meticulous testing (Fig. 8B). Treatment with osimertinib
(AZD9291; 80 mg/day) and bevacizumab (7.5 mg/kg; every three weeks
as one cycle) was continued, whereas the colorectal
cancer-associated chemotherapy was discontinued as no colorectal
cancer cells were found in the liver lesion.
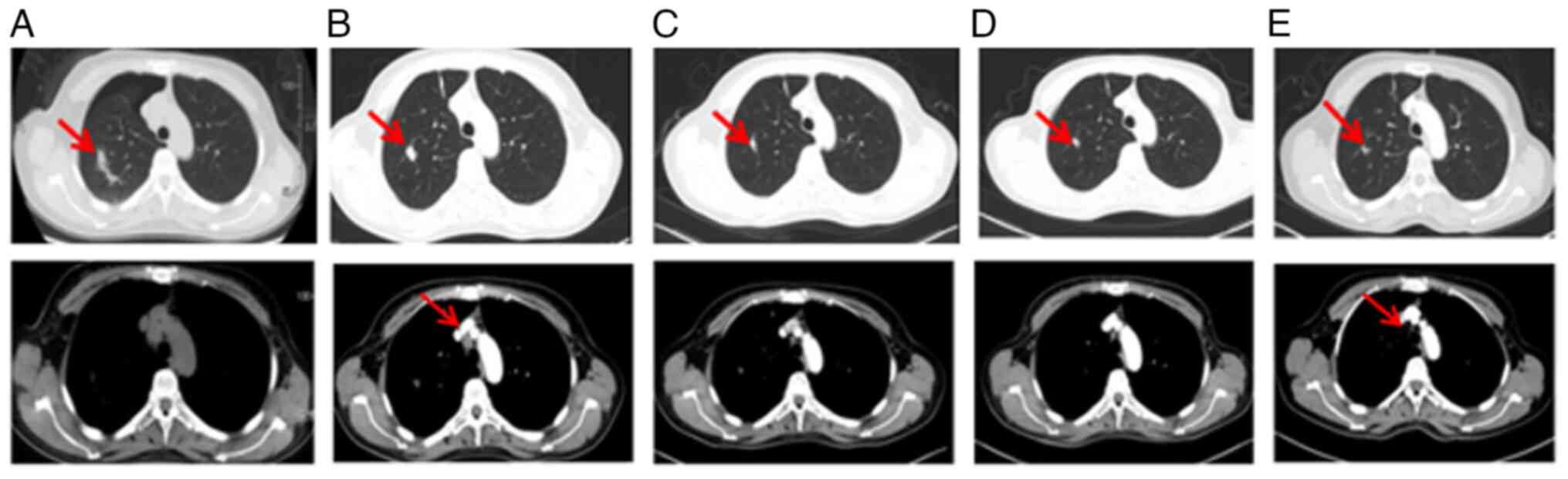
A subsequent thoracic contrast-enhanced CT scan in
May 2021 revealed the presence of several newly enlarged small
pulmonary nodules in both lungs (Fig.
9A and B) compared with the last scan in January 2021. In
addition, an abdominal and pelvic MRI identified a new abnormal
focus in the right posterior lobe of the liver, indicating the
presence of metastatic tumors (Fig.
9C). In May 2021, a percutaneous thoracic needle biopsy guided
by MRI followed by subsequent ablation was performed to obtain
tissue specimens from the largest lung nodule (Fig. 9B) which presented complete ablation
in a thoracic contrast-enhanced CT in February 2022 (Fig. 9D). The biopsy revealed a small
amount of adenocarcinoma tissue. IHC analysis showed weak
expression of caudal type homeobox 2 (CDX2), negative expression of
cytokeratin 7 (CK7), positive expression of CK20, negative Napsin A
expression, partial Special AT-rich sequence-binding protein 2
(SATB2) expression and negative thyroid transcription factor 1
(TTF1) expression (Fig. 10). Based
on these IHC results, the diagnosis was enteric adenocarcinoma,
although the possibility of metastasis could not be ruled out.
Unfortunately, the biopsy specimen contained an insufficient
quantity of tumor cells for the NGS test. The patient continued to
receive treatment with oxaliplatin (200 mg on day 1), raltitrexed
(5 mg on day 1) and bevacizumab (75 mg/kg; on day 1) every three
weeks as one cycle for six cycles. Subsequently, the treatment
regimen was adjusted to include raltitrexed, bevacizumab and
osimertinib.
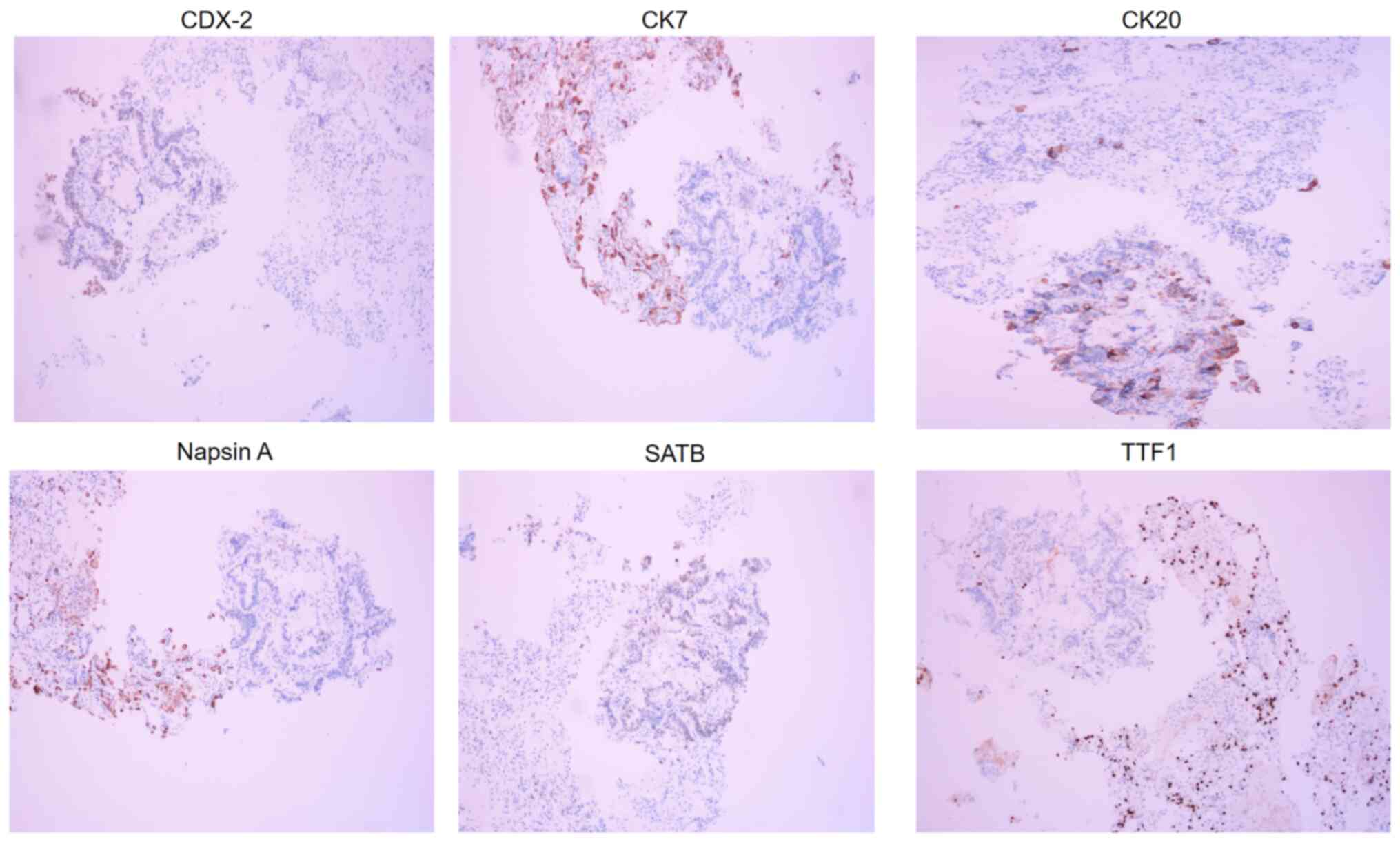 | Figure 10.Immunohistochemical analysis of the
specimen from the MRI-guided percutaneous thoracic needle biopsy
after maintaining bevacizumab therapy for 16 months (May 2021)
revealed CDX2+ (weak), CK7 (−), CK20 (+), napsin A (−), SATB2+
(partial) and TTF1 (−) staining patterns (magnification, ×200),
which supported metastatic enteric adenocarcinoma. CDX2, caudal
type homeobox 2; CK7, cytokeratin 7; SATB2, SATB homeobox 2; TTF1,
thyroid transcription factor 1. |
In February 2022, a small pulmonary nodule in the
left lung showed slight enlargement in a thoracic contrast-enhanced
CT scan (Fig. 11A) compared with
the previous scan in May (Fig. 8A)
and in November 2021, prompting an ablation procedure and a
subsequent biopsy (Fig. 11B and
C). The pathology report confirmed adenocarcinoma, although the
primary site of origin could not be determined because of
insufficient histopathologic slides for IHC and NGS. Following
this, the patient continued treatment with AZD9291, bevacizumab and
capecitabine up to the present time. Of note, the liver metastasis
(Fig. 9C and 12A) was shown to have disappeared on the
basis of an MRI examination conducted in November 2022 (Fig. 12) and at the time of conclusion of
the present study (March 2023), the patient has remained alive with
regular follow-up of laboratory test and CT/MRI scan.
Materials and methods
Pathology
Pathological assessment was performed on the
formalin-fixed, paraffin-embedded (FFPE) tissue block of
operational specimens or tissue puncture needle biopsy specimens
stained with hematoxylin and eosin (HE) according to standard
procedures (cat. no. ab245880; Abcam) (12).
Patient and samples
Pathological samples from the patient were collected
from operational specimens or puncture needle biopsy specimens.
Pathological assessment was performed on tissue sections cut from
FFPE tissue blocks. Tissue slides were stained with HE according to
standard procedures (cat. no. ab245880; Abcam) for morphological
observation.
IHC procedure
For the IHC analysis, tumor samples were obtained
from the operation or using a tissue puncture needle. Tissues were
immersed in 4% paraformaldehyde (Sigma-Aldrich; Merck KGaA) for 3–4
h. Dehydration was performed using a series of alcohol solutions:
75, 85 and 95% followed by anhydrous ethanol. The dehydrated
tissues were embedded in paraffin and 5-µm sections were cut as
required. Paraffin sections were heated at 60°C for 20–30 min,
followed by sequential treatments with xylene and an ethanol
gradient. Sections were treated with a permeabilization solution
(40 ml PBS + 120 µl TritonX-100 + 400 µl 30%
H2O2) that had been pre-warmed to 37°C for 30
min. Antigen retrieval was performed in a solution of 0.01M sodium
citrate (pH 6.0) at 90°C for 20 min. Endogenous enzyme activity was
deactivated with 3% H2O2 for 10 min at room
temperature. Non-specific loci were blocked with goat serum
blocking solution (cat. no. WE0320; Beijing Baiolaibo Technology
Co., Ltd.) for 30 min at 37°C. Primary antibodies (e.g., anti-PD-L1
antibody; 1:1,000 dilution; cat. no. JSZ1800077; Dako North
America, Inc.) were applied and incubated at 37°C for 1–2 h. After
washing, HRP-conjugated secondary antibodies (HRP anti-rabbit IgG
antibody; 1:500 dilution; cat. no. ab288151; Abcam) were added and
incubated at 37°C for 1–2 h. Diaminobenzidine (cat. no. D12384;
Sigma-Aldrich; Merck KGaA) was added for 10 min at room temperature
and the reaction was observed and confirmed by microscopy.
Restaining with hematoxylin, differentiation with
hydrochloric acid alcohol and dehydration were performed. Results
were observed under a light microscope at ×200 magnification
(RX50M; Sunny Optical Technology Co., Ltd.).
For the IHC analysis with antibodies to CDX2 (cat.
no. 20180310), ER (cat. no. 20173404076), CK7 (cat. no. 20180128),
CK20 (cat. no. 20180074), Napsin A (cat. no. 20180102), SATB2 (cat.
no. 20190103), TTF1 (cat. no. 20180077), Villin (cat. no.
20180094), MSH2 (cat. no. 20180514), MSH6 (cat. no. 20180088), PMS2
(cat. no. 20180266), MLH1 (cat. no. 20180092; all from Shanghai
Gentech Co., Ltd.) and PDL1 (cat. no. JSZ1800077; Dako North
America, Inc.), 5-µm-thick sections from formalin-fixed
paraffin-embedded tissues were utilized. Deparaffinization,
rehydration, antigen retrieval and staining procedures followed a
standard protocol (13), and
negative controls were processed without the primary antibody.
DNA extraction and EGFR
amplification-refractory mutation system (ARMS) mutation
analysis
Genomic DNA was extracted utilizing the QIAamp DNA
FFPE Tissue Kit (cat. no. 56404; Qiagen GmbH) from FFPE sections
and smear slides in accordance with the manufacturer's protocols.
The elution was performed with 50 µl Tris/Acetate/EDTA, and
subsequent quantification of genomic DNA was performed using a Nano
UV spectrophotometer (BSNA-101; Biolab Scientific Ltd.), ensuring
optical density values at 260/280 nm fell within the range of
1.8–2.0.
For mutation analysis of the EGFR gene, an AmoyDx
EGFR 29 mutations detection kit (cat. no. ADx-EG0X; Amoy
Diagnostics Co., Ltd.) was employed. The DNA template, adjusted to
a concentration of 2 ng/µl, underwent analysis on a real-time PCR
instrument (PF1457N; Stratagene Mx3005P; Thermo Fisher Scientific,
Inc.), following the manufacturer's instructions. Result
interpretation was carried out by a technician trained to identify
AmoyDx EGFR mutations. It is noteworthy that both the DNA
extraction process and the ARMS method for EGFR analysis had been
previously validated within our laboratory (14). Routine analysis of positive and
negative controls was conducted to ensure the integrity of lab
procedures.
NGS
All FFPE samples underwent independent assessment by
experienced pathologists, ensuring a minimum tumor content of 20%.
Subsequently, FFPE tissue sections and matched blood specimens from
each patient were collected and separately processed to yield
50–250 ng of DNA per sample. Following extraction, libraries were
constructed and targeted genomic regions were captured using
hybridization techniques. Sequence reads were then generated using
instruments from Illumina, Inc. Tumor tissue sample DNA variant
testing was performed in our laboratory using published methods
(14). In addition, germline
mutation comparison testing was conducted on the patient's paired
leukocyte DNA. Sequencing was carried out using the Cancer
Sequencing YS panel, which covers the entire exonic sequences of
450 genes, the TERT promoter and introns of 39 genes. Tumor DNA was
sequenced at a depth of 1,000×, while paired leukocyte DNA was
sequenced at a depth of 300×. Somatic variant types included
mutations, copy number variations, as well as rearrangements and
fusion events, detected using the MuTect, Pindel and EXCAVATOR
tools for single nucleotide variants, insertions/deletions and copy
number variations, respectively. Gene rearrangements or fusions
were identified using internally developed algorithms. All variants
were manually reviewed on the Integrative Genomics Viewer to ensure
accuracy.
Discussion
EGFR-TKIs are the standard first-line therapy for
patients with advanced or metastatic NSCLC harboring sensitive EGFR
mutations (15). Osimertinib, a
third-generation EGFR-TKI, has been shown to be effective as a
therapy in the majority of cases of EGFR-mutated NSCLC. According
to a study by Ramalingam et al (16), patients with exon 19Del or an L858R
allele who received first-line osimertinib had a median OS of 38.6
months compared with 31.8 months in the comparative group treated
with gefitinib or erlotinib, while PFS is 18.9 months compared with
10.2 months. When used as second-line treatment in patients with
the T790M mutation after having received first- or
second-generation EGFR-TKIs, osimertinib resulted in a PFS time of
10.1 months for T790M-positive disease (17). The median OS time is 31.8 after
first-generation (16) and 36.7
months after second-generation EGFR-TKIs (18), respectively. However, almost all
patients who initially responded to an EGFR-TKI eventually
developed drug resistance To extend the survival rates of patients,
a promising approach is to use combination therapy of
first-generation EGFR-TKIs with other therapeutic methods (19). Several prospective phase III studies
have shown that this combination of chemotherapy and TKI treatment
leads to a significant improvement in PFS, objective response rates
and quality of life, surpassing the benefits observed with
chemotherapy or EGFR-TKI alone (20).
In the present case report, the treatments were
aligned with the recommendations outlined in the 2016 Guidelines of
the Chinese Society of Clinical Oncology (21), which advocate for the continuation
of TKI therapy for T790M-negative patients during the slowly
progressive and oligoprogressive stages. The patient of the present
study has undergone two chemotherapy regimens, before and after the
initial administration of the first-generation TKI. Subsequently,
the patient was treated with a second first-generation TKI,
resulting in a 3-month PFS outcome with the emergence of a new
lesion. Furthermore, a previous study reported that 21% of
T790M-negative patients exhibit good efficacy when treated with
AZD9291 (22). Consequently, it was
chosen to administer this treatment to the patient and the results
obtained were highly positive. Of note, osimertinib treatment
beyond the second line of EGFR-TKI therapy showed a remarkable
benefit of PFS of 63 months at the time of conclusion of the
present study (March 2023) while the patient has remained alive
without progression, surpassing the findings of PFS and OS in the
previous studies (16,17).
Prior to 2016, in China, medical conditions
prevailed where NGS genetic testing was not readily available for
the majority of patients, and RT-qPCR analysis remained the primary
method for checking the EGFR mutation status. In the present case,
chemotherapy was administered to reverse 1st EGFR-TKI resistance
without the secondary EGFR T790M mutation ever detected by RT-qPCR
(23). Subsequently, erlotinib was
used, resulting in a PFS of 3 months. Of note, it has been reported
that-21% of patients with T790M-negative NSCLC also exhibit
sensitivity to second-line osimertinib (22). Therefore, when osimertinib became
available in China, the patient of the present study was switched
to osimertinib chemotherapy. The remarkable response to osimertinib
prompted us to confirm the T790M-negative status. NGS was performed
on the previous gefitinib- or erlotinib-resistant tumor specimens
obtained from the patient, which confirmed the presence of the EGFR
19Del and T790M mutations. Of note, no other resistance genes
associated with EGFR-TKI were identified by the NGS test during the
course of the patient's treatment.
MWA, as a local treatment for oligoprogressive
lesions, shows promise in eradicating insensitive and
drug-resistant clones, potentially restoring treatment sensitivity.
Furthermore, compared with surgery, MWA offers the advantage of
preserving more of the normal tissue, resulting in an improved
quality of life and maintaining lung function for post-procedural
patients (24). The present case
report provides compelling evidence that the patient benefitted
from undergoing five sessions of MWA combined with
chemotherapy/EGFR-TKI treatment, leading to an exceptional survival
period of 110 months, far exceeding what has been reported in the
literature (16,17).
The prevalence of multiple primary malignancies in
cancer patients, whether in the same or different organ systems,
ranges from 2–17% (25). Multiple
malignancies can be categorized as synchronous or metachronous,
based on their timing relative to the initial cancer diagnosis
(26). In the case of the present
study, the patient developed metachronous colon cancer, followed by
subsequent liver and lung metastases. The occurrence of colon
metastasis in lung cancer is relatively rare, with a previous study
reporting only 10 cases (0.19%) out of a total of 5,239 patients
with lung cancer with metastases to the colon and rectum (27). Pathology results from a colon biopsy
confirmed the presence of primary colon cancer and lung metastases
in this case. Radical surgery is the preferred treatment for
colorectal cancer. In the present case, a synchronous liver
metastasis was detected 43 days following colon cancer surgery.
Following neoadjuvant chemotherapy combined with bevacizumab, the
patient achieved a pathological complete response, indicating the
effectiveness of the chemotherapy regimen against colon cancer.
Subsequently, two oligometastases in the lung were identified
during the maintenance phase of bevacizumab treatment, occurring at
16 months (May 2021) and 28 months (May 2022), respectively. The
first oligometastasis was confirmed as metastatic enteric
adenocarcinoma through pathological examination, whereas it could
not be determined whether the second originated from the lung or
intestine due to its small size. Another liver metastasis arose in
May 2021. To supplement the maintenance therapy of bevacizumab and
osimertinib, the patient underwent six cycles of double-drug
chemotherapy. Subsequently, in line with the patient's preference,
treatment continued with osimertinib, bevacizumab and capecitabine
up to the present time. No sign of liver metastases was observed
during the MRI examination conducted in November 2022. Throughout
the disease progression, the patient underwent repeated biopsies to
assess changes in tumor pathology and to conduct genotyping for
targeted therapies. As of the latest follow-up in March 2023, no
recurrence has been detected in the patient.
While the reported case is promising, the diversity
observed in tumor characteristics among different patients raises
the need for caution in generalizing these findings to the broader
population. Tumor heterogeneity encompasses variations in genetic,
phenotypic and microenvironmental factors, influencing responses to
treatment.
To guide clinical practice effectively, further
analysis is essential to determine the degree of representativeness
of the patient of the present study. This analysis should involve a
larger and more diverse patient cohort, considering different tumor
subtypes and clinical scenarios. In addition, exploring potential
factors contributing to the observed longevity, such as specific
genetic mutations or treatment responses, will provide a more
comprehensive understanding. In the future, more cases will be
accumulated to validate our clinical results and, where possible,
conduct additional clinical research to obtain higher levels of
evidence.
Of note, the present case report did have several
limitations. The utilization of MWA in clinical practice for lung
cancer remains limited. Thus, there is an urgent need for clinical
trials to be conducted that compare the effectiveness of local
ablation with other therapies, such as radiotherapy and surgery,
when combined with systemic treatment for advanced cancers. The
present case study, however, has offered valuable insight into the
optimal approach for patients with lung cancer who have developed
acquired resistance to EGFR TKIs.
In conclusion, the combination of MWA and systemic
therapies has demonstrated its potential to effectively manage
in situ oligoprogression or ex situ oligoprogression,
and to enhance the efficacy of chemotherapy/TKI therapy in patients
with NSCLC with EGFR 19Del mutations and metachronous colon
adenocarcinoma. Repeated histologic biopsies and genetic testing
serve as valuable indicators for adjusting treatment regimens
accordingly. In addition, in cases of advanced lung cancer with a
chronic course, physicians should remain vigilant regarding the
occurrence and treatment of double primary carcinomas. Timely and
accurate adjustments to treatment plans are expected to be of
significant benefit to patients in terms of treatment efficacy and
their overall quality of life.
Acknowledgements
Not applicable.
Funding
The preparation of this study was funded by the project of the
investigation of factors associated with the planning system for
percutaneous pulmonary microwave ablation surgery (grant no.
lj007).
Availability of data and materials
The raw data of NGS can be obtained via accession
no. GVM000687 under the following link: (https://bigd.big.ac.cn/gvm/getProjectDetail?Project=GVM000687).
Authors' contributions
YL was responsible for the conceptualization of the
present study and writing the manuscript. YX, SC, JiL, FR, CX and
PL acquired the majority of the data, analyzed the data, performed
research of the literature and prepared the original draft. JuL was
in charge of the full treatment of the patient and responsible for
editing and critical review of the manuscript. YL and JuL checked
and confirmed the authenticity of the raw data. All authors have
read and approved the final manuscript for publication.
Ethics approval and consent to
participate
The study involving a human participant was reviewed
and approved by Shandong Provincial Hospital Affiliated to Shandong
First Medical University, Shandong, China (approval no. 2022–061;
Jinan, China). None of the treatments were experimental and written
informed consent for each treatment was obtained from the patient
and all procedures were conducted in accordance with the
Declaration of Helsinki.
Patient consent for publication
The patient provided consent for publication of
their case data and images.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Thandra KC and Barsouk A, Saginala K,
Aluru JS and Barsouk A: Epidemiology of lung cancer. Contemp Oncol
(Pozn). 25:45–52. 2021.PubMed/NCBI
|
|
2
|
Schad F, Thronicke A, Steele ML, Merkle A,
Matthes B, Grah C and Matthes H: Overall survival of stage IV
non-small cell lung cancer patients treated with Viscum album L. in
addition to chemotherapy, a real-world observational multicenter
analysis. PLoS One. 13:e02030582018. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Billing DL and Rimner A: Results of
radiation therapy as local ablative therapy for oligometastatic
non-small cell lung cancer. Cancers (Basel). 13:57732021.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wei Y, Cui Y, Guo Y, Li L and Zeng L: A
lung adenocarcinoma patient with a rare EGFR E709_T710delinsD
mutation showed a good response to afatinib treatment: A case
report and literature review. Front Oncol. 11:7003452021.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Liang H, Li C, Zhao Y, Zhao S, Huang J,
Cai X, Cheng B, Xiong S, Li J, Wang W, et al: Concomitant mutations
in EGFR 19Del/L858R mutation and their association with response to
EGFR-TKIs in NSCLC patients. Cancer Manag Res. 12:8653–8662. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ettinger DS, Wood DE, Aisner DL, Akerley
W, Bauman JR, Bharat A, Bruno DS, Chang JY, Chirieac LR, D'Amico
TA, et al: Non-small cell lung cancer, version 3.2022, NCCN
clinical practice guidelines in oncology. J Natl Compr Canc Netw.
20:497–530. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Pisano C, De Filippis M, Jacobs F, Novello
S and Reale ML: Management of oligoprogression in patients with
metastatic NSCLC harboring ALK rearrangements. Cancers (Basel).
14:7182022. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Nguyen KT, Sakthivel G, Milano MT, Qiu H
and Singh DP: Oligoprogression in non-small cell lung cancer: A
narrative review. J Thorac Dis. 14:4998–5011. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Shang Y, Li G, Zhang B, Wu Y, Chen Y, Li
C, Zhao W and Liu J: Image-guided percutaneous ablation for lung
malignancies. Front Oncol. 12:10202962022. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wei Z, Ye X, Yang X, Zheng A, Huang G, Li
W, Wang J, Han X, Meng M and Ni Y: Microwave ablation combined with
EGFR-TKIs versus only EGFR-TKIs in advanced NSCLC patients with
EGFR-sensitive mutations. Oncotarget. 8:56714–56725. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Mirsadraee S, Oswal D, Alizadeh Y, Caulo A
and van Beek E Jr: The 7th lung cancer TNM classification and
staging system: Review of the changes and implications. World J
Radiol. 4:128–134. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Feldman AT and Wolfe D: Tissue processing
and hematoxylin and eosin staining. Methods Mol Biol. 1180:31–43.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Magaki S, Hojat SA, Wei B, So A and Yong
WH: An introduction to the performance of immunohistochemistry.
Methods Mol Biol. 1897:289–298. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Liu J, Zhao R and Zhang J and Zhang J:
ARMS for EGFR mutation analysis of cytologic and corresponding lung
adenocarcinoma histologic specimens. J Cancer Res Clin Oncol.
141:221–227. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Nan X, Xie C, Yu X and Liu J: EGFR TKI as
first-line treatment for patients with advanced EGFR
mutation-positive non-small-cell lung cancer. Oncotarget.
8:75712–75726. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ramalingam SS, Vansteenkiste J, Planchard
D, Cho BC, Gray JE, Ohe Y, Zhou C, Reungwetwattana T, Cheng Y,
Chewaskulyong B, et al: Overall survival with osimertinib in
untreated, EGFR-mutated advanced NSCLC. N Engl J Med. 382:41–50.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Mok TS, Wu YL, Ahn MJ, Garassino MC, Kim
HR, Ramalingam SS, Shepherd FA, He Y, Akamatsu H, Theelen WS, et
al: Osimertinib or platinum-pemetrexed in EGFR T790M-positive lung
cancer. N Engl J Med. 376:629–640. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Mok TS, Cheng Y, Zhou X, Lee KH, Nakagawa
K, Niho S, Lee M, Linke R, Rosell R, Corral J, et al: Improvement
in overall survival in a randomized study that compared dacomitinib
with gefitinib in patients with advanced non-small-cell lung cancer
and EGFR-activating mutations. J Clin Oncol. 36:2244–2250. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhou Q, Xu CR, Cheng Y, Liu YP, Chen GY,
Cui JW, Yang N, Song Y, Li XL, Lu S, et al: Bevacizumab plus
erlotinib in Chinese patients with untreated, EGFR-mutated,
advanced NSCLC (ARTEMIS-CTONG1509): A multicenter phase 3 study.
Cancer Cell. 39:1279–1291.e3. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wu Q, Luo W, Li W, Wang T, Huang L and Xu
F: First-generation EGFR-TKI plus chemotherapy versus EGFR-TKI
alone as first-line treatment in advanced NSCLC with EGFR
activating mutation: A systematic review and meta-analysis of
randomized controlled trials. Front Oncol. 11:5982652021.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Chinese Society of Clinical Oncology, .
Guidelines of Chinese Society of Clinical Oncology (CSCO) Primary
Lung Cancer 2016.V1. People's Medical Publishing House, Beijing,
China. https://meeting.csco.org.cn/pdf/web/viewer.html?file=/upload/Periodical/201811/201811965213.pdfAccessed
November 9, 2018 (In Chinese).
|
|
22
|
Jänne PA, Yang JC, Kim DW, Planchard D,
Ohe Y, Ramalingam SS, Ahn MJ, Kim SW, Su WC, Horn L, et al: AZD9291
in EGFR inhibitor-resistant non-small-cell lung cancer. N Engl J
Med. 372:1689–1699. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Xia GH, Zeng Y, Fang Y, Yu SR, Wang L, Shi
MQ, Sun WL, Huang XE, Chen J and Feng JF: Effect of EGFR-TKI
retreatment following chemotherapy for advanced non-small cell lung
cancer patients who underwent EGFR-TKI. Cancer Biol Med.
11:270–726. 2014.PubMed/NCBI
|
|
24
|
Lin M, Eiken P and Blackmon S: Image
guided thermal ablation in lung cancer treatment. J Thorac Dis.
12:7039–7047. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Vogt A, Schmid S, Heinimann K, Frick H,
Herrmann C, Cerny T and Omlin A: Multiple primary tumours:
Challenges and approaches, a review. ESMO Open. 2:e0001722017.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Pan SY, Huang CP and Chen WC:
Synchronous/metachronous multiple primary malignancies: Review of
associated risk factors. Diagnostics (Basel). 12:19402022.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Gonzalez-Tallon AI, Vasquez-Guerrero J and
Garcia-Mayor MA: Colonic metastases from lung carcinoma: A case
report and review of the literature. Gastroenterology Res. 6:29–33.
2013.PubMed/NCBI
|