Introduction
Colorectal cancer (CRC) currently has the fourth
highest incidence of all cancer types and is the second leading
cause of cancer-related mortality in the US (1). Given that the 5-year overall survival
(OS) rate of patients with CRC is ~52.37% (2), it is necessary to identify more
effective treatments for these patients. At present, the standard
first-line chemotherapy regimens are combinations of 5-fluorouracil
and oxaliplatin (CAPEOX/FOLFOX) or 5-fluorouracil and irinotecan
(FOLFIRI). If one first-line treatment fails, the other regimen is
used for second-line treatment. However, the reported incidence of
fluoropyrimidine-related cardiac toxicity varies from 1.5–18%
(3). It is therefore necessary to
develop effective alternatives to fluoropyrimidines.
The ARCTIC study reported that raltitrexed, alone or
in combination with oxaliplatin or irinotecan, provided a safe
option for patients who had previously developed cardiac toxicity
from 5-fluorouracil or capecitabine (3). Raltitrexed has been recommended by the
European Society for Medical Oncology, National Institute for
Health and Clinical Excellence and Chinese Society of Clinical
Oncology as an alternative to fluoropyrimidines and is widely used
as such (4,5). A phase II study of irinotecan combined
with raltitrexed (TOMIRI) as second-line therapy for CRC was
conducted in 2003 (6). The same
group reported a median OS (mOS) of 11.9 months and median
progression-free survival (mPFS) of 4.6 months. The main adverse
events (AEs) of this regimen were diarrhea, weakness, vomiting,
infection and neutropenia. Other studies have also reported the
efficacy of this combination for treating CRC (7,8).
However, the efficacy of TOMIRI chemotherapy in the real world
still needs to be further elucidated.
Thus, in the present study, clinical experience with
TOMIRI chemotherapy in a real-world setting was summarized.
Furthermore, a series of possible prognostic factors were assessed
to identify those factors that predicted beneficial outcomes.
Materials and methods
Study cohort
The cohort of the present single-institution,
retrospective and observational study included 205 patients with
advanced CRC who had received TOMIRI chemotherapy at Harbin Medical
University Cancer Hospital (Harbin, China) between January 2017 and
December 2019 and had provided written informed consent to this
treatment. The inclusion criteria were as follows: i) Histological
diagnosis of advanced CRC; and ii) patients had received TOMIRI
chemotherapy with or without targeted therapy. The exclusion
criteria were as follows: i) History of other malignancies; and ii)
patients without efficacy evaluation every 3–4 cycles. In addition,
50 patients who had received FOLFIRI as second-line chemotherapy
were used as controls. The inclusion criteria were as follows: i)
Histological diagnosis of advanced CRC in Harbin Medical University
Cancer Hospital; and ii) patients had received FOLFIRI chemotherapy
with or without targeted therapy between May 2014 to December
2020.
Efficacy was compared between patients who had
received TOMIRI as a first-line and third- or later-line treatment,
after which the clinical efficacy of TOMIRI in patients who had
received it as second-line chemotherapy was focused on. A flow
chart of the study is presented in Fig. S1.
Data collection
Basic patient characteristics, such as chemotherapy
regimen, treatment cycles, clinical efficacy and AEs, were
independently extracted from medical records by two physicians, as
were findings from imaging examinations, including abdomen, chest
and pelvis enhanced computed tomography, and liver and rectal
nuclear magnetic resonance imaging. These had been performed every
two or three cycles as necessary to evaluate the efficacy of
chemotherapy. Laboratory findings were collected to analyze AEs
after treatment with TOMIRI, including white blood cell count, red
blood cell count, hemoglobin, platelet count, urinalysis, liver
function tests, renal function tests and electrocardiograms.
Carcinoembryonic antigen (CEA) and carbohydrate antigen 19-9
(CA19-9) concentrations were recorded prior to the first cycle of
TOMIRI. Age and pathologic tumor-node-metastasis (pTNM) stage at
the time of initial diagnosis were recorded. The last follow-up was
in December 2021.
Data assessment
The primary endpoints in the present study were OS,
PFS, objective response rate (ORR) and disease control rate (DCR).
The secondary endpoint was AEs. Tumor responses were assessed in
accordance with the Response Evaluation Criteria in Solid Tumors
version 1.1 (RECIST 1.1) (9),
namely complete remission (CR), partial remission (PR), stable
disease (SD) and progressive disease (PD), which were evaluated by
two independent physicians. ORR was defined as the sum of patients
with complete and partial remissions. The DCR was calculated as the
sum of patients with complete and partial remissions and SD. PFS
was defined as the time from the start of TOMIRI chemotherapy to
detection of any form of disease progression. OS was defined as the
time from the start of TOMIRI chemotherapy to mortality from any
cause. Treatment-related toxicity was graded according to the
Common Terminology Criteria for Adverse Events version 5.0
(10).
Statistical analysis
Basic patient characteristics were analyzed by
descriptive statistics. Survival curves were constructed using the
Kaplan-Meier method and compared using the log-rank test. In the
event of late-stage crossover of curves, the two-stage test was
performed using the R package ‘TSHRC’ (Version 0.1.6; R Foundation
for Statistical Computing) (11).
Pairwise comparisons were performed using the log-rank test with
the R package ‘survminer’ (Version 3.5.5; R Foundation for
Statistical Computing) and R package ‘survival’ (Version 0.4.9; R
Foundation for Statistical Computing). Age, sex, tumor stage,
treatment cycles, the use of targeted therapy, surgery (yes or no),
tumor location, treatment interval, local treatment (yes or no),
RAS, BRAF, first-line PFS, CEA and CA 19-9 were included in the Cox
proportional hazards model for univariate and multivariate
analysis. Statistical analysis was performed using R version 4.3.1
(R Foundation for Statistical Computing), GraphPad Prism Software
Prism Version 7 for Windows (GraphPad Software; Dotmatics) and SPSS
18.0 (SPSS, Inc.). P<0.05 was considered to indicate a
statistically significant difference.
Results
Patient characteristics
After excluding the cases that did not meet the
inclusion criteria, 205 patients who had received TOMIRI were
included in the retrospective analysis. TOMIRI chemotherapy was
used as a first-line treatment in 23 patients, as a second-line
treatment in 164 patients and as a third- or later-line treatment
in 18 patients (Fig. S1). The
baseline characteristics of all 205 patients are summarized in
Table SI.
Comparison of efficacy
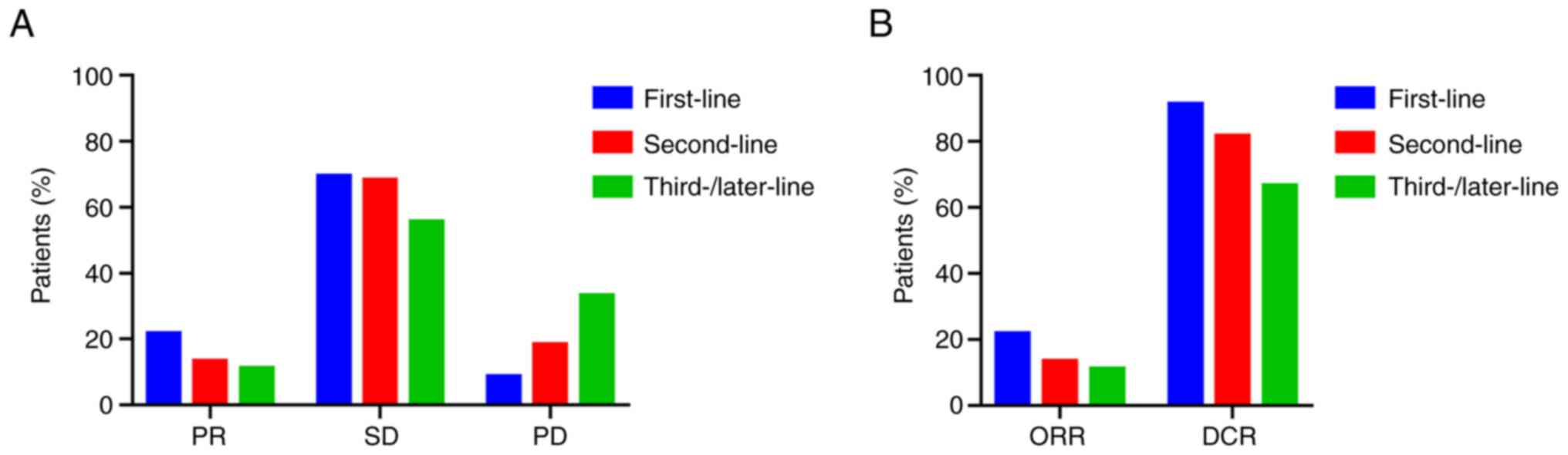
The efficacy of TOMIRI as a first, second and third-
or later-line chemotherapy was compared (Fig. 1). The ratios of PR, SD and PD are
provided in Fig. 1A. The ORRs of
patients who received TOMIRI as a first, second and third- or
later-line treatment was 21.7, 13.4 and 11.1%, respectively
(Fig. 1B). DCRs were 91.3, 81.7 and
66.7%, respectively, as presented in Fig. 1B. The median PFS of TOMIRI as
first-line chemotherapy was 9 months (95% CI, 5.3–12.7), whereas
for TOMIRI as a second-line therapy, it was 7 months (95% CI,
6.2–7.8), and as third- or later-line, it was 6 months (95% CI,
4.8–7.2). The median OS was 37 months for TOMIRI as first-line
chemotherapy (95% CI, 16.3–57.7), 21 months for TOMIRI as
second-line therapy (95% CI, 16.6–25.4) and 17 months for TOMIRI as
third- or later-line therapy (95% CI, 10.1–23.9). Earlier treatment
with TOMIRI was associated with greater efficacy, suggesting that
administering this regimen earlier may improve patient
outcomes.
To further evaluate the efficacy of TOMIRI as a
second-line treatment for CRC, differences in outcomes between the
TOMIRI and FOLFIRI regimens were compared. As indicated in Fig. S2, the differences in PFS and OS
were not statistically significant (Fig. S2A and B). However, TOMIRI with targeted therapy
achieved longer mOS (25 months) than FOLFIRI with targeted therapy
(20 months).
Details of the second-line treatment
group
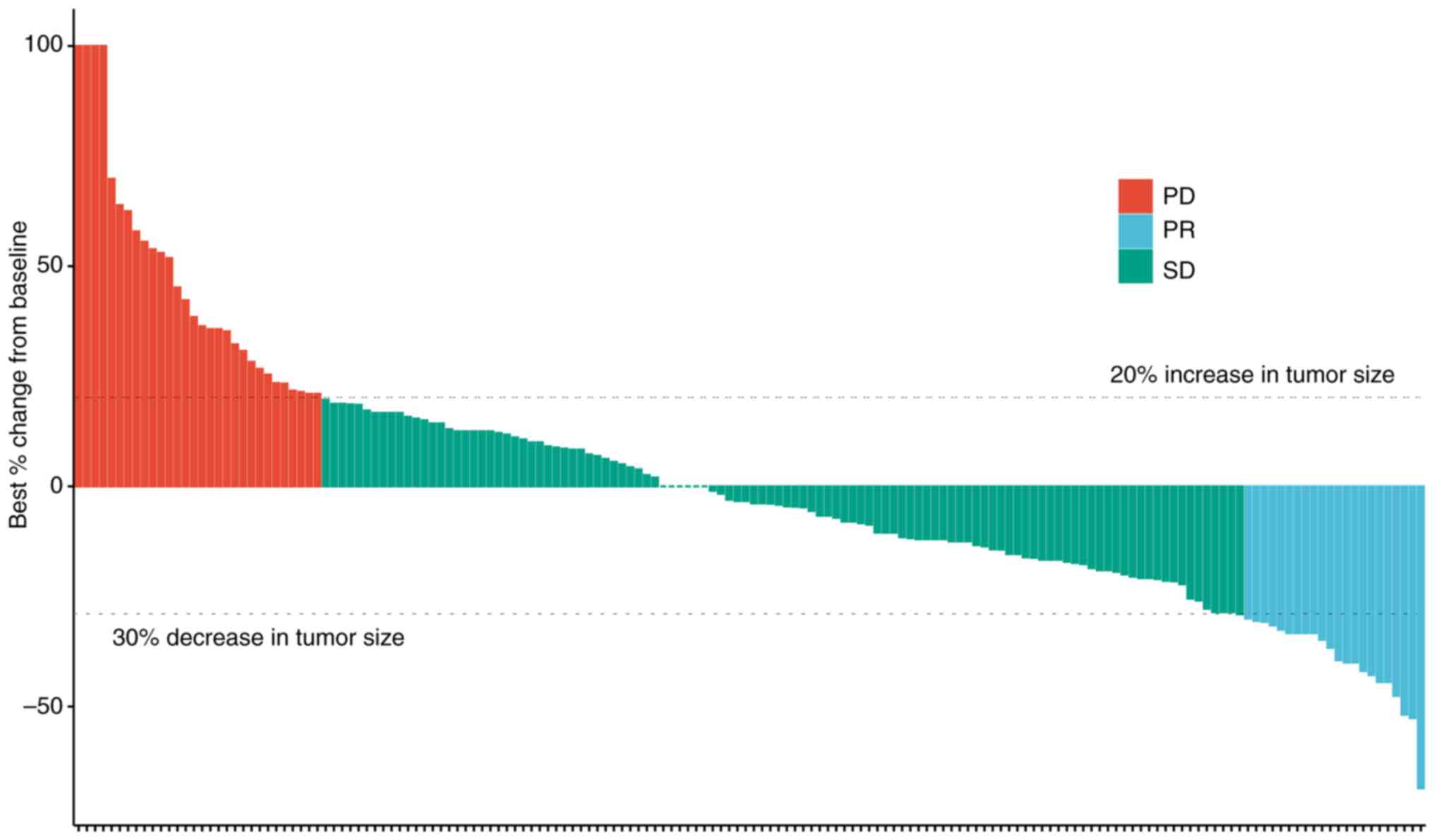
The efficacy of TOMIRI as a second-line chemotherapy
treatment was further assessed (Fig.
2). The waterfall plot showed the efficacy of patients
receiving TOMIRI, and most patients experienced tumor shrinkage
after application. Details of previous treatments are indicated in
Table SII. A total of 87 patients
had undergone radical surgery, 36 palliative surgery and 15 local
radical surgery without excision of metastases. CAPEOX and FOLFOX
were administered as first-line chemotherapy in most patients
(CAPEOX, 81.1%; FOLFOX, 11.6%). Table
SIII presents the details and outcomes of second-line
treatments. When TOMIRI was administered as a second-line therapy,
PR, SD and PD was achieved in 13.4, 68.3 and 18.3% of patients,
respectively. Patients received a median of four cycles of
treatment (range, 2–27). Localized lesions had been resected or
subjected to radiotherapy or radiofrequency ablation during
second-line therapy in 11.0% of patients. The most common third- or
later-line treatment was regorafenib (11.6%).
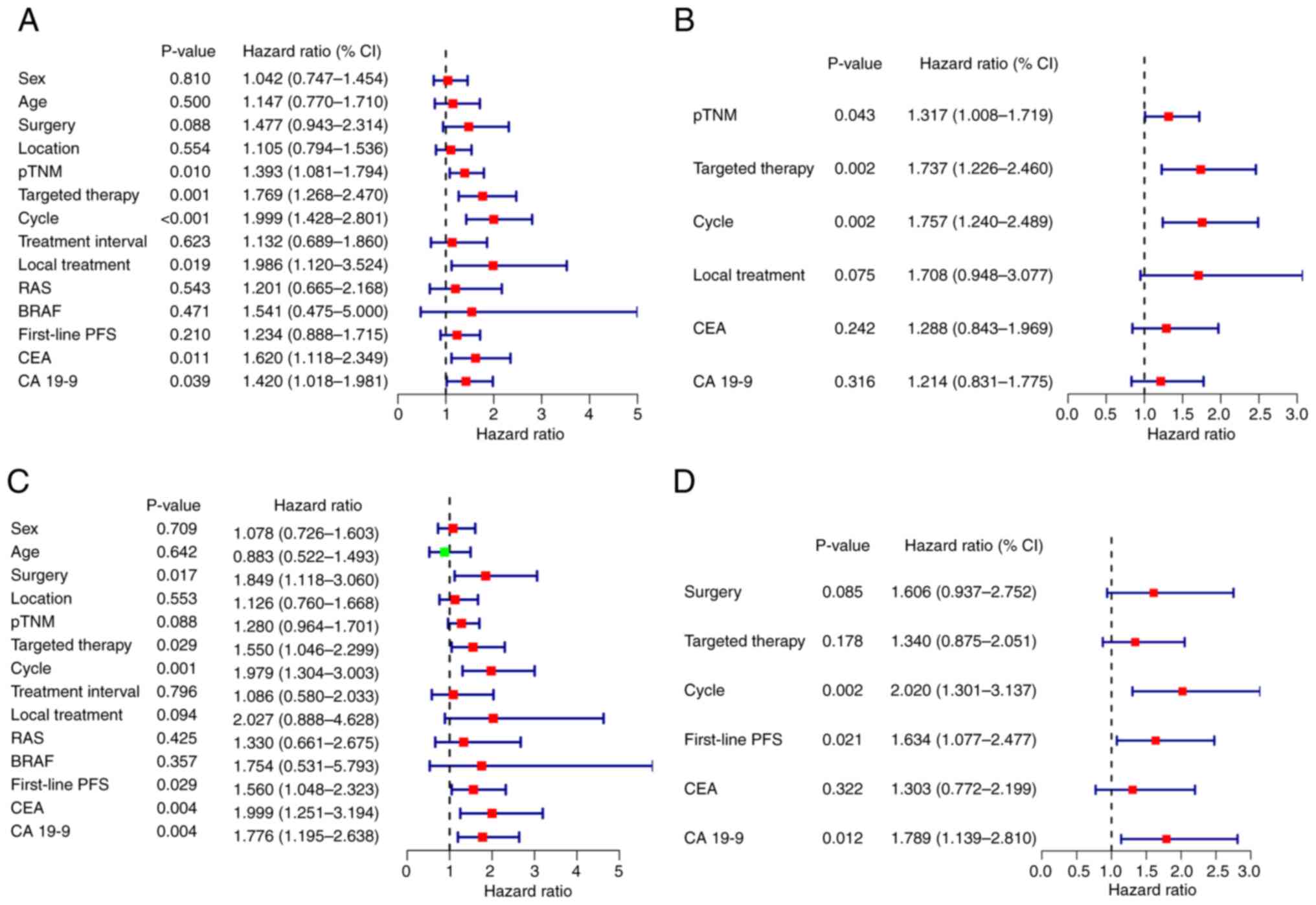
Prognostic factors
Univariate and multivariate analysis were performed
to evaluate prognostic factors (Fig.
3). Multivariate analysis demonstrated that pTNM stage,
targeted therapy and the number of treatment cycles were
independent prognostic factors for PFS, and treatment cycle,
first-line PFS and CA19-9 concentration were independent prognostic
factors for OS.
Whether or not surgery had been performed on the
primary tumor had no significant impact on PFS after second-line
treatment (P=0.17; Fig. S3A).
However, as shown in Fig. S3B, the
mOS for no surgery, local surgery, palliative surgery and radical
surgery was 14, 19, 22 and 25 months, respectively (P=0.091).
Similarly, it was found that early diagnosis was associated with a
significantly longer PFS. OS showed a similar trend; however, it
was not statistically significant (PFS: P=0.006; OS: P=0.210;
Fig. S3C and D). Fig.
S4 shows the separate impacts of T, N and M stage on prognosis.
It was demonstrated that T stage had no significant association
with prognosis (PFS: P=0.94; OS: P=0.93), whereas N stage was
significantly associated with OS (P=0.0012) and M stage with PFS
(P=0.0089). This highlights the importance of N and M stage for
prognosis of patients with advanced-stage disease.
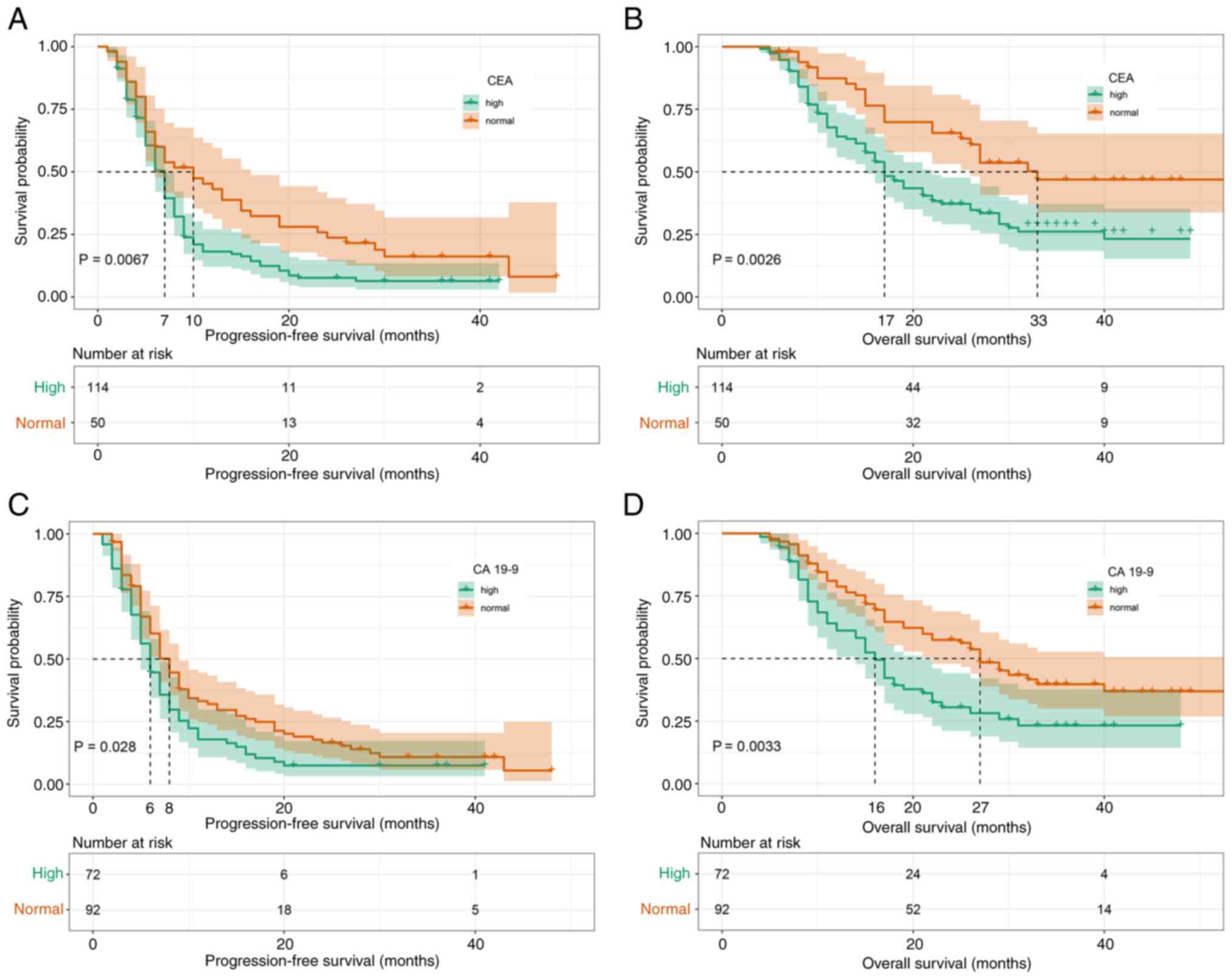
Subsequently, the prognostic effect of
pre-second-line therapy CEA and CA19-9 concentrations were
analyzed. It was found that high CEA concentrations were
significantly associated with a shorter PFS (mPFS, 7 vs. 10 months;
P=0.0067; Fig. 4A) and OS (mOS, 17
vs. 33 months; P=0.0026; Fig. 4B).
The same was true for pre-second-line therapy CA19-9 concentrations
[mPFS, 6 vs. 8 months; P=0.028 (Fig.
4C); and mOS, 16 vs. 27 months; P=0.0033 (Fig. 4D)].
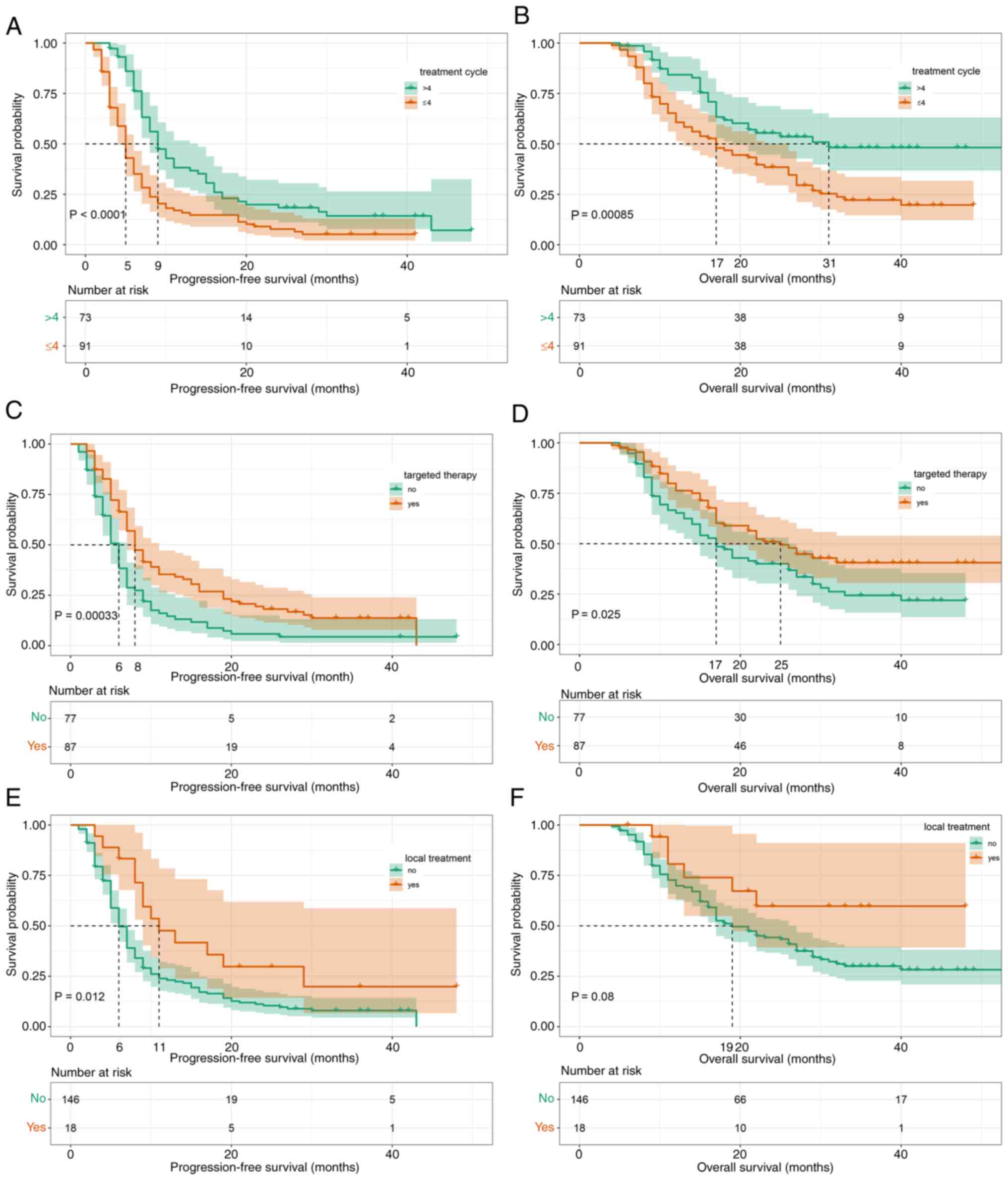
The impact of selected factors on efficacy during
second-line therapy was further assessed and significant
associations were demonstrated between the number of treatment
cycles and longer PFS (mPFS, 5 vs. 9 months; P<0.0001; Fig. 5A) and OS (mOS, 17 vs. 31 months;
P=0.00085; Fig. 5B). As recommended
by the Chinese Society of Clinical Oncology clinical guidelines
(12), 87 patients received
bevacizumab or cetuximab combined with chemotherapy, whereas the
remaining 77 patients received TOMIRI alone. It was found that
targeted therapy had significant positive effects on PFS (mPFS, 6
vs. 8 months; P=0.00033; Fig. 5C)
and OS (mOS, 17 vs. 25 months; P=0.025; Fig. 5D). A total of 18 patients had
undergone local treatment (resection of metastases, radiofrequency
ablation or radiation therapy) during second-line chemotherapy.
Local treatment had a significant association with favourable
prognosis (Fig. 5E and F),
particularly with regard to PFS (mPFS, 6 vs. 11 months;
P=0.012).
There were no differences in survival time between
patients who had twice-weekly vs. thrice-weekly treatment cycles
(Fig. S5). Similarly, the efficacy
of first-line treatment was not significantly associated with
improved PFS or OS (Fig. S6).
However, first-line PFS was significantly associated with improved
OS (mOS, 17 vs. 27 months; P=0.024).
To evaluate the importance of prognostic factors in
TOMIRI therapy, random forest regression was performed using the
following factors: pTNM stage, CEA, CA19-9, treatment cycle, local
treatment and targeted therapy. Each of these factors had been
identified as prognostic factors in TOMIRI therapy by univariate
analysis. The results are demonstrated in Fig. S7, which shows that the number of
treatment cycles and the use of targeted therapy were the most
important prognostic factors.
Safety
The occurrence of AEs for first-line, second-line
and third- or later-line treatment with TOMIRI therapy is presented
in Table SIV. Overall, treatment
was well tolerated and most hematological toxicities were grade
(G)1 or G2 (neutropenia, 12.2%; thrombocytopenia, 10.2%; anemia,
27.3%). Proteinuria (38.1%) and hematuria (21.0%) were also
commonly encountered AEs. Hepatic dysfunction was the most commonly
reported AE for both G1-2 (55.1%) and G3-4 (7.3%) categories. No
cases of gastrointestinal perforation or severe heart failure were
identified in the cohort.
Discussion
Irinotecan in combination with raltitrexed has been
widely used in patients with CRC who have developed
5-fluoropyrimidine-associated cardiac toxicity (3,5,13). The
retrospective analysis in the present study was conducted to
evaluate the clinical benefit of TOMIRI chemotherapy. Data from 205
patients who had been treated with this regimen were analyzed.
These patients were divided into three groups according to when
TOMIRI had been administered: As a first-line treatment (n=23),
second-line treatment (n=164) and third- or later-line treatment
(n=18). The clinical benefits were evaluated using the primary
endpoints of PFS, OS, ORR and DCR.
It was found that TOMIRI chemotherapy was most
effective when administered as first-line treatment (ORR, 21.7%;
DCR, 91.3%; mPFS, 10 months; and mOS, 37 months). The results for
its administration as second-line treatment (ORR, 13.4%; DCR,
81.7%; mPFS, 7 months; and mOS, 21 months) indicated that this
regimen may be more beneficial to administer earlier. This finding
is supported by a previous meta-analysis demonstrating that, when
administered as a first-line therapy, this combination had an ORR
of 34.1%, mPFS of 6.7 months and mOS of 14.2 months (4). In another study, 75 patients were
treated with raltitrexed alone, oxaliplatin + raltitrexed or TOMIRI
with or without bevacizumab. The mPFS and mOS were 10.6 (95% CI,
8.2–13.1) and 27.4 months (95% CI, 24.1–38.1), respectively
(14). Although the present study
did not reach the ORR reported in the meta-analysis, the results
for mPFS and mOS were improved as compared to a previous study.
This discrepancy may be attribuTable to insufficient numbers of
patients and different decades of treatment.
Most of the patients in the present study were in
the second-line treatment group according to the relevant
guidelines. The mOS and mPFS for FOLFIRI as a second-line therapy
were 15.4 and 6.2 months, respectively, which is consistent with
findings of previous studies (15).
In another study, the mPFS was 6.4 months with bevacizumab/FOLFOX
and 6.9 months with bevacizumab/FOLFIRI. As for mOS, it was 14.1
months with bevacizumab/FOLFOX and 15.7 months with
bevacizumab/FOLFIRI (16). In a
phase II trial, twice-weekly TOMIRI as a second-line therapy for
metastatic CRC achieved a median PFS of 4.5 months (95% CI,
3.8–5.2) and a median OS of 12.0 months (95% CI, 8.5–15.5)
(17). Thus, TOMIRI chemotherapy
achieved greater benefits in the patients in the present study than
in previous studies (15,16). In the present study, TOMIRI achieved
noninferiority PFS and OS benefit as compared with FOFIRI. TOMIRI
with targeted therapy achieved longer mOS than FOLFIRI with
targeted therapy. As the sample of the present study was relatively
small (more patients in the oncology centers of Harbin Medical
University Cancer Hospital were either administered TOMIRI or had
been enrolled in clinical trials), the findings need to be
confirmed by further clinical studies.
Furthermore, factors that identified the patients
that were most likely to benefit from TOMIRI administered as a
second-line therapy after recurrence or progression after standard
first-line chemotherapy were assessed. Kaplan-Meier curves on
variables identified as significant by univariate analysis were
analyzed, and then the results of the two statistical analyses were
combined to reach the following conclusions: Surgical resection,
even palliative surgery on the primary tumor, significantly and
positively impacted long-term survival, which is consistent with
previously published data (18,19).
However, it had no effect on the outcomes of second-line treatment.
Of note, tumor stage at first diagnosis was a greater predictor of
efficacy of second-line treatment. However, N and M stage, but not
T stage, were associated with a greater duration of survival. As
reported in a previous study, the prognosis after second-line
therapy varied considerably according to tumor biomarker status
(20). Results from the present
study demonstrated that patients with normal and high CEA
concentrations before second-line treatment achieved an mPFS of 7
and 10 months, respectively, whereas they achieved an mOS of 17 and
31 months, respectively. Normal CA19-9 concentrations were also
associated with a significant survival advantage compared with high
CA19-9 concentrations (mPFS, 6 vs. 8 months; mOS, 17 vs. 33
months). Previous analyses have shown that patients who have better
responses to first-line chemotherapy or longer PFS are more likely
to benefit from second-line chemotherapy (21,22).
In the present study, a comprehensive analysis of tumor responses
demonstrated that patients who had longer PFS with first-line
chemotherapy had longer OS, but not PFS, with second-line
chemotherapy.
The efficacy of second-line chemotherapy is highly
dependent on the combination used. Results of the present study
showed that administration of targeted therapy, whether bevacizumab
or cetuximab, was associated with prolongation of the survival time
(OS, 25 vs. 17 months). However, as so few patients received
targeted therapy, it was not possible to distinguish between the
impacts of bevacizumab vs. cetuximab when used as a component of
second-line treatment. Furthermore, the PRODIGE18 study reported a
non-significant difference between bevacizumab and cetuximab with
chemotherapy when administered to patients with wild-type RAS
metastatic CRC (23). However,
certain patients who underwent local treatment of metastases,
including resection, radiofrequency ablation and radiation therapy,
had improved outcomes. These findings need to be further validated
by large prospective trials. Furthermore, the present study found
no significant difference in efficacy and safety between
twice-weekly and thrice-weekly cycles, which is consistent with
previously reported findings (24,25).
The present study did not reveal any significant
association between treatment time-line and clinical AEs. Overall,
the incidence of G3/4 AEs was low. However, the patients did
present with higher incidences of myelosuppression and liver
dysfunction than previously reported (6). This indicates that it is important to
protect liver function and prevent myelosuppression whilst using
TOMIRI chemotherapy.
In conclusion, the findings of the present study
suggest that irinotecan in combination with raltitrexed
chemotherapy may be a superior choice than FOLFIRI for second-line
chemotherapy in patients with CRC.
Supplementary Material
Supporting Data
Supporting Data
Acknowledgements
Not applicable.
Funding
The present work was supported by the National Natural Science
Foundation of China (grant no. U20A20376), Beijing Award Foundation
(grant no. YXJL-2020-0818-0478), Heilongjiang Province Postdoctoral
Science Foundation (grant no. LBHZ21189), Harbin Medical University
Innovative Science Research Funded Project (grant no.
2022-KYYWF-0289) and China Postdoctoral Science Foundation (grant
no. 2022MD713747).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
JL, XP, BX and HL conceived and designed the
analysis. JL, RW, XW, XP, BX, ST and JS collected the data. JL, RW
and XW performed the analysis. JL and HL wrote the manuscript. All
authors have read and approved the final manuscript. JL and HL
confirm the authenticity of all the raw data.
Ethics approval and consent to
participate
The research protocol was approved by Harbin Medical
University Cancer Hospital (approval no. KY2017-19) and the study
was conducted in accordance with the principles of the Declaration
of Helsinki. Written informed consent for treatment with the TOMIRI
or FOLFIRI regimen was obtained from the patients or their parents
before the start of treatment.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Siegel RL, Miller KD, Fuchs HE and Jemal
A: Cancer statistics, 2021. CA Cancer J Clin. 71:7–33. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Li X, Zhou Y, Luo Z, Gu Y, Chen Y, Yang C,
Wang J, Xiao S, Sun Q, Qian M and Zhao G: The impact of screening
on the survival of colorectal cancer in Shanghai, China: A
population based study. BMC Public Health. 19:10162019. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ransom D, Wilson K, Fournier M, Simes RJ,
Gebski V, Yip D, Tebbutt N, Karapetis CS, Ferry D, Gordon S and
Price TJ: Final results of Australasian gastrointestinal trials
group ARCTIC study: An audit of raltitrexed for patients with
cardiac toxicity induced by fluoropyrimidines. Ann Oncol.
25:117–121. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Barni S, Ghidini A, Coinu A, Borgonovo K
and Petrelli F: A systematic review of raltitrexed-based first-line
chemotherapy in advanced colorectal cancer. Anticancer Drugs.
25:1122–1128. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Batra A, Rigo R, Hannouf MB and Cheung WY:
Real-world safety and efficacy of raltitrexed in patients with
metastatic colorectal cancer. Clin Colorectal Cancer. 20:e75–e81.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Aparicio J, Vicent JM, Maestu I, Garcerá
S, Busquier I, Bosch C, Llorca C, Díaz R, Fernández-Martos C and
Galán A: Multicenter phase II trial evaluating a three-weekly
schedule of irinotecan plus raltitrexed in patients with
5-fluorouracil-refractory advanced colorectal cancer. Ann Oncol.
14:1121–1125. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Chiara S, Nobile MT, Tomasello L, Acquati
M, Taveggia P, Murolo C, Percivale P and Rosso R: Phase II trial of
irinotecan and raltitrexed in chemotherapy-naive advanced
colorectal cancer. Anticancer Res. 25:1391–1396. 2005.PubMed/NCBI
|
|
8
|
Aparicio J, de las Peñas R, Vicent JM,
Garcerá S, Llorca C, Maestu I, Yuste AL and Farrés J: Multicenter
phase I study of irinotecan plus raltitrexed in patients with
5-fluorouracil-refractory advanced colorectal cancer. Oncology.
63:42–47. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Schwartz LH, Seymour L, Litière S, Ford R,
Gwyther S, Mandrekar S, Shankar L, Bogaerts J, Chen A, Dancey J, et
al: RECIST 1.1-standardisation and disease-specific adaptations:
Perspectives from the RECIST working group. Eur J Cancer.
62:138–145. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Basch E, Dueck AC, Rogak LJ, Mitchell SA,
Minasian LM, Denicoff AM, Wind JK, Shaw MC, Heon N, Shi Q, et al:
Feasibility of implementing the patient-reported outcomes version
of the common terminology criteria for adverse events in a
multicenter trial: NCCTG N1048. J Clin Oncol. 36:JCO20187886202018.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Li H, Han D, Hou Y, Chen H and Chen Z:
Statistical inference methods for two crossing survival curves: A
comparison of methods. PLoS One. 10:e01167742015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Dong C, Ding Y, Weng S, Li G, Huang Y, Hu
H, Zhang Z, Zhang S and Yuan Y: Update in version 2021 of CSCO
guidelines for colorectal cancer from version 2020. Chin J Cancer
Res. 33:302–307. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Fohlen A, Bordji K, Assenat E, Gongora C,
Bazille C, Boulonnais J, Naveau M, Breuil C, Pérès EA, Bernaudin M
and Guiu B: Anticancer drugs for intra-arterial treatment of
colorectal cancer liver metastases: In-vitro screening after short
exposure time. Pharmaceuticals (Basel). 14:6392021. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Gallois C, Hafliger E, Auclin E, Perret A,
Coutzac C, Turpin A, Pellat A, Randrian V, Basile D, Faroux R, et
al: First-line chemotherapy with raltitrexed in metastatic
colorectal cancer: An association des gastro-entérologues
oncologues (AGEO) multicentre study. Dig Liver Dis. 54:684–691.
2022. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Clarke SJ, Yip S, Brown C, van Hazel GA,
Ransom DT, Goldstein D, Jeffrey GM, Tebbutt NC, Buck M, Lowenthal
RM, et al: Single-agent irinotecan or FOLFIRI as second-line
chemotherapy for advanced colorectal cancer; results of a
randomised phase II study (DaVINCI) and meta-analysis [corrected].
Eur J Cancer. 47:1826–1836. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Bendell JC, Tournigand C, Swieboda-Sadlej
A, Barone C, Wainberg ZA, Kim JG, Pericay C, Pastorelli D, Tarazi
J, Rosbrook B, et al: Axitinib or bevacizumab plus FOLFIRI or
modified FOLFOX-6 after failure of first-line therapy for
metastatic colorectal cancer: A randomized phase II study. Clin
Colorectal Cancer. 12:239–247. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Cheng K, Zhou YW, Chen Y, Li ZP, Qiu M and
Liu JY: Biweekly raltitrexed combined with irinotecan as
second-line therapy for patients with metastatic colorectal cancer:
A phase II trial. Cancer Control. 29:107327482210803322022.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Arhin ND, Shen C, Bailey CE, Matsuoka LK,
Hawkins AT, Holowatyj AN, Ciombor KK, Hopkins MB, Geiger TM, Kam
AE, et al: Surgical resection and survival outcomes in metastatic
young adult colorectal cancer patients. Cancer Med. 10:4269–4281.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wong EYT, Tan GHC, Ng DWJ, Koh TPT, Kumar
M and Teo MCC: Surgical management of metastatic colorectal cancer:
A single-centre experience on oncological outcomes of pulmonary
resection vs cytoreductive surgery and HIPEC. J Gastrointest
Cancer. 48:353–360. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Yoshino T, Obermannová R, Bodoky G,
Garcia-Carbonero R, Ciuleanu T, Portnoy DC, Kim TW, Hsu Y, Ferry D,
Nasroulah F and Tabernero J: Baseline carcinoembryonic antigen as a
predictive factor of ramucirumab efficacy in RAISE, a second-line
metastatic colorectal carcinoma phase III trial. Eur J Cancer.
78:61–69. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Palmieri LJ, Fihri A, Doat S, Dubreuil O,
Manceau G, Karoui M, Wagner M, Lucidarme O and Bachet JB:
Tumor-size responses to first-line is a predictor of overall
survival in metastatic colorectal cancer. Eur Radiol. 29:3871–3880.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Lavacchi D, Roviello G, Giommoni E, Dreoni
L, Derio S, Brugia M, Amedei A, Pillozzi S and Antonuzzo L:
Aflibercept plus FOLFIRI as second-line treatment for metastatic
colorectal cancer: A single-institution real-life experience.
Cancers (Basel). 13:38632021. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Bennouna J, Hiret S, Bertaut A, Bouché O,
Deplanque G, Borel C, François E, Conroy T, Ghiringhelli F, des
Guetz G, et al: Continuation of bevacizumab vs cetuximab plus
chemotherapy after first progression in KRAS wild-type metastatic
colorectal cancer: The UNICANCER PRODIGE18 randomized clinical
trial. JAMA Oncol. 5:83–90. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Matsuda C, Honda M, Tanaka C, Fukunaga M,
Ishibashi K, Munemoto Y, Hata T, Bando H, Oshiro M, Kobayashi M, et
al: Multicenter randomized phase II clinical trial of oxaliplatin
reintroduction as a third- or later-line therapy for metastatic
colorectal cancer-biweekly versus standard triweekly XELOX (the
ORION study). Int J Clin Oncol. 21:566–572. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Hurwitz H, Mitchell E, Cartwright T, Kwok
A, Hu S, McKenna E and Patt YZ: A randomized, phase II trial of
standard triweekly compared with dose-dense biweekly capecitabine
plus oxaliplatin plus bevacizumab as first-line treatment for
metastatic colorectal cancer: XELOX-A-DVS (dense versus standard).
Oncologist. 17:937–946. 2012. View Article : Google Scholar : PubMed/NCBI
|