Introduction
Lung cancer remains a leading cause of
cancer-related mortality globally, with small cell lung cancer
(SCLC) representing ~13% of all cases (1). The United States Department of
Veterans Affairs categorizes SCLC into two stages: Limited-stage
(LS)-SCLC and extensive-stage (ES)-SCLC (2). In China, SCLC accounts for 13–15% of
all lung cancer cases, with ~180,000 new cases reported annually
(3).
SCLC is characterized by rapid proliferation and
early onset of distant metastasis, with ~70% of patients diagnosed
at the extensive stage (4). The
brain is frequently affected by distant metastasis in SCLC, with
10–24% of patients exhibiting brain metastases (BM) at diagnosis
and >50% developing them during the disease (5).
Recent advancements in comprehensive treatment have
incrementally improved SCLC survival rates, subsequently increasing
the incidence of BM. Within 2 years of achieving complete or
partial remission, 67% of patients with LS-SCLC experience BM, with
survival extending >2 years in 50–80% of cases (6). Prophylactic cranial irradiation (PCI)
significantly reduces the risk of BM and enhances overall survival
(OS), thus becoming the standard post-radiotherapy and chemotherapy
treatment for LS-SCLC (7,8). Nevertheless, certain patients still
develop BM post-PCI, underscoring the need for further refinement
in selecting candidates for this intervention.
Advancements in therapeutic strategies, including
enhanced chemoradiotherapy protocols and precise radiation
techniques, have significantly improved the management of LS-SCLC
(9,10). Nevertheless, the aggressive nature
of SCLC, characterized by rapid cell division and early metastasis,
remains challenging. Brain metastases are especially problematic
due to the blood-brain barrier, which limits the effectiveness of
many systemic therapies, thus necessitating the use of PCI as a
preventive measure (11).
Identifying patients at higher risk for BM is
crucial for optimizing treatment protocols and improving outcomes.
Previous studies have emphasized the significance of factors such
as tumor size and treatment response in predicting BM (12). Larger tumors and partial responses
to treatment are associated with an increased risk of BM. Research
into molecular and genetic markers, such as circulating tumor cells
(CTCs) and specific gene mutations, holds promise for more
accurately predicting BM risk (13). Integrating these biomarkers into
clinical practice could lead to more personalized treatment
approaches, thereby improving survival rates and quality of life
for LS-SCLC patients (14).
In the present study, a retrospective analysis was
performed of clinical data from 290 patients with LS-SCLC who
achieved complete remission (CR)/partial remission (PR) following
PCI at Chengde Central Hospital (Chengde, China) and Hebei Cangzhou
Hospital of Integrated Traditional Chinese and Western Medicine
(Cangzhou, China). The aim was to elucidate the clinical
characteristics that influence the risk of developing BM and
prognosis after PCI.
Patients and methods
Clinical data
The present study gathered clinical data from 290
patients diagnosed with LS-SCLC who received PCI after achieving CR
or PR. The data collection spanned from January 2015 to December
2023 at Chengde Central Hospital and Hebei Cangzhou Hospital of
Integrated Traditional Chinese and Western Medicine. The time of
collecting the statistical data was the same for both hospitals.
The present study is based entirely on previously recorded patient
data. All patients had a confirmed diagnosis of SCLC, either
pathologically or cytologically, and were free of secondary primary
malignancies. Restaging was performed using the American Joint
Committee on Cancer (AJCC) Lung Cancer 8th Edition
tumor-node-metastasis (TNM) clinical staging criteria (15) and the Department of Veterans Affairs
two-stage system (2). The factors
analyzed in the present study included age, sex, performance status
(PS) score, initial tumor maximum diameter and treatment
modalities. The inclusion criteria were as follows: i) Histological
or cytological confirmation of SCLC; ii) initial diagnosis of
LS-SCLC staged according to the 8th edition of the AJCC Cancer
Staging Manual and the Veterans Administration Lung Study Group
two-tier system (2); and iii)
initial treatment with curative intent chemoradiotherapy (CRT;
concurrent or sequential), followed by PCI after achieving CR or
PR. The exclusion criteria were as follows: i) Presence of a second
primary malignancy or other histological types of cancer; ii)
diagnosis of ES-SCLC; iii) loss to follow-up or incomplete clinical
data; and iv) absence of brain magnetic imagining resonance (MRI)
data prior to PCI to exclude BM; vi) those who had surgical
interventions.
In the present study, levels of carcinoembryonic
antigen (CEA) and neuron-specific enolase (NSE) were measured from
blood samples collected from patients at the two medical centers.
Assessments were performed using the Cobas® E 601 module
analyzer (Roche Diagnostics) using the electrochemiluminescence
method, with Elecsys® CEA and NSE assay kits (Roche
Diagnostics, Elecsys® CEA Assay Kit: Cat. no.
11731629322; Elecsys® NSE Assay Kit: Cat. no.
04827021190). To maintain data integrity and accuracy, a dedicated
Laboratory Data Collection Team was formed, which was responsible
for the collection and verification of laboratory data from both
centers, ensuring uniformity in reference ranges. Established
reference ranges for CEA and NSE were set at 0–5 and 0–16 ng/ml,
respectively.
Treatment
All patients underwent standard chemotherapy and PCI
according to the Chinese Society of Clinical Oncology guidelines
(12,16). The preferred modality was concurrent
CRT, with sequential CRT used when the former was not tolerable;
54.5% received concurrent treatment. Those who had surgical
interventions were excluded from the analysis. Chemotherapy
comprised 4–6 cycles of etoposide combined with cisplatin or
carboplatin, with concurrent and induction chemotherapy involving
2–3 cycles and 1–3 cycles, respectively (8).
Chemotherapy regimen
All patients underwent standard chemotherapy
consisting of etoposide and platinum-based drugs (cisplatin or
carboplatin). Etoposide was administered intravenously at a dose of
100 mg/m2 on days 1 to 3 of each cycle. Cisplatin was
administered intravenously at a dose of 75 mg/m2 on day
1, or carboplatin was administered intravenously at an area under
the curve (AUC) of 5 on day 1. Each chemotherapy cycle lasted 21
days, and patients typically received 4 to 6 cycles of
chemotherapy. Thoracic radiotherapy was administered either as 45
Gy in 30 fractions twice daily or as 54–70 Gy in 28–30 fractions
once daily. Response to CRT was evaluated using the Response
Evaluation Criteria in Solid Tumours 1.1 criteria (17). Patients achieving a CR or PR
proceeded with PCI. Brain MRI was performed prior to PCI in all
cases to rule out metastases. PCI typically commenced 4–6 weeks
post-CRT, delivered as 25 Gy in 5 weekly fractions over 2 weeks
(18). Hippocampal delineation
adhered to the RTOG0933 principles (19), ensuring a maximum dose to the
hippocampus of <17 Gy and an average dose of <10 Gy (20). Dose constraints for high-risk organs
were set as follows: Brainstem, ≤54 Gy; spinal cord, ≤45 Gy;
temporal lobe, ≤65 Gy; optic chiasm and nerve, ≤54 Gy; pituitary,
mean dose ≤45 Gy; eye, ≤50 Gy or mean dose, ≤35 Gy; lens, ≤9 Gy;
mandible and temporomandibular joint, ≤70 Gy; parotid gland mean
dose, ≤26 Gy, and V30, ≤50% (at least unilaterally) or D20cc, ≤20
Gy (bilaterally), with average doses kept at <10 Gy and maximum
doses of <17 Gy.
Follow-up and efficacy evaluation
Efficacy evaluation was performed 1 month following
the completion of CRT. Patients underwent follow-up assessments
every 3 months for the first 2 years post-treatment, every 6 months
until the fifth year, and annually thereafter. These assessments
included chest and abdominal CT scans. In instances of headaches or
neurological symptoms, an immediate brain MRI was administered.
Follow-up methods comprised patient revisits, telephone
consultations and reviews of registration data. Survival metrics,
such as OS, were calculated from the onset of treatment to death or
the last follow-up. The time to BM was measured from initiation of
treatment to confirmation via imaging. As of January 2024,
follow-up data was up-to-date, with a median duration of 55 months,
ranging from 11–102 months.
Statistical analysis
Statistical analyses were performed using SPSS
software, version 27.0 (IBM Corp.). Survival data were analyzed
using the Kaplan-Meier method coupled with the log-rank test.
Single-factor and multifactorial risk factors impacting BM and OS
were assessed using Cox regression analysis. All tests performed
were two-tailed and P<0.05 was considered to indicate a
statistically significant difference.
Results
Analysis of clinical
characteristics
At the time of follow-up, basic clinical data were
collected from 290 patients involved in this study. The median age
was 58 years, ranging from 42–74 years. A total of 44.5% of these
patients (129 cases) presented with an initial tumor diameter of
>5 cm at the onset of treatment. The clinical characteristic of
the patients are presented in Table
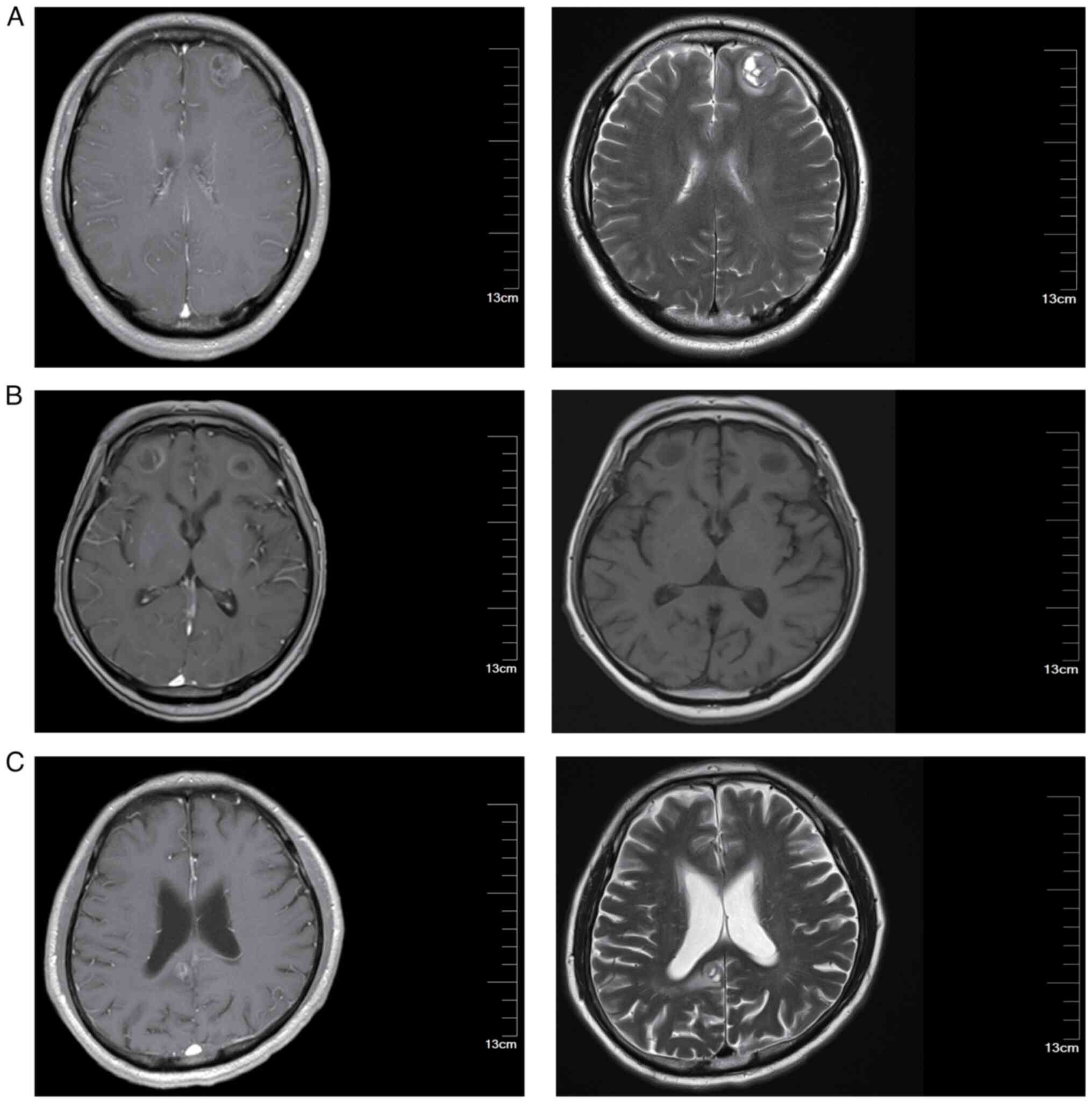
I. Representative brain MRI images are shown in Fig. 1. The MRI images presented in this
study are representative images taken when brain metastases were
first detected in the patients. These images provide a visual
representation to help readers better understand the typical
appearance and progression of brain metastases in patients with
LS-SCLC.
 | Table I.Clinical characteristics of 290
patients with limited stage-small cell lung cancer. |
Table I.
Clinical characteristics of 290
patients with limited stage-small cell lung cancer.
| Characteristic | n (%) |
|---|
| Age, years |
|
|
<60 | 169 (58.3) |
|
≥60 | 121 (41.7) |
| Sex |
|
|
Male | 136 (46.9) |
|
Female | 154 (53.1) |
| PS |
|
|
0-1 | 214 (73.8) |
| 2 | 76 (26.2) |
| Smoking |
|
| No | 153 (52.8) |
|
Yes | 137 (47.2) |
| Initial tumor
maximum diameter, cm |
|
| ≤5 | 161 (55.5) |
|
>5 | 129 (44.5) |
| N stage |
|
| N0 | 41 (14.1) |
| N1 | 33 (11.4) |
| N2 | 69 (23.8) |
| N3 | 147 (50.7) |
| Clinical stage |
|
| I | 24 (8.3) |
| II | 34 (11.7) |
|
III | 232 (80.0) |
| Treatment |
|
|
Concurrent | 158 (54.5) |
|
Sequential | 132 (45.5) |
| Response |
|
| CR | 53 (18.3) |
| PR | 237 (81.7) |
Factors associated with BM
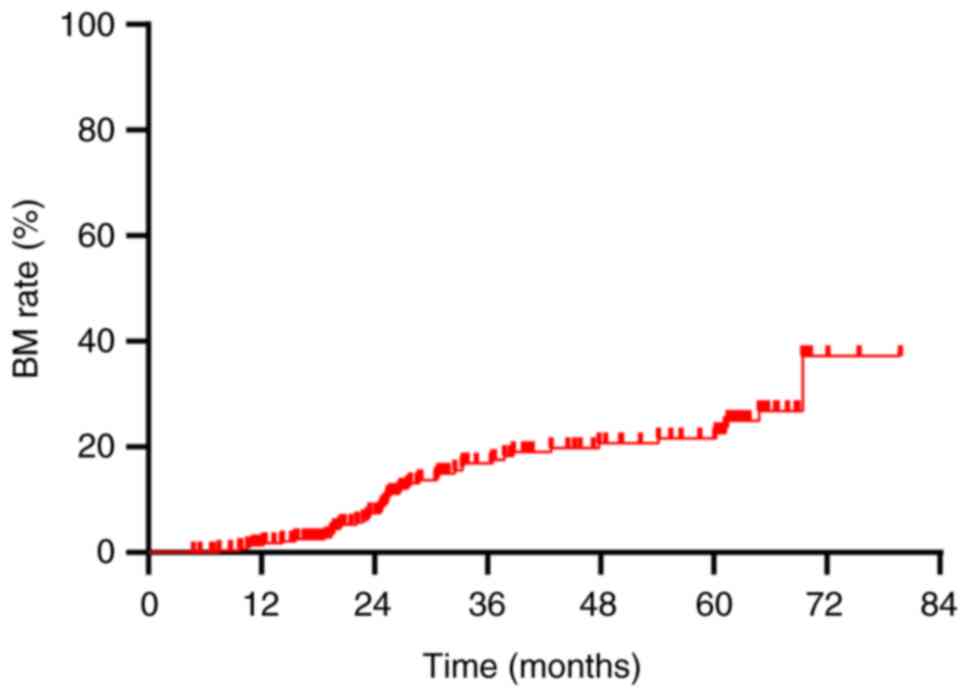
The overall BM rate was demonstrated to be 16.6%
(48/290). Annual rates of BM at 1, 2 and 3 years post-diagnosis
were 1.4, 6.6 and 12.8%, respectively (Fig. 2). This study established a tumor
size of 5 cm as the initial standard, grounded in the AJCC staging
criteria for lung cancer, where T3 is defined as a tumor greater
than 5 cm. To validate this standard, statistical analyses were
conducted using various tumor sizes as classification criteria, and
the results were compared. The analyses revealed no statistically
significant differences in BM and OS when 3,4,6 and 7 cm were used
as classification criteria (P>0.05) (Table II). This finding further supports
the statistical and clinical significance of using 5 cm as the
grouping standard. A detailed analysis of factors influencing BM
highlighted significant associations in univariate analysis:
Notably, the maximum diameter of the initial tumor [hazard ratio
(HR)=13.276; 95% confidence interval (CI): 5.248–33.586;
P<0.001], type of treatment administered (HR=2.149; 95% CI:
1.199–3.851; P=0.010) and treatment response (HR=2.981; 95% CI:
1.231–7.223; P=0.016) were significantly associated with an
increased risk of BM following Prophylactic cranial irradiation
(PCI) (Table III). Multivariable
Cox regression analysis identified that an initial tumor maximum
diameter of >5 cm was an independent risk factor for BM post-PCI
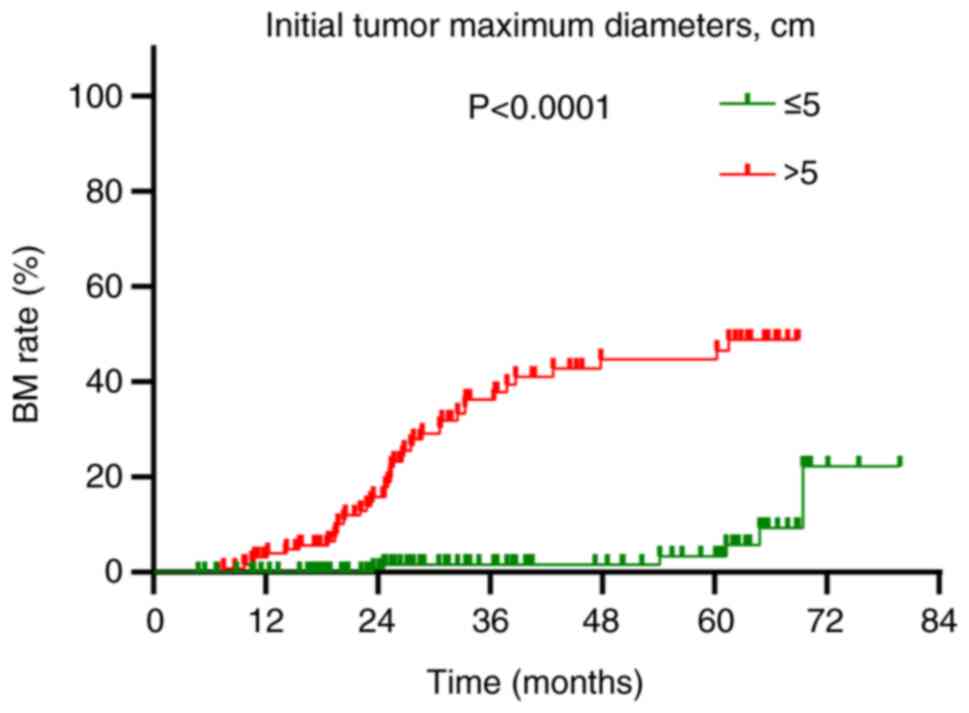
(HR=15.031; 95% CI: 5.610–40.270; P<0.001; Table III). Patients with tumors >5 cm
in diameter experienced a BM rate of 32.6% (42/129), which was
significantly higher than the 3.7% (6/161) observed in patients
with tumors of ≤5 cm in diameter (P<0.001; Fig. 3).
 | Table II.Effect of initial tumor size in
relation to brain metastases and overall survival. |
Table II.
Effect of initial tumor size in
relation to brain metastases and overall survival.
| A, Effect on brain
metastases |
|---|
|
|---|
| Initial tumor
maximum diameter, cm | HR | 95% CI | P-value |
|---|
| >3 (n=181) vs.
≤3 (n=109) | 1.451 | 0.879–2.397 | 0.145 |
| >4 (n=154) vs.
≤4 (n=136) | 1.395 | 0.864–2.251 | 0.173 |
| >6 (n=92) vs. ≤6
(n=198) | 1.462 | 0.902–2.371 | 0.123 |
| >7 (n=53) vs. ≤7
(n=237) | 1.334 | 0.818–2.175 | 0.249 |
|
| B, Effect on
overall survival |
|
| Initial tumor
maximum diameter, cm | HR | 95% CI | P-value |
|
| >3 (n=181) vs.
≤3 (n=109) | 1.164 | 0.905–1.497 | 0.238 |
| >4 (n=154) vs.
≤4 (n=136) | 1.052 | 0.824–1.343 | 0.684 |
| >6 (n=92) vs. ≤6
(n=198) | 1.225 | 5.248–3.586 | 0.111 |
| >7 (n=53) vs. ≤7
(n=237) | 1.194 | 0.922–1.546 | 0.179 |
 | Table III.Analysis of factors affecting brain
metastasis in 290 patients with limited stage-small cell lung
cancer. |
Table III.
Analysis of factors affecting brain
metastasis in 290 patients with limited stage-small cell lung
cancer.
|
| Univariate
analysis | Multifactorial Cox
analysis |
|---|
|
|
|
|
|---|
| Characteristic | HR | 95% CI | P-value | HR | 95% CI | P-value |
|---|
| Age (<60 vs. ≥60
years) | 1.006 | 0.563–1.795 | 0.985 |
|
|
|
| Sex (female vs.
male) | 1.482 | 0.837–2.623 | 0.177 |
|
|
|
| PS (2 vs. 0–1) | 1.416 | 0.769–2.608 | 0.264 |
|
|
|
| Smoking (yes vs.
no) | 1.387 | 0.787–2.445 | 0.258 |
|
|
|
| Initial tumor
maximum diameter (>5 vs. ≤5 cm) | 13.276 | 5.248–33.586 | <0.001 | 15.031.000 | 5.610–40.270 | <0.001 |
| N stage (N+ vs.
N0) | 1.300 | 0.619–2.729 | 0.488 |
|
|
|
| Clinical stage
(IIA-III vs. I–IIA) | 2.780 | 0.829–9.322 | 0.098 |
|
|
|
| Treatment
(sequential vs. concurrent) | 2.149 | 1.199–3.851 | 0.010 | 0.638 | 0.340–1.196 | 0.161 |
| Response (PR vs.
CR) | 2.981 | 1.231–7.223 | 0.016 | 1.697 | 0.665–4.239 | 0.273 |
| CEA (raised vs.
normal) | 1.677 | 0.887–3.172 | 0.112 |
|
|
|
| NSE (raised vs.
normal) | 1.761 | 0.748–4.145 | 0.195 |
|
|
|
Factors associated with OS
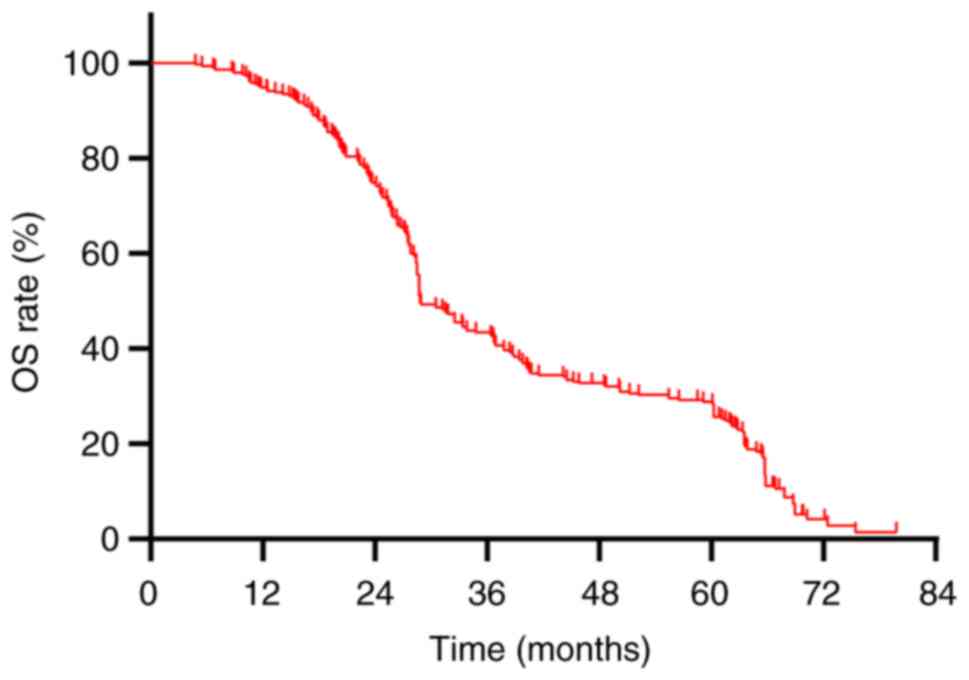
The median OS for the cohort of 290 patients was
recorded at 28.8 months, accompanied by a 5-year OS rate of 27.9%
(Fig. 4). A comparative analysis
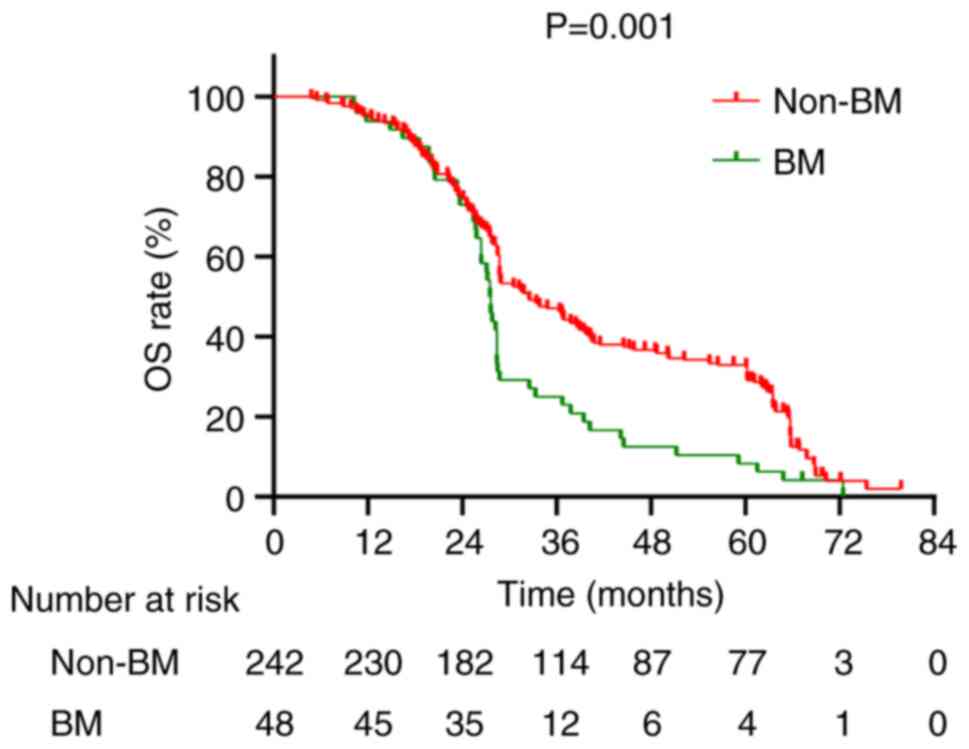
between patients with BM and those without revealed median OS
values of 27.55 months and 32.5 months, respectively, with
corresponding 5-year OS rates of 8.3 and 31.8%, respectively
(P=0.001; Fig. 5). The median OS
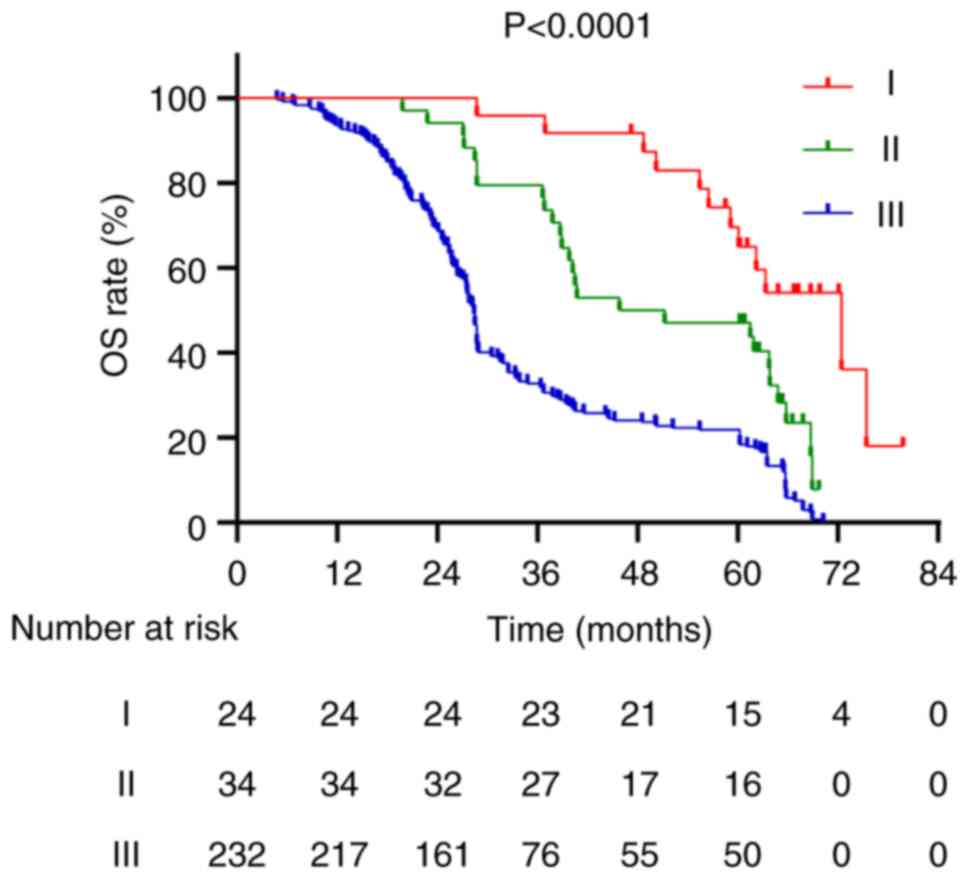
for stage I, II and III patients was 61.15, 48.5 and 28.4 months,
respectively, with 5-year OS rates of 62.5, 47.1 and 21.6%,
respectively (P<0.001; Fig. 6).
Univariate analysis revealed several factors significantly
associated with OS, including initial tumor maximum diameter
(P=0.003), N staging (P<0.001), clinical staging (P<0.001),
treatment modality (P=0.002), treatment response (P<0.001), and
the presence of BM (P=0.001) (Table
IV). Multivariate Cox regression analysis revealed that the
presence of BM (HR=1.934; 95% CI: 1.358–2.764; P<0.001) and
clinical staging (HR=1.741; 95% CI: 11.102–2.750; P=0.018) as
significant independent risk factors for OS (Table IV).
 | Table IV.Cox proportional risk model analysis
affecting overall survival in patients with limited stage-small
cell lung cancer. |
Table IV.
Cox proportional risk model analysis
affecting overall survival in patients with limited stage-small
cell lung cancer.
|
| Univariate
analysis | Cox multifactorial
analysis |
|---|
|
|
|
|
|---|
| Characteristic | HR | 95% CI | P-value | HR | 95% CI | P-value |
|---|
| Age (<60 vs. ≥60
years) | 1.159 | 0.907–1.482 | 0.238 |
|
|
|
| Sex (female vs.
male) | 1.19 | 0.931–1.522 | 0.166 |
|
|
|
| PS (2 vs. 0–1) | 1.110 | 0.841–1.465 | 0.461 |
|
|
|
| Smoking (yes vs.
no) | 1.181 | 0.926–1.506 | 0.181 |
|
|
|
| Initial tumor
maximum diameter (>5 vs. ≤5 cm) | 1.451 | 1.134–1.855 | 0.003 | 0.978 | 0.704–1.359 | 0.895 |
| N stage (N+ vs.
N0) | 3.254 | 2.126–4.982 | <0.001 | 1.709 | 0.953–3.065 | 0.072 |
| Clinical stage
(IIA-III vs. I–IIA) | 5.053 | 2.660–9.602 | <0.001 | 1.741 | 1.102–2.750 | 0.018 |
| Treatment
(sequential vs. concurrent) | 1.471 | 1.151–1.881 | 0.002 | 1.155 | 0.864–1.544 | 0.330 |
| Response (PR vs.
CR) | 3.078 | 2.122–4.465 | <0.001 | 1.985 | 0.816–4.827 | 0.130 |
| CEA (raised vs.
normal) | 1.195 | 0.924–1.544 | 0.174 |
|
|
|
| NSE (raised vs.
normal) | 1.036 | 0.757–1.418 | 0.825 |
|
|
|
| BM (yes vs.
no) | 1.692 | 1.228–2.331 | 0.001 | 1.934 | 1.358–2.754 | <0.001 |
Discussion
For patients with LS-SCLC who exhibit a favorable
initial response to treatment, PCI is recommended as a class I
intervention according to the National Comprehensive Cancer Network
guidelines (21). In the modern MRI
era, studies have reported that patients with LS-SCLC who did not
receive PCI experienced a 1- and 3-year BM rate of 23.8 and 41.3%,
respectively (22,23). Conversely, the 3-year BM rate among
patients who underwent PCI was reported to be notably lower at
11.2% (24), and the 5-year
progression-free rate for BM was 69% (25). The results of the present study
revealed a 3-year BM rate of 12.8% post-PCI in patients with
LS-SCLC, aligning closely with the outcomes observed in the
aforementioned research.
The findings of the present study demonstrate that
patients with an initial tumor maximum diameter of >5 cm at the
time of initial diagnosis exhibit a substantially elevated risk of
BM following PCI. This observation is consistent with prior
research indicating that higher clinical stages, which consider
local spread and tumor size, are associated with an increased risk
of BM development. Levy et al (26) reported an association between the
volume of the primary tumor in the thorax and the subsequent risk
of BM in patients with LS-SCLC. Similarly, Chen et al
(27) performed a retrospective
analysis on 550 patients with LS-SCLC and reported that an initial
tumor maximum diameter of >5 cm was a notable risk factor for
BM. This increased risk may be attributed to larger tumors
dispersing more malignant cells into the circulatory system, which
then potentially seed metastases in distant organs (28).
In the present study, further analysis was performed
on the factors affecting the prognosis of patients with SCLC
following PCI. It was observed that patients developing BM post-PCI
exhibited a significantly lower OS compared with those without BM,
with a median OS of 27.55 months vs. 32.5 months, and five-year OS
rates at 8.3 and 31.8%, respectively (P=0.001). Cen et al
(29) reported that BM serve as an
independent risk factor for the prognosis of patients with SCLC
post-PCI. Moreover, the present study identified clinical staging
as an independent risk factor influencing the OS of patients with
LS-SCLC after PCI. Kim et al (30) noted that in patients aged ≥65 with
stage II–III disease, PCI did not confer marked survival
advantages. Similarly, Farooqi et al (31) reported no improvement in OS for
individuals aged ≥70 with tumor diameters of ≥5 cm following PCI.
Furthermore, the size of the tumor at initial diagnosis in the
present study was not significantly associated with a worse OS.
However, previous research suggests that larger tumor size may
indicate a more aggressive phenotype, elevating the risk of
metastasis, especially BM. Patients in advanced stages may exhibit
higher rates of extracranial disease progression, potentially
masking the survival benefits of PCI (27,31).
This highlights the crucial role of utilizing TNM clinical staging
more extensively for guiding clinical decisions (32).
The preventive role of PCI in reducing BM risk for
patients with LS-SCLC achieving CR after CRT is well-established.
Despite this, 16.6% of patients still developed BM post-PCI in the
present study, suggesting that a fraction of patients with LS-SCLC
who receive curative CRT may not benefit from PCI. Further research
is necessary to delineate the characteristics of these patients.
Given the limitations of traditional imaging methods such as CT and
MRI in assessing early therapeutic effectiveness and prognostic
outcomes, the exploration of molecular biomarkers for the early
prediction of BM and evaluation of PCI efficacy represents a vital
research direction. Slotman et al (33) examined the effectiveness of PCI in
ES-SCLC, categorizing patients into a brain radiotherapy group and
a control group, each consisting of 143 patients. The brain
radiotherapy group received different dosages: 20 Gy in 5 fractions
(n=89), 30 Gy in 10 fractions (n=23), 30 Gy in 12 fractions (n=9)
and 25 Gy in 10 fractions (n=7). The results revealed symptomatic
BM in 16.8% (n=24) of the radiotherapy group compared with 41.3%
(n=59) in the control group (P<0.001). The cumulative risk of BM
at 6 and 12 months for the radiotherapy group was 4.4 and 14.6%,
respectively, compared with 32.0 and 40.4%, respectively, for the
control group. Median disease-free survival was 14.7 weeks in the
radiotherapy group and 12.0 weeks in the control group (P=0.02),
with median OS at 6.7 and 5.4 months, respectively (P=0.003). The
1-year OS rate was 27.1% in the radiotherapy group and 13.3% in the
control group. These findings underscore that PCI can enhance
survival and reduce the incidence of subsequent BM in patients with
ES-SCLC who respond well to systemic chemotherapy and thoracic
radiotherapy.
Moreover, a Phase III randomized controlled trial
performed in Japan by Takahashi et al (34) provided contrasting outcomes. This
study included patients with ES-SCLC who had responded to
platinum-based chemotherapy and exhibited no signs of BM on MRI.
Participants were divided into two groups: A PCI group consisting
of 113 patients and an observation group of 111 patients. The PCI
group received a total of 25 Gy administered in 10 fractions. The
findings revealed that the median OS was 11.6 months for the PCI
group compared with 13.7 months for the observation group
(P=0.094). The 1- and 2-year OS rates were 48.4 and 15.0% for the
PCI group, respectively, compared with 53.6 and 18.8% for the
observation group, respectively. Furthermore, the cumulative
incidence of BM at 6, 12 and 18 months was markedly lower in the
PCI group (15.0, 32.9 and 40.1%, respectively) compared with the
observation group (46.2, 59.0 and 63.8%, respectively). Despite a
significant reduction in the incidence of intracranial metastases
(48 vs. 69%; P<0.0001), PCI did not provide a survival
advantage.
PCI is implicated in the onset of delayed
neurotoxicity, particularly when administered at doses of >3 Gy
per fraction and/or in conjunction with CRT (31). Consequently, PCI is contraindicated
for patients exhibiting a poor PS of 3–4 or compromised
neurocognitive function (35).
Additionally, a higher incidence of chronic neurotoxicity is
observed in individuals of >60 years (36). The conflicting data from several
clinical trials and the growing concerns regarding the use of PCI
(33,37,38)
led to the initiation of the SWOG S1827/MAVERICK trial in the
United States (39). This
randomized study evaluated the efficacy of exclusive brain MRI
monitoring against the combination of brain MRI and PCI in managing
both advanced and early-stage SCLC. Participants were randomly
allocated to either the MRI-only group or the combined MRI and PCI
group. The primary outcome measure was OS, with secondary outcomes
including survival free from cognitive decline, survival free from
BM, and rates of adverse events. Although the results are pending,
this trial is expected to yield significant insight and data for
the future management of SCLC (39). Moreover, the study by Chen et
al (27) assessed this subject;
however, the present study differs in several key aspects: The
present study is based on data from a dual-center collaboration
between the Hebei Province Cangzhou Hospital of Integrated
Traditional and Western Medicine and the Chengde City Central
Hospital, which offers more accurate and reliable statistical
outcomes than single-center studies; the enrolled patients were
re-staged using the 8th edition of the AJCC Cancer Staging Manual
and the Veterans Administration Lung Study Group two-tier system,
unlike the study by Chen et al (27), which used the 7th edition,
potentially affecting the comparability of stage-related outcomes.
The adoption of the widely applied 8th edition staging system
enhances the credibility of the results of the present study; in
addition to analyzing the incidence of BM post-PCI in LS-SCLC, the
present study further assessed the high-risk factors and identified
high-risk individuals, providing a clinical basis for tailored
monitoring and treatment; and finally, the present study
reclassified the nodal status and clinical staging into two groups
for statistical analysis, differing from the grouping method of the
study by Chen et al (27),
thus ensuring data consistency and enhancing the reliability of the
results of the present study.
In conclusion, retrospective analyses of patients
with LS-SCLC indicate that an initial maximum tumor diameter of
>5 cm serves as an independent risk factor for BM following PCI.
Furthermore, both BM and clinical staging independently influence
OS in these patients post-PCI. Presently, research into the risk
factors for BM post-PCI remains sparse and predominantly
retrospective. According to the Chinese Society of Clinical
Oncology guidelines, concurrent CRT is the standard treatment for
patients with LS-SCLC of stage >T1-2N0 (40). If patients cannot tolerate this
regimen, sequential CRT is also an option (41). In the present study of 290 patients,
80% were Stage III (n=232), with 50.7% at N3 (n=147). Treatment
plans were tailored for each patient using a multidisciplinary team
approach, taking into account functional status, laboratory
findings and imaging data. Considering the significant adverse
reactions from concurrent CRT in Stage III (N3) patients, which
many find intolerable, a portion opted for sequential CRT,
resulting in a lower proportion of patients undergoing concurrent
treatment (54.5%). Therefore, there is a compelling need for more
prospective studies to further assess these associations.
Acknowledgements
Not applicable.
Funding
Funding was received from the Scientific Research Fund Program
of Hebei Provincial Health and Wellness Commission (grant no.
20211577).
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
GY and JZ performed the data analysis and manuscript
writing. RL was responsible for the research design and guided the
revision of the manuscript. JD provided the enrolled cases and made
substantial contributions to the conception or design of the work.
All authors have read and approved the final manuscript. GY and RL
confirm the authenticity of all the raw data.
Ethics approval and consent to
participate
The current study was performed in accordance with
the Declaration of Helsinki and approved by the local ethics
committee of the Cangzhou Hospital of Integrated Traditional
Chinese and Western Medicine-Hebei Province (Cangzhou, China;
approval no. 2021-KY-062.1) and the Chengde Central Hospital
(Chengde, China; approval no. CDCHLL2023-407). Each patient
provided written informed consent for participation.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Bray F, Ferlay J, Soerjomataram I, Siegel
RL, Torre LA and Jemal A: Global cancer statistics 2018: GLOBOCAN
estimates of incidence and mortality worldwide for 36 cancers in
185 countries. CA Cancer J Clin. 68:394–424. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Micke P, Faldum A, Metz T, Beeh KM,
Bittinger F, Hengstler JG and Buhl R: Staging small cell lung
cancer: Veterans Administration Lung Study Group versus
International Association for the Study of Lung Cancer-what limits
limited disease? Lung Cancer Amst Neth. 37:271–276. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Wang B, Guo H, Xu H, Yu H, Chen Y and Zhao
G: Research progress and challenges in the treatment of central
nervous system metastasis of non-small cell lung cancer. Cells.
10:26202021. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Chauhan AF and Liu SV: Small cell lung
cancer: Advances in diagnosis and management. Semin Respir Crit
Care Med. 41:435–446. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Chu X, Li S, Xia B, Chu L, Yang X, Ni J,
Zou L, Li Y, Xie C, Lin J and Zhu Z: Patterns of brain metastasis
immediately before prophylactic cranial irradiation (PCI):
implications for PCI optimization in limited-stage small cell lung
cancer. Radiat Oncol Lond Engl. 14:1712019. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wu Q and Chen M, Peng F, Zhang Q, Kong Y,
Bao Y, Xu Y, Hu X and Chen M: A study of the prognosis of patients
with limited-stage small cell lung cancer who did or did not
receive prophylactic cranial irradiation after effective
chemoradiotherapy. Front Oncol. 13:11183712023. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Tai P, Assouline A, Joseph K, Stitt L and
Yu E: Prophylactic cranial irradiation for patients with
limited-stage small-cell lung cancer with response to
chemoradiation. Clin Lung Cancer. 14:40–44. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Aupérin A, Arriagada R, Pignon JP, Le
Péchoux C, Gregor A, Stephens RJ, Kristjansen PE, Johnson BE, Ueoka
H, Wagner H and Aisner J: Prophylactic cranial irradiation for
patients with small-cell lung cancer in complete remission.
Prophylactic Cranial Irradiation Overview Collaborative Group. N
Engl J Med. 341:476–484. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Chun SG, Simone CB II, Amini A, Chetty IJ,
Donington J, Edelman MJ, Higgins KA, Kestin LL, Movsas B, Rodrigues
GB, et al: American Radium Society Appropriate Use Criteria:
Radiation Therapy for Limited-Stage SCLC 2020. J Thorac Oncol.
16:66–75. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Couñago F, de la Pinta C, Gonzalo S,
Fernández C, Almendros P, Calvo P, Taboada B, Gómez-Caamaño A,
Guerra JLL, Chust M, et al: GOECP/SEOR radiotherapy guidelines for
small-cell lung cancer. World J Clin Oncol. 12:115–143. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Moreno AC and Lin SH: The optimal
treatment approaches for stage I small cell lung cancer. Transl
Lung Cancer Res. 8:88–96. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Xu C, Li M, Cai X, Yuan S, Cao J, Zhu S,
Chen M, Bi N, Hu X, Li J, et al: Practice patterns of treatment
strategy of limited-stage small-cell lung cancer: Survey of Chinese
Oncologists. Front Oncol. 12:8723242022. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Yuan M, Zhao Y, Arkenau HT, Lao T, Chu L
and Xu Q: Signal pathways and precision therapy of small-cell lung
cancer. Signal Transduct Target Ther. 7:1872022. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Patel SR and Das M: Small cell lung
cancer: Emerging targets and strategies for precision therapy.
Cancers (Basel). 15:40162023. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Rami-Porta R, Asamura H, Travis WD and
Rusch VW: Lung cancer-major changes in the American Joint Committee
on Cancer eighth edition cancer staging manual. CA Cancer J Clin.
67:138–155. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Chen Y, Wang Y, Ren F, Huang Z, Tan B,
Zhao Z, Yu X, Dong P, Yu J and Meng X: Prophylactic cranial
irradiation (PCI) versus active surveillance in patients with
limited-stage small cell lung cancer: A retrospective, multicentre
study. Respir Res. 23:2742022. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Eisenhauer EA, Therasse P, Bogaerts J,
Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S,
Mooney M, et al: New response evaluation criteria in solid tumours:
Revised RECIST guideline (version 1.1). Eur J Cancer. 45:228–247.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Gondi V, Deshmukh S, Brown PD, Wefel JS,
Armstrong TS, Tome WA, Gilbert MR, Konski A, Robinson CG, Bovi JA,
et al: Sustained preservation of cognition and prevention of
patient-reported symptoms with hippocampal avoidance during
whole-brain radiation therapy for brain metastases: Final results
of NRG oncology CC001. Int J Radiat Oncol Biol Phys. 117:571–580.
2023. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Gondi V, Tome WA, Marsh J, Struck A, Ghia
A, Turian JV, Bentzen SM, Kuo JS, Khuntia D and Mehta MP: Estimated
risk of perihippocampal disease progression after hippocampal
avoidance during whole-brain radiotherapy: Safety profile for RTOG
0933. Radiother Oncol. 95:327–331. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Gondi V, Pugh SL, Tome WA, Caine C, Corn
B, Kanner A, Rowley H, Kundapur V, DeNittis A, Greenspoon JN, et
al: Preservation of memory with conformal avoidance of the
hippocampal neural stem-cell compartment during whole-brain
radiotherapy for brain metastases (RTOG 0933): A phase II
multi-institutional trial. J Clin Oncol. 32:3810–3816. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Bogart JA, Waqar SN and Mix MD: Radiation
and systemic therapy for limited-stage small-cell lung cancer. J
Clin Oncol. 40:661–670. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Pan L, Fan X, Wang L, Wang Y, Li Y, Cui Y,
Zheng H, Yi Q and Wu K: Prophylactic cranial irradiation for
limited-stage small-cell lung cancer in the magnetic resonance
imaging era. Cancer Med. 12:2484–2492. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Held MK, Hansen O, Schytte T, Hansen KH,
Bahij R, Nielsen M, Nielsen TB and Jeppesen SS: Outcomes of
prophylactic cranial irradiation in patients with small cell lung
cancer in the modern era of baseline magnetic resonance imaging of
the brain. Acta Oncol. 61:185–192. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Pezzi TA, Fang P, Gjyshi O, Feng L, Liu S,
Komaki R and Lin SH: Rates of overall survival and intracranial
control in the magnetic resonance imaging era for patients with
limited-stage small cell lung cancer with and without prophylactic
cranial irradiation. JAMA Netw Open. 3:e2019292020. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Lim YJ, Song C and Kim HJ; Korean
Association for Lung Cancer, Korea Central Cancer Registry, :
Survival impact of prophylactic cranial irradiation in small-cell
lung cancer in the modern era of magnetic resonance imaging
staging. Radiat Oncol. 17:262022. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Levy A, Le Péchoux C, Mistry H,
Martel-Lafay I, Bezjak A, Lerouge D, Padovani L, Taylor P and
Faivre-Finn C: Prophylactic cranial irradiation for limited-stage
small-cell lung cancer patients: Secondary findings from the
prospective Randomized phase 3 CONVERT trial. J Thorac Oncol.
14:294–297. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Chen MY, Ji Y, Hu X and Chen M: Factors
affecting the risk of brain metastasis in limited-stage small cell
lung cancer after prophylactic cranial irradiation. Cancer Manag
Res. 14:1807–1814. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Wang X, Ma K, Yang Z, Cui J, He H, Hoffman
AR, Hu JF and Li W: Systematic correlation analyses of circulating
tumor cells with clinical variables and tumor markers in lung
cancer patients. J Cancer. 8:3099–3104. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Cen M, Jin J, Ji Y, Hu X and Chen M: Risk
assessment of brain metastases after prophylactic brain irradiation
in 550 patients with limited-stage small cell lung cancer who
achieved remission through chemoradiotherapy. Chin J Radiat Oncol.
31:138–142. 2022.
|
|
30
|
Kim TG, Pyo H, Ahn YC, Noh JM and Oh D:
Role of prophylactic cranial irradiation for elderly patients with
limited-disease small-cell lung cancer: Inverse probability of
treatment weighting using propensity score. J Radiat Res.
60:630–638. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Farooqi AS, Holliday EB, Allen PK, Wei X,
Cox JD and Komaki R: Prophylactic cranial irradiation after
definitive chemoradiotherapy for limited-stage small cell lung
cancer: Do all patients benefit? Radiother Oncol. 122:307–312.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Wolfson AH, Bae K, Komaki R, Meyers C,
Movsas B, Le Pechoux C, Werner-Wasik M, Videtic GM, Garces YI and
Choy H: Primary analysis of a phase II randomized trial Radiation
Therapy Oncology Group (RTOG) 0212: Impact of different total doses
and schedules of prophylactic cranial irradiation on chronic
neurotoxicity and quality of life for patients with limited-disease
small-cell lung cancer. Int J Radiat Oncol Biol Phys. 81:77–84.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Slotman B, Faivre-Finn C, Kramer G, Rankin
E, Snee M, Hatton M, Postmus P, Collette L, Musat E and Senan S;
EORTC Radiation Oncology Group and Lung Cancer Group, :
Prophylactic cranial irradiation in extensive small-cell lung
cancer. N Engl J Med. 357:664–672. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Takahashi T, Yamanaka T, Seto T, Harada H,
Nokihara H, Saka H, Nishio M, Kaneda H, Takayama K, Ishimoto O, et
al: Prophylactic cranial irradiation versus observation in patients
with extensive-disease small-cell lung cancer: A multicentre,
randomised, open-label, phase 3 trial. Lancet Oncol. 18:663–671.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Choi M, Lee Y, Moon SH, Han JY, Kim HT and
Lee JS: Effect of accurate staging using positron emission
tomography on the outcomes of prophylactic cranial irradiation in
patients with limited stage small-cell lung cancer. Clin Lung
Cancer. 18:77–84. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Zeng H, Hendriks LEL, van Geffen WH,
Witlox WJA, Eekers DBP and De Ruysscher DKM: Risk factors for
neurocognitive decline in lung cancer patients treated with
prophylactic cranial irradiation: A systematic review. Cancer Treat
Rev. 88:1020252020. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Mehta MP: Models support prophylactic
cranial irradiation. J Clin Oncol. 24:3524–3526. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Witlox WJA, Ramaekers BLT, Lacas B, Le
Pechoux C, Pignon JP, Sun A, Wang SY, Hu C, Redman M, van der Noort
V, et al: Individual patient data meta-analysis of prophylactic
cranial irradiation in locally advanced non-small cell lung cancer.
Radiother Oncol. 158:40–47. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Taylor JM, Rusthoven CG and Moghanaki D:
Prophylactic cranial irradiation or MRI surveillance for extensive
stage small cell lung cancer. J Thorac Dis. 12:6225–6233. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Zeng H, De Ruysscher DKM, Hu X, Zheng D,
Yang L, Ricardi U, Kong FMS and Hendriks LEL: Radiotherapy for
small cell lung cancer in current clinical practice guidelines. J
Natl Cancer Cent. 2:113–125. 2022. View Article : Google Scholar
|
|
41
|
Takada M, Fukuoka M, Kawahara M, Sugiura
T, Yokoyama A, Yokota S, Nishiwaki Y, Watanabe K, Noda K, Tamura T,
et al: Phase III study of concurrent versus sequential thoracic
radiotherapy in combination with cisplatin and etoposide for
limited-stage small-cell lung cancer: Results of the Japan Clinical
Oncology Group Study 9104. J Clin Oncol. 20:3054–3060. 2002.
View Article : Google Scholar : PubMed/NCBI
|