Introduction
Breast cancer (BC) is the most frequently diagnosed
malignancy, affecting 2.3 million women and causing nearly half a
million deaths in 2022, thus representing one in four cancer cases
and one in six cancer-associated deaths among women worldwide
(1). Neoadjuvant chemotherapy (NAC)
is the standard therapeutic method for locally advanced BC (LABC).
NAC confers several advantages, including tumor downstaging to
increase the viability of conservative surgery, aiding tumor
response assessment and demonstrating comparable effectivity to
adjuvant treatment to boost overall survival (OS) (2–4).
Achieving a pathological complete response (pCR) following NAC
serves as an important prognostic factor, especially when
determining improved long-term outcomes (5–8).
Conversely, residual disease would indicate the need for adjuvant
therapy, particularly in triple-negative BC (TNBC) (9) and human epidermal growth factor
receptor 2 (HER2)-positive disease (10).
The neutrophil-to-lymphocyte ratio (NLR) is a
measurement that reflects tumor effect and host immune and
inflammatory responses to the tumor, which has been shown to yield
prognostic significance among various solid cancer types, including
BC (11,12). A previous study has suggested that
the NLR can be potentially used as an independent prognostic marker
for disease-free survival (DFS) and OS in patients with BC, and
should be assessed before surgical or medical treatment (13). However, conflicting findings
regarding the association between pre-treatment NLR levels and pCR
exist. A number of studies have revealed a significant association
between lower NLR and higher rates of pCR regardless of molecular
subtype (14,15). By contrast, others have identified
an association with specific molecular subtypes, e.g., TNBC and
HER2-positive disease (16–20), while other studies have found no
such association between the NLR and pCR (21–29).
Due to the diversity in ethnic backgrounds, the optimal NLR cut-off
in the literature spans from 2.1 to 3.3, indicating the lack of a
universally applicable threshold for the NLR. Furthermore, specific
medical conditions, e.g., chronic inflammatory and autoimmune
diseases, can influence NLR levels. Hence, it is vital to employ a
receiver operating characteristic (ROC) curve for NLR determination
and adherence to eligibility criteria when selecting cases to
evaluate the prognostic significance of the NLR (15,30).
Since the NLR is a readily measurable parameter that can
potentially aid in the management of patients with localized BC,
further investigation is warranted.
Therefore, the present study aimed to evaluate the
prognostic role of the pre-treatment NLR in the NAC response in
patients with LABC. Specifically, the primary objective was to
determine whether the NLR serves as a predictive variable for the
pCR in patients with LABC. The secondary objective was to determine
whether the pre-treatment NLR is associated with DFS and OS in
patients with LABC.
Patients and methods
Patients
A retrospective review was conducted on patients who
underwent standard NAC for LABC at King Feisal Specialist Hospital
and Research Center (Riyadh, Saudi Arabia) between January 2005 and
December 2014. The study protocol was approved by the Medical
Ethics Committee and the Research Advisory Council (RAC) of the
King Faisal Specialist Hospital and Research Center as part of the
Breast Cancer Research Project (approval no. RAC#2051-029).
Informed consent was waived by the Medical Ethics Committee owing
to the retrospective nature of the study with assurance that all
participants remained anonymous, where no identifying information
or protected health data were collected. The study adhered to the
Declaration of Helsinki and to the REMARK guidelines (31,32).
Eligible patients include those with LABC who underwent NAC and
breast surgery, were aged 18–70 years and had complete medical
records (including surgical pathology assessment data and pre-NAC
differential leukocyte count). Exclusion criteria were the presence
of inflammatory BC, pregnancy-related BC, autoimmune diseases,
hematological disorders, active infection, chronic obstructive
pulmonary disease requiring treatment, chronic liver disease,
end-stage renal disease, other malignancies, a history of
cerebrovascular accidents, use of steroidal medication or missing
clinical data.
The data was retrieved from a BC prospective
database at King Faisal Specialist Hospital and Research Center.
Two investigators independently reviewed the medical records of
each patient. Collected data included age at diagnosis, menopausal
status, tumor histology, grade, nodal stages, tumor size,
immunohistochemistry results for estrogen and progesterone, HER2
upregulation, neoadjuvant chemotherapy protocol, type of surgery
and radiation therapy.
Pathology slides were confirmed by a specialized BC
pathologist at the institution, and IHC and fluorescence in
situ hybridization analyses were performed as previously
described. Estrogen or progesterone positivity was defined as ≥1%
of the cells having nuclear receptors on immunohistochemical
analysis (33). HER2 status was
determined by immunohistochemical scoring. HER2 would be considered
negative if the score was 0 or 1+ and positive in scores 3+ or 2+,
and results were confirmed by fluorescence in situ
hybridization. HER2 positivity would be deemed if the
HER2-to-chromosome enumeration probe (CEP) 17 ratio was >2 with
a HER2 copy number of ≥4 (34,35).
The clinical staging of BC followed the Tumor-Node-Metastasis
classification system from the American Joint Committee on Cancer
and the Union for International Cancer Control, 8th edition
(36). White blood cell
differential count was obtained post-BC diagnosis and pre-NAC. A
pCR was defined in cases of complete absence of viable invasive
tumor cells upon pathological examination of both breast and axilla
specimens, including surgical margins (ypT0 ypN0). DFS time was
defined as the time span between histological diagnosis and disease
recurrence or death, whereas OS time was defined as the period
between histological diagnosis and the date of death.
Statistical analysis
Continuous variables are presented as median values
with interquartile ranges and were compared using the Mann-Whitney
U test. Categorical variables are presented as n (%) and were
compared using the χ2 test or Fisher's exact test. The
NLR cut-off point was determined using the ROC curve. The study
population was stratified into low and high NLR groups. Univariate
and multivariate logistic regression analyses were used to estimate
the associations between various parameters and a pCR. The
Kaplan-Meier method was used to estimate probabilities for DFS and
OS, and log-rank analysis was used to compare the groups. The
hazard ratio (HR) was estimated using Cox regression analysis.
P<0.05 was considered to indicate a statistically significant
difference. All statistical analyses were performed using SPSS for
Mac (v28; IBM Corp.).
Results
The present study included 465 consecutive patients
who underwent NAC between January 2005 and December 2014. The flow
diagram for the study population is illustrated in Fig. 1. The median age at diagnosis was 44
years (interquartile range, 38–50 years), where the median NLR was
1.91 (interquartile range, 1.27–2.65). The pre-treatment NLR was
found to be significantly associated with pCR as a continuous
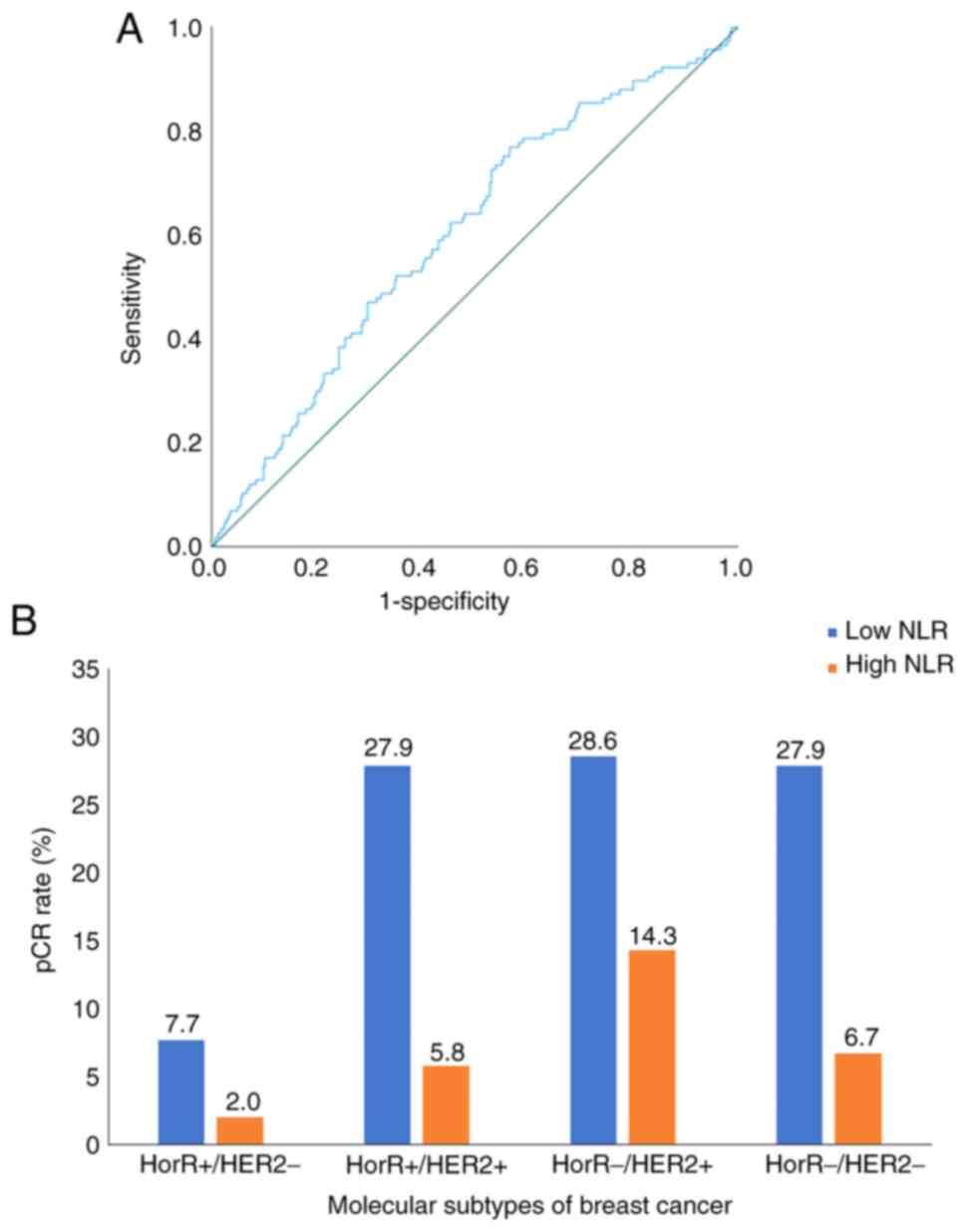
variable (P<0.001), with a cut-off point of 2.2, 77% sensitivity
and 57% specificity (Fig. 2A). The
patients were thereafter stratified into low NLR (≤2.2; 291
patients; 62.6%) and high NLR (>2.2; 174 patients; 37.4%)
groups. No significant differences were observed between the two
groups in terms of patient demographics, disease and management
characteristics (Table I). However,
the pCR rate was higher in the low NLR group (30.9%) compared with
that in the high NLR group (15.5%) (P<0.001).
 | Table I.Patient characteristics stratified
based on a low and high NLR. |
Table I.
Patient characteristics stratified
based on a low and high NLR.
| Variables | Total | High NLR | Low NLR | P-value |
|---|
| Age,
yearsa | 44 (38–50) | 44 (38–49) | 45 (38–50) | 0.50b |
| Age groups, n
(%) | 465 (100.0) | 174 (37.4) | 291 (62.6) | 0.34c |
| ≤40
years | 169 (36.3) | 68 (39.1) | 101 (34.7) |
|
| >40
years | 296 (63.7) | 106 (60.9) | 190 (65.3) |
|
| Menopausal status,
n (%) | 463 (100.0) | 173 (37.4) | 290 (62.6) | 0.08c |
|
Pre-menopausal | 333 (71.9) | 134 (77.5) | 199 (68.6) |
|
|
Peri-menopausal | 12 (2.6) | 5 (2.9) | 7 (2.4) |
|
|
Post-menopausal | 118 (25.5) | 34 (19.6) | 84 (29.0) |
|
| Histology, n
(%) | 465 (100.0) | 175 (37.6) | 290 (62.4) | 0.57d |
|
Invasive ductal carcinoma | 287 (83.4) | 149 (85.6) | 238 (82.0) |
|
|
Invasive lobular
carcinoma | 75 (16.2) | 24 (13.8) | 51 (17.6) |
|
|
Others | 3 | 2 (1.1) | 1 (0.3) |
|
| Grade, n (%) | 448 (100.0) | 170 (37.9) | 278 (62.1) | 0.97d |
| 1 | 5 (1.1) | 2 (1.2) | 3 (1.1) |
|
| 2 | 221 (49.3) | 83 (48.8) | 138 (49.6) |
|
| 3 | 222 (49.6) | 85 (50) | 137 (49.3) |
|
| ER, n (%) | 464 (100.0) | 174 (37.1) | 291 (62.6) | 0.41c |
|
Positive | 281 (60.4) | 101 (58.0) | 180 (61.9) |
|
|
Negative | 184 (39.6) | 73 (42.0) | 111 (38.1) |
|
| PR, n (%) | 465 (100.0) | 174 (37.4) | 291 (62.6) | 0.85c |
|
Positive | 227 (48.8) | 84 (48.3) | 143 (49.1) |
|
|
Negative | 238 (51.2) | 90 (51.7) | 148 (50.9) |
|
| HER2, n (%) | 464 (100.0) | 174 (37.1) | 291 (62.6) | 0.76c |
|
Positive | 167 (35.9) | 61 (35.1) | 106 (36.4) |
|
|
Negative | 298 (64.1) | 113 (64.9) | 185 (63.6) |
|
| T stage, n (%) | 463 (100.0) | 174 (37.6) | 289 (62.4) | 0.09d |
| 1 | 8 (1.7) | 2 (1.1) | 6 (2.1) |
|
| 2 | 124 (26.8) | 45 (25.9) | 79 (27.3) |
|
| 3 | 176 (38) | 57 (32.8) | 119 (41.2) |
|
| 4 | 155 (33.5) | 70 (40.2) | 85 (29.4) |
|
| Clinical N stage, n
(%) | 463 (100.0) | 173 (37.4) | 290 (62.6) | 0.25c |
| 0 | 49 (10.6) | 15 (8.7) | 34 (11.7) |
|
| 1 | 275 (59.4) | 97 (56.0) | 178 (61.4) |
|
| 2 | 106 (22.9) | 46 (26.6) | 60 (20.7) |
|
| 3 | 33 (7.1) | 15 (8.7) | 18 (6.2) |
|
| Molecular subtypes,
n (%) | 463 (100.0) | 174 (37.6) | 289 (62.4) |
|
|
HorR+/HER2- | 196 (42.2) | 76 (43.7) | 120 (41.5) | 0.39c |
|
HorR+/HER2+ | 86 (18.5) | 26 (14.9) | 60 (20.8) |
|
|
HorR−/HER2+ | 77 (16.6) | 33 (19.0) | 44 (15.2) |
|
|
HorR−/HER2- | 104 (22.4) | 39 (22.4) | 65 (22.5) |
|
| Pathological
complete response | - | - | - |
<0.001c |
| cPR
Axilla, n (%) | 216 (46.5) | 58 (33.3) | 158 (54.3) |
|
| cPR
Breast, n (%) | 165 (35.5) | 48 (27.6) | 117 (40.2) |
|
| cPR
Both, n (%) | 117 (25.2) | 27 (15.5) | 90 (30.9) |
|
| Neoadjuvant
chemotherapy, n (%) | 465 (100.0) | 174 (37.4) | 291 (62.6) | 0.18c |
|
Anthracycline/taxane-based | 252 (54.2) | 86 (49.4) | 166 (57) |
|
|
Anthracycline based | 90 (8.4) | 39 (22.4) | 51 (17.5) |
|
|
Anthracycline/taxane-based and
Platinum | 63 (13.5) | 27 (15.5) | 36 (12.4) |
|
| Taxane
and others | 41 (8.8) | 18 (10.3) | 23 (7.9) |
|
|
Others | 19 (4.1) | 4 (2.3) | 15 (5.2) |
|
| Surgery, n (%) | 465 (100) | 182 (39.1) | 283 (60.9) | 0.33c |
| Breast
conservative surgery | 64 (13.8) | 26 (14.3) | 38 (13.4) |
|
|
Modified radical
mastectomy | 360 (77.4) | 136 (74.7) | 224 (79.2) |
|
|
Skin-sparing mastectomy | 24 (5.2) | 16 (8.8) | 8 (2.8) |
|
| Simple
mastectomy | 17 (3.6) | 4 (2.2) | 13 (4.6) |
|
| Axillary status, n
(%) | 365 (100.0) | 174 (37.4) | 291 (62.6) | 0.20c |
|
ALND | 396 (85.2) | 153 (88.0) | 243 (83.5) |
|
|
SLNB | 44 (9.4) | 14 (8.0) | 30 (10.3) |
|
| SLNB +
ALND | 25 (5.4) | 7 (4.0) | 18 (6.2) |
|
| Adjuvant therapy, n
(%) | - | - | - |
|
|
Chemotherapy | 59 (12.8) | 28 (16.2) | 31 (10.7) | 0.08c |
|
Radiation | 449(97.2) | 169 (97.7) | 280 (96.9) | 0.61c |
|
Hormonal | 274 (61.4) | 98 (60.1) | 176 (62.2) | 0.66c |
|
Anti-HER2 | 168 (36.4) | 63 (37.5) | 105 (36.5) | 0.95c |
Overall, the pCR rate was 25.2%, varying among
molecular subtypes as follows: Hormone receptor (HorR)-/HER2+
disease (42.9%), HorR+/HER2+ (33.7%), HorR-/HER2-(34.6%) and
HorR+/HER2-(9.7%). The pCR rate of molecular subtypes as
stratified by NLR groups is presented in Fig. 2B. Multivariate analysis showed that
pCR was independently associated with the NLR (OR, 2.59; 95% CI,
1.52–4.38; P<0.001), along with molecular subtype (HorR+/HER2+:
OR, 5.64; 95% CI, 2.77–11.48; P<0.001; HorR-/HER2+: OR, 9.58;
95% CI, 4.58–20.01; P<0.001; HorR-/HER2-: OR, 6.25; 95% CI,
3.10–12.60; P<0.001) and N stage (OR, 0.51; 95% CI, 0.29–0.88;
P=0.01) (Table II).
 | Table II.Univariate and multivariate binary
logistic regression of variables associated with pathological
complete response in patients with locally advanced breast
cancer. |
Table II.
Univariate and multivariate binary
logistic regression of variables associated with pathological
complete response in patients with locally advanced breast
cancer.
|
| Univariate | Multivariate |
|---|
|
|
|
|
|---|
| Variables | Odds ratio | 95% Confidence
interval | P-value | Odds ratio | 95% Confidence
interval | P-value |
|---|
| Age |
|
|
|
|
|
|
| ≤40
years | Reference |
|
| Reference |
|
|
| >40
years | 1.19 | 0.76–1.85 | 0.43 | 1.11 | 0.64–1.92 | 0.68 |
| Menopausal
Status |
|
|
|
|
|
|
|
Premenopausal | Reference |
|
| Reference |
|
|
|
Postmenopausal | 1.2 | 0.75–1.93 | 0.43 | 0.99 | 0.55–1.78 | 0.99 |
| Molecular
subtypes |
|
|
|
|
|
|
|
HorR+/HER2- | Reference |
|
|
|
|
|
|
HorR+/HER2+ | 4.7 | 2.47–9.08 | <0.001 | 5.64 | 2.77–11.48 | <0.001 |
|
HorR-/HER2+ | 6.9 | 3.63–13.43 | <0.001 | 9.58 | 4.58–20.01 | <0.001 |
|
HorR-/HER2- | 4.9 | 2.64–9.18 | <0.001 | 6.25 | 3.10–12.60 | <0.001 |
| Grade |
|
|
|
|
|
|
|
≤G2 | Reference |
|
| Reference |
|
|
| G3 | 1.23 | 0.80–1.89 | 0.32 | 0.81 | 0.49–1.34 | 0.42 |
| T stage |
|
|
|
|
|
|
|
≤T2 | Reference |
|
| Reference |
|
|
|
≥T3 | 0.88 | 0.55–1.40 | 0.59 | 0.8 | 0.47–1.36 | 0.41 |
| N stage |
|
|
|
|
|
|
|
≤N1 | Reference |
|
| Reference |
|
|
|
≥N2 | 0.57 | 0.34–0.93 | 0.02 | 0.51 | 0.29–0.88 | 0.017 |
|
Neutrophil-to-lymphocyte ratio |
|
|
|
|
|
|
| High
(>2.21) | Reference |
|
| Reference |
|
|
| Low
(≤2.21) | 2.43 | 1.50–3.93 | <0.001 | 2.59 | 1.52–4.38 | <0.001 |
| Neoadjuvant
chemotherapy protocol |
|
|
|
|
|
|
|
Anthracycline/taxane-based | Reference |
|
| Reference |
|
|
|
Anthracycline based | 0.69 | 0.38–1.27 | 0.24 | 0.65 | 0.33–1.30 | 0.23 |
|
Anthracycline/taxane-based and
Platinum | 1.72 | 0.95–3.10 | 0.06 | 2.14 | 1.08–4.22 | 0.02 |
| Taxane
and others | 0.84 | 0.38–1.86 | 0.67 | 0.75 | 0.31–1.78 | 0.52 |
|
Others | 1.07 | 0.37–3.09 | 0.89 | 1.04 | 0.29–3.69 | 0.95 |
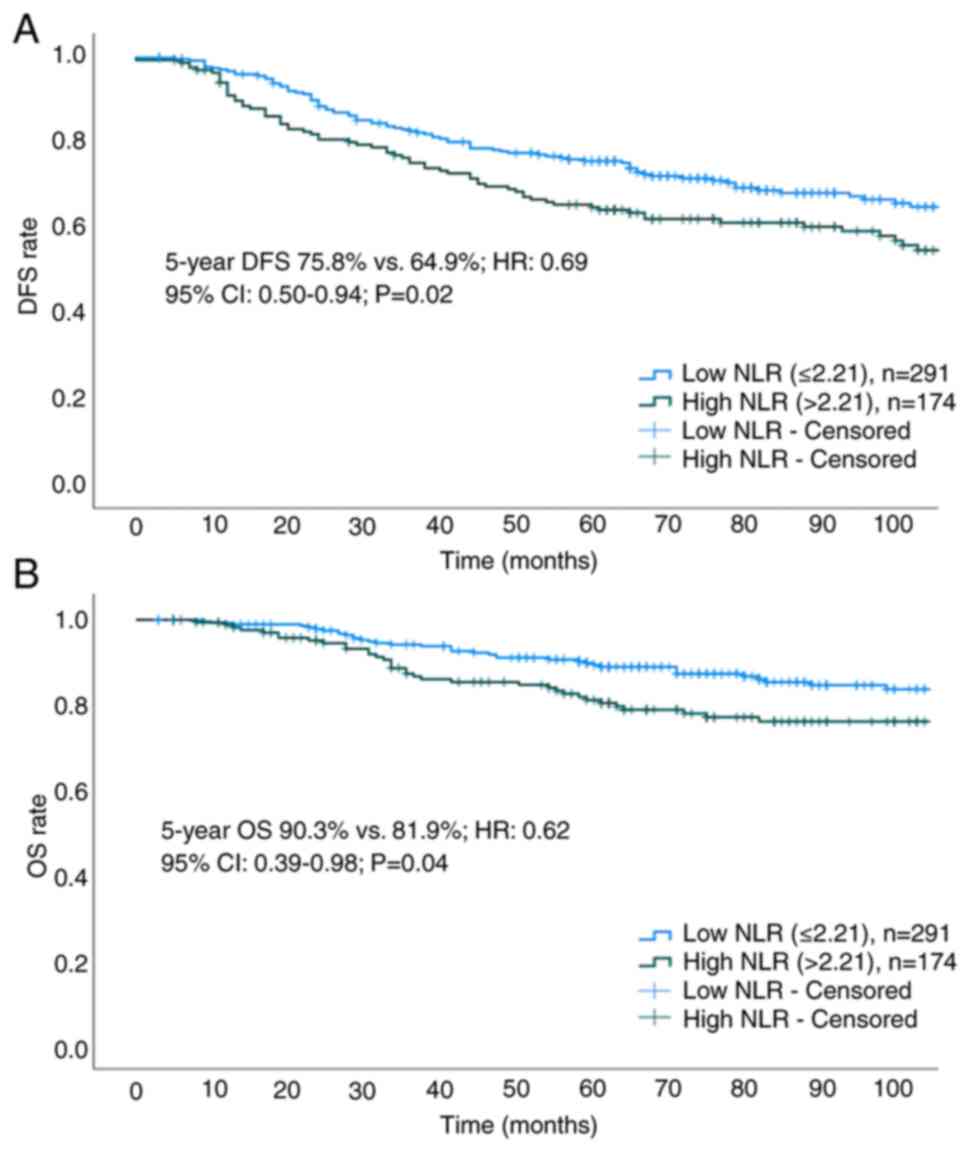
The median duration of follow-up was 77 months
(interquartile range, 55–110 months). The 5-year DFS (75.8 vs.
64.9%; HR, 0.69; 95% CI, 0.50–0.94; P=0.02) and OS (90.3 vs. 81.9%;
HR, 0.62; 95% CI, 0.39–0.98; P=0.04) rates were found to be higher
in the low NLR group compared with those in the high NLR group
(Fig. 3A and B). Cox regression
analysis showed that a low NLR, T stage, N1 disease and pCR were
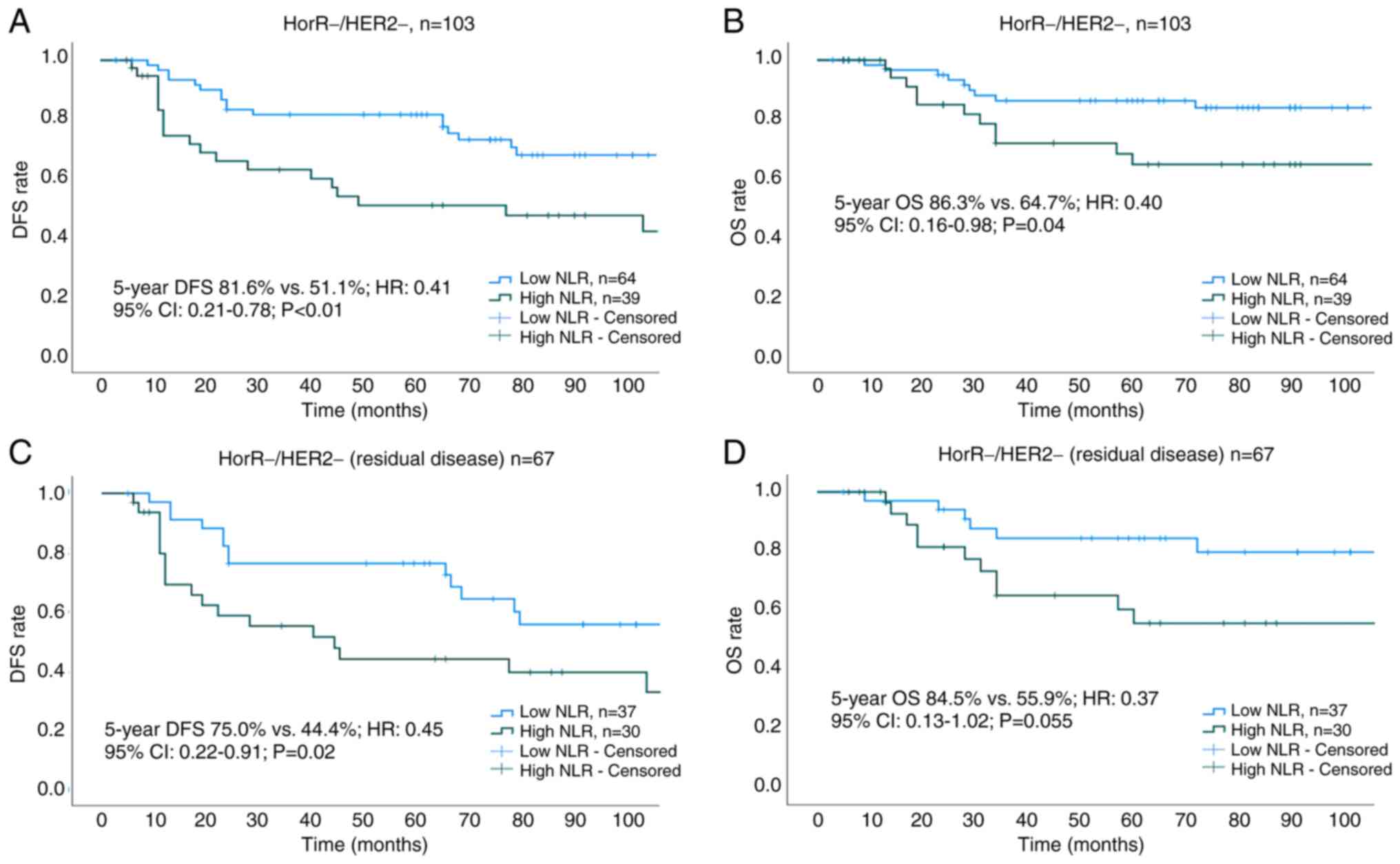
associated with prolonged DFS and OS (Table III). Subgroup analysis of survival
outcomes among molecular subtypes revealed statistical significance
only in patients with TNBC (Fig. 4A and
B). The 5-year DFS and OS rates for low vs. high NLR groups
across all molecular subtypes were as follows: HorR+/HER2- (DFS:
73.4 vs. 70.6%; P=0.71; OS: 88.4 vs. 87.4%; P=0.88; Fig. 5A and B), HorR+/HER2+ (DFS: 78.5 vs.
73.1%; P=0.68; OS: 97.7 vs. 80.1%; P=0.06; Fig. 5C and D), HorR-/HER2+ (DFS: 69.3 vs.
55.7%; P=0.35; OS: 83.6 vs. 79.4%; P=0.36; Fig. 5E and F), and HorR-/HER2- (DFS: 81.6
vs. 51.1%; P<0.01; OS: 86.3 vs. 64.7%; P=0.04; Fig. 4A and B). Notably, among patients
with TNBC with residual disease (no-pCR), those with a high
pre-treatment NLR exhibited lower 5-year DFS (44.4 vs. 75.0%;
P=0.02) and 5-year OS (55.9 vs. 84.5%, P=0.055) rates compared with
those patients with a low pre-treatment NLR (Fig. 4C and D).
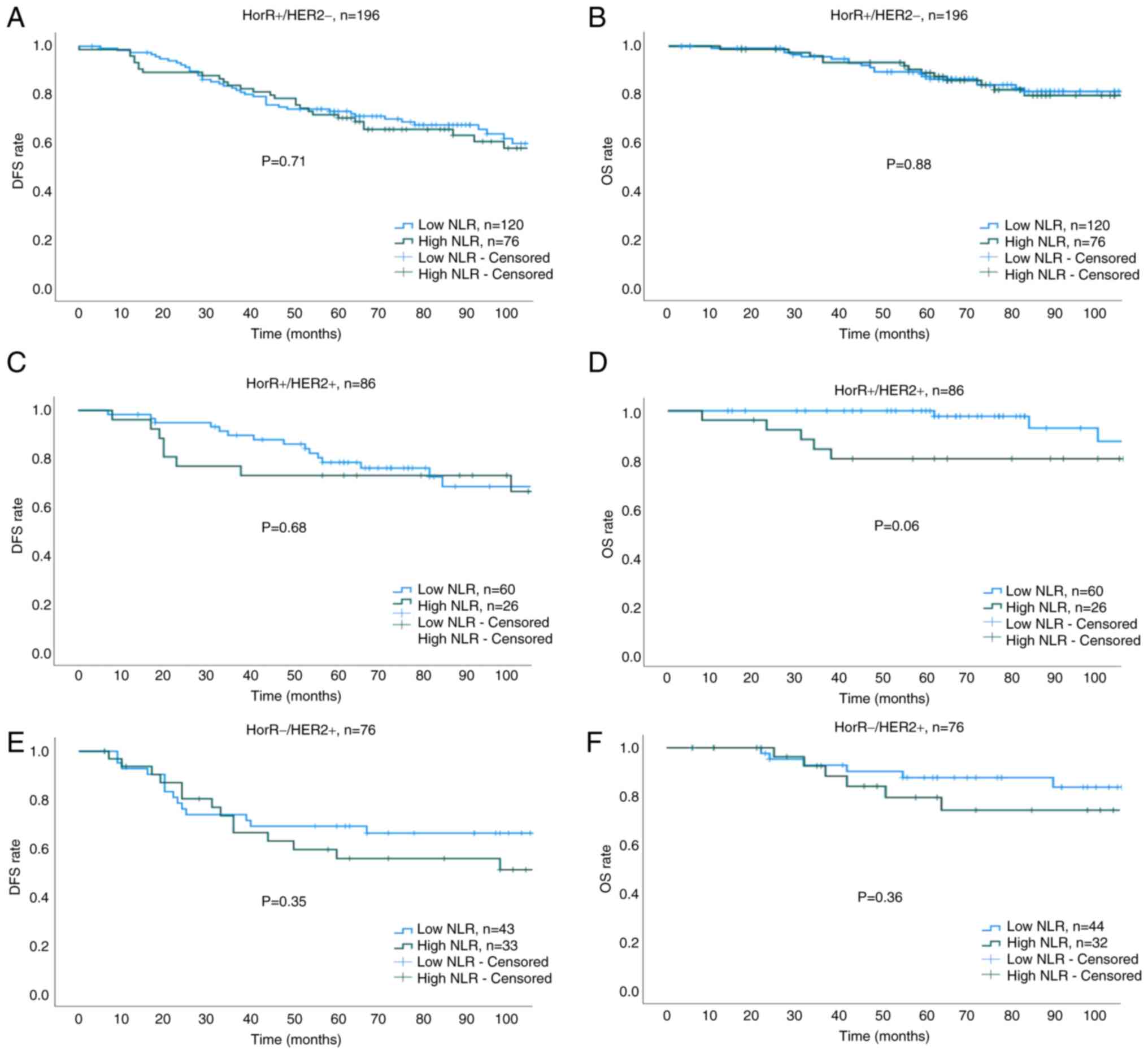 | Figure 5.Kaplan-Meier survival curves across
all molecular subtypes. (A) DFS of HorR+/HER2-, (B) OS of
HorR+/HER2-, (C) DFS of HorR+/HER2+, (D) OS of HorR+/HER2+, (E) DFS
of HorR-/HER2+ and (F) OS of HorR-/HER2+ subtype, as stratified
using the pre-treatment NLR. HorR, hormone receptor; HER2, human
epidermal growth factor receptor 2; NLR, neutrophil-to-lymphocyte
ratio; DFS, disease-free survival; OS, overall survival. |
 | Table III.Cox regression analysis of variables
associated with DFS and OS in patients with localized breast cancer
who received neoadjuvant chemotherapy. |
Table III.
Cox regression analysis of variables
associated with DFS and OS in patients with localized breast cancer
who received neoadjuvant chemotherapy.
|
| DFS | OS |
|---|
|
|
|
|
|---|
| Variables | Hazard ratio | 95% Confidence
interval | P-value | Hazard ratio | 95% Confidence
interval | P-value |
|---|
| Age |
|
|
|
|
|
|
| ≤40
years | Reference |
|
| Reference |
|
|
| >40
years | 0.85 | 0.62–1.17 | 0.33 | 0.72 | 0.46–1.15 | 0.17 |
| Menopausal
status |
|
|
|
|
|
|
|
Pre/perimenopausal | Reference |
|
| Reference |
|
|
|
Postmenopausal | 0.91 | 0.63–1.32 | 0.63 | 0.93 | 0.54–1.60 | 0.81 |
| Grade |
|
|
|
|
|
|
| G3 | Reference |
|
| Reference |
|
|
|
≤G2 | 0.94 | 0.69–1.30 | 0.75 | 0.64 | 0.55–1.43 | 0.64 |
| T stage |
|
|
|
|
|
|
|
≥T3 | Reference |
|
| Reference |
|
|
|
≤T2 | 0.53 | 0.36–0.78 | 0.002 | 0.42 | 0.22–0.79 | 0.007 |
| N stage |
|
|
|
|
|
|
|
≥N2 | Reference |
|
| Reference |
|
|
|
<N2 | 0.46 | 0.33–0.63 | <0.001 | 0.4 | 0.25–0.64 | <0.001 |
|
Neutrophil-to-lymphocyte ratio |
|
|
|
|
|
|
| High
(>2.21) | Reference |
|
| Reference |
|
|
| Low
(≤2.21) | 0.69 | 0.50–0.94 | 0.02 | 0.62 | 0.39–0.98 | 0.04 |
| pCR |
|
|
|
|
|
|
| No
(residual disease) | Reference |
|
| Reference |
|
|
| Yes
(pCR) | 0.28 | 0.17–0.46 | <0.001 | 0.27 | 0.12–0.58 | 0.001 |
Discussion
The present study aimed to evaluate the prognostic
significance of the pre-treatment NLR for predicting a pCR
following NAC in patients with LABC, in addition to its association
with DFS and OS. The pre-treatment NLR was found to serve as a
viable independent predictor of pCR after NAC in patients with LABC
and as a prognostic factor for DFS and OS in patients with TNBC
(including those with residual disease). This finding emphasizes
the potential value of the NLR in BC prognostics, particularly in
patients with TNBC, who frequently exhibit poorer clinical outcomes
and have limited targeted treatment options.
Although numerous studies have previously explored
the association between the NLR and pathological response to NAC,
results have been inconsistent (14–28).
This is possibly due to the retrospective study designs, variations
in sample sizes, factors influencing the NLR and heterogenous NLR
cut-off values (15,30). In addition, the lack of a
standardized NLR cut-off is a major limitation in its use as a
prognostic factor; it is typically influenced by multiple factors,
including ethnicity, age, comorbidities and medications, e.g.,
steroids, that affect neutrophil and lymphocyte counts. Therefore,
in the present study, an NLR cut-off of 2.2 was derived from the
study population using the ROC curve of the 465 patients. This was
found to be in the range of 1.8–4 reported by previous studies
(5,16,30,37–39).
Achieving a pCR in BC has been extensively studied.
A pCR was found to be associated with an improved prognosis, which
can guide adjuvant treatment planning and serve as a surrogate
endpoint in clinical trials (5,8,39,40).
Several prognostic factors associated with increased pCR in BC,
including smaller tumor size, TNBC, HER2-positive disease, higher
tumor grade, younger patient age, high tumor-infiltrating
lymphocyte (TIL) count, and use of anthracycline and taxane-based
regimens (5,8,41,42).
In particular, a high number of TILs, among other immune cells, has
emerged as an important indicator of chemotherapy response and
survival in BC (42). The
immunogenic nature of TNBC underscores its distinct
characteristics, as evidenced by significantly increased pCR rates
when pembrolizumab is added to a NAC protocol with four cycles of
paclitaxel and carboplatin, and four cycles of doxorubicin and
cyclophosphamide, compared with a placebo (64.8 vs. 51.2%),
irrespective of programmed death-ligand 1 expression (43). These factors highlight the complex
interplay among tumor biology, host immune response and treatment
strategies in determining the likelihood of achieving a pCR in BC.
Therefore, recognizing and comprehending these factors can
facilitate the optimization of treatment strategies, potentially
leading to improved patient outcomes. However, it is imperative to
acknowledge that individual responses to treatment may vary.
Although achieving a pCR is of importance, it is not the sole
determinant of long-term survival.
The present study observed an overall pCR rate of
25.2%, with the highest rate observed in HER2-positive and TNBC
subtypes, which is consistent with previous research (30,39,44).
However, the pre-treatment NLR was independently associated with
pCR across all molecular subtypes, highlighting its significance.
Furthermore, the high pre-treatment NLR was associated with poor
survival outcomes (DFS and OS), which aligns with the result of a
previous meta-analysis that included 42 studies (45). Subgroup analysis revealed a
statistically significant association between the NLR and survival
outcomes only in patients with TNBC. The underlying mechanisms
linking the NLR to pCR in BC subtypes, particularly TNBC, remain
unclear. Hypotheses based on preclinical and clinical studies
suggest that elevated neutrophil levels and reduced lymphocyte
levels can contribute to tumor growth, invasion and metastasis
(46,47). TNBC is characterized by an
inflammatory microenvironment that can promote tumor progression
through mechanisms such as angiogenesis, extracellular matrix
remodeling, immune evasion and development of treatment-resistance
(5,38–50).
Additionally, TNBC frequently exhibits high levels of TILs, which
is associated with increased pCR rates in response to neoadjuvant
therapy (42). Furthermore, results
from the present study align with those from the previous reports
on the association between the pre-treatment NLR and survival
outcomes in patients with residual TNBC (16,27).
This finding may have implications for guiding further treatment
decisions in the adjuvant setting of patients with residual disease
TNBC.
While the present study primarily investigated the
association between the pre-treatment NLR and pCR, the importance
of distinguishing the unique implications of both preoperative and
postoperative NLR values on patient prognosis cannot be overlooked.
The postoperative NLR holds notable prognostic significance and
merits attention in oncological investigations; it can reflect the
systemic inflammatory response following tumor removal, providing
insights into the host immune response, modulation of the tumor
microenvironment and residual disease burden. Previous studies
reported that elevated NLR levels, both immediately within 1 week
postoperatively and later, 5 years after diagnosis, were associated
with poorer survival outcomes in BC (51–53).
The major strengths of the present study include the
utilization of a large sample size of patients with LABC who
received NAC and data obtained from a prospective database,
allowing for a comprehensive analysis of the NLR as a prognostic
factor. Additionally, potential confounding factors that may impact
the NLR were mitigated by following defined eligibility criteria
and the NLR cut-off derived from the study population. However,
limitations remain. The retrospective nature of the present study
may introduce selection bias. In addition, the duration of
follow-up may have been insufficient to fully capture long-term
survival outcomes in patients with BC, particularly in molecular
subtypes with a less aggressive disease course. The present study
population was also drawn from a single institution, which may have
limited the generalizability of the findings. Furthermore, the
absence of tumor tissue data is a notable limitation, as it
prevented the inclusion of TIL analysis, which could have provided
additional insights into the prognostic landscape.
In conclusion, the present findings suggest that the
pre-treatment NLR can serve as an independent prognostic factor for
a pCR after NAC in patients with LABC and for survival outcomes,
particularly in patients with TNBC (including those with residual
disease). This highlights the potential of the NLR as a prognostic
marker to identify patients who benefit most from NAC, particularly
in TNBC, guiding treatment planning and patient counseling.
However, further prospective multicenter studies are warranted to
validate such findings and explore the underlying mechanisms
linking the NLR and treatment response in different BC
subtypes.
Acknowledgements
The early results of this study were presented as an
E-Poster at the European Society for Medical Oncology (ESMO) 2020.
The abstract was published in the Annals of Oncology as Supplement
S344, available at http://www.annalsofoncology.org/article/S0923-7534(20)40069-9/fulltext.
Funding
Funding: No funding was received.
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
BA conceptualized the present study. MAE, MA, NF,
AB, AA, KS and BA performed data collection. AA, KS and TAT were
involved in designing and managing the patient and BC database. BA
and TAT confirm the authenticity of all of the raw data. BA and TAT
performed a literature review and wrote the first draft of the
manuscript. TE and BA performed the analysis and prepared tables
and figures. All authors edited and approved the final version of
the manuscript.
Ethics approval and consent to
participate
The study was approved by the RAC of King Faisal
Specialist Hospital and Research Centre (Riyadh, Saudi Arabia;
approval no. 2051-029). The RAC committee at King Faisal Specialist
Hospital and Research Centre waived the requirement for informed
consent. All methods were performed in accordance with relevant
guidelines and regulations.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
HER2
|
human epidermal growth factor receptor
2
|
|
HR
|
hazard ratio
|
|
HorR
|
hormone receptor
|
|
LABC
|
locally advanced breast cancer
|
|
NAC
|
neoadjuvant chemotherapy
|
|
NLR
|
neutrophil-to-lymphocyte ratio
|
|
OS
|
overall survival
|
|
pCR
|
pathological complete response
|
|
RAC
|
Research Advisory Council
|
|
ROC
|
receiver operating characteristic
|
|
TIL
|
tumor-infiltrating lymphocyte
|
|
TNBC
|
triple-negative breast cancer
|
References
|
1
|
Bray F, Laversanne M, Sung H, Ferlay J,
Siegel RL, Soerjomataram I and Jemal A: Global cancer statistics
2022: GLOBOCAN estimates of incidence and mortality worldwide for
36 cancers in 185 countries. CA Cancer J Clin. 74:229–263. 2024.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kaufmann M, Hortobagyi GN, Goldhirsch A,
Scholl S, Makris A, Valagussa P, Blohmer JU, Eiermann W, Jackesz R,
Jonat W, et al: Recommendations from an international expert panel
on the use of neoadjuvant (primary) systemic treatment of operable
breast cancer: An update. J Clin Oncol. 24:1940–1949. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Rastogi P, Anderson SJ, Bear HD, Geyer CE,
Kahlenberg MS, Robidoux A, Margolese RG, Hoehn JL, Vogel VG, Dakhil
SR, et al: Preoperative chemotherapy: Updates of national surgical
adjuvant breast and bowel project protocols B-18 and B-27. J Clin
Oncol. 26:778–785. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Early Breast Cancer Trialists'
Collaborative Group (EBCTCG), . Long-term outcomes for neoadjuvant
versus adjuvant chemotherapy in early breast cancer: Meta-analysis
of individual patient data from ten randomised trials. Lancet
Oncol. 19:27–39. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
von Minckwitz G, Untch M, Blohmer JU,
Costa SD, Eidtmann H, Fasching PA, Gerber B, Eiermann W, Hilfrich
J, Huober J, et al: Definition and impact of pathologic complete
response on prognosis after neoadjuvant chemotherapy in various
intrinsic breast cancer subtypes. J Clin Oncol. 30:1796–1804. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Fayanju OM, Ren Y, Thomas SM, Greenup RA,
Plichta JK, Rosenberger LH, Tamirisa N, Force J, Boughey JC, Hyslop
T and Hwang ES: The clinical significance of breast-only and
node-only pathologic complete response (PCR) after neoadjuvant
chemotherapy (NACT): A review of 20,000 breast cancer patients in
the national cancer data base (NCDB). Ann Surg. 268:591–601. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Viale G: Characterization and clinical
impact of residual disease after neoadjuvant chemotherapy. Breast.
22 (Suppl 2):S88–S91. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Cortazar P, Zhang L, Untch M, Mehta K,
Costantino JP, Wolmark N, Bonnefoi H, Cameron D, Gianni L,
Valagussa P, et al: Pathological complete response and long-term
clinical benefit in breast cancer: The CTNeoBC pooled analysis.
Lancet. 384:164–172. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Masuda N, Lee SJ, Ohtani S, Im YH, Lee ES,
Yokota I, Kuroi K, Im SA, Park BW, Kim SB, et al: Adjuvant
capecitabine for breast cancer after preoperative chemotherapy. N
Engl J Med. 376:2147–2159. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Von Minckwitz G, Huang CS, Mano MS, Loibl
S, Mamounas EP, Untch M, Wolmark N, Rastogi P, Schneeweiss A,
Redondo A, et al: Trastuzumab emtansine for residual invasive
HER2-positive breast cancer. N Engl J Med. 380:617–628. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Templeton AJ, McNamara MG, Šeruga B,
Vera-Badillo FE, Aneja P, Ocaña A, Leibowitz-Amit R, Sonpavde G,
Knox JJ, Tran B, et al: Prognostic role of neutrophil-to-lymphocyte
ratio in solid tumors: A systematic review and meta-analysis. J
Natl Cancer Inst. 106:dju1242014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Paramanathan A, Saxena A and Morris DL: A
systematic review and meta-analysis on the impact of pre-operative
neutrophil lymphocyte ratio on long term outcomes after curative
intent resection of solid tumours. Surg Oncol. 23:31–39. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Faria SS, Fernandes PC Jr, Silva MJ, Lima
VC, Fontes W, Freitas-Junior R, Eterovic AK and Forget P: The
neutrophil-to-lymphocyte ratio: A narrative review.
Ecancermedicalscience. 10:7022016.PubMed/NCBI
|
|
14
|
Qian Y, Tao J, Li X, Chen H, Lu Q, Yang J,
Pan H, Wang C, Zhou W and Liu X: Peripheral inflammation/immune
indicators of chemosensitivity and prognosis in breast cancer
patients treated with neoadjuvant chemotherapy. Onco Targets Ther.
11:1423–1432. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Chen Y, Chen K, Xiao X, Nie Y, Qu S, Gong
C, Su F and Song E: Pretreatment neutrophil-to-lymphocyte ratio is
correlated with response to neoadjuvant chemotherapy as an
independent prognostic indicator in breast cancer patients: A
retrospective study. BMC Cancer. 16:3202016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Bae SJ, Cha YJ, Yoon C, Kim D, Lee J, Park
S, Cha C, Kim JY, Ahn SG, Park HS, et al: Prognostic value of
neutrophil-to-lymphocyte ratio in human epidermal growth factor
receptor 2-negative breast cancer patients who received neoadjuvant
chemotherapy. Sci Rep. 10:130782020. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Asano Y, Kashiwagi S, Onoda N, Noda S,
Kawajiri H, Takashima T, Ohsawa M, Kitagawa S and Hirakawa K:
Predictive value of neutrophil/lymphocyte ratio for efficacy of
preoperative chemotherapy in triple-negative breast cancer. Ann
Surg Oncol. 23:1104–1110. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Chae S, Kang KM, Kim HJ, Kang E, Park SY,
Kim JH, Kim SH, Kim SW and Kim EK: Neutrophil-lymphocyte ratio
predicts response to chemotherapy in triple-negative breast cancer.
Curr Oncol. 25:e113–e119. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Rao JS, Hanumappa HK, Joseph EP, Chowdappa
RG and Ramesh R: Elevated neutrophil-lymphocyte ratio in
luminal-type locally advanced breast cancer to circumvent
neo-adjuvant chemotherapy. Indian J Surg Oncol. 10:454–459. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Rivas M, Acevedo F, Dominguez F, Galindo
H, Camus M, Oddo D, Villarroel A, Razmilic D, Peña J, Munoz Medel
M, et al: The neutrophil to lymphocyte ratio predicts the response
to neoadjuvant chemotherapy in luminal B breast cancer. Asian Pac J
Cancer Prev. 20:2209–2212. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Coleman N, Rom FM, Brennan A, Bennett K,
Jayaram AK and Kennedy MJ: Does baseline absolute
neutrophil-to-lymphocyte ratio (Nlr) correlate with pathological
complete response (Pcr) in neoadjuvant breast cancer (Bc)? Ann
Oncol. 25 (Suppl 4):IV1132014. View Article : Google Scholar
|
|
22
|
Eryilmaz MK, Mutlu H, Salim DK, Musri FY,
Tural D and Coskun HS: The neutrophil to lymphocyte ratio has a
high negative predictive value for pathologic complete response in
locally advanced breast cancer patients receiving neoadjuvant
chemotherapy. Asian Pac J Cancer Prev. 15:7737–7740. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Koh YW, Lee HJ, Ahn JH, Lee JW and Gong G:
Prognostic significance of the ratio of absolute neutrophil to
lymphocyte counts for breast cancer patients with ER/PR-positivity
and HER2-negativity in neoadjuvant setting. Tumour Biol.
35:9823–9830. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Suppan C, Bjelic-Radisic V, La Garde M,
Groselj-Strele A, Eberhard K, Samonigg H, Loibner H, Dandachi N and
Balic M: Neutrophil/lymphocyte ratio has no predictive or
prognostic value in breast cancer patients undergoing preoperative
systemic therapy. BMC Cancer. 15:10272015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Graziano V, Grassadonia A, Iezzi L, Vici
P, Pizzuti L, Barba M, Quinzii A, Camplese A, Di Marino P, Peri M,
et al: Combination of peripheral neutrophil-to-lymphocyte ratio and
platelet-to-lymphocyte ratio is predictive of pathological complete
response after neoadjuvant chemotherapy in breast cancer patients.
Breast. 44:33–38. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Marín Hernández C, Piñero Madrona A, Gil
Vázquez PJ, Galindo Fernández PJ, Ruiz Merino G, Alonso Romero JL
and Parrilla Paricio P: Usefulness of lymphocyte-to-monocyte,
neutrophil-to-monocyte and neutrophil-to-lymphocyte ratios as
prognostic markers in breast cancer patients treated with
neoadjuvant chemotherapy. Clin Transl Oncol. 20:476–483. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Muñoz-Montaño W, Cabrera-Galeana P,
Alvarado-Miranda A, Villarreal-Garza C, Mohar A, Olvera A,
Bargallo-Rocha E, Lara-Medina F and Arrieta O: Prognostic value of
the pretreatment neutrophil-to-lymphocyte ratio in different
phenotypes of locally advanced breast cancer during neoadjuvant
systemic treatment. Clin Breast Cancer. 20:307–316.e1. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Peng Y, Chen R, Qu F, Ye Y, Fu Y, Tang Z,
Wang Y, Zong B, Yu H, Luo F and Liu S: Low pretreatment
lymphocyte/monocyte ratio is associated with the better efficacy of
neoadjuvant chemotherapy in breast cancer patients. Cancer Biol
Ther. 21:189–196. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Bulut G and Ozdemir ZN: Significance of
neutrophil-lymphocyte ratio and Trombocyte-lymphocyte ratio in
predicting complete pathological response in patients with local
advanced breast cancer. Eurasian J Med Invest. 61:78–83. 2022.
|
|
30
|
Cullinane C, Creavin B, O'Leary DP,
O'Sullivan MJ, Kelly L, Redmond HP and Corrigan MA: Can the
neutrophil to lymphocyte ratio predict complete pathologic response
to neoadjuvant breast cancer treatment? A systematic review and
meta-analysis. Clin Breast Cancer. 20:e675–e681. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
McShane LM, Altman DG, Sauerbrei W, Taube
SE, Gion M and Clark GM; Statistics Subcommittee of NCI-EORTC
Working Group on Cancer Diagnostics, : REporting recommendations
for tumor MARKer prognostic studies (REMARK). Breast Cancer Res
Treat. 100:229–235. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Sauerbrei W, Taube SE, McShane LM,
Cavenagh MM and Altman DG: Reporting recommendations for tumor
marker prognostic studies (REMARK): An abridged explanation and
elaboration. J Natl Cancer Inst. 110:803–811. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Hammond ME, Hayes DF, Wolff AC, Mangu PB
and Temin S: American society of clinical oncology/college of
american pathologists guideline recommendations for
immunohistochemical testing of estrogen and progesterone receptors
in breast cancer. J Oncol Pract. 6:195–197. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Wolff AC, Hammond ME, Schwartz JN, Hagerty
KL, Allred DC, Cote RJ, Dowsett M, Fitzgibbons PL, Hanna WM, Langer
A, et al: American society of clinical oncology/college of American
pathologists guideline recommendations for human epidermal growth
factor receptor 2 testing in breast cancer. J Clin Oncol.
25:118–145. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Wolff AC, Hammond ME, Hicks DG, Dowsett M,
McShane LM, Allison KH, Allred DC, Bartlett JM, Bilous M,
Fitzgibbons P, et al: Recommendations for human epidermal growth
factor receptor 2 testing in breast cancer: American society of
clinical oncology/college of American pathologists clinical
practice guideline update. J Clin Oncol. 31:3997–4013. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Amin MB, Edge SB, Greene FL, Byrd DR,
Brookland RK, Washington MK, Gershenwald JE, Compton CC, Hess KR,
Sullivan DC, et al: AJCC cancer staging manual. 8th edition.
Springer; 2017
|
|
37
|
Liu X, Qu JK, Zhang J, Yan Y, Zhao XX,
Wang JZ, Qu HY, Liu L, Wang JS and Duan XY: Prognostic role of
pretreatment neutrophil to lymphocyte ratio in breast cancer
patients: A meta-analysis. Medicine (Baltimore). 96:e81012017.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Ethier JL, Desautels D, Templeton A, Shah
PS and Amir E: Prognostic role of neutrophil-to-lymphocyte ratio in
breast cancer: A systematic review and meta-analysis. Breast Cancer
Res. 19:22017. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Spring LM, Fell G, Arfe A, Sharma C,
Greenup R, Reynolds KL, Smith BL, Alexander B, Moy B, Isakoff SJ,
et al: Pathologic complete response after neoadjuvant chemotherapy
and impact on breast cancer recurrence and survival: A
comprehensive meta-analysis. Clin Cancer Res. 26:2838–2848. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Zhou Q, Dong J, Sun Q, Lu N, Pan Y and Han
X: Role of neutrophil-to-lymphocyte ratio as a prognostic biomarker
in patients with breast cancer receiving neoadjuvant chemotherapy:
A meta-analysis. BMJ Open. 11:e0479572021. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Mieog JSD, Van Der Hage JA and Van De
Velde CJH: Preoperative chemotherapy for women with operable breast
cancer. Cochrane Database Syst Rev. 2007:CD0050022007.PubMed/NCBI
|
|
42
|
Denkert C, von Minckwitz G, Darb-Esfahani
S, Lederer B, Heppner BI, Weber KE, Budczies J, Huober J, Klauschen
F, Furlanetto J, et al: Tumour-infiltrating lymphocytes and
prognosis in different subtypes of breast cancer: A pooled analysis
of 3771 patients treated with neoadjuvant therapy. Lancet Oncol.
19:40–50. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Schmid P, Cortes J, Dent R, Pusztai L,
McArthur H, Kümmel S, Bergh J, Denkert C, Park YH, Hui R, et al:
Event-free survival with pembrolizumab in early triple-negative
breast cancer. N Engl J Med. 386:556–567. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
von Au A, Shencoru S, Uhlmann L, Mayer L,
Michel L, Wallwiener M, Hennigs A, Deutsch T, Riedel F, Heil J, et
al: Predictive value of neutrophil-to-lymphocyte-ratio in
neoadjuvant-treated patients with breast cancer. Arch Gynecol
Obstet. 307:1105–1113. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Savioli F, Morrow ES, Dolan RD, Romics L,
Lannigan A, Edwards J and McMillan DC: Prognostic role of
preoperative circulating systemic inflammatory response markers in
primary breast cancer: Meta-analysis. Br J Surg. 109:1206–1215.
2022. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Hanahan D and Weinberg RA: Hallmarks of
cancer: The next generation. Cell. 144:646–674. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Mantovani A, Romero P, Palucka AK and
Marincola FM: Tumour immunity: Effector response to tumour and role
of the microenvironment. Lancet. 371:771–783. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Quail DF and Joyce JA: Microenvironmental
regulation of tumor progression and metastasis. Nat Med.
19:1423–1437. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
DeNardo DG, Brennan DJ, Rexhepaj E,
Ruffell B, Shiao SL, Madden SF, Gallagher WM, Wadhwani N, Keil SD,
Junaid SA, et al: Leukocyte complexity predicts breast cancer
survival and functionally regulates response to chemotherapy.
Cancer Discov. 1:54–67. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Larionova I, Cherdyntseva N, Liu T,
Patysheva M, Rakina M and Kzhyshkowska J: Interaction of
tumor-associated macrophages and cancer chemotherapy.
Oncoimmunology. 8:15960042019. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Ren Z, Yang J, Liang J, Xu Y, Lu G, Han Y,
Zhu J, Tan H, Xu T and Ren M: Monitoring of postoperative
neutrophil-to-lymphocyte ratio, D-dimer, and CA153 in: Diagnostic
value for recurrent and metastatic breast cancer. Front Surg.
9:9274912023. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Lee SK, Choi MY, Bae SY, Lee JH, Lee HC,
Kil WH, Lee JE, Kim SW and Nam SJ: Immediate postoperative
inflammation is an important prognostic factor in breast cancer.
Oncology. 88:337–344. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Moon G, Noh H, Cho IJ, Lee JI and Han A:
Prediction of late recurrence in patients with breast cancer:
Elevated neutrophil to lymphocyte ratio (NLR) at 5 years after
diagnosis and late recurrence. Breast Cancer. 27:54–61. 2020.
View Article : Google Scholar : PubMed/NCBI
|