Introduction
The management of patients with malignant lymphoma
entails developing a treatment strategy based on clinical factors,
including age and prognostic factors (1,2), as
well as incorporating treatment evidence from patient backgrounds
and guidelines. Clinical management approaches range from immediate
treatment with chemotherapy or radiation therapy, even without
symptoms, to a watch-and-wait (W&W) approach, where treatment
is initiated when symptoms manifest (3,4).
Before the advent of chemotherapy, patients with diffuse large
B-cell lymphoma often succumbed to the disease within weeks to
months of diagnosis (5). In the
1970s, the introduction of cyclophosphamide, doxorubicin,
vincristine, and prednisolone (CHOP) chemotherapy marked a major
advancement, achieving a complete response in approximately 50% of
patients and disease-free survival in 30–40% of patients (6).
With the considerable prolongation of survival
periods through chemotherapy, the emphasis on quality of life (QOL)
increased (7–9). Accordingly, with the development of
new drugs, measuring QOL as a secondary endpoint (10) and comparing the QOL between patients
receiving chemotherapy and those managed with the W&W approach
(11) have become integral research
components. Such studies show that long-term survival may be
achieved at the cost of a gradual decrease in QOL (12). However, to the best of our
knowledge, no study has examined the effect of initial chemotherapy
on QOL or the early cost-effectiveness of chemotherapy for
malignant lymphoma, despite the high response rates and early
treatment effects (13). The
transition from everyday life to an altered state because of
treatment represents a major aspect of initial chemotherapy for
patients with malignant lymphoma. This transition can influence QOL
owing to restrictions in daily activities, anxiety related to
treatment, and discomfort caused by adverse effects (14).
Economic issues also affect health-related QOL in
non-Hodgkin lymphoma survivors (15). Thus, healthcare providers must
monitor and respond to changes in the QOL of patients, particularly
because rising healthcare costs impose heavier societal burdens
(16). Although CHOP has been a
universal first-line treatment since its effectiveness was
established in the 1970s, there are no reports on its
cost-effectiveness. In clinical practice, it is crucial to
prioritize the efficacy of chemotherapy; however, the landscape of
treatment has drastically changed since the efficacy of CHOP was
first established. In recent years, the inclusion of
cost-effectiveness calculations in the review process has become
standard practice when applying for approval of a new drug in other
countries. In addition, considering the challenging financial
situation of Japan's public healthcare services, it is imperative
to allocate the limited available budgets appropriately based on
cost-effectiveness of treatments. Thus, clarifying the
cost-effectiveness of established treatments is crucial.
Additionally, long-term declines in QOL (17–19)
have not captured changes in the QOL of patients undergoing
traumatic life changes. Understanding the changes that occur during
periods of considerable change in the lives of patients can help
healthcare providers improve the quality of care. Access to
real-world analysis results that reflect the cost-effectiveness
associated with substantial life changes remains crucial. In this
milieu, we conducted a prospective patient-reported QOL survey to
clarify the effect of initial chemotherapy on QOL and its
cost-effectiveness in patients with malignant lymphoma.
Patients and methods
Participants
Patients with malignant lymphoma who received
initial chemotherapy at Gifu Municipal Hospital from January 2021
to December 2022 were included. Inclusion criteria comprised
patients diagnosed with all types of lymphoma and those scheduled
to begin chemotherapy via intravenous infusion. Exclusion criteria
comprised patients diagnosed with lymphoma but treated with oral
anticancer agents and patients who did not receive chemotherapy,
such as those managed using the W&W approach. Consent to
participate in the study was obtained after the treatment plan was
determined and before the first dose of chemotherapy was
administered. We also administered pre- and post-treatment
questionnaires. The pretreatment questionnaire was administered
before the start of chemotherapy on day 1, and the post-treatment
questionnaire was administered on the last day of each regimen's
defined treatment period (e.g., for a 3-week regimen, pre- and
post-treatment questionnaires were administered on days 1 and 21,
respectively).
Patient characteristics
Data on patient characteristics, including sex, age,
performance status, cancer stage, B symptom, classification,
treatment regimen, administration date, days from administration to
discharge, drugs used, prescribed drug dose, and administered drug
dose, were retrieved from electronic medical records. Pain
management during treatment involved the use of analgesics when the
pain was intense and based on patient's request. For psychological
symptoms, such as depression, patients are assessed by the medical
staff. If severe depression is diagnosed, counselors are made
available under the guidance of the attending physician; however,
counselors were not available for this study. These procedures for
psychological symptoms and pain are standard methods in daily
medical practice and were adhere to by the participants in our
study. No special, unique interventions were applied to the
participants.
Regimen
Chemotherapy involved administering drug therapy
based on a predetermined treatment plan (regimen) that detailed the
dosage, administration method, administration date, and treatment
intervals. Certain regimens were considered clinically equivalent,
with similar effectiveness, and were classified accordingly. CHOP;
CHOP + rituximab (R); and pirarubicin, cyclophosphamide,
vincristine, prednisolone were classified as CHOP ± R therapy. The
relative dose intensity (RDI) was calculated by dividing the
administered drug dose by the prescribed drug dose during the
specified period. In addition, the molecular-targeted drugs
rituximab, obinutuzumab, and polatuzumab vedotin were administered
to all patients prior to the administration of antihistamines,
antipyretic analgesics, and corticosteroids, as specified in the
package insert to reduce infusion reactions. As an antiemetic
measure for cytotoxic anticancer drugs, 5-hydroxytryptamine and
corticosteroids were administered to all patients according to the
risk classification of The Japan Society of Clinical Oncology
Clinical Practice Guidelines for Antiemesis (20). These antiemetics were administered
in advance to all patients, and anticancer agents were administered
after them. If nausea and vomiting occurred despite these
premedications, prochlorperazine was added at the discretion of the
attending physician.
Quality of life
We conducted a self-reported patient questionnaire
survey using EuroQol-5 dimensions (EQ-5D) (21) before and after initial chemotherapy.
The utility values and percentage of issues in each of the five
dimensions were compared before and after chemotherapy. Due to the
varying adverse effects across different regimens that affect the
QOL, the analysis was stratified by regimen. As chemotherapy
involves the selection of a regimen tailored to the conditions of
individual patients, we analyzed CHOP ± R therapy and all regimens
(all patients) as the target for analysis; regimens administered to
<20% of the total patients were excluded from the analysis owing
to a lack of accuracy in utility value changes and average cost
values. This exclusion minimizes potential errors in comparing
regimens, which may compromise the precision of the analysis.
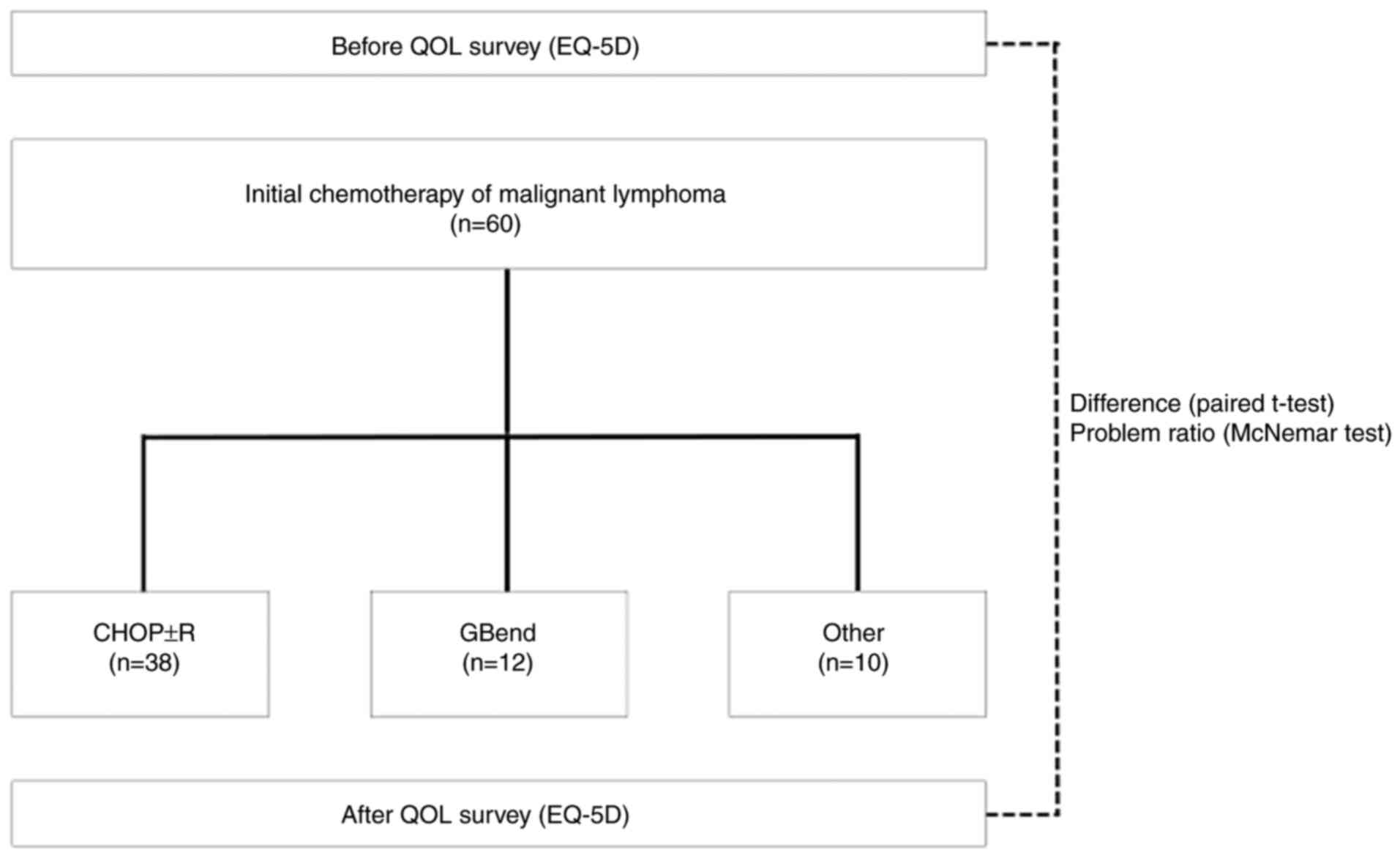
Fig. 1 shows a schematic for the
experimental design.
Cost-effectiveness
We utilized the official drug prices, and the
analysis was conducted from the perspective of the cost payer (the
standpoint of public healthcare) for cost calculation of the
administered drug dose. In Japan, the Ministry of Health, Labour
and Welfare (MHLW) recommends the use of generic drugs, including
cytotoxic anticancer drugs. Generic drugs are products that contain
the identical amount of the active ingredient found in the patented
products (brand-name drug), offering the same efficacy, dosage, and
method of administration. They were approved by the MHLW as
therapeutically ‘equivalent to’ and ‘substitutable for’ the
patented drug. The Japanese MHLW recommends the use of generic
drugs for all applications except when only brand-name drugs are
available on the market. This is widely practiced in the Japanese
medical field as a measure to control healthcare costs, as national
healthcare costs in Japan continue to increase. The MHLW also
recommends the use of biosimilars for molecular-targeted drugs. At
our institution, we use generic drugs and biosimilars when they are
available in the Japanese market, but when they are not available,
we use brand-name drugs in our daily practice. In Japan, generic
drugs and biosimilars are manufactured under Good Manufacturing
Practices as stipulated by the MHLW, which assures their quality
and that the efficacy and safety of both drugs are equivalent. As a
result, several medical institutions in Japan use generic drugs,
leading to the widespread use of such drugs in Japan. In addition,
due to the uniform pricing of generic drugs among several
pharmaceutical manufacturers for the same ingredients and
specifications, the price of generic drugs is almost consistent.
Because of these factors, the price of the least expensive version
of the drug is selected for the commonly used version of the drug,
and the estimates in this study also reflect these conditions. The
cost-utility analysis was employed for cost-effectiveness analysis.
Incremental cost-effectiveness ratio (ICER) was calculated to
determine the cost required per quality-adjusted life year (QALY).
The ICER threshold was set at 7.5 million yen, a value recognized
by the Japanese MHLW as indicating favorable cost-effectiveness for
anticancer drugs (22). In cases
where the cost was ~7.5 million yen, sensitivity analysis was
conducted to account for potential errors. However, if the cost
significantly exceeded 7.5 million yen (such as by more than three
times), sensitivity analysis was not performed as the regimen was
considered not to be cost-effective. Owing to variations in
policies regarding hospitalization, supportive therapy, and
monitoring among facilities, the cost calculation solely focused on
the administered anticancer drugs to eliminate differences in the
surrounding environment. As no control group was established, we
considered the scenario of chemotherapy non-administration,
evaluating cost-effectiveness under the assumption of no change in
QOL during the same period as chemotherapy administration.
Sensitivity analysis
Classifying patients receiving chemotherapy as a
control group without treatment was considered unethical.
Therefore, the control group was established under the assumption
of no treatment and no change in utility values. Sensitivity
analysis was conducted assuming that QOL changes would occur in the
control group under the hypothetical conditions. Moreover, the
drugs used and doses administered were considered to rarely change
as they are specified in the regimen. Additionally, drug costs
remain relatively stable as they were based on the publicly set
prices by the MHLW. As there have been reports on changes in QOL
and as these reports mostly specified standard deviations, QOL
values were used as parameters in the sensitivity analysis. The
previously described QOL change values (23) were adopted for this analysis. The
QOL change values were converted to 0.0025 and double of that value
(0.005), for 3 weeks, the same duration as for most regimens. These
values were varied on the ascending and descending sides,
respectively. We compared the results with the scenario of no
reported change in QOL to analyze the effect of these
variations.
Statistical analysis
The McNemar test was employed to compare the
proportion of issues in the EQ-5D before and after chemotherapy. A
paired t-test was used to compare the utility values of the EQ-5D
before and after chemotherapy. Results with a significance level
<5% were considered statistically significant. Statistical
analyses were performed using SPSS Statistics 29.0.
Ethics
This study was approved by the research ethics
committees of Gifu Municipal Hospital (approval number 663) and
Gifu Pharmaceutical University (approval number 2–23).
Participation was restricted to patients who provided written
informed consent for their enrollment after receiving a written
description of the study.
Results
Patient characteristics
This study involved 60 patients (33 male and 27
female individuals) aged 69.7±10.9 years [mean ± standard deviation
(SD)]. Seventeen patients had performance status 0, 23 had stage IV
tumor, 51 showed B symptom absence, 28 had diffuse large B-cell
lymphoma, 51 had B-cell type, and 33 had aggressive lymphoma
(Table I).
 | Table I.Demographics of the patients included
in the present study. |
Table I.
Demographics of the patients included
in the present study.
|
Characteristics | Value |
|---|
| Age, years (mean ±
SD) | 69.7±10.9 |
| Hospitalization
period, days (mean ± SD) | 16.7±4.9 |
| Sex, n |
|
|
Male | 34 |
|
Female | 26 |
| Performance status,
n |
|
| 0 | 17 |
| 1 | 16 |
| 2 | 0 |
| 3 | 1 |
|
Unknown | 26 |
| Stage, n |
|
| I | 6 |
| II | 12 |
|
III | 3 |
| IV | 23 |
|
Unknown | 16 |
| B symptom, n |
|
| + | 9 |
| - | 51 |
| Cell
classification, n |
|
| B
cells | 51 |
| Natural
killer T cells | 9 |
| Clinical
classification, n |
|
|
Indolent lymphoma | 24 |
|
Aggressive lymphoma | 33 |
| Highly
aggressive lymphoma | 3 |
Regimen
The RDI for all the regimens was 90.8%. The most
common regimen was CHOP ± R therapy (cyclophosphamide 750
mg/m2 (day 2), doxorubicin 50 mg/m2 (day 2),
vincristine 1.4 mg/m2 (day 2), prednisolone, and
rituximab 375 mg/m2 (day 1)), administered to 38
patients, with an RDI of 88.4±11%. The second most common regimen
was obinutuzumab and bendamustine therapy [GBend; obinutuzumab
1,000 mg/body (days 1, 8, and 15) and bendamustine 90
mg/m2 (days 1 and 2)], administered to 12 patients, with
an RDI of 99.0±0.9%. Drugs with different adverse effect profiles
from the cytotoxic anticancer drug (inhibits cell division and
proliferation) included the molecularly targeted agents (specific
action on unique target molecules involved in cancer cell growth)
rituximab (anti-CD20 monoclonal antibody), obinutuzumab (humanized
anti-CD20 monoclonal antibody), brentuximab vedotin (anti-CD30
monoclonal antibody), and polatuzumab vedotin (anti-CD79b
monoclonal antibody), which were used in combination with the
cytotoxic anticancer drug (Table
II). The mean cost of the administered drugs was 267,577 yen
for all regimens and 90,568 yen for CHOP ± R therapy (Table III).
 | Table II.Tumor subtype and regimen. |
Table II.
Tumor subtype and regimen.
| Tumor subtype (WHO
classification) | Patients, n | Regimen | Patients, n | Period, days | Administration,
days | Drug name, drug
dosage and administration day |
|---|
| Diffuse large
B-cell lymphoma, NOS | 27 | RCHOP | 25 | 21 | 1,2 | Rituximab (375
mg/m2; day 1), cyclophosphamide (750 mg/m2;
day 2), doxorubicin (50 mg/m2; day 2), vincristine (1.4
mg/m2; day 2), prednisolone (p.o. 100 mg/body; day
2–6) |
|
|
| PolaRCHP | 1 | 21 | 1,2 | Polatuzumab vedotin
(1.8 mg/kg; day 2), rituximab (375 mg/m2; day 1),
cyclophosphamide (750 mg/m2; day 2), doxorubicin (50
mg/m2; day 2), prednisolone (p.o. 100 mg/body; day
2–6) |
|
|
| Bend | 1 | 21 | 1,2 | Bendamustine (120
mg/m2; day 1, 2) |
| Follicular
lymphoma | 15 | GBend | 12 | 28 | 1,2,8,15 | Obinutuzumab (1,000
mg/body; day 1, 8, 15), bendamustine (90 mg/m2; day 1,
2) |
|
|
| RCHOP | 2 | 21 | 1,2 | Rituximab (375
mg/m2; day 1), cyclophosphamide (750 mg/m2;
day 2), doxorubicin (50 mg/m2; day 2), vincristine (1.4
mg/m2; day 2), prednisolone (p.o. 100 mg/body; day
2–6) |
|
|
| CHOP | 1 | 21 | 1 | Cyclophosphamide
(750 mg/m2; day 1), doxorubicin (50 mg/m2;
day 1), vincristine (1.4 mg/m2; day 1), prednisolone
(p.o. 100 mg/body; day 1–5) |
| Peripheral T-cell
lymphoma, NOS | 5 | THPCOP | 4 | 21 | 1 | Pirarubicin (50
mg/m2; day 1), cyclophosphamide (750 mg/m2;
day 1), vincristine (1.4 mg/m2; day 1) |
|
|
| A-CHP | 1 | 21 | 1 | Brentuximab vedotin
(1.8 mg/kg; day 1), cyclophosphamide (750 mg/m2; day 1),
doxorubicin (50 mg/m2; day 1), prednisolone (p.o. 100
mg/body; day 1–5) |
|
Enteropathy-associated T-cell
lymphoma | 2 | CHOP | 1 | 21 | 1 | Cyclophosphamide
(750 mg/m2; day 1), doxorubicin (50 mg/m2;
day 1), vincristine (1.4 mg/m2; day 1), prednisolone
(p.o. 100 mg/body; day 1–5) |
|
|
| THPCOP | 1 | 21 | 1 | Pirarubicin (50
mg/m2; day 1), cyclophosphamide (750 mg/m2;
day 1), vincristine (1.4 mg/m2; day 1), prednisolone
(p.o. 100 mg/body; day 1–5) |
| Mantle cell
lymphoma | 2 | RHyperCVAD | 2 | 21 | 1-5,12 | Rituximab (375
mg/m2; day 1), cyclophosphamide (300 mg/m2;
day 2–4), doxorubicin (50 mg/m2; day 5), vincristine
(1.4 mg/m2; day 5, 12), dexamethasone (33 mg/body; day
2–5, day 12–15) |
| Anaplastic large
cell lymphoma, ALK-positive | 1 | A-CHP | 1 | 21 | 1 | Brentuximab vedotin
(1.8 mg/kg; day 1), cyclophosphamide (750 mg/m2; day 1),
doxorubicin (50 mg/m2; day 1), prednisolone (p.o. 100
mg/body; day 1–5) |
| Angioimmunoblastic
T-cell lymphoma | 1 | THPCOP | 1 | 21 | 1 | Pirarubicin (50
mg/m2; day 1), cyclophosphamide (750 mg/m2;
day 1), vincristine (1.4 mg/m2; day 1), prednisolone
(p.o. 100 mg/body; day 1–5) |
| Burkitt
lymphoma | 1 | CODOX-M | 1 | 21 | 1-5,8,10,15 | Cyclophosphamide
(800 mg/m2; day 1; 200 mg/m2; day 2–5),
doxorubicin (40 mg/m2; day 1), vincristine (1.5
mg/m2; day 1, 8, 15), methotrexate (536 mg/m2
+ 2,461 mg/m2; day10) |
| Extranodal natural
killer/T-cell lymphoma, nasal type | 1 | Devic | 1 | 21 | 1,2,3 | Carboplatin (300
mg/m2; day 1), ifosfamide (150 mg/m2; day
1–3), etoposide (100 mg/m2; day 1–3), dexamethasone (33
mg/body; day 1–3; p.o. 8 mg/body; day 4) |
| High-grade B-cell
lymphoma, NOS | 1 | EPOCHR | 1 | 21 | 1-6 | Etoposide (50
mg/m2; day 2–5), vincristine (0.4 mg/m2; day
2–5), cyclophosphamide (750 mg/m2; day 6), doxorubicin
(10 mg/m2; day 2–5), prednisolone (p.o. 60
mg/m2; day 1–5), rituximab (375 mg/m2; day
1) |
| Intravascular large
B-cell lymphoma | 1 | RCHOP | 1 | 21 | 1,2 | Rituximab (375
mg/m2; day 1), cyclophosphamide (750 mg/m2;
day 2), doxorubicin (50 mg/m2; day 2), vincristine (1.4
mg/m2; day 2), prednisolone (p.o. 100 mg/body; day
2–6) |
| Lymphoplasmacytic
lymphoma | 1 | RBend | 1 | 28 | 1,2,3 | Rituximab (375
mg/m2; day 1), bendamustine (90 mg/m2; day
2–3) |
| Primary cutaneous
follicle center lymphoma | 1 | RCHOP | 1 | 21 | 1,2 | Rituximab (375
mg/m2; day 1), cyclophosphamide (750 mg/m2;
day 2), doxorubicin (50 mg/m2; day 2), vincristine (1.4
mg/m2; day 2), prednisolone (p.o. 100 mg/body; day
2–6) |
|
T-cell/histiocyte-rich large B-cell
lymphoma | 1 | RCHOP | 1 | 21 | 1,2 | Rituximab (375
mg/m2; day 1), cyclophosphamide (750 mg/m2;
day 2), doxorubicin (50 mg/m2; day 2), vincristine (1.4
mg/m2; day 2), prednisolone (p.o. 100 mg/body; day
2–6) |
 | Table III.Regimen and utility value. |
Table III.
Regimen and utility value.
| Regimen | Patients, n | Relative dose
intensity, % | Cost of drugs,
yen | Before utility
value (A) | After utility value
(B) | Difference in
utility value (B-A) | P-value (B vs.
A) |
|---|
| All regimens | 60 | 90.9±10.9 |
267,577±300,974 | 0.853±0.149 | 0.868±0.123 | 0.016±0.142 | 0.397 |
| CHOP ± R | 38 | 88.4±11.3 | 90,568±30,539 | 0.841±0.135 | 0.876±0.117 | 0.035±0.103 | 0.043a |
| GBend | 12 | 99.0±0.9 | 597,694±21,800 | 0.889±0.103 | 0.895±0.092 | 0.006±0.107 | 0.843 |
| A-CHP | 2 | 99.3±0.3 |
1,218,066±33,599 | 0.868±0.187 | 1.000 | 0.132±0.187 | 0.500 |
| RHyperCVAD | 2 | 97.3±3.0 | 104,672±14,200 | 1.000 | 0.839±0.078 | −0.161±0.078 | 0.211 |
| CODOX-M | 1 | 88.8 | 12,239 | 1.000 | 0.461 | −0.539 | - |
| RBend | 1 | 100 | 192,814 | 0.823 | 0.867 | 0.044 | - |
| EPOCHR | 1 | 99.4 | 108,198 | 0.895 | 0.782 | −0.113 | - |
| PolaRCHP | 1 | 84.8 | 1,287,351 | 0.245 | 0.659 | 0.414 | - |
| Bend | 1 | 71.2 | 119,828 | 1.000 | 0.889 | −0.111 | - |
| Devic | 1 | 68.9 | 9,260 | 0.823 | 0.710 | −0.113 | - |
Quality of life
The utility values before chemotherapy were
0.853±0.149 (mean ± SD) for all regimens and 0.841±0.135 for CHOP ±
R therapy. After chemotherapy, the utility values were 0.868±0.123
for all regimens and 0.876±0.117 for CHOP ± R therapy. The
differences in utility values before and after chemotherapy were
0.016±0.142 (P=0.397) for all regimens and 0.035±0.103 (P=0.043)
for CHOP ± R therapy (Table III).
For each dimension of the EQ-5D, the proportion of patients
answering ‘having issues’ before and after chemotherapy is
presented below (the McNemar test was used). For all regimens, the
proportions of ‘Mobility’ were 21.7 and 30% (P=0.302), ‘Self-care’
were 6.7 and 6.7% (P=1.000), ‘Usual activities’ were 20 and 35%
(P=0.049), ‘Pain/Discomfort’ were 43.3 and 31.7% (P=0.167), and
‘Anxiety/Depression’ were 46.7 and 28.3% (P=0.003). For CHOP ± R
therapy, the proportions for ‘Mobility’ were 26.3 and 31.6%
(P=0.754), ‘Self-care’ were 5.3 and 7.9% (P=1.000), ‘Usual
activities’ were 26.3 and 28.9% (P=1.000), ‘Pain/Discomfort’ were
39.5 and 28.9% (P=0.344), and ‘Anxiety/Depression’ were 52.6 and
31.6% (P=0.008) (Table IV).
 | Table IV.Major problems of each dimension in
EuroQol-5 dimensions. |
Table IV.
Major problems of each dimension in
EuroQol-5 dimensions.
| Regimen | Dimension | Patients with
problems before (%) | Patients with
problems after (%) | P-value |
|---|
| All regimen
(n=60) | Mobility | 21.7 | 30.0 | 0.302 |
|
| Personal care | 6.7 | 6.7 | >0.999 |
|
| Usual
activities | 20.0 | 35.0 | 0.049a |
|
|
Pain/discomfort | 43.3 | 31.7 | 0.167 |
|
|
Anxiety/depression | 46.7 | 28.3 | 0.003a |
| CHOP ± R
(n=38) | Mobility | 26.3 | 31.6 | 0.754 |
|
| Personal care | 5.3 | 7.9 | >0.999 |
|
| Usual
activities | 26.3 | 28.9 | >0.999 |
|
|
Pain/discomfort | 39.5 | 28.9 | 0.344 |
|
|
Anxiety/depression | 52.6 | 31.6 | 0.008a |
Cost-effectiveness
Under the conditions of assumption, the ICER was
34,323,051 yen/QALY for all regimens and 5,152,457 yen/QALY for
CHOP ± R therapy (Table V).
 | Table V.Incremental cost-effectiveness ratio
and probabilistic sensitivity. |
Table V.
Incremental cost-effectiveness ratio
and probabilistic sensitivity.
|
|
| Change of utility
value for probabilistic sensitivity analysis (yen/QALY) |
|---|
|
|
|
|
|---|
| Regimen | ICER
(yen/QALY) | 0.0025 Up | 0.0025 Down | 0.005 Up | 0.005 Down |
|---|
| All regimens | 34,323,051 | 40,877,422 | 29,580,115 | 50,525,908 | 25,988,842 |
| CHOP ± R | 5,152,458 | 5,546,916 | 4,810,377 | 6,006,779 | 4,510,891 |
Sensitivity analysis
Varying the utility values caused the ICER to
fluctuate from 25,988,842 to 50,525,908 yen/QALY for all regimens
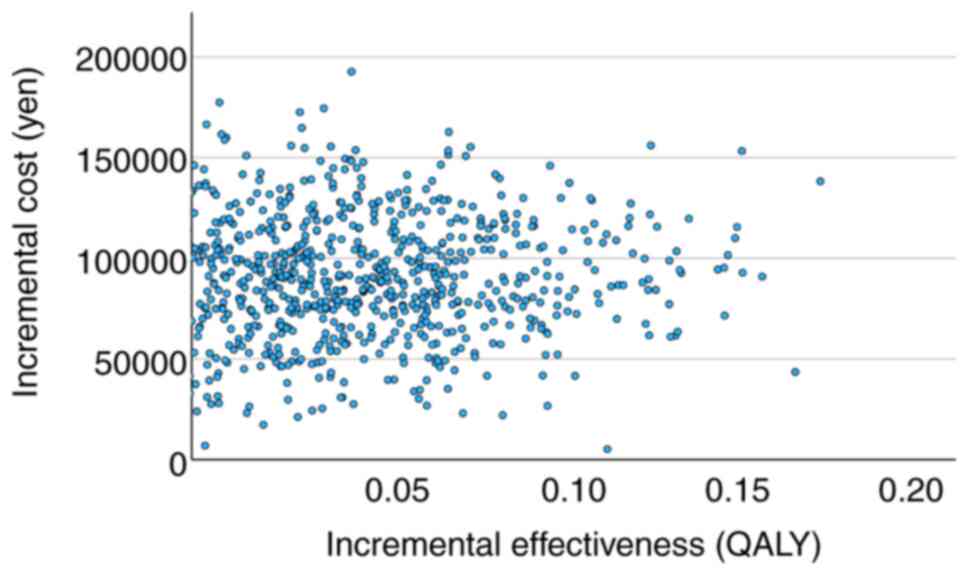
and 4,510,890 to 6,006,778 yen/QALY for CHOP ± R therapy (Table IV). Fig. 2 shows the relationship between
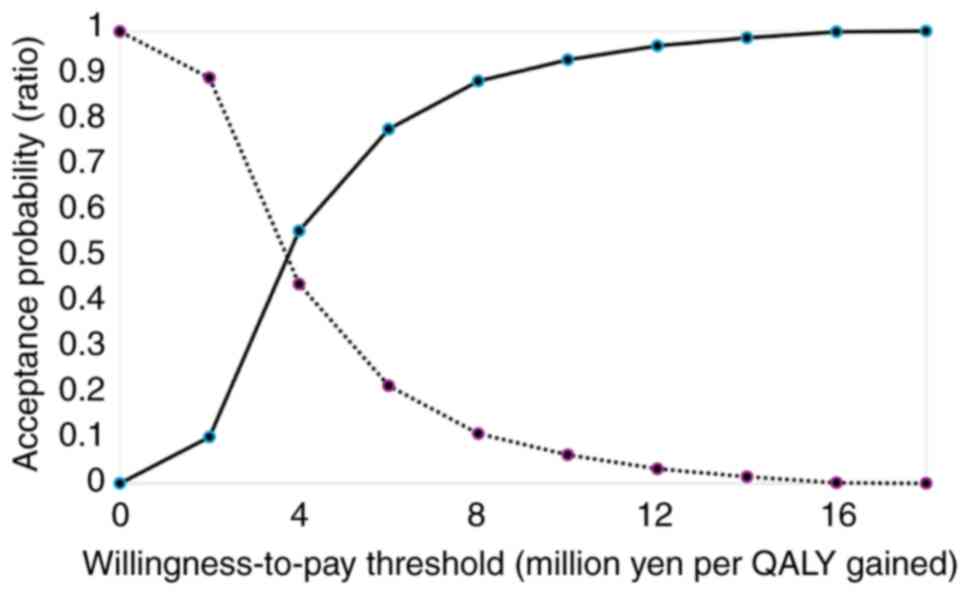
incremental utility and incremental cost and Fig. 3 shows the relationship between
willingness to pay and cost-effectiveness based on data from the
study on CHOP ± R therapy.
Discussion
We conducted a prospective patient-reported QOL
survey to clarify the effect of initial chemotherapy on QOL and its
cost-effectiveness. The male-to-female ratio and mean age of
patients for all regimens did not significantly differ from the
epidemiological survey results in the JSH Practical Guidelines for
Hematological Malignancies 2018 (24), and the tumor subtypes and regimens
used for treatment generally tended to be consistent with the
treatment strategies outlined in the guidelines. After initial
chemotherapy for patients with malignant lymphoma, the utility
values tended to increase compared with those before
administration. In particular, CHOP ± R therapy showed a
significant increase in utility values. For regimens other than
CHOP ± R therapy, the baseline utility values were high, permitting
limited room for further increase because of chemotherapy. These
regimens include GBend therapy, which is not expected to
significantly increase the utility values of chemotherapy.
Additionally, there are more potent regimens, including those
administered to prospective transplant recipients (25–27)
and those used for treating extranodal natural killer/T-cell
lymphoma, nasal type (ENKL), and Burkitt lymphoma. These potent
regimens (particularly those used for ENKL and Burkitt lymphoma)
often involve administering anticancer drugs over multiple days,
dispersed (split) within a single cycle. In contrast, the CHOP ± R
regimen typically specifies early administration within a single
cycle. Anticancer drugs administered over multiple days and
dispersed (split) are associated with a higher incidence of certain
adverse effects, such as gastrointestinal symptoms and fatigue
(28). As chemotherapy was
administered at a high RDI, a higher proportion of patients
responded that they had problems with their ‘Usual activities’ in
the questionnaire survey after chemotherapy, which is thought to
have contributed to the decrease in utility values.
In the five dimensions of the EQ-5D 5 questionnaire
used for the QOL survey, the ‘Mobility’ and ‘Usual activities’
domain showed a worsening trend, whereas the ‘Personal care’
domains showed negligible changes. A trend toward decreased
prevalence of ‘Pain/Discomfort’ was observed after chemotherapy
compared with that before treatment, whereas the proportion of
patients reporting ‘Anxiety/Depression’ significantly decreased.
The effectiveness of chemotherapy for malignant lymphoma, which
shows high response rates and rapid treatment effects (13), is thought to alleviate
patient-perceived symptoms, such as pain from swelling or tumors at
the lesion site, fever from B-symptoms due to disease progression,
weight loss, and night sweats. Patients may experience relief from
disease-related symptoms and decreased anxiety (29,30),
potentially arising from the awareness of symptoms and a clear
understanding of the disease. Moreover, improvement in
‘Anxiety/Depression’ may increase the motivation and expectations
of a patient to resume daily life like that before the onset of the
disease. However, all patients with malignant lymphoma who
initiated intravenously administered anticancer therapy at our
facility were treated as inpatients. This approach was necessary
for comprehensive disease management and effectively addressing
adverse effects of chemotherapy, such as febrile neutropenia.
Chemotherapy administered within an inpatient setting is preferred
in Japan (31), leading to
restrictions in ‘Usual activities’, such as work, study, household
chores, family, and leisure activities that patients engage in when
at home. Because the mean hospitalization period exceeding 16 days,
a gap between expectations and reality, significantly increased the
number of patients reporting problems with their ‘Usual
activities’. It is suggested that if healthcare providers offer
assistance and support in the area of ‘Usual activities’ to
patients undergoing initial chemotherapy, preventing a decline in
QOL and maintaining it while administering chemotherapy treatment
is possible.
Similar to the above findings, the proportion of
patients reporting ‘Pain/Discomfort’ and ‘Anxiety/Depression’
decreased among patients receiving CHOP ± R therapy. Although the
proportion of patients perceiving issues with ‘Usual activities’
significantly increased for all regimens, it only slightly
increased for CHOP ± R therapy. This difference may be attributed
to the inclusion of potent regimens with multiple days of dispersed
(split) administration of anticancer drugs for all regimens,
contrasting with CHOP ± R therapy, which involves early
administration on a single day.
The ICER for the initial chemotherapy in patients
with malignant lymphoma was 34.3 million yen/QALY for all regimens
and 5.15 million yen/QALY for CHOP ± R therapy. CHOP ± R remains
widely used in the treatment of malignant lymphoma because it is
the standard treatment and has been approved for use for
approximately 60 years (6). The
drugs comprising CHOP ± R therapy were introduced in Japan several
decades ago, with cyclophosphamide in 1962, doxorubicin in 1975,
vincristine in 1968, prednisolone in 1956, and the most expensive,
rituximab, in 2001. In Japan, the MHLW establishes official drug
prices that decrease (discount) with time following a drug's
launch. However, the prices of drugs have remained consistent for
many years, reaching the lowest price to date except for rituximab.
Furthermore, the use of biosimilars for rituximab, as recommended
by the MHLW, has helped maintain the cost of CHOP ± R therapy low.
The drug costs for polatuzumab vedotin with rituximab,
cyclophosphamide, doxorubicin, and prednisolone (Pola-R-CHP)
therapy for one person and brentuximab vedotin with
cyclophosphamide, doxorubicin, and prednisolone (A-CHP) therapy for
two people exceed one million yen per person. When these two
regimens (three patients) were excluded, the mean drug cost for all
regimens was approximately 190,000 yen, with an ICER of
approximately 25 million yen/QALY. When the 12 patients with the
next highest-cost GBend therapy were excluded, the mean drug cost
for all regimens was approximately 160,000 yen and the ICER was 18
million yen/QALY. When the 15 patients with the three highest-cost
regimens were excluded, the average drug cost for all regimens was
approximately 100,000 yen and the ICER was 10 million yen/QALY.
Thus, the drug cost must be reduced to approximately 100,000 yen to
maintain the ICER within the threshold based on the increase in
utility values. The high cost of recently introduced drugs (such as
molecular-targeted drugs) for treating malignant lymphoma is
considered a factor contributing to the increased ICER threshold.
However, the threshold for the ICER varies from country to country,
and its acceptability depends on the healthcare system and its
policies. The cost-effectiveness calculations in this study were
based on drug costs alone, without including the costs associated
with hospitalization. Therefore, it is possible to divert the
cost-effectiveness results even for outpatient chemotherapy because
the results of this study apply to patients receiving R-CHOP
therapy, which is administered worldwide, if the conditions with
equivalent drug costs and equivalent patient backgrounds are met.
In some countries, such as the United Kingdom, it is common to
incorporate an economic evaluation into insurance coverage and drug
pricing decisions, with ICER quantifying the costs required to
improve one QALY unit, which is used to determine whether a drug
can be covered by the insurance and its price. In Japan, the
insurance coverage and drug prices are not determined on the basis
of the ICER. Because an economic evaluation is not mandatory in
Japan for insurance coverage and determination of drug price, there
is a lack of evidence using QALYs, and cost-effectiveness has not
been examined for the treatment of patients with malignant
lymphoma. QALYs can be analyzed in terms of health-related QOL and
can be compared across treatments, including those of different
diseases. It can be used to make policy decisions on the selection
of cost-effective treatment, such as selecting a lower cost
treatment when there are multiple treatments with the same
effectiveness, or selecting a treatment with higher effectiveness
when multiple treatments are available at the same cost. In
addition, incremental cost-effectiveness costs by QALYs can be
compared to thresholds set by each country and can be used to
inform healthcare policy decisions such as insurance reimbursement.
Health economic evaluation based on QALY provides information that
will lead to the selection of more effective and cost-effective
treatments, thereby improving patients' QOL and optimizing medical
finances. We believe that the results obtained from the
patient-reported outcomes in this study may provide useful
information for comparison and consideration of existing and future
new treatment options.
In the sensitivity analysis, where the utility
values varied by ±0.0025 and ±0.005 in both upward and downward
directions, the ICERs for all regimens considerably exceeded the
threshold. This finding is consistent with the survey results.
However, the ICER remained within the Japanese threshold of 7.5
million yen per the survey results for CHOP ± R therapy,
demonstrating its robustness.
Following initial chemotherapy, improved QOL was
observed; however, this improvement is not expected to continue
throughout repeated chemotherapy. In other words, initial
chemotherapy may have contributed to an improvement in discomfort
symptoms caused by the illness (32), with the recovery from
chemotherapy-related adverse effects likely influencing the
observed trend in QOL improvement.
This study has certain limitations. First, it was a
single-center study; therefore, institutional policies may have
influenced the treatment choices and treatment environment.
Furthermore, the study duration had to be extended to increase the
number of cases owing to the low incidence of malignant lymphoma
compared with that of solid tumors. This constraint arises from the
evolving treatment approaches over time, necessitating a
constrained study period. Therefore, the small number of patients
and short follow-up period may have influenced our findings.
This study sheds light on the dramatic changes in
QOL experienced by patients receiving initial chemotherapy, which
was not evident in previously reported long-term QOL studies.
Hence, the findings provide valuable insights into potentially
beneficial changes that can be implemented by healthcare providers
throughout the treatment course of a patient. Furthermore, CHOP ± R
was found to have superior cost-effectiveness, considering the new
findings on QOL changes from real-world data. Collectively, this
study provides valuable insights to guide patient treatment options
and influence national healthcare policies.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
KT, MY, TT, SI, JK, YN, HT and SK designed the
study. KT and MY collected EuroQol 5 dimensions questionnaires. KT,
TT and YT collected clinical data. KT and YT confirm the
authenticity of all the raw data. KT, MY, TT, YI, YT and TY
performed statistical analysis. KT, MY, YI and TY generated figures
and tables. KT, MY, YI and TY validated and visualized the study.
KT, MY and SK wrote the first draft of the manuscript. TT, YT, SI,
YI, JK, YN, TY and HT critically revised the manuscript and
provided valuable feedback. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
The present study was approved by the Gifu Municipal
Hospital Clinical Research Review Board (approval number 663; Gifu,
Japan) and Gifu Pharmaceutical University Ethics Committee
(approval number 2–23; Gifu, Japan). Participation was restricted
to patients who, after receiving a written description of the
study, provided written informed consent for their enrollment.
Patient consent for publication
Written informed consent for publication of the
paper was obtained from patients at the time of study
participation.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
QOL
|
quality of life
|
|
EQ-5D
|
EuroQol 5 dimensions
|
|
CHOP
|
cyclophosphamide, doxorubicin,
vincristine, prednisolone
|
|
RDI
|
relative dose intensity
|
|
MHLW
|
Ministry of Health, Labour and
Welfare
|
|
ICER
|
incremental cost-effectiveness
ratio
|
|
QALY
|
quality-adjusted life year
|
|
GBend
|
obinutuzumab, bendamustine
|
|
ENKL
|
extranodal natural killer/T-cell
lymphoma, nasal type
|
References
|
1
|
Zhou Z, Sehn LH, Rademaker AW, Gordon LI,
Lacasce AS, Crosby-Thompson A, Vanderplas A, Zelenetz AD, Abel GA,
Rodriguez MA, et al: An enhanced international prognostic index
(NCCN-IPI) for patients with diffuse large B-cell lymphoma treated
in the rituximab era. Blood. 123:837–842. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Solal-Celigny P: Follicular lymphoma
international prognostic index. Curr Treat Options Oncol.
7:270–275. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ardeshna KM, Smith P, Norton A, Hancock
BW, Hoskin PJ, MacLennan KA, Marcus RE, Jelliffe A, Vaughan G,
Hudson, et al: Long-term effect of a watch and wait policy versus
immediate systemic treatment for asymptomatic advanced-stage
non-Hodgkin lymphoma: A randomised controlled trial. Lancet.
362:516–522. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Martin P, Chadburn A, Christos P, Weil K,
Furman RR, Ruan J, Elstrom R, Niesvizky R, Ely S, Diliberto M, et
al: Outcome of deferred initial therapy in mantle-cell lymphoma. J
Clin Oncol. 27:1209–1213. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
DeVita VT Jr, Canellos GP, Chabner B,
Schein P, Hubbard SP and Young RC: Advanced diffuse histiocytic
lymphoma, a potentially curable disease. Lancet. 1:248–250. 1975.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Coltman CA, Dahlberg S and Jones SE: CHOP
is curative in thirty percent of patients with large cell lymphoma:
A twelve-year Southwest oncology group follow-up. Advances in
cancer chemotherapy: Update on treatment for diffuse large cell
lymphoma. Skarin AT: Wiley; New York, NY: pp. 71–77. 1986
|
|
7
|
Johnsen AT, Tholstrup D, Petersen MA,
Pedersen L and Groenvold M: Health related quality of life in a
nationally representative sample of haematological patients. Eur J
Haematol. 83:139–148. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Montgomery C, Pocock M, Titley K and Lloyd
K: Individual quality of life in patients with leukaemia and
lymphoma. Psychooncology. 11:239–243. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Parker PA, Baile WF, de Moor CD and Cohen
L: Psychosocial and demographic predictors of quality of life in a
large sample of cancer patients. Psychooncology. 12:183–193. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Friedberg JW, Thompson CA, Trněný M,
Morschhauser F, Salles G, Reagan PM, Hertzberg M, Smolewski P,
Zhang H, Thieblemont C, et al: Health-related quality of life
(HRQoL) in patients with diffuse large B-cell lymphoma (DLBCL)
treated with polatuzumab vedotin, rituximab, cyclophosphamide,
doxorubicin and prednisone (Pola-R-CHP) versus rituximab,
cyclophosphamide, doxorubicin, vincristine and prednisone (R-CHOP)
in the Phase III POLARIX Study. Blood. 140 (Suppl 1):S6623–S6626.
2022. View Article : Google Scholar
|
|
11
|
Ardeshna KM, Qian W, Smith P, Braganca N,
Lowry L, Patrick P, Warden J, Stevens L, Pocock CFE, Miall F, et
al: Rituximab versus a watch-and-wait approach in patients with
advanced-stage, asymptomatic, non-bulky follicular lymphoma: An
open-label randomised phase 3 trial. Lancet Oncol. 15:424–435.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Brandt J, Dietrich S, Meissner J, Neben K,
Ho AD and Witzens-Harig M: Quality of life of long-term survivors
with Hodgkin lymphoma after high-dose chemotherapy, autologous stem
cell transplantation, and conventional chemotherapy. Leuk Lymphoma.
51:2012–2020. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Coiffier B, Lepage E, Briere J, Herbrecht
R, Tilly H, Bouabdallah R, Morel P, Van Den Neste E, Salles G,
Gaulard P, et al: CHOP chemotherapy plus rituximab compared with
CHOP alone in elderly patients with diffuse large-B-cell lymphoma.
N Engl J Med. 346:235–242. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
El Haidari RE, Anota A, Dabakuyo-Yonli TS,
Guillemin F, Conroy T, Velten M, Jolly D, Causeret S, Cuisenier J,
Graesslin O, et al: Utility values and its time to deterioration in
breast cancer patients after diagnosis and during treatments. Qual
Life Res. 31:3077–3085. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wasse SK, Mounier M, Assogba E, Rossi C,
Adnet J, Gauthier S, Girard S, Atsou KM, Dabakuyo-Yonli TS and
Maynadie M: Factors affecting health-related quality of life among
survivors of non-Hodgkin lymphoma: A population-based study.
Cancers (Basel). 15:38852023. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Sasaki T, Izawa M and Okada Y: Current
trends in health insurance systems: OECD countries vs Japan. Neurol
Med Chir (Tokyo). 55:267–275. 2015. View Article : Google Scholar
|
|
17
|
Paunescu AC, Copie CB, Malak S, Gouill SL,
Ribrag V, Bouabdallah K, Sibon D, Rumpold G, Preau M, Mounier N, et
al: Quality of life of survivors 1 year after the diagnosis of
diffuse large B-cell lymphoma: A LYSA study. Ann Hematol.
101:317–332. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Oerlemans S, Issa DE, van den Broek EC,
Nijziel MR, Coebergh JWW, Huijgens PC, Mols F and van de
Poll-Franse LV: Health-related quality of life and persistent
symptoms in relation to (R-)CHOP14, (R-)CHOP21, and other therapies
among patients with diffuse large B-cell lymphoma: Results of the
population-based PHAROS-registry. Ann Hematol. 93:1705–1715. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Mounier N, Anthony S, Busson R,
Thieblemont C, Ribrag V, Tilly H, Haioun C, Casasnovas RO,
Morschhauser F, Feugier P, et al: Long-term fatigue in survivors of
non-Hodgkin lymphoma: The lymphoma study association SIMONAL
cross-sectional study. Cancer. 125:2291–2299. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Aogi K, Takeuchi H, Saeki T, Aiba K,
Tamura K, Iino K, Imamura CK, Okita K, Kagami Y, Tanaka R, et al:
Optimizing antiemetic treatment for chemotherapy-induced nausea and
vomiting in Japan: Update summary of the 2015 Japan society of
clinical oncology clinical practice guidelines for antiemesis. Int
J Clin Oncol. 26:1–17. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Herdman M, Gudex C, Lloyd A, Janssen M,
Kind P, Parkin D, Bonsel G and Badia X: Development and preliminary
testing of the new five-level version of EQ-5D (EQ-5D-5L). Qual
Life Res. 20:1727–1736. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Hasegawa M, Komoto S, Shiroiwa T and
Fukuda T: Formal implementation of cost-effectiveness evaluations
in Japan: A unique health technology assessment system. Value
Health. 23:43–51. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Doorduijn J, Buijt I, Holt B, Steijaert M,
Uyl-de Groot C and Sonneveld P: Self-reported quality of life in
elderly patients with aggressive non-Hodgkin's lymphoma treated
with CHOP chemotherapy. Eur J Haematol. 75:116–123. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Omachi K: JSH practical guidelines for
hematological malignancies, 2018: II. Lymphoma-overview. Int J
Hematol. 110:3–10. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wilson WH, Grossbard ML, Pittaluga S, Cole
D, Pearson D, Drbohlav N, Steinberg SM, Little RF, Janik J,
Gutierrez M, et al: Dose-adjusted EPOCH chemotherapy for untreated
large B-cell lymphomas: A pharmacodynamic approach with high
efficacy. Blood. 99:2685–2693. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kantarjian HM, O'Brien S, Smith TL, Cortes
J, Giles FJ, Beran M, Pierce S, Huh Y, Andreeff M, Koller C, et al:
Results of treatment with hyper-CVAD, a dose-intensive regimen, in
adult acute lymphocytic leukemia. J Clin Oncol. 18:547–561. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Romaguera JE, Fayad L, Rodriguez MA,
Broglio KR, Hagemeister FB, Pro B, McLaughlin P, Younes A,
Samaniego F, Goy A, et al: High rate of durable remissions after
treatment of newly diagnosed aggressive mantle-cell lymphoma with
rituximab plus hyper-CVAD alternating with rituximab plus high-dose
methotrexate and cytarabine. J Clin Oncol. 23:7013–7023. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Markman M: Toxicities of the platinum
antineoplastic agents. Expert Opin Drug Saf. 2:597–607. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Devlen J, Maguire P, Phillips P, Crowther
D and Chambers H: Psychological problems associated with diagnosis
and treatment of lymphomas. I: Retrospective study. Br Med J (Clin
Res Ed). 295:953–954. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Oerlemans S, Mols F, Nijziel MR, Zijlstra
WP, Coebergh JWW and van de Poll-Franse LV: The course of anxiety
and depression for patients with Hodgkin's lymphoma or diffuse
large B cell lymphoma: A longitudinal study of the PROFILES
registry. J Cancer Surviv. 8:555–564. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Ohno S, Shoji A, Hatake K, Oya N and
Igarashi A: Cost-effectiveness analysis of treatment regimens with
obinutuzumab plus chemotherapy in Japan for untreated follicular
lymphoma patients. J Med Econ. 23:1130–1141. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Tang AL and Thomas SJ: Relationships
between depressive symptoms, other psychological symptoms, and
quality of life. Psychiatry Res. 289:1130492020. View Article : Google Scholar : PubMed/NCBI
|