Introduction
Neoadjuvant chemoradiation therapy followed by
surgery is the first-line therapeutic option for locally advanced
rectal cancer (LARC) (1). Numerous
studies have been performed to evaluate the risk factors for
predicting outcomes in patients with rectal cancer following
radical surgery (2,3). After neoadjuvant chemoradiation
therapy, pathological lymph node status has been found to be one of
the most effective risk factors for recurrence (4). In addition, achieving a pathological
complete response (pCR) has been associated with superior outcomes
following radical resection (5).
The prognosis of LARC is associated with the physiological makeup
of the patient pre-treatment, especially whether systemic
inflammatory diseases were present and the immuno-nutritional
status. The systemic inflammatory response serves a role in cancer
development, progression, treatment response, and prognosis
(6,7). This response can be measured using
various indicators of hematological parameter changes, including
the systemic immune-inflammation index (SII; neutrophil count X
plated count/lymphocyte count), neutrophil-to-lymphocyte ratio
(NLR), platelet-to-lymphocyte ratio (PLR) and the
lymphocyte-to-monocyte ratio (LMR) (8). Systemic inflammatory response markers,
such as increased NLR and PLR, has been reported to ably predict an
unfavorable prognosis in rectal cancer following neoadjuvant
chemoradiotherapy (9,10). By contrast, increased LMR has been
associated with superior survival outcomes in patients with LARC
treated with neoadjuvant therapy followed by surgery (8). SII is a relatively novel marker
compared with other inflammatory markers. It is calculated based on
the neutrophil, platelet and lymphocyte counts (11). A previous meta-analysis reported its
prognostic ability to predict poor overall survival and
progression-free survival in colorectal cancer (11).
The prognostic nutritional index (PNI; PNI=10 ×
albumin levels + 0.005 × total lymphocyte count) is a marker that
can be used to reflect the nutritional and immunological status and
is calculated based on a number inflammatory parameters and albumin
levels (12). PNI has been reported
to be accurate for predicting prognosis, with low PNI associated
with worse overall survival and disease-free survival in patients
with colorectal cancer (12,13).
However, whilst a number of studies have previously explored the
prognostic value of individual biomarkers such as NLR, PLR and SII
in patients with LARC who have received neoadjuvant
chemoradiotherapy, to the best of our knowledge studies that have
collectively investigated and compared the prognostic value of
these biomarkers remain scarce (14,15).
Therefore, the present study aimed to
comprehensively analyze and compare the prognostic value of
nutritional and immune-related biomarkers in patients with LARC
treated with neoadjuvant therapy followed by surgery to devise
strategies to optimize their individualized management
protocols.
Patients and methods
Patients
A retrospective review of the medical records of
patients with LARC who underwent radical resection at our Ajou
University Hospital (Suwon, Korea) from January 2010 to June 2019.
The present study included all patients diagnosed with LARC using
endoscopy and radiological evidence (clinical stage II and III) who
underwent surgery following neoadjuvant chemoradiotherapy.
Histopathological staining is typically not necessary when the
combination of endoscopic and radiological findings provides a
clear and definitive diagnosis. This approach allows for prompt and
appropriate treatment planning for patients. All patients were aged
>18 years and had no history of other primary cancers. Patients
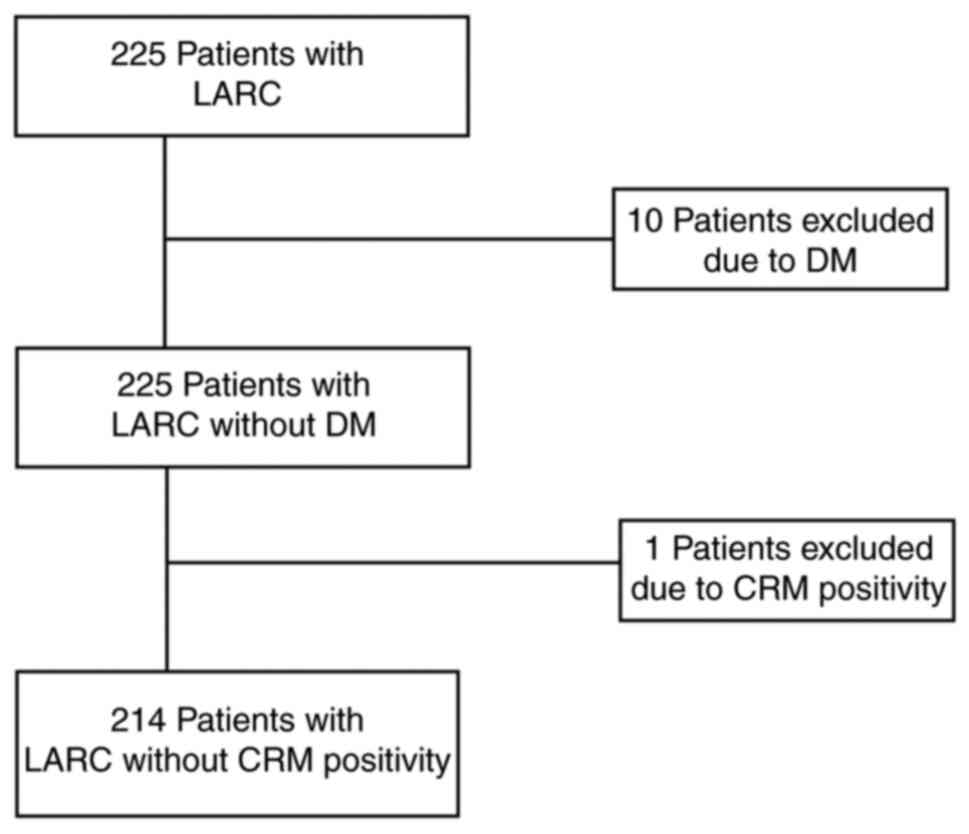
diagnosed with distant metastases (to the liver, lung or
peritoneum) during the initial operation (n=10) or those with whom
no radical resection was performed following neoadjuvant
chemoradiotherapy (n=1) were excluded (Fig. 1). The institutional review board of
Ajou University Hospital (Suwon, Korea) approved the present study
(approval no. AJOUIRB-MDB-2022-109). Informed consent was not
required for the present study because the present study was a
medical record review.
Tables I and
II shows the demographic and
clinical data collected. The present study included 214 patients
(147 males and 67 females) with LARC treated with neoadjuvant
therapy followed by surgery between 2010 and 2019. The median age
of the patients was 59 years (range, 26–87 years). Staging was
performed according to the tumor-node-metastasis classification of
the American Joint Committee on Cancer (7th edition) (16). The distance from the anal verge to
the lower margin of the tumor was measured preoperatively using
rigid proctoscopy. Lower rectal cancer is characterized by lesions
within 5 cm of the anal verge, whilst middle rectal cancer is
characterized by tumors located 5–10 cm from the anal verge.
 | Table I.Clinicopathological characteristics
(n=214). |
Table I.
Clinicopathological characteristics
(n=214).
| Variables | N (%) |
|---|
| Sex |
|
| Male | 147 (68.7) |
|
Female | 67 (31.3) |
| Age, years |
|
|
<60 | 108 (50.5) |
| ≥60 | 106 (49.5) |
| American Society of
Anesthesiology score |
|
| 1 | 112 (52.3) |
| 2 | 90 (42.1) |
| 3 | 12 (5.6) |
| Body-mass index,
kg/m2 |
|
| ≤25 | 155 (72.4) |
|
>25 | 59 (27.6) |
| Radiotherapy
interval, weeks |
|
| ≤8 | 124 (57.9) |
|
>8 | 90 (42.1) |
| Location |
|
|
Mid | 110 (51.4) |
|
Low | 104 (48.6) |
| Clinical T
stage |
|
|
cT1-2 | 18 (8.4) |
|
cT3-4 | 196 (91.6) |
| Clinical N
stage |
|
|
cN0 | 21 (9.8) |
|
cN1-2 | 193 (90.2) |
| Tumor
circumference |
|
|
Non-encircling | 175 (81.8) |
|
Encircling | 39 (18.2) |
 | Table II.Tumor response data. |
Table II.
Tumor response data.
| Variables | Initial | Post-CRT |
|---|
| Carcinoembryonic
antigen, ng/ml |
|
|
| ≤5 | 106 (49.5) | 178 (83.2) |
|
>5 | 108 (50.5) | 36 (16.8) |
| Serum albumin,
g/l | 4.4 (2.0–5.1) | 4.4 (3.2–5.2) |
| Hemoglobin,
g/dl | 13.4
(5.4–17.2) | 12.8
(5.9–16.5) |
| Lymphocyte count,
109/l | 1,993
(1026–4014) | 1,003.4
(280–3115) |
| Neutrophil count,
109/l | 4,494
(1315–8904) | 3,564
(1323–9298) |
| Platelet count,
109/l | 274 (124–649) | 243 (78–506) |
|
Neutrophil-lymphocyte ratio | 2.28
(0.62–5.60) | 3.52
(0.89–22.07) |
| Platelet-lymphocyte
ratio | 140.4
(51.3–392.5) | 240.8
(49.2–958.9) |
| Lymphocyte-monocyte
ratio | 4.1 (1.5–13.2) | 2.2 (0.7–9.3) |
| Systemic
immune-inflammatory index | 598.4
(118.9–3191.2) | 848.3
(119.3–5914.7) |
| Prognostic
nutritional index | 53.5
(31.4–67.0) | 49.3
(37.7–61.1) |
Hemoglobin, platelet count, lymphocyte count (LC),
neutrophil count (NC), NLR, PLR, LMR, SII, PNI, carcinoembryonic
antigen (CEA) and body mass index were all collected to develop a
prognostic model. Baseline blood samples were collected 2 weeks
before chemoradiotherapy, while preoperative samples were collected
2 weeks before surgery.
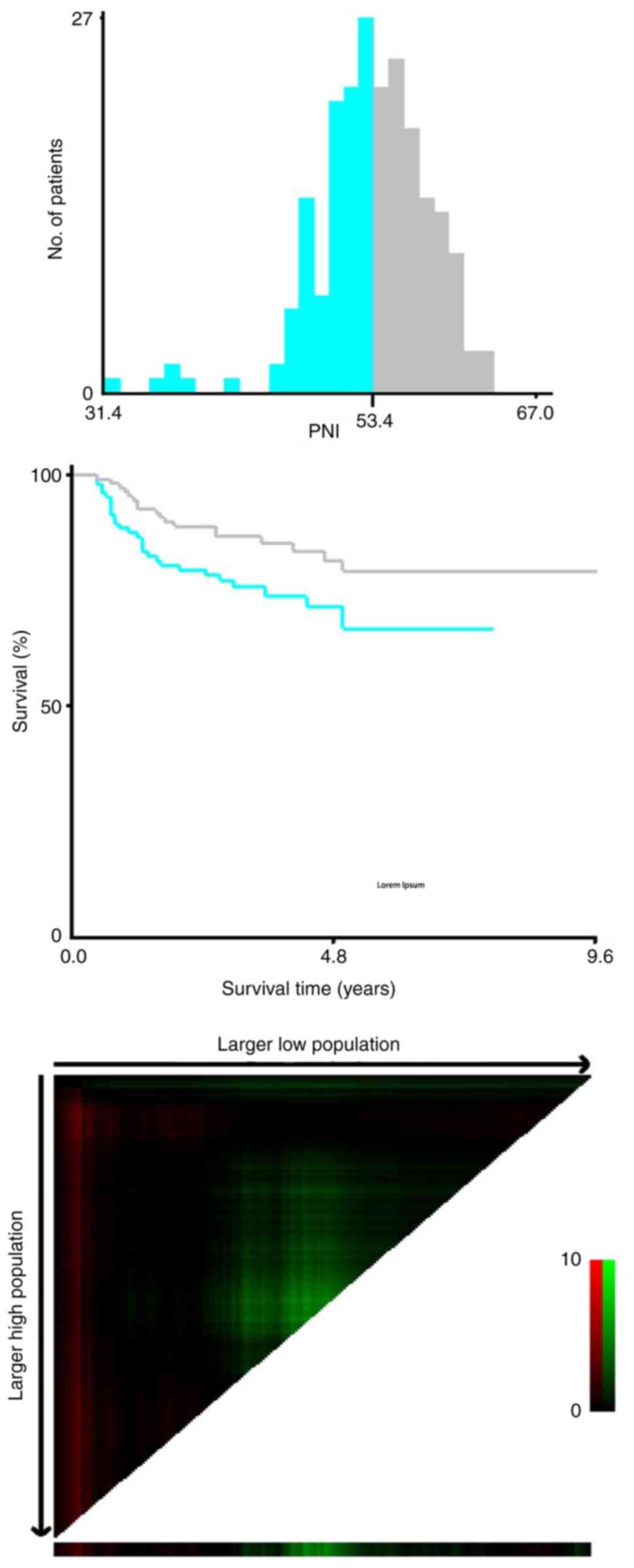
The cut-off values for platelet count, LC, NC, NLR,
PLR, LMR, PNI and SII were calculated and determined using the
X-tile 3.6.1 software (Fig. 2)
(17). A brief overview of how the
program was used to determine these values includes: Data input,
statistical analysis, cut-off value selection and validation. A
common method was applied within the program for all parameters.
This means that the same approach for determining and validating
the cut-off values was consistently used for platelet count, LC,
NC, NLR, PLR, LMR, PNI and SII. Cut-off values for hemoglobin, CEA,
albumin and body mass index were determined based on standard
clinical values. Patients were classified into low and high groups
based on the cut-off values of the parameters.
Preoperative radiotherapy was administered to the
pelvis of each patient 5 days weekly for 5 weeks, with each daily
dose 1.8 Gy. In addition, a booster dose of 5.4 Gy was delivered to
the tumor site. Concomitant chemotherapy was administered during
radiotherapy. The chemotherapy regimen selected was 5-fluorouracil
or capecitabine. For the 5-fluorouracil regimen, patients received
225 mg/m2 intravenously over 24 h, daily. This
administration occurred consecutively for 5 days each week,
spanning a period of 5 weeks. Capecitabine was administered orally
at a dose of 825 mg/m2 twice daily on weekdays
(Monday-Friday), spanning a period of 28–30 days. Surgery took
place 6–8 weeks following chemoradiotherapy completion.
Subsequently, patients were scheduled for outpatient follow-up
visits every 3–6 months during the initial 2 years after surgery,
which was set to every 6 months for the subsequent 3 years and then
decreased to once-annual follow-ups thereafter. Physical
examinations and serum CEA measurements were performed at each
visit. Annually, chest radiography, chest and abdominopelvic CT and
colonoscopy were performed if recurrence was suspected. PET would
also be performed if recurrence was suspected. Cancer recurrence
was identified using a combination of imaging data and CEA
measurements, which were subsequently confirmed by pathological
examination.
Patients were followed up until they succumbed or
reached the designated cut-off date of December 31, 2021 (the last
followed up date), whichever came first. The median follow-up time
was 57.0 months (4.0-143.0 months). Disease-free survival (DFS) was
defined as the time from irradiation initiation to disease
recurrence or the last follow-up date. Overall survival (OS) was
defined as the time from irradiation initiation to mortality.
Statistical analysis
Differences in the clinicopathological features were
compared using χ2 test. The data in Table II were evaluated for normality
using the Kolmogorov-Smirnov test to determine whether they
followed a normal distribution, which informed the decision to
apply parametric or non-parametric statistical test for subsequent
analysis. However, none of the data in the present study were found
to be normally distributed. Therefore, Wilcoxon signed-rank test
was used to analyze variables before and after chemoradiotherapy.
Quantitative data that did not to the normal distribution are
expressed as the median (interquartile range). Survival curves were
evaluated using the Kaplan-Meier method and were compared using the
log-rank test. Independent predictors were determined using
multivariate Cox regression analyses of OS and DFS. Variables with
P<0.05 in univariate analysis were included in the following
multivariate analysis. P<0.05 was considered to indicate a
statistically significant difference. Statistical analyses were
performed using SPSS version 25.0 (IBM Corp.) and GraphPad Prism 9
(Dotmatics).
Results
Clinicopathological
characteristics
For the present study, the X-tile program was used
to compare the survival outcomes between groups categorized as low
and high based on the parameters' cut-off values. The X-tile
program identifies cut-off points that can maximize predefined
criteria including maximizing the difference in survival between
groups, maximizing the statistical significance of the difference
(log-rank test), minimizing the P-value, maximizing the hazard
ratio, balancing group sizes and optimizing clinical relevance. The
criterion chosen for generating the cut-off point for the present
study was maximizing the statistical significance of the
difference. This criterion was selected because it ensures that the
identified cut-off points are statistically robust, providing a
more reliable basis for comparing the survival outcomes between the
low and high groups. The optimal cut-off values for LC, NC,
platelet count, NLR, PLR, LMR, PNI and SII before neoadjuvant
chemoradiotherapy were 1,530.0, 5,072.0, 326.0, 2.2, 126.7, 2.8,
53.4, and 508.8, respectively. The optimal cut-off values for LC,
NC, platelet count, NLR, PLR, LMR, PNI and SII after neoadjuvant
chemoradiotherapy were 776.0, 4,012.8, 278.0, 1.8, 213.9, 1.2, 46.4
and 722.9, respectively.
Univariate and multivariate analyses
for DFS and OS
Upon evaluation of the levels of nutrition and
immune-related biomarkers calculated before and after neoadjuvant
chemoradiotherapy, no significant difference was observed in DFS
and OS between the high and low groups of each parameter tested,
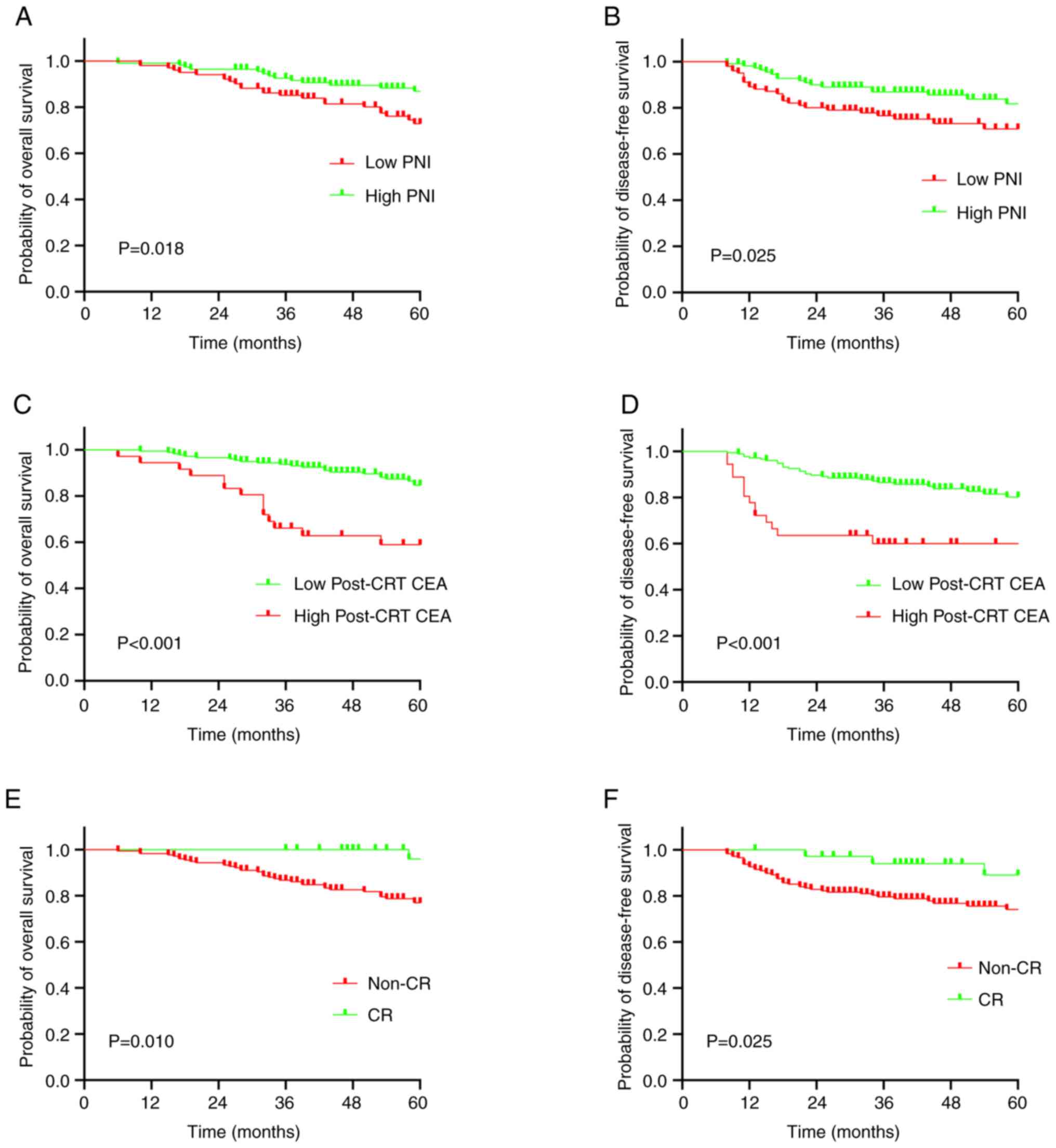
except for between the high and low PNI groups (Tables III and IV). Low PNI was found to be significantly
associated with decreased 5-year DFS and OS rates (72.9 vs. 87.0%;
P=0.018) compared with high PNI (65.6 vs. 79.7%; P=0.025; Fig. 3). In addition, high
post-chemoradiotherapy CEA levels were significantly associated
with decreased 5-year OS (61.1 vs. 87.1%; P<0.001) and DFS (61.1
vs. 82.0%; P<0.001) (Fig. 3).
Non-complete response status was also significantly associated with
decreased 5-year OS (79.8 vs. 97.2%; P=0.010) and DFS (75.8 vs.
91.7%; P=0.025; Fig. 3).
Multivariate analysis for cancer recurrence demonstrated that PNI
[hazard ratio (HR), 1.993; 95% CI, 1.099–3.614; P=0.023] and
post-chemoradiotherapy CEA level (HR, 3.003; 95% CI, 1.596–5.649;
P=0.001) were significant predictors of 5-year cancer recurrence
(Table III). Multivariate
analysis of mortality demonstrated that PNI (HR, 2.030; 95% CI,
1.032–3.992; P=0.040) and post-chemoradiotherapy CEA level (HR,
3.052; 95% CI, 1.560–5.971; P=0.001) were significant predictors of
the 5-year mortality (Table
IV).
 | Table III.Univariate and multivariate analyses
for factors predicting cancer recurrence. |
Table III.
Univariate and multivariate analyses
for factors predicting cancer recurrence.
|
| Univariate | Multivariate |
|---|
|
|
|
|
|---|
| Variables | HR | 95% CI | P-value | HR | 95% CI | P-value |
|---|
| Sex, female vs.
male | 1.134 | 0.612–2.103 | 0.689 |
|
|
|
| Age, >60 vs. ≤60
years | 1.238 | 0.693–2.213 | 0.471 |
|
|
|
| American Society of
Anesthesiology score, 1 vs. 2–3 | 1.358 | 0.755–2.443 | 0.307 |
|
|
|
| Body mass index,
<25 vs. ≥25 kg/m2 | 2.653 | 1.125–6.259 | 0.026 |
|
|
|
| Radiotherapy
interval, ≤8 vs. >8 weeks | 1.048 | 0.571–1.925 | 0.879 |
|
|
|
| Location, low vs.
high | 1.019 | 0.566–1.837 | 0.949 |
|
|
|
| Clinical stage, III
vs. II | 2.696 | 0.653–11.131 | 0.171 |
|
|
|
| Tumor
circumference, non-encircling vs. encircling | 1.003 | 0.467–2.153 | 0.993 |
|
|
|
| Initial CEA, >5
vs. ≤5 ng/ml | 1.785 | 0.986–3.231 | 0.056 |
|
|
|
|
Post-chemoradiotherapy CEA, >5 vs. ≤5
ng/ml | 2.966 | 1.579–5.570 | 0.001 | 3.003 | 1.596–5.649 | 0.001 |
| Tumor response,
non-complete response vs. complete | 3.517 | 1.090–11.348 | 0.035 |
|
|
|
| response |
|
|
|
|
|
|
| Prognostic
nutritional index, <53.4 vs. ≥53.4 | 1.968 | 1.087–3.563 | 0.025 | 1.993 | 1.099–3.614 | 0.023 |
|
Neutrophil-lymphocyte ratio, >2.2 vs.
≤2.2 | 1.603 | 0.881–2.917 | 0.123 |
|
|
|
| Platelet-lymphocyte
ratio, >126.7 vs. ≤126.7 | 1.319 | 0.718–2.421 | 0.372 |
|
|
|
| Lymphocyte-monocyte
ratio, >2.8 vs. ≤2.8 | 1.502 | 0.594–3.803 | 0.390 |
|
|
|
| Systemic
immune-inflammatory index, >508.8 vs. | 1.750 | 0.888–3.449 | 0.106 |
|
|
|
| ≤508.8 | |
|
|
|
|
|
 | Table IV.Univariate and multivariate analyses
for factors predicting mortality. |
Table IV.
Univariate and multivariate analyses
for factors predicting mortality.
|
| Univariate | Multivariate |
|---|
|
|
|
|
|---|
| Variables | HR | 95% CI | P-value | HR | 95% CI | P-value |
|---|
| Sex, female vs.
male | 1.413 | 0.727–2.746 | 0.308 |
|
|
|
| Age, >60 vs. ≤60
years | 1.997 | 1.017–3.923 | 0.045 |
|
|
|
| American Society of
Anesthesiology score, 2–3 vs. 1 | 1.181 | 0.620–2.251 | 0.612 |
|
|
|
| Body mass index,
vs. ≥25 kg/m2 | 1.588 | 0.698–3.616 | 0.270 |
|
|
|
| Radiotherapy
interval, ≤8 vs. >8 weeks | 1.103 | 0.560–2.175 | 0.776 |
|
|
|
| Location, low vs.
high | 1.039 | 0.544–1.985 | 0.907 |
|
|
|
| Clinical stage, III
vs. II | 1.010 | 0.358–2.855 | 0.984 |
|
|
|
| Tumor
circumference, non-encircling vs. complete response | 1.149 | 0.479–2.755 | 0.755 |
|
|
|
| Initial CEA, >5
vs. ≤5 ng/ml | 1.551 | 0.804–2.992 | 0.190 |
|
|
|
|
Post-chemoradiotherapy CEA, >5 vs. ≤5
ng/ml | 3.738 | 1.920–7.277 | <0.001 | 3.052 | 1.560–5.971 | 0.001 |
| Tumor response,
non-complete response vs. complete response | 8.639 | 1.184–63.031 | 0.033 |
|
|
|
| Prognostic
nutritional index, <54.3 vs. ≥53.4 | 2.204 | 1.122–4.329 | 0.022 | 2.030 | 1.032–3.992 | 0.040 |
|
Neutrophil-lymphocyte ratio, >2.2 vs.
≤2.2 | 1.174 | 0.612–2.250 | 0.629 |
|
|
|
| Platelet-lymphocyte
ratio, >126.7 vs. ≤126.7 | 0.867 | 0.452–1.662 | 0.668 |
|
|
|
| Lymphocyte-monocyte
ratio, >2.8 vs. ≤2.8 | 2.228 | 0.684–7.255 | 0.184 |
|
|
|
| Systemic
immune-inflammatory index, >508.8 vs. ≤508.8 | 1.076 | 0.541–2.143 | 0.834 |
|
|
|
Comparison of clinicopathological
characteristics between low PNI and high PNI
No significant associations could be observed
between any of the clinicopathological features and PNI, except for
age and body mass index (Table
V).
 | Table V.Association between each of the
clinicopathological characteristics and PNI. |
Table V.
Association between each of the
clinicopathological characteristics and PNI.
| Variables | Total (n=214) | PNI <53.4
(n=102) | PNI >53.4
(n=112) | P-value |
|---|
| Sex |
|
|
| 0.542 |
|
Male | 147 | 68 | 79 |
|
|
Female | 67 | 34 | 33 |
|
| Age, years |
|
|
| <0.001 |
|
<60 | 108 | 37 | 71 |
|
|
≥60 | 106 | 65 | 41 |
|
| ASA score |
|
|
| 0.080 |
| 1 | 112 | 47 | 65 |
|
|
2-3 | 102 | 55 | 47 |
|
| Body-mass index,
kg/m2 |
|
|
| 0.013 |
|
≤25 | 155 | 82 | 73 |
|
|
>25 | 59 | 20 | 39 |
|
| Radiotherapy
interval, weeks |
|
|
| 0.977 |
| ≤8 | 124 | 59 | 65 |
|
|
>8 | 90 | 43 | 47 |
|
| Location |
|
|
| 0.348 |
|
Mid | 110 | 49 | 61 |
|
|
Low | 104 | 53 | 51 |
|
| Clinical T
stage |
|
|
| 0.484 |
|
cT1-2 | 18 | 10 | 8 |
|
|
cT3-4 | 196 | 92 | 104 |
|
| Clinical N
stage |
|
|
| 0.997 |
|
cN0 | 21 | 10 | 11 |
|
|
cN1-2 | 193 | 92 | 101 |
|
| Tumor
circumference |
|
|
| 0.617 |
|
Non-encircling | 175 | 82 | 93 |
|
|
Encircling | 39 | 20 | 19 |
|
| Initial CEA,
ng/ml |
|
|
| 0.677 |
| ≤5 | 106 | 49 | 57 |
|
|
>5 | 108 | 53 | 55 |
|
|
Post-chemoradiotherapy CEA, ng/ml |
|
|
| 0.501 |
| ≤5 | 178 | 83 | 95 |
|
|
>5 | 36 | 19 | 17 |
|
| Tumor response |
|
|
| 0.430 |
| CR | 36 | 15 | 21 |
|
|
Non-CR | 178 | 87 | 91 |
|
Discussion
The present study investigated the prognostic impact
of nutrition and immune-related biomarkers combined with various
clinicopathological features in patients with LARC treated with
neoadjuvant therapy followed by surgery. Although it was found that
the calculated cut-off values for SII, PLR, LMR and NLR were
similar compared with those in previous studies (11,18),
when the prognostic value of these biomarkers was assessed, none
emerged as an independent predictor in the present cohort. Only the
initial PNI strongly predicted disease recurrence and survival in
patients with LARC treated with neoadjuvant therapy followed by
surgery.
Previous studies have demonstrated that malnutrition
and the immunological status serve a role in cancer development
(19,20). Therefore, several scoring systems
such as PNI, SII and NLR based on the nutritional and immunological
status have been established to predict colorectal cancer
prognosis. Among those, PNI appears to be the most accessible,
since it can be readily calculated from albumin levels and total
LC. In addition, PNI has been documented to be an independent
predictor of colorectal cancer outcomes (21,22).
The present study focused on the prognostic value of
nutrition and immune-related biomarkers in patients with LARC
treated with neoadjuvant therapy followed by surgery. Notably, two
previous studies have reported the prognostic ability of PNI in
patients with LARC (13,23). Okugawa et al (13) attempted to use the patient
nutritional status to predict long-term oncological outcomes, which
was reflected by applying the PNI. It was found that a low
pre-chemoradiotherapy PNI was significantly associated with shorter
DFS and OS in 114 patients with LARC (13). Similarly, Wang et al
(23) previously measured various
systemic inflammatory response markers, nutrition and
immune-related biomarkers to examine the chemoradiotherapy response
and long-term oncological outcomes in 273 patients with LARC. This
previous study reported that PLR and PNI independently predicted
responses to chemoradiotherapy, where PNI was also an independent
predictor of DFS and OS in patients with LARC (23). The present study explored the
utility of various systemic inflammatory response markers,
including SII and nutrition and immune-related biomarkers, to
examine the response to chemoradiotherapy and long-term oncological
outcomes in 214 patients with LARC. However, an association between
the tested biomarkers and pathological response could not be found,
though PNI and pCR were found to be independent predictors of DFS
and OS. This is consistent with previous observations that LARC
with pCR results in highly favorable oncological outcomes (5,24).
Hu et al (25) previously described SII based on NC,
LC and platelet counts. A subsequent study demonstrated the
prognostic value of SII in colorectal cancer (26). SII was previously revealed to be an
independent predictive factor for pCR in patients with LARC
receiving chemoradiotherapy (14).
To the best of our knowledge, SII has not yet been reported to be
associated with cancer recurrence and mortality in patients with
LARC. Therefore, SII was included in the present analysis to
determine whether it was associated with oncologic outcomes.
However, no association could be found between SII and cancer
recurrence or mortality.
CEA is produced and secreted by colorectal cancer
cells (27). Serum CEA is a
representative tumor marker for colorectal cancer and has been
widely used for surveillance after colorectal cancer surgery
(28). Previously, serum CEA levels
in rectal cancer before and after neoadjuvant chemoradiotherapy
have been reported to be associated with treatment response and
survival (29,30). The multivariate analysis performed
in the present study revealed that post-chemoradiotherapy CEA
levels were strongly associated with DFS and OS in patients with
LARC treated with preoperative chemoradiotherapy.
The present study has a number of limitations.
Potential selection bias may persist, due to its retrospective
design and the inclusion of a small cohort from a single
institution. Therefore, there needs to be more prospective
validation. Nevertheless, these data were systematically collected
from an electronic database that were regularly updated. Therefore,
the data used for the present analysis were reflective of current
clinical practices, thereby providing a solid foundation for the
findings. The present study was conducted using a single cohort,
focusing on patients with LARC treated with neoadjuvant therapy
followed by surgery. Therefore, in the inclusion and exclusion
criteria mainly pertained to clinical stage. Other inclusion and
exclusion criteria such as performance status, biomarkers status,
nutritional status and comorbidities, should be considered for
future studies. Preoperative PNI in patients undergoing curative
surgery for rectal cancer served as a simple and cost-effective
method to identify individuals with a potentially unfavorable
prognosis. Early detection of recurrence may enable the resection
of metastatic lesions in patients with rectal cancer, and
understanding the risk factors that can predict recurrence would be
beneficial for managing LARC. However, the optimal PNI threshold
for predicting recurrence remains to be determined. Therefore,
further assessment in lager, multi-center studies is required to
evaluate the robustness of prediction models.
In conclusion, results from the present study
suggest that PNI is a robust predictive factor for disease
recurrence and survival in patients with LARC treated with
neoadjuvant therapy followed by surgery. These findings may
facilitate the risk stratification of patients and the selection of
optimal personalized treatment plans for patients with LARC.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
SYO and GP contributed to the design of this paper,
data analysis and revising this paper. Both authors read and
approved the final version of the manuscript. SYO and GP confirm
the authenticity of all the raw data.
Ethics approval and consent to
participate
The present study received full ethical approval
from the Institutional Review Board of Ajou University School of
Medicine (approval no. AJOUIRB-MDB-2022-109; Suwon, Korea).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
LARC
|
Locally Advanced Rectal Cancer
|
|
PNI
|
Prognostic Nutritional Index
|
|
pCR
|
Pathologic Complete Response
|
|
SII
|
Systemic Immune-Inflammation Index
|
|
NLR
|
Neutrophil-to-Lymphocyte Ratio
|
|
PLR
|
Platelet-to-Lymphocyte ratio
|
|
LMR
|
Lymphocyte-to-Monocyte Ratio
|
|
LC
|
Lymphocyte Count
|
|
NC
|
Neutrophil Count
|
|
DFS
|
Disease-free Survival
|
|
OS
|
Overall Survival
|
|
HR
|
Hazard ratio
|
References
|
1
|
Benson AB, Venook AP, Al-Hawary MM, Azad
N, Chen YJ, Ciombor KK, Cohen S, Cooper HS, Deming D,
Garrido-Laguna I, et al: Rectal Cancer, Version 2.2022, NCCN
clinical practice guidelines in oncology. J Natl Compr Canc Netw.
20:1139–1167. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kim S, Huh JW, Lee WY, Yun SH, Kim HC, Cho
YB, Park Y and Shin JK: Predicting survival in locally advanced
rectal cancer with effective chemoradiotherapy response. Eur J Surg
Oncol. 50:1083612024. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Guan B, Huang X, Xia H, Guan G and Xu B:
Prognostic value of mesorectal package area in patients with
locally advanced rectal cancer following neoadjuvant
chemoradiotherapy: A retrospective cohort study. Front Oncol.
12:9417862022. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Mirbagheri N, Kumar B, Deb S, Poh BR, Dark
JG, Leow CC and Teoh WM: Lymph node status as a prognostic
indicator after preoperative neoadjuvant chemoradiotherapy of
rectal cancer. Colorectal Dis. 16:O339–O346. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Maas M, Nelemans PJ, Valentini V, Das P,
Rödel C, Kuo LJ, Calvo FA, García-Aguilar J, Glynne-Jones R,
Haustermans K, et al: Long-term outcome in patients with a
pathological complete response after chemoradiation for rectal
cancer: A pooled analysis of individual patient data. Lancet Oncol.
11:835–844. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Sethi G, Shanmugam MK, Ramachandran L,
Kumar AP and Tergaonkar V: Multifaceted link between cancer and
inflammation. Biosci Rep. 32:1–15. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Karki R, Man SM and Kanneganti TD:
Inflammasomes and cancer. Cancer Immunol Res. 5:94–99. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Yamamoto A, Toiyama Y, Okugawa Y, Oki S,
Ide S, Saigusa S, Araki T and Kusunoki M: Clinical implications of
pretreatment: lymphocyte-to-monocyte ratio in patients with rectal
cancer receiving preoperative chemoradiotherapy. Dis Colon Rectum.
62:171–180. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ke TM, Lin LC, Huang CC, Chien YW, Ting WC
and Yang CC: High neutrophil-to-lymphocyte ratio and
platelet-to-lymphocyte ratio predict poor survival in rectal cancer
patients receiving neoadjuvant concurrent chemoradiotherapy.
Medicine (Baltimore). 99:e198772020. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Nagasaki T, Akiyoshi T, Fujimoto Y,
Fujimoto Y, Konishi T, Nagayama S, Fukunaga Y and Ueno M:
Prognostic impact of neutrophil-to-lymphocyte ratio in patients
with advanced low rectal cancer treated with preoperative
chemoradiotherapy. Dig Surg. 32:496–503. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Dong M, Shi Y, Yang J, Zhou Q, Lian Y,
Wang D, Ma T, Zhang Y, Mi Y, Gu X and Fan R: Prognostic and
clinicopathological significance of systemic immune-inflammation
index in colorectal cancer: A meta-analysis. Ther Adv Med Oncol.
12:17588359209374252020. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Jian-Hui C, Iskandar EA, Cai ShI, Chen CQ,
Wu H, Xu JB and He YL: Significance of Onodera's prognostic
nutritional index in patients with colorectal cancer: A large
cohort study in a single Chinese institution. Tumor Biol.
37:3277–3283. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Okugawa Y, Toiyama Y, Oki S, Ide S,
Yamamoto A, Ichikawa T, Kitajima T, Fujikawa H, Yasuda H, Saigusa
S, et al: Feasibility of assessing prognostic nutrition index in
patients with rectal cancer who receive preoperative
chemoradiotherapy. JPEN J Parenter Enteral Nutr. 42:998–1007. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Eraslan E, Adas YG, Yildiz F, Gulesen AI,
Karacin C and Arslan UY: Systemic immune-inflammation index (SII)
predicts pathological complete response to neoadjuvant
chemoradiotherapy in locally advanced rectal cancer. J Coll
Physicians Surg Pak. 31:399–404. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Partl R, Paal K, Stranz B, Hassler E,
Magyar M, Brunner TB and Langsenlehner T: The Pre-Treatment
Platelet-to-Lymphocyte Ratio as a Prognostic Factor for
Loco-Regional Control in Locally Advanced Rectal Cancer.
Diagnostics (Basel). 13:6792023. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Edge SB and Compton CC: The American Joint
Committee on Cancer: the 7th edition of the AJCC cancer staging
manual and the future of TNM. Ann Surg Oncol. 17:1471–1474. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Camp RL, Dolled-Filhart M and Rimm DL:
X-tile: A new bio-informatics tool for biomarker assessment and
outcome-based cut-point optimization. Clin Cancer Res.
10:7252–7259. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kim TG, Park W, Kim H, Choi DH, Park HC,
Kim SH, Cho YB, Yun SH, Kim HC, Lee WY, et al: Baseline
neutrophil-lymphocyte ratio and platelet-lymphocyte ratio in rectal
cancer patients following neoadjuvant chemoradiotherapy. Tumori.
105:434–440. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zitvogel L, Pietrocola F and Kroemer G:
Nutrition, inflammation and cancer. Nat Immunol. 18:843–850. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Alwarawrah Y, Kiernan K and MacIver NJ:
Changes in nutritional status impact immune cell metabolism and
function. Front Immunol. 9:10552018. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Buzby GP, Mullen JL, Matthews DC, Hobbs CL
and Rosato EF: Prognostic nutritional index in gastrointestinal
surgery. Am J Surg. 139:160–167. 1980. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Sun G, Li Y, Peng Y, Lu D, Zhang F, Cui X,
Zhang Q and Li Z: Impact of the preoperative prognostic nutritional
index on postoperative and survival outcomes in colorectal cancer
patients who underwent primary tumor resection: A systematic review
and meta-analysis. Int J Colorectal Dis. 34:681–689. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wang Y, Chen L, Zhang B, Song W, Zhou G,
Xie L and Yu D: Pretreatment inflammatory-nutritional biomarkers
predict responses to neoadjuvant chemoradiotherapy and survival in
locally advanced rectal cancer. Front Oncol. 11:6399092021.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Runau F, Collins A, Fenech GA, Ford E,
Dimitriou N, Chaudhri S and Yeung JM: A single institution's
long-term follow-up of patients with pathological complete response
in locally advanced rectal adenocarcinoma following neoadjuvant
chemoradiotherapy. Int J Colorectal Dis. 32:341–348. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Hu B, Yang XR, Xu Y, Sun YF, Sun C, Guo W,
Zhang X, Wang WM, Qiu SJ, Zhou J and Fan J: Systemic
immune-inflammation index predicts prognosis of patients after
curative resection for hepatocellular carcinoma. Clin Cancer Res.
20:6212–6122. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Chen JH, Zhai ET, Yuan YJ, Wu KM, Xu JB,
Peng JJ, Chen CQ, He YL and Cai SR: Systemic immune-inflammation
index for predicting prognosis of colorectal cancer. World J
Gastroenterol. 23:6261–6272. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Gold P and Freedman SO: Specific
carcinoembryonic antigens of the human digestive system. J Exp Med.
122:467–481. 1965. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Park IJ, Choi GS, Lim KH, Kang BM and Jun
SH: Serum carcinoembryonic antigen monitoring after curative
resection for colorectal cancer: Clinical significance of the
preoperative level. Ann Surg Oncol. 16:3087–3093. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Lee JH, Kim DY, Kim SH, Cho HM, Shim BY,
Kim TH, Kim SY, Baek JY, Oh JH, Nam TK, et al: Carcinoembryonic
antigen has prognostic value for tumor downstaging and recurrence
in rectal cancer after preoperative chemoradiotherapy and curative
surgery: A multi-institutional and case-matched control study of
KROG 14–12. Radiother Oncol. 116:202–208. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Perez RO, são Julião GP, Habr-Gama A, Kiss
D, Proscurshim I, Campos FG, Gama-Rodrigues JJ and Cecconello I:
The role of carcinoembriogenic antigen in predicting response and
survival to neoadjuvant chemoradiotherapy for distal rectal cancer.
Dis Colon Rectum. 52:1137–1143. 2009. View Article : Google Scholar : PubMed/NCBI
|