Introduction
Despite the importance of adverse events associated
with cancer treatments and the broad range of mitigating
interventions, limited systematic efforts have been made to
identify, appraise and summarize the totality of evidence on the
effectiveness of such interventions. In particular, the burden of
these adverse events remains high, which is associated with
considerable rates of morbidity and mortality, in addition to the
high cost involved for the patient. All the aforementioned factors
contribute to a negative effect on the physical, emotional and
social wellbeing of the patient (1,2).
However, the trajectory of adverse toxicities associated with
cancer treatments is unique to each cancer type, and dependent on
the physiology of each individual patient. Therefore, particular
attention must be paid to the management of adverse events during
anti-tumour treatment. Chemotherapy is one of the most common
cancer treatment options, and immune checkpoint inhibitors (ICIs)
have transformed the treatment of cancer in recent years.
ICI treatment combined with chemotherapy has been
widely used for various types of cancer in clinical practice.
However, this type of combined therapy increases the difficulty of
identifying adverse events, especially when they are rare or
atypical. Oxaliplatin is a third-generation platinum agent approved
for the treatment of gastric, colorectal and other types of cancer.
Among known oxaliplatin-induced dose-limiting toxicities are common
neurological (paraesthesia and dysaesthesia of the hands, feet and
perioral region), haematopoietic and gastrointestinal toxicities,
and more rarely, hypersensitivity reactions (HSRs) (3). These HSRs are often mild or moderate,
but occasionally can be serious and lead to patient mortalities. In
the present report, a male patient who was misdiagnosed with severe
and atypical oxaliplatin-related HSR following chemotherapy
combined with programmed cell death protein-1 (PD-1) inhibitor
immunotherapy was documented.
Case report
A 67-year-old man was diagnosed with gastric stump
cancer with local liver invasion and underwent resection in the
general surgery department of Binhaiwan Central Hospital of
Dongguan (Dongguan, China) in February 2023. The postoperative
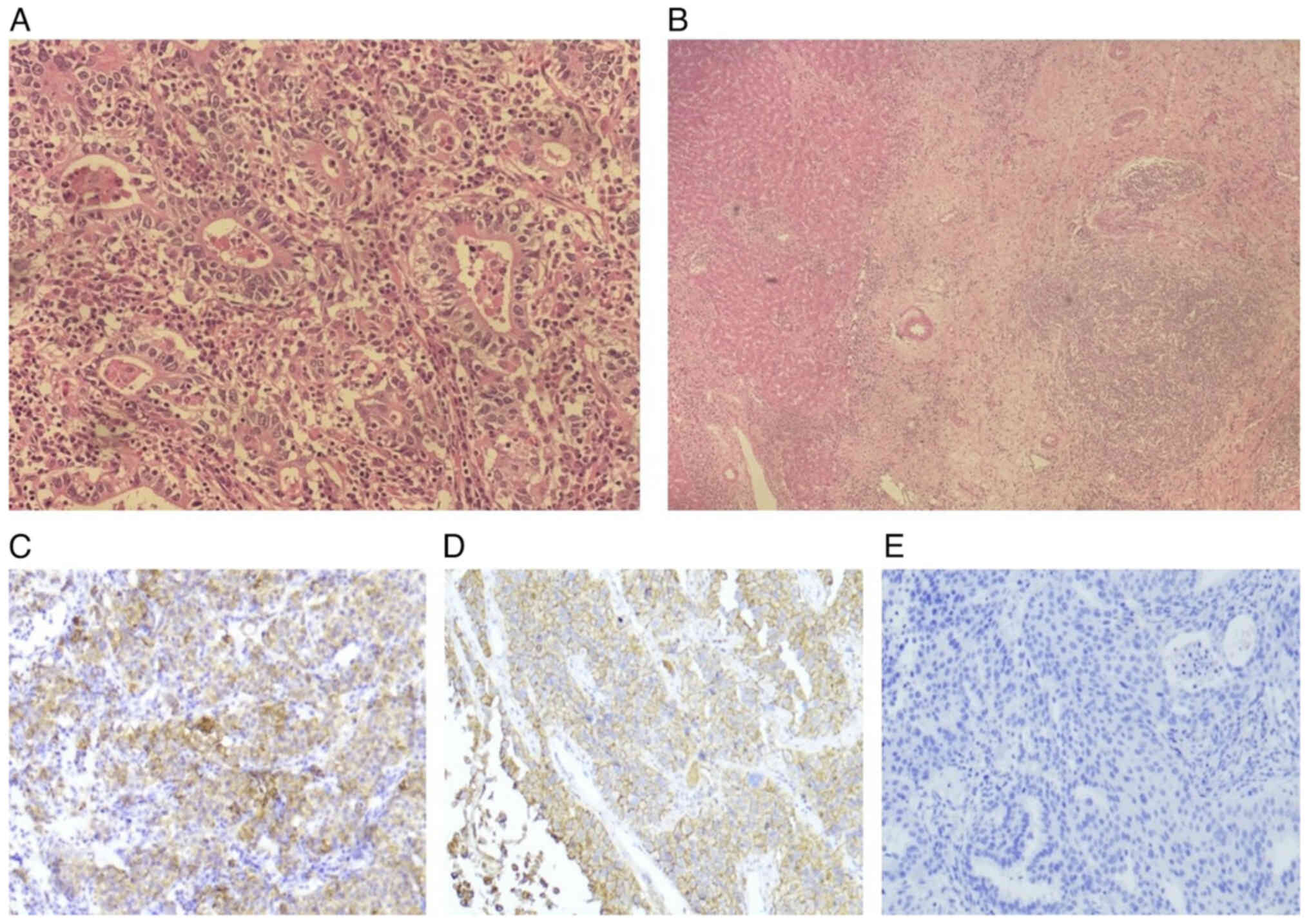
pathology results were as follows: i) Poorly differentiated
adenocarcinoma; ii) pT4b N3a M0; iii) stage IIIc; iv)
microsatellite stable; and v) programmed death-ligand 1 (PD-L1)
combined positive score (CPS) of 15 (IHC 22C3 pharmDx assay)
(Fig. 1). The patient recovered
well and came to the oncology department of the same hospital for
further treatment. Historically, the patient had undergone Billroth
II gastrectomy due to gastric bleeding in 2013.
An attraction-5 study showed that, although
oxaliplatin and capecitabine (CAPEOX regimen) chemotherapy combined
with PD-1 inhibitor could not reduce the 3-year rate of
relapse-free survivals of postoperative stage III
gastric/gastroesophageal junction cancer compared with CAPEOX
regimen chemotherapy alone, clinical benefits were observed in
subgroups of patients with either stage IIIc or PD-L1 CPS >1
when chemotherapy was combined with PD-1 inhibitor (4). Following comprehensive discussions
with the patient, he requested chemotherapy combined with PD-1
inhibitor immunotherapy and signed the informed consent.
In March 2023, the patient was treated with the
CAPEOX chemotherapy regimen (oxaliplatin, 130 mg/m2 on
day 1; capecitabine, 1,000 mg/m2 on days 1–14) combined
with the PD-1 inhibitor (tislelizumab, 200 mg on day 1) for the
first course. At 3 days after treatment, the liver function of the
patient was slightly impaired but recovered spontaneously after 1
week. Other laboratory test results were near normal. In April
2023, the patient was treated with a second course of the same
regimen. Chills and high fever occurred in the patient following
treatment on day 1. The patient noted that several family members,
with whom he maintained close interactions, had contracted
influenza. Blood test results on days 4 and 7 from treatment were
as follows: i) Common Terminology Criteria for Adverse Events
(CTCAE) grade II neutropenia, thrombocytopenia and liver function
impairment; ii) CTCAE grade I cardiac and renal function
impairment; iii) elevation of inflammatory marker procalcitonin
(PCT) levels; and iv) no microbial growth in blood culture. The
aforementioned results led to the diagnosis of side effects of
chemotherapy combined with influenza. The patient was therefore
treated with anti-bacterial, anti-viral and organ-protective drugs,
and recovered after 2 weeks.
In May 2023, the patient was treated with a third
course of the same regimen. After treatment on day 1, the patient
suffered from sudden chills, high fever (40.2°C) and facial
flushing. After anti-pyretic drug treatment, his fever was slightly
alleviated (38–39°C). However, 8 h later, the mental state of the
patient deteriorated, exhibiting somnolence. His blood pressure and
blood oxygen saturation were immediately measured, and were found
to be 55/36 mmHg and 65% respectively; the patient was therefore
diagnosed with shock. Rapid fluid infusion and dopamine were
administered to raise his blood pressure, alongside high-flow
oxygen inhalation. The shock was then rectified and his mental
state improved, but dopamine was required to maintain his blood
pressure. Blood test results were as follows: i) PCT, 52.48 ng/ml
[normal range (NR), 0.00–0.05 ng/ml]; ii) IL-6, >2,500.00 pg/ml
(NR, 0–7 pg/ml); iii) creatinine, 214.30 µmol/l (NR, 46–104
µmol/l); iv) alanine transaminase, 234.40 U/l (NR, 10–40 U/l); v)
aspartate transaminase, 383.00 U/l (NR, 10–40 U/l); vi) pro-brain
natriuretic peptide (pro-BNP), 7,732 pg/ml (NR, 0–125 pg/ml); vii)
troponin T, 23.4 pg/ml (NR, 0–14 pg/ml); viii) triiodothyronine,
0.619 nmol/l (NR, 1.2–3.1 nmol/l); and ix) thyroxine, 42.9 nmol/l
(NR, 66–181 nmol/l). Electrocardiogram and cardiac ultrasound
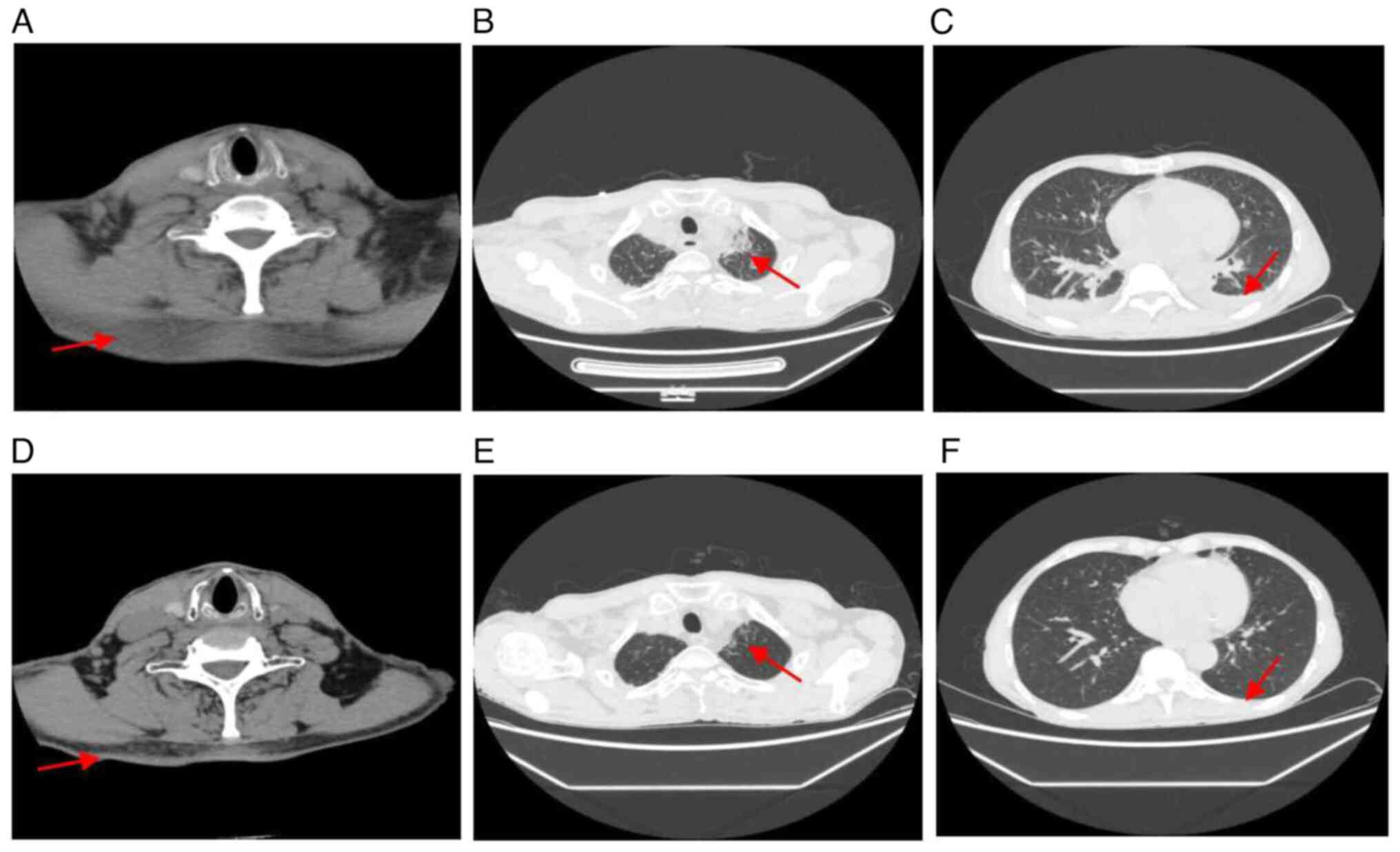
results did not reveal abnormalities. Computed tomography scans
revealed the following findings: i) No abnormalities in the brain;
ii) diffuse exudative changes in the neck; iii) patchy shadows in
both sides of the lungs, most likely inflammation; iv) small amount
of pleural effusion in both sides; and v) a small amount of ascites
(Fig. 2).
Since similar symptoms had occurred in this patient
on treatment day 1 of the two previous courses, and the symptoms
during the third course were notably more severe compared with
those during the second course, the symptoms of both courses were
suspected to be immune-related adverse events (irAEs) induced by
tislelizumab, causing multiple organ damage. The adverse events of
the third course involved the heart, lungs, liver, kidneys and the
thyroid gland, which were classified into CTCAE grades IV, I, III,
II and I, respectively. In addition, cytokine release syndrome
(CRS) was also suspected, since this patient had high fever with
markedly increased IL-6 levels. However, since the PCT level was
also markedly elevated in this patient, the possibility of severe
bacterial infection could not be ruled out. The patient was then
treated with methylprednisolone (200 mg on days 1–7) and
immunoglobulin (20 g on days 1–7) plus tocilizumab (160 mg on days
1–2), to suppress the immune response, combined with plasma
exchange and anti-bacterial and organ-protective drugs. The high
fever in the patient subsequently dissipated and the patient no
longer needed dopamine to maintain blood pressure.
A review of the various indicators revealed
significant improvements, with no microbial growth observed in the
blood culture. However, during treatment the patient developed
atrial fibrillation and his cardiac function deteriorated [pro-BNP,
12,334 pg/ml (NR, 0–125 pg/ml); left ventricular ejection fraction,
48% (NR, 50–70%)], which were rectified by cedilanid treatment. The
aforementioned events were then verified as irAEs associated with
CRS, instead of bacterial infection. The patient therefore
continued with the methylprednisolone treatment (dosage was reduced
step by step for 6 weeks) regimen, and his condition was stable
during follow-up. The results of the computed tomography (CT) scan
showed that the cervical exudation, patchy shadows of the lung and
pleural effusion had been absorbed (Fig. 2).
Due to the occurrence of severe adverse events
induced by tislelizumab during the third course, tislelizumab was
permanently discontinued for the patient. In June 2023, the patient
underwent a fourth course of the CAPEOX regimen. Following
treatment on day 1, the patients developed chills, high fever and
facial flushing again. The patient was immediately treated with
methylprednisolone (200 mg) plus immunoglobulin (15 g), which
relieved the symptoms. However, 2 h later, shock re-occurred and
had to be treated with rapid fluid infusion, dopamine and oxygen
inhalation. Blood test results also showed multiple organ damage,
coupled with the significant elevation of IL-6 and PCT levels,
albeit the symptoms were less severe compared with those in the
third course. The patient developed similar symptoms following the
discontinuation of tislelizumab. Comprehensive analysis of clinical
data during the three recent courses found that the symptom onset
time was 1–7 h after oxaliplatin infusion (6 h and 34 min, 3 h and
52 min, and 1 h and 15 min, respectively) and the interval time was
progressively shorter (Table I).
Given that anti-allergic treatment was effective for these symptoms
and no allergic reaction was observed following the administration
of combined drugs in the past, it was adjudged that the
aforementioned symptoms in the three recent courses were due to
oxaliplatin-related HSRs (CTCAE grade II, IV and IV, respectively).
The patient was therefore treated with gradually reducing doses of
methylprednisolone (the duration was 2 weeks) and his condition was
stable during follow-up. Between July 2023 and January 2024,
tegafur/gimeracil/oteracil potassium capsule (S-1) chemotherapy was
administered instead for the patient, and his condition was also
stable. On last follow-up in February 2024, the patient's cancer
remained stable without recurrence.
 | Table I.Laboratory test results between the
first and fourth courses. |
Table I.
Laboratory test results between the
first and fourth courses.
| Parameter | First course
highest/lowest value | Second course
highest/lowest value | Third course
highest/lowest value | Fourth course
highest/lowest value | Range of normal
values |
|---|
| Pro-brain natriuretic
peptide, pg/ml | 532 | 2,875 | 12,334 | 3,800 | 0-125 |
| Cardiac troponin T,
pg/ml | 9.49 | 17.92 | 23.40 | 12.50 | 0-14 |
| Left ventricle
ejection fraction | 70% | - | 48% | 60% | 50-70% |
| Alanine transaminase,
U/l | 47.3 | 146.9 | 234.2 | 78.0 | 10-40 |
| Aspartate
transaminase, U/l | 45.0 | 186.0 | 383.0 | 81.0 | 10-40 |
| Creatinine,
µmol/l | 82.7 | 147.2 | 214.3 | 104.1 | 46-104 |
| Triiodothyronine,
nmol/l | 1.33 | - | 0.42 | 0.69 | 1.2–3.1 |
| Thyroxine,
nmol/l | 66.59 | - | 36.40 | 44.25 | 66-181 |
| Procalcitonin,
ng/ml | <0.05 | 9.28 | 52.48 | 26.30 | 0.00–0.05 |
| IL-6, pg/ml | - | - | >2,500 | >2,500 | 0-7 |
Discussion
The mechanism underlying oxaliplatin-related HSR
remains unknown. Stahl et al (5) previously found that almost all
examined patients experience oxaliplatin-related HSRs after
multiple infusions, suggesting that sensitization to oxaliplatin is
required during the initial courses. In addition, IgE-mediated type
I HSRs were suspected (5). Another
hypothesis of oxaliplatin-related HSR is that platinum-based drugs
can act as ‘superantigens’ on peripheral blood mononuclear cells to
induce the expansion of T lymphocytes, which in turn release large
quantities of proinflammatory cytokines, such as IL-6, TNF-α and
IFN-γ (6). Data from 10
oxaliplatin-related HSR studies over the past 20 years have
subsequently been analysed and summarized. Briefly, the incidence
has been revealed to be mostly 10–20%, with that of grade IV being
<2%. However, pre-medication with steroids and anti-histamines
seems ineffective for its prevention. The median occurrence time is
within 1 h from the start of oxaliplatin infusion, with the main
symptoms being cutaneous and respiratory symptoms. The treatment
method for oxaliplatin-related HSR is anti-allergic treatment. The
prognosis for the majority of patients was favourable, since deaths
were rare (Table II) (7–16).
 | Table II.Characteristics of oxaliplatin-related
HSR in previous clinical studies. |
Table II.
Characteristics of oxaliplatin-related
HSR in previous clinical studies.
| Study | Patients | Patients with HSR, n
(%) | Patients with grade
III/IV HSR, n (%) | Median occurrence
courses, range | Median onset time
from start of infusion, min | Premedication | Main symptoms | Prognosis | (Refs.) |
|---|
| Brandi et al,
2003 | 124 | 17 (13.7) | N/A | 9 (2–17) | 10-20 | N/A | Cutaneous and
respiratory symptoms | Recovery | (7) |
| André et al,
2004 | 1,108 | 114 (10.3) | Grade III, 25 (2.3)
Grade IV, 7 (0.6) | N/A | N/A | N/A | N/A | N/A | (8) |
| Siu et al,
2006 | 180 | 27 (15.0) | 4 (2.2) | 9 (1–18) | N/A | N/A | Cutaneous and
respiratory symptoms | Recovery | (9) |
| Shao et al,
2010 | 383 | 47 (12.3) | N/A | 10 (2–19) | 40 | Steroids
Antihistamines | Cutaneous and
respiratory symptoms | 1 death | (10) |
| Parel et al,
2014 | 191 | 17 (8.9) | Grade III, 3 (1.6)
Grade IV, 0 (0) | 3 (1–13) | N/A | N/A | Cutaneous and
respiratory symptoms | Recovery | (11) |
| Okayama et
al, 2015 | 162 | 28 (17.2) | Grade III, 9 (5.5)
Grade IV, 1 (0.6) | 8 (5–17) | Grade III, 54 Grade
IV, 18 | N/A | N/A | Recovery | (12) |
| Shen et al,
2018 | 291 | 39 (13.4) | Grade III, 9 (3.1)
Grade IV, 7 (1.0) | 8 (4–15) | N/A | Steroids
Antihistamines | N/A | Recovery | (13) |
| Sohn et al,
2018 | 679 | 103 (15.2) | Grade III, 8 (1.2)
Grade IV, 2 (0.3) | 4.72±2.73 | N/A | N/A | N/A | Recovery | (14) |
| Barbin et
al, 2022 | 153 | 17 (11.1) | Grade III, 12 (7.8)
Grade IV, 1 (0.6) | 2 (1–11) | N/A | Steroids
Antihistamines | Cutaneous and
respiratory symptoms | Recovery | (15) |
| Selcuk et
al, 2023 | 57 | 11 (19.3) | N/A | 4 (1–7) | N/A | Steroids
Antihistamines | N/A | Recovery | (16) |
IrAEs are distinct types of toxicities that are
caused by the non-specific activation of the immune system, which
can damage almost any organ. The precise mechanism of irAE
pathogenesis remains unclear, although several inflammatory cell
types, such as Th17, have been reported to be involved
(17). However, CRS is not
universally defined and is considered to be a phenomenon of immune
hyperactivation, whereby lymphocytes (such as B cells, T cells and
natural killer cells) and bone marrow cells (such as macrophages,
dendritic cells and monocytes) are activated to release
pro-inflammatory cytokines, including IL-6, IL-10 and IFN-γ
(18). This effect has been
commonly observed following various immunotherapies, such as
chimeric antigen receptor-T cells and monoclonal antibodies. In
addition, this phenomenon has been reported in the field of viral
infection therapy, such as H1N1 and Coronavirus disease 2019
(18–20).
It is not common for oxaliplatin-related HSR to
cause multiple organ damage unless it is particularly severe or not
treated in a timely manner, due to the ensuing cytokine storm
(19,21). The causes of misdiagnosis in the
present patient were therefore investigated. The present patient
had no common HSR symptoms, such as cutaneous and respiratory
symptoms, while exhibiting serious multiple organ damage, which was
misdiagnosed as irAEs. In addition, the occurrence time of the most
severe symptoms was on day 52 of tislelizumab immunotherapy during
the third course, which coincided with the high incidence time of
fatal toxic effects associated with PD-1 inhibitor (22,23).
PCT levels were markedly elevated in this patient with high fever,
which was misdiagnosed as severe bacterial infection. PCT is a
common biomarker of bacterial infection or sepsis. Although
non-infectious diseases can also cause systemic inflammation and
increase PCT, supporting data remain limited (24). The relationship between PCT and HSR
was then assessed through a literature review, although no
definitive reports could be found. The possibility of severe
bacterial infection prevented the early use of glucocorticoid for
this patient during the third course when oxaliplatin-related HSR
occurred. There were 15 h between the onset of HSR and the use of
glucocorticoid, which delayed the treatment and aggravated the
damage to the patient. During the fourth course, glucocorticoid was
applied early when HSR occurred, with the symptoms then becoming
less severe.
Admittedly, irAEs caused by tislelizumab following
HSR occurrence during the third course could not be completely
ruled out. However, due to similar symptoms occurring during the
third and fourth courses, coupled with cardiac toxicities
manifesting as heart failure rather than myocardial damage, irAEs
caused by tislelizumab became less likely in the third course. In
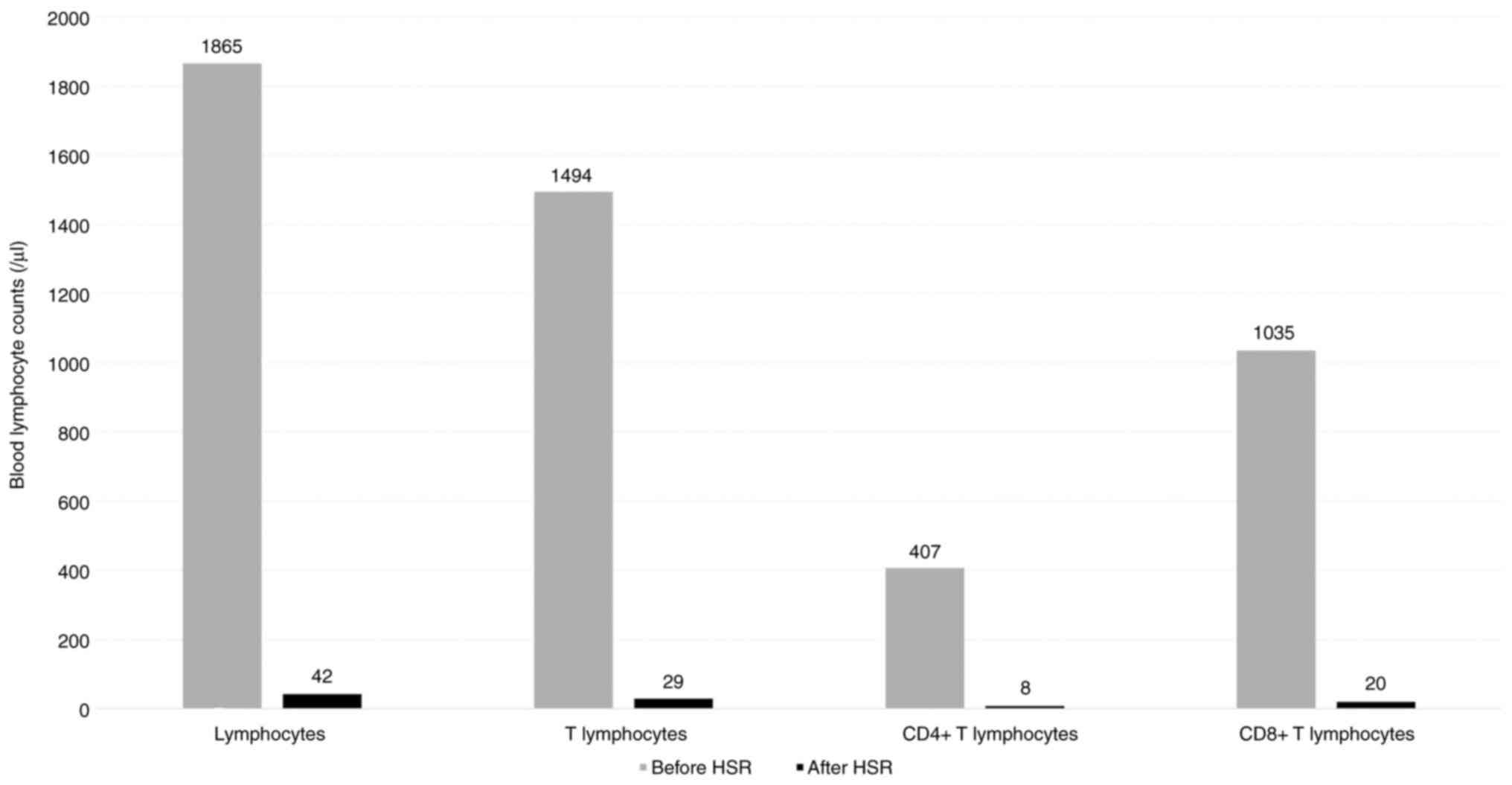
addition, a significant decrease in the blood T lymphocyte counts
following HSRs was observed in the patient during the third course
(Fig. 3), further supporting this
viewpoint. To date, several studies have reported that an increased
T lymphocyte count is associated with irAEs (25–27).
However, a previous study has found that patients with irAEs have
lower levels of T and B lymphocyte subsets, and higher levels of
IL-6, compared with those without irAEs (28); this requires further investigation.
The lower severity and quicker control of the condition of the
patient during the fourth course compared with that during the
third course was attributed to the early intervention of
glucocorticoid when HSR occurred.
In conclusion, severe oxaliplatin-related HSR is
rare and at times atypical. It can cause serious multiple organ
damage and significant increases in PCT levels, which often leads
to misdiagnosis and a delay in treatment, particularly when
oxaliplatin chemotherapy is combined with other treatments.
Furthermore, given that combination therapy for cancer can increase
therapeutic efficacy through multiple mechanisms (29) and become increasingly popular in
clinical practice, we hypothesise that complex adverse events such
as the ones that occurred in the present patient will increase,
which is something that requires vigilance.
Acknowledgements
Not applicable.
Funding
This study was supported by the Science and Technology Project
of Dongguan Social Development (grant no. 20231800937182).
Availability of data and materials
The data generated in the present study are included
in the figures and/or tables of this article.
Authors' contributions
MS and SH conceived and designed this study. MS, LC
and GX contributed to the analysis and interpretation of data. MS
wrote the manuscript. SH and LC supervised this study and
critically reviewed the manuscript for important intellectual
content. MS and SH confirm the authenticity of all the raw data.
All authors read and approved the final version of the
manuscript.
Ethics approval and consent to
participate
The study was approved by the Ethics Committee of
Binhaiwan Central Hospital of Dongguan (approval no. 2023048) and
was conducted in accordance with the Declaration of Helsinki.
Patient consent for publication
Written informed consent for publication was
obtained from the patient.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Lustberg MB, Kuderer NM, Desai A, Bergerot
C and Lyman GH: Mitigating long-term and delayed adverse events
associated with cancer treatment: Implications for survivorship.
Nat Rev Clin Oncol. 20:527–542. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kuderer NM, Desai A, Lustberg MB and Lyman
GH: Mitigating acute chemotherapy-associated adverse events in
patients with cancer. Nat Rev Clin Oncol. 19:681–697. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Gowda A, Goel R, Berdzik J, Leichman CG
and Javle M: Hypersensitivity reactions to oxaliplatin: Incidence
and management. Oncology (Williston Park). 18:1671–1675.
167616801683–1684. 2004.PubMed/NCBI
|
|
4
|
Terashima M, Kang YK, Kim YW, Boku N,
Chung HCC, Chen JS, Ji J, Yeh TS, ChenL T, Ryu MH, et al:
ATTRACTION-5: A phase 3 study of nivolumab plus chemotherapy as
postoperative adjuvant treatment for pathological stage III (pStage
III) gastric or gastroesophageal junction (G/GEJ) cancer. J Clin
Oncol. 41 (Suppl 16):S40002023. View Article : Google Scholar
|
|
5
|
Stahl M, Köster W and Wilke H: Reaction
after oxaliplatin-prevention with corticosteroids? Ann Oncol.
12:8742001. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Santini D, Tonini G, Salerno A, Vincenzi
B, Patti G, Battistoni F, Dicuonzo G and Labianca R: Idiosyncratic
reaction after oxaliplatin infusion. Ann Oncol. 12:132–133. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Brandi G, Pantaleo MA, Galli C, Falcone A,
Antonuzzo A, Mordenti P, Di Marco MC and Biasco G: Hypersensitivity
reactions related to oxaliplatin (OHP). Br J Cancer. 89:477–481.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
André T, Boni C, Mounedji-Boudiaf L,
Navarro M, Tabernero J, Hickish T, Topham C, Zaninelli M, Clingan
P, Bridgewater J, et al: Oxaliplatin, fluorouracil, and leucovorin
as adjuvant treatment for colon cancer. N Engl J Med.
350:2343–2351. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Siu SWK, Chan RTT and Au GKH:
Hypersensitivity reactions to oxaliplatin: Experience in a single
institute. Ann Oncol. 17:259–261. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Shao YY, Hu FC, Liang JT, Chiu WT, Cheng
AL and Yang CH: Characteristics and risk factors of
oxaliplatin-related hypersensitivity reactions. J Formos Med Assoc.
109:362–368. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Parel M, Ranchon F, Nosbaum A, You B,
Vantard N, Schwiertz V, Gourc C, Gauthier N, Guedat MG, He S, et
al: Hypersensitivity to oxaliplatin: Clinical features and risk
factors. BMC Pharmacol Toxicol. 15:12014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Okayama T, Ishikawa T, Sugatani K, Yoshida
N, Kokura S, Matsuda K, Tsukamoto S, Ihara N, Kuriu Y, Nakanishi M,
et al: Hypersensitivity reactions to oxaliplatin: Identifying the
risk factors and judging the efficacy of a desensitization
protocol. Clin Ther. 37:1259–1269. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Shen Y, Li C, Liu W, Mao W, Qian H, Wang H
and Xu Q: Clinical analysis of hypersensitivity reactions to
oxaliplatin among colorectal cancer patients. Oncol Res.
26:801–807. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Sohn KH, Kang DY, Kim JY, Lee SY, Lee KH,
Han SW and Kang HR: Incidence and risk of oxaliplatin-induced
hypersensitivity in patients with asymptomatic prior exposure: A
prospective observational study. J Allergy Clin Immunol Pract.
6:1642–1648.e2. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Barbin F, Ghidini M, Panichi A, Tomasello
G, Bareggi C, Galassi B, Denaro N, Ruatta F, Cauchi C, Rossino MG
and Garrone O: Oxaliplatin-related hypersensitivity reactions: A
single institution series and literature review. Biomedicines.
10:32752022. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Selcuk A and Yıldız B: Oxaliplatin-induced
hypersensitivity reactions: Risk factors and management. Eur Rev
Med Pharmacol Sci. 27:2640–2645. 2023.PubMed/NCBI
|
|
17
|
Puzanov I, Diab A, Abdallah K, Bingham CO
III, Brogdon C, Dadu R, Hamad L, Kim S, Lacouture ME, LeBoeuf NR,
et al: Managing toxicities associated with immune checkpoint
inhibitors: Consensus recommendations from the Society for
Immunotherapy of Cancer (SITC) toxicity management working group. J
Immunother Cancer. 5:952017. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Lee DW, Gardner R, Porter DL, Louis CU,
Ahmed N, Jensen M, Grupp SA and Mackall CL: Current concepts in the
diagnosis and management of cytokine release syndrome. Blood.
124:188–195. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Fajgenbaum DC and June CH: Cytokine storm.
N Engl J Med. 383:2255–2273. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Rotz SJ, Leino D, Szabo S, Mangino JL,
Turpin BK and Pressey JG: Severe cytokine release syndrome in a
patient receiving PD-1-directed therapy. Pediatr Blood Cancer.
64:e266422017. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Polyzos A, Tsavaris N, Gogas H, Souglakos
J, Vambakas L, Vardakas N, Polyzos K, Tsigris C, Mantas D,
Papachristodoulou A, et al: Clinical features of hypersensitivity
reactions to oxaliplatin: A 10-year experience. Oncology. 76:36–41.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Mahmood SS, Fradley MG, Cohen JV, Nohria
A, Reynolds KL, Heinzerling LM, Sullivan RJ, Damrongwatanasuk R,
Chen CL, Gupta D, et al: Myocarditis in patients treated with
immune checkpoint inhibitors. J Am Coll Cardiol. 71:1755–1764.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wang DY, Salem JE, Cohen JV, Chandra S,
Menzer C, Ye F, Zhao S, Das S, Beckermann KE, Ha L, et al: Fatal
toxic effects associated with immune checkpoint inhibitors: A
systematic review and meta-analysis. JAMA Oncol. 4:1721–1728. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Neeser O, Branche A, Mueller B and Schuetz
P: How to: Implement procalcitonin testing in my practice. Clin
Microbiol Infect. 25:1226–1230. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Oh DY, Cham J, Zhang L, Fong G, Kwek SS,
Klinger M, Faham M and Fong L: Immune toxicities elicted by CTLA-4
blockade in cancer patients are associated with early
diversification of the T-cell repertoire. Cancer Res. 77:1322–1330.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Diehl A, Yarchoan M, Hopkins A, Jaffee E
and Grossman SA: Relationships between lymphocyte counts and
treatment-related toxicities and clinical responses in patients
with solid tumors treated with PD-1 checkpoint inhibitors.
Oncotarget. 8:114268–114280. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Subudhi SK, Aparicio A, Gao J, Zurita AJ,
Araujo JC, Logothetis CJ, Tahir SA, Korivi BR, Slack RS, Vence L,
et al: Clonal expansion of CD8 T cells in the systemic circulation
precedes development of ipilimumab-induced toxicities. Proc Natl
Acad Sci USA. 113:11919–11924. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Yu Y, Wang S, Su N, Pan S, Tu B, Zhao J,
Shen Y, Qiu Q, Liu X, Luan J, et al: Increased circulating levels
of CRP and IL-6 and decreased frequencies of T and B lymphocyte
subsets are associated with immune-related adverse events during
combination therapy with PD-1 inhibitors for liver cancer. Front
Oncol. 12:9068242022. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Melero I, Berman DM, Aznar MA, Korman AJ,
Pérez Gracia JL and Haanen J: Evolving synergistic combinations of
targeted immunotherapies to combat cancer. Nat Rev Cancer.
15:457–472. 2015. View
Article : Google Scholar : PubMed/NCBI
|