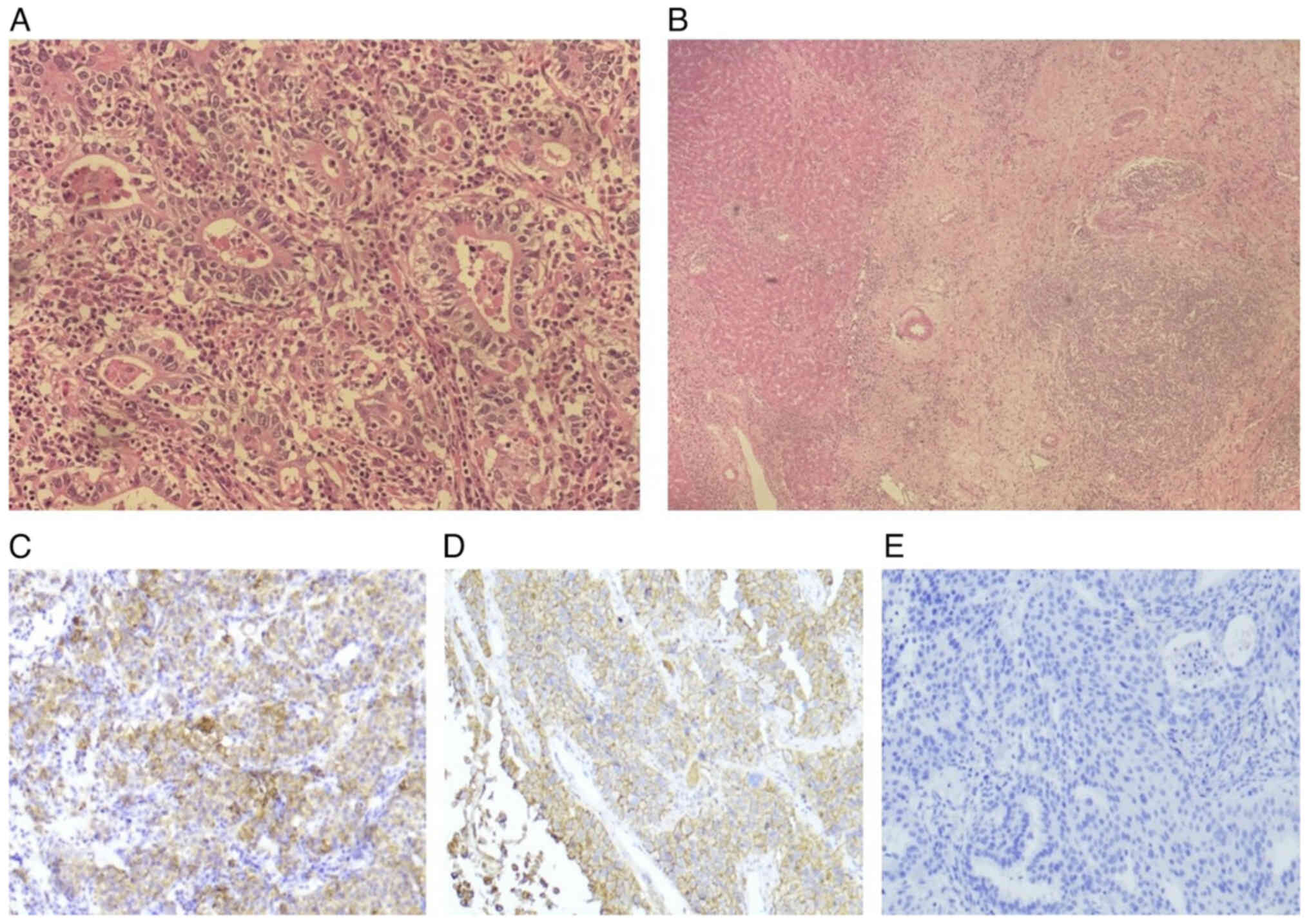
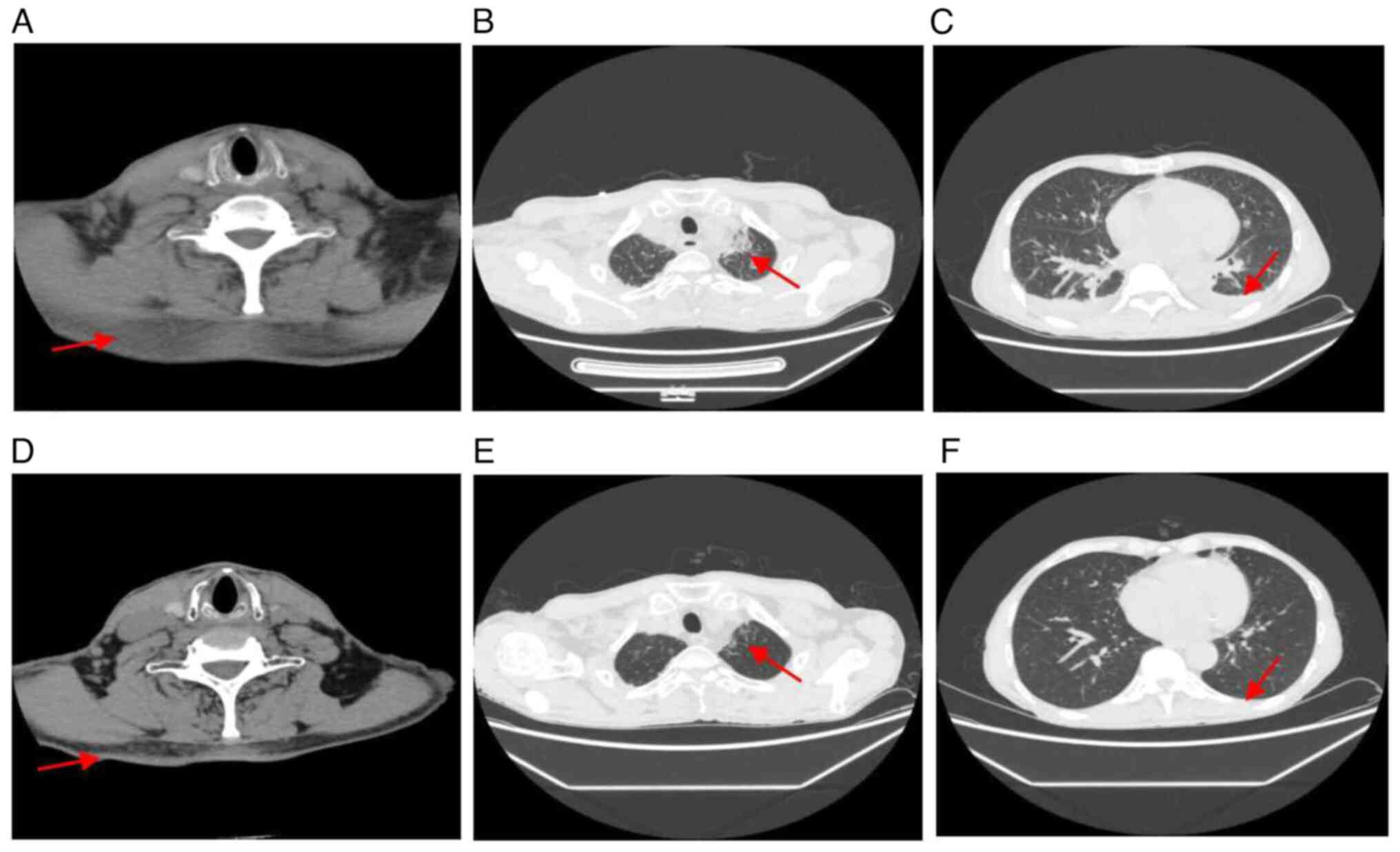
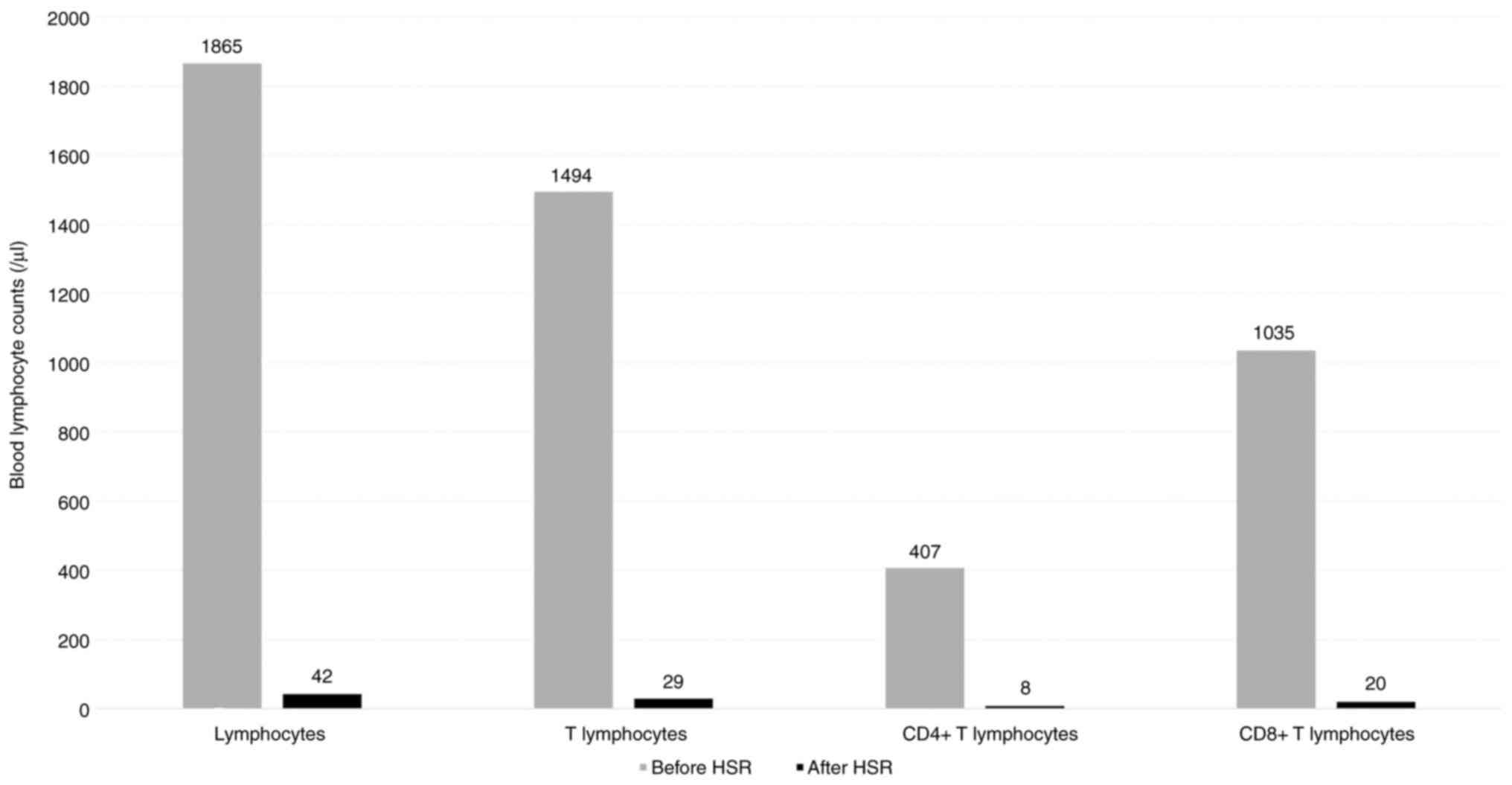
|
1
|
Lustberg MB, Kuderer NM, Desai A, Bergerot
C and Lyman GH: Mitigating long-term and delayed adverse events
associated with cancer treatment: Implications for survivorship.
Nat Rev Clin Oncol. 20:527–542. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kuderer NM, Desai A, Lustberg MB and Lyman
GH: Mitigating acute chemotherapy-associated adverse events in
patients with cancer. Nat Rev Clin Oncol. 19:681–697. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Gowda A, Goel R, Berdzik J, Leichman CG
and Javle M: Hypersensitivity reactions to oxaliplatin: Incidence
and management. Oncology (Williston Park). 18:1671–1675.
167616801683–1684. 2004.PubMed/NCBI
|
|
4
|
Terashima M, Kang YK, Kim YW, Boku N,
Chung HCC, Chen JS, Ji J, Yeh TS, ChenL T, Ryu MH, et al:
ATTRACTION-5: A phase 3 study of nivolumab plus chemotherapy as
postoperative adjuvant treatment for pathological stage III (pStage
III) gastric or gastroesophageal junction (G/GEJ) cancer. J Clin
Oncol. 41 (Suppl 16):S40002023. View Article : Google Scholar
|
|
5
|
Stahl M, Köster W and Wilke H: Reaction
after oxaliplatin-prevention with corticosteroids? Ann Oncol.
12:8742001. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Santini D, Tonini G, Salerno A, Vincenzi
B, Patti G, Battistoni F, Dicuonzo G and Labianca R: Idiosyncratic
reaction after oxaliplatin infusion. Ann Oncol. 12:132–133. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Brandi G, Pantaleo MA, Galli C, Falcone A,
Antonuzzo A, Mordenti P, Di Marco MC and Biasco G: Hypersensitivity
reactions related to oxaliplatin (OHP). Br J Cancer. 89:477–481.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
André T, Boni C, Mounedji-Boudiaf L,
Navarro M, Tabernero J, Hickish T, Topham C, Zaninelli M, Clingan
P, Bridgewater J, et al: Oxaliplatin, fluorouracil, and leucovorin
as adjuvant treatment for colon cancer. N Engl J Med.
350:2343–2351. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Siu SWK, Chan RTT and Au GKH:
Hypersensitivity reactions to oxaliplatin: Experience in a single
institute. Ann Oncol. 17:259–261. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Shao YY, Hu FC, Liang JT, Chiu WT, Cheng
AL and Yang CH: Characteristics and risk factors of
oxaliplatin-related hypersensitivity reactions. J Formos Med Assoc.
109:362–368. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Parel M, Ranchon F, Nosbaum A, You B,
Vantard N, Schwiertz V, Gourc C, Gauthier N, Guedat MG, He S, et
al: Hypersensitivity to oxaliplatin: Clinical features and risk
factors. BMC Pharmacol Toxicol. 15:12014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Okayama T, Ishikawa T, Sugatani K, Yoshida
N, Kokura S, Matsuda K, Tsukamoto S, Ihara N, Kuriu Y, Nakanishi M,
et al: Hypersensitivity reactions to oxaliplatin: Identifying the
risk factors and judging the efficacy of a desensitization
protocol. Clin Ther. 37:1259–1269. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Shen Y, Li C, Liu W, Mao W, Qian H, Wang H
and Xu Q: Clinical analysis of hypersensitivity reactions to
oxaliplatin among colorectal cancer patients. Oncol Res.
26:801–807. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Sohn KH, Kang DY, Kim JY, Lee SY, Lee KH,
Han SW and Kang HR: Incidence and risk of oxaliplatin-induced
hypersensitivity in patients with asymptomatic prior exposure: A
prospective observational study. J Allergy Clin Immunol Pract.
6:1642–1648.e2. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Barbin F, Ghidini M, Panichi A, Tomasello
G, Bareggi C, Galassi B, Denaro N, Ruatta F, Cauchi C, Rossino MG
and Garrone O: Oxaliplatin-related hypersensitivity reactions: A
single institution series and literature review. Biomedicines.
10:32752022. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Selcuk A and Yıldız B: Oxaliplatin-induced
hypersensitivity reactions: Risk factors and management. Eur Rev
Med Pharmacol Sci. 27:2640–2645. 2023.PubMed/NCBI
|
|
17
|
Puzanov I, Diab A, Abdallah K, Bingham CO
III, Brogdon C, Dadu R, Hamad L, Kim S, Lacouture ME, LeBoeuf NR,
et al: Managing toxicities associated with immune checkpoint
inhibitors: Consensus recommendations from the Society for
Immunotherapy of Cancer (SITC) toxicity management working group. J
Immunother Cancer. 5:952017. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Lee DW, Gardner R, Porter DL, Louis CU,
Ahmed N, Jensen M, Grupp SA and Mackall CL: Current concepts in the
diagnosis and management of cytokine release syndrome. Blood.
124:188–195. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Fajgenbaum DC and June CH: Cytokine storm.
N Engl J Med. 383:2255–2273. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Rotz SJ, Leino D, Szabo S, Mangino JL,
Turpin BK and Pressey JG: Severe cytokine release syndrome in a
patient receiving PD-1-directed therapy. Pediatr Blood Cancer.
64:e266422017. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Polyzos A, Tsavaris N, Gogas H, Souglakos
J, Vambakas L, Vardakas N, Polyzos K, Tsigris C, Mantas D,
Papachristodoulou A, et al: Clinical features of hypersensitivity
reactions to oxaliplatin: A 10-year experience. Oncology. 76:36–41.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Mahmood SS, Fradley MG, Cohen JV, Nohria
A, Reynolds KL, Heinzerling LM, Sullivan RJ, Damrongwatanasuk R,
Chen CL, Gupta D, et al: Myocarditis in patients treated with
immune checkpoint inhibitors. J Am Coll Cardiol. 71:1755–1764.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wang DY, Salem JE, Cohen JV, Chandra S,
Menzer C, Ye F, Zhao S, Das S, Beckermann KE, Ha L, et al: Fatal
toxic effects associated with immune checkpoint inhibitors: A
systematic review and meta-analysis. JAMA Oncol. 4:1721–1728. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Neeser O, Branche A, Mueller B and Schuetz
P: How to: Implement procalcitonin testing in my practice. Clin
Microbiol Infect. 25:1226–1230. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Oh DY, Cham J, Zhang L, Fong G, Kwek SS,
Klinger M, Faham M and Fong L: Immune toxicities elicted by CTLA-4
blockade in cancer patients are associated with early
diversification of the T-cell repertoire. Cancer Res. 77:1322–1330.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Diehl A, Yarchoan M, Hopkins A, Jaffee E
and Grossman SA: Relationships between lymphocyte counts and
treatment-related toxicities and clinical responses in patients
with solid tumors treated with PD-1 checkpoint inhibitors.
Oncotarget. 8:114268–114280. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Subudhi SK, Aparicio A, Gao J, Zurita AJ,
Araujo JC, Logothetis CJ, Tahir SA, Korivi BR, Slack RS, Vence L,
et al: Clonal expansion of CD8 T cells in the systemic circulation
precedes development of ipilimumab-induced toxicities. Proc Natl
Acad Sci USA. 113:11919–11924. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Yu Y, Wang S, Su N, Pan S, Tu B, Zhao J,
Shen Y, Qiu Q, Liu X, Luan J, et al: Increased circulating levels
of CRP and IL-6 and decreased frequencies of T and B lymphocyte
subsets are associated with immune-related adverse events during
combination therapy with PD-1 inhibitors for liver cancer. Front
Oncol. 12:9068242022. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Melero I, Berman DM, Aznar MA, Korman AJ,
Pérez Gracia JL and Haanen J: Evolving synergistic combinations of
targeted immunotherapies to combat cancer. Nat Rev Cancer.
15:457–472. 2015. View
Article : Google Scholar : PubMed/NCBI
|