Introduction
Serum creatine kinase (s-CK) is one of the routine
blood tests in patients with cancer. s-CK is an enzyme consumed in
energy metabolism in various tissues and has been reported to be
involved in cell division and the immune system (1,2).
Importantly, studies have reported low s-CK levels as a poor
prognostic factor in esophageal (3), gastric (4), breast (5), and lung cancers (6,7).
The mechanisms of low s-CK activity in patients with
cancer are not fully understood. Tumor cells may consume s-CK to
generate energy needed for increased proliferation, leading to a
decline in s-CK levels. Cancer-related inflammation and cachexia
may also lead to the loss of muscle mass, and muscle wasting in
patients with cancer is considered to impact the decrease in s-CK
levels.
Hepatocellular carcinoma (HCC) is the sixth most
common cancer and the third most common cause of cancer-related
deaths (8). The prognosis of
patients with HCC is unsatisfactory because of the high incidence
of tumor recurrence and distant metastasis (9). Early detection of HCC recurrence is
associated with improved prognosis due to the better response of
smaller tumors to potentially curative treatments such as liver
resection, radiofrequency ablation, and transcatheter arterial
chemoembolization (10–12). Therefore, the development of
biomarkers that can predict HCC recurrence is important (13). s-CK has long been known to be highly
active in cancers, including HCC, suggesting its potential as a
prognostic factor (14). However,
no report to date has demonstrated the clinicopathologic and
prognostic significance of s-CK in patients with HCC.
Based on our hypothesis that low s-CK level was
associated with adverse outcomes in HCC, we aimed to evaluate the
prognostic significance of preoperative s-CK levels in patients
with HCC.
Materials and methods
Study population
This was a retrospective study including 163
patients (127 male and 36 female patients) with a median age of 69
(range, 40–88) who were diagnosed with HCC and underwent radical
liver resection between January 2004 and December 2021 in Toho
University Omori Medical Center. This study protocol was approved
by the Ethics Committee of Toho University Omori Medical Center
(The approval number is M22223, the approval date is November 30,
2022.) and conducted in accordance with the Declaration of
Helsinki. Information about the study was disclosed on the
institutional website and the potential participants were given the
opportunity to opt-out.
Study design
The following clinicopathologic factors were
included to evaluate their association with preoperative and
postoperative s-CK levels: age, sex, presence of cirrhosis, tumor
size, stage [Japanese Classification of Hepatocellular Carcinoma;
2019 (The 6th Edition)], indocyanine green retention (ICG) rate at
15 min, white blood cell, platelet counts, levels of α-fetoprotein
(AFP) and levels of protein induced by vitamin K absence or
antagonist-II (PIVKA-II).
Preoperative s-CK levels measured during routine
blood tests within seven days before surgery and postoperative s-CK
levels measured at discharge or at one-month follow-up after
surgery were included in the present study. Cut-off preoperative
and postoperative s-CK levels were determined using receiver
operating characteristic curve (ROC) analysis, with death due to
any case as the dependent variable. Then, patients were categorized
into those with high and low preoperative s-CK levels based on the
cutoff preoperative s-CK level to evaluate the association of
preoperative s-CK levels with clinicopathologic factors, overall
survival (OS), and recurrence-free survival (RFS).
In the present study, OS was defined as the interval
from the date of surgery to the date of death or last follow-up.
RFS was defined as the interval from the date of surgery to the
date of known recurrence.
Statistical analysis
Student's t-test (unpaired) and Fisher's exact
probability tests were used for two-group comparisons. OS and RFS
were calculated using the Kaplan-Meier method, and differences
between the groups were evaluated using the log-rank test.
Univariate and multivariate analyses were performed using Cox
proportional hazards regression. All statistical analyses were
performed using EZR (15). A
two-sided P-value of <0.05 was considered to denote statistical
significance.
Results
Patient categorization according to
preoperative s-CK levels
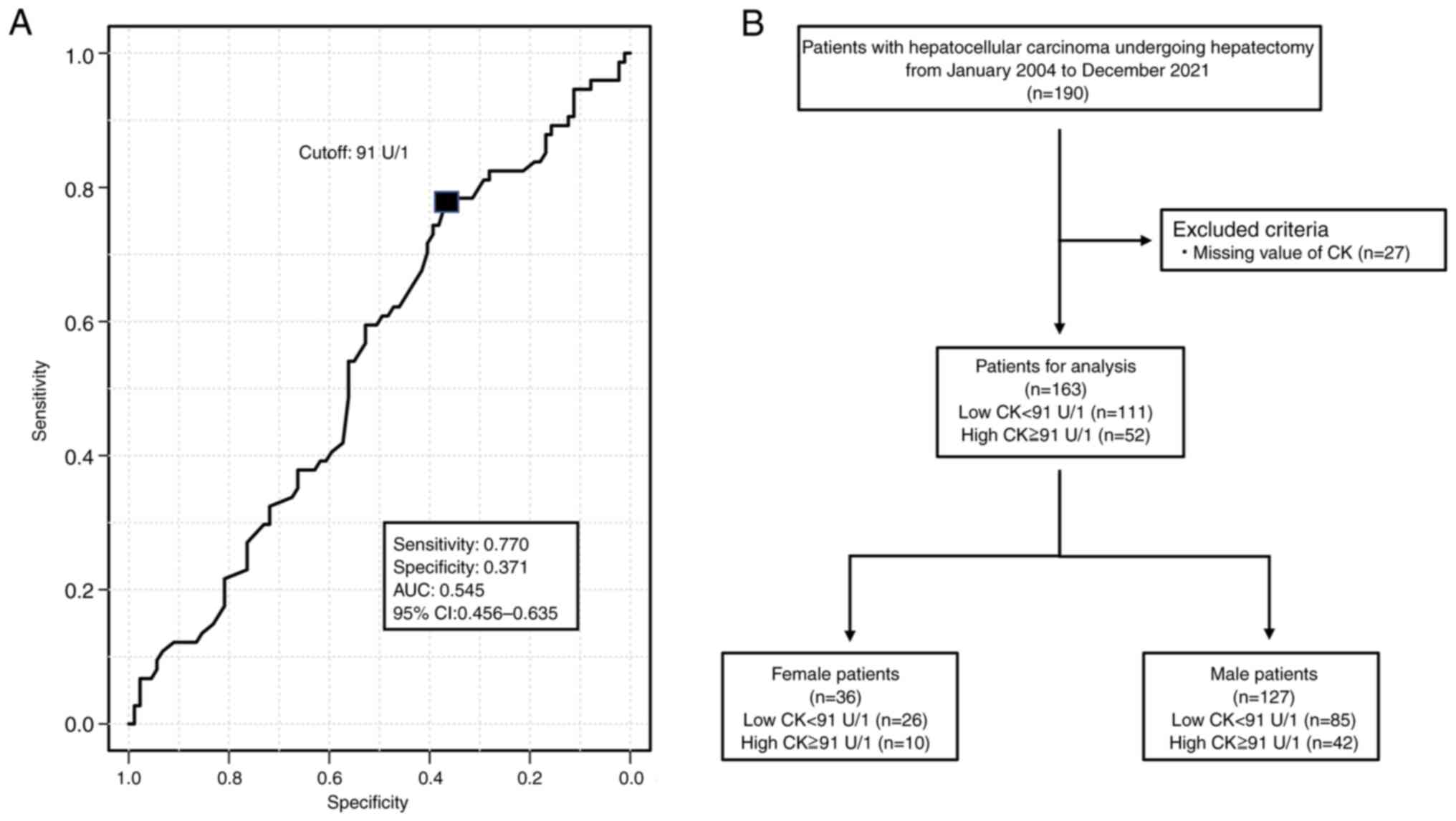
Based on the cutoff preoperative s-CK level of 91
U/l according to the ROC analysis (Fig.
1A), the 127 males were divided into the high CK group (n=85)
and low CK group (n=42). The 36 females were divided into the high
CK group (n=26) and the low CK group (n=10) (Fig. 1B).
Association of preoperative s-CK levels with
clinicopathologic factors.
Four patients did not undergo the ICG test because
of allergy to the contrast medium. No clinicopathological factors
were significantly associated with the rate of patients with low
preoperative s-CK levels (Table
I).
 | Table I.Univariate analysis of
clinicopathological factors of the low and high preoperative
creatine kinase groups in patients with hepatocellular
carcinoma. |
Table I.
Univariate analysis of
clinicopathological factors of the low and high preoperative
creatine kinase groups in patients with hepatocellular
carcinoma.
| Variables | Groups | No. of patients | Low preoperative
(n=163) creatine kinase, <91 U/l (n=111) | High preoperative
creatine kinase, ≥91 U/l (n=52) | P-valuea |
|---|
| Sex | Female | 36 | 26 | 10 | 0.686 |
|
| Male | 127 | 85 | 42 |
|
| Age, years | <65 | 55 | 35 | 20 | 0.477 |
|
| ≥65 | 108 | 76 | 32 |
|
| Cirrhotic liver | Negative | 62 | 41 | 21 | 0.730 |
|
| Positive | 101 | 70 | 31 |
|
| Tumor size, mm | <20 | 42 | 31 | 11 | 0.443 |
|
| ≥20 | 121 | 80 | 41 |
|
| Stage | I/II | 111 | 71 | 40 | 0.108 |
|
| III/IV | 52 | 40 | 12 |
|
| ICG R15, % | <10 | 73 | 51 | 22 | 0.733 |
|
| ≥10 | 86 | 57 | 29 |
|
| White blood cell,
/µl | <8,000 | 154 | 105 | 49 | 1.000 |
|
| ≥8,000 | 9 | 6 | 3 |
|
| Platelet, /µl | <150,000 | 69 | 49 | 20 | 0.614 |
|
| ≥150,000 | 94 | 62 | 32 |
|
| AFP, ng/ml | Negative | 105 | 72 | 33 | 0.863 |
|
| Positive | 58 | 39 | 19 |
|
| PIVKA-II, mAU/ml | Negative | 80 | 54 | 26 | 1.000 |
|
| Positive | 83 | 57 | 26 |
|
Comparison of OS between patients with
low and high preoperative s-CK levels
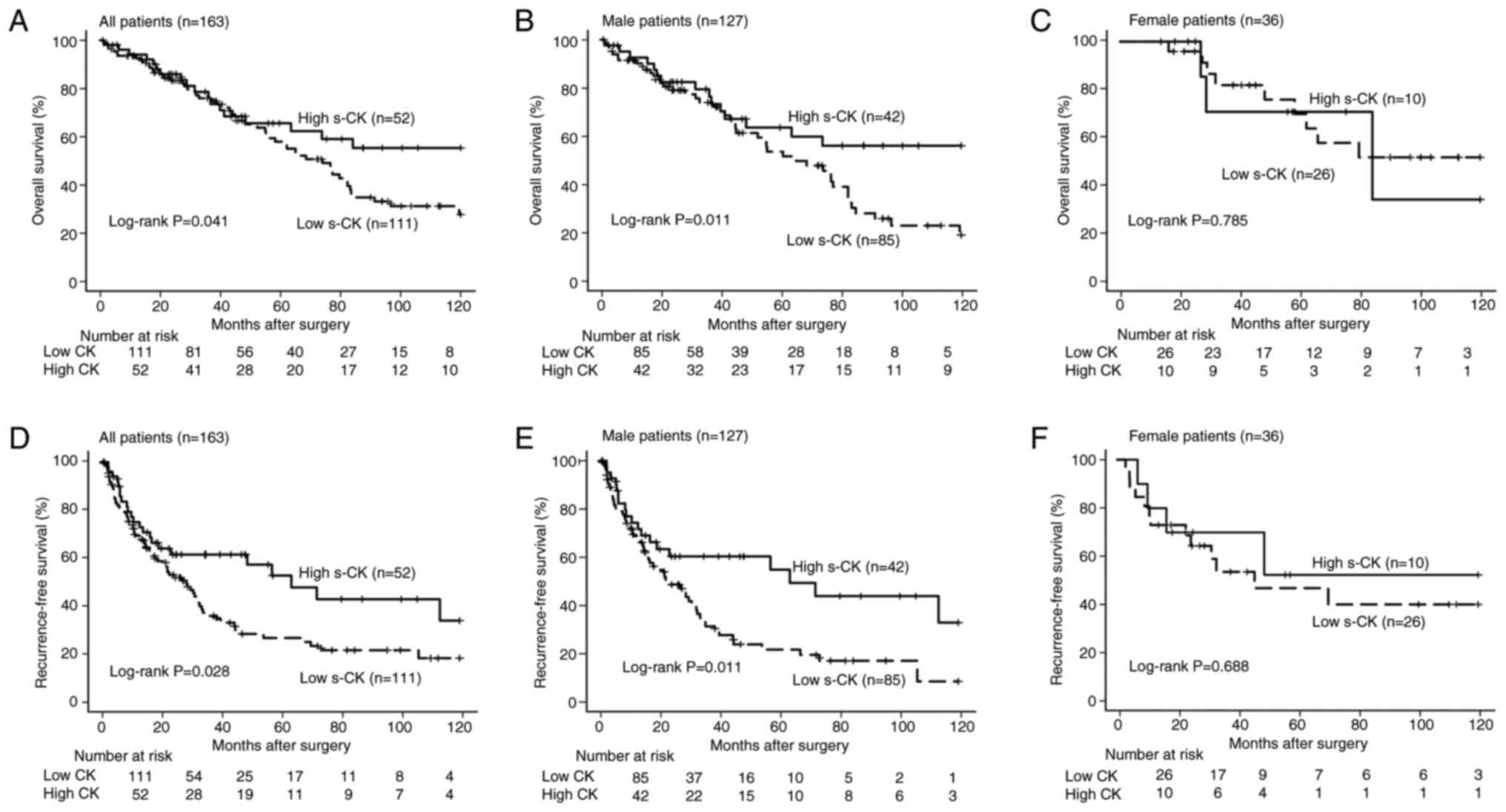
In the overall cohort, the OS was significantly
worse in the low preoperative s-CK group than in the high
preoperative s-CK group (P=0.043, Fig.
2A). Similarly, the OS was significantly worse in the low
preoperative s-CK group than in the high preoperative s-CK group
among the male patients (P=0.011, Fig.
2B). However, a significant difference in OS was not found
between the female patients with low and high preoperative s-CK
levels (P=0.785, Fig. 2C).
Univariate analysis revealed that the OS was
significantly worse in patients with low preoperative s-CK levels,
those aged over 65 years, those with an ICG retention rate of ≥10%
at 15 min, and those with high α-fetoprotein and PIVKA-II levels
compared to others (P<0.05, Table
II). Multivariate analysis revealed that a low preoperative
s-CK level, ICG retention rate of ≥10% at 15 min, and high PIVKA-II
level were independent poor prognostic factors for OS (P<0.05,
Table II).
 | Table II.Univariate and multivariate analysis
of clinicopathological factors for predicting overall survival of
patients with hepatocellular carcinoma. |
Table II.
Univariate and multivariate analysis
of clinicopathological factors for predicting overall survival of
patients with hepatocellular carcinoma.
|
|
| Univariate
analysis | Multivariate
analysis |
|---|
|
|
|
|
|
|---|
| Variables | No. patients
(n=163) | Hazard ratio (95%
confidence interval) |
P-valuea | Hazard ratio (95%
confidence interval) |
P-valuea |
|---|
| Sex |
|
|
|
|
|
|
Male/Female | 127/36 | 1.756
(0.946–3.259) | 0.074 |
|
|
| Age (years) |
|
|
|
|
|
|
≥65/<65 | 108/55 | 1.744
(1.031–2.950) | 0.038 | 1.502
(0.884–2.552) | 0.133 |
| Cirrhotic
liver |
|
|
|
|
|
|
Positive/negative | 101/62 | 0.897
(0.559–1.437) | 0.650 |
|
|
| Tumor size, mm |
|
|
|
|
|
|
≥20/<20 | 121/42 | 1.262
(0.724–2.198) | 0.412 |
|
|
| Stage |
|
|
|
|
|
| I
II/III IV | 111/52 | 0.781
(0.483–1.263) | 0.313 |
|
|
| ICG R15, % |
|
|
|
|
|
|
≥10/<10 | 86/73 | 1.996
(1.223–3.258) | 0.005 | 2.077
(1.264–3.414) | 0.004 |
| White blood cell,
/µl |
|
|
|
|
|
|
≥8,000/<8,000 | 9/154 | 0.618
(0.195–1.969) | 0.416 |
|
|
| Platelet, /µl |
|
|
|
|
|
|
≥150,000/<150,000 | 94/69 |
1.221(0.769–1.936) | 0.397 |
|
|
| AFP, ng/ml |
|
|
|
|
|
|
Positive/negative | 58/105 | 1.668
(1.053–2.643) | 0.029 | 1.336
(0.819–2.179) | 0.247 |
| PIVKA-II,
mAU/ml |
|
|
|
|
|
|
Positive/negative | 83/80 | 2.389
(1.473–3.875) | 0.001 | 2.115
(1.263–3.541) | 0.004 |
| Preoperative serum
creatine kinase, U/l |
|
|
|
|
|
|
<91/≥91 | 111/52 | 1.739
(1.017–2.973) | 0.043 | 1.946
(1.115–3.397) | 0.019 |
Comparison of RFS between patients
with low and high preoperative s-CK levels
The RFS of the low preoperative s-CK group was
significantly worse than that of the high preoperative s-CK group
in the overall cohort (P=0.028, Fig.
2D) and among the male patients (P=0.011, Fig. 2E). Similar to our findings regarding
OS, the RFS did not significantly differ between the female
patients with low and high preoperative s-CK levels (P=0.688,
Fig. 2F).
Univariate analysis revealed that the RFS was
significantly worse in patients with low preoperative s-CK levels,
those with an ICG retention rate of ≥10% at 15 min, and those with
high PIVKA-II levels compared to others (P<0.05, Table III). Multivariate analysis
revealed that low preoperative s-CK level, an ICG retention rate of
≥10% at 15 min, and high PIVKA-II level were independent poor
prognostic factors for RFS (P<0.05, Table III).
 | Table III.Univariate and multivariate analysis
of clinicopathological factors for predicting recurrence-free
survival of patients with hepatocellular carcinoma. |
Table III.
Univariate and multivariate analysis
of clinicopathological factors for predicting recurrence-free
survival of patients with hepatocellular carcinoma.
|
|
| Univariate
analysis | Multivariate
analysis |
|---|
|
|
|
|
|
|---|
| Variables | No. patients
(n=163) | Hazard ratio (95%
confidence interval) |
P-valuea | Hazard ratio (95%
confidence interval) |
P-valuea |
|---|
| Sex |
|
|
|
|
|
|
Male/female | 127/36 | 1.651
(0.974–2.798) | 0.063 |
|
|
| Age, years |
|
|
|
|
|
|
≥65/<65 | 108/55 | 1.127
(0.732–1.737) | 0.587 |
|
|
| Cirrhotic
liver |
|
|
|
|
|
|
Positive/negative | 101/62 | 1.356
(0.879–2.091) | 0.587 |
|
|
| Tumor size, mm |
|
|
|
|
|
|
≥20/<20 | 121/42 | 1.085
(0.676–1.739) | 0.736 |
|
|
| Stage |
|
|
|
|
|
| I
II/III IV | 111/52 | 0.875
(0.566–1.354) | 0.549 |
|
|
| ICG R15, % |
|
|
|
|
|
|
≥10/<10 | 86/73 | 1.514
(0.991–2.312) | 0.050 | 1.625
(1.061–2.491) | 0.026 |
| White blood cell,
/µl |
|
|
|
|
|
|
≥8,000/<8,000 | 9/154 | 0.803
(0.326–1.979) | 0.634 |
|
|
| Platelet, /µl |
|
|
|
|
|
|
≥150,000/<150,000 | 94/69 | 1.452
(0.965–2.184) | 0.073 |
|
|
| AFP, ng/ml |
|
|
|
|
|
|
Positive/Negative | 58/105 | 1.295
0.850–1.972) | 0.228 |
|
|
| PIVKA-II,
mAU/ml |
|
|
|
|
|
|
Positive/Negative | 83/80 | 1.746
(1.155–2.639) | 0.008 | 1.802
(1.180–2.752) | 0.006 |
| Preoperative serum
creatine kinase, U/l |
|
|
|
|
|
|
<91/≥91 | 111/52 | 1.689
(1.053–2.709) | 0.029 | 1.843
(1.122–3.000) | 0.014 |
Discussion
In the present study, although preoperative s-CK
level was not associated with any clinicopathological factors, low
preoperative s-CK level was an independent risk factor for poor OS
and RFS in patients with HCC.
In the previous reports, cutoff values have varied
by cancer type, and there seems to be no unified view regarding how
the cutoff should be determined. The cutoff values have varied by
the median value (3), the ROC curve
(4), the mean value (5), and the upper or lower limit of normal
(6). Our study first examined
cutoff values based on the ROC curve, median, and quartile. Among
them, only the cutoff value based on the ROC curve showed
significant differences both in the results of log-rank tests for
OS and RFS and the results of univariate and multivariate analyses
of prognostic factors for OS and RFS. The area under the curve in
this ROC curve is 0.545, indicating low accuracy. Although this is
a weak point of our study, further discussion of the cutoff value
for s-CK in HCC is difficult because no other reports showed it. In
the future, we plan to conduct a multi-center collaborative study
by recruiting other facilities that agree with this paper's
objectives.
Regarding to the clinicopathological significance of
s-CK level, low s-CK level was related to the tumor progression in
other cancers. Reduction of s-CK level was found in the deep tumor
in esophageal (3) and gastric
cancer (4), large tumor in breast
cancer (5), and bone and lymph node
metastases in lung cancer (7).
However, in the present study on HCC, preoperative s-CK levels were
not associated with any of the analyzed clinicopathologic factors.
These findings suggest that clinicopathologic factors might have
limited association with prognosis in HCC and that the malignancy
potential of the tumor itself might be related to prognosis.
Regarding to the OS, the low and high s-CK groups
showed similar survival curves until five years after surgery.
However, the survival difference appeared at five years after
surgery in OS. This observation was not previously reported in
other cancer types and might be unique to HCC. This difference
might be due to the availability of various treatment options for
HCC recurrence, including repeat liver resection, radiofrequency
ablation, transcatheter arterial chemoembolization, chemotherapy,
and immune checkpoint inhibitors, which might have contributed to
improved survival after recurrence for up to five years. Although
the tendency of RFS was similar to that of OS, the differences in
RFS between the two groups were obvious even within five years. The
RFS was significantly different between the patients with low and
high preoperative s-CK levels five years after liver resection.
The potential role of estrogen should be considered
regarding the observed sex difference in the impact of low
preoperative s-CK levels on OS. In females, estrogen, a major
female hormone, stabilizes skeletal fascia by suppressing the
release of s-CK into the bloodstream (16,17).
Therefore, s-CK levels are lower in females than in males, and the
difference in OS between the female patients with low and high s-CK
levels could have been similar to the difference in OS observed
between the male patients in the absence of estrogen's effect on
s-CK levels. Because our study included only 36 females, prognostic
factors were not examined separately for males and females.
Estrogen suppression of s-CK release may have the same effect as in
gastric cancer (4), but the small
number of female cases makes it difficult to draw a definite
conclusion. Further research is necessary to determine if s-CK is a
potential prognostic factor in females.
Our multivariate analysis also revealed that low
preoperative s-CK level was an independent poor risk factor OS and
RFS, similar to that observed in esophageal (3) and gastric cancers (4). Although low s-CK levels were
associated with disease progression in esophageal and gastric
cancers, a similar finding was not previously reported in HCC. We
speculate that tumor volume might be associated with s-CK
consumption in esophageal and gastric cancers. However, we did not
observe an association between tumor volume and low s-CK levels in
the current cohort of patients with HCC and low s-CK levels in HCC
might strongly reflect malignant potential. It was suggested that
the higher the cancer cells consume s-CK, the higher the malignant
potential.
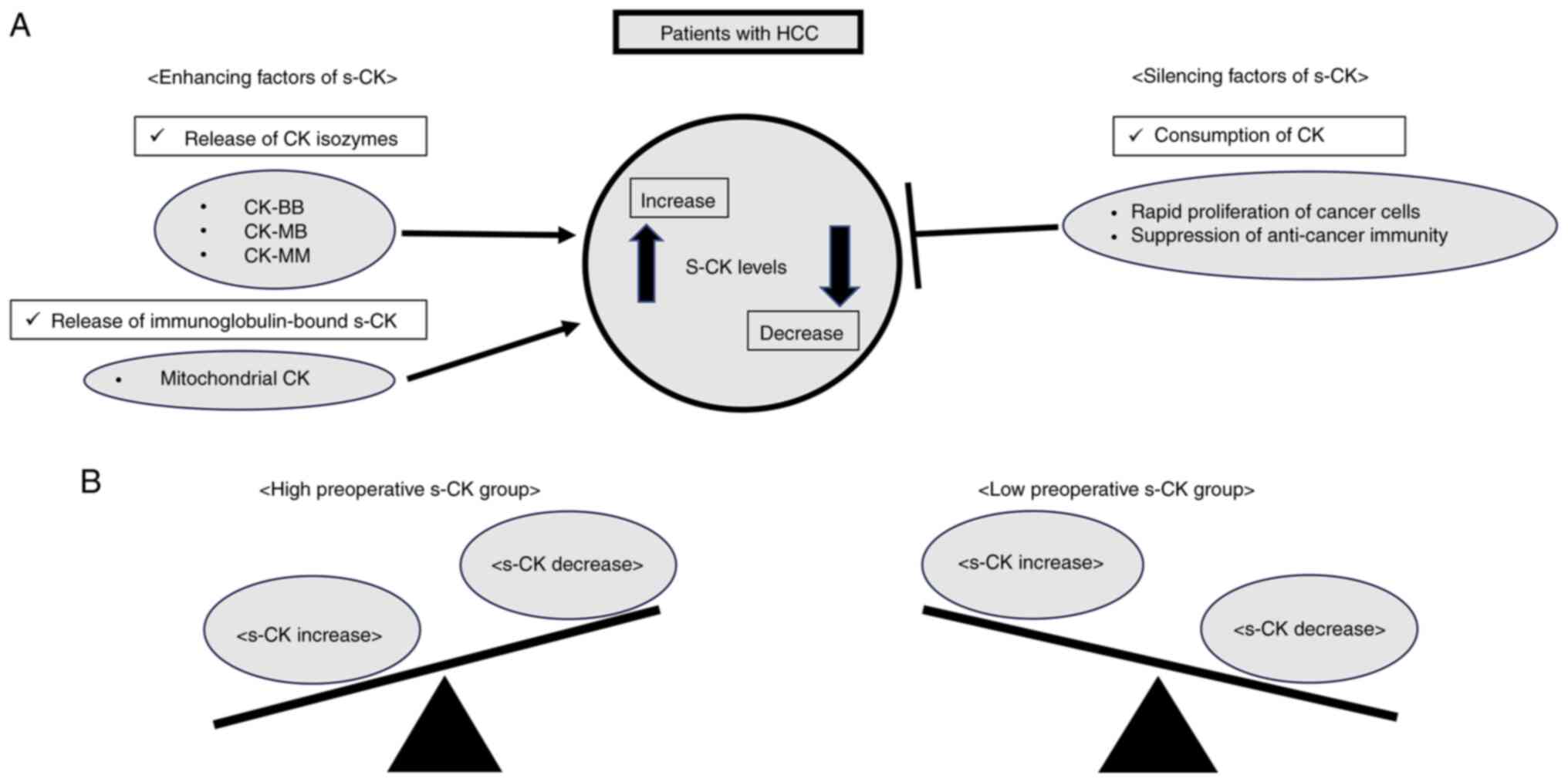
Based on our present study, in the patients with
HCC, various factors are supposed to effect s-CK levels. There were
several factors that increased or decreased s-CK in the patients
with HCC (Fig. 3A). According to
Yan YB's report (1), enhancing
factors of s-CK include CK isozymes (e.g., CK-BB, CK-MB, CK-MM) and
immunoglobulin-bound forms (e.g., mitochondrial CK), which may be
associated with s-CK increase. On the other hand, silencing factors
of s-CK include rapid cancer proliferation and decreased
anti-cancer immunity, which may be associated with s-CK decrease.
We think that the rapid proliferation of cancer cells and the
suppression of anti-cancer immunity could be explained by cancer
cells' enhancement of creatine metabolism (2). Highly active cancer cells need energy
for rapid proliferation. Therefore, we assume cancer cells obtain
energy by consuming s-CK and converting adenosine triphosphate to
adenosine diphosphate. T cells play an essential role in
anti-cancer defense. And creatine metabolism has the function of
anti-tumor T-cell immunity (2). In
rapidly proliferated cancer cells, T cells and cancer cells compete
for creatine kinase in the blood. As a result, T cell production is
reduced, leading to decreased anti-tumor immunity. The preoperative
high s-CK group is a condition where CK increase exceeds decrease,
and the preoperative low s-CK group is a condition where CK
decrease exceeds increase (Fig.
3B). Therefore, the preoperative low s-CK group may reflect a
highly malignant potential of HCC.
The present study has several limitations. First,
since this was the first study to evaluate the association between
s-CK levels and HCC, a cut-off value for S-CK was determined using
a test cohort of all patients. We expect further reports from other
centers on the validation cohort regarding the significance of the
cut-off value in the future. Second, the sample size was relatively
small in this single-center study and future studies with larger
cohort sizes are warranted to confirm the current study findings.
Third, CK has three isozymes: CK-MM, CK-MB, and CK-BB. A previous
study reported that the CK-MB/total CK ratio was useful in
detecting pancreatic adenocarcinoma (18), suggesting that the determination of
all isozymes might be considered to more accurately evaluate the
role of CK as a prognostic factor compared to total s-CK levels.
Unfortunately, the retrospective study design precluded the
determination of the levels of all three isozymes. Fourth,
underlying diseases such as dermatomyositis and cardiovascular
disease, which could potentially impact preoperative s-CK levels,
were not excluded. We aim to resolve these limitations in the
future through a multicenter study.
In conclusion, low preoperative s-CK level was an
independent risk factor for poor OS and RFS after surgery in
patients with HCC. Our present study showed promising early
findings regarding the association between low s-CK levels and poor
OS in HCC. Further study with a larger sample size is required to
make the findings more statistically reliable. Long-term follow-up
of patients is crucial to ensure the impact of low s-CK levels on
poor OS and RFS. Rigorous statistical analysis will ensure the
strength of the conclusions drawn. It is also important to compare
the prognostic value of low s-CK levels with existing standard
treatments to establish their relative importance. These efforts
will help determine whether low s-CK levels can be reliably used as
a prognostic biomarker in the clinical management of HCC.
Acknowledgements
The authors would like to thank Professor Matsuura
H. and Mr. Ota T. (Department of Medical Information Center, Toho
University School of Medicine, Tokyo, Japan) for collecting
laboratory data.
Funding
Funding: No funding was received.
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
YM and HS designed this study. YM, YO, RO, YI, KK,
JI, TM, MT and KF were involved in its conception, design and data
collection. YM, RO and YI confirm the authenticity of all the raw
data. YM and HS wrote the manuscript. All authors read and approved
the final manuscript.
Ethics approval and consent to
participate
The study protocol was approved by the Ethics
Committee of Toho University Omori Medical Center (approval no.
M22223; November 2022), and we provided means of opting out for
patients.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
s-CK
|
serum creatine kinase
|
|
HCC
|
hepatocellular carcinoma
|
|
ICG
|
indocyanine green retention
|
|
AFP
|
α-fetoprotein
|
|
PIVKA-II
|
vitamin K absence or antagonist-II
|
|
ROC
|
receiver operating characteristic
curve
|
|
OS
|
overall survival
|
|
RFS
|
recurrence-free survival
|
References
|
1
|
Yan YB: Creatine kinase in cell cycle
regulation and cancer. Amino Acids. 48:1775–1784. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kazak L and Cohen P: Creatine metabolism:
Energy homeostasis, immunity and cancer biology. Nat Rev
Endocrinol. 16:421–436. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Murayama K, Suzuki T, Yajima S, Oshima Y,
Nanami T, Shiratori F and Shimada H: Preoperative low serum
creatine kinase is associated with poor overall survival in the
male patients with esophageal squamous cell carcinoma. Esophagus.
12:105–112. 2022. View Article : Google Scholar
|
|
4
|
Yamazaki N, Oshima Y, Shiratori F, Nanami
T, Suzuki T, Yajima S, Funahashi K and Shimada H: Prognostic
significance of preoperative low serum creatine kinase levels in
gastric cancer. Surg Today. 52:1551–1559. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Pan H, Xia K, Zhou W, Xue J, Liang X, Chen
L, Wu N, Liang M, Wu D, Ling L, et al: Low serum creatine kinase
levels in breast cancer patients: A case-control study. PLoS One.
8:e621122013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Liu L, He Y, Ge G, Li L, Zhou P, Zhu Y,
Tang H, Huang Y, Li W and Zhang: Lactate dehydrogenase and creatine
kinase as poor prognostic factors in lung cancer: A retrospective
observational study. PLoS One. 12:e01821682017. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Takamori S, Toyokawa G, Shimokawa M,
Kinoshita F, Kazuma Y, Matsubara T, Haratake N, Akamine T, Hirai F,
Tagawa T, et al: A novel prognostic marker in patients with
non-small cell lung cancer: Musculo- immuno-nutritional score
calculated by controlling nutritional status and creatine kinase. J
Thorac Dis. 11:927–935. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Forner A, Reig M and Bruix J:
Hepatocellular carcinoma. Lancet. 391:1301–1314. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Qin LX and Tang ZY: The prognostic
significance of clinical and pathological features in
hepatocellular carcinoma. World J Gastroenterol. 8:193–199. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Befeler AS and Di Bisceglie AM:
Hepatocellular carcinoma: Diagnosis and treatment.
Gastroenterology. 122:1609–1610. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ho EY, Cozen ML, Shen H, Lerrigo R,
Trimble E, Ryan JC, Covera CU and Monto A; HOVAS Group
(Hepatocellular Carcinoma Treatment Outcome at VA San Francisco), :
Expanded use of aggressive therapies improves survival in early and
intermediate hepatocellular carcinoma. HPB (Oxford). 16:758–767.
2015. View Article : Google Scholar
|
|
12
|
Liu Y, Shi M, Chen S, Wan W, Shen L, Shen
B, Qui H, Cao F, Wu Y, Huang T, et al: Intermediate stage
hepatocellular carcinoma: Comparison of the value of
inflammation-based scores in predicting progression-free survival
of patients receiving transarterial chemoembolization. J Cancer Res
Ther. 17:740–748. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Yanagaki M, Haruki K, Taniai T, Igarashi
Y, Yasuda J, Furukawa K, Onda S, Shirai Y and Tsunematsu M: The
significance of osteosarcopenia as a predictor of the long-term
outcomes in hepatocellular carcinoma after hepatic resection. J
Hepatobiliary Pancreat Sci. 30:453–461. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kanemitsu F, Kawanishi I, Mizushima J and
Okigaki T: Mitochondrial creatine kinase as a tumor-associated
marker. Clin Chim Acta. 138:175–183. 1984. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kanda Y: Investigation of the freely
available easy-to-use software ‘EZR’ for medical statistics. Bone
Marrow Transplant. 48:452–458. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Neal RC, Ferdinand KC, Ycas J and Miller
E: Relationship of ethnic origin, gender, and age to blood creatine
kinase levels. Am J Med. 122:73–78. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Hackney AC, Kallman AL and Ağgön E: Female
sex hormones and the recovery from: Menstrual cycle phase affects
responses. Biomed Hum Kinet. 11:87–89. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Chen C, Lin X, Lin R, Huang H and Lu F: A
high serum creatine kinase (CK)-MB-to-total-CK ratio in patients
with pancreatic cancer: A novel application of a traditional marker
in predicting malignancy of pancreatic masses? World J Surg Oncol.
21:132023. View Article : Google Scholar : PubMed/NCBI
|