Introduction
Interleukin-10 (IL-10) is a pleiotropic cytokine
that regulates immunological homeostasis through anti-inflammatory
and/or immunostimulatory functions (1–3). IL-10
has been implicated in immunopathogenesis during tumor development
and progression. Accumulating evidence indicates that IL-10 plays a
key role in establishing and maintaining a protumor
microenvironment as a potent immunosuppressive cytokine. In
particular, major immunosuppressive cells, such as regulatory T
cells (Tregs), myeloid-derived suppressor cells, and
tumor-associated macrophages, in the tumor microenvironment utilize
IL-10 as one of the multiple immunosuppressive mechanisms (4,5).
Conversely, IL-10 can also exhibit immunostimulatory properties,
including the induction of proliferation and cytotoxic activity of
CD8+ T cells. Emmerich et al demonstrated that
treatment with IL-10 could activate tumor-resident CD8+
T cells and suppress well-established large tumors in mouse tumor
models (6). Guo et al also
revealed that the IL-10-Fc fusion protein expands and enhances the
cytotoxic function of terminally exhausted CD8+
tumor-infiltrating lymphocytes that do not respond to immune
checkpoint inhibitors (7). Thus,
IL-10 possesses diverse roles in tumor immunology and immunotherapy
(8,9). In addition, the amount and timing of
IL-10 exposure may critically impact its function in antitumor
immunity.
IL-10 is produced by various cell types, including
CD4+ T cells. Among CD4+ T cells, T helper
(Th)1, Th2, Th17, and T regulatory type 1 (Tr1) cells are notable
producers of IL-10 (10,11). Simultaneously, IL-10 can directly
and/or indirectly suppress Th responses following specific antigen
stimulation and potentially induce the formation of a negative
feedback loop to regulate immune responses. To date, several
reports have shown that tumor antigen-specific regulatory T cells
that produce IL-10 exist in the peripheral circulation, as well as
in the tumor microenvironment (12–14).
Conversely, tumor antigen-specific Th effector cells producing
IL-10 in patients with cancer have received considerably less
attention. Regarding IL-10 expression in Th effector cells, IL-10
secretion from Th2 cells is stable, whereas that from Th1 and Th17
cells was found to be unstable and conditional (10). Thus, the role of tumor
antigen-specific IL-10-producing T cells in antitumor responses is
more complex than that of CD8+ cytotoxic T
lymphocytes.
In the present study, we identified circulating
tumor antigen-specific IL-10-producing T cells in patients with
head and neck squamous cell carcinoma (HNSCC) and explored factors
influencing the immunodynamics of IL-10-producing T cells.
Materials and methods
Patients and blood collection
During March 2019 to April 2021, blood samples were
obtained at Gunma University Hospital from 18 patients with HNSCC
who did not receive any anticancer drugs, radiotherapy, or surgery
prior to blood collection. Patients with autoimmune diseases,
severe infections, or receiving steroid treatment were excluded in
this study. The median age of patients was 63.5 years (range: 48–77
years). Peripheral blood mononuclear cells (PBMCs) were isolated
using density gradient centrifugation, followed by
cryopreservation. This study was approved by the Ethics Committee
of Gunma University Hospital (approval no. HS2017-152). Written
informed consent was obtained from all patients.
Immunohistochemical expression of
tumor antigens, p53 and MAGE-A4 in HNSCC
During March 2019 to April 2021, HNSCC samples were
obtained by biopsy or surgical resection at Gunma University
Hospital from the same patients who provided blood samples. The use
of HNSCC samples was also approved by the Ethics Committee and
patient consent was obtained. Immunohistochemical analysis of p53
and MAGE-A4 expression in tumor specimens was performed as
described previously (15).
Briefly, formalin-fixed paraffin-embedded specimens sectioned at 3
µm were deparaffinized. Antigen retrieval was achieved by boiling
samples at 98°C for 30 min with 20% zinc sulfate solution and
citrate buffer (pH 6.0) for p53 and MAGE-A4 staining, respectively.
After blocking, slides were incubated for 2 h with primary
antibodies (anti-p53 antibody, NCL-L-p53-DO7, NOVOCASTRA;
anti-MAGE-A4 antibody, clone 57 B, MERCK), followed by overnight
incubation at 4°C. Subsequently, slides were incubated with a
secondary antibody (Histofine Simple Stain MAX-PO (MULTI),
Nichirei), and the reaction products were detected with
3,3′-diaminobenzidine (DAB, DOJINDO, Kumamoto, Japan). Sections
were counterstained with Mayer's hematoxylin.
The sections were evaluated by two independent,
blinded researchers (H. Tada and K.C.). For p53, specific staining
in >10% of tumor cells was defined as positive expression. For
MAGE-A4, each specimen was considered positive if specific staining
was present.
In vitro sensitization and interferon
(IFN)-γ/IL-10 double-color enzyme-linked immunosorbent spot
(ELISPOT) assay
Thawed PBMCs were cultured with recombinant tumor
antigen protein (10 µg/ml of p53 or MAGE-A4) in a final volume of
0.5 ml AIM-V medium, supplemented with 10 IU/ml IL-2 and 5 ng/ml
IL-7 in a 48-well tissue culture plate. After 4 days, AIM-V medium
(0.5 ml) containing 10 IU/ml IL-2 was added to each well. After
three days of culture, PBMCs were harvested as effector cells and
examined for IFN-γ/IL-10 production using the ELISPOT assay. For
blocking assay, mouse control IgG1κ (P3.6.2.8.1; eBioscience) or
anti-lymphocyte activation gene 3 (Lag-3) antibodies (17B4;
AdipoGen, Liestal, Switzerland) (10 µg/ml each) were added
throughout the culture period.
ELISPOT assays were performed using the Human
IFN-γ/IL-10 double-color ELISPOT kit (Cellular Technology Ltd.,
Cleveland, OH, USA) according to manufacturer protocol. Briefly, a
96-well plate was precoated with IFN-γ/IL-10 capture antibody and
incubated at 4°C overnight. Harvested effector cells
(1–5×104 cells/well) were plated into a precoated plate
and co-cultured with PBMCs (1×105 cells/well) in the
presence of p53 or MAGE-A4 protein (10 µg/ml each). The plates were
incubated at 37°C for 24 h. After incubation, the plates were
washed and developed with anti-human IFN-γ (FITC) and
FITC-horseradish peroxidase and IL-10 (Biotin) and
streptavidin-alkaline phosphatase, respectively. The number of
spot-forming cells (SFC) in each well was counted using a
CTL-ImmunoSpot Analyzer (Cellular Technology Ltd.). The mean number
of spots in control wells (no protein) was subtracted from the mean
number of spots in the experimental wells, and the results were
expressed as SFC per 5×104 cells, as described
previously (15). A T-cell response
to a given tumor antigen was considered to be positive if at least
10 cells per 5×104 responder cells secreted IFN-γ or
IL-10.
Flow cytometric analysis
Flow cytometry was performed using a FACSVerse flow
cytometer (BD Biosciences) to analyze the proportion of T cells
expressing immune checkpoint molecules in PBMCs, as reported
previously (16). Briefly,
cryopreserved PBMCs were thawed, blocked using BD Fc Block (BD
Bioscience, San Jose, CA, USA), and stained with antibodies
specific for CD3, CD4, CD8, programmed cell death-1 (PD-1),
cytotoxic T-lymphocyte-associated antigen-4 (CTLA-4), T-cell
immunoglobulin and mucin-domain containing-3 (Tim-3), and Lag-3. As
a negative control, cells were stained with a mouse IgG isotype
control (BD Biosciences). The data were analyzed using FlowJo
software (TreeStar, Ashland, OR, USA). The gating strategy is
illustrated in Fig. S1.
Statistical analysis
Statistical analyses were performed using GraphPad
Prism software (version 8.0; GraphPad Software, San Diego, CA,
USA). Unpaired two-tailed t-test was performed to determine the
presence of a significant difference between the number of SFC in
protein-stimulated and unstimulated wells, as described previously
(17). Fisher's exact test of
independence was used to determine differences in categorical
variables. Kaplan-Meier curves were plotted and compared using
log-rank tests to compare survival curves between patients with and
without p53-specific IL-10 production. P<0.05 was considered to
indicate a statistically significant difference.
Results
Patients' characteristics and p53 and
MAGE-A4 expression
Table I summarizes
the characteristics of the included patients. The primary tumor
sites included the larynx (n=6), oropharynx (n=5), and hypopharynx
(n=7). Immunohistochemical analyses were performed on 17 available
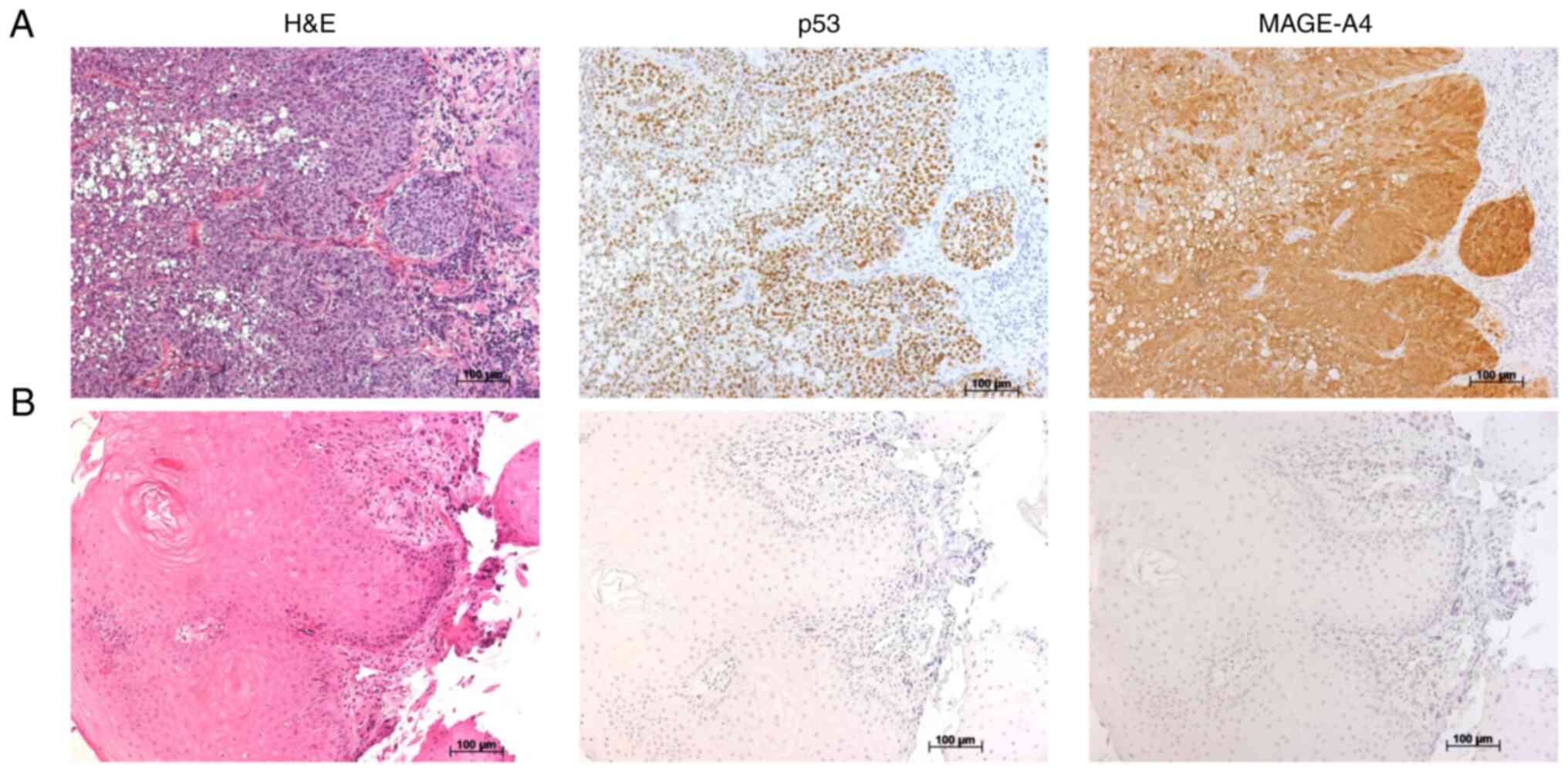
tumor specimens. Fig. 1A and B show
representative immunohistochemical staining results for p53 and
MAGE-A4. p53 and MAGE-A4 were detected in 8 (47.1%) and 13 (76.5%)
patients, respectively (Table
I).
 | Table I.Patient characteristics and T-cell
response to p53 and MAGE-A4 proteins. |
Table I.
Patient characteristics and T-cell
response to p53 and MAGE-A4 proteins.
|
|
|
|
|
|
|
|
|
| T-cell response
p53 |
| T-cell response
MAGE-A4 |
|---|
| Patient no. | Age, years | Sex | Primary site | T | N | M | Stage | (p53) staining |
| (MAGE-A4)
staining |
|
|---|
| IFN-γ | IL-10 | IFN-γ | IL-10 |
|---|
| 1 | 72 | M | Larynx | 4a | 0 | 0 | IVA | + | - | + | + | + | - |
| 2 | 61 | M | Oro | 2 | 2b | 0 | IVA | - | - | + | + | - | + |
| 3 | 59 | M | Hypo | 4b | 3b | 0 | IVB | - | - | + | + | ND | ND |
| 4 | 72 | M | Oro | 4a | 3b | 1 | IVC | + | - | + | + | - | - |
| 5 | 62 | M | Hypo | 1 | 0 | 0 | I | - | - | - | + | - | - |
| 6 | 77 | M | Hypo | 4a | 1 | 0 | IVA | + | - | + | + | - | - |
| 7 | 76 | M | Larynx | 4a | 2c | 0 | IVA | - | - | - | + | + | - |
| 8 | 48 | M | Hypo | 4b | 1 | 0 | IVB | + | - | - | + | + | + |
| 9 | 69 | M | Hypo | 4a | 3b | 0 | IVB | - | - | - | - | - | - |
| 10 | 73 | M | Larynx | 3 | 0 | 0 | III | + | - | - | - | - | - |
| 11 | 62 | M | Oro | 4a | 3b | 0 | IVB | - | - | + | + | - | + |
| 12 | 57 | M | Larynx | 2 | 0 | 0 | II | - | + | + | - | ND | ND |
| 13 | 63 | M | Oro | 4a | 2b | 0 | IVA | + | - | - | + | ND | ND |
| 14 | 56 | M | Hypo | 3 | 0 | 0 | III | + | - | - | + | ND | ND |
| 15 | 74 | M | Hypo | 2 | 2c | 0 | IVA | - | - | + | - | - | + |
| 16 | 74 | M | Oro | 4a | 2c | 0 | IVA | NA | + | - | NA | + | + |
| 17 | 64 | M | Larynx | 3 | 0 | 0 | III | + | - | + | + | - | + |
| 18 | 58 | M | Larynx | 3 | 0 | 0 | III | - | - | - | + | - | + |
Tumor antigen-specific IFN-γ/IL-10
production
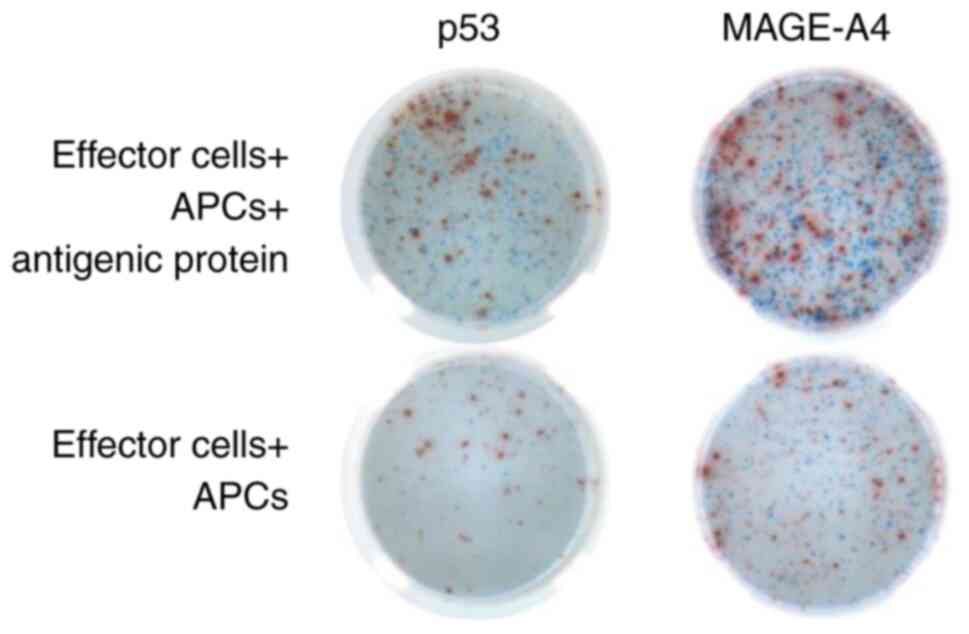
In vitro, PBMCs stimulated with p53 or
MAGE-A4 protein were evaluated using IFN-γ/IL-10 double-color
ELISPOT assays (Fig. 2). Of the 18
patients with HNSCC, 2 (11.1%) and 9 (50.0%) patients showed
p53-specific IFN-γ and IL-10 production, respectively. Meanwhile,
MAGE-A4 specific IFN-γ and IL-10 production were detected in 4
(28.6%) and 7 (50.0%) of 14 patients (Table I). Three patients (pt-8, 12, 16)
exhibited both IFN-γ and IL-10 production in response to the same
tumor antigen. In the p53-specific responses, IL-10-producing T
cells were observed in significantly more patients than IFN-γ
producing T cells (P=0.0275, Table
II). There was no significant correlation between clinical
factors and tumor antigen-specific IL-10 production (Table SI). To evaluate the prognostic
significance of p53-specific IL-10 production, Kaplan-Meier
survival analyses were performed for patients with and without
p53-specific IL-10 production (Fig.
S2). Although patients with p53-specific IL-10 production
appeared to have a better prognosis for overall survival, the
difference was non-significant (overall survival, P=0.2518;
relapse-free survival, P=0.5868).
 | Table II.Tumor antigen-specific T-cell
responses in patients with HNSCC. |
Table II.
Tumor antigen-specific T-cell
responses in patients with HNSCC.
|
|
| T-cell
responses |
|
|---|
|
|
|
|
|
|---|
| Tumor antigen | Cytokine | Positive | Negative | P-value |
|---|
| p53 | IFN-γ | 2 | 16 | 0.0275 |
|
| IL-10 | 9 | 9 |
|
| MAGE-A4 | IFN-γ | 4 | 10 | 0.4401 |
|
| IL-10 | 7 | 7 |
|
Comparison of proportions of T-cells
expressing immune checkpoint molecules
To further elucidate the immunological context
underlying tumor antigen-specific IL-10 production, we focused on
p53-specific IL-10 production and determined the proportion of T
cells expressing immune checkpoint molecules in the peripheral
blood. In both CD4+ and CD8+ T cells, the
proportion of T cells expressing Lag-3 was significantly lower in
patients who exhibited p53-specific IL-10 production than in those
who did not, as shown in Fig.
3.
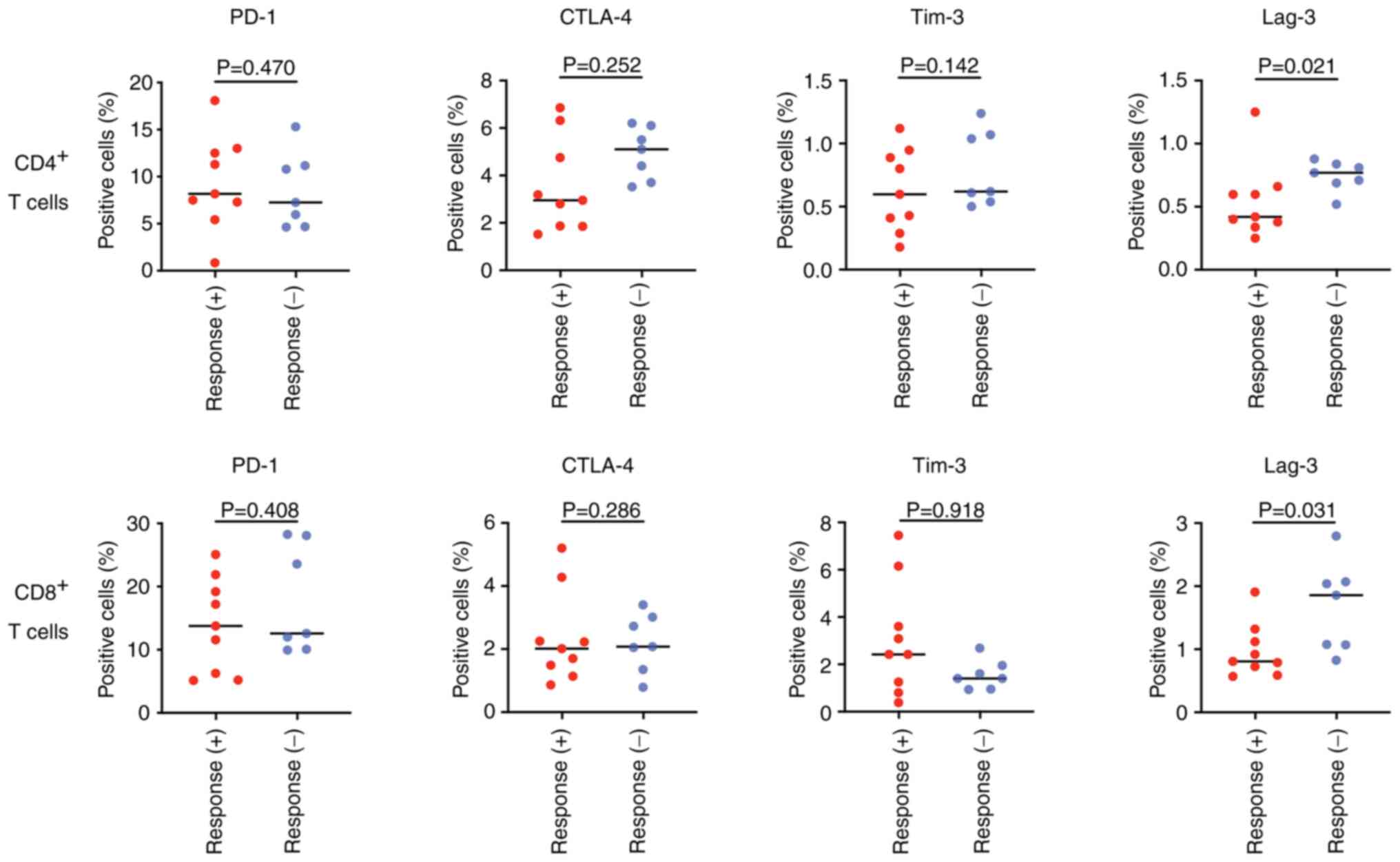 | Figure 3.Comparison of the proportion of the
immune checkpoint molecules, PD-1, CTLA-4, Tim-3 and Lag-3
expressing T cells, for each CD4+ and CD8+ T
cells. Response (+), positive for p53-specific IL-10 production;
Response (−), negative for p53-specific IL-10 production. PD-1,
programmed cell death-1; CTLA-4, cytotoxic T-lymphocyte-associated
antigen-4; Tim-3, T-cell immunoglobulin and mucin-domain
containing-3; Lag-3, lymphocyte activation gene-3. |
Enhancement of tumor antigen-specific
IL-10 production by blockade of Lag-3
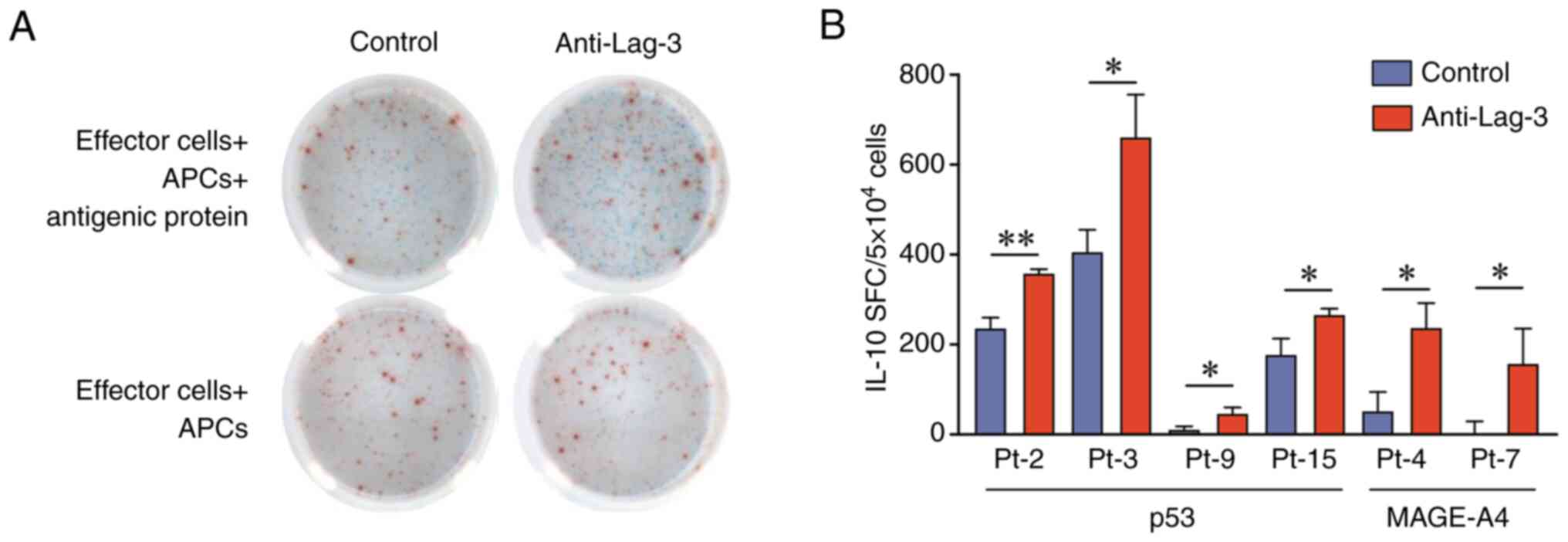
Finally, we investigated whether anti-Lag-3
antibodies enhanced tumor antigen-specific IL-10 production. In
some patients, Lag-3 blockade significantly enhanced tumor
antigen-specific IL-10 production (Fig.
4A and B). Meanwhile, Lag-3 blockade failed to enhance p53 and
MAGE-A4-specific IFN-γ production in all patients (Fig. S3).
Discussion
In the current study, we demonstrated that tumor
antigen-specific IL-10-producing T cells circulate in the
peripheral blood of patients with HNSCC, and their detection rate
was significantly higher than that of IFN-γ-producing T cells. In
patients with HNSCC, similar to other types of cancers, various
immunosuppressive mechanisms are activated, and the functions of
relevant effector cells are suppressed not only within the tumor
sites but also at systemic sites, including the peripheral blood,
bone marrow, and lymph nodes (18–20).
Our results may reflect the systemic immunosuppressive status of
patients with HNSCC. In some patients whose tumors did not express
p53 or MAGE-A4, we detected the presence of tumor antigen-specific
T-cells. Consistently, Heusinkveld et al (21) and Hoffmann et al (22,23)
reported similar findings regarding p53. The authors discussed the
possibility that p53-negative tumors represent immune escape
variants and/or harbor p53 mutations that do not result in
overexpression. There was no significant association between tumor
antigen-specific IL-10-producing T cells and clinical factors,
including prognosis, mirroring the high heterogeneity of
IL-10-producing T cells and the dual function of IL-10 in tumor
promotion and suppression. To date, high serum IL-10 levels have
been associated with poor prognosis in several malignancies,
including gastric cancer (24),
malignant myeloma (25), and lung
cancer (26). In contrast, studies
have shown that serum IL-10 levels do not correlate with prognosis
(27–29). More recently, a relationship between
IL-10 and the clinical benefits of immune checkpoint inhibitors has
been reported (30,31). The level of serum IL-10 and
percentage of CD4+ IL-10+ PBMCs were
associated with prognosis and treatment response in patients
treated with immune checkpoint inhibitors, respectively. However,
IL-10 is produced not only by tumor antigen-specific T cells but
also by several immune cells, including dendritic cells,
macrophages, B cells, and neutrophils (32). To clarify the clinical significance
of tumor antigen-specific IL-10 production in patients with cancer,
it is necessary to consider the types of tumor antigens and
CD4+ T cell subsets that produce IL-10. Furthermore, it
is also essential to elucidate the relationship between
IL-10-producing T cells within the tumor microenvironment and tumor
characteristics such as PD-L1 expression, tumor mutation burden,
and microsatellite instability.
Notably, the proportion of Lag-3+ T-cells
was significantly lower in patients with p53-specific
IL-10-producing T cells. Lag-3 is an immune inhibitory checkpoint
expressed on exhausted CD4+ and CD8+ T cells
in the context of persistent tumor antigen stimulation, as well as
on immune regulatory cells, including Tregs and Tr1 cells (33,34).
Therefore, in addition to the exhausted status of CD4+
and CD8+ T cells, Lag-3+ regulatory cells may
be involved in tumor antigen-specific IL-10-producing T-cell
responses. Particularly, CD4+ Tr1 cells are induced in
the periphery upon antigen stimulation, producing high amounts of
IL-10 and exhibiting robust immunosuppressive effects (35). As expected, the blockade of Lag-3
could reinvigorate tumor antigen-specific IL-10 production in some
patients, suggesting the existence of a mechanism through which
IL-10 production from T cells may be suppressed by IL-10 from
Lag-3+ regulatory cells. Conversely, tumor antigen-specific IFN-γ
production was not induced. Matsuzaki et al have reported
that CD8+Lag-3+PD-1+ T cells were
more impaired in IFN-γ/tumor necrosis factor (TNF)-α production
than Lag-3+PD-1− or
Lag-3−PD-1− T cell subsets in
NY-ESO-1-specific CD8+ T cells derived from patients
with ovarian cancer; therefore, dual blockade of PD-1 and Lag-3
efficiently augmented cytokine production of tumor antigen-specific
CD8+ T cells (36).
Thus, Lag-3 blockade alone may be insufficient to induce and
activate tumor antigen-specific IFN-γ production.
The present study has several limitations other than
the small number of cases. As naïve CD4+ Th cells
differentiate into different subsets of Th cells depending on the
cytokine milieu, the Th cell balance continuously changes depending
on the immune status and/or composition of the tumor
microenvironment. Moreover, Bonertz et al demonstrated that
the repertoires of tumor antigens recognized by Tregs and
effector/memory T cells differ in patients with colorectal cancer
(14). Thus, tumor antigen-specific
T cells that produce IL-10 may exhibit distinct behaviors depending
on whether they are effector or regulatory T cells. To identify the
type of Th cells or Tr1 producing IL-10 in response to tumor
antigens, further analyses, such as single-cell proteomic analysis,
are required.
To the best of our knowledge, this is the first
report to indicate that tumor antigen-specific IL-10-producing T
cells are present in the peripheral blood of patients with HNSCC.
Nevertheless, it remains unclear whether these tumor
antigen-specific IL-10-producing T cells function as effectors or
regulatory cells. Lag-3+ T cells play an important role
in modulating IL-10-producing T cells. These findings provide new
insights into the roles of IL-10 and Lag-3 in mediating antitumor
immune responses.
Supplementary Material
Supporting Data
Supporting Data
Acknowledgements
Not applicable.
Funding
This work was supported in part by a Grant-in-Aid for Scientific
Research (grant nos. 22K16894 to HTad, 23K08956 to YT and 20H03834
to KC) from the Ministry of Education, Culture, Sports, Science,
and Technology, Japan.
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
KC conceived and designed the study. MH, KM, HTak
and HTad acquired the data. KC and ST confirm the authenticity of
all the raw data. KC, HTak, YT, MM, TO, ST and KC performed data
analysis and interpretation. KC wrote the manuscript. All authors
have read and approved the final version of the manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of Gunma University Hospital (approval no. HS2017-152).
Written informed consent was obtained from all patients.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
IL-10
|
interleukin-10
|
|
PBMC
|
peripheral blood mononuclear cell
|
|
IFN-γ
|
interferon-γ
|
|
ELISPOT
|
enzyme-linked immunosorbent spot
|
|
Lag-3
|
lymphocyte activation gene-3
|
|
HNSCC
|
head and neck squamous cell
carcinoma
|
|
Treg
|
regulatory T-cell
|
|
Th
|
T helper
|
|
Tr1
|
T regulatory type 1
|
|
SFC
|
spot-forming-cells
|
|
PD-1
|
programmed cell death-1
|
|
CTLA-4
|
cytotoxic T-lymphocyte-associated
antigen-4
|
|
Tim-3
|
T-cell immunoglobulin and mucin-domain
containing-3
|
|
TNF-α
|
tumor necrosis factor-α
|
References
|
1
|
Carlini V, Noonan DM, Abdalalem E, Goletti
D, Sansone C, Calabrone L and Albini A: The multifaceted nature of
IL-10: Regulation, role in immunological homeostasis and its
relevance to cancer, COVID-19 and post-COVID conditions. Front
Immunol. 14:11610672023. View Article : Google Scholar
|
|
2
|
Mocellin S, Panelli MC, Wang E, Nagorsen D
and Marincola FM: The dual role of IL-10. Trends Immunol. 24:36–43.
2003. View Article : Google Scholar
|
|
3
|
Saraiva M, Vieira P and O'Garra A: Biology
and therapeutic potential of interleukin-10. J Exp Med.
217:e201904182020. View Article : Google Scholar
|
|
4
|
Fujimura T, Kambayashi Y and Aiba S:
Crosstalk between regulatory T cells (Tregs) and myeloid derived
suppressor cells (MDSCs) during melanoma growth. Oncoimmunology.
1:1433–1434. 2012. View Article : Google Scholar
|
|
5
|
Pan Y, Yu Y, Wang X and Zhang T:
Tumor-associated macrophages in tumor immunity. Front Immunol.
11:5830842020. View Article : Google Scholar
|
|
6
|
Emmerich J, Mumm JB, Chan IH, LaFace D,
Truong H, McClanahan T, Gorman DM and Oft M: IL-10 directly
activates and expands tumor-resident CD8(+) T cells without de novo
infiltration from secondary lymphoid organs. Cancer Res.
72:3570–3581. 2012. View Article : Google Scholar
|
|
7
|
Guo Y, Xie YQ, Gao M, Zhao Y, Franco F,
Wenes M, Siddiqui I, Bevilacqua A, Wang H, Yang H, et al: Metabolic
reprogramming of terminally exhausted CD8+ T cells by
IL-10 enhances anti-tumor immunity. Nat Immunol. 22:746–756. 2021.
View Article : Google Scholar
|
|
8
|
Oft M: IL-10: Master switch from
tumor-promoting inflammation to antitumor immunity. Cancer Immunol
Res. 2:194–199. 2014. View Article : Google Scholar
|
|
9
|
Mannino MH, Zhu Z, Xiao H, Bai Q,
Wakefield MR and Fang Y: The paradoxical role of IL-10 in immunity
and cancer. Cancer Lett. 367:103–107. 2015. View Article : Google Scholar
|
|
10
|
Jankovic D, Kugler DG and Sher A: IL-10
production by CD4+ effector T cells: A mechanism for
self-regulation. Mucosal Immunol. 3:239–246. 2010. View Article : Google Scholar
|
|
11
|
Roncarolo MG, Gregori S, Bacchetta R,
Battaglia M and Gagliani N: The biology of T regulatory type 1
cells and their therapeutic application in immune-mediated
diseases. Immunity. 49:1004–1019. 2018. View Article : Google Scholar
|
|
12
|
Vence L, Palucka AK, Fay JW, Ito T, Liu
YJ, Banchereau J and Ueno H: Circulating tumor antigen-specific
regulatory T cells in patients with metastatic melanoma. Proc Natl
Acad Sci USA. 104:20884–20889. 2007. View Article : Google Scholar
|
|
13
|
Wang HY, Peng G, Guo Z, Shevach EM and
Wang RF: Recognition of a new ARTC1 peptide ligand uniquely
expressed in tumor cells by antigen-specific CD4+ regulatory T
cells. J Immunol. 174:2661–2670. 2005. View Article : Google Scholar
|
|
14
|
Bonertz A, Weitz J, Pietsch DHK, Rahbari
NN, Schlude C, Ge Y, Juenger S, Vlodavsky I, Khazaie K, Jaeger D,
et al: Antigen-specific Tregs control T cell responses against a
limited repertoire of tumor antigens in patients with colorectal
carcinoma. J Clin Invest. 119:3311–3321. 2009.
|
|
15
|
Yamada K, Masuda K, Ida S, Tada H, Bando
M, Abe K, Tatematsu KI, Sezutsu H, Oyama T, Chikamatsu K and Takeda
S: In vitro assessment of antitumor immune responses using tumor
antigen proteins produced by transgenic silkworms. J Mater Sci
Mater Med. 32:582021. View Article : Google Scholar
|
|
16
|
Tada H, Takahashi H, Yamada K, Masuda K,
Nagata Y, Uchida M, Shino M, Ida S, Mito I, Matsuyama T, et al:
Dynamic alterations of circulating T lymphocytes and the clinical
response in patients with head and neck squamous cell carcinoma
treated with nivolumab. Cancer Immunol Immunother. 71:851–863.
2022. View Article : Google Scholar
|
|
17
|
Nagorsen D, Keilholz U, Rivoltini L,
Schmittel A, Letsch A, Asemissen AM, Berger G, Buhr HJ, Thiel E and
Scheibenbogen C: Natural T-cell response against MHC class I
epitopes of epithelial cell adhesion molecule, her-2/neu, and
carcinoembryonic antigen in patients with colorectal cancer. Cancer
Res. 60:4850–4854. 2000.
|
|
18
|
Duray A, Demoulin S, Hubert P, Delvenne P
and Saussez S: Immune suppression in head and neck cancers: A
review. Clin Dev Immunol. 2010:7016572010.
|
|
19
|
Kostecki KL, Iida M, Crossman BE, Salgia
R, Harari PM, Bruce JY and Wheeler DL: Immune escape strategies in
head and neck cancer: Evade, resist, inhibit, recruit. Cancers
(Basel). 16:3122024. View Article : Google Scholar
|
|
20
|
Elmusrati A, Wang J and Wang CY: Tumor
microenvironment and immune evasion in head and neck squamous cell
carcinoma. Int J Oral Sci. 13:242021. View Article : Google Scholar
|
|
21
|
Heusinkveld M, Goedemans R, Briet RJP,
Gelderblom H, Nortier JWR, Gorter A, Smit VTHBM, Langeveld APM,
Jansen JC and van der Burg SH: Systemic and local human
papillomavirus 16-specific T-cell immunity in patients with head
and neck cancer. Int J Cancer. 131:E74–E85. 2012. View Article : Google Scholar
|
|
22
|
Hoffmann TK, Donnenberg AD, Finkelstein
SD, Donnenberg VS, Friebe-Hoffmann U, Myers EN, Appella E, DeLeo AB
and Whiteside TL: Frequencies of tetramer+ T cells specific for the
wild-type sequence p53(264–272) peptide in the circulation of
patients with head and neck cancer. Cancer Res. 62:3521–3529.
2002.
|
|
23
|
Hoffmann TK, Nakano K, Elder EM, Dworacki
G, Finkelstein SD, Appella E, Whiteside TL and DeLeo AB: Generation
of T cells specific for the wild-type sequence p53(264–272) peptide
in cancer patients: Implications for immunoselection of epitope
loss variants. J Immunol. 165:5938–5944. 2000. View Article : Google Scholar
|
|
24
|
Ikeguchi M, Hatada T, Yamamoto M, Miyake
T, Matsunaga T, Fukumoto Y, Yamada Y, Fukuda K, Saito H and Tatebe
S: Serum interleukin-6 and −10 levels in patients with gastric
cancer. Gastric Cancer. 12:95–100. 2009. View Article : Google Scholar
|
|
25
|
Wang H, Wang L, Chi PD, Wang WD, Chen XQ,
Geng QR, Xia ZJ and Lu Y: High level of interleukin-10 in serum
predicts poor prognosis in multiple myeloma. Br J Cancer.
114:463–468. 2016. View Article : Google Scholar
|
|
26
|
De Vita F, Orditura M, Galizia G, Romano
C, Roscigno A, Lieto E and Catalano G: Serum interleukin-10 levels
as a prognostic factor in advanced non-small cell lung cancer
patients. Chest. 117:365–373. 2000. View Article : Google Scholar
|
|
27
|
Cortes JE, Talpaz M, Cabanillas F, Seymour
JF and Kurzrock R: Serum levels of interleukin-10 in patients with
diffuse large cell lymphoma: Lack of correlation with prognosis.
Blood. 85:2516–2520. 1995. View Article : Google Scholar
|
|
28
|
Evans C, Morrison I, Heriot AG, Bartlett
JB, Finlayson C, Dalgleish AG and Kumar D: The correlation between
colorectal cancer rates of proliferation and apoptosis and systemic
cytokine levels; plus their influence upon survival. Br J Cancer.
94:1412–1419. 2006. View Article : Google Scholar
|
|
29
|
Green VL, Irune E, Prasai A, Alhamarneh O,
Greenman J and Stafford ND: Serum IL10, IL12 and circulating
CD4+CD25high T regulatory cells in relation to long-term clinical
outcome in head and neck squamous cell carcinoma patients. Int J
Oncol. 40:833–839. 2012.
|
|
30
|
Kim Y, Yang H, Lee WS, Cheon J, Sang YB,
Kang B, Chon HJ and Kim C: High levels of baseline serum IL-10 are
associated with reduced clinical benefit from first-line immune
checkpoint inhibitor therapy in advanced renal cell carcinoma. J
Cancer. 14:935–942. 2023. View Article : Google Scholar
|
|
31
|
Giunta EF, Barra G, De Falco V, Argenziano
G, Napolitano S, Vitale P, Zanaletti N, Terminiello M, Martinelli
E, Morgillo F, et al: Baseline IFN-γ and IL-10 expression in PBMCs
could predict response to PD-1 checkpoint inhibitors in advanced
melanoma patients. Sci Rep. 10:176262020. View Article : Google Scholar
|
|
32
|
Rutz S and Ouyang W: Regulation of
interleukin-10 expression. Adv Exp Med Biol. 941:89–116. 2016.
View Article : Google Scholar
|
|
33
|
Huo JL, Wang YT, Fu WJ, Lu N and Liu ZS:
The promising immune checkpoint LAG-3 in cancer immunotherapy: From
basic research to clinical application. Front Immunol.
13:9560902022. View Article : Google Scholar
|
|
34
|
Joller N and Kuchroo VK: Tim-3, Lag-3, and
TIGIT. Curr Top Microbiol Immunol. 410:127–156. 2017.
|
|
35
|
Gagliani N, Magnani CF, Huber S, Gianolini
ME, Pala M, Licona-Limon P, Guo B, Herbert DR, Bulfone A, Trentini
F, et al: Coexpression of CD49b and LAG-3 identifies human and
mouse T regulatory type 1 cells. Nat Med. 19:739–746. 2013.
View Article : Google Scholar
|
|
36
|
Matsuzaki J, Gnjatic S, Mhawech-Fauceglia
P, Beck A, Miller A, Tsuji T, Eppolito C, Qian F, Lele S, Shrikant
P, et al: Tumor-infiltrating NY-ESO-1-specific CD8+ T cells are
negatively regulated by LAG-3 and PD-1 in human ovarian cancer.
Proc Natl Acad Sci USA. 107:7875–7880. 2010. View Article : Google Scholar
|