Introduction
Gastric cancer (GC) is the third leading cause of
cancer-related mortality worldwide, with a particularly high
incidence in East Asia (men, 32.5%; women, 13.2%) (1). Despite recent advances in the
diagnosis and treatment of GC, a poor prognosis for unresectable
advanced GC and metastatic or recurrent GC persists (2,3).
Sarcopenia, characterized by the progressive loss of
skeletal muscle mass and function, has emerged as a novel
prognostic factor of patients with cancer (4). The association of sarcopenia with a
worse prognosis of GC has been reported across several types of
cancers and treatment modalities (5,6).
Common methods for assessing skeletal muscle index (SMI) and
quality include dual-energy X-ray absorptiometry (7) and bioelectrical impedance analysis
(8). Furthermore, novel methods
using computed tomography (CT) to measure CT-derived SMI and
skeletal muscle radiodensity (SMD) have been reported (9,10).
Furthermore, several studies have highlighted SMI and SMD as
prognostic indicators in patients with cancer (11,12).
Thus, the combination of SMI and SMD may serve as a prognostic
factor or indicate the risk of comorbidities by assessing total
muscle mass and quality (13,14).
However, the relationship between the combination of SMI and SMD
and prognosis in patients with GC has not been fully investigated.
Therefore, the present study aimed to determine the relationship
between preoperative SMI and SMD and prognosis in patients with
GC.
Materials and methods
Patients
In total, 540 patients with GC were enrolled in the
present study at the Kanagawa Cancer Center (Yokohama, Japan) from
December 2013 to November 2017. Eligibility criteria for patients
were as follows: i) Age >20 years; ii) no history of cancer;
iii) pathologically confirmed gastric adenocarcinoma or
gastroesophageal junction adenocarcinoma; iv) no treatment before
surgery; v) Eastern Cooperative Oncology Group performance status
(15) of 0–2; vi) CT scans
performed within 1 month before surgery; and vii) gastrectomy with
R0 resection, ensuring complete removal of all cancerous tissue
with no visible or microscopic residual tumor at the primary site.
The exclusion criteria were as follows: i) Essential data were
missing; ii) gastrectomy with R0 resection was not performed; iii)
pathological assessment revealed neuroendocrine tumor involvement;
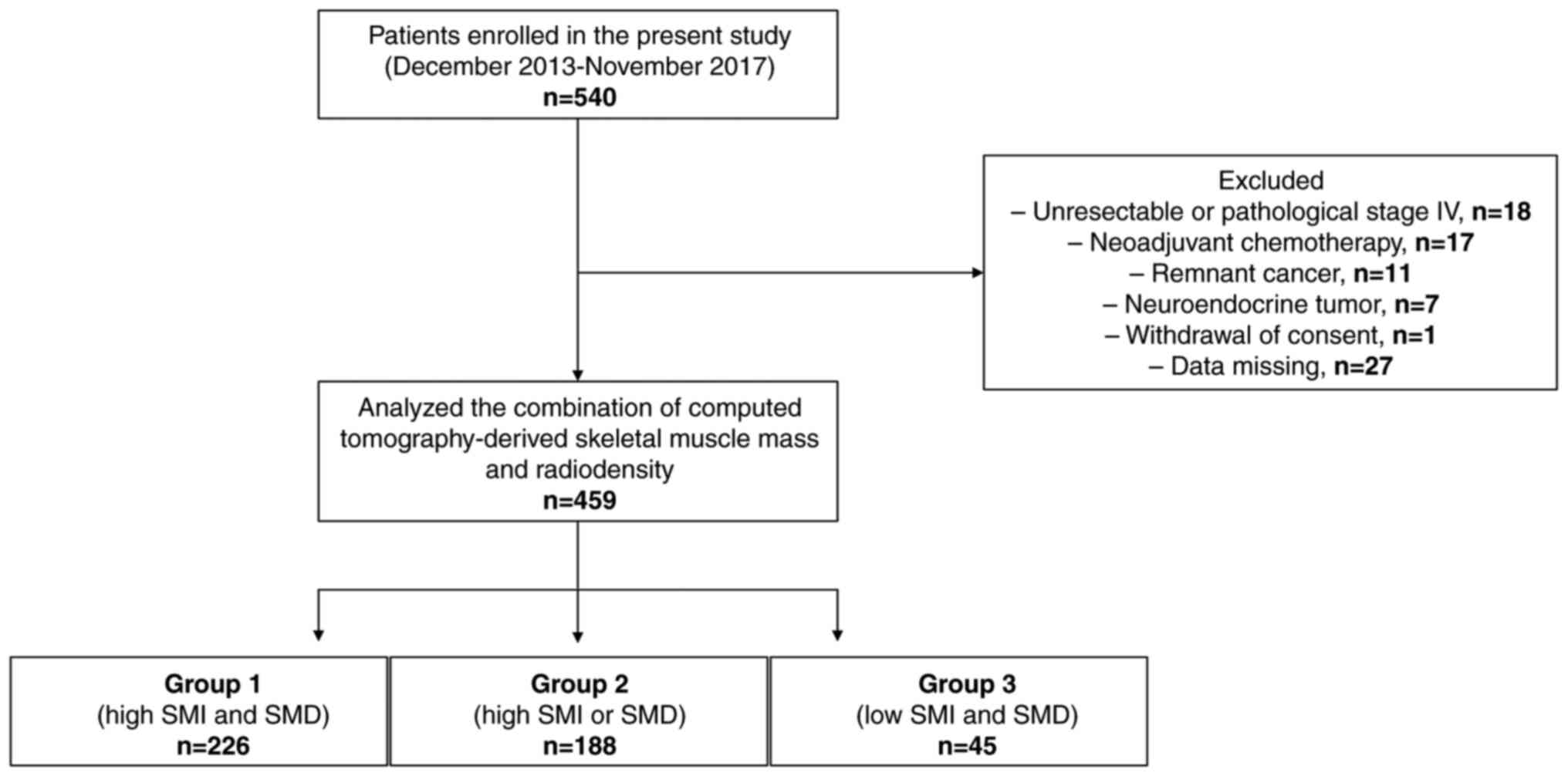
and iv) consent was withdrawn. Of the 540 patients enrolled, 81
were excluded and 459 (300 men and 159 women) were included in the
present study (Fig. 1). The median
age was 68 years (range, 32–90 years).
The present study was approved by the Ethics
Committee of Kanagawa Cancer Center (Yokohama, Japan; approval no.
25 Research-20). All patients provided informed consent, and this
study adhered to the ethical guidelines outlined in the 1996
Declaration of Helsinki.
Image analysis
In accordance with previous studies (16,17),
the SliceOmatic 5.0, Revision 9 graphics program (Tomovision) and
ABACS (version 9; Voronoi Health Analytics Incorporated) were used
to analyze skeletal muscle mass and radiodensity from preoperative
CT images (Aquilion 64 CT Scanner; Canon Medical Systems
Corporation). The threshold range was −29-150 Hounsfield units (HU)
for skeletal muscle. The SMI was calculated based on patient height
(m2). The SMD was calculated as the average HU of all
skeletal muscles at the level of L3.
Cutoff values for SMI and SMD
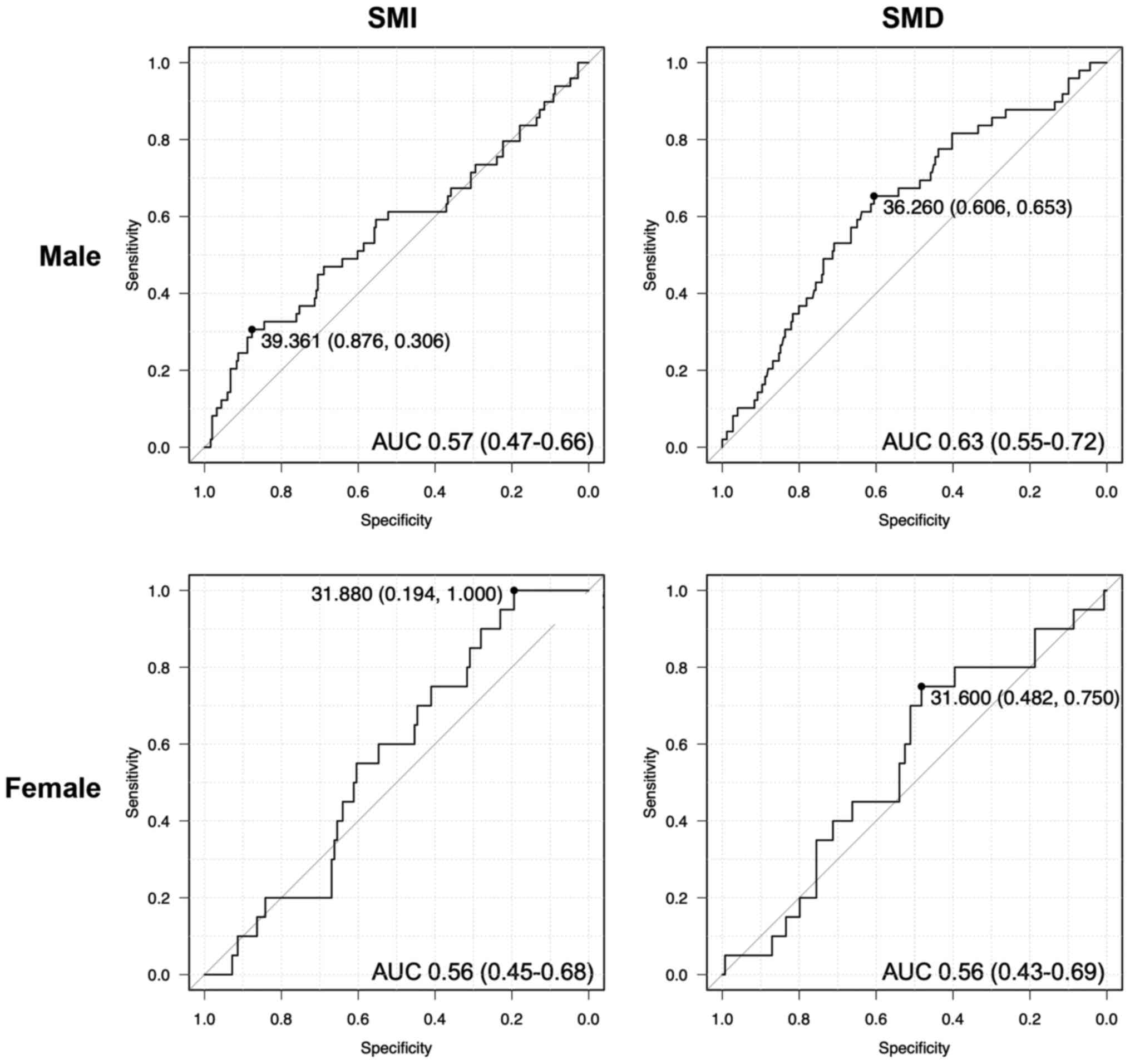
The SMI and SMD values demonstrate marked sex
differences (18). Therefore, using
receiver operating characteristic curve analysis of 5-year survival
and mortality outcome data, sex-specific cutoff values were
calculated. The cutoff values for SMI were 39.4 for men [area under
the curve (AUC), 0.57; 95% confidence interval (CI), 0.47–0.66] and
31.9 for women (AUC, 0.56; 95% CI, 0.45–0.68; Fig. 2). The cut-off values for SMD were
36.3 for men (AUC, 0.63; 95% CI, 0.55–0.72) and 31.6 for women
(AUC, 0.56; 95% CI, 0.43–0.69; Fig.
2). Based on these values, patients were categorized into the
following groups based on high and low SMI and SMD: Group 1, high
SMI and SMD; Group 2, high SMI or SMD; and Group 3, low SMI and
SMD.
Statistical analysis
Continuous variables are presented as median ±
standard deviation and were evaluated nonparametrically using the
Kruskal-Wallis test and the Steel-Dwass test. Categorical variables
were analyzed using the χ2 test or Fisher's exact test,
as appropriate. Correlation between SMI and SMD was analyzed using
Spearman's rank correlation test. Kaplan-Meier analysis and the
log-rank test were used to assess overall survival (OS) and
relapse-free survival (RFS). Statistically significant variables
(P<0.05) in the univariate analysis were included in
multivariate regression analysis, with results reported as hazard
ratios (HR) and 95% CIs. P<0.05 was considered to indicate a
statistically significant difference. EZR (version 1.68, Saitama
Medical Center, Jichi Medical University), a graphical user
interface for R (The R Foundation for Statistical Computing), was
used for all statistical analyses.
Results
Correlation between SMI and SMD
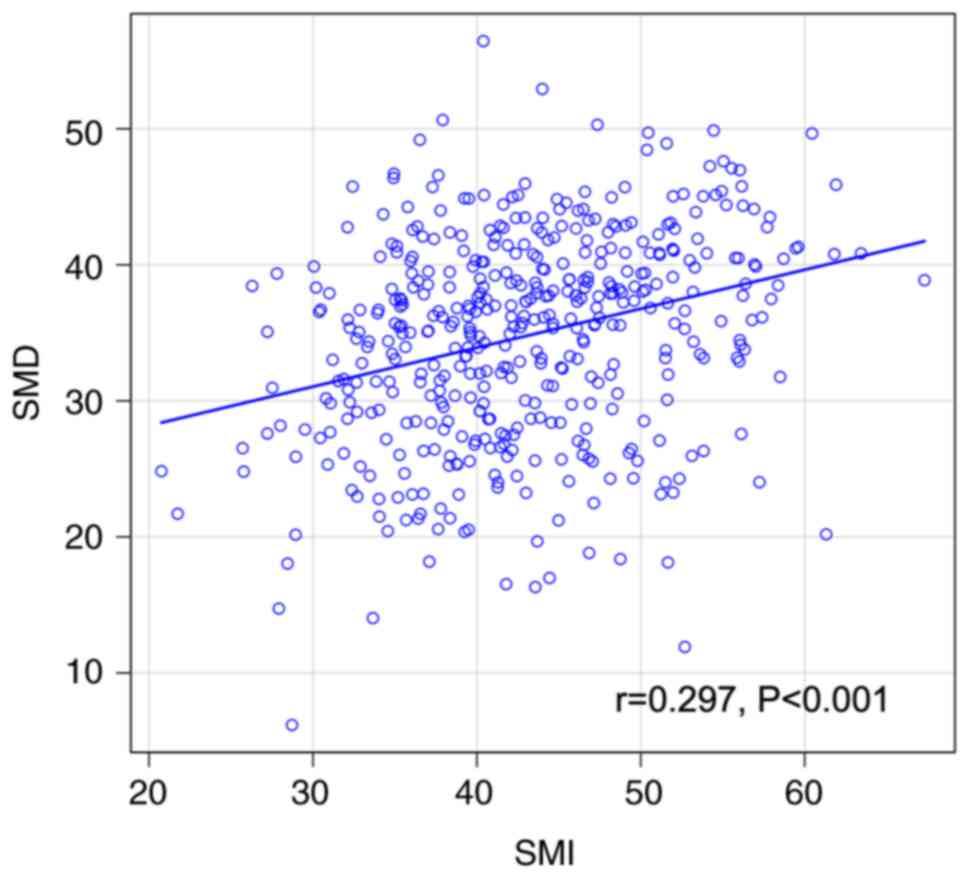
The results revealed a significant but weak positive
correlation between SMI and SMD (r=0.297; P<0.001; Fig. 3).
OS and RFS based on SMI and SMD after
gastrectomy
OS rates were notably lower in the low-SMI group
than in the high-SMI group; however, the difference was not
significant (79.1% vs. 87.8%, respectively; P=0.06; Fig. 4A). However, OS rates were
significantly lower in the low-SMD group than in the high-SMD group
(83.4% vs. 88.8%, respectively; P=0.04; Fig. 4B). There was no significant
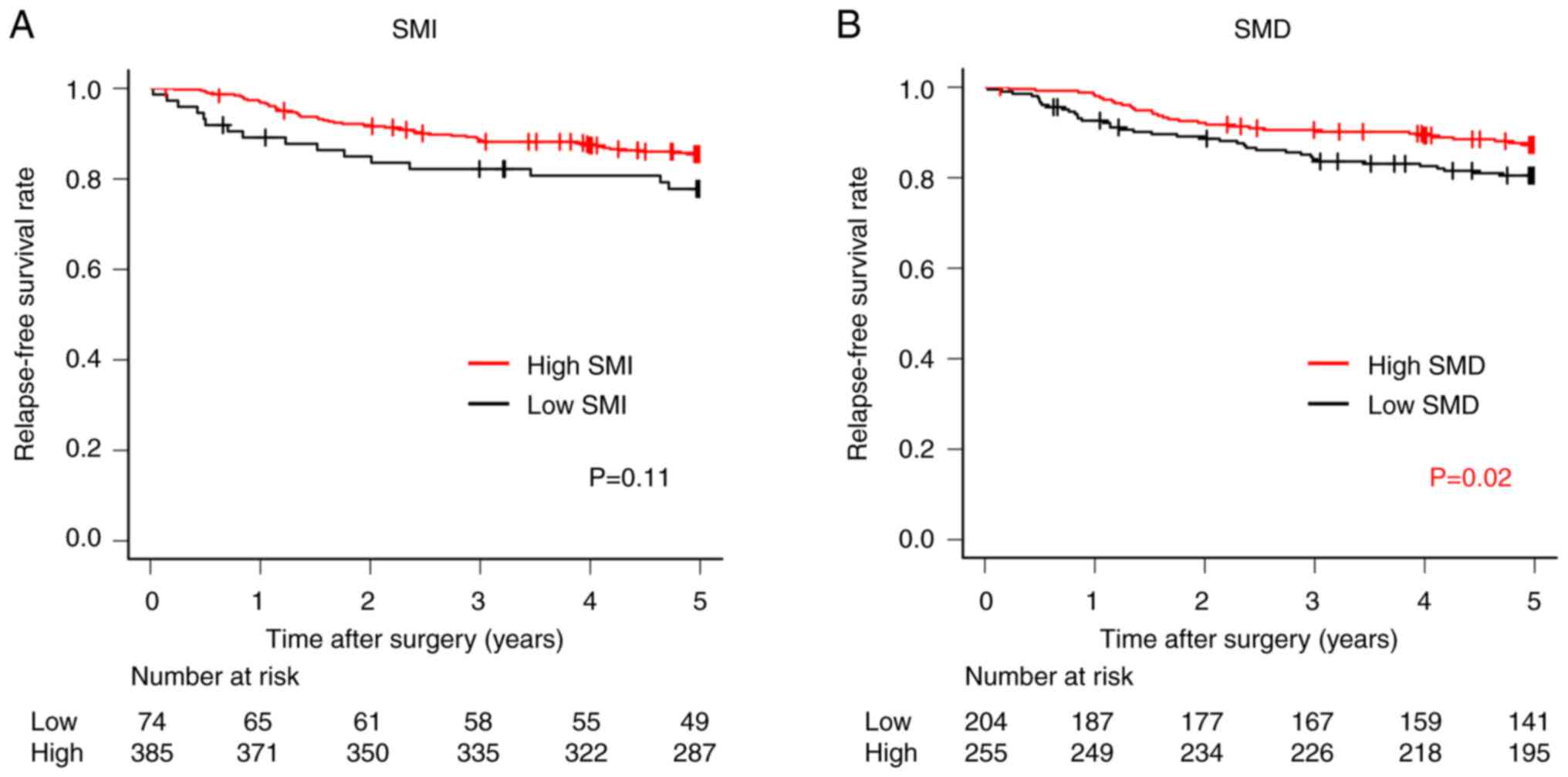
difference in RFS rates between the high- and low-SMI groups (77.8%
vs. 85.5%, respectively; P=0.11; Fig.
5A). However, RFS rates were significantly lower in the low-SMD
group than in the high-SMD group (80.5% vs. 87.2%, respectively;
P=0.02; Fig. 5B).
Combined analysis of SMI and SMD
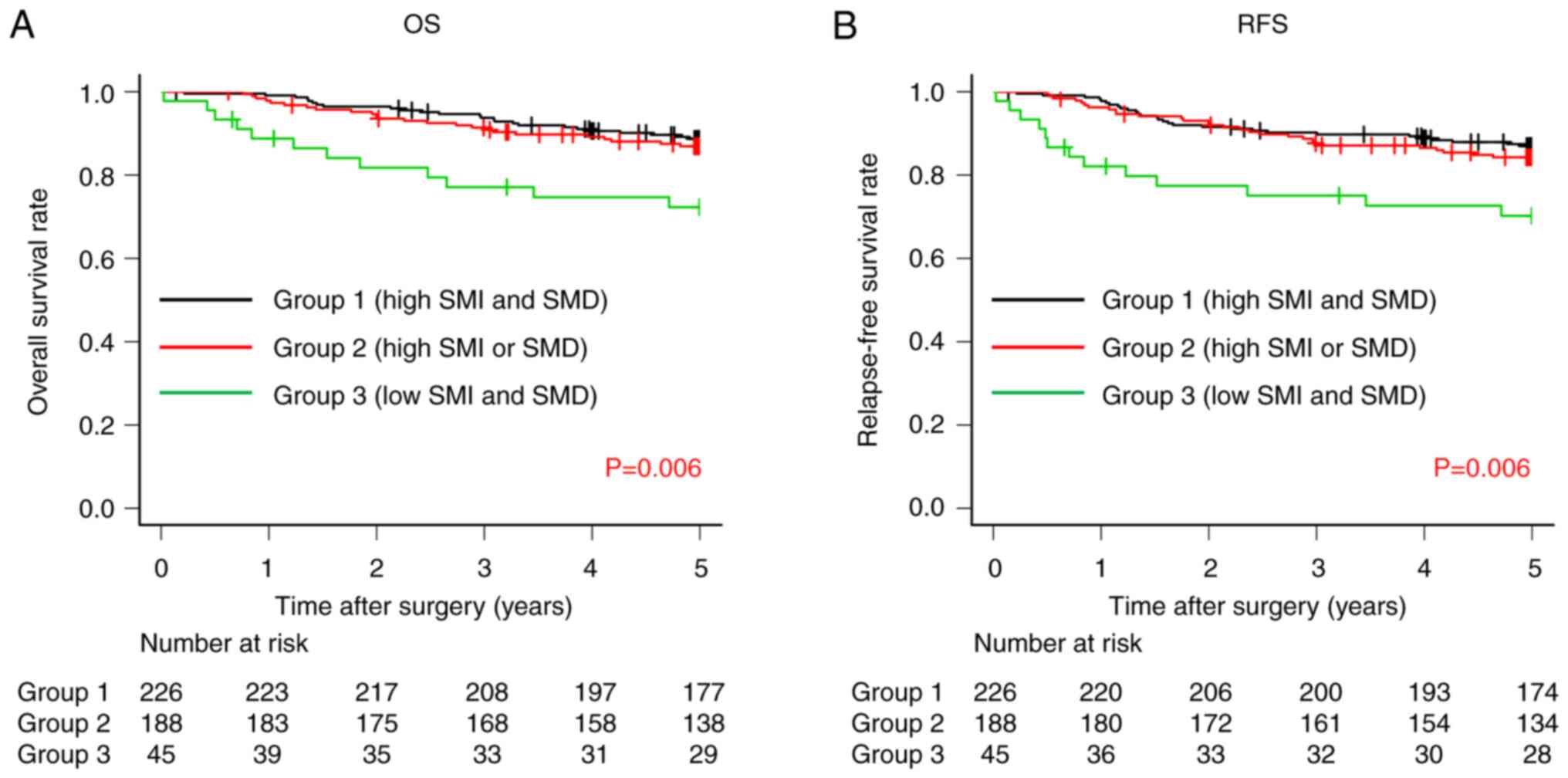
Both OS and RFS rates were significantly lower in
Group 3 compared with Groups 2 and 1 (OS, 72.3% vs. 86.9% vs.
88.7%, respectively; P=0.006; Fig.
6A and RFS, 70.2% vs. 84.3% vs. 87.0%, respectively; P=0.006;
Fig. 6B).
Comparison of the association between
clinicopathologic factors and SMI and SMD between groups
Table I presents the
clinicopathologic factors and SMI and SMD between groups. The
results revealed that patients in Group 3 were significantly older
(P<0.001), had a significantly lower body mass index (BMI;
P<0.001), significantly lower preoperative albumin levels
(P<0.001), significantly lower preoperative Prognostic
Nutritional Index (PNI) values (P<0.001), and significantly
worse histological type (P<0.001) than those in Groups 1 and
2.
 | Table I.Association between
clinicopathological factors and the combination of computed
tomography-derived skeletal muscle index and radiodensity. |
Table I.
Association between
clinicopathological factors and the combination of computed
tomography-derived skeletal muscle index and radiodensity.
| Variable | Group 1
(n=226) | Group 2
(n=188) | Group 3 (n=45) | P-value |
|---|
| Age |
|
|
| <0.001 |
| <65
years | 100 (44.2) | 33 (17.6) | 7 (15.6) |
|
| ≥65
years | 126 (55.8) | 155 (82.4) | 38 (84.4) |
|
| Sex |
|
|
| 0.527 |
|
Male | 149 (65.9) | 125 (66.5) | 26 (57.8) |
|
|
Female | 77 (34.1) | 63 (33.5) | 19 (42.2) |
|
| BMI |
|
|
| <0.001 |
|
<18.5 kg/m2 | 24 (10.6) | 12 (6.4) | 11 (24.4) |
|
| ≤18.5,
<25.0 kg/m2 | 159 (70.4) | 119 (63.3) | 33 (73.3) |
|
| ≥25
kg/m2 | 43 (19.0) | 57 (30.3) | 1 (2.2) |
|
| Pre Alb, median
(SD) | 4.2 (0.3) | 4.0
(0.4)a | 4.0
(0.4)a | <0.001 |
| Pre PNI, median
(SD) | 50.5 (4.7) | 48.7
(5.0)a | 47.3
(4.8)a | <0.001 |
| Pre NLR, median
(SD) | 2.0 (1.2) | 2.0 (1.8) | 2.3 (1.6) | 0.067 |
| Pre CRP, median
(SD) | 0.06 (0.16) | 0.09
(0.21)a | 0.10 (0.37) | 0.006 |
| Total
gastrectomy |
|
|
| 0.177 |
| No | 180 (79.6) | 135 (71.8) | 34 (75.6) |
|
|
Yes | 46 (20.4) | 53 (28.2) | 11 (24.4) |
|
| Tumor size |
|
|
| 0.032 |
| ≤30
mm | 125 (55.3) | 84 (44.7) | 28 (62.2) |
|
| >30
mm | 101 (44.7) | 104 (55.3) | 17 (37.8) |
|
| Histological
type |
|
|
| <0.001 |
| Well
moderate | 93 (41.2) | 113 (60.1) | 20 (44.4) |
|
|
Poorly | 133 (58.8) | 75 (39.9) | 25 (55.6) |
|
| Lymphatic
invasion |
|
|
| 0.340 |
| No | 163 (72.1) | 124 (66.0) | 33 (73.3) |
|
|
Yes | 63 (27.9) | 64 (34.0) | 12 (26.7) |
|
| Venous
invasion |
|
|
| 0.340 |
| No | 140 (61.9) | 108 (57.4) | 23 (51.1) |
|
|
Yes | 86 (38.1) | 80 (42.6) | 22 (48.9) |
|
| pStage |
|
|
| 0.874 |
| I | 158 (69.9) | 128 (68.1) | 30 (66.7) |
|
|
II/III | 68 (30.1) | 60 (31.9) | 15 (33.3) |
|
| Surgical
complications |
|
|
| 0.430 |
| No | 195 (86.3) | 153 (81.8) | 38 (86.4) |
|
|
Yes | 31 (13.7) | 34 (18.2) | 6 (13.6) |
|
Univariate and multivariate analysis
of OS and RFS
Multivariate analyses for OS demonstrated that PNI
<40 [Hazard Ratio (HR), 2.22; 95% CI, 1.03–4.76; P=0.041],
pStage II–III (HR, 2.56; 95% CI, 1.35–4.84; P=0.004) and low SMI
and SMD (Group 3; HR, 2.32; 95% CI, 1.17–4.59; P=0.016) were
independent prognostic factors (Table
II). Multivariate analyses for RFS demonstrated that PNI <40
(HR, 2.63; 95% CI, 1.27–5.56; P=0.010), lymphatic invasion (HR,
2.01; 95% CI, 1.20–3.39; P=0.009), pStage II–III (HR, 2.40; 95% CI,
1.33–4.33; P=0.004) and low SMI and SMD (Group 3; HR, 2.28; 95% CI,
1.19–4.37; P=0.013) were independent prognostic factors (Table III).
 | Table II.Univariate and multivariate analyses
of clinicopathological factors and the combination of computed
tomography-derived skeletal muscle index and radiodensity for
overall survival. |
Table II.
Univariate and multivariate analyses
of clinicopathological factors and the combination of computed
tomography-derived skeletal muscle index and radiodensity for
overall survival.
|
| Univariate |
| Multivariate |
|
|---|
|
|
|
|
|
|
|---|
| Factor | HR | 95% CI | P-value | HR | 95% CI | P-value |
|---|
| Age |
|
|
|
|
|
|
| ≥65
years | 1 |
|
| 1 |
|
|
| ≥65
years | 2.05 | 1.12–3.74 | 0.020 | 1.76 | 0.93–3.33 | 0.080 |
| Sex |
|
|
|
|
|
|
|
Male | 1 |
|
|
|
|
|
|
Female | 0.77 | 0.46–1.30 | 0.325 |
|
|
|
| BMI |
|
|
|
|
|
|
|
<18.5 kg/m2 | 1 |
|
|
|
|
|
| ≤18.5,
<25.0 kg/m2 | 0.68 | 0.33–1.39 | 0.287 |
|
|
|
| ≥25
kg/m2 | 0.87 | 0.39–1.94 | 0.733 |
|
|
|
| PNI |
|
|
|
|
|
|
|
≥40 | 1 |
|
| 1 |
|
|
|
<40 | 4.35 | 2.04–9.09 | <0.001 | 2.22 | 1.03–4.76 | 0.041 |
| Total
gastrectomy |
|
|
|
|
|
|
| No | 1 |
|
| 1 |
|
|
|
Yes | 2.17 | 1.34–3.52 | 0.002 | 1.65 | 0.99–2.75 | 0.052 |
| Tumor size |
|
|
|
|
|
|
| ≤30
mm | 1 |
|
| 1 |
|
|
| >30
mm | 1.97 | 1.21–3.22 | 0.007 | 0.93 | 0.52–1.63 | 0.790 |
| Histological
type |
|
|
|
|
|
|
|
Well/moderate | 1 |
|
|
|
|
|
|
Poorly | 1.16 | 0.72–1.86 | 0.548 |
|
|
|
| Lymphatic
invasion |
|
|
|
|
|
|
| No | 1 |
|
| 1 |
|
|
|
Yes | 3.39 | 2.10–5.46 | <0.001 | 1.72 | 0.99–2.99 | 0.054 |
| Venous
invasion |
|
|
|
|
|
|
| No | 1 |
|
| 1 |
|
|
|
Yes | 3.92 | 2.33–6.60 | <0.001 | 1.75 | 0.94–3.25 | 0.075 |
| pStage |
|
|
|
|
|
|
| I | 1 |
|
| 1 |
|
|
|
II–III | 4.66 | 2.84–7.66 | <0.001 | 2.56 | 1.35–4.84 | 0.004 |
| Surgical
complications |
|
|
|
|
|
|
| No | 1 |
|
|
|
|
|
|
Yes | 0.76 | 0.37–1.53 | 0.435 |
|
|
|
| Combination of SMI
and SMD |
|
|
|
|
|
|
| Group
1 | 1 |
|
| 1 |
|
|
| Group
2 | 1.24 | 0.73–2.09 | 0.426 | 0.90 | 0.52–1.56 | 0.700 |
| Group
3 | 2.80 | 1.45–5.41 | 0.002 | 2.32 | 1.17–4.59 | 0.016 |
 | Table III.Univariate and multivariate analyses
of clinicopathological factors and the combination of computed
tomography-derived skeletal muscle index and radiodensity for
relapse free survival. |
Table III.
Univariate and multivariate analyses
of clinicopathological factors and the combination of computed
tomography-derived skeletal muscle index and radiodensity for
relapse free survival.
|
| Univariate |
| Multivariate |
|
|---|
|
|
|
|
|
|
|---|
| Factor | HR | 95% CI | P-value | HR | 95% CI | P-value |
|---|
| Age |
|
|
|
|
|
|
| <65
years | 1 |
|
| 1 |
|
|
| ≥65
years | 1.85 | 1.07–3.20 | 0.028 | 1.50 | 0.84–2.70 | 0.170 |
| Sex |
|
|
|
|
|
|
|
Male | 1 |
|
|
|
|
|
|
Female | 0.83 | 0.52–1.35 | 0.453 |
|
|
|
| BMI |
|
|
|
|
|
|
|
<18.5 kg/m2 | 1 |
|
|
|
|
|
|
≤18.5–25.0
kg/m2 | 0.66 | 0.34–1.31 | 0.239 |
|
|
|
| ≥25
kg/m2 | 0.94 | 0.45–1.99 | 0.877 |
|
|
|
| PNI |
|
|
|
|
|
|
|
≥40 | 1 |
|
| 1 |
|
|
|
<40 | 4.55 | 2.27–9.09 | <0.001 | 2.63 | 1.27–5.56 | 0.010 |
| Total
gastrectomy |
|
|
|
|
|
|
| No | 1 |
|
| 1 |
|
|
|
Yes | 1.93 | 1.22–3.06 | 0.005 | 1.50 | 0.93–2.42 | 0.097 |
| Tumor size |
|
|
|
|
|
|
| ≤30
mm | 1 |
|
| 1 |
|
|
| >30
mm | 2.06 | 1.30–3.27 | 0.002 | 1.03 | 0.60–1.75 | 0.920 |
| Histological
type |
|
|
|
|
|
|
|
Well/moderate | 1 |
|
|
|
|
|
|
Poorly | 1.19 | 0.77–1.86 | 0.434 |
|
|
|
| Lymphatic
invasion |
|
|
|
|
|
|
| No | 1 |
|
| 1 |
|
|
|
Yes | 3.45 | 2.21–5.39 | <0.001 | 2.01 | 1.20–3.39 | 0.009 |
| Venous
invasion |
|
|
|
|
|
|
| No | 1 |
|
| 1 |
|
|
|
Yes | 2.94 | 1.85–4.66 | <0.001 | 1.25 | 0.71–2.19 | 0.430 |
| pStage |
|
|
|
|
|
|
| I | 1 |
|
| 1 |
|
|
|
II–III | 4.21 | 2.67–6.64 | <0.001 | 2.40 | 1.33–4.33 | 0.004 |
| Surgical
complications |
|
|
|
|
|
|
| No | 1 |
|
|
|
|
|
|
Yes | 0.92 | 0.50–1.71 | 0.801 |
|
|
|
| Combination of SMI
and SMD |
|
|
|
|
|
|
| Group
1 | 1 |
|
| 1 |
|
|
| Group
2 | 1.26 | 0.77–2.05 | 0.352 | 0.91 | 0.55–1.52 | 0.720 |
| Group
3 | 2.68 | 1.43–5.03 | 0.002 | 2.28 | 1.19–4.37 | 0.013 |
Comparison of causes of death between
groups of SMI and SMD
Group 3 had significantly more intercurrent disease
death than Groups 2 and 1 (P=0.002; Table IV).
 | Table IV.Association between the cause of
death and the combination of computed tomography-derived skeletal
muscle mass and radiodensity. |
Table IV.
Association between the cause of
death and the combination of computed tomography-derived skeletal
muscle mass and radiodensity.
| Event | Group 1
(n=226) | Group 2
(n=188) | Group 3 (n=45) | P-value |
|---|
| Gastric
cancer-specific death | 18 (8.0) | 14 (7.4) | 4 (8.9) | 0.945 |
| Intercurrent
disease death | 11 (4.9) | 15 (8.0) | 9 (20.0) | 0.002 |
Discussion
The purpose of the present study was to assess the
clinical impact of preoperative SMI and SMD on long-term survival
outcomes of patients with GC. SMI and SMD were quantified using CT
and their impact on 5-year OS and 5-year RFS was evaluated. The
findings revealed that patients in Group 3 (low SMI and SMD group)
had significantly lower 5-year OS and RFS rates than those in Group
2 (high SMI or SMD group) and Group 1 (high SMI and SMD group).
Additionally, the combination of low SMI and low SMD was identified
as an independent predictor of lower 5-year OS and RFS rates.
The significance of assessing the combination of SMI
and SMD lies in the ability of these parameters to provide a more
comprehensive assessment of sarcopenia in patients with cancer,
where SMI and SMD reflect muscle mass and muscle function,
respectively (4,19–21).
Sarcopenia is associated with poor prognosis (22,23)
and a high risk of cancer (24–27).
Although the association between low SMI and poor prognosis in
patients with GC is well known (24,25),
the clinical significance of low SMD has been inadequately
explored, despite studies linking it with a poor prognosis
(26,27). Furthermore, the combined evaluation
of SMI and SMD has demonstrated prognostic significance in patients
with colorectal cancer (28). Low
SMI is a recognized hallmark of sarcopenia (11), whereas low SMD indicates adiposity
and muscle fibrosis, signifying reduced muscle quality and function
(29,30). Decreased muscle quality and function
are caused by aging (31),
inflammation (30,32) and malnutrition (33), all of which are poor prognostic
indicators in patients with cancer (34,35).
Furthermore, the combined evaluation of SMI and SMD allows for the
detection of patients with a poor prognosis preoperatively. The
findings of the present study indicate that patients with low SMI
and SMD are often older, have a lower BMI and exhibit lower PNI
values. Although these patients are more likely to die from other
causes, perioperative rehabilitation (36), enhanced nutritional support
(36) and proactive management of
comorbidities (37) have shown
promise in improving prognosis.
Nonetheless, the present study had certain
limitations. First, it was a single-center retrospective study with
a limited sample size. Thus, further validation through a
multicenter study is required. Moreover, although SMI and SMD have
been reported as prognostic factors of patients with cancer
(13,14), there is no consensus on how to
determine cutoff values; thus, this requires further
investigation.
In conclusion, the results of the present study
indicate the potential of the combined evaluation of preoperative
SMI and SMD as a significant prognostic indicator after gastrectomy
in patients with GC. Incorporating this index into preoperative
screening and implementing interventions such as intensified
nutritional support and comorbidity management based on it may
offer opportunities to enhance patient outcomes.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
IH and TOs had full access to all the data in the
study and take responsibility for the integrity and accuracy of the
data analysis. IH and TOs confirm the authenticity of all the raw
data. IH, KK, YM, SN, TK, TA, TH, TYa, TS, TOg, HC, TYo, NY, YR, AS
and TOs conceptualized and designed the study. IH, KK, YM, SN, TK,
TA, TH, TYa, TS, TOg, HC, TYo, NY, YR, AS and TOs collected the
data and performed the literature search. IH and TOs prepared the
draft manuscript and figures. All authors have read and approved
the final manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of Kanagawa Cancer Center (Yokohama, Japan; approval no.
25 Research-20). Written informed consent was obtained from all
patients in the present study.
Patient consent for publication
Not applicable.
Competing interests
The authors declared that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
CT
|
computed tomography
|
|
SMI
|
skeletal muscle index
|
|
SMD
|
skeletal muscle radiodensity
|
|
GC
|
gastric cancer
|
|
OS
|
overall survival
|
|
RFS
|
recurrence-free survival
|
|
HU
|
Hounsfield units
|
|
HR
|
hazard ratios
|
|
CI
|
confidence interval
|
|
BMI
|
body mass index
|
|
PNI
|
Prognostic Nutritional Index
|
References
|
1
|
Sung H, Ferlay J, Siegel RL, Laversanne M,
Soerjomataram I, Jemal A and Bray F: Global cancer statistics 2020:
GLOBOCAN estimates of incidence and mortality worldwide for 36
cancers in 185 countries. CA Cancer J Clin. 71:209–249. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Smyth EC, Nilsson M, Grabsch HI, van
Grieken NC and Lordick F: Gastric cancer. Lancet. 396:635–648.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Machlowska J, Baj J, Sitarz M, Maciejewski
R and Sitarz R: Gastric cancer: Epidemiology, risk factors,
classification, genomic characteristics and treatment strategies.
Int J Mol Sci. 21:40122020. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Cruz-Jentoft AJ, Bahat G, Bauer J, Boirie
Y, Bruyère O, Cederholm T, Cooper C, Landi F, Rolland Y, Sayer AA,
et al: Sarcopenia: Revised European consensus on definition and
diagnosis. Age Ageing. 48:6012019. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zheng ZF, Lu J, Zheng CH, Li P, Xie JW,
Wang JB, Lin JX, Chen QY, Lin M and Huang CM: A novel prognostic
scoring system based on preoperative sarcopenia predicts the
long-term outcome for patients after R0 resection for gastric
cancer: Experiences of a high-volume center. Ann Surg Oncol.
24:1795–1803. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Yang M, Shen Y, Tan L and Li W: Prognostic
value of sarcopenia in lung cancer: A systematic review and
meta-analysis. Chest. 156:101–111. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Masanés F, Rojano I, Luque X, Salvà A,
Serra-Rexach JA, Artaza I, Formiga F, Cuesta F, López Soto A, Ruiz
D and Cruz-Jentoft AJ: Cut-off points for muscle mass-not grip
strength or gait speed-determine variations in sarcopenia
prevalence. J Nutr Health Aging. 21:825–829. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Rossi AP, Fantin F, Micciolo R, Bertocchi
M, Bertassello P, Zanandrea V, Zivelonghi A, Bissoli L and Zamboni
M: Identifying sarcopenia in acute care setting patients. J Am Med
Dir Assoc. 15:303.e7–12. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Derstine BA, Holcombe SA, Ross BE, Wang
NC, Su GL and Wang SC: Skeletal muscle cutoff values for sarcopenia
diagnosis using T10 to L5 measurements in a healthy US population.
Sci Rep. 8:113692018. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Rollins KE, Gopinath A, Awwad A, Macdonald
IA and Lobo DN: Computed tomography-based psoas skeletal muscle
area and radiodensity are poor sentinels for whole L3 skeletal
muscle values. Clin Nutr. 39:2227–2232. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Shachar SS, Williams GR, Muss HB and
Nishijima TF: Prognostic value of sarcopenia in adults with solid
tumours: A meta-analysis and systematic review. Eur J Cancer.
57:58–67. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
McGovern J, Dolan RD, Horgan PG, Laird BJ
and McMillan DC: Computed tomography-defined low skeletal muscle
index and density in cancer patients: Observations from a
systematic review. J Cachexia Sarcopenia Muscle. 12:1408–1417.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Martin L, Birdsell L, Macdonald N, Reiman
T, Clandinin MT, McCargar LJ, Murphy R, Ghosh S, Sawyer MB and
Baracos VE: Cancer cachexia in the age of obesity: Skeletal muscle
depletion is a powerful prognostic factor, independent of body mass
index. J Clin Oncol. 31:1539–1547. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Caan BJ, Meyerhardt JA, Kroenke CH,
Alexeeff S, Xiao J, Weltzien E, Feliciano EC, Castillo AL,
Quesenberry CP, Kwan ML and Prado CM: Explaining the obesity
paradox: The association between body composition and colorectal
cancer survival (C-SCANS Study). Cancer Epidemiol Biomarkers Prev.
26:1008–1015. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Oken MM, Creech RH, Tormey DC, Horton J,
Davis TE, McFadden ET and Carbone PP: Toxicity and response
criteria of the Eastern Cooperative Oncology Group. Am J Clin
Oncol. 5:649–655. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Irving BA, Weltman JY, Brock DW, Davis CK,
Gaesser GA and Weltman A: NIH ImageJ and Slice-O-Matic computed
tomography imaging software to quantify soft tissue. Obesity
(Silver Spring). 15:370–376. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Dennis RA, Long DE, Landes RD, Padala KP,
Padala PR, Garner KK, Wise JN, Peterson CA and Sullivan DH:
Tutorial for using SliceOmatic to calculate thigh area and
composition from computed tomography images from older adults. PLoS
One. 13:e02045292018. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Lee JK, Park YS, Lee K, Youn SI, Won Y,
Min SH, Ahn SH, Park DJ and Kim HH: Prognostic significance of
surgery-induced sarcopenia in the survival of gastric cancer
patients: A sex-specific analysis. J Cachexia Sarcopenia Muscle.
12:1897–1907. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Heymsfield SB, Gallagher D, Visser M,
Nuñez C and Wang ZM: Measurement of skeletal muscle: Laboratory and
epidemiological methods. J Gerontol A Biol Sci Med Sci. 50:Spec No.
23–29. 1995.PubMed/NCBI
|
|
20
|
Heymsfield SB, Adamek M, Gonzalez MC, Jia
G and Thomas DM: Assessing skeletal muscle mass: Historical
overview and state of the art. J Cachexia Sarcopenia Muscle.
5:9–18. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Williams GR, Deal AM, Muss HB, Weinberg
MS, Sanoff HK, Nyrop KA, Pergolotti M and Shachar SS: Skeletal
muscle measures and physical function in older adults with cancer:
Sarcopenia or myopenia? Oncotarget. 8:33658–33665. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Deng HY, Hou L, Zha P, Huang KL and Peng
L: Sarcopenia is an independent unfavorable prognostic factor of
non-small cell lung cancer after surgical resection: A
comprehensive systematic review and meta-analysis. Eur J Surg
Oncol. 45:728–735. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Jin SB, Tian ZB, Ding XL, Guo YJ, Mao T,
Yu YN, Wang KX and Jing X: The impact of preoperative sarcopenia on
survival prognosis in patients receiving neoadjuvant therapy for
esophageal cancer: A systematic review and meta-analysis. Front
Oncol. 11:6195922021. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Sakurai K, Kubo N, Tamura T, Toyokawa T,
Amano R, Tanaka H, Muguruma K, Yashiro M, Maeda K, Hirakawa K and
Ohira M: Adverse effects of low preoperative skeletal muscle mass
in patients undergoing gastrectomy for gastric cancer. Ann Surg
Oncol. 24:2712–2719. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zheng ZF, Lu J, Xie JW, Wang JB, Lin JX,
Chen QY, Cao LL, Lin M, Tu RH, Zheng CH, et al: Preoperative
skeletal muscle index vs the controlling nutritional status score:
Which is a better objective predictor of long-term survival for
gastric cancer patients after radical gastrectomy? Cancer Med.
7:3537–3547. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Lin J, Zhang W, Chen W, Huang Y, Wu R,
Chen X, Shen X and Zhu G: Muscle mass, density, and strength are
necessary to diagnose sarcopenia in patients with gastric cancer. J
Surg Res. 241:141–148. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Dong QT, Cai HY, Zhang Z, Zou HB, Dong WX,
Wang WB, Song HN, Luo X, Chen XL and Huang DD: Influence of body
composition, muscle strength, and physical performance on the
postoperative complications and survival after radical gastrectomy
for gastric cancer: A comprehensive analysis from a large-scale
prospective study. Clin Nutr. 40:3360–3369. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Dolan RD, Almasaudi AS, Dieu LB, Horgan
PG, McSorley ST and McMillan DC: The relationship between computed
tomography-derived body composition, systemic inflammatory
response, and survival in patients undergoing surgery for
colorectal cancer. J Cachexia Sarcopenia Muscle. 10:111–122. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Lieber RL and Ward SR: Cellular mechanisms
of tissue fibrosis. 4. Structural and functional consequences of
skeletal muscle fibrosis. Am J Physiol Cell Physiol. 305:C241–C252.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Chu MP, Lieffers J, Ghosh S, Belch A, Chua
NS, Fontaine A, Sangha R, Turner RA, Baracos VE and Sawyer MB:
Skeletal muscle density is an independent predictor of diffuse
large B-cell lymphoma outcomes treated with rituximab-based
chemoimmunotherapy. J Cachexia Sarcopenia Muscle. 8:298–304. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Larsson L, Degens H, Li M, Salviati L, Lee
YI, Thompson W, Kirkland JL and Sandri M: Sarcopenia: Aging-related
loss of muscle mass and function. Physiol Rev. 99:427–511. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Schaap LA, Pluijm SM, Deeg DJ, Harris TB,
Kritchevsky SB, Newman AB, Colbert LH, Pahor M, Rubin SM, Tylavsky
FA, et al: Higher inflammatory marker levels in older persons:
Associations with 5-year change in muscle mass and muscle strength.
J Gerontol A Biol Sci Med Sci. 64:1183–1189. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Landi F, Camprubi-Robles M, Bear DE,
Cederholm T, Malafarina V, Welch AA and Cruz-Jentoft AJ: Muscle
loss: The new malnutrition challenge in clinical practice. Clin
Nutr. 38:2113–2120. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Zhang X, Tang T, Pang L, Sharma SV, Li R,
Nyitray AG and Edwards BJ: Malnutrition and overall survival in
older adults with cancer: A systematic review and meta-analysis. J
Geriatr Oncol. 10:874–883. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Ruan GT, Zhang Q, Zhang X, Tang M, Song
MM, Zhang XW, Li XR, Zhanf KP, Ge YZ, Yang M, et al: Geriatric
nutrition risk index: Prognostic factor related to inflammation in
elderly patients with cancer cachexia. J Cachexia Sarcopenia
Muscle. 12:1969–1982. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Yamamoto K, Nagatsuma Y, Fukuda Y, Hirao
M, Nishikawa K, Miyamoto A, Ikeda M, Nakamori S, Sekimoto M,
Fujitani K and Tsujinaka T: Effectiveness of a preoperative
exercise and nutritional support program for elderly sarcopenic
patients with gastric cancer. Gastric Cancer. 20:913–918. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Morishima T, Matsumoto Y, Koeda N, Shimada
H, Maruhama T, Matsuki D, Nakata K, Ito Y, Tabuchi T and Miyashiro
I: Impact of comorbidities on survival in gastric, colorectal, and
lung cancer patients. J Epidemiol. 29:110–115. 2019. View Article : Google Scholar : PubMed/NCBI
|