Introduction
Carcinoma of the thyroid gland, which is the most
common endocrine malignant tumor (1), has experienced an increasing incidence
globally in the past decades. In 2011, the incidence of thyroid
carcinoma has accounted for 1 to 2% of all emerging tumors in the
world (2). Papillary thyroid
carcinoma (PTC) is the major subtype, which accounts for 80% of all
malignant thyroid tumors (3).
Inflammation is an important component of the tumor
microenvironment that contributes to cancer development and
progression. Selected chronic inflammatory conditions enhance the
risk of developing cancer (4).
These inflammation-driven markers significantly contribute to tumor
occurrence, growth and progression (5). The focus on establishing novel
non-invasive and sensitive predictive indicators from hematological
parameters for inflammatory diseases and tumors has been
continuously increasing in recent years.
Although a complete blood count can be routinely
obtained from patients for clinicians, the roles of some parameters
in the diagnosis and treatment of malignancy, such as red blood
cell distribution width (RDW) and platelet parameters, including
mean platelet volume (MPV) and platelet distribution width (PDW),
remain obscure. RDW is a laboratory parameter widely used for
diagnosing anemia (6). However,
recent studies have indicated that RDW can be applied as a
laboratory diagnostic parameter for inflammatory diseases and
tumors, such as atherosclerosis, inflammatory bowel diseases, lung
cancer and breast cancer (7–10). PDW
is a measurement of change in platelet size and is a direct
platelet volume flow cytometric measurement. PDW has been assessed
as a biomarker for determining platelet morphology and activation
(11,12). A previous study has also shown the
association of PDW with complete blood count and C-reactive
protein, and indicated the broad association between platelets and
inflammation (13). MPV, another
hematological parameter that serves as a marker of platelet size
and activity, has been considered as a potential biomarker of
platelet function and activation (14). MPV is associated with
inflammation-induced arterial and venous thrombosis (15), and large platelets are considered to
be more reactive than small platelets, as they are more easily
stimulated to release chemical mediators (16). Therefore, the present study aimed to
assess whether the three laboratory parameters could be biomarkers
used to evaluate disease diagnosis through retrospective analysis
of the association between the values of RDW, MPV and PDW, and the
clinical data of patients with PTC.
Materials and methods
Study population
The clinicopathological data of 780 patients with
PTC, including 542 patients with PTMC (defined as a PTC ≤10 mm in
maximal diameter), with a mean age of 46 years and a male:female
ratio of 1:3, who had undergone clinical thyroid examination and
fine-needle aspiration biopsy (FNAB) for diagnosis were
retrospectively analyzed. The data of 400 healthy subjects, with a
mean age of 43 years and a male:female ratio of 1:3, were included
as the control. All individuals were assessed at Shandong
Provincial Hospital Affiliated to Shandong First Medical University
(Jinan, China) between January 2017 and December 2018. The
diagnosis of papillary thyroid carcinoma/microcarcinoma in all
patients was histopathologically reconfirmed by reassessment of
biospsy slides by two pathologists. Patients were included in this
study based on the following inclusion criteria: A confirmed
histopathological diagnosis of PTC/PTMC; a complete whole blood
count assessment prior to FNAB; and available clinicopathological
data. Patients with clinical signs of acute infection, anemia,
hematological disease, diabetes mellitus, cirrhosis, kidney
disease, autoimmune disease, severe coronary heart and artery
disease, and other malignant tumors were excluded. All
clinicopathological data were obtained from medical records. All
patients were staged based on the American Joint Committee on
Cancer (AJCC) Staging Guidelines (8th edition) (17). Considering the potential for certain
patients with PTMC to be overlooked, caution was taken when
choosing the healthy controls. The 400 healthy controls were
selected from the Medical Examination Center of Shandong Provincial
Hospital Affiliated to Shandong First Medical University, and were
subjects whose examination results were normal, including blood
test, B-ultrasound and computed tomography examination results.
This study was approved by the Ethics Committee of Shandong
Provincial Hospital Affiliated to Shandong First Medical University
(approval no. 2018-456) and written informed consent was obtained
from all participants.
Blood sampling and laboratory
assays
The RDW, MPV and PDW laboratory parameter values of
the patients were assessed 1 week before FNAB with the fully
automated hematological analyzer XE-2100 (Sysmex Corporation).
Thyroid functional biomarkers, such as free triiodothyronine (FT3),
free thyroxine (FT4), anti-thyroglobulin antibodies,
anti-thyroperoxidase antibody (anti-TPO) and anti-thyrotropin
receptor antibody (TRAB) were also detected within 1 month of FNAB
using a Cobas e601 analyzer (Roche Diagnostics). The median values
of RDW, MPV and PDW were calculated in all 780 patients with PTC
and in the 542 patients with PTMC, respectively. The median values
were applied as cut-off values to divide patients into higher and
lower RDW, MPV and PDW groups.
Statistical analysis
All statistical analyses were performed using SPSS
software version 20.0 (IBM Corp.). The normal distributions of
continuous data are displayed as the mean ± standard deviation, and
the non-normal distributions of continuous data are expressed as
the median. Categorical variables are presented as percentages.
Unpaired Student's t-test was performed for comparisons of the mean
differences in RDW, MPV and PDW between groups. The χ2
test was performed to assess the differences between categories of
each clinicopathological feature. Receiver operating characteristic
(ROC) curves were applied to evaluate the diagnostic accuracy.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Differences between patients with PTC
or PTMC and the healthy controls
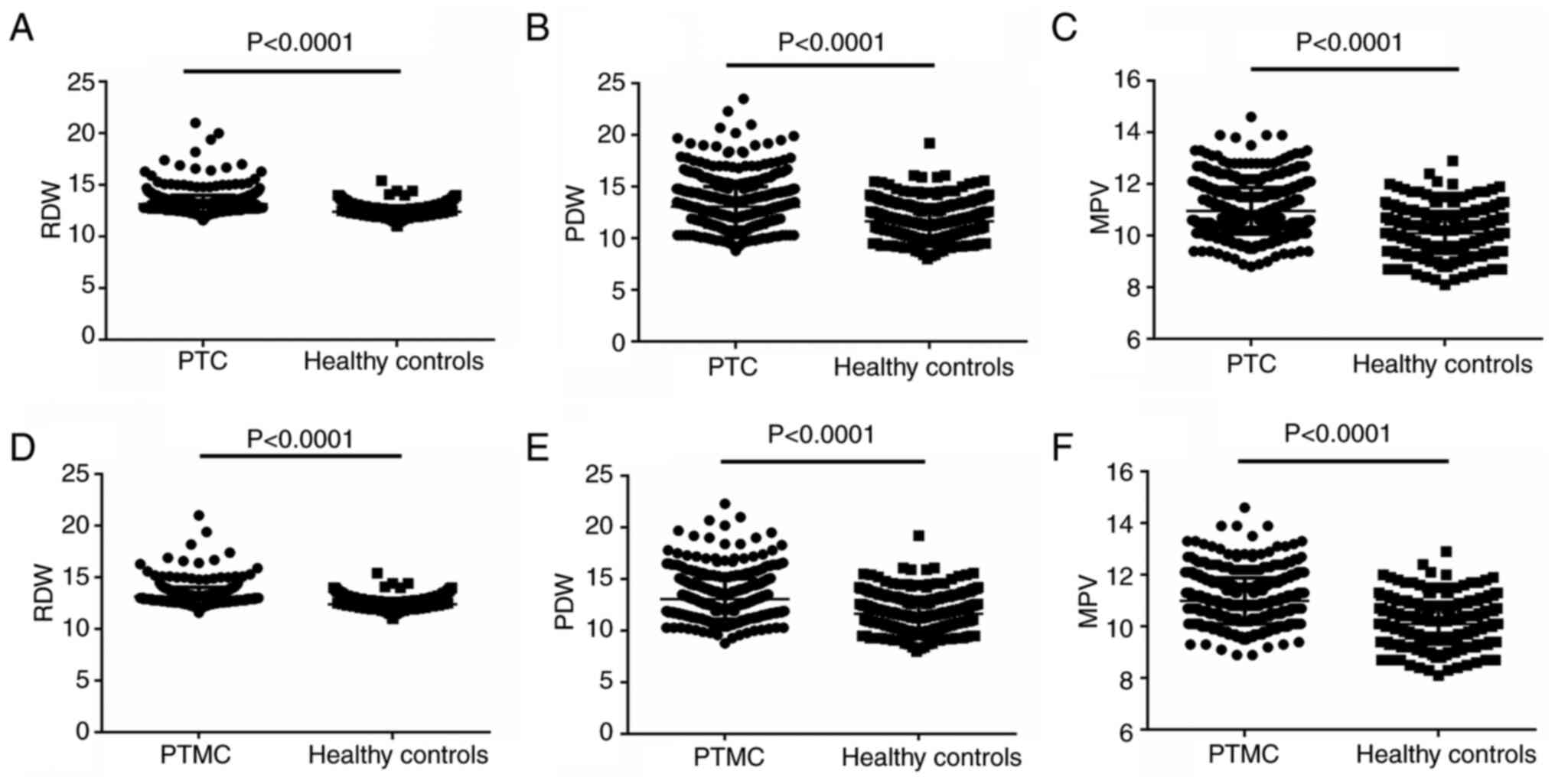
The preoperative RDW values in the 780 patients with
PTC and the 400 healthy controls were 13.13±0.8550 and
12.40±0.5820%, respectively. The RDW value in the PTC group was
significant higher than that in the control group (P<0.001;
Fig. 1A). The preoperative PDW
value in the PTC group was 13.05±1.963%, which was significantly
higher than the 11.65±1.591% in the control group (P<0.001;
Fig. 1B). The preoperative MPV
values in the PTC and control groups were 10.96±0.8945 and
10.08±0.7724%, respectively. Similar to the other laboratory
parameters, there was a significant difference in the MPV values
between the two groups (P<0.001; Fig. 1C).
The values of RDW, PDW and MPV were also analyzed in
the 542 patients with PTMC, which were recorded as 13.16±0.8845,
13.08±1.938 and 10.99±0.8887%, respectively. Significant
differences were noted when the values of each group were compared
with those of the control group (all P<0.001; Fig. 1D-F).
Diagnostic accuracy of RDW, MPV and
PDW
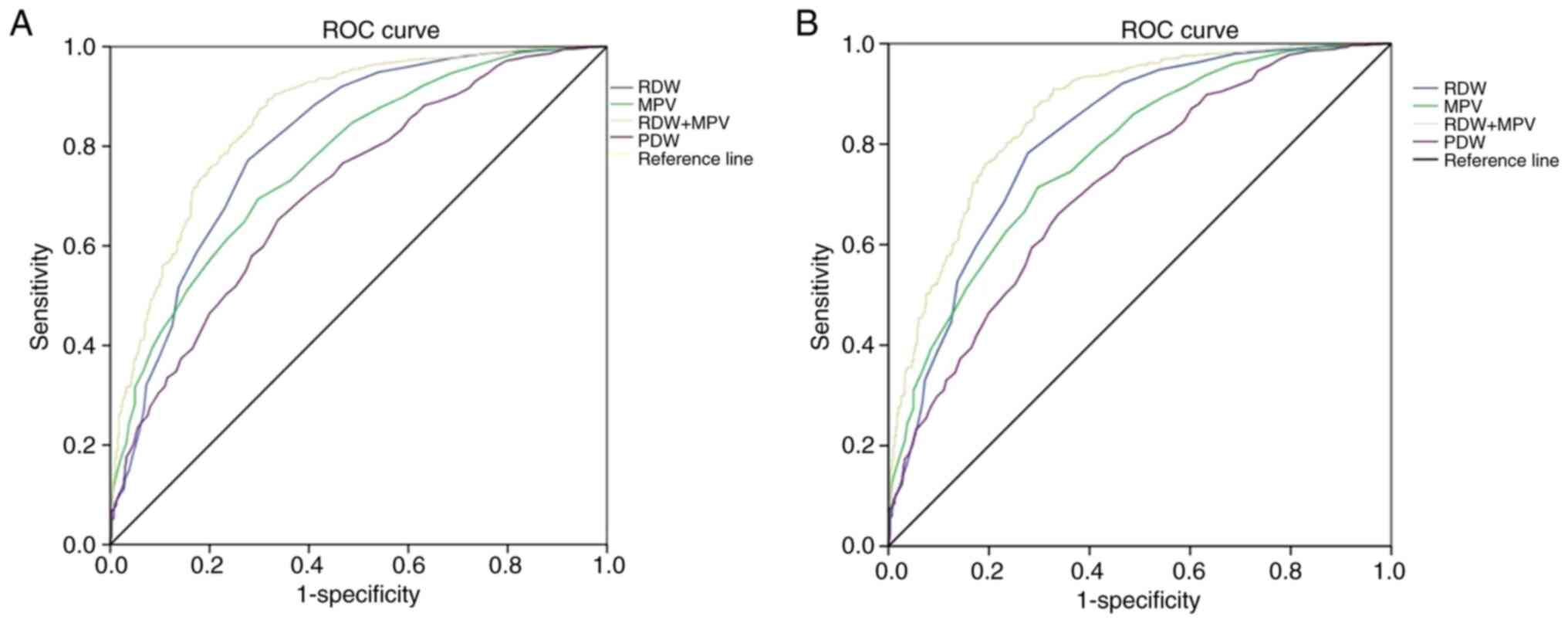
ROC curves were used to verify the ability of RDW,
PDW and MPV in predicting the presence of PTC and PTMC. For PTC,
the diagnostic values of RDW, MPV and PDW for differentiating
patients with PTC or PTMC from healthy controls are shown in
Table I and Fig. 2A. When the optimal cut-off point was
12.65, RDW had sensitivity (SEN) and specificity (SPE) values of
0.772 and 0.722, respectively. When the optimal cut-off point was
10.45, MPV had SEN and SPE values of 0.694 and 0.702, respectively.
When the optimal cut-off point was 12.15, PDW had SEN and SPE
values of 0.652 and 0.662, respectively. RDW, MPV and PDW had AUC
(95% CI) values of 0.808 (0.780–0.835), 0.771 (0.743–0.799) and
0.711 (0.681–0.742), respectively (Fig.
2A). When RDW and MPV were combined together, the AUC (95% CI)
value was enhanced to 0.858 (0.835–0.881).
 | Table I.Evaluation of diagnostic values
(normal and papillary thyroid carcinoma or papillary thyroid
microcarcinoma). |
Table I.
Evaluation of diagnostic values
(normal and papillary thyroid carcinoma or papillary thyroid
microcarcinoma).
| Variables | Cut-off point | Sensitivity | Specificity | AUC (95% CI) |
|---|
| RDW | 12.65 | 0.772 | 0.722 | 0.808
(0.780–0.835) |
| MPV | 10.45 | 0.694 | 0.702 | 0.771
(0.743–0.799) |
| PDW | 12.15 | 0.652 | 0.662 | 0.711
(0.681–0.742) |
| RDW + MPV |
| 0.894 | 0.682 | 0.858
(0.835–0.881) |
For PTMC, the diagnostic values of RDW, MPV and PDW
for differentiating between the patients with PTMC and the healthy
controls are shown in Table II and
Fig. 2B. When the optimal cut-off
point was 12.65, RDW had SEN and SPE values of 0.782 and 0.723,
respectively. When the optimal cut-off point was 10.45, MPV had SEN
and SPE values of 0.714 and 0.703, respectively. When the optimal
cut-off point was 12.15, PDW had SEN and SPE values of 0.661 and
0.663, respectively. RDW, MPV and PDW had AUC (95% CI) values of
0.812 (0.783–0.840), 0.779 (0.749–0.808) and 0.718 (0.685–0.751),
respectively. When RDW and MPV were combined together, the AUC (95%
CI) value was enhanced to 0.858 (0.835–0.881).
 | Table II.Evaluation of diagnostic values
(normal and papillary thyroid microcarcinoma). |
Table II.
Evaluation of diagnostic values
(normal and papillary thyroid microcarcinoma).
| Variables | Cut-off point | Sensitivity | Specificity | AUC (95% CI) |
|---|
| RDW | 12.65 | 0.782 | 0.723 | 0.812
(0.783–0.840) |
| MPV | 10.45 | 0.714 | 0.703 | 0.779
(0.749–0.808) |
| PDW | 12.15 | 0.661 | 0.663 | 0.718
(0.685–0.751) |
| RDW + MPV |
| 0.894 | 0.682 | 0.858
(0.835–0.881) |
Association between RDW, MPV, PDW and
clinicopathological characteristics in patients with PTC or
PTMC
The differences in the clinicopathological
characteristics between higher and lower groups in patients with
PTC based on RDW, PDW and MPV are summarized in Table III. A higher RDW value was
significantly associated with being female (P=0.0003), deeper tumor
infiltration (P=0.0472), and normal FT3 (P=0.0076) and FT4
(P=0.0490) levels. A higher PDW value was only significantly
associated with an elevated TRAB level (P=0.0119). A higher MPV
value was only significantly associated with an elevated TRAB level
(P=0.0032).
 | Table III.Comparison of clinicopathological
parameters of 780 patients with papillary thyroid carcinoma or
papillary thyroid microcarcinoma between high and low groups in
terms of RDW, PDW and MPV. |
Table III.
Comparison of clinicopathological
parameters of 780 patients with papillary thyroid carcinoma or
papillary thyroid microcarcinoma between high and low groups in
terms of RDW, PDW and MPV.
|
|
| RDW | PDW | MPV |
|---|
|
|
|
|
|
|
|---|
|
Characteristics | Cases, n | <13, n (%) | ≥13, n (%) |
P-valuea | <12.8, n
(%) | ≥12.8, n (%) |
P-valuea | <10.9, n
(%) | ≥10.9, n (%) |
P-valuea |
|---|
| Sex |
|
|
| 0.0003 |
|
| 0.2539 |
|
| 0.8810 |
|
Male | 184 | 110 (29.3) | 74 (18.3) |
| 85 (21.9) | 99 (25.3) |
| 91 (23.8) | 93 (23.4) |
|
|
Female | 596 | 266 (70.7) | 330 (81.7) |
| 304 (78.1) | 292 (74.7) |
| 291 (76.2) | 305 (76.6) |
|
| Age, years |
|
|
| 0.1170 |
|
| 0.3865 |
|
| 0.3137 |
|
<46 | 379 | 191 (50.8) | 188 (46.5) |
| 187 (48.1) | 192 (49.1) |
| 189 (49.5) | 190 (47.7) |
|
|
≥46 | 401 | 185 (49.2) | 216 (53.5) |
| 202 (51.9) | 199 (50.9) |
| 193 (50.5) | 208 (52.3) |
|
| Depth of tumor |
|
|
| 0.0472 |
|
| 0.5594 |
|
| 0.9308 |
| T1 | 726 | 357 (94.9) | 369 (91.3) |
| 360 (92.5) | 366 (93.6) |
| 354 (92.7) | 372 (93.5) |
|
| T2 + T3
+ T4 | 54 | 19 (5.1) | 35 (8.7) |
| 29 (7.5) | 25 (6.4) |
| 28 (7.3) | 26 (6.5) |
|
| Lymph node
metastasis |
|
|
| 0.0571 |
|
| 0.3860 |
|
| 0.1368 |
| N0 | 584 | 270 (71.8) | 314 (77.7) |
| 286 (73.5) | 298 (76.2) |
| 277 (72.5) | 307 (77.1) |
|
| N1 | 196 | 106 (28.2) | 90 (22.3) |
| 103 (26.5) | 93 (23.8) |
| 105 (27.5) | 91 (22.9) |
|
| Number of foci |
|
|
| 0.7183 |
|
| 0.2416 |
|
| 0.2107 |
|
Unifocal | 497 | 242 (64.4) | 255 (63.1) |
| 240 (61.7) | 257 (65.7) |
| 235 (61.5) | 262 (65.8) |
|
|
Multifocal | 283 | 134 (35.6) | 149 (36.9) |
| 149 (38.3) | 134 (34.3) |
| 147 (38.5) | 136 (34.2) |
|
| pStage |
|
|
| 0.9559 |
|
| 0.4087 |
|
| 0.3319 |
| I | 743 | 358 (95.2) | 385 (95.3) |
| 373 (95.9) | 370 (94.6) |
| 365 (95.5) | 378 (95.0) |
|
| II | 37 | 18 (4.8) | 19 (4.7) |
| 16 (4.1) | 21 (5.4) |
| 17 (4.5) | 20 (5.0) |
|
| FT3 |
|
|
| 0.0076 |
|
| 0.9898 |
|
| 0.9187 |
|
≤6.01 | 756 | 358 (95.2) | 398 (98.5) |
| 377 (96.9) | 379 (96.9) |
| 370 (96.9) | 386 (97.0) |
|
|
>6.01 | 24 | 18 (4.8) | 6 (1.5) |
| 12 (3.1) | 12 (3.1) |
| 12 (3.1) | 12 (3.0) |
|
| FT4 |
|
|
| 0.0490 |
|
| 0.7174 |
|
| 0.6361 |
|
≤19.05 | 746 | 354 (94.1) | 392 (97.0) |
| 371 (95.4) | 375 (95.9) |
| 364 (95.3) | 382 (96.0) |
|
|
>19.05 | 34 | 22 (5.9) | 12 (3.0) |
| 18 (4.6) | 16 (4.1) |
| 18 (4.7) | 16 (4.0) |
|
| Anti-AG |
|
|
| 0.1801 |
|
| 0.8986 |
|
| 0.8327 |
|
≤115 | 645 | 318 (84.6) | 327 (80.9) |
| 321 (82.5) | 324 (82.9) |
| 317 (83.0) | 328 (82.4) |
|
|
>115 | 135 | 58 (15.4) | 77 (19.1) |
| 68 (17.5) | 67 (17.1) |
| 65 (17.0) | 70 (17.6) |
|
| Anti-TPO |
|
|
| 0.4796 |
|
| 0.2596 |
|
| 0.6973 |
|
≤34 | 534 | 262 (69.7) | 272 (67.3) |
| 259 (66.6) | 275 (70.3) |
| 259 (67.8) | 275 (69.1) |
|
|
>34 | 246 | 114 (30.3) | 132 (32.7) |
| 130 (33.4) | 116 (29.7) |
| 123 (32.2) | 123 (30.9) |
|
| Anti-TRAB |
|
|
| 0.5152 |
|
| 0.0119 |
|
| 0.0032 |
|
≤1.22 | 764 | 367 (97.6) | 397 (98.3) |
| 386 (99.2) | 378 (96.7) |
| 380 (99.5) | 384 (96.5) |
|
|
>1.22 | 16 | 9 (2.4) | 7 (1.7) |
| 3 (0.8) | 13 (3.3) |
| 2 (0.5) | 14 (3.5) |
|
The differences in the clinicopathological
characteristics between higher and lower groups based on RDW, PDW
and MPV in patients with PTMC are summarized in Table IV. A higher RDW value was only
significantly associated with being female (0.0108). A higher PDW
value was significantly associated with being female (P=0.0267) and
an elevated TRAB level (P=0.0202). A higher MPV value was also
significantly associated with elevated TRAB (P=0.0047).
 | Table IV.Comparison of clinicopathological
parameters of 542 patients with PTMC between high and low groups in
terms of RDW, PDW and MPV. |
Table IV.
Comparison of clinicopathological
parameters of 542 patients with PTMC between high and low groups in
terms of RDW, PDW and MPV.
|
|
| RDW | PDW | MPV |
|---|
|
|
|
|
|
|
|---|
|
Characteristicsa | Cases, n | <13, n (%) | ≥13, n (%) |
P-valuea | <12.8, n
(%) | ≥12.8, n (%) |
P-valuea | <10.9, n
(%) | ≥10.9, n (%) |
P-valueb |
|---|
| Sex |
|
|
| 0.0108 |
|
| 0.0267 |
|
| 0.3626 |
|
Male | 122 | 70 (27.3) | 52 (18.2) |
| 50 (18.5) | 72 (26.5) |
| 55 (20.8) | 67 (24.1) |
|
|
Female | 420 | 186 (72.7) | 234 (81.8) |
| 220 (81.5) | 200 (73.5) |
| 209 (79.2) | 211 (75.9) |
|
| Age, years |
|
|
| 0.6526 |
|
| 0.7274 |
|
| 0.6275 |
|
<46 | 257 | 124 (48.4) | 133 (46.5) |
| 126 (46.7) | 131 (48.2) |
| 128 (48.5) | 129 (46.4) |
|
|
>46 | 285 | 132 (51.6) | 153 (53.5) |
| 144 (53.3) | 141 (51.8) |
| 136 (51.5) | 149 (53.6) |
|
| Lymph node
metastasis |
|
|
| 0.5393 |
|
| 0.1708 |
|
| 0.1634 |
| N0 | 442 | 206 (80.5) | 236 (82.5) |
| 214 (79.3) | 228 (83.8) |
| 209 (79.2) | 233 (83.8) |
|
| N1 | 100 | 50 (19.5) | 50 (17.5) |
| 56 (20.7) | 44 (16.2) |
| 55 (20.8) | 45 (16.2) |
|
| Number of foci |
|
|
| 0.6368 |
|
| 0.2546 |
|
| 0.1785 |
|
Unifocal | 348 | 167 (65.2) | 181 (63.3) |
| 167 (61.9) | 181 (66.5) |
| 162 (61.4) | 186 (66.9) |
|
|
Multifocal | 194 | 89 (34.8) | 105 (36.7) |
| 103 (38.1) | 91 (33.5) |
| 102 (38.6) | 92 (33.1) |
|
| FT3 |
|
|
| 0.0514 |
|
| 0.2377 |
|
| 0.0654 |
|
≤6.01 | 530 | 247 (96.5) | 283 (99.0) |
| 262 (97.0) | 268 (98.5) |
| 255 (96.6) | 275 (98.9) |
|
|
>6.01 | 12 | 9 (3.5) | 3 (1.0) |
| 8 (3.0) | 4 (1.5) |
| 9 (3.4) | 3 (1.1) |
|
| FT4 |
|
|
| 0.2438 |
|
| 0.1664 |
|
| 0.3033 |
|
≤19.05 | 522 | 244 (95.3) | 278 (97.2) |
| 257 (95.2) | 265 (97.4) |
| 252 (95.5) | 270 (97.1) |
|
|
>19.05 | 20 | 12 (4.7) | 8 (2.8) |
| 13 (4.8) | 7 (2.6) |
| 12 (4.5) | 8 (2.9) |
|
| Anti-AG |
|
|
| 0.3810 |
|
| 0.7050 |
|
| 0.9508 |
|
≤115 | 447 | 215 (84.0) | 232 (81.1) |
| 221 (81.9) | 226 (83.1) |
| 218 (82.6) | 229 (82.4) |
|
|
>115 | 95 | 41 (16.0) | 54 (18.9) |
| 49 (18.1) | 46 (16.9) |
| 46 (17.4) | 49 (17.6) |
|
| Anti-TPO |
|
|
| 0.9646 |
|
| 0.9533 |
|
| 0.4854 |
|
≤34 | 370 | 175 (68.4) | 195 (68.2) |
| 184 (68.1) | 186 (68.4) |
| 184 (69.7) | 186 (66.9) |
|
|
>34 | 172 | 81 (31.6) | 91 (31.8) |
| 86 (31.9) | 86 (31.6) |
| 80 (30.3) | 92 (33.1) |
|
| Anti-TRAB |
|
|
| 0.6961 |
|
| 0.0202 |
|
| 0.0047 |
|
≤1.22 | 530 | 251 (98.0) | 279 (97.6) |
| 268 (99.3) | 262 (96.3) |
| 263 (99.6) | 267 (96.0) |
|
|
>1.22 | 12 | 5 (2.0) | 7 (2.4) |
| 2 (0.7) | 10 (3.7) |
| 1 (0.4) | 11 (4.0) |
|
Discussion
Studies have suggested that cancer may be either
causative or a result of chronic inflammation, which has moved the
research focus towards the connection between inflammation and
malignant tumors (18,19). Cancer-associated inflammatory
changes have a significant effect on carcinogenesis and tumor
progression (18,19). The potential mechanism behind this
may involve the association between inflammation and malnutrition,
immune dysfunction, angiogenesis, and activation of cytokines and
platelets (20,21).
Tumor-infiltrating inflammatory cells mediate a
series of processes that are associated with progression, invasion
and metastasis (22). The immune
regulatory cytokines that are secreted in a pro-inflammatory
environment can also promote tumor growth and metastases (23). Certain evidence indicates that
inflammation markers such as neutrophil/lymphocyte ratio and
platelet/lymphocyte ratio play a vital role during the differential
diagnosis of PTC (24). The
associated inflammatory markers, such as lymphocyte-to-monocyte
ratio and IL-6, have been shown to play an important role in PTC
(25,26). As a simple index of the inflammatory
system, blood parameters have been widely applied as the predictive
markers for the prognosis of some types of malignancy, such as
colorectal cancer, nasopharyngeal carcinoma and gastric cancer
(27–29). In the last two decades, increasing
evidence has suggested that a high RDW value increases the overall
and disease-specific mortality rate in patients who have
inflammatory bowel disease, acute myocardial infarction and
prostate cancer (7,8,30,31).
Studies have also shown that RDW can be applied as a diagnostic and
prognostic biomarker in various types of solid cancer. For example,
the RDW value was significantly higher in patients with breast
cancer compared with that in patients with fibroadenomas, and it
was also highly correlated with the size of the primary tumor and
the number of metastatic axillary lymph nodes (9). Furthermore, RDW was reported to be
associated with malnutrition, which has been proven to be
associated with a lesser response to treatment, and a poorer
prognosis and quality of life (32,33).
Platelets regulate neoangiogenesis, diffusion and tumor cell growth
(34). Nevertheless, the activation
of platelets is more closely associated with their size rather than
their count. MPV is an indicator of platelet activation, while PDW
displays variation in platelet size, and both of these parameters
have been applied to predict the prognosis of various cancer types,
such as gastric and breast cancer (29,35).
As one of the cancer types with the most quickly
changing basis of understanding in the past decade, thyroid
carcinoma has undergone significant staging system revisions based
on the eighth version of the AJCC Staging Guidelines (17). Several studies have attempted to
discover the possibility of preoperatively predicting malignancy
(36,37), which is largely advocated to
establish a tailored surgery, thus preventing a diagnostic
thyroidectomy. Gambardella et al (38) analyzed the role of the
neutrophil-to-lymphocyte ratio (NLR), the platelet-to-lymphocyte
ratio and the lymphocyte-to-monocyte ratio as prognostic factors of
malignancy for indeterminate thyroid nodules, and found that the
NLR is an easy and reproducible inflammatory biomarker that is able
to improve the accuracy of preoperative prognostication for
malignancies (38).
In the present study, the values of RDW, PDW and MPV
in patients with PTC were identified as significantly higher than
those in healthy controls through comparison between the two
groups. In order to verify whether the RDW, PDW and MPV values
could be used as differential diagnosis indicators for PTMC, the
patients with PTMC were also analyzed, and the differences between
the patient and control were also significant. These results
suggested that RDW, PDW and MPV could be applied as screening
indicators for PTC or PTMC. Jin et al (37) reported that the PDW value was higher
in patients with PTC than that in patients with benign thyroid
nodules or healthy controls, which was consistent with the present
result. The present study also showed that RDW, PDW and MPV alone
had the ability to differentiate PTC or PTMC from healthy controls.
In addition, combining the RDW and MPV values could enhance their
diagnostic power, which suggests their ability to be clinically
accessible indicators. Low levels of MPV and PDW were considered as
indicators coexisting with Hashimoto's thyroiditis in patients with
PTC (39). Young patients with PTC
are more likely to suffer from Hashimoto's thyroiditis than elderly
patients with PTC, who are considered to have a poor prognosis.
Hashimoto's thyroiditis was associated with a smaller primary tumor
and less lymph node involvement at presentation, and was also
predictive of a lower rate of lymph node involvement and persistent
disease at the end of follow-up, which indicated that Hashimoto's
thyroiditis may have a protective effect on patients with PTC
(40,41). Decreased MPV and PDW were prognostic
of coexistence with Hashimoto's thyroiditis in elderly patients
with PTC, which indicated that lower MPV and PDW might be
protective indicators for patients with PTC (39).
The associations between RDW, PDW and MPV, and
clinicopathological features, were investigated in the present
study. A higher RDW was significantly associated with the female
sex and deeper tumor infiltration, but had no association with
lymph node metastasis. High RDW level has a prognostic value for
lower survival time in patients with gastric, lung, renal and
hematological tumors (42,43). However, few studies have reported
the association between RDW and the prognosis of PTC. This may be
associated with the disease state of PTC, a relatively indolent
malignancy (44,45), which is different from other
malignant tumors.
Despite gaining some new data, the present study
still has some limitations. Firstly, the main limitation of the
study is the decision method for the cut-off values. The current
literatures confirmed the optimal cut-off values for RDW, MPV and
PDW by using ROC curves, median value or previous studies. Only the
median values were applied as cut-off values for analysis, and the
cutoff values according to ROC and previous studies were not used.
Secondly, patients with PTC usually have long survival times;
therefore, it is quite difficult to conduct a survival analysis and
the standardization of follow-up is a major problem. Thirdly, the
study is retrospective in nature and only conducted in a single
center. Therefore, similar to most retrospectively designed
studies, potential bias and inaccuracies are inevitable in the data
collection process. Further prospective randomized controlled
studies that include large cohorts and multiple centers are
therefore needed to validate these findings in the future.
In summary, RDW, PDW and MPV, as available and
convenient biomarkers with diagnostic capabilities, can
differentiate patients with PTC or PTMC from healthy controls. In
addition, combining the RDW and MPV values can increase this
diagnostic power. To the best of our knowledge, this is the first
study to verify the utility of preoperative RDW combined with MPV
for the diagnosis of PTC or PTMC.
Acknowledgements
Not applicable.
Funding
This study was supported by grants from the National Natural
Science Foundation of China (no. 81802097), and the Clinical
Medical Science and Technology Innovation Program of Jinan City
(no. 201805068).
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
JH, JW, QW, JZ and HS conceived and designed the
experiments, analyzed and interpreted the data, and wrote the
manuscript. JH, JW, QW, YL and TL performed the experiments, and
collected and analyzed the data. JH, JW, JZ and HS confirm the
authenticity of all the raw data. All authors read and approved the
final version of the manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of Shandong Provincial Hospital Affiliated to Shandong
First Medical University (Jinan, China; approval no. 2018-456), and
each participant provided written informed consent.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Chen AY, Jemal A and Ward EM: Increasing
incidence of differentiated thyroid cancer in the United States,
1988–2005. Cancer. 115:3801–3807. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Gimm O, Castellone MD, Hoang-Vu C and
Kebebew E: Biomarkers in thyroid tumor research: New diagnostic
tools and potential targets of molecular-based therapy. J Thyroid
Res. 2011:6315932011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Schneider DF and Chen H: New developments
in the diagnosis and treatment of thyroid cancer. CA Cancer J Clin.
63:374–394. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Mantovani A, Barajon I and Garlanda C: IL
−1 and IL −1 regulatory pathways in cancer progression and therapy.
Immunol Rev. 281:57–61. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Pease NA, Wise-Draper T and Privette
Vinnedge L: Dissecting the Potential Interplay of DEK Functions in
Inflammation and Cancer. J Oncol. 2015:1065172015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Förhécz Z, Gombos T, Borgulya G, Pozsonyi
Z, Prohászka Z and Jánoskuti L: Red cell distribution width in
heart failure: Prediction of clinical events and relationship with
markers of ineffective erythropoiesis, inflammation, renal
function, and nutritional state. Am Heart J. 158:659–666. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Yeşil A, Şenateş E, Bayoğlu İV, Erdem ED,
Demirtunç R and Kurdaş Övünç AO: Red Cell Distribution Width: A
Novel Marker of Activity in Inflammatory Bowel Disease. Gut Liver.
5:460–467. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Gunebakmaz O, Kaya MG, Duran M, Akpek M,
Elcik D and Eryol NK: Red Blood Cell Distribution Width in
‘Non-Dippers’ versus ‘Dippers.’. Cardiology. 123:154–159. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Seretis C, Seretis F, Lagoudianakis E,
Gemenetzis G and Salemis NS: Is red cell distribution width a novel
biomarker of breast cancer activity? Data from a pilot study. J
Clin Med Res. 5:121–126. 2013.PubMed/NCBI
|
|
10
|
Koma Y, Onishi A, Matsuoka H, Oda N,
Yokota N, Matsumoto Y, Koyama M, Okada N, Nakashima N, Masuya D, et
al: Increased red blood cell Distribution Width Associates with
Cancer Stage and Prognosis in Patients with Lung Cancer. PLoS One.
8:e802402013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Bath PM, Missouris CG, Buckenham T and
MacGregor GA: Increased Platelet Volume and Platelet Mass in
Patients with Atherosclerotic Renal Artery Stenosis. Clin Sci
(Lond). 87:253–257. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Bath PM and Butterworth RJ: Platelet size:
Measurement, physiology and vascular disease. Blood Coagul
Fibrinolysis. 7:157–161. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Santimone I, Di Castelnuovo A, De Curtis
A, Spinelli M, Cugino D, Gianfagna F, Zito F, Donati MB, Cerletti
C, de Gaetano G, et al: White blood cell count, sex and age are
major determinants of heterogeneity of platelet indices in an adult
general population: Results from the MOLI-SANI project.
Haematologica. 96:1180–1188. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lee JH, Park M, Han S, Hwang JJ, Park SH
and Park SY: An increase in mean platelet volume during admission
can predict the prognoses of patients with pneumonia in the
intensive care unit: A retrospective study. PLoS One.
13:e02087152018. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Gasparyan AY, Ayvazyan L, Mikhailidis DP
and Kitas GD: Mean platelet volume: A link between thrombosis and
inflammation? Curr Pharm Des. 17:47–58. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Thompson CB, Jakubowski JA, Quinn PG,
Deykin D and Valeri CR: Platelet size and age determine platelet
function independently. Blood. 63:1372–1375. 1984. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Amin MB, Edge S, Greene F, Byrd DR,
Brookland RK, Washington MK, Gershenwald JE, Compton CC, Hess KR,
Sullivan DC, et al: AJCC Cancer Staging Manual. 8th Edition.
Springer; New York, NY: 2017
|
|
18
|
Mantovani A, Allavena P, Sica A and
Balkwill F: Cancer-related inflammation. Nature. 454:436–444. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Pollard JW: Tumour-educated macrophages
promote tumour progression and metastasis. Nat Rev Cancer. 4:71–78.
2004. View
Article : Google Scholar : PubMed/NCBI
|
|
20
|
Balkwill F and Mantovani A: Inflammation
and cancer: Back to Virchow? Lancet. 357:539–545. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Schreiber RD, Old LJ and Smyth MJ: Cancer
immunoediting: Integrating immunity's roles in cancer suppression
and promotion. Science. 331:1565–1570. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Orozco-Morales M, Soca-Chafre G,
Barrios-Bernal P, Hernández-Pedro N and Arrieta O: Interplay
between cellular and molecular inflammatory mediators in lung
cancer. Mediators Inflamm. 2016:34946082016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Barrera L, Montes-Servín E, Barrera A,
Ramírez-Tirado LA, Salinas-Parra F, Bañales-Méndez JL,
Sandoval-Ríos M and Arrieta Ó: Cytokine profile determined by
data-mining analysis set into clusters of non-small-cell lung
cancer patients according to prognosis. Ann Oncol. 26:428–435.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zhang X, Li S, Wang J, Liu F and Zhao Y:
Relationship between serum inflammatory factor levels and
differentiated thyroid carcinoma. Technol Cancer Res Treat.
20:15330338219900552021.PubMed/NCBI
|
|
25
|
Yokota M, Katoh H, Nishimiya H, Kikuchi M,
Kosaka Y, Sengoku N, Watanabe M and Yamashita K:
Lymphocyte-monocyte ratio significantly predicts recurrence in
papillary thyroid cancer. J Surg Res. 246:535–543. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kobawala TP, Trivedi TI, Gajjar KK, Patel
DH, Patel GH and Ghosh NR: Significance of interleukin-6 in
papillary thyroid carcinoma. J Thyroid Res. 2016:61789212016.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Chan JC, Chan DL, Diakos CI, Engel A,
Pavlakis N, Gill A and Clarke SJ: The lymphocyte-to-monocyte ratio
is a superior predictor of overall survival in comparison to
established biomarkers of resectable colorectal cancer. Ann Surg.
265:539–546. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
He JR, Shen GP, Ren ZF, Qin H, Cui C,
Zhang Y, Zeng YX and Jia WH: Pretreatment levels of peripheral
neutrophils and lymphocytes as independent prognostic factors in
patients with nasopharyngeal carcinoma. Head Neck. 34:1769–1776.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Cheng S, Han F, Wang Y, Xu Y, Qu T, Ju Y
and Lu Z: The red distribution width and the platelet distribution
width as prognostic predictors in gastric cancer. BMC
Gastroenterol. 17:1632017. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Karabulut A and Uzunlar B: Correlation
between red cell distribution width and coronary ectasia in the
acute myocardial infarction. Clin Appl Thromb Hemost. 18:551–552.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Albayrak S, Zengin K, Tanik S, Bakirtas H,
Imamoglu A and Gurdal M: Red cell distribution width as a predictor
of prostate cancer progression. Asian Pac J Cancer Prev.
15:7781–7784. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
McMillan DC: Systemic inflammation,
nutritional status and survival in patients with cancer. Curr Opin
Clin Nutr Metab Care. 12:223–226. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Hébuterne X, Lemarié E, Michallet M, De
Montreuil CB, Schneider SM and Goldwasser F: Prevalence of
malnutrition and current use of nutrition support in patients with
cancer. JPEN J Parenter Enteral Nutr. 38:196–204. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Jiang L, Luan Y, Miao X, Sun C, Li K,
Huang Z, Xu D, Zhang M, Kong F and Li N: Platelet releasate
promotes breast cancer growth and angiogenesis via VEGF-integrin
cooperative signalling. Br J Cancer. 117:695–703. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Tanriverdi O, Menekse S, Teker F, Oktay E,
Nur Pilanc K, Gunaldi M, Kocar M, Kacan T, Bahceci A, Avci N, et
al: The mean platelet volume may predict the development of
isolated bone metastases in patients with breast cancer: A
retrospective study of the Young Researchers Committee of the
Turkish Oncology Group (TOG). J BUON. 21:840–850. 2016.PubMed/NCBI
|
|
36
|
Marotta V, Sciammarella C, Capasso M,
Testori A, Pivonello C, Chiofalo MG, Gambardella C, Grasso M,
Antonino A, Annunziata A, et al: Germline Polymorphisms of the VEGF
pathway predict recurrence in nonadvanced differentiated thyroid
cancer. J Clin Endocrinol Metab. 102:661–671. 2017.PubMed/NCBI
|
|
37
|
Jin J, Wu G, Ruan C, Ling H, Zheng X, Ying
C and Zhang Y: Preoperative platelet distribution width-to-platelet
ratio combined with serum thyroglobulin may be objective and
popularizable indicators in predicting papillary thyroid carcinoma.
J Clin Lab Anal. 36:e244432022. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Gambardella C, Mongardini FM, Paolicelli
M, Bentivoglio D, Cozzolino G, Ruggiero R, Pizza A, Tolone S, Del
Genio G, Parisi S, et al: Role of inflammatory biomarkers (NLR,
LMR, PLR) in the prognostication of malignancy in indeterminate
thyroid nodules. Int J Mol Sci. 24:64662023. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Wen W, Wu P, Li J, Wang H, Sun J and Chen
H: Predictive values of the selected inflammatory index in elderly
patients with papillary thyroid cancer. J Transl Med. 16:2612018.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Dvorkin S, Robenshtok E, Hirsch D, Strenov
Y, Shimon I and Benbassat CA: Differentiated thyroid cancer is
associated with less aggressive disease and better outcome in
patients with coexisting hashimotos thyroiditis. J Clin Endocrinol
Metab. 98:2409–2414. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Lee JH, Kim Y, Choi JW and Kim YS: The
association between papillary thyroid carcinoma and histologically
proven Hashimoto's thyroiditis: A meta-analysis. Eur J Endocrinol.
168:343–349. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Życzkowski M, Rajwa P, Gabrys E,
Jakubowska K, Jantos E and Paradysz A: The relationship between red
cell distribution width and cancer-specific survival in patients
with renal cell carcinoma treated with partial and radical
nephrectomy. Clin Genitourin Cancer. 16:e677–e683. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Ai L, Mu S and Hu Y: Prognostic role of
RDW in hematological malignancies: A systematic review and
meta-analysis. Cancer Cell Int. 18:612018. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Haugen BR, Alexander EK, Bible KC, Doherty
GM, Mandel SJ, Nikiforov YE, Pacini F, Randolph GW, Sawka AM,
Schlumberger M, et al: 2015 American Thyroid Association Management
Guidelines for Adult Patients with Thyroid Nodules and
Differentiated Thyroid Cancer: The American Thyroid Association
Guidelines Task Force on Thyroid Nodules and Differentiated Thyroid
Cancer. Thyroid. 26:1–133. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Perrier ND, Brierley JD and Tuttle RM:
Differentiated and anaplastic thyroid carcinoma: Major changes in
the American Joint Committee on Cancer eighth edition cancer
staging manual. CA Cancer J Clin. 68:55–63. 2018. View Article : Google Scholar : PubMed/NCBI
|