Introduction
The emergence of immunotherapy has marked a
significant turning point in the prognosis of patients with various
types of advanced cancer (1). In
2017, the results of the phase III clinical trial ‘ATTRACTION-2’
indicated that nivolumab, an immune checkpoint inhibitor (ICI),
significantly prolonged overall survival (OS) compared to placebo,
making nivolumab monotherapy the standard of care for patients with
advanced gastric cancer (GC) after ≥2 chemotherapy regimens
(2,3).
Advanced gastric cancer is characterized by enhanced
cell proliferation through various proliferation signals, such as
transforming growth factor-beta signaling (4). The human epidermal growth factor
receptor 2 (HER2) is among the most common oncogenes
involved in this process. The HER2 gene, which has a
structure similar to that of the epidermal growth factor receptor
gene, is involved in cell proliferation and differentiation.
ATTRACTION2 subgroup analysis showed prolonged OS with nivolumab
alone compared to placebo, regardless of HER2 status or whether
patients received anti-HER2 drugs (5).
Furthermore, in 2021, the CheckMate 649 and
ATTRACTION-4 trials demonstrated an add-on effect of nivolumab on
chemotherapy, making this combination therapy the standard
first-line treatment for these patients (6,7).
Although clinical trials have demonstrated the efficacy of
nivolumab, a critical medical need persists to determine the
patient population that would derive the most significant advantage
from its use.
Recent studies have reported a direct effect of
concomitant medications on ICI treatment (8–10). In
particular, antibiotic administration during ICI treatment exhibits
a poor prognosis in various carcinomas (10–12).
However, few studies have compared the prognostic value of ICI
therapy and concurrent drugs for gastric cancer.
Immunotherapy primarily exerts antitumor effects by
modulating the interactions between cancer cells and immune cells,
such as T lymphocytes, natural killer cells, and dendritic cells
(DCs), which are responsible for recognizing and attacking cancer
cells. Specifically, immunotherapy targets key signaling pathways,
such as PD-1/PD-L1 and cytotoxic T-lymphocyte-associated protein 4,
which regulate immune responses to cancer cells (1). Thus, the success of immunotherapy
against cancer depends heavily on the activation of immune
cells.
The circadian rhythm has a well-established impact
on the number of circulating immune cells in the bloodstream
(13). This rhythm influences the
immune parameters of these cells, such as their functional activity
and transport capacity, which vary throughout the day. Therefore,
the effectiveness of cancer immunosurveillance may vary at
different times of the day, emphasizing the significance of the
timing of immunotherapy administration (14). Previous research has proposed that
patients with advanced solid tumors who receive ICI infusions in
the morning may experience longer OS than those who receive
infusions in the afternoon (15–19).
Currently, no information is available regarding the association
between the timing of ICI administration and its clinical
effectiveness in patients with advanced GC.
This study aimed to evaluate the optimal timing of
nivolumab administration in patients with advanced GC to determine
its clinical efficacy.
Materials and methods
Study design and patients
We retrospectively evaluated 58 consecutive patients
with advanced GC (stage IV) who received nivolumab monotherapy
after ≥2 chemotherapy regimens at Kurume University Hospital
(Kurume, Japan) between October 2017 and December 2023. All
patients had histologically confirmed advanced GC with a
performance status (PS) of ≤2. The following data were collected
from medical charts and reviewed: age, sex, PS, previous
gastrectomy, liver metastasis, peritoneal dissemination, number of
organs with metastases, HER2 status, occurrence of immune-related
adverse events (irAEs), serum levels of C-reactive protein (CRP),
carcinoembryonic antigen (CEA), carbohydrate antigen 19-9, and
neutrophil-to-lymphocyte ratio (NLR), treatment method, and
outcome. We defined drug administration (e.g., proton pump
inhibitors (PPIs), nonsteroidal anti-inflammatory drugs (NSAIDs),
opioid analgesics, probiotics, and antibiotics) as drug use during
treatment and/or within 6 months before the date of the first
nivolumab treatment.
The median of all cases was used to stratify the
cutoff for this administration time. For example, if only one cycle
of nivolumab was administered for each case, the time was defined
as the administration time. In cases where multiple cycles of
nivolumab were administered, the median value was defined as the
administration time. The median of all cases was analyzed as the
cutoff value. Patients were divided into two groups according to
the median timing of nivolumab administration: (I) those who
received nivolumab earlier than the median start time (before 11:41
a.m) (early-timing group) and (II) those who received nivolumab
later (after 11:41 a.m) (late-timing group).
This retrospective study was performed at the Kurume
University Hospital (Kurume, Japan). This study conformed to the
principles of the Declaration of Helsinki and was approved by the
Ethics Committee of Kurume University (approval number 21118). An
opt-out approach was employed to obtain informed consent from
patients, and personal information was protected during data
collection.
Evaluation of therapeutic response and
definition of progression-free survival (PFS) and OS
Therapeutic response was evaluated at 8-week
intervals using dynamic computed tomography or magnetic resonance
imaging scans following the Response Evaluation Criteria in Solid
Tumors version 1.1 (20). PFS was
defined as the time from the enrollment date to disease progression
with a 20% or greater increase in the diameters of target lesions
or the appearance of new lesions during treatment or to death from
any cause. OS was defined as the duration from enrollment to death
from any cause.
Nivolumab dosing regimen and
schedule
The patients were intravenously administered
nivolumab at a dose of 240 mg for 30 min once every 2 weeks,
following the clinical guidelines for advanced GC (21). This dose was continued until disease
progression, deterioration of the patient's condition, the onset of
intolerable adverse effects, or the patient's decision to
discontinue treatment.
Statistical analysis
Categorical variables were compared using the
χ2 test or the Fisher's exact test, while continuous
variables were compared using the Wilcoxon rank sum test.
Univariate and multivariate analyses were conducted using the Cox
regression model to identify risk factors associated with OS or
PFS. OS and PFS were calculated using the Kaplan-Meier method and
assessed using the log-rank test. To evaluate the optimal cutoff
value for administered time, we employed the Cutoff Finder
application (22). The optimal
cutoff was defined as the point with the most significant split
(log-rank test). P<0.05 was considered to indicate statistical
significance. Data analysis was performed using JMP Pro version
16.0 and SAS 9.4 (both from the SAS Institute, Inc., NC, USA).
Results
Baseline patient characteristics
In this study, the median timing of nivolumab
administration was 11:41 a.m. Subsequently, we divided the patients
into early- and late-timing groups, comprising a total of 29
patients. The early group received nivolumab between 9:40 and 11:40
a.m., whereas the late group received it between 12:20 and 3:30
p.m. The histograms depict the administration times for both groups
(Fig. S1). In the early timing
group, most cases were administered nivolumab from 10:00 to 11:41
a.m. In contrast, the late timing group received it any time after
11:41 a.m. Baseline patient characteristics are summarized in
Table I. No substantial differences
were observed in age, PS, previous gastrectomy, liver metastasis,
peritoneal dissemination, number of organs with metastases, HER2
status, irAEs, NLR, administration of proton pump inhibitors,
opioid analgesics, NSAIDs, probiotics, antibiotics, serum CRP
levels, or CEA levels between the two groups. However, the
prevalence of male sex was significantly higher in the early-timing
group than in the late-timing group (Table I).
 | Table I.Baseline patient characteristics. |
Table I.
Baseline patient characteristics.
| Characteristic | Early timing
(n=29) | Late timing
(n=29) | P-value |
|---|
| Median time of
administration (IQR) | 10:48 a.m. (9:40
a.m. −11:38 a.m., 9:33 a.m.-11:21 a.m.) | 12:57 p.m. (11:45
a.m. −3:30 p.m., 12:03 p.m.-1:40 p.m.) |
|
| Median age, years
(IQR) | 69 (67–73) | 65 (57–74) | 0.22 |
| Sex,
female/male | 10%/90% (3/26) | 24%/76% (7/22) | 0.30 |
| Performance status,
0/1/2 | 45%/45%/10%
(13/13/3) | 38%/52%/10%
(11/15/3) | 0.86 |
| Previous
gastrectomy, no/yes | 72%/28% (21/8) | 59%/21%
(17/12) | 0.27 |
| Liver metastasis,
absence/presence | 52%/48%
(15/14) | 69%/31% (20/9) | 0.18 |
| Peritoneal
dissemination, absence/presence | 52%/48%
(15/14) | 28%/72% (8/21) | 0.06 |
| Number of organs
with metastases, <2/≥2 | 62%/38%
(18/11) | 62%/38%
(18/11) | 1.00 |
| HER2 status,
negative/positive | 72%/28% (21/8) | 83%/17% (24/5) | 0.34 |
| irAEs, no/yes | 73%/17% (24/5) | 79%/21% (23/6) | 0.73 |
| Median C-reactive
protein, mg/dl (IQR) | 0.30
(0.04–9.03) | 0.39
(0.04–6.04) | 0.27 |
| Median CEA, ng/dl
(IQR) | 3.3 (1.2–82.2) | 5.7 (1.6–226) | 0.14 |
| Median CA19-9, ng/d
(IQR) | 43.8
(2.3–1,537) | 16.5 (2–8,477) | 0.94 |
| Median
neutrophil-to-lymphocyte ratio (IQR) | 2.58
(0.82–6.72) | 3.22
(0.77–18.3) | 0.09 |
| Medicine |
|
|
|
| Proton
pump inhibitor, no/yes | 34%/66%
(10/19) | 28%/72% (8/21) | 0.57 |
| NSAIDs,
no/yes | 55%/45%
(16/13) | 31%/69% (9/20) | 0.06 |
| Opioid
analgesics, no/yes | 62%/38%
(18/11) | 59%/41%
(17/12) | 0.79 |
|
Probiotics, no/yes | 83%/17% (24/5) | 79%/21% (23/6) | 0.74 |
|
Antibiotic, no/yes | 69%/31% (20/9) | 67%/33%
(19/10) | 0.78 |
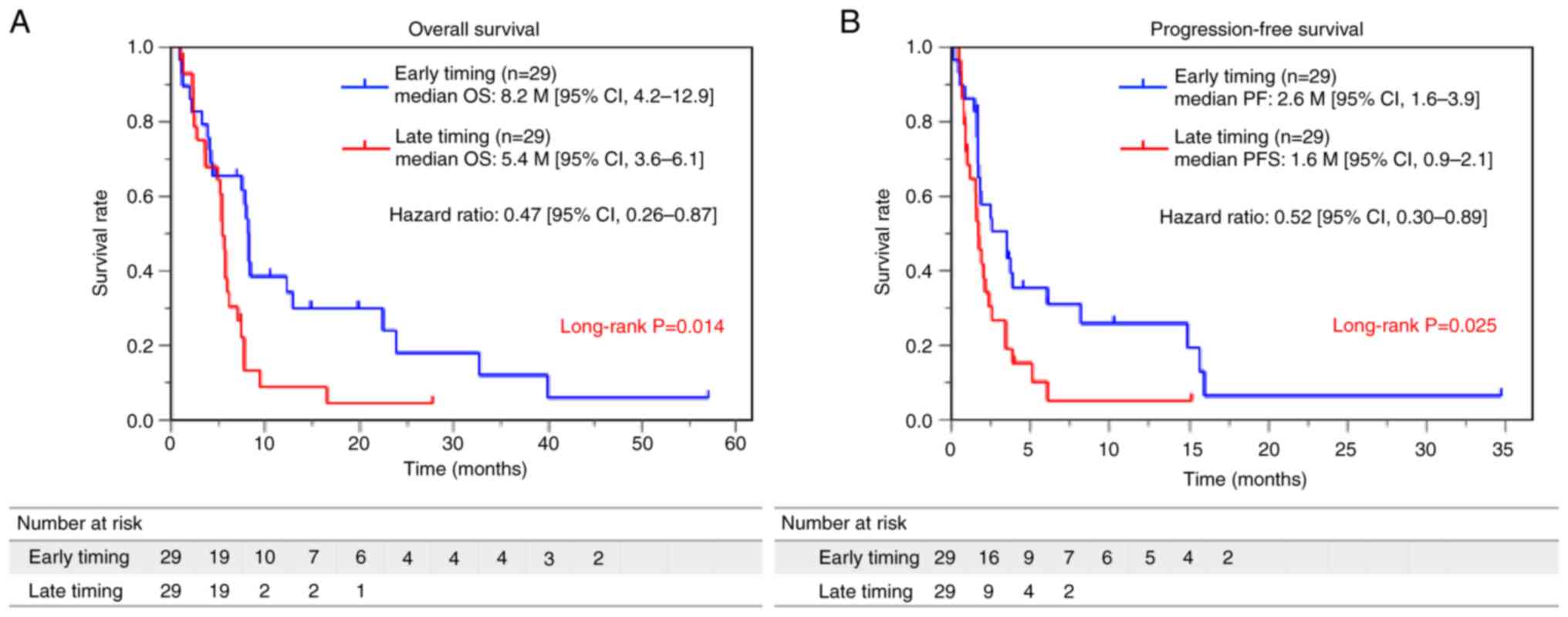
Patients in the early-timing group had
a long PFS and OS with a better therapeutic response
To assess the effect of the timing of drug
administration on PFS and OS, we plotted Kaplan-Meier curves for
the two groups (Fig. 1A and B). The
early-timing group demonstrated a significantly longer OS compared
to the late-timing group (median OS 8.2 months [95% confidence
interval (CI), 4.2–12.9] vs. 5.4 months; 95% CI, 3.6–6.1: hazard
ratio (HR) 0.47; 95% CI, 0.26–0.87) (Fig. 1A). Moreover, patients in the
early-timing group demonstrated a substantially longer PFS than
those in the late-timing group (median PFS 2.6 months, with a 95%
CI of 1.3–3.9 months vs. median PFS 1.6 months, with a 95% CI of
0.9–2.1 months). The HR for the early-timing group was 0.52, with a
95% CI of 0.30–0.89 (Fig. 1B).
The overall response rate (ORR) was greater in the
early-timing group, with three patients achieving a complete or
partial response (17.2%), than in the late-timing group (3.4%)
(Fig. 2). The ORR was higher in the
early-timing group than in the late-timing group (17.2% vs. 3.4%)
(Fig. 2). The disease control rate
was higher in the early-timing group than in the late-timing group
(48.3% vs. 31.0%) (Fig. 2).
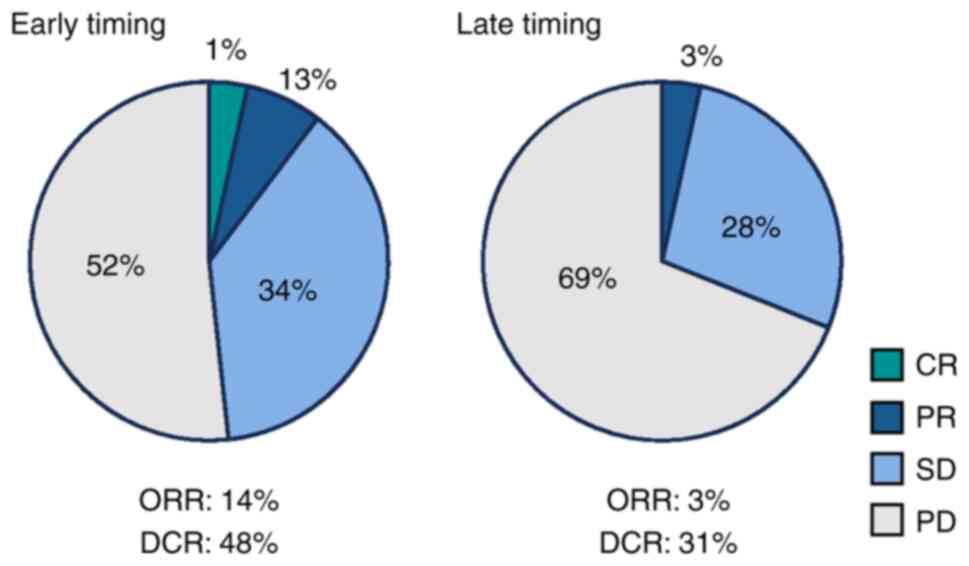 | Figure 2.Best overall response between the
early- and late-timing groups. Pie graphs indicate the percentages
of patients who achieved CR (green), PR (blue), SD (light blue), or
PD (gray). The ORR and DCR were determined as the proportions of
patients who achieved CR, PR, CR, PR, and SD, respectively. CR,
complete response; PR, partial response; SD, stable disease; PD,
progressive disease; ORR, overall response rate; DCR, disease
control rate. |
Evaluation of concomitant medications
and prognosis in treatment with nivolumab
We used Kaplan-Meier survival curves to assess the
effect of concomitant medications with nivolumab on OS (Fig. S2). The group not administered
NSAIDs had a significantly better prognosis than the
NSAID-administered group (median OS 8.2 months [95% CI, 5.4–32.7]
vs. 5.4 months [95% CI, 3.3–6.1]). However, the use of concomitant
medications, such as PPIs, opioid analgesics, probiotics, and
antibiotics, did not affect prognosis.
Univariate Cox model analyses for PFS
and OS
A univariate analysis was performed to identify
factors associated with OS and PFS. In the analysis, the
early-timing group, irAEs, and NSAID administration were associated
with a longer OS. Additionally, univariate analysis showed that
early timing, irAEs, and NSAID administration were associated with
longer PFS (Table II).
 | Table II.Univariate analysis of OS and PFS
using the Cox regression model (n=58). |
Table II.
Univariate analysis of OS and PFS
using the Cox regression model (n=58).
|
|
| OS | PFS |
|---|
|
|
|
|
|
|---|
| Characteristic | Cut-off | HR | 95% CI | P-value | HR | 95% CI | P-value |
|---|
| Age | >65 years | 1.31 | 0.74–2.33 | 0.36 | 1.18 | 0.69–2.01 | 0.53 |
| Sex | Female | 1.26 | 0.59–2.70 | 0.55 | 1.01 | 0.51–2.01 | 0.97 |
| Performance
status | >1 | 1.25 | 0.70–2.24 | 0.46 | 1.07 | 0.63–1.83 | 0.80 |
| Nivolumab
administration timing | Early | 0.47 | 0.26–0.87 | 0.02 | 0.52 | 0.30–0.89 | 0.02 |
| Number of
metastatic sites | ≥2 | 0.92 | 0.51–1.65 | 0.78 | 0.73 | 0.42–1.25 | 0.25 |
| Liver
metastasis | Yes | 0.93 | 0.52–1.66 | 0.80 | 0.88 | 0.51–1.50 | 0.63 |
| Peritoneal
dissemination | Yes | 1.65 | 0.90–3.04 | 0.11 | 1.42 | 0.82–2.44 | 0.20 |
| Previous
gastrectomy | Yes | 1.24 | 0.67–2.30 | 0.50 | 1.51 | 0.86–2.63 | 0.16 |
| IrAE | Yes | 0.27 | 0.12–0.62 | <0.01 | 0.31 | 0.15–0.64 | <0.01 |
| C-reactive
protein | >0.4 mg/dl | 1.11 | 0.63–1.99 | 0.71 | 1.13 | 0.63–2.00 | 0.69 |
| CEA | >5.0 ng/dl | 0.85 | 0.48–1.52 | 0.59 | 1.13 | 0.67–1.91 | 0.65 |
| CA19-9 | >37 ng/dl | 0.87 | 0.49–1.55 | 0.64 | 1.06 | 0.63–1.78 | 0.83 |
|
Neutrophil-to-lymphocyte ratio | >3.0 | 1.10 | 0.62–1.95 | 0.75 | 0.93 | 0.55–1.57 | 0.92 |
| Medicine |
|
|
|
|
|
|
|
| Proton
pump inhibitor | Yes | 0.83 | 0.45–1.54 | 0.56 | 0.70 | 0.37–1.30 | 0.26 |
|
NSAIDs | Yes | 2.66 | 1.42–4.97 | <0.01 | 2.50 | 1.40–4.60 | <0.01 |
| Opioid
analgesics | Yes | 1.31 | 0.73–2.36 | 0.36 | 1.33 | 0.75–2.38 | 0.33 |
|
Probiotics | Yes | 1.45 | 0.71–2.95 | 0.30 | 1.56 | 0.77–3.20 | 0.22 |
|
Antibiotic | Yes | 1.46 | 0.81–2.63 | 0.21 | 1.55 | 0.86–2.79 | 0.14 |
Multivariate analysis and optimizing
the correlation with OS
We performed multivariable Cox regression analysis
to adjust for baseline patient background in the early- and
late-timing groups (Table III).
The early-timing group had significantly better PFS than the
late-timing group in model 1, adjusted for age, sex, and PS (HR,
0.50; 95% CI, 0.29–0.86). In addition, the early-timing group had
significantly better OS than the late-timing group in model 1,
adjusted for age, sex, and PS (HR, 0.45; 95% CI, 0.24–0.85). Next,
in model 2, adjusted for irAEs and NSAIDs, which were identified as
prognostic factors in this study, PFS/OS was significantly longer
in the early-timing group than in the late-timing group (HR 0.42;
95% CI, 0.24–0.76; P=0.004/HR 0.39; 95% CI, 0.19–0.77; P=0.001),
respectively. Finally, in model 3, adjusted for age, sex, PS,
irAEs, and NSAIDs, the early-timing group had significantly longer
PFS than the late-timing group (HR 0.36; 95% CI, 0.20–0.67). In
addition, in model 3, adjusted for age, sex, PS, irAE, and NSAIDs,
the early-timing group had significantly better OS than the
late-timing group (HR 0.34; 95% CI, 0.17–0.69) (Table III).
 | Table III.Multivariate Cox regression analysis
to adjust for baseline patient background. |
Table III.
Multivariate Cox regression analysis
to adjust for baseline patient background.
|
|
| PFS | OS |
|---|
|
|
|
|
|
|---|
| Model | Adjusted value | HR | 95% CI | P-value | HR | 95% CI | P-value |
|---|
| 0 | Unadjusted | 0.52 | 0.30–0.89 | 0.017 | 0.47 | 0.26–0.87 | 0.016 |
| 1 | Age, sex, PS | 0.50 | 0.29–0.86 | 0.013 | 0.45 | 0.24–0.85 | 0.013 |
| 2 | IrAEs, NSAIDs | 0.42 | 0.24–0.76 | 0.004 | 0.39 | 0.19–0.77 | 0.001 |
| 3 | Age, sex, PS,
irAEs, NSAIDs | 0.36 | 0.20–0.67 | 0.001 | 0.34 | 0.17–0.69 | 0.003 |
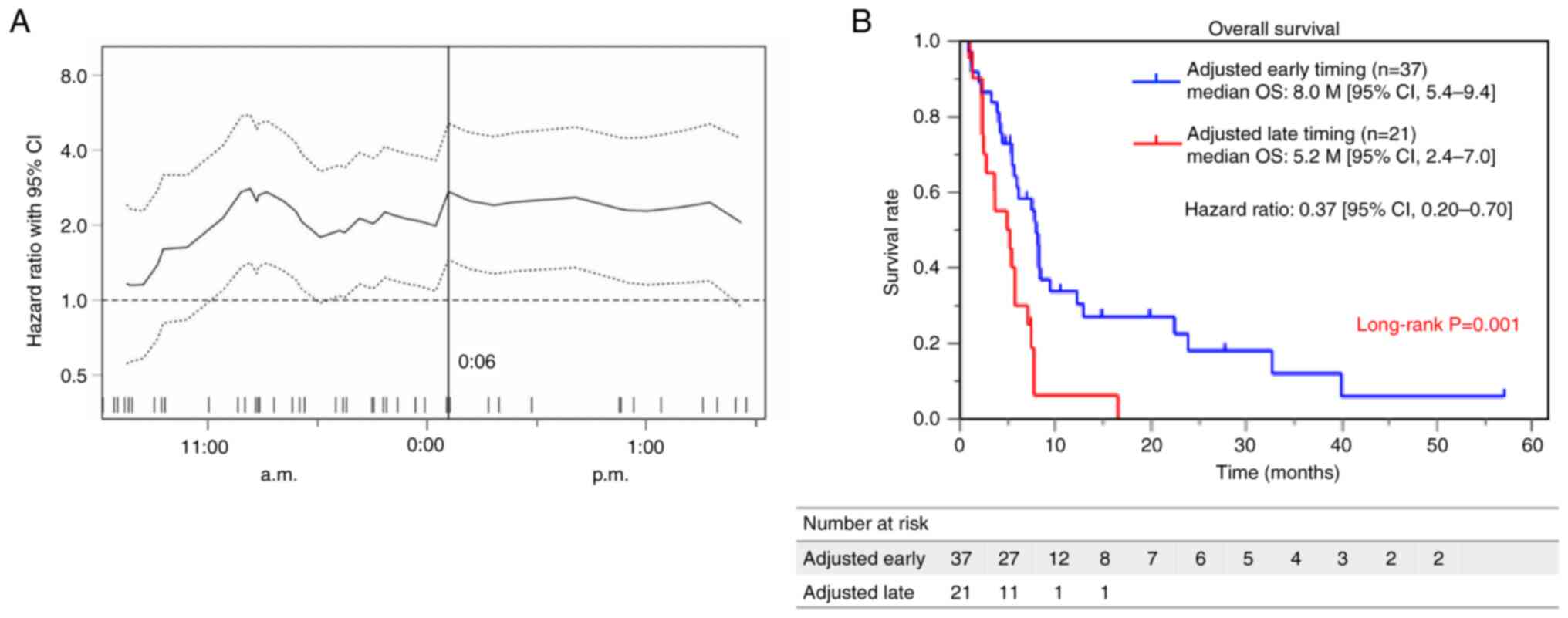
The optimal cutoff value for survival, determined
using the Cutoff Finder application, was 12:06 p.m. (Fig. 3A). We compared the OS between the
two groups, which were divided by this cutoff, using the
Kaplan-Meier curve. Patients in the early-timing group demonstrated
a substantially longer OS than those in the late-timing group
(median OS 8.0 months, with a 95% CI of 5.4–9.4 months vs. median
of 5.2 months, with a 95% CI of 2.4–7.0 months, P=0.001) (Fig. 3B).
Discussion
To the best of our knowledge, this is the first
report on patients with advanced GC stratified according to the
timing of nivolumab infusion. The results of this study indicate
that individuals who received nivolumab infusion earlier in the
day, specifically between 9:40 and 11:40 a.m., experienced
significantly better outcomes in terms of ORR, PFS, and OS than
those who received the infusion later in the day, between 12:20 and
3:30 p.m. These results remained consistent after multivariable Cox
regression analysis. Moreover, Cutoff Finder analysis revealed that
the optimal timing of nivolumab administration for achieving better
outcomes was before 12:06 p.m. The findings of these studies
collectively provide evidence for the enhanced efficacy of
nivolumab administration in the morning.
The relationship between circadian rhythms and the
adaptive immune system is an area of growing research interest,
with potential implications for ICI treatment. The influence of
specific time-of-day patterns of ICI injections on therapeutic
efficacy is highly debated. Several studies have been conducted on
various solid tumors, including melanoma, non-small cell lung
cancer (NSCLC), and colorectal, head and neck, breast, urinary,
renal, and pancreatic cancer. A recent study reported that
pembrolizumab monotherapy for advanced esophageal cancer resulted
in a better prognosis when treatment was initiated in the morning
than in the afternoon (19). These
studies suggest that administering ICIs early in the morning rather
than in the evening improves clinical outcomes (15–19).
Thus, our findings align with a growing body of evidence that
adaptive immune responses are more robust when initially stimulated
in the morning than in the evening. The circadian oscillation of
lymphocytes is believed to be an underlying mechanism. According to
research conducted by Wang et al, the capacity of immune
cells to identify and eradicate cancer cells depends on their
biological clock (23). According
to various experiments and human studies, CD8+ T
lymphocytes promote blood circulation at night and distinguish
cancer antigens in the early morning to eradicate target cells
based on the serum level of cortisol (24,25).
In cellular experiments on melanoma and colorectal cancer,
treatment with anti-PD-1 antibody therapy was more effective in the
morning than in the evening (26).
This variation in efficacy based on timing was critically dependent
on CD8+ T cells, and anti-PD-1 therapy was ineffective in the
evening because CD8+ T cells were depleted (26). According to recent studies, DCs and
CD8+ T cells are crucial in regulating tumor volume
through a circadian antitumor function. Additionally, the rhythmic
migration of DCs to lymph nodes draining from the tumor appears to
be a key factor in the circadian response of tumor antigen-specific
CD8+ T lymphocytes, which is dependent on the circadian
expression of the costimulatory molecule CD80 (23). Another study found that T
lymphocytes did not migrate from the blood to the lymph nodes in
mice during the latter part of the nocturnal active period
(27). This corresponds to the
afternoon and evening in humans, which may explain the reason for
the observed differences in nivolumab efficacy. In a phase I study
of nivolumab, pharmacokinetic parameters of nivolumab
administration at 10 mg/kg, with ≥1 dose administered, showed a
Tmax of 3 h (1.0–9.0 h) (28).
Therefore, nivolumab therapy in the morning, when CD8+ T cells are
activated, is likely to reach an effective blood concentration and
reflect the therapeutic effect.
Consistent with our previous report using decision
tree analysis, which identified irAE development as the first
divergence variable and as a prognostic factor in patients with
advanced GC (29), we also found
that irAE development was a good prognostic factor. The extended
observation period in this study was considered to clarify whether
irAE development was correlated with the prolongation of PFS in
addition to OS. IrAE development has been reported to be closely
linked to favorable outcomes in patients with GC (30). Although the exact reason for this
association remains unclear, recent studies have proposed potential
mechanisms, including ICI-activated CD8+ T lymphocytes that target
common antigens in tumors and healthy tissues, leading to irAEs and
antitumor efficacy (31–33). Consequently, the more potent
immune-mediated anti-tumor effects of ICIs imply a similar
potential for the development of irAEs. However, the current study
found no significant differences in the frequency of irAEs,
regardless of whether the timing of nivolumab administration was
early or late.
In our study results, PPIs, opioid analgesics,
probiotics, and antibiotics did not affect prognosis, whereas
concomitant use of NSAIDs exhibited poor prognosis. When
administering ICI treatment, considering the influence of
concurrent medications on prognosis is essential. Several studies
have indicated a dismal outlook on administering antibiotics before
administering ICIs for cancer treatment (10–12).
This may be because antibiotics affect cytokine release and immune
responses by altering the composition of the gut microbiota
(34). The impact of the timing of
antibiotic exposure on the gut microbiota and response to ICIs has
been found to be significant, with negative consequences reported
when exposure occurs within a year before ICI administration
(12). Our findings, which are
inconsistent with those of previous research (20), suggest that antibiotic exposure has
no impact on OS when ICIs are administered. However, our study only
evaluated antibiotic exposure within 6 months prior to treatment,
and the sample size was limited. No adjustments were made for
potential confounding factors. Inconsistent with reports on
patients with NSCLC and renal cell carcinoma treated with ICIs
(9,35), the use of NSAIDs was identified as a
poor prognostic factor in patients with advanced GC treated with
ICIs. The underlying mechanisms are thought to be that NSAIDs
increase the intratumoral accumulation of CD8+ T
lymphocytes and alter the tumor inflammatory environment to favor T
cell activation by inhibiting the cyclooxygenase-2 pathway
(36). However, a recent
meta-analysis showed that the use of NSAIDs did not affect the
prognosis (37). Therefore, the
relationship between NSAID use and the prognosis of ICI-treated
patients remains controversial. These results should be interpreted
with caution and may change with the inclusion of additional cases
in future studies. This study had several limitations. First, the
sample size was relatively small, and the study was conducted
retrospectively at a single institution in Japan. Additionally, the
role of circadian rhythms in CD8+ T cells and the impact of
circadian rhythms on other immune functions remain unclear.
Furthermore, only the results of nivolumab-only treatment were
available in this study. Finally, in this study, the median start
time of treatment for all patients was employed to determine
whether administration time was earlier or later than the median.
The median was adopted because few patients received treatment
extremely late in the afternoon or early in the morning; however,
future studies should investigate randomizing administration
between the two groups, early in the morning and late in the
afternoon. To address these limitations, future studies should
include large-scale clinical trials and detailed immunological
evaluations.
In conclusion, we demonstrated that nivolumab
administration in the morning, particularly before 12:06 p.m., was
a favorable factor associated with longer OS and PFS in patients
with advanced GC. These outcomes contribute to the expanding body
of research on the early administration of ICIs. Furthermore, since
the combination of chemotherapy and ICIs is now the mainstream
therapy, we believe that it would be worthwhile to examine whether
the treatment effect of chemotherapy plus ICI therapy also varies
with administration time.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
TT and HS conceived the study and wrote the
manuscript. SY, YS and SN collected the clinical data, and made
substantial contributions to the conception and design of this
study. KMu, FF, KMi and TK were involved in raw data analysis. All
authors discussed the results and contributed to the final
manuscript. TT and KMu confirm the authenticity of all the raw
data. All authors have read and approved the final version of the
manuscript.
Ethics approval and consent to
participate
This study was conducted in accordance with the
Declaration of Helsinki, and reviewed and approved by the Ethics
Committee of Kurume University (approval no. 21118; Kurume, Japan).
An opt-out approach was employed to obtain informed consent from
patients, and personal information was protected during data
collection.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
ICI
|
immune checkpoint inhibitor
|
|
GC
|
gastric cancer
|
|
CI
|
confidence interval
|
|
PFS
|
progression-free survival
|
|
HER2
|
human epidermal growth factor receptor
2
|
|
DCs
|
dendritic cells
|
|
PS
|
performance status
|
|
irAEs
|
immune-related adverse events
|
|
CRP
|
C-reactive protein
|
|
CEA
|
carcinoembryonic antigen
|
|
NLR
|
neutrophil-to-lymphocyte ratio
|
|
NSAIDs
|
nonsteroidal anti-inflammatory
drugs
|
|
HR
|
hazard ratio
|
|
ORR
|
overall response rate
|
|
NSCLC
|
non-small cell lung cancer
|
|
OS
|
overall survival
|
References
|
1
|
Mellman I, Coukos G and Dranoff G: Cancer
immunotherapy comes of age. Nature. 480:480–489. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kang YK, Boku N, Satoh T, Ryu MH, Chao Y,
Kato K, Chung HC, Chen JS, Muro K, Kang WK, et al: Nivolumab in
patients with advanced gastric or gastro-oesophageal junction
cancer refractory to, or intolerant of, at least two previous
chemotherapy regimens (ONO-4538-12, ATTRACTION-2): A randomised,
double-blind, placebo-controlled, phase 3 trial. Lancet.
390:2461–2471. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kato K, Satoh T, Muro K, Yoshikawa T,
Tamura T, Hamamoto Y, Chin K, Minashi K, Tsuda M, Yamaguchi K, et
al: A subanalysis of Japanese patients in a randomized,
double-blind, placebo-controlled, phase 3 trial of nivolumab for
patients with advanced gastric or gastro-esophageal junction cancer
refractory to, or intolerant of, at least two previous chemotherapy
regimens (ONO-4538-12, ATTRACTION-2). Gastric Cancer. 22:344–354.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Gao S, Tan H and Li D: Oridonin suppresses
gastric cancer SGC-7901 cell proliferation by targeting the
TNF-alpha/androgen receptor/TGF-beta signalling pathway axis. J
Cell Mol Med. 27:2661–2674. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Satoh T, Kang YK, Chao Y, Ryu MH, Kato K,
Cheol Chung H, Chen JS, Muro K, Ki Kang W, Yeh KH, et al:
Exploratory subgroup analysis of patients with prior trastuzumab
use in the ATTRACTION-2 trial: A randomized phase III clinical
trial investigating the efficacy and safety of nivolumab in
patients with advanced gastric/gastroesophageal junction cancer.
Gastric Cancer. 23:143–153. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Janjigian YY, Shitara K, Moehler M,
Garrido M, Salman P, Shen L, Wyrwicz L, Yamaguchi K, Skoczylas T,
Campos Bragagnoli A, et al: First-line nivolumab plus chemotherapy
versus chemotherapy alone for advanced gastric, gastro-oesophageal
junction, and oesophageal adenocarcinoma (CheckMate 649): A
randomised, open-label, phase 3 trial. Lancet. 398:27–40. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kang YK, Chen LT, Ryu MH, Oh DY, Oh SC,
Chung HC, Lee KW, Omori T, Shitara K, Sakuramoto S, et al:
Nivolumab plus chemotherapy versus placebo plus chemotherapy in
patients with HER2-negative, untreated, unresectable advanced or
recurrent gastric or gastro-oesophageal junction cancer
(ATTRACTION-4): A randomised, multicentre, double-blind,
placebo-controlled, phase 3 trial. Lancet Oncol. 23:234–247. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Rizzo A, Santoni M, Mollica V, Ricci AD,
Calabrò C, Cusmai A, Gadaleta-Caldarola G, Palmiotti G and Massari
F: The impact of concomitant proton pump inhibitors on
immunotherapy efficacy among patients with urothelial Carcinoma: A
meta-analysis. J Pers Med. 12:8422022. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Sebastian NT, Stokes WA, Behera M, Jiang
R, Gutman DA, Huang Z, Burns A, Sukhatme V, Lowe MC, Ramalingam SS,
et al: The association of improved overall survival with NSAIDs in
non-small cell lung cancer patients receiving immune checkpoint
inhibitors. Clin Lung Cancer. 24:287–294. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wilson BE, Routy B, Nagrial A and Chin VT:
The effect of antibiotics on clinical outcomes in immune-checkpoint
blockade: A systematic review and meta-analysis of observational
studies. Cancer Immunol Immunother. 69:343–354. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lurienne L, Cervesi J, Duhalde L, de
Gunzburg J, Andremont A, Zalcman G, Buffet R and Bandinelli PA:
NSCLC immunotherapy efficacy and antibiotic use: A systematic
review and meta-analysis. J Thorac Oncol. 15:1147–1159. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Eng L, Sutradhar R, Niu Y, Liu N, Liu Y,
Kaliwal Y, Powis ML, Liu G, Peppercorn JM, Bedard PL and
Krzyzanowska MK: Impact of antibiotic exposure before immune
checkpoint inhibitor treatment on overall survival in older adults
with cancer: A population-based study. J Clin Oncol. 41:3122–3134.
2023. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Balachandran DD, Bashoura L, Sheshadri A,
Manzullo E and Faiz SA: The impact of immunotherapy on sleep and
circadian rhythms in patients with cancer. Front Oncol.
13:12952672023. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Man K, Loudon A and Chawla A: Immunity
around the clock. Science. 354:999–1003. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Catozzi S, Assaad S, Delrieu L, Favier B,
Dumas E, Hamy AS, Latouche A, Crochet H, Blay JY, Mullaert J, et
al: Early morning immune checkpoint blockade and overall survival
of patients with metastatic cancer: An In-depth chronotherapeutic
study. Eur J Cancer. 199:1135712024. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Yeung C, Kartolo A, Tong J, Hopman W and
Baetz T: Association of circadian timing of initial infusions of
immune checkpoint inhibitors with survival in advanced melanoma.
Immunotherapy. 15:819–826. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Karaboue A, Collon T, Pavese I, Bodiguel
V, Cucherousset J, Zakine E, Innominato PF, Bouchahda M, Adam R and
Lévi F: Time-Dependent efficacy of checkpoint inhibitor nivolumab:
Results from a pilot study in patients with metastatic
non-small-cell lung cancer. Cancers (Basel). 14:8962022. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Qian DC, Kleber T, Brammer B, Xu KM,
Switchenko JM, Janopaul-Naylor JR, Zhong J, Yushak ML, Harvey RD,
Paulos CM, et al: Effect of immunotherapy time-of-day infusion on
overall survival among patients with advanced melanoma in the USA
(MEMOIR): A propensity score-matched analysis of a single-centre,
longitudinal study. Lancet Oncol. 22:1777–1786. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Nomura M, Hosokai T, Tamaoki M, Yokoyama
A, Matsumoto S and Muto M: Timing of the infusion of nivolumab for
patients with recurrent or metastatic squamous cell carcinoma of
the esophagus influences its efficacy. Esophagus. 20:722–731. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Eisenhauer EA, Therasse P, Bogaerts J,
Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S,
Mooney M, et al: New response evaluation criteria in solid tumours:
Revised RECIST guideline (version 1.1). Eur J Cancer. 45:228–247.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ajani JA, D'Amico TA, Bentrem DJ, Chao J,
Cooke D, Corvera C, Das P, Enzinger PC, Enzler T, Fanta P, et al:
Gastric cancer, version 2.2022, NCCN clinical practice guidelines
in oncology. J Natl Compr Canc Netw. 20:167–192. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Budczies J, Klauschen F, Sinn BV, Győrffy
B, Schmitt WD, Darb-Esfahani S and Denkert C: Cutoff Finder: A
comprehensive and straightforward Web application enabling rapid
biomarker cutoff optimization. PLoS One. 7:e518622012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wang C, Barnoud C, Cenerenti M, Sun M,
Caffa I, Kizil B, Bill R, Liu Y, Pick R, Garnier L, et al:
Dendritic cells direct circadian anti-tumour immune responses.
Nature. 614:136–143. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Lemmer B, Scheidel B and Behne S:
Chronopharmacokinetics and chronopharmacodynamics of cardiovascular
active drugs. Propranolol, organic nitrates, nifedipine. Ann N Y
Acad Sci. 618:166–181. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Besedovsky L, Born J and Lange T: Blockade
of mineralocorticoid receptors enhances naive T-helper cell counts
during early sleep in humans. Brain Behav Immun. 26:1116–1121.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Wang C, Zeng Q, Gul ZM, Wang S, Pick R,
Cheng P, Bill R, Wu Y, Naulaerts S, Barnoud C, et al: Circadian
tumor infiltration and function of CD8(+) T cells dictate
immunotherapy efficacy. Cell. 187:2690–2702. e172024. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Druzd D, Matveeva O, Ince L, Harrison U,
He W, Schmal C, Herzel H, Tsang AH, Kawakami N, Leliavski A, et al:
Lymphocyte circadian clocks control lymph node trafficking and
adaptive immune responses. Immunity. 46:120–132. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Yamamoto N, Nokihara H, Yamada Y, Shibata
T, Tamura Y, Seki Y, Honda K, Tanabe Y, Wakui H and Tamura T: Phase
I study of Nivolumab, an anti-PD-1 antibody, in patients with
malignant solid tumors. Invest New Drugs. 35:207–216. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Tanaka T, Miwa K, Shimotsuura Y, Nagasu S,
Shigyou H, Hirota K, Koya S, Akagi Y and Kawaguchi T: High
intramuscular adipose tissue content was a favorable prognostic
factor in patients with advanced gastric cancer treated with
nivolumab monotherapy. J Gastroenterol Hepatol. 38:1760–1767. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Hara Y, Baba Y, Toihata T, Harada K, Ogawa
K, Iwatsuki M, Iwagami S, Miyamoto Y, Yoshida N and Baba H:
Immune-related adverse events and prognosis in patients with upper
gastrointestinal cancer treated with nivolumab. J Gastrointest
Oncol. 13:2779–2788. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Ding P, Liu P, Meng L and Zhao Q:
Mechanisms and biomarkers of immune-related adverse events in
gastric cancer. Eur J Med Res. 28:4922023. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Wang Y, Zou J, Li Y, Jiao X, Wang Y, Zhuo
N, Gao M, Gong J, Li J, Zhang X, et al: Serological biomarkers
predict immune-related adverse events and clinical benefit in
patients with advanced gastrointestinal cancers. Front Immunol.
13:9875682022. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Ando T, Ueda A, Ogawa K, Motoo I, Kajiura
S, Nakajima T, Hirano K, Okumura T, Tsukada K, Hara T, et al:
Prognosis of immune-related adverse events in patients with
advanced gastric cancer treated with nivolumab or pembrolizumab: A
multicenter retrospective analysis. In Vivo. 35:475–482. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Pinato DJ, Howlett S, Ottaviani D, Urus H,
Patel A, Mineo T, Brock C, Power D, Hatcher O, Falconer A, et al:
Association of prior antibiotic treatment with survival and
response to immune checkpoint inhibitor therapy in patients with
cancer. JAMA Oncol. 5:1774–1778. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Zhang Y, Kumar P, Adashek JJ, Skelton WP
IV, Li J, Vosoughi A, Chahoud J, Manley BJ and Spiess PE: Adding
cyclooxygenase inhibitors to immune checkpoint inhibitors did not
improve outcomes in metastatic renal cell carcinoma. Cells.
11:25052022. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Pelly VS, Moeini A, Roelofsen LM, Bonavita
E, Bell CR, Hutton C, Blanco-Gomez A, Banyard A, Bromley CP,
Flanagan E, et al: Anti-Inflammatory drugs remodel the tumor immune
environment to enhance immune checkpoint blockade efficacy. Cancer
Discov. 11:2602–2619. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Mao Z, Jia X, Jiang P, Wang Q, Zhang Y, Li
Y, Fu X, Jiao M, Jiang L, Liu Z and Guo H: Effect of concomitant
use of analgesics on prognosis in patients treated with immune
checkpoint inhibitors: A systematic review and meta-analysis. Front
Immunol. 13:8617232022. View Article : Google Scholar : PubMed/NCBI
|