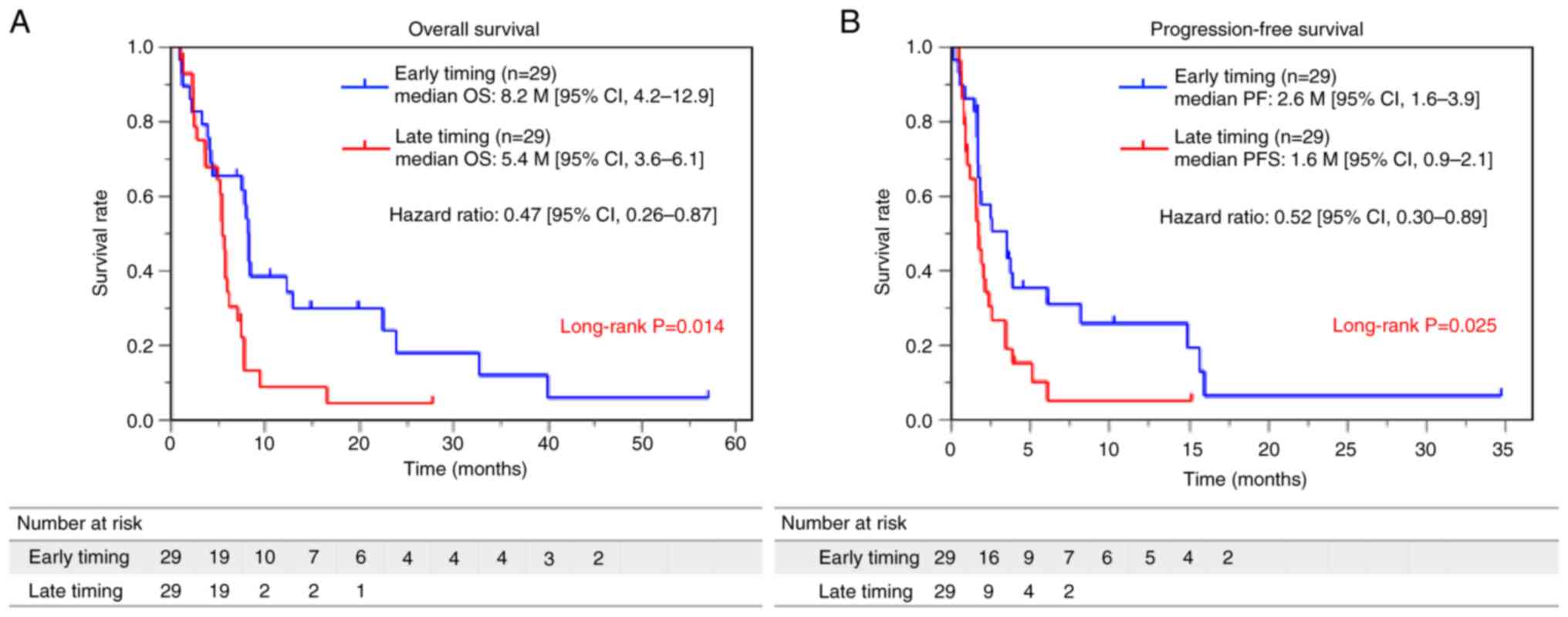
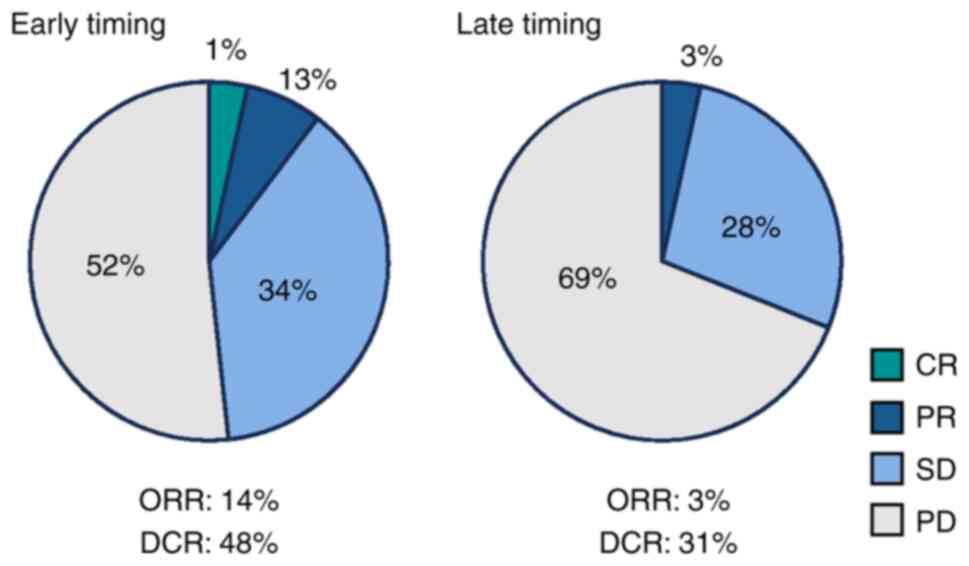
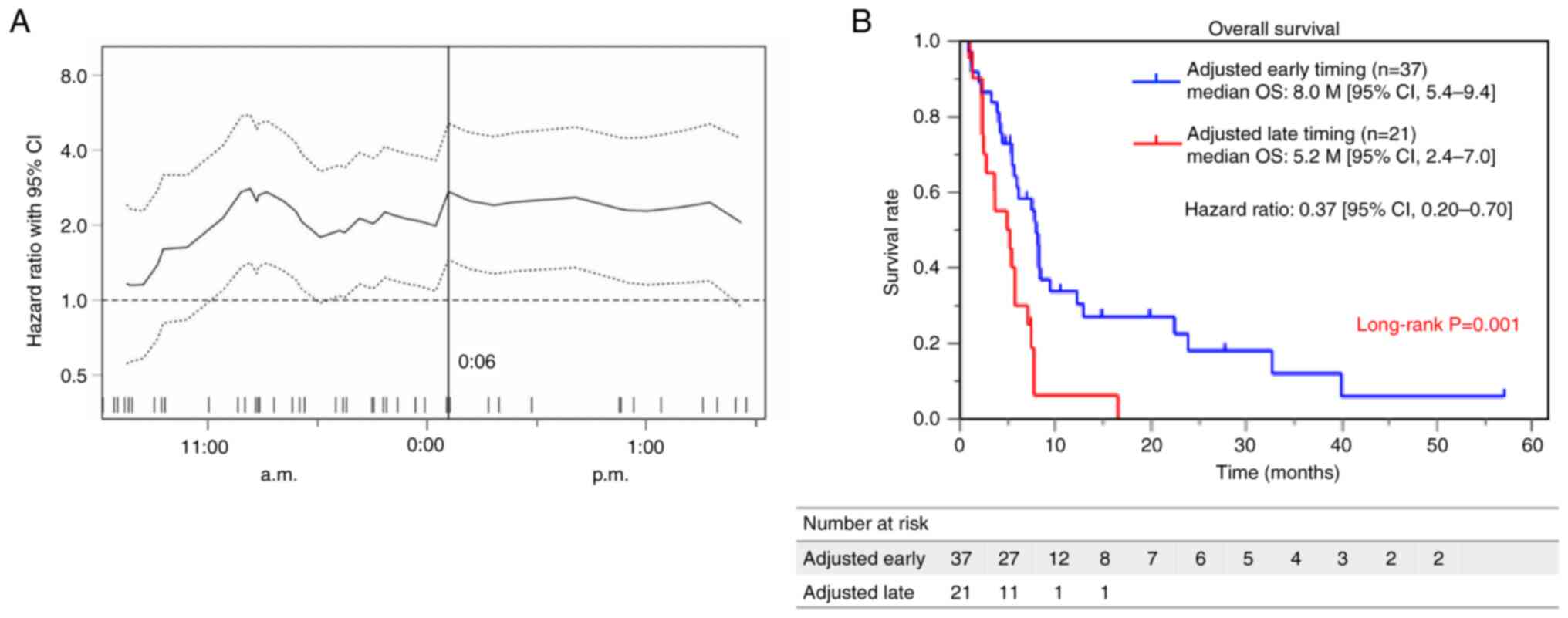
|
1
|
Mellman I, Coukos G and Dranoff G: Cancer
immunotherapy comes of age. Nature. 480:480–489. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kang YK, Boku N, Satoh T, Ryu MH, Chao Y,
Kato K, Chung HC, Chen JS, Muro K, Kang WK, et al: Nivolumab in
patients with advanced gastric or gastro-oesophageal junction
cancer refractory to, or intolerant of, at least two previous
chemotherapy regimens (ONO-4538-12, ATTRACTION-2): A randomised,
double-blind, placebo-controlled, phase 3 trial. Lancet.
390:2461–2471. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kato K, Satoh T, Muro K, Yoshikawa T,
Tamura T, Hamamoto Y, Chin K, Minashi K, Tsuda M, Yamaguchi K, et
al: A subanalysis of Japanese patients in a randomized,
double-blind, placebo-controlled, phase 3 trial of nivolumab for
patients with advanced gastric or gastro-esophageal junction cancer
refractory to, or intolerant of, at least two previous chemotherapy
regimens (ONO-4538-12, ATTRACTION-2). Gastric Cancer. 22:344–354.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Gao S, Tan H and Li D: Oridonin suppresses
gastric cancer SGC-7901 cell proliferation by targeting the
TNF-alpha/androgen receptor/TGF-beta signalling pathway axis. J
Cell Mol Med. 27:2661–2674. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Satoh T, Kang YK, Chao Y, Ryu MH, Kato K,
Cheol Chung H, Chen JS, Muro K, Ki Kang W, Yeh KH, et al:
Exploratory subgroup analysis of patients with prior trastuzumab
use in the ATTRACTION-2 trial: A randomized phase III clinical
trial investigating the efficacy and safety of nivolumab in
patients with advanced gastric/gastroesophageal junction cancer.
Gastric Cancer. 23:143–153. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Janjigian YY, Shitara K, Moehler M,
Garrido M, Salman P, Shen L, Wyrwicz L, Yamaguchi K, Skoczylas T,
Campos Bragagnoli A, et al: First-line nivolumab plus chemotherapy
versus chemotherapy alone for advanced gastric, gastro-oesophageal
junction, and oesophageal adenocarcinoma (CheckMate 649): A
randomised, open-label, phase 3 trial. Lancet. 398:27–40. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kang YK, Chen LT, Ryu MH, Oh DY, Oh SC,
Chung HC, Lee KW, Omori T, Shitara K, Sakuramoto S, et al:
Nivolumab plus chemotherapy versus placebo plus chemotherapy in
patients with HER2-negative, untreated, unresectable advanced or
recurrent gastric or gastro-oesophageal junction cancer
(ATTRACTION-4): A randomised, multicentre, double-blind,
placebo-controlled, phase 3 trial. Lancet Oncol. 23:234–247. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Rizzo A, Santoni M, Mollica V, Ricci AD,
Calabrò C, Cusmai A, Gadaleta-Caldarola G, Palmiotti G and Massari
F: The impact of concomitant proton pump inhibitors on
immunotherapy efficacy among patients with urothelial Carcinoma: A
meta-analysis. J Pers Med. 12:8422022. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Sebastian NT, Stokes WA, Behera M, Jiang
R, Gutman DA, Huang Z, Burns A, Sukhatme V, Lowe MC, Ramalingam SS,
et al: The association of improved overall survival with NSAIDs in
non-small cell lung cancer patients receiving immune checkpoint
inhibitors. Clin Lung Cancer. 24:287–294. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wilson BE, Routy B, Nagrial A and Chin VT:
The effect of antibiotics on clinical outcomes in immune-checkpoint
blockade: A systematic review and meta-analysis of observational
studies. Cancer Immunol Immunother. 69:343–354. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lurienne L, Cervesi J, Duhalde L, de
Gunzburg J, Andremont A, Zalcman G, Buffet R and Bandinelli PA:
NSCLC immunotherapy efficacy and antibiotic use: A systematic
review and meta-analysis. J Thorac Oncol. 15:1147–1159. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Eng L, Sutradhar R, Niu Y, Liu N, Liu Y,
Kaliwal Y, Powis ML, Liu G, Peppercorn JM, Bedard PL and
Krzyzanowska MK: Impact of antibiotic exposure before immune
checkpoint inhibitor treatment on overall survival in older adults
with cancer: A population-based study. J Clin Oncol. 41:3122–3134.
2023. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Balachandran DD, Bashoura L, Sheshadri A,
Manzullo E and Faiz SA: The impact of immunotherapy on sleep and
circadian rhythms in patients with cancer. Front Oncol.
13:12952672023. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Man K, Loudon A and Chawla A: Immunity
around the clock. Science. 354:999–1003. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Catozzi S, Assaad S, Delrieu L, Favier B,
Dumas E, Hamy AS, Latouche A, Crochet H, Blay JY, Mullaert J, et
al: Early morning immune checkpoint blockade and overall survival
of patients with metastatic cancer: An In-depth chronotherapeutic
study. Eur J Cancer. 199:1135712024. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Yeung C, Kartolo A, Tong J, Hopman W and
Baetz T: Association of circadian timing of initial infusions of
immune checkpoint inhibitors with survival in advanced melanoma.
Immunotherapy. 15:819–826. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Karaboue A, Collon T, Pavese I, Bodiguel
V, Cucherousset J, Zakine E, Innominato PF, Bouchahda M, Adam R and
Lévi F: Time-Dependent efficacy of checkpoint inhibitor nivolumab:
Results from a pilot study in patients with metastatic
non-small-cell lung cancer. Cancers (Basel). 14:8962022. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Qian DC, Kleber T, Brammer B, Xu KM,
Switchenko JM, Janopaul-Naylor JR, Zhong J, Yushak ML, Harvey RD,
Paulos CM, et al: Effect of immunotherapy time-of-day infusion on
overall survival among patients with advanced melanoma in the USA
(MEMOIR): A propensity score-matched analysis of a single-centre,
longitudinal study. Lancet Oncol. 22:1777–1786. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Nomura M, Hosokai T, Tamaoki M, Yokoyama
A, Matsumoto S and Muto M: Timing of the infusion of nivolumab for
patients with recurrent or metastatic squamous cell carcinoma of
the esophagus influences its efficacy. Esophagus. 20:722–731. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Eisenhauer EA, Therasse P, Bogaerts J,
Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S,
Mooney M, et al: New response evaluation criteria in solid tumours:
Revised RECIST guideline (version 1.1). Eur J Cancer. 45:228–247.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ajani JA, D'Amico TA, Bentrem DJ, Chao J,
Cooke D, Corvera C, Das P, Enzinger PC, Enzler T, Fanta P, et al:
Gastric cancer, version 2.2022, NCCN clinical practice guidelines
in oncology. J Natl Compr Canc Netw. 20:167–192. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Budczies J, Klauschen F, Sinn BV, Győrffy
B, Schmitt WD, Darb-Esfahani S and Denkert C: Cutoff Finder: A
comprehensive and straightforward Web application enabling rapid
biomarker cutoff optimization. PLoS One. 7:e518622012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wang C, Barnoud C, Cenerenti M, Sun M,
Caffa I, Kizil B, Bill R, Liu Y, Pick R, Garnier L, et al:
Dendritic cells direct circadian anti-tumour immune responses.
Nature. 614:136–143. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Lemmer B, Scheidel B and Behne S:
Chronopharmacokinetics and chronopharmacodynamics of cardiovascular
active drugs. Propranolol, organic nitrates, nifedipine. Ann N Y
Acad Sci. 618:166–181. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Besedovsky L, Born J and Lange T: Blockade
of mineralocorticoid receptors enhances naive T-helper cell counts
during early sleep in humans. Brain Behav Immun. 26:1116–1121.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Wang C, Zeng Q, Gul ZM, Wang S, Pick R,
Cheng P, Bill R, Wu Y, Naulaerts S, Barnoud C, et al: Circadian
tumor infiltration and function of CD8(+) T cells dictate
immunotherapy efficacy. Cell. 187:2690–2702. e172024. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Druzd D, Matveeva O, Ince L, Harrison U,
He W, Schmal C, Herzel H, Tsang AH, Kawakami N, Leliavski A, et al:
Lymphocyte circadian clocks control lymph node trafficking and
adaptive immune responses. Immunity. 46:120–132. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Yamamoto N, Nokihara H, Yamada Y, Shibata
T, Tamura Y, Seki Y, Honda K, Tanabe Y, Wakui H and Tamura T: Phase
I study of Nivolumab, an anti-PD-1 antibody, in patients with
malignant solid tumors. Invest New Drugs. 35:207–216. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Tanaka T, Miwa K, Shimotsuura Y, Nagasu S,
Shigyou H, Hirota K, Koya S, Akagi Y and Kawaguchi T: High
intramuscular adipose tissue content was a favorable prognostic
factor in patients with advanced gastric cancer treated with
nivolumab monotherapy. J Gastroenterol Hepatol. 38:1760–1767. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Hara Y, Baba Y, Toihata T, Harada K, Ogawa
K, Iwatsuki M, Iwagami S, Miyamoto Y, Yoshida N and Baba H:
Immune-related adverse events and prognosis in patients with upper
gastrointestinal cancer treated with nivolumab. J Gastrointest
Oncol. 13:2779–2788. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Ding P, Liu P, Meng L and Zhao Q:
Mechanisms and biomarkers of immune-related adverse events in
gastric cancer. Eur J Med Res. 28:4922023. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Wang Y, Zou J, Li Y, Jiao X, Wang Y, Zhuo
N, Gao M, Gong J, Li J, Zhang X, et al: Serological biomarkers
predict immune-related adverse events and clinical benefit in
patients with advanced gastrointestinal cancers. Front Immunol.
13:9875682022. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Ando T, Ueda A, Ogawa K, Motoo I, Kajiura
S, Nakajima T, Hirano K, Okumura T, Tsukada K, Hara T, et al:
Prognosis of immune-related adverse events in patients with
advanced gastric cancer treated with nivolumab or pembrolizumab: A
multicenter retrospective analysis. In Vivo. 35:475–482. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Pinato DJ, Howlett S, Ottaviani D, Urus H,
Patel A, Mineo T, Brock C, Power D, Hatcher O, Falconer A, et al:
Association of prior antibiotic treatment with survival and
response to immune checkpoint inhibitor therapy in patients with
cancer. JAMA Oncol. 5:1774–1778. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Zhang Y, Kumar P, Adashek JJ, Skelton WP
IV, Li J, Vosoughi A, Chahoud J, Manley BJ and Spiess PE: Adding
cyclooxygenase inhibitors to immune checkpoint inhibitors did not
improve outcomes in metastatic renal cell carcinoma. Cells.
11:25052022. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Pelly VS, Moeini A, Roelofsen LM, Bonavita
E, Bell CR, Hutton C, Blanco-Gomez A, Banyard A, Bromley CP,
Flanagan E, et al: Anti-Inflammatory drugs remodel the tumor immune
environment to enhance immune checkpoint blockade efficacy. Cancer
Discov. 11:2602–2619. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Mao Z, Jia X, Jiang P, Wang Q, Zhang Y, Li
Y, Fu X, Jiao M, Jiang L, Liu Z and Guo H: Effect of concomitant
use of analgesics on prognosis in patients treated with immune
checkpoint inhibitors: A systematic review and meta-analysis. Front
Immunol. 13:8617232022. View Article : Google Scholar : PubMed/NCBI
|