Introduction
Non-small cell lung cancer (NSCLC) accounts for ~85%
of all diagnosed cases of lung cancer, which is a predominant cause
of cancer-related deaths worldwide (1). Currently, the treatment approaches for
NSCLC include surgery, chemotherapy, radiotherapy, immunotherapy
and targeted therapy, which have achieved notable advances
(2). However, NSCLC remains an
incurable disease and a proportion of patients with NSCLC cannot
benefit from the aforementioned treatment strategies, thus
resulting in disease progression and severely affecting patient
prognosis (3–5). In addition, the 5-year survival rate
in patients with NSCLC, which ranges between 20 and 50%, remains
poor (6–8). Therefore, exploring potential
pharmacological agents to attenuate NSCLC progression to improve
patient prognosis is of great importance.
Mucosa-associated lymphoid tissue lymphoma
translocation protein 1 (MALT1) is a type of paracaspase, which is
associated with the pathogenesis and progression of several types
of cancer, including lung cancer (9,10). A
previous study indicated that MALT1 silencing can inhibit the
proliferation, migration, invasion and tumor-formation abilities of
hepatocellular carcinoma (HCC) cells via hindering the nuclear
factor-κB (NF-κB) pathway (11).
Furthermore, another study showed that MALT1 enhances the
proliferation and colony-forming abilities, and inhibits the
apoptosis of prostate cancer cells, possibly due to its regulation
of the NF-κB, Wnt/β-catenin and transforming growth factor β
pathways (12). Additionally, a
study illustrated that biperiden, a potent MALT1 inhibitor,
inhibits the proliferation and accelerates the apoptosis of
pancreatic ductal adenocarcinoma cells (13). In terms of lung cancer, a previous
study demonstrated that MALT1 knockdown diminishes A431 and HCC827
cell migration and motility (14).
Furthermore, as an integral subunit of the caspase recruitment
domain family member 11/B-cell lymphoma (BCL)10/MALT1 (CBM)
complex, MALT1 is a key activator of the NF-κB pathway (9). Activation of the NF-κB pathway can
facilitate inflammation, and cancer cell proliferation and
metastasis, which may accelerate NSCLC progression (15). Therefore, it has been hypothesized
that MALT1 inhibitors may induce a suppressive effect on NSCLC.
MI-2 is a strong MALT1 inhibitor that works by directly binding to
MALT1 and inhibiting its protease function, and it is effective in
slowing the progression of several types of cancer, such as
glioblastoma multiforme and chronic lymphocytic leukemia (16–18).
However, the effect of MI-2 on NSCLC is unclear and should be
further explored.
The present study aimed to investigate the effect of
a MALT1 inhibitor, namely MI-2, on the behavior of NSCLC cells, as
well as its underlying mechanism of action.
Materials and methods
Cell culture
The BEAS-2B human normal lung epithelial cell line
was provided by Beyotime Institute of Biotechnology, and the cells
were cultured in Dulbecco's modified Eagle's medium (Beyotime
Institute of Biotechnology) supplemented with 10% fetal bovine
serum (FBS; HyClone; Cytiva). The NSCLC cell lines, NCI-H1299,
NCI-H1650, HCC827, A549 and NCI-H23, were obtained from iCell
Bioscience, Inc. NCI-H1299, H1650, HCC827 and NCI-H23 cells were
maintained in RPMI-1640 medium (iCell Bioscience, Inc.), and A549
cells were maintained in Ham's F-12K medium (Procell Life Science
& Technology Co., Ltd.). All media were supplemented with 10%
FBS. All cells were grown in media supplemented with 1%
penicillin/streptomycin solution (iCell Bioscience, Inc.) at 37°C
in an incubator containing 5% CO2.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
The mRNA expression levels of MALT1 were detected by
RT-qPCR. Briefly, BEAS-2B and NSCLC cells were cultured for 24 h
and RNA was then extracted using the MolPure® Cell RNA
Kit (Shanghai Yeasen Biotechnology Co., Ltd.). RT-qPCR was
performed using the HiScript II One Step RT-qPCR Kit (Vazyme
Biotech Co., Ltd.), according to the manufacturer's instructions.
The thermocycling conditions were as follows: 55°C for 20 min, 1
cycle; 94°C for 3 min, 1 cycle; 94° for 30 sec, 61°C for 30
sec,72°C for 30 sec, 35 cycles; 72°C for 5 min, 1 cycle. The
2−ΔΔCq method was utilized to quantify mRNA expression
levels (19). The specific primer
sequences (5′-3′) used were as follows: MALT1, forward
TCTTGGCTGGACAGTTTGTGA and reverse, GCTCTCTGGGATGTCGCAA; and GAPDH,
forward CCATCACCATCTTCCAGGAG and reverse CCTGCTTCACCACCTTCTTG.
Assessment of the sensitivity of NSCLC
cells to MI-2
The sensitivity of NCI-H1650 and A549 cells to MI-2
(MedChemExpress), a MALT1 inhibitor, was assessed using the Cell
Counting Kit-8 (CCK-8) assay (MedChemExpress). Briefly, cells were
seeded into a 96-well plate at a density of 3×103
cells/well and were then cultured in medium supplemented with 0,
0.25, 0.5, 1, 2, 4 or 8 µmol/l MI-2 for 24 h at 37°C. Subsequently,
cells were incubated with 10 µl CCK-8 reagent for an additional 2
h. Cell viability was calculated by measuring the optical density
(OD) in each well using the iMark microplate reader (Bio-Rad
Laboratories, Inc.). Subsequently, the half-maximal inhibitory
concentration (IC50) of MI-2 in NCI-H1650 and A549 cells
was calculated using GraphPad Prism 9.0 (Dotmatics).
Cell proliferation assay
Cell proliferation was assessed using CCK-8 and
5-ethynyl-2′-deoxyuridine (EdU) staining assays. Briefly, NCI-H1650
and A549 cells were cultured and stimulated with 1 or 2 µmol/l
MI-2, respectively, based on the IC50 value. A total of
0, 24, 48 and 72 h after stimulation at 37°C, the cells were
supplemented with CCK-8 reagent and the OD value was measured as
aforementioned. In addition, EdU staining was performed using the
BeyoClick™ EdU-488 Kit (Beyotime Institute of
Biotechnology). Briefly, cells were stimulated with 1 or 2 µmol/l
MI-2 for 24 h at 37°C and were then stained with EdU working
mixture at 37°C for 24 h, followed by fixing with paraformaldehyde
(Sangon Biotech Co., Ltd.) at room temperature for 10 min and
permeabilization using Triton X-100 (Beyotime Institute of
Biotechnology) at room temperature for 3 min. Subsequently, the
cells were successively stained with an EdU reaction mixture for 30
min and Hoechst 33342 (both from Beyotime Institute of
Biotechnology) for 10 min at room temperature, according to the
manufacturer's protocol. An inverted fluorescence microscope (Motic
Incorporation, Ltd.) was applied to capture images.
Cell apoptosis assay
NCI-H1650 and A549 cells were stimulated with 1 or 2
µmol/l MI-2 for 24 h at 37°C and the cell apoptosis rate was
determined using the Annexin V–IF488/PI Cell Apoptosis Detection
Kit (Wuhan Servicebio Technology Co., Ltd.). Briefly, cells were
washed and were then incubated with 5 µl Annexin V and 5 µl
propidium iodide for 20 min at room temperature in the dark.
Finally, cells were collected and cell apoptosis was assessed using
the FACSCanto II flow cytometer (BD Biosciences) and analyzed by
Flowjo X (FlowJo, LLC).
Cell migration and invasion
assays
For the cell migration assay, NCI-H1650 and A549
cells were cultured to near confluence and a wound was created on
the cell monolayer using a 10-µl pipette tip. Cells were then
washed to remove unattached cells prior to culturing for 24 h in
serum-free medium with 1 or 2 µmol/l MI-2. Images of the wound area
were captured by an inverted light microscope (Motic Incorporation,
Ltd.) and the cell migration rate was then calculated using the
following formula: 1-wound area at 24 h/wound area at 0 h.
Additionally, cell invasion was assed using a
Transwell assay. Briefly, NCI-H1650 and A549 cells at a density of
3×104 cells/well in serum-free medium supplemented with
1 or 2 µmol/l MI-2 were seeded into 24-well plates pre-coated with
Matrigel, at 37°C for 1 h, on the upper inserts (Corning, Inc.) of
the Transwell chamber. The lower chamber was filled with complete
medium. Following incubation for 24 h, the invasive cells were
stained with 0.1% crystal violet (Beyotime Institute of
Biotechnology) at room temperature for 10 min and images were
captured using an inverted light microscope (Motic Incorporation,
Ltd.).
Western blot analysis
The BEAS-2B and NSCLC cell lines were cultured and
the protein expression levels of MALT1 were determined by western
blotting. In addition, NCI-H1650 and A549 cells were stimulated
with 1 or 2 µmol/l of MI-2 for 24 h at 37°C, and the protein
expression levels of BCL2, BCL2-associated X-protein (BAX)
phosphorylated (p)-c-JUN N-terminal kinase (JNK), JNK, p-c-JUN,
c-JUN and GAPDH were detected by western blot analysis. Briefly,
total proteins were extracted with a Cell Lysis Buffer (Cell
Signaling Technology, Inc.) and were quantified using the Total
Protein Assay Kit (Nanjing Jiancheng Bioengineering Institute).
Subsequently, 20 µg proteins were separated by SDS-PAGE on 4–20%
gels and were electrotransferred onto PVDF membranes. The membranes
were then incubated with 5% non-fat powdered milk (Beyotime
Institute of Biotechnology) at 37°C for 90 min and with primary
antibodies (Affinity Biosciences) against the aforementioned
proteins at an appropriate dilution, according to the supplier's
instructions, at 4°C overnight. Following incubation with the
corresponding secondary antibodies (Affinity Biosciences) at 37°C
for 2 h, the protein bands were visualized using the Super ECL
Detection Reagent (Shanghai Yeasen Biotechnology Co., Ltd.). The
proteins were semi-quantified by ImageJ 1.8 (National Institutes of
Health). The antibodies used were as follows: Anti-BCL2 (cat. no.
AF6139; 1:2,000), anti-BAX (cat. no. AF0120; 1:2,000), anti-p-JNK
(cat. no. AF3318; 1:2,000); anti-JNK (cat. no. AF6318; 1:1,500),
anti-c-JUN (cat. no. AF6090; 1:2,000), anti-p-c-JUN (cat. no.
AF3095; 1:2,000), anti-MALT1 (cat. no. DF6867; 1:3,000), anti-GAPDH
(cat. no. AF7021; 1:3,000), and Goat Anti-Rabbit IgG (H+L) HRP
(cat. no. S0001; 1:5,000) (all from Affinity Biosciences).
Anisomycin treatment assay
Anisomycin (MedChemExpress), an activator of the JNK
pathway, was adopted to validate the modulation of the JNK pathway
by MALT1. Briefly, NCI-H1650 and A549 cells were cultured and
co-stimulated with 1 or 2 µmol/l MI-2 and 0.1 µmol/l anisomycin
alone or in combination; the concentration of anisomycin (0.1
µmol/l) was determined based on a previous study (20). Following treatment for 24 h at 37°C,
cells were collected for western blotting, EdU staining and cell
apoptosis assays, which were conducted as aforementioned. Notably,
the CCK-8 assay was carried out after 0, 24, 48 and 72 h of
stimulation.
Statistical analysis
All analyses were performed using SPSS 22.0 software
(IBM Corp.). Experiments were repeated in triplicate and data are
presented as the mean ± standard deviation. The differences among
multiple groups were compared by one-way ANOVA followed by
Dunnett's or Tukey's multiple comparisons test. The differences
between two groups were compared by unpaired Student's t-test.
P<0.05 was considered to indicate a statistically significant
difference.
Results
MALT1 expression in NSCLC cell
lines
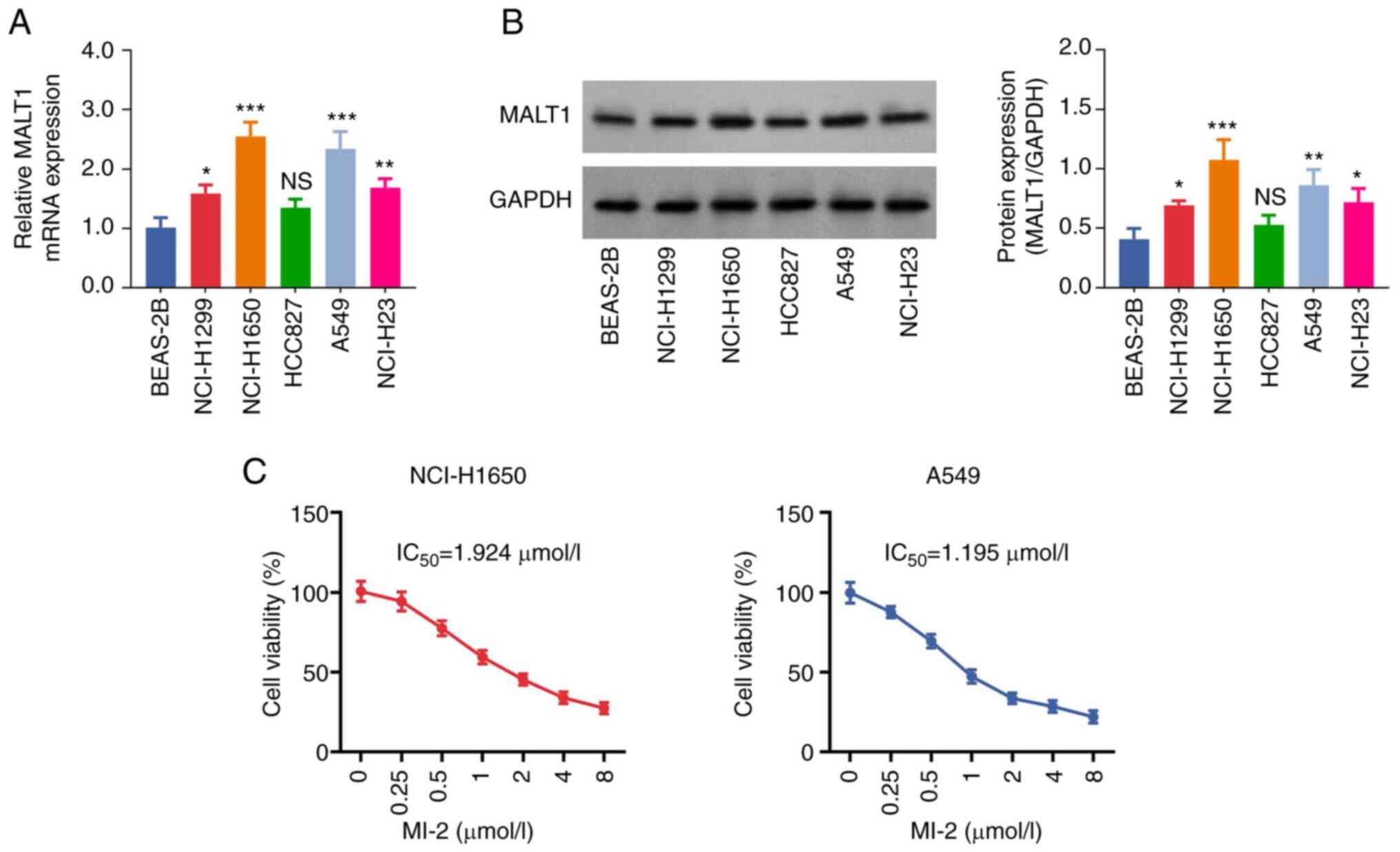
The results showed that the mRNA expression levels
of MALT1 were significantly increased in NCI-H1299 (P<0.05),
NCI-H1650 (P<0.001), A549 (P<0.001) and NCI-H23 (P<0.01)
cells compared with those in the BEAS-2B cell line. However, no
significant difference was observed in the expression levels of
MALT1 between HCC827 and BEAS-2B cells (P>0.05; Fig. 1A). Consistent with the RT-qPCR
analysis results, western blot analysis indicated that the protein
expression levels of MALT1 were increased in NSCLC cells
(P<0.05; Fig. 1B). Notably, the
mRNA and protein expression levels of MALT1 were highest in
NCI-H1650 and A549 cells; therefore, these cell lines were chosen
for subsequent experiments. Additionally, the CCK-8 assay
demonstrated that the viability of MI-2-treated NCI-H1650 and A549
cells was decreased in a dose-dependent manner. The IC50
values of MI-2 in NCI-H1650 and A549 cells were 1.924 and 1.195
µmol/l, respectively (Fig. 1C).
Effect of MI-2 on NCI-H1650 and A549
cell proliferation, apoptosis, migration and invasion
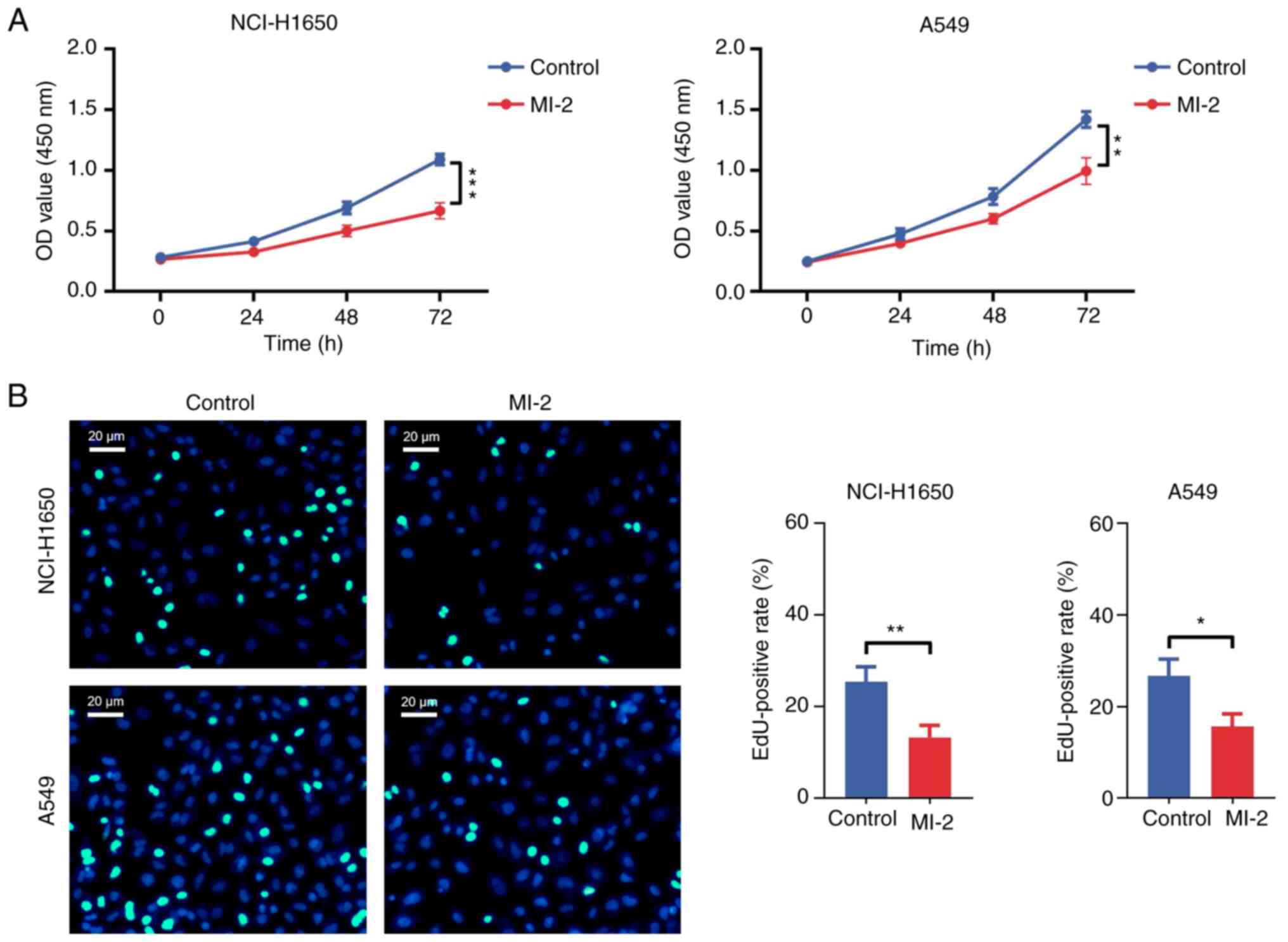
The CCK-8 assay results revealed that the OD value
in NCI-H1650 (P<0.001) and A549 (P<0.01) cells treated with
MI-2 for 72 h was significantly decreased compared with that in the
control group (Fig. 2A). In
addition, the EdU staining assay showed that the EdU-positive rate
was reduced in MI-2-treated NCI-H1650 (P<0.01) and A549
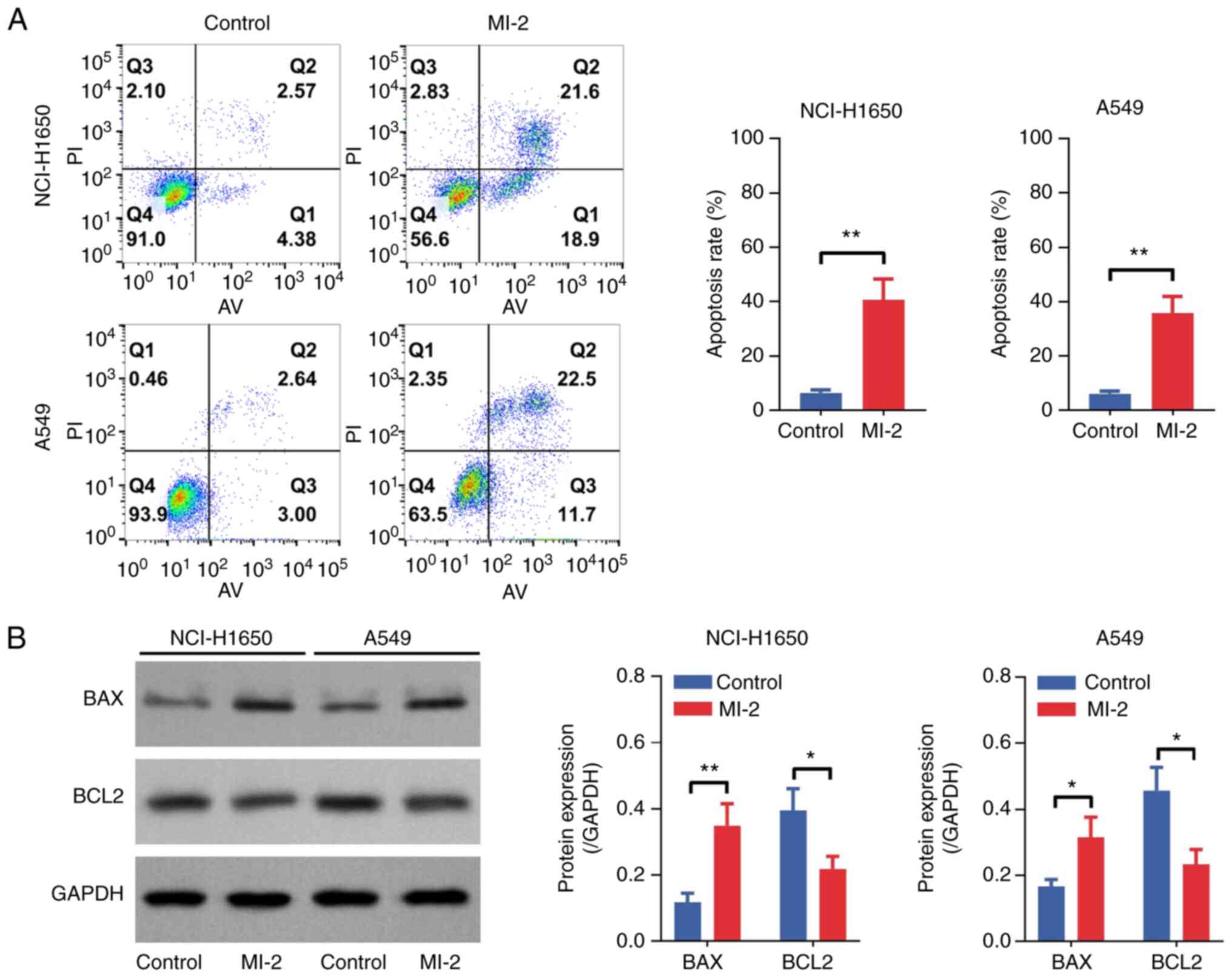
(P<0.05) cells compared with that in untreated cells (Fig. 2B). Furthermore, an Annexin
V–IF488/PI Cell Apoptosis Detection Kit was used to determine cell
apoptosis. As shown in Fig. 3A, the
cell apoptosis rate was increased by MI-2 in NCI-H1650 (P<0.01)
and A549 (P<0.01) cells. Furthermore, western blot analysis
revealed that BAX was upregulated and BCL2 was downregulated in
MI-2-treated NCI-H1650 and A549 cells compared with those in the
control group (all P<0.05; Fig.
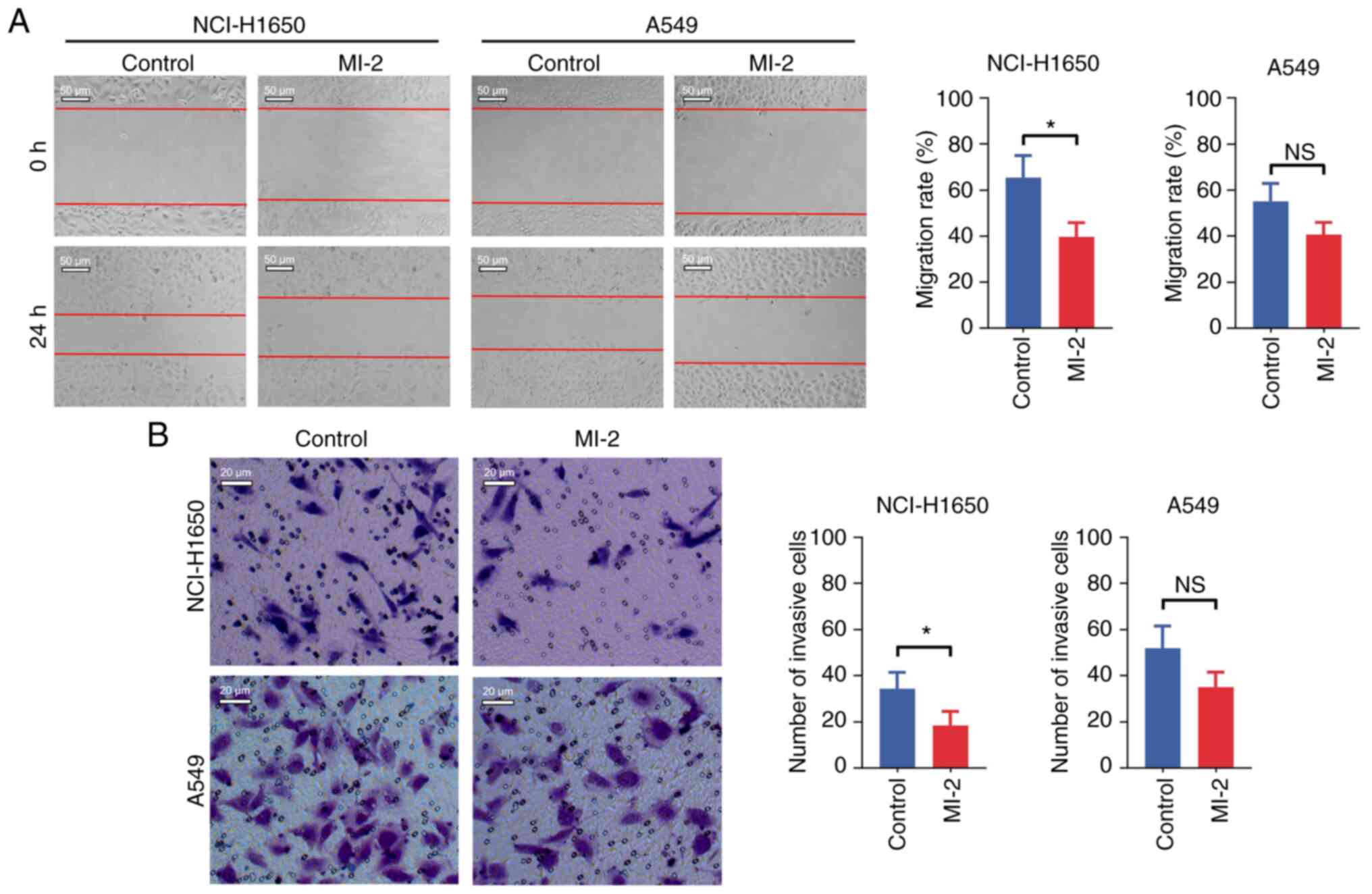
3B). In addition, the cell migration assay demonstrated that
MI-2 attenuated the migration of NCI-H1650 cells (P<0.05), but
not that of A549 cells (P>0.05), compared with in untreated
cells (Fig. 4A). Finally, the
invasive ability of NCI-H1650 and A549 cells was assessed by
Transwell assays. The results indicated that MI-2 reduced the
invasion of NCI-H1650 cells (P<0.05), but not that of A549 cells
(P>0.05), compared with in the control group (Fig. 4B).
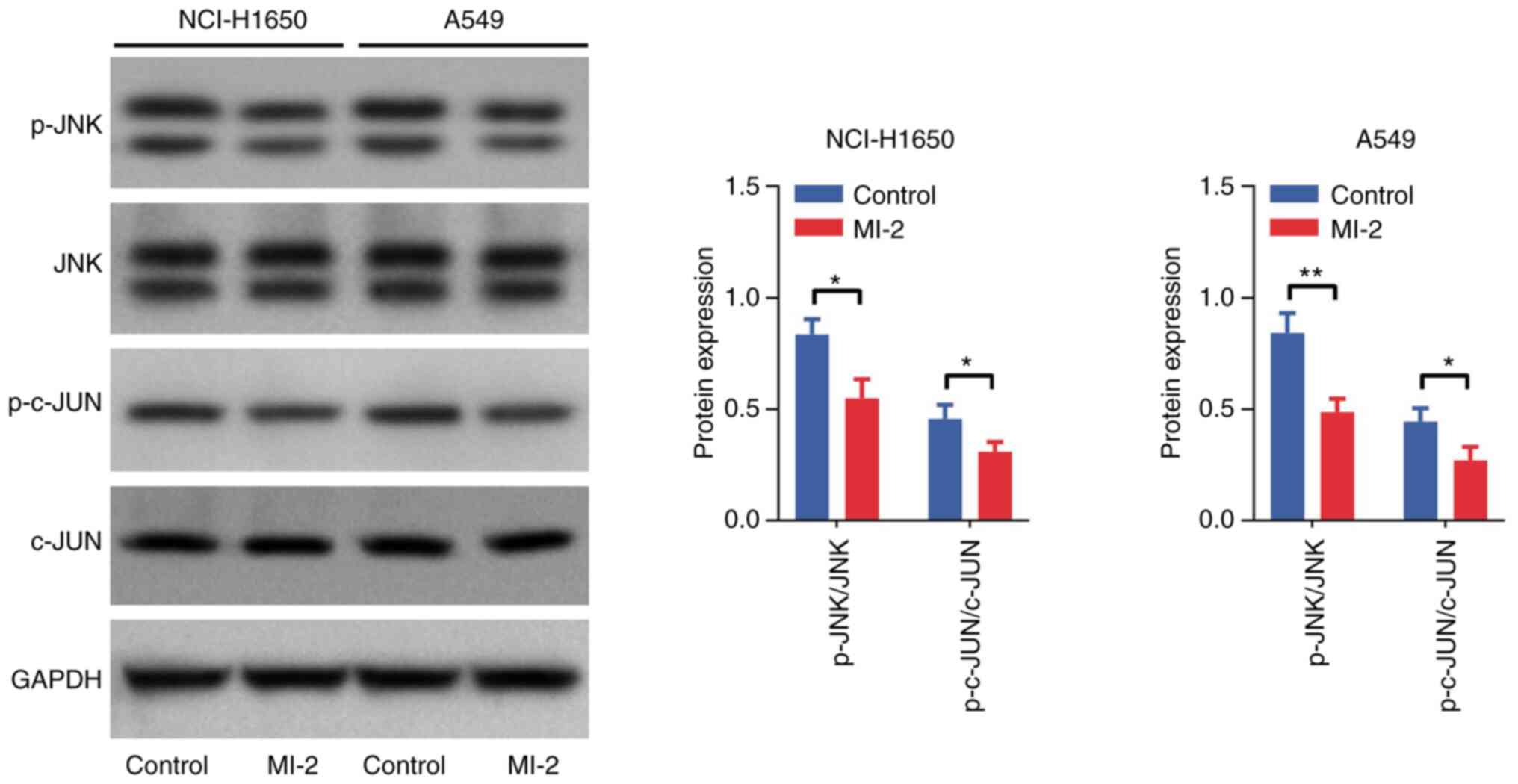
Effect of MI-2 on the JNK/c-JUN
pathway in NCI-H1650 and A549 cells
Western blot analysis was performed to detect the
protein expression levels of p-JNK, JNK, p-c-JUN and c-JUN in
NCI-H1650 and A549 cells. The results demonstrated that the
p-JNK/JNK and p-c-JUN/c-JUN ratios were decreased in NCI-H1650 and
A549 cells treated with MI-2 compared with those in the control
group (all P<0.05; Fig. 5).
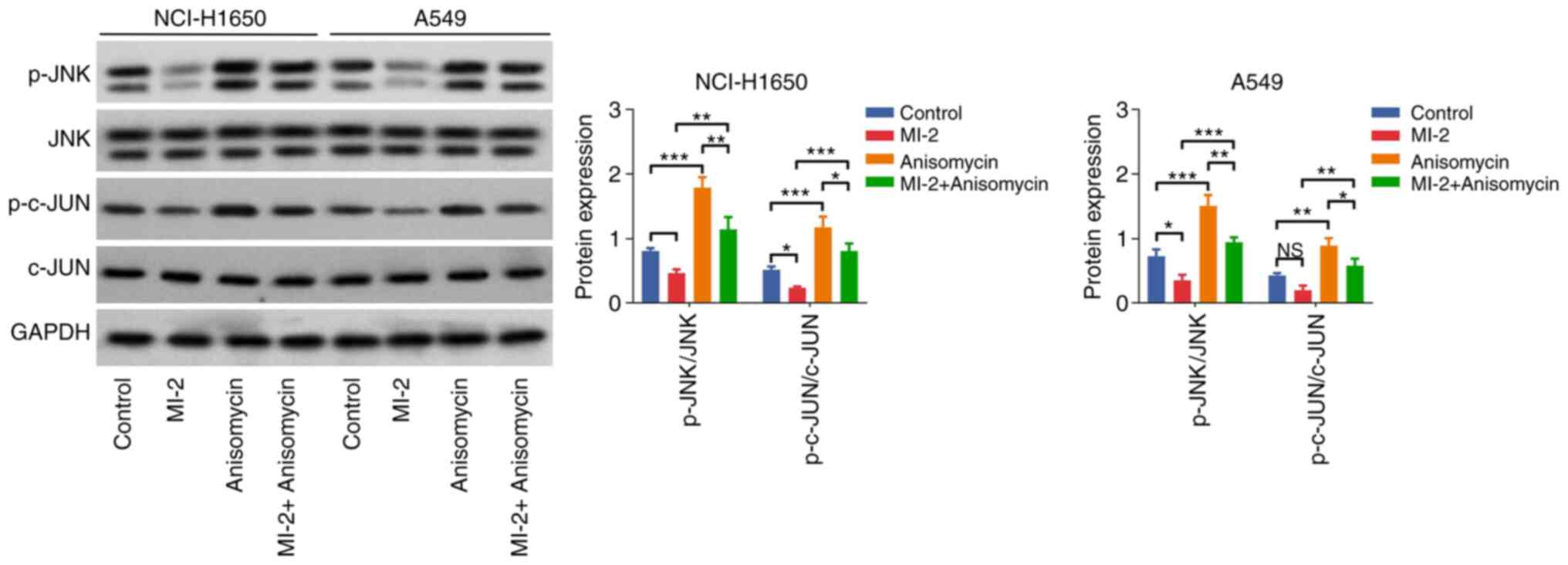
Effect of anisomycin on the
MI-2-mediated JNK/c-JUN pathway in NCI-H1650 and A549 cells
Furthermore, western blot analysis indicated that
the p-JNK/JNK and p-c-JUN/c-JUN ratios were elevated in the
anisomycin group compared with those in the control group (both
P<0.001). In addition, these protein ratios were enhanced in the
MI-2 + anisomycin group vs. the MI-2 group in NCI-H1650 and A549
cells (all P<0.01; Fig. 6).
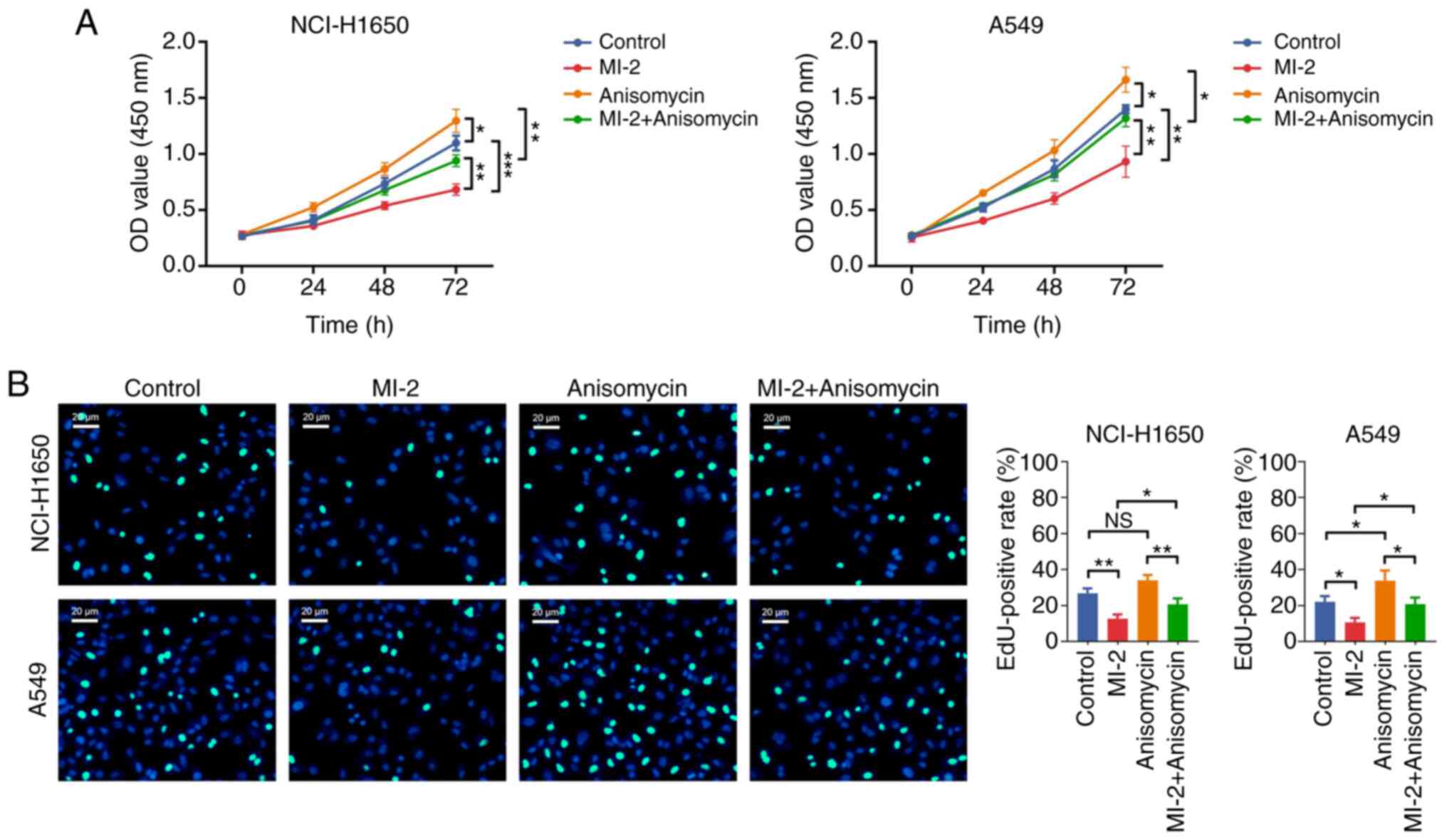
Effect of anisomycin on MI-2-mediated
NCI-H1650 and A549 cell proliferation and apoptosis
Subsequently, the CCK-8 assay revealed that the OD
value was increased in the anisomycin group vs. the control group
(both P<0.05), and in the MI-2 + anisomycin group vs. the MI-2
group (both P<0.01), in NCI-H1650 and A549 cells (Fig. 7A). The EdU staining assay
demonstrated that the EdU-positive rate was not affected by
anisomycin in NCI-H1650 cells (P>0.05), but it was increased in
the anisomycin group compared with the control group in A549 cells
(P<0.05). Consistently, the EdU-positive rate was elevated in
the MI-2 + anisomycin group compared with that in the MI-2 group in
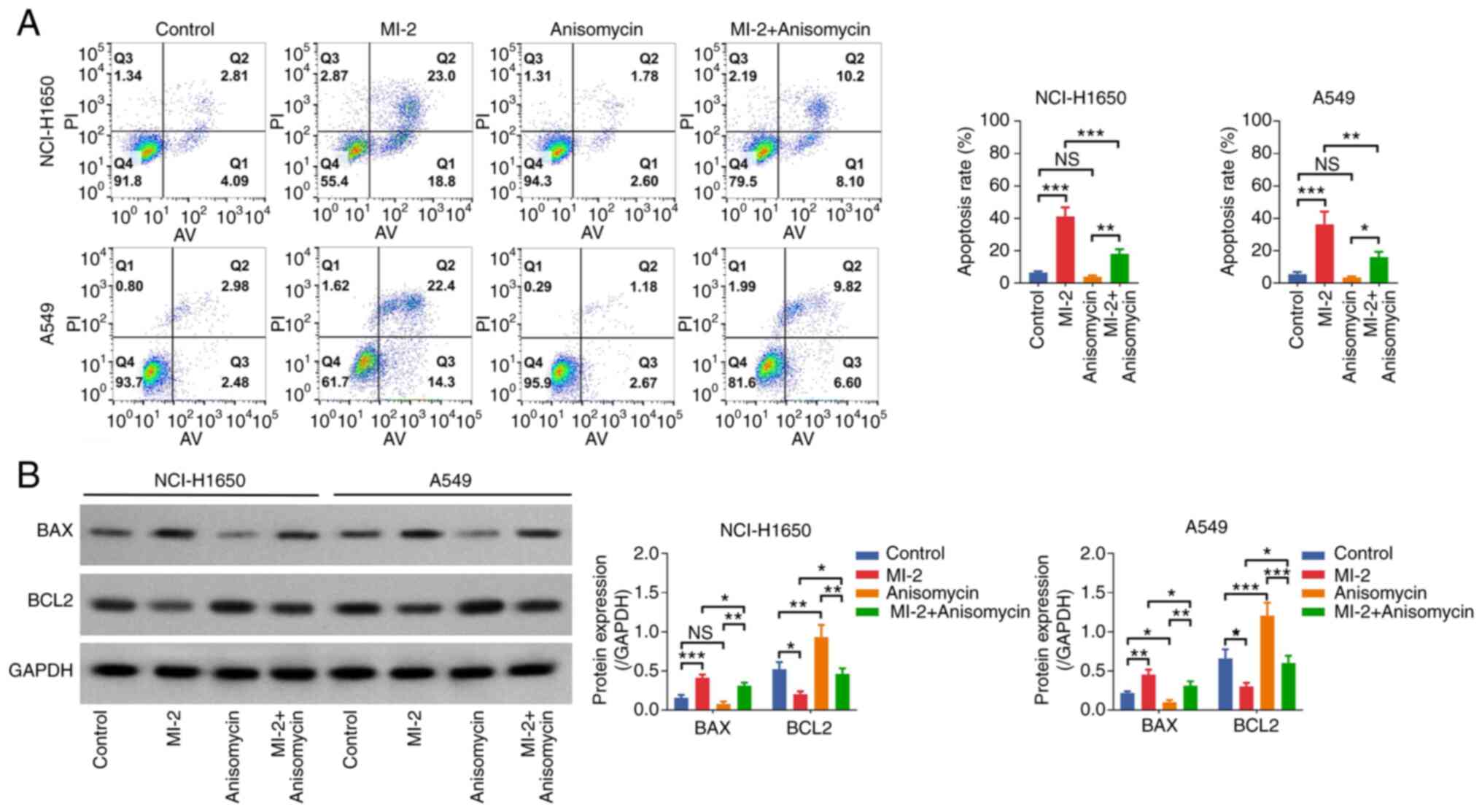
both NCI-H1650 and A549 cells (all P<0.05; Fig. 7B). Additionally, the Annexin
V–IF488/PI Cell Apoptosis Detection Kit showed that the cell
apoptosis rate was not affected by anisomycin (both P>0.05), but
it was reduced in the MI-2 + anisomycin group compared with that in
the MI-2 group in both NCI-H1650 and A549 cells (both P<0.01;
Fig. 8A). Western blot analysis
indicated that BAX was only downregulated by anisomycin in A549
cells (P<0.05), whereas BCL2 was upregulated in both
anisomycin-treated NCI-H1650 (P<0.01) and A549 (P<0.001)
cells compared with in the control group. Furthermore, BAX was
downregulated and BCL2 was upregulated in the MI-2 + anisomycin
group compared with in the MI-2 group in both NCI-H1650 and A549
cells (all P<0.05; Fig. 8B).
These findings indicated that anisomycin may enhance proliferation
but attenuate apoptosis in NCI-H1650 and A549 cells. Furthermore,
anisomycin weakened the effect of MI-2 on inhibiting proliferation
and facilitating apoptosis in NCI-H1650 and A549 cells.
Discussion
MALT1 is an indispensable unit of the CBM complex,
which regulates malignant tumor cell survival, proliferation and
metastasis, and is thus involved in the pathology and progression
of some types of cancer (9). It has
been reported that MALT1 is upregulated in several types of cancer
(11,21). For example, a previous study
elucidated that MALT1 is abundantly expressed in prostate cancer
tissues compared with in normal tissues (22). Another study illustrated that MALT1
is elevated in colorectal cancer (CRC) tissues compared with in
adjacent normal tissues (21). In
the present study, the results showed that both the mRNA and
protein expression levels of MALT1 were increased in NCI-H1299,
NCI-H1650, A549 and NCI-H23 cells compared with in BEAS-2B cells.
These finding could be due to the fact that MALT1 promotes cell
proliferation, thus suggesting that it could be involved in the
malignant proliferation of NSCLC cells (11,23,24).
Therefore, MALT1 was overexpressed in NSCLC cell lines compared
with in normal lung epithelial cells. The identification of novel
biomarkers is fundamental for the early diagnosis of NSCLC, which
assists in improving the prognosis of patients with NSCLC (25–27).
The present findings provided a theoretical reference that MALT1
was highly expressed in NSCLC cells compared with in normal cells,
which suggested that MALT1 may possess the potential to serve as a
biomarker for patients with NSCLC. However, this hypothesis should
be validated by subsequent studies.
Targeting MALT1 is a potential strategy for
attenuating cancer progression. Therefore, the role of MALT1
inhibitors, such as MI-2, on the progression of several types of
cancer has been widely investigated (11,17,28,29).
For example, a previous study reported that MI-2 can reduce A2058
and A375 melanoma cell proliferation and migration (29). Another study elucidated that MI-2
attenuates the migration, invasion and tumor-forming abilities of
HCC cells, possibly via inhibiting the NF-κB pathway (11). Additionally, MI-2 can effectively
inhibit glioblastoma multiforme cell proliferation, survival,
migration and invasion (17), and
can diminish the proliferation and migration of CRC cells (21). In the present study, the results
suggested that MI-2 could reduce NSCLC cell proliferation,
migration and invasion, and enhance cell apoptosis. These findings
could be attributed to the possible effect of MI-2 on suppressing
the activation of several signaling pathways, including the NF-κB,
B cell receptor, phosphoinositide 3-kinase/protein kinase
B/mammalian target of rapamycin and extracellular-signal-regulated
kinase/mitogen-activated protein kinase pathways, to affect NSCLC
cell proliferation, apoptosis, migration and invasion (18,24,30,31).
Notably, MI-2 could only reduce migration and invasion in NCI-H1650
cells, but not in A549 cells; the reasons for this may be: i)
According to a previous study, metastatic cell lines with high
MALT1 expression are particularly sensitive to MI-2 (29). In addition, according to the
American Type Culture Collection, NCI-H1650 cells were derived from
the metastatic sites of the lung, whereas A549 cells were not.
Therefore, the inhibitory effect of MALT1 by MI-2 might have a
limited effect on reducing A549 cell invasion and migration
compared with NCI-H1650 cells. However, further experiments are
required to validate this speculation. ii) Since the number of
experimental repeats was only three, the statistical power would be
affected. To further confirm the findings, more experimental
repeats are recommended, preferably at least five. Moreover, it
should be clarified that MI-2 is a potent MALT1 inhibitor, which
acts by directly binding to MALT1 and inhibiting its protease
function (16). Thus, it was
hypothesized that MI-2 could only inhibit the protease function of
MALT1, but it could not affect its expression, which was confirmed
by a previous study (32). However,
this speculation should be validated by subsequent experiments.
Emerging evidence has suggested that the JNK/c-JUN
pathway is closely involved in the pathology and progression of
NSCLC (33–36). For example, a previous study showed
that the activated JNK/c-JUN pathway promotes the growth and
metastasis of lung adenocarcinoma (33). Additionally, inhibition of the
JNK/c-JUN pathway accelerates NSCLC cell apoptosis (36). Furthermore, another study found that
activation of the JNK/c-JUN pathway could induce NSCLC cell
proliferation and migration, and attenuate NSCLC cell apoptosis
(35). Regarding the regulatory
effect of MALT1 on the JNK/c-JUN pathway, a previous study
indicated that MALT1 activates the JNK/c-JUN pathway to facilitate
melanoma cell proliferation and motility (29). However, whether the regulatory
effect of MALT1 on the JNK/c-JUN pathway also participates in the
progression of NSCLC remains unclear. In the current study, the
results demonstrated that MI-2 suppressed the JNK/c-JUN pathway in
NSCLC cells. This could be due to the fact that MI-2 can degrade
the CBM complex or regulate cleavage of the deubiquitinase
cylindromatosis (CYLD), thus further inactivating JNK/c-JUN
signaling (23,37). In addition, the present study showed
that co-treatment of NSCLC cell with anisomycin and MI-1 abrogated
the effects of MI-2 on the JNK/c-JUN signaling pathway, along with
its effect on NSCLC cell proliferation and apoptosis. As
aforementioned, it was hypothesized that MI-2 could affect the CBM
complex or CYLD cleavage, thus inactivating the JNK/c-JUN signaling
pathway, ultimately attenuating NSCLC cell proliferation and
promoting NSCLC cell apoptosis (23,37).
However, whether the regulatory effect of MI-2 on JNK/c-JUN
signaling is direct or indirect should be verified by further
experiments.
In conclusion, the present study demonstrated that
MI-2, a MALT1 inhibitor, could impair NSCLC cell proliferation,
migration and invasion, and promote NSCLC cell apoptosis by
suppressing the JNK/c-JUN pathway, which could be involved in
attenuating NSCLC progression. To the best of our knowledge, this
is the first study that explores the involvement of MI-2 in NSCLC,
and our findings suggested that MI-2 may be helpful in attenuating
NSCLC progression. Based on the findings of this study, further
studies could consider exploring the effect of MI-2 on other types
of cancer. Meanwhile, other potential pathways involved in the
regulation of MI-2 on NSCLC could be further investigated.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
CW, WG and YW contributed to the study design,
collected and analyzed data, and contributed to the paper writing
and reviewing. CW and WG performed the experiments. WG and YW
confirm the authenticity of all the raw data. All authors have read
and approved the final manuscript.
Ethical approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Thai AA, Solomon BJ, Sequist LV, Gainor JF
and Heist RS: Lung cancer. Lancet. 398:535–554. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ettinger DS, Wood DE, Aisner DL, Akerley
W, Bauman JR, Bharat A, Bruno DS, Chang JY, Chirieac LR, DeCamp M,
et al: NCCN guidelines® insights: Non-small cell lung
cancer, version 2.2023. J Natl Compr Canc Netw. 21:340–350. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Miller M and Hanna N: Advances in systemic
therapy for non-small cell lung cancer. BMJ. 375:n23632021.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wu J and Lin Z: Non-small cell lung cancer
targeted therapy: Drugs and mechanisms of drug resistance. Int J
Mol Sci. 23:150562022. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wang M, Herbst RS and Boshoff C: Toward
personalized treatment approaches for non-small-cell lung cancer.
Nat Med. 27:1345–1356. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Rhodin KE, Rucker AJ, Ready NE, D'Amico TA
and Antonia SJ: The immunotherapeutic landscape in non-small cell
lung cancer and its surgical horizons. J Thorac Cardiovasc Surg.
159:1616–1623. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ganti AK, Klein AB, Cotarla I, Seal B and
Chou E: Update of incidence, prevalence, survival, and initial
treatment in patients with non-small cell lung cancer in the US.
JAMA Oncol. 7:1824–1832. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Sadeghirad H, Bahrami T, Layeghi SM,
Yousefi H, Rezaei M, Hosseini-Fard SR, Radfar P, Warkiani ME,
O'Byrne K and Kulasinghe A: Immunotherapeutic targets in non-small
cell lung cancer. Immunology. 168:256–272. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
O'Neill TJ, Tofaute MJ and Krappmann D:
Function and targeting of MALT1 paracaspase in cancer. Cancer Treat
Rev. 117:1025682023. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Gomez Solsona B, Schmitt A,
Schulze-Osthoff K and Hailfinger S: The paracaspase MALT1 in
cancer. Biomedicines. 10:3442022. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kurden-Pekmezci A, Cakiroglu E, Eris S,
Mazi FA, Coskun-Deniz OS, Dalgic E, Oz O and Senturk S: MALT1
paracaspase is overexpressed in hepatocellular carcinoma and
promotes cancer cell survival and growth. Life Sci. 323:1216902023.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Tan H, Xie Y, Zhang X, Wu S, Zhao H, Wu J,
Wang W and Lin C: Integrative analysis of MALT1 as a potential
therapeutic target for prostate cancer and its immunological role
in pan-cancer. Front Mol Biosci. 8:7149062021. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Konczalla L, Perez DR, Wenzel N,
Wolters-Eisfeld G, Klemp C, Lüddeke J, Wolski A, Landschulze D,
Meier C, Buchholz A, et al: Biperiden and mepazine effectively
inhibit MALT1 activity and tumor growth in pancreatic cancer. Int J
Cancer. 146:1618–1630. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Pan D, Jiang C, Ma Z, Blonska M, You MJ
and Lin X: MALT1 is required for EGFR-induced NF-κB activation and
contributes to EGFR-driven lung cancer progression. Oncogene.
35:919–928. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Dimitrakopoulos FD, Kottorou AE, Kalofonou
M and Kalofonos HP: The fire within: NF-κB involvement in non-small
cell lung cancer. Cancer Res. 80:4025–4036. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Fontan L, Yang C, Kabaleeswaran V, Volpon
L, Osborne MJ, Beltran E, Garcia M, Cerchietti L, Shaknovich R,
Yang SN, et al: MALT1 small molecule inhibitors specifically
suppress ABC-DLBCL in vitro and in vivo. Cancer Cell. 22:812–824.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Liu X, Yue C, Shi L, Liu G, Cao Q, Shan Q,
Wang Y, Chen X, Li H, Wang J, et al: MALT1 is a potential
therapeutic target in glioblastoma and plays a crucial role in
EGFR-induced NF-κB activation. J Cell Mol Med. 24:7550–7562. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Saba NS, Wong DH, Tanios G, Iyer JR,
Lobelle-Rich P, Dadashian EL, Liu D, Fontan L, Flemington EK,
Nichols CM, et al: MALT1 inhibition is efficacious in both naïve
and ibrutinib-resistant chronic lymphocytic leukemia. Cancer Res.
77:7038–7048. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Hsieh KY, Wei CK and Wu CC: YC-1 prevents
tumor-associated tissue factor expression and procoagulant activity
in hypoxic conditions by inhibiting p38/NF-κB signaling pathway.
Int J Mol Sci. 20:2442019. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Qian R, Niu X, Wang Y, Guo Z, Deng X, Ding
Z, Zhou M and Deng H: Targeting MALT1 suppresses the malignant
progression of colorectal cancer via miR-375/miR-365a-3p/NF-κB
axis. Front Cell Dev Biol. 10:8450482022. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Tsui KH, Chang KS, Sung HC, Hsu SY, Lin
YH, Hou CP, Yang PS, Chen CL, Feng TH and Juang HH:
Mucosa-associated lymphoid tissue 1 is an oncogene inducing cell
proliferation, invasion, and tumor growth via the upregulation of
NF-κB activity in human prostate carcinoma cells. Biomedicines.
9:2502021. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Knies N, Alankus B, Weilemann A, Tzankov
A, Brunner K, Ruff T, Kremer M, Keller UB, Lenz G and Ruland J:
Lymphomagenic CARD11/BCL10/MALT1 signaling drives malignant B-cell
proliferation via cooperative NF-κB and JNK activation. Proc Natl
Acad Sci USA. 112:E7230–E7238. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Chiba T, Soeno Y, Shirako Y, Sudo H,
Yagishita H, Taya Y, Kawashiri S, Okada Y and Imai K: MALT1
inhibition of oral carcinoma cell invasion and ERK/MAPK activation.
J Dent Res. 95:446–452. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Adams E, Sepich-Poore GD,
Miller-Montgomery S and Knight R: Using all our genomes:
Blood-based liquid biopsies for the early detection of cancer. View
(Beijing). 3:202001182022.PubMed/NCBI
|
|
26
|
Wang L, Zhang M, Pan X, Zhao M, Huang L,
Hu X, Wang X, Qiao L, Guo Q, Xu W, et al: Integrative serum
metabolic fingerprints based multi-modal platforms for lung
adenocarcinoma early detection and pulmonary nodule classification.
Adv Sci (Weinh). 9:e22037862022. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Yang J, Yin X, Zhang L, Zhang X, Lin Y,
Zhuang L, Liu W, Zhang R, Yan X, Shi L, et al: Defective Fe
metal-organic frameworks enhance metabolic profiling for
high-accuracy diagnosis of human cancers. Adv Mater.
34:e22014222022. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Hamp I, O'Neill TJ, Plettenburg O and
Krappmann D: A patent review of MALT1 inhibitors (2013-present).
Expert Opin Ther Pat. 31:1079–1096. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Wang Y, Zhang G, Jin J, Degan S, Tameze Y
and Zhang JY: MALT1 promotes melanoma progression through JNK/c-Jun
signaling. Oncogenesis. 6:e3652017. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Jiang VC, Liu Y, Lian J, Huang S, Jordan
A, Cai Q, Lin R, Yan F, McIntosh J, Li Y, et al: Cotargeting of BTK
and MALT1 overcomes resistance to BTK inhibitors in mantle cell
lymphoma. J Clin Invest. 133:e1656942023. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
McAuley JR, Bailey KM, Ekambaram P, Klei
LR, Kang H, Hu D, Freeman TJ, Concel VJ, Hubel NE, Lee JL, et al:
MALT1 is a critical mediator of PAR1-driven NF-κB activation and
metastasis in multiple tumor types. Oncogene. 38:7384–7398. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Zhang H, Sun G, Li X, Fu Z, Guo C, Cao G,
Wang B, Wang Q, Yang S, Li D, et al: Inhibition of MALT1
paracaspase activity improves lesion recovery following spinal cord
injury. Sci Bull (Beijing). 64:1179–1194. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Zhang D, Jiang Q, Ge X, Shi Y, Ye T, Mi Y,
Xie T, Li Q and Ye Q: RHOV promotes lung adenocarcinoma cell growth
and metastasis through JNK/c-Jun pathway. Int J Biol Sci.
17:2622–2632. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Jiang T, Wu H, Lin M, Yin J, Tan L, Ruan Y
and Feng M: B4GALNT1 promotes progression and metastasis in lung
adenocarcinoma through JNK/c-Jun/Slug pathway. Carcinogenesis.
42:621–630. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Luo Z, Han Z, Shou F, Li Y and Chen Y:
LINC00958 accelerates cell proliferation and migration in non-small
cell lung cancer through JNK/c-JUN signaling. Hum Gene Ther
Methods. 30:226–234. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Tanimura K, Yamada T, Horinaka M, Katayama
Y, Fukui S, Morimoto K, Nakano T, Tokuda S, Morimoto Y, Iwasaku M,
et al: Inhibition of c-Jun N-terminal kinase signaling increased
apoptosis and prevented the emergence of ALK-TKI-tolerant cells in
ALK-rearranged non-small cell lung cancer. Cancer Lett.
522:119–128. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Staal J, Driege Y, Bekaert T, Demeyer A,
Muyllaert D, Van Damme P, Gevaert K and Beyaert R: T-cell
receptor-induced JNK activation requires proteolytic inactivation
of CYLD by MALT1. EMBO J. 30:1742–1752. 2011. View Article : Google Scholar : PubMed/NCBI
|