Introduction
Prostate cancer (PCa), the second most prevalent
malignancy in men worldwide, caused ~34,500 deaths in the United
States in 2022 (1), and research
has indicated that an estimated 15% of men receive a diagnosis of
PCa during their lifetime (2).
Obesity influences the pathogenesis of prostate disease, including
benign prostate hyperplasia (BPH) and PCa (3–5).
Notably, obesity can lead to alterations in endocrine status,
including increased levels of estradiol due to the expression of
the enzyme P450 aromatase in adipose tissue, which converts
androgens to estrogen; this results in gonadotropin suppression and
favors the development of BPH (6).
Obesity also enhances sympathetic nervous activity, which impacts
the severity of urinary voiding dysfunction, contributing to BPH
(7,8). Additionally, obesity induces
inflammatory processes, promoting microvascular disease and leading
to tissue ischemia and oxidative stress, creating a favorable
intraprostatic environment for hyperplastic growth and potential
precancerous transformation (9).
Low serum testosterone levels are associated with
aggressive PCa, since testosterone has a regulatory role in
maintaining normal prostate cell growth (10), and men with low testosterone levels
often exhibit a more aggressive PCa phenotype (11). Trials of 5α-reductase inhibitors,
which inhibit the conversion of testosterone into
dihydrotestosterone (a hormone that plays a crucial role in
prostate growth), have shown a decreased overall risk of PCa but a
higher Gleason score, reflecting the association between low serum
testosterone and aggressive PCa (12). Insulin resistance, which is commonly
associated with obesity, has been shown to promote PCa by
increasing circulating levels of bioactive IGF-1, a growth factor
implicated in numerous types of cancer (13). Among adipokines, leptin, which is
elevated in central obesity, promotes angiogenesis in human PCa
cell lines, thereby supporting cancer growth (14). Conversely, adiponectin, which has
antitumor effects by inhibiting cancer cell proliferation and
metastasis, has been detected at reduced levels in central obesity
(15). Notably, multiple studies
have reported an association between PCa and obesity (16,17),
and three meta-analyses have reported a positive correlation
between the incidence of PCa and obesity (18–20).
It has also been indicated that obesity can affect the outcomes of
PCa. A systematic review and meta-analysis identified that for
every 5-kg/m2 increase in the body mass index (BMI) of
patients undergoing radical prostatectomy, the risk of biochemical
recurrence of PCa increased by 20% and the risk of PCa-specific
mortality increased by 15% (21).
Most studies on obesity define the condition and
measure its degree using BMI (17–20);
however, BMI is an inaccurate measure because it fails to directly
account for the amount of adipose tissue in the body. Thus,
researchers are increasingly considering the role of body
composition, including the distribution of fat and lean tissue, in
PCa. Several small-scale studies have implicated visceral and
subcutaneous fat in the initiation and progression of PCa. Duong
et al (22) demonstrated
that adipocytes, the main cellular component of adipose tissue,
were involved in solid tumor progression. Adipose tissue is
considered to be more metabolically active than other tissues and
to serve a prominent role in prostate tumorigenesis. Visceral fat
cells produce multiple hormones and cytokines, including
interleukin-6 (IL-6), tumor necrosis factor-α (TNF-α), leptin and
adiponectin (2,23). von Hafe et al (24) used computed tomography (CT) to
determine that visceral obesity may be a risk factor for PCa, which
could be explained by the activities of adipokines secreted by
visceral fat cells, as well as the elevated levels of insulin and
disturbances in steroid hormone homeostasis associated with
visceral obesity. Furthermore, Zimmermann et al (25) demonstrated that patients with higher
visceral fat volumes and densities exhibited more favorable
biochemical outcomes after radical prostatectomy and postoperative
radiotherapy.
Periprostatic adipose tissue (PPAT), a type of
visceral adipose tissue, serves a key role in PCa; the
extracapsular extension of cancer cells into PPAT has been reported
to be associated with poor prognosis (26). PPAT is anatomically defined as the
local adipose tissue that surrounds the prostate gland in the
pelvic cavity. Spatial imaging, such as CT or magnetic resonance
imaging (MRI), enables the measurement of distinct areas of adipose
tissue (27). In addition to
secreting multiple hormones and other protein factors, including
adiponectin, leptin and IL-6, similar to general visceral adipose
tissue, PPAT has been found to be correlated with PCa
aggressiveness (28). van Roermund
et al (28) demonstrated
that fat-related parameters of PPAT, including area and density,
were directly correlated with the aggressiveness of PCa and may
serve as better markers of obesity than BMI. Multiple studies have
also reported that PCa cells may alter adipocyte biology around the
prostate gland, while in vivo studies have demonstrated the
crosstalk between tumors and adipocytes (29–34).
The current medical treatments for PCa primarily
target the hormonal pathway, with the immune checkpoint and
homologous recombination pathways serving as secondary targets.
Although current medical treatments have demonstrated promising
efficacy, they face some limitations (35,36).
Notably, current treatments for localized PCa, such as radical
prostatectomy or radiation therapy, can achieve a high cure rate
and low PCa-related mortality rate; however, there has been limited
advancement in curing advanced PCa. Targeted therapies, such as
androgen receptor (AR)-signaling inhibitors (ARSIs), poly
(ADP-ribose) polymerase (PARP) inhibitors and radiopharmaceuticals
[prostate-specific membrane antigen (PSMA) Lu-177 or radium-223],
are novel treatments for advanced metastatic PCa aimed at disease
control rather than cure (37,38).
Notably, the treatment efficacy of these novel agents for advanced
or metastatic PCa is limited by their targeting mechanisms. PARP
inhibitors are effective in PCa with germline or somatic homologous
recombination repair deficiencies; however, these mutations,
including BRCA2, ATM, CDK12 and CHEK2, only account
for one in four patients (39).
ARSIs can achieve better chemical castration levels than
conventional androgen deprivation therapy (ADT) with
gonadotropin-releasing hormone agonist or antagonist, but patients
may eventually progress to castration refractory PCa (CRPC), mainly
due to AR mutation, splice variant formation, amplification, or
progression to neuroendocrine PCa (40). Regarding radiopharmaceuticals, the
effectiveness of PSMA-based treatments depends on PSMA expression
in the tumor; however, the innate heterogeneity of PSMA expression
is a notable limitation. Furthermore, downregulation of PSMA
expression is widespread in patients with advanced metastatic CRPC,
primarily due to lineage plasticity (41,42).
The treatment efficacy of radium-223 is limited to metastatic bone
lesions only, due to its bone-seeking calcium mimetic nature
(43). Therefore, novel treatment
strategies are warranted. Elucidating the interactions between PPAT
and PCa may enable the design of novel anticancer strategies
targeting different pathways.
The present study investigated the association
between pelvic adipose tissue (PAT) distribution and PCa
aggressiveness, as well as the underlying mechanisms. MRI was used
to evaluate clinical morphological characteristics. In addition,
PPAT collected during robot-assisted radical prostatectomy (RaRP)
was used to prepare conditioned medium (CM), the effects of which
were investigated on two PCa cell lines and one prostate epithelial
cell line.
Patients and methods
Patient data and tissue
collection
Between January 2009 and December 2021, patients
were consecutively enrolled at a single medical center, Linkou
Chang Gung Memorial Hospital (Taoyuan, Taiwan). A total of 50
patients with localized PCa who underwent RaRP were included. All
patients were reviewed and discussed at a multidisciplinary
uro-oncological meeting. RaRP was indicated for patients with
localized or selected locally advanced PCa. Before surgery, shared
decision-making about the treatment plan, along with other
alternative modalities, was discussed with the patients. Patients
that had previously been treated with ADT, radiation therapy,
chemotherapy or pelvic surgery were excluded, as were patients who
were not willing to provide informed consent. Patients underwent
pelvic MRI, with the results used to determine cancer stage and
treatment plan before surgery. All treatments were administered in
accordance with relevant regulations and guidelines. The medical
records of the patients were retrospectively reviewed to obtain
data regarding their general characteristics. PCa-related data were
also collected, including the serum level of initial
prostate-specific antigen (iPSA), Gleason score and pathological
stage (44).
To standardize the study, only patients harboring
tumors that were pathologically staged as T2 were selected. A total
of 1 g each of PPAT and perivesical adipose tissue (PVAT) were
obtained during RaRP and served as pericancerous adipose tissue and
normal adipose tissue, respectively. For studying the
characteristics of PPAT, only patients with pathological T2 stage
were selected because T3 stage represents extracapsular invasion,
meaning that tissue collected from patients with T3 stage or higher
may have potential PCa cell contamination in the PPAT specimens.
According to the final pathological report of the prostate
specimen, T2 refers to organ-confined disease, whereas T1 stage is
not applicable for PCa based on the 8th edition of the American
Joint Committee on Cancer (AJCC) TNM Staging System for Prostate
Cancer (44). This is because the
clinical T1 stage indicates a clinical inapparent tumor that is not
palpable. However, in the AJCC guideline for pathological T
staging, only T2 or higher stages are defined. All adipose tissues
were collected under the premise of noninterference with
pathological diagnoses. Only paired PPAT and PVAT tissues were used
for the preparation of CM and further studies, whereas patients
with insufficient or inadequate tissue quality were excluded. The
present study was approved by the Chang Gung Medical Foundation
Institutional Review Board. Written informed consent was obtained
from the patients for tissue collection.
Image analysis
Body composition, including the volume of PAT, PPAT
and perirectal adipose tissue (PRAT), was determined from the MRI
scans used for cancer staging by a single radiologist who was
blinded to the clinical information of the patients. All MRI scans
were performed using a 1.5-T or 3-T system according to the method
described by Chien et al (45). The MRI results were analyzed using
OsiriX (OsiriX MD, v10.0; Pixmeo SARL) by a single radiologist who
was informed that the patients had PCa and underwent subsequent
RaRP.
From the axial T1-weighted images of the pelvis, the
pelvic cavity, bladder, prostate gland, seminal vesicles, rectum
and perirectal space were manually segmented from the level of the
prostate base to the apex. The representative estimated volumes of
the regions are depicted in Fig. 1.
Subsequently, PAT, PRAT and PPAT volumes were calculated using the
following formulae: i) PAT volume=(pelvic cavity volume)-(bladder
volume)-(prostate volume)-(seminal vesicle volume)-(rectum volume);
ii) PRAT volume=(perirectal space volume)-(rectum volume); iii)
PPAT volume=(pelvic cavity volume)-(bladder volume)-(prostate
volume)-(seminal vesicle volume)-(perirectal space volume).
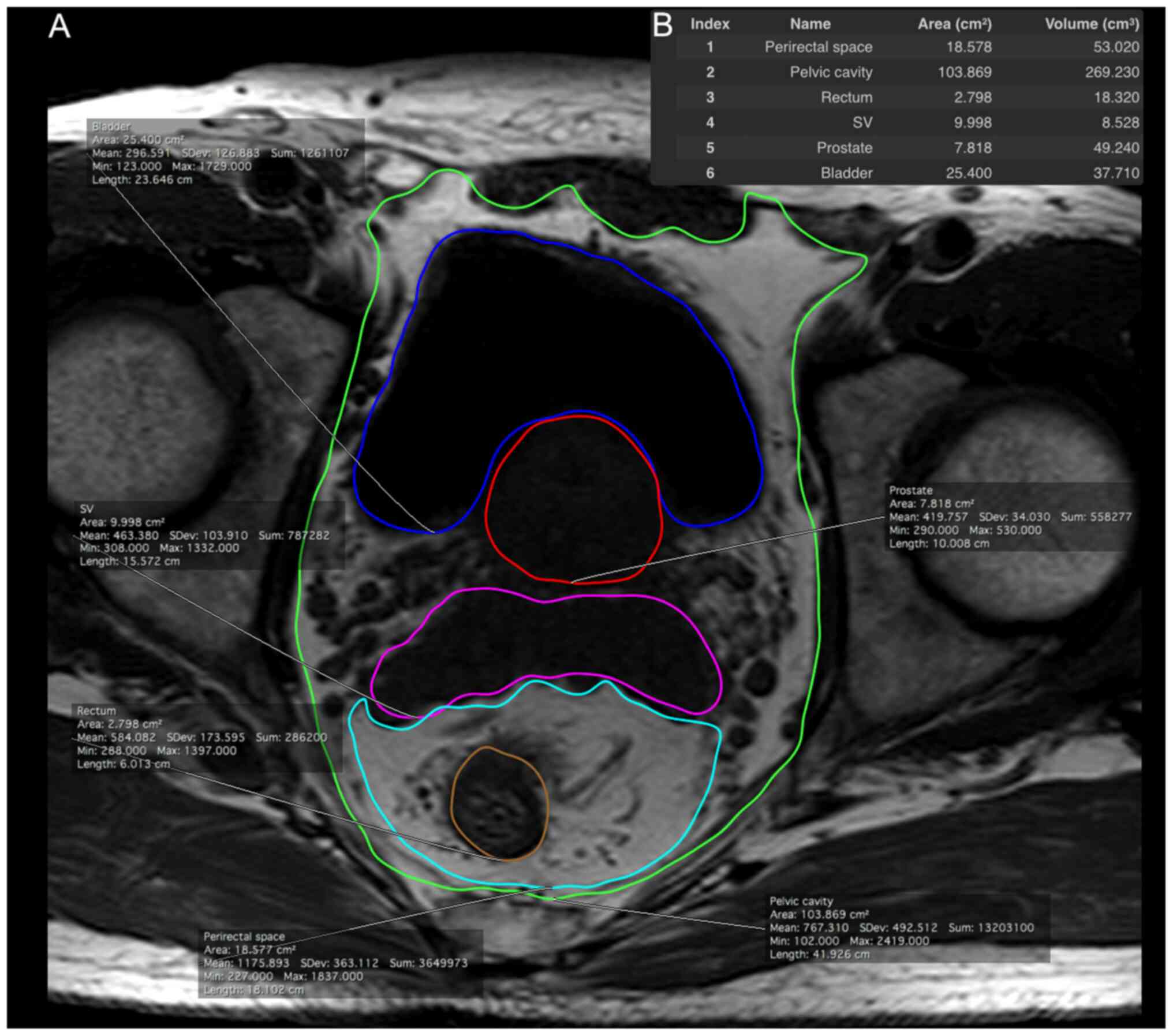 | Figure 1.Adipose tissue distribution in the
pelvic cavity was measured from T1-weighted magnetic resonance
imaging scans. (A) Each area is illustrated with a different color:
perirectal space (1, cyan), pelvic cavity (2, green), rectum (3,
orange), SV (4, pink), prostate (5, red) and bladder (6, blue). (B)
Measurement was performed from the level of the prostate base to
the apex. SV, seminal vesicles. |
Primary adipose tissue cultures and
preparation of CM
The collected PPAT or PVAT specimens were first
washed three times with prechilled phosphate-buffered saline (137
mM NaCl, 2.7 mM KCl, 10 mM Na2HPO4, 1.8 mM
KH2PO4, pH 7.4) to eliminate cell debris, and
were then weighed. The tissue specimens were minced using sterile
scissors and incubated in a T75 culture flask for 1 h at 37°C in 5%
CO2 with M199 culture medium (Gibco; Thermo Fisher
Scientific, Inc.; 1 g tissue in 10 ml medium) supplemented with
gentamicin (50 µg/ml). After 1 h, the medium was discarded, and the
minced tissue was incubated in fresh M199 medium for a further 24
h. The medium in the flask was collected and centrifuged 5 min at
400 × g at 4°C to remove cell pellets and debris. The resulting
supernatant was labeled as CM and stored at −80°C. In total, 25
pairs of PPAT and PVAT were collected for CM preparation.
Additionally, a control was created by collecting serum-free M199
medium after 24 h of incubation without adipose tissue in a T75
flask at 37°C in 5% CO2.
Cell lines and cell culture
The human PCa cell lines LNCaP and C4-2 were
obtained from the Bioresource Collection and Research Centre (BCRC)
and the American Type Culture Collection, respectively. The human
prostate epithelial cell line PZ-HPV-7 was obtained from the BCRC.
The LNCaP cells were maintained in RPMI 1640 medium (Gibco; Thermo
Fisher Scientific, Inc.) supplemented with 2 mM L-glutamine, 100
U/ml penicillin, 100 µg/ml streptomycin, 1 mM sodium pyruvate and
10% fetal bovine serum (FBS; Gibco; Thermo Fisher Scientific,
Inc.). The C4-2 cells were maintained in DMEM/F12 (4:1; Gibco;
Thermo Fisher Scientific, Inc.) supplemented with 0.1 µg/ml insulin
(Gibco; Thermo Fisher Scientific, Inc.), 275 ng/ml triiodothyronine
(Sigma-Aldrich; Merck KGaA), 88.6 ng/ml apo-Transferrin
(Sigma-Aldrich; Merck KGaA), 4.9 ng/ml d-Biotin (Sigma-Aldrich;
Merck KGaA), 251.8 ng/ml adenine (Sigma-Aldrich; Merck KGaA) and
10% FBS. The PZ-HPV-7 cells were maintained in keratinocyte
serum-free medium (Gibco; Thermo Fisher Scientific, Inc.)
supplemented with 0.05 ng/ml bovine pituitary extract (Gibco;
Thermo Fisher Scientific, Inc.), 5 ng/ml epidermal growth factor
(Gibco; Thermo Fisher Scientific, Inc.), 100 U/ml penicillin G and
100 µg/ml streptomycin. All cells were cultured at 37°C in a
humidified atmosphere of 5% CO2.
Cell proliferation and cell apoptosis
assay
The cells were seeded in 6-well plates (Corning,
Inc.) at a density of 3×105 cells/well and were
incubated at 37°C for 24 h in complete medium. Subsequently, the
spent medium was replaced with fresh serum-free medium containing
50% control-CM, PPAT-CM (tumor) or PVAT-CM (normal) and a final
concentration of 2% FBS. The cells were incubated at 37°C for 72 h,
trypsinized and counted after trypan blue staining. The staining
protocol included 0.4% trypan blue (Gibco; Thermo Fisher
Scientific, Inc.), a staining duration of 3 min at room
temperature, and visualization using a light microscope. Cell
apoptosis was assessed using the Annexin V-PE/7-AAD apoptosis assay
kit (BD Biosciences) with a BD LSRFortessa flow cytometer (BD
Biosciences) in accordance with the manufacturer's instructions.
FlowJo software (v10.4; FlowJo LLC) was used for data analysis.
RNA sequencing (RNA-seq) and pathway
analysis
RNA was extracted from LNCaP cells cultured in
control-CM, PPAT-CM or PVAT-CM for 72 h using the RNeasy mini kit
(Qiagen, Inc.) in accordance with the manufacturer's instructions.
The extracted RNA was sequenced to enable a comparison of the RNA
expression between the three groups of cultured cells. RNA-seq
libraries were prepared using 1 µg total RNA with the KAPA mRNA
HyperPrep Kit (cat. no. 08098123702; KAPA Biosystems; Roche
Diagnostics) following the manufacturer's recommendations, with
index codes added to attribute sequences to each sample. Short
double-stranded cDNA fragments were constructed and ligated to
sequencing adaptors, and the library fragments were purified using
the KAPA Pure Beads system (KAPA Biosystems; Roche Diagnostics).
The library, carrying appropriate adapter sequences at both ends,
was amplified using KAPA HiFi HotStart ReadyMix (KAPA Biosystems;
Roche Diagnostics) along with library amplification primers. The
strand marked with dUTP was not amplified, allowing for
strand-specific sequencing. PCR products were purified using the
KAPA Pure Beads system and assessed on the Qubit® 2.0
Fluorometer (Thermo Fisher Scientific, Inc.) and Agilent
Bioanalyzer 5400 system (Agilent Technologies, Inc.). Finally,
paired-end sequencing was performed using the NovaSeq 6000 S4
Reagent Kit v1.5 (300 cycles; cat. no. 20028312; Illumina, Inc.) on
the Illumina NovaSeq 6000 platform (cat. no. 20012850; Illumina,
Inc.) with the type of sequencing being 150 bp paired-end. The
loading concentration of the final library was 400 pM, with
concentrations measured by Q-PCR and Qubit® 2.0
Fluorometer. Gene Set Enrichment Analysis (GSEA) was used to
analyze the enriched pathways, with the C5 ontology gene sets in
the Molecular Signatures Database (v7.5.1) serving as a reference
(46–48).
Statistical analysis
Data are presented as the mean ± standard deviation.
Pearson correlation analysis was used to analyze the correlation
between PAT distribution and PCa aggressiveness. Intergroup
differences were analyzed using one-way ANOVA, followed by a post
hoc Bonferroni test, or an unpaired Student's t-test when
appropriate. All statistical analyses were performed using SPSS
software (v22.0; IBM Corporation). All tests were two-tailed, and
P≤0.05 was considered to indicate a statistically significant
difference. All in vitro experiments were performed as three
independent replicates.
Results
Baseline characteristics
The average age of the 50 patients enrolled in the
present study was 65.18±5.94 years. Their mean body weight, BMI and
serum iPSA levels were 70.39±9.55 kg, 25.92±3.17 kg/m2
and 16.64±12.84 ng/ml, respectively. The most common biopsy Gleason
score was 7 (42%), followed by 6 (38%) and 9 (14%), and the most
common pathological stage was T2c (46%), followed by T2a (24%) and
T3a (20%). Detailed information is presented in Table I.
 | Table I.General characteristics of the
patients (n=50). |
Table I.
General characteristics of the
patients (n=50).
| Characteristic | Value |
|---|
| Age, years | 65.18±5.94
(52–76) |
| Body weight,
kg | 70.39±9.55
(53.1–93.4) |
| BMI,
kg/m2 | 25.92±3.17
(20.8–34.6) |
| TRUS volume,
cm3 | 40.02±28.19
(13–137) |
| iPSA, ng/ml | 16.64±12.84
(4.16–58.08) |
| Gleason score |
|
| 5 | 1 (2) |
| 6 | 19 (38) |
| 7 | 21 (42) |
| 8 | 2 (4) |
| 9 | 7 (14) |
| Clinical T
stage |
|
| 2a | 12 (24) |
| 2b | 1 (2) |
| 2c | 23 (46) |
| 3a | 10 (20) |
| 3b | 3 (6) |
| 4 | 1 (2) |
| Pelvic adipose
tissue volume, ml | 114.05±48.31
(38.05–254.36) |
| Periprostatic
adipose tissue volume, ml | 69.78±31.48
(22.71–164.32) |
| Perirectal adipose
tissue volume, ml | 44.28±24.54
(1.16–119.61) |
Body composition and tumor
aggressiveness
Serum iPSA levels were significantly correlated with
the volumes of PAT (Pearson's r=0.404, P=0.006) and PPAT (Pearson's
r=0.436, P=0.003), and were not significantly correlated with the
volume of PRAT (Pearson's r=0.280, P=0.062) (Table II). Gleason scores were not
correlated with any PAT-related factor. The volume of PPAT was
significantly higher in patients exhibiting extracapsular extension
(pT3 or higher stage, P=0.031; Table
III).
 | Table II.Correlation between factors of
prostate cancer aggression and pelvic adipose distribution
(n=50). |
Table II.
Correlation between factors of
prostate cancer aggression and pelvic adipose distribution
(n=50).
|
| iPSA | Gleason score |
|---|
|
|
|
|
|---|
| Volume | Pearson correlation
coefficient | P-value | Pearson correlation
coefficient | P-value |
|---|
| Pelvic adipose
tissue volume | 0.404 | 0.006a | 0.220 | 0.146 |
| Periprostatic
adipose tissue volume | 0.436 | 0.003a | 0.175 | 0.251 |
| Perirectal adipose
tissue volume | 0.280 | 0.062 | 0.202 | 0.184 |
 | Table III.Association of extracapsular
extension with pelvic adipose distribution. |
Table III.
Association of extracapsular
extension with pelvic adipose distribution.
|
| Mean volume,
ml |
|
|---|
|
|
|
|
|---|
| Variable | With extracapsular
extension | Without
extracapsular extension | P-value |
|---|
| Pelvic adipose
tissue | 126.88 | 101.79 | 0.082 |
| Periprostatic
adipose tissue | 80.16 | 59.86 | 0.031a |
| Perirectal adipose
tissue | 46.73 | 41.93 | 0.518 |
Prostate cell proliferation in CM
Proliferation assays were performed on LNCaP and
C4-2 cells, as well as on PZ-HPV-7 cells, cultured in control-CM,
PVAT-CM (normal) or PPAT-CM (tumor) (Fig. 2). A total of 25 pairs of PPAT-CM and
PVAT-CM were used in the experiment. Initially, morphological
changes were observed after 3 days of culture in CM, with the cells
cultured in PPAT-CM exhibiting the most severe shrinkage among the
experimental groups (Fig. 2A). The
relative proliferation rate of the LNCaP cells was significantly
lower in the PPAT-CM group than in the control- and PVAT-CM groups
(P=0.0216 and 0.0343, respectively; Fig. 2B). Similar trends in proliferation
rates were observed for C4-2 cells (P=0.0074 and 0.0266 compared
with the control- and PVAT-CM groups, respectively) and PZ-HPV-7
cells (P=0.0063 compared with the control-CM group) cultured in
PPAT-CM. When comparing the cell proliferation rates between the
PVAT- and PPAT-CM groups, the cells in the PPAT-CM group showed a
trend of slower growth, but the difference was not statistically
significant (Fig. 2C). These
results indicated that PPAT may inhibit prostate cell
proliferation, and such impacts appear to be more pronounced in PCa
cells used in this study.
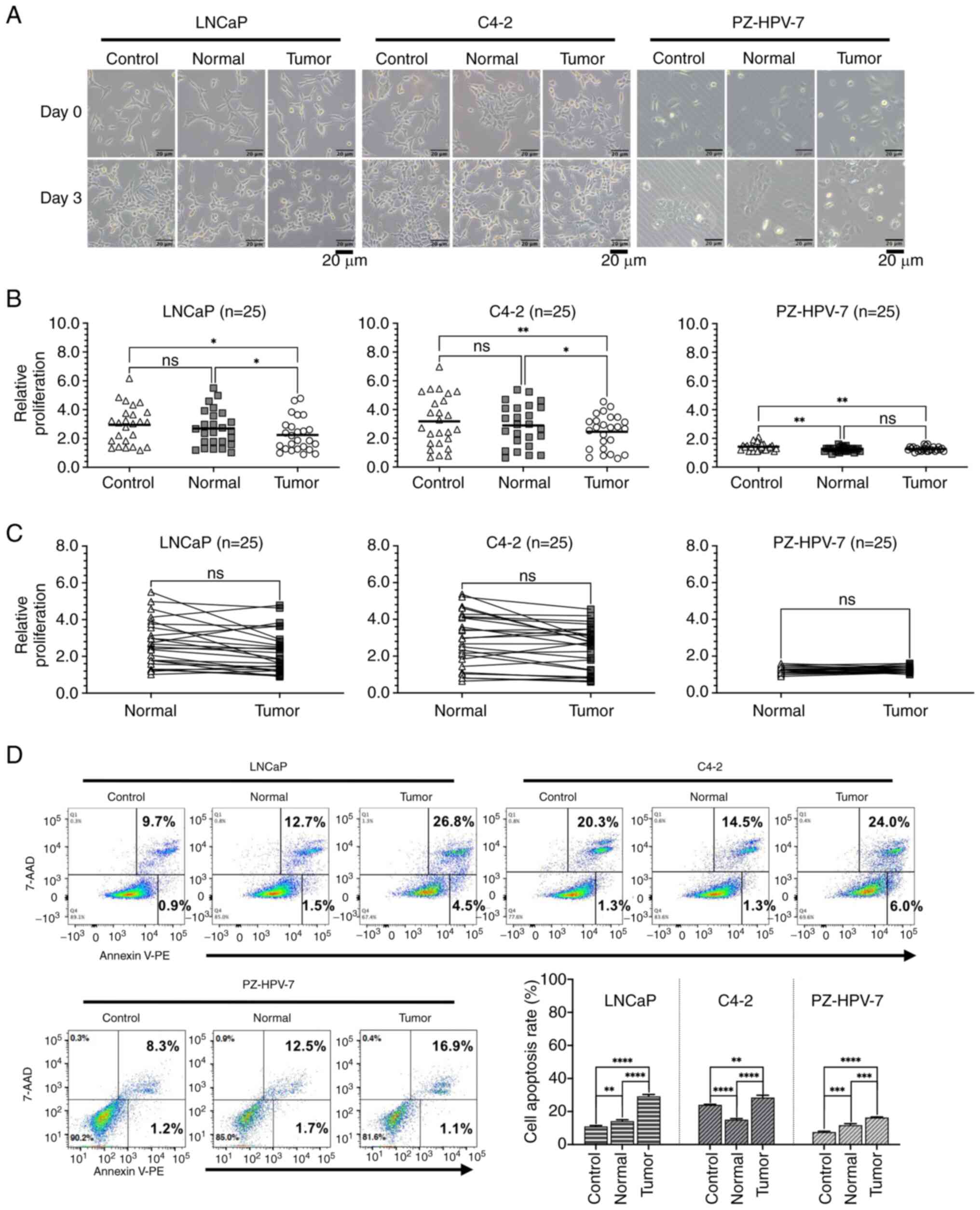 | Figure 2.Effect of different CM on the
proliferation and apoptosis of LNCaP and C4-2 prostate cancer cell
lines, and the PZ-HPV-7 prostate epithelial cell line. Cells were
seeded in 6-well culture plates. After 24 h, the medium was changed
to serum-free RPMI 1640 medium containing 50% M199 medium
(Control), periprostatic adipose tissue-CM (Tumor) or perivesicle
adipose tissue-CM (Normal) with a final concentration of 2% FBS.
After 72 h of incubation, cell morphology, cell proliferation and
cell apoptosis were assessed. (A) Representative cell morphology
(original magnification, ×100; scale bar, 20 µm). (B) Scatter plots
and (C) paired scatter plots representing the relative fold-change
in cell proliferation, calculated by dividing the total cell count
at day 3 by the total cell count at day 0 (n=25). (D) Apoptosis was
analyzed by Annexin V-PE/7-AAD staining using the BD LSRFortessa
System. Apoptotic cells were compared between various groups, with
three independent replicates. *P≤0.05, **P≤0.01, ***P≤0.001,
****P≤0.0001. CM, conditioned medium. |
Prostate cell apoptosis in CM
To verify whether the attenuated proliferation rate
in the PPAT-CM group was due to the induction of cell apoptosis, a
cell apoptosis assay was performed using flow cytometry. The extent
of apoptosis in all three cell lines was higher in the PPAT-CM
group than in the control- and PVAT-CM groups (Fig. 2D), suggesting that some factors in
PPAT may induce the apoptosis of prostate cells.
RNA-seq of PCa cells cultured in
CM
GSEA was used to compare the RNA expression profiles
of LNCaP cells cultured in control-CM, PVAT-CM and PPAT-CM, since
LNCaP cells exhibit significantly differential responses to CM,
particularly in cell apoptosis. The results, according to the C5
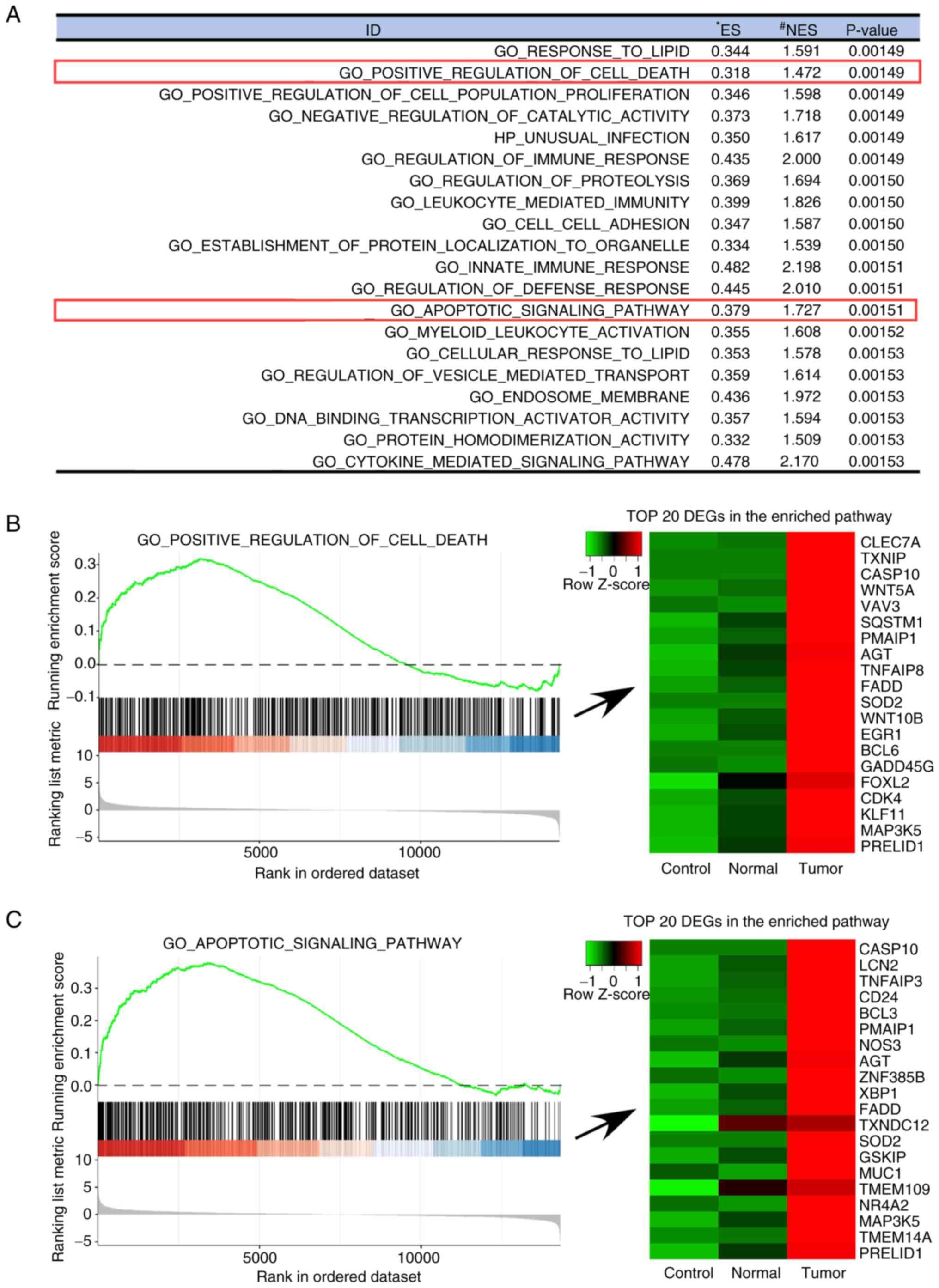
ontology gene sets, are presented in Fig. 3. The following pathways were more
activated in the PPAT-CM-cultured LNCaP cells than in the
control-CM- or PVAT-CM-cultured cells: ‘Regulation of immune
response’ (P=0.00149), ‘Leukocyte-mediated immunity’ (P=0.00150),
‘Cytokine-mediated signaling pathways’ (P=0.00153), ‘Innate immune
response’ (P=0.00151), ‘Regulation of defense response’
(P=0.00151), ‘Positive regulation of cell death’ (P=0.00149) and
‘Apoptotic signaling pathway’ (P=0.00151). These results indicate
that the presence of factors in PPAT-CM that induce immune related
responses and cell apoptosis. Specifically, several genes
associated with cell death and cell apoptosis, such as SOD2,
FADD and CASP10, were upregulated in the
PPAT-CM-cultured LNCaP cells, as shown in the ‘Positive regulation
of cell death’ and ‘Apoptotic signaling pathway’ (Fig. 3B and C).
Discussion
According to the literature, PPAT can markedly
influence the progression of PCa. Woo et al (49) studied 190 patients with PCa who
underwent MRI before radical prostatectomy and demonstrated that
PPAT thickness, as determined through MRI, was significantly
correlated with Gleason score and was an independent predictor of
high-grade PCa. In addition, Zhang et al (50) discovered that periprostatic
adiposity was significantly associated with Gleason scores and
clinical stage after evaluating the MRI scans of 184 patients with
PCa who underwent radical prostatectomy. Bhindi et al
(51) demonstrated that PPAT
volume, as estimated through transrectal ultrasound, can predict
the presence and the grade of PCa. PPAT area and density have also
been reported to be more strongly correlated with PCa
aggressiveness than other obesity-related parameters, including
waist circumference and BMI (28,49,50).
In addition, the correlations of PPAT thickness with BMI and weight
have been shown to be nonsignificant or weak (49–54).
Therefore, measurements of PPAT may serve as independent predictors
of PCa aggressiveness. In the current study, the volumes of PAT,
PRAT and PPAT were measured using MRI to identify associations
between PAT distribution and PCa aggressiveness. The present study
revealed that serum iPSA levels were significantly associated with
higher PAT and PPAT volumes. Furthermore, a higher PPAT volume was
correlated with extracapsular extension, which is consistent with
the findings of a study investigating the association between PPAT
volume and tumor aggressiveness (55).
Although clinical findings have indicated an
association between PPAT and PCa, the mechanisms underlying this
association remain unclear. PPAT may provide a favorable
microenvironment for aggressive PCa or may passively accumulate fat
content in response to local PCa progression. Furthermore, PPAT may
affect PCa progression and pathogenesis by providing cancer cells
with fatty acids and other mitogens. A previous study identified
factors secreted by both PPAT and PCa cells potentially capable of
mediating bidirectional communication between them (56). Ribeiro et al (31) reported that PPAT-CM stimulated PC3
and LNCaP cell migration but inhibited LNCaP proliferation.
Conversely, another study reported that PPAT-CM did not
significantly affect the proliferation and motility of LNCaP or PC3
cells (57). In addition, a
previous study indicated that the proliferation of PC3 cells was
increased when cocultured with rat epididymal adipocytes (58), but these findings were not observed
in a later study (33). These
conflicting results are likely due to differences in the
characteristics of the experimental methodologies and cell lines
that were used.
The present study used the LNCaP and C4-2 cell lines
as the experimental models because the present clinical analysis
focused on patients with T2 stage PCa, ensuring that the adipose
tissue was free from potential contamination by PCa cells. LNCaP
cells, known for their well-established use in PCa research, have
lower malignancy and AR positivity, representing earlier-stage PCa,
compared to other commonly used PCa cell lines, such as PC-3, DU145
and CWR22R-v1 (59). By contrast,
C4-2 cells, derived from LNCaP cells, exhibit higher invasiveness
and metastatic potential, representing a more advanced stage of PCa
(60,61). While C4-2 cells indeed exhibit
higher invasiveness and metastatic potential compared with LNCaP
cells, they still inherently express AR, representing relatively
early stages of PCa. Conversely, other commonly used PCa cell
lines, such as PC-3, DU145 and CWR22R-v1, demonstrate higher
malignancy, representing advanced stages of PCa. In addition, PC-3
and DU145 cells lack AR expression, with DU145 further classified
as a CRPC cell line (62–64). Similarly, although CWR22R-v1 is
AR-positive, it is also classified as a CRPC cell line (65). Therefore, to mimic the T2 tumor
stage of the clinical specimens, C4-2 cells were chosen as another
PCa cell line for investigation. This selection allows for the
study of differential responses between less and more aggressive
PCa cells, and may improve understanding of the impact of the
adipose tissue secretome on tumors at different stages of
malignancy. Additionally, the present study incorporated
experiments using the prostate normal epithelial cell line PZ-HPV-7
to provide a comprehensive comparison and to observe the effects on
normal prostate cells. The proliferation rate of prostate cells was
significantly lower in the PPAT-CM group compared with that in the
control- and PVAT-CM groups. However, when comparing the cell
proliferation rates between the PVAT- and PPAT-CM groups, the cells
in the PPAT-CM group showed a trend of slower growth, but the
difference was not statistically significant, possibly due to the
insufficient number of groups used in the experiment. The un-paired
Student's t-test did not show statistical significance, likely due
to individual variations among the samples. Collecting more cases
could reduce these individual differences and achieve statistical
significance. Furthermore, the cells cultured in PPAT-CM exhibited
more pronounced apoptosis than those cultured in control- and
PVAT-CM did. Compared with the PCa cell lines, although the CM had
a slight effect on the prostate normal epithelial cell line, the
impact on PCa cells appeared to be more pronounced. RNA-seq and
analysis revealed that immune responses, and the cell death and
apoptosis pathways were more activated in PPAT-CM-cultured cells
than in PVAT-CM-cultured cells. These findings indicated that the
cytokines and other factors secreted from PPAT-CM may have induced
PCa cell apoptosis.
Evidence has indicated that adipose tissue can act
as an energy reservoir, and it is a metabolically active organ
(66) that produces growth factors,
hormones and adipokines (67). The
secretions of adipose tissue affect both physiological cellular
responses, and the paracrine and autocrine signaling networks,
especially in tumor microenvironments, where hormonal dependence
mediates cancer progression (32,57,68–71).
Several cytokines and adipokines, such as IL-6, leptin and vascular
endothelial growth factor, have been associated with tumor
progression (72,73); however, several others, including
adiponectin (74), suppress tumor
growth, whereas the effects of factors such as TNF-α remain unclear
(75).
The secretome of adipose tissue may vary across
different stages of PCa. Sacca et al (76) analyzed PPAT-CM from patients either
with PCa (including stage T2 and T3 tumors) or from those with
benign diseases using liquid chromatography-mass spectrometry-based
proteomics. The results observed that the PPAT from patients with
cancer exhibited stronger immune responses. Moreover, the PPAT from
patients with stage T3 PCa was rich in catalytic proteins, whereas
that from patients with stage T2 PCa was rich in defense and immune
response proteins. These findings indicated that, in the early
stages of localized PCa, the secretome of PPAT, including
cytokines, may activate immune defense responses and induce cell
apoptosis. This mechanism potentially acts as a first line of
defense in the early stages of PCa. However, when the disease
progresses and cancer cells extend to adipose tissue, their
crosstalk with the tissue may induce catalytic activity and alter
the tumor microenvironment, triggering progression or invasion.
The present study explored the role of PPAT in the
early stages of PCa and revealed that the secretome of PPAT could
inhibit cell proliferation by inducing apoptosis. However, this
study has several limitations. First, PPAT was obtained only from
patients harboring early-stage tumors (T2); samples from patients
with more advanced PCa are required to further validate that PPAT
inhibits cell proliferation. Second, different PCa cell lines
cultured with PPAT should be used to thoroughly understand the
influence of adipose tissue on cancer cells. Finally, the mechanism
underlying the changes in the secretome of PPAT with the
progression of PCa and its related signaling pathways remain to be
elucidated.
In conclusion, the present study revealed that PPAT
was significantly associated with extracapsular PCa extension.
Furthermore, in vitro experiments revealed that PPAT could
inhibit PCa cell proliferation by secreting factors that activated
immune responses and could thereby promote cancer cell apoptosis.
This mechanism may act as a first line of defense in the early
stages of PCa. The mechanisms underlying further crosstalk between
PPAT and PCa cells remain to be elucidated.
Acknowledgements
Not applicable.
Funding
The present study was supported by grants from the Chang Gung
Memorial Hospital Research Foundation (grant no. CMRPG3N0041).
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author. The RNA-seq data generated
in the present study may be found in the National Center for
Biotechnology Information-Gene Expression Omnibus database under
accession number GSE267084 or at the following URL: https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE267084.
Authors' contributions
IHS, CTW, CHH and STP conceived and designed the
study. IHS, YiHC, LKH, YCC, HCK, PHL, KJY, MLH, CKC, CTW and STP
participated in data acquisition. IHS, THC, YHH, TWS and YuHC
participated in the collection and assembly of data. TWS, LJW and
YuHC participated in image analysis. IHS, THC, YHH and CHH
participated in data analysis and interpretation. IHS, THC, CHH and
STP participated in manuscript writing. IHS, CHH and STP confirmed
the authenticity of all raw data. All authors read and approved the
final version of the manuscript.
Ethics approval and consent to
participate
The present study was conducted in accordance with
the ethical principles outlined in The Declaration of Helsinki
(2013). This study was approved by the Chang Gung Medical
Foundation Institutional Review Board (IRB no. 1912230098). Written
informed consent was obtained from the patients for tissue
collection.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Siegel RL, Miller KD, Fuchs HE and Jemal
A: Cancer statistics, 2022. CA Cancer J Clin. 72:7–33. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Bray F, Ferlay J, Soerjomataram I, Siegel
RL, Torre LA and Jemal A: Global cancer statistics 2018: GLOBOCAN
estimates of incidence and mortality worldwide for 36 cancers in
185 countries. CA Cancer J Clin. 68:394–424. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
López Fontana C, Maselli ME, Pérez
Elizalde R, Di Milta N, Corica Alberto P and López Laur JD: Obesity
modifies prostatic specific antigen in men over 45 years. Arch Esp
Urol. 64:35–42. 2011.PubMed/NCBI
|
|
4
|
Parikesit D, Mochtar CA, Umbas R and Hamid
ARAH: The impact of obesity towards prostate diseases. Prostate
Int. 4:1–6. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Cataño JG, Ramos-Hernández A, Bravo-Balado
A, Mariño-Álvarez AM, Caicedo JI, Trujillo CG and Plata M: Obesity
and radical prostatectomy: The enigma continues. Arch Esp Urol.
71:517–522. 2018.(In Spanish). PubMed/NCBI
|
|
6
|
Williams G: Aromatase up-regulation,
insulin and raised intracellular oestrogens in men, induce
adiposity, metabolic syndrome and prostate disease, via aberrant
ER-α and GPER signalling. Mol Cell Endocrinol. 351:269–278. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Caine M, Raz S and Zeigler M: Adrenergic
and cholinergic receptors in the human prostate, prostatic capsule
and bladder neck. Br J Urol. 47:193–202. 1975. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Giovannucci E, Rimm EB, Chute CG, Kawachi
I, Colditz GA, Stampfer MJ and Willett WC: Obesity and benign
prostatic hyperplasia. Am J Epidemiol. 140:989–1002. 1994.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hamid ARAH, Umbas R and Mochtar CA: Recent
role of inflammation in prostate diseases: Chemoprevention
development opportunity. Acta Med Indones. 43:59–65.
2011.PubMed/NCBI
|
|
10
|
Alukal JP and Lepor H: Testosterone
deficiency and the prostate. Urol Clin North Am. 43:203–208. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Schnoeller T, Jentzmik F, Rinnab L,
Cronauer MV, Damjanoski I, Zengerling F, Ghazal AA, Schrader M and
Schrader AJ: Circulating free testosterone is an independent
predictor of advanced disease in patients with clinically localized
prostate cancer. World J Urol. 31:253–259. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Theoret MR, Ning YM, Zhang JJ, Justice R,
Keegan P and Pazdur R: The risks and benefits of 5α-reductase
inhibitors for prostate-cancer prevention. N Engl J Med. 365:97–99.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Gallagher EJ and LeRoith D: The
proliferating role of insulin and insulin-like growth factors in
cancer. Trends Endocrinol Metab. 21:610–618. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ribeiro R, Lopes C and Medeiros R: Leptin
and prostate: Implications for cancer prevention-overview of
genetics and molecular interactions. Eur J Cancer Prev. 13:359–368.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Izadi V, Farabad E and Azadbakht L: Serum
adiponectin level and different kinds of cancer: A review of recent
evidence. ISRN Oncol. 2012:9827692012.PubMed/NCBI
|
|
16
|
Amling CL, Riffenburgh RH, Sun L, Moul JW,
Lance RS, Kusuda L, Sexton WJ, Soderdahl DW, Donahue TF, Foley JP,
et al: Pathologic variables and recurrence rates as related to
obesity and race in men with prostate cancer undergoing radical
prostatectomy. J Clin Oncol. 22:439–445. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Rodriguez C, Freedland SJ, Deka A, Jacobs
EJ, McCullough ML, Patel AV, Thun MJ and Calle EE: Body mass index,
weight change, and risk of prostate cancer in the cancer prevention
study II nutrition cohort. Cancer Epidemiol Biomarkers Prev.
16:63–69. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Renehan AG, Tyson M, Egger M, Heller RF
and Zwahlen M: Body-mass index and incidence of cancer: A
systematic review and meta-analysis of prospective observational
studies. Lancet. 371:569–578. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
MacInnis RJ and English DR: Body size and
composition and prostate cancer risk: Systematic review and
meta-regression analysis. Cancer Causes Control. 17:989–1003. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Bergström A, Pisani P, Tenet V, Wolk A and
Adami HO: Overweight as an avoidable cause of cancer in Europe. Int
J Cancer. 91:421–430. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Cao Y and Ma J: Body mass index, prostate
cancer-specific mortality, and biochemical recurrence: A systematic
review and meta-analysis. Cancer Prev Res (Phila). 4:486–501. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Duong MN, Geneste A, Fallone F, Li X,
Dumontet C and Muller C: The fat and the bad: Mature adipocytes,
key actors in tumor progression and resistance. Oncotarget.
8:57622–57641. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
van Roermund JG and Witjes JA: The impact
of obesity on prostate cancer. World J Urol. 25:491–497. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
von Hafe P, Pina F, Pérez A, Tavares M and
Barros H: Visceral fat accumulation as a risk factor for prostate
cancer. Obes Res. 12:1930–1935. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zimmermann M, Delouya G, Barkati M,
Campeau S, Rompotinos D and Taussky D: Impact of visceral fat
volume and fat density on biochemical outcome after radical
prostatectomy and postoperative radiotherapy. Horm Mol Biol Clin
Investig. 26:173–178. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Cheng L, Darson MF, Bergstralh EJ, Slezak
J, Myers RP and Bostwick DG: Correlation of margin status and
extraprostatic extension with progression of prostate carcinoma.
Cancer. 86:1775–1782. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Miladinovic D, Cusick T, Mahon KL, Haynes
AM, Cortie CH, Meyer BJ, Stricker PD, Wittert GA, Butler LM,
Horvath LG and Hoy AJ: Assessment of periprostatic and subcutaneous
adipose tissue lipolysis and adipocyte size from men with localized
prostate cancer. Cancers (Basel). 12:13852020. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
van Roermund JG, Hinnen KA, Tolman CJ, Bol
GH, Witjes JA, Bosch JL, Kiemeney LA and van Vulpen M:
Periprostatic fat correlates with tumour aggressiveness in prostate
cancer patients. BJU Int. 107:1775–1779. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Laurent V, Guérard A, Mazerolles C, Le
Gonidec S, Toulet A, Nieto L, Zaidi F, Majed B, Garandeau D,
Socrier Y, et al: Periprostatic adipocytes act as a driving force
for prostate cancer progression in obesity. Nat Commun.
7:102302016. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Laurent V, Toulet A, Attané C, Milhas D,
Dauvillier S, Zaidi F, Clement E, Cinato M, Le Gonidec S, Guérard
A, et al: Periprostatic adipose tissue favors prostate cancer cell
invasion in an obesity-dependent manner: Role of oxidative stress.
Mol Cancer Res. 17:821–835. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Ribeiro R, Monteiro C, Cunha V, Oliveira
MJ, Freitas M, Fraga A, Príncipe P, Lobato C, Lobo F, Morais A, et
al: Human periprostatic adipose tissue promotes prostate cancer
aggressiveness in vitro. J Exp Clin Cancer Res. 31:322012.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Finley DS, Calvert VS, Inokuchi J, Lau A,
Narula N, Petricoin EF, Zaldivar F, Santos R, Tyson DR and Ornstein
DK: Periprostatic adipose tissue as a modulator of prostate cancer
aggressiveness. J Urol. 182:1621–1627. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Kaneko A, Satoh Y, Tokuda Y, Fujiyama C,
Udo K and Uozumi J: Effects of adipocytes on the proliferation and
differentiation of prostate cancer cells in a 3-D culture model.
Int J Urol. 17:369–376. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Estève D, Roumiguié M, Manceau C, Milhas D
and Muller C: Periprostatic adipose tissue: A heavy player in
prostate cancer progression. Curr Opin Endocr Metab Res. 10:29–35.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Kulasegaran T and Oliveira N: Metastatic
castration-resistant prostate cancer: Advances in treatment and
symptom management. Curr Treat Options Oncol. 25:914–931. 2024.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Venkatachalam S, McFarland TR, Agarwal N
and Swami U: Immune checkpoint inhibitors in prostate cancer.
Cancers (Basel). 13:21872021. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Gourdin T: Highlighting recent progress in
the treatment of men with advanced prostate cancer. Curr Opin
Oncol. 36:174–179. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Gillette CM, Yette GA, Cramer SD and
Graham LS: Management of advanced prostate cancer in the precision
oncology era. Cancers (Basel). 15:25522023. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Abida W, Armenia J, Gopalan A, Brennan R,
Walsh M, Barron D, Danila D, Rathkopf D, Morris M, Slovin S, et al:
Prospective Genomic profiling of prostate cancer across disease
states reveals germline and somatic alterations that may affect
clinical decision making. JCO Precis Oncol. 2017.PO.17.00029, 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Zhang Y, Ming A, Wang J, Chen W and Fang
Z: PROTACs targeting androgen receptor signaling: Potential
therapeutic agents for castration-resistant prostate cancer.
Pharmacol Res. 205:1072342024. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Sallam M, Nguyen NT, Sainsbury F, Kimizuka
N, Muyldermans S and Benešová-Schäfer M: PSMA-targeted
radiotheranostics in modern nuclear medicine: Then, now, and what
of the future? Theranostics. 14:3043–3079. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Current K, Meyer C, Magyar CE, Mona CE,
Almajano J, Slavik R, Stuparu AD, Cheng C, Dawson DW, Radu CG, et
al: Investigating PSMA-targeted radioligand therapy efficacy as a
function of cellular PSMA levels and intratumoral PSMA
heterogeneity. Clin Cancer Res. 26:2946–2955. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Isaacsson Velho P, Qazi F, Hassan S,
Carducci MA, Denmeade SR, Markowski MC, Thorek DL, DeWeese TL, Song
DY, Tran PT, et al: Efficacy of radium-223 in bone-metastatic
castration-resistant prostate cancer with and without homologous
repair gene defects. Eur Urol. 76:170–176. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Buyyounouski MK, Choyke PL, McKenney JK,
Sartor O, Sandler HM, Amin MB, Kattan MW and Lin DW: Prostate
cancer-major changes in the American joint committee on cancer
eighth edition cancer staging manual. CA Cancer J Clin. 67:245–253.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Chien YH, Hsieh ML, Sheng TW, Chang YH,
Wang LJ, Chuang CK, Pang ST, Wu CT and Shao IH: Body composition
and pelvic fat distribution are associated with prostate cancer
aggressiveness and can predict biochemical recurrence. Medicine
(Baltimore). 101:e310762022. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Subramanian A, Tamayo P, Mootha VK,
Mukherjee S, Ebert BL, Gillette MA, Paulovich A, Pomeroy SL, Golub
TR, Lander ES and Mesirov JP: Gene set enrichment analysis: a
knowledge-based approach for interpreting genome-wide expression
profiles. Proc Natl Acad Sci U S A. 102:15545–15550. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Mootha VK, Lindgren CM, Eriksson KF,
Subramanian A, Sihag S, Lehar J, Puigserver P, Carlsson E,
Ridderstråle M, Laurila E, et al: PGC-1alpha-responsive genes
involved in oxidative phosphorylation are coordinately
downregulated in human diabetes. Nat Genet. 34:267–273. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Liberzon A, Birger C, Thorvaldsdóttir H,
Ghandi M, Mesirov JP and Tamayo P: The molecular signatures
database (MSigDB) hallmark gene set collection. Cell Syst.
1:417–425. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Woo S, Cho JY, Kim SY and Kim SH:
Periprostatic fat thickness on MRI: Correlation with Gleason score
in prostate cancer. AJR Am J Roentgenol. 204:W43–W47. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Zhang Q, Sun LJ, Qi J, Yang ZG, Huang T
and Huo RC: Periprostatic adiposity measured on magnetic resonance
imaging correlates with prostate cancer aggressiveness. Urol J.
11:1793–1799. 2014.PubMed/NCBI
|
|
51
|
Bhindi B, Trottier G, Elharram M,
Fernandes KA, Lockwood G, Toi A, Hersey KM, Finelli A, Evans A, van
der Kwast TH and Fleshner NE: Measurement of peri-prostatic fat
thickness using transrectal ultrasonography (TRUS): A new risk
factor for prostate cancer. BJU Int. 110:980–986. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Taussky D, Barkati M, Campeau S, Zerouali
K, Nadiri A, Saad F and Delouya G: Changes in periprostatic adipose
tissue induced by 5α-reductase inhibitors. Andrology. 5:511–515.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Tiberi D, Gruszczynski N, Meissner A,
Delouya G and Taussky D: Influence of body mass index and
periprostatic fat on rectal dosimetry in permanent seed prostate
brachytherapy. Radiat Oncol. 9:932014. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
van Roermund JG, Bol GH, Witjes JA, Ruud
Bosch JL, Kiemeney LA and van Vulpen M: Periprostatic fat measured
on computed tomography as a marker for prostate cancer
aggressiveness. World J Urol. 28:699–704. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Valastyan S and Weinberg RA: Tumor
metastasis: Molecular insights and evolving paradigms. Cell.
147:275–292. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Nassar ZD, Aref AT, Miladinovic D, Mah CY,
Raj GV, Hoy AJ and Butler LM: Peri-prostatic adipose tissue: The
metabolic microenvironment of prostate cancer. BJU Int. 121 (Suppl
3):S9–S21. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Sacca PA, Creydt VP, Choi H, Mazza ON,
Fletcher SJ, Vallone VB, Scorticati C, Chasseing NA and Calvo JC:
Human periprostatic adipose tissue: Its influence on prostate
cancer cells. Cell Physiol Biochem. 30:113–122. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Tokuda Y, Satoh Y, Fujiyama C, Toda S,
Sugihara H and Masaki Z: Prostate cancer cell growth is modulated
by adipocyte-cancer cell interaction. BJU Int. 91:716–720. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
59
|
König JJ, Kamst E, Hagemeijer A, Romijn
JC, Horoszewicz J and Schröder FH: Cytogenetic characterization of
several androgen responsive and unresponsive sublines of the human
prostatic carcinoma cell line LNCaP. Urol Res. 17:79–86. 1989.
View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Wu HC, Hsieh JT, Gleave ME, Brown NM,
Pathak S and Chung LW: Derivation of androgen-independent human
LNCaP prostatic cancer cell sublines: Role of bone stromal cells.
Int J Cancer. 57:406–412. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Liu AY, Brubaker KD, Goo YA, Quinn JE,
Kral S, Sorensen CM, Vessella RL, Belldegrun AS and Hood LE:
Lineage relationship between LNCaP and LNCaP-derived prostate
cancer cell lines. Prostate. 60:98–108. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Kaighn ME, Narayan KS, Ohnuki Y, Lechner
JF and Jones LW: Establishment and characterization of a human
prostatic carcinoma cell line (PC-3). Invest Urol. 17:16–23.
1979.PubMed/NCBI
|
|
63
|
Stone KR, Mickey DD, Wunderli H, Mickey GH
and Paulson DF: Isolation of a human prostate carcinoma cell line
(DU 145). Int J Cancer. 21:274–281. 1978. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Alimirah F, Chen J, Basrawala Z, Xin H and
Choubey D: DU-145 and PC-3 human prostate cancer cell lines express
androgen receptor: Implications for the androgen receptor functions
and regulation. FEBS Lett. 580:2294–2300. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Sramkoski RM, Pretlow TG II, Giaconia JM,
Pretlow TP, Schwartz S, Sy MS, Marengo SR, Rhim JS, Zhang D and
Jacobberger JW: A new human prostate carcinoma cell line, 22Rv1. In
Vitro Cell Dev Biol Anim. 35:403–409. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Wu Y, Kim JY, Zhou S and Smas CM:
Differential screening identifies transcripts with depot-dependent
expression in white adipose tissues. BMC Genomics. 9:3972008.
View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Mistry T, Digby JE, Desai KM and Randeva
HS: Obesity and prostate cancer: A role for adipokines. Eur Urol.
52:46–53. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Fletcher SJ, Sacca PA, Pistone-Creydt M,
Coló FA, Serra MF, Santino FE, Sasso CV, Lopez-Fontana CM, Carón
RW, Calvo JC and Pistone-Creydt V: Human breast adipose tissue:
Characterization of factors that change during tumor progression in
human breast cancer. J Exp Clin Cancer Res. 36:262017. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Karagiannis GS, Pavlou MP and Diamandis
EP: Cancer secretomics reveal pathophysiological pathways in cancer
molecular oncology. Mol Oncol. 4:496–510. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Kim KY, Baek A, Park YS, Park MY, Kim JH,
Lim JS, Lee MS, Yoon SR, Lee HG, Yoon Y, et al: Adipocyte culture
medium stimulates invasiveness of MDA-MB-231 cell via CCL20
production. Oncol Rep. 22:1497–1504. 2009.PubMed/NCBI
|
|
71
|
Schnäbele K, Roser S, Rechkemmer G, Hauner
H and Skurk T: Effects of adipocyte-secreted factors on cell cycle
progression in HT29 cells. Eur J Nutr. 48:154–161. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Culig Z and Puhr M: Interleukin-6 and
prostate cancer: Current developments and unsolved questions. Mol
Cell Endocrinol. 462:25–30. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Lopez Fontana CM, Maselli Artola ME, Di
Milta Monaco N, Recalde Rincon GM, Vanrell Rodriguez MC, Uvilla
Recupero A, Messina Lombino D, Perez Elizalde RF and Lopez Laur JD:
Influence of leptin and adiponectin on prostate cancer. Arch Esp
Urol. 62:103–108. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Hu X, Hu C, Zhang C, Zhang M, Long S and
Cao Z: Role of adiponectin in prostate cancer. Int Braz J Urol.
45:220–228. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Balkwill F: Tumour necrosis factor and
cancer. Nat Rev Cancer. 9:361–371. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Sacca PA, Mazza ON, Scorticati C,
Vitagliano G, Casas G and Calvo JC: Human periprostatic adipose
tissue: secretome from patients with prostate cancer or benign
prostate hyperplasia. Cancer Genomics Proteomics. 16:29–58. 2019.
View Article : Google Scholar : PubMed/NCBI
|