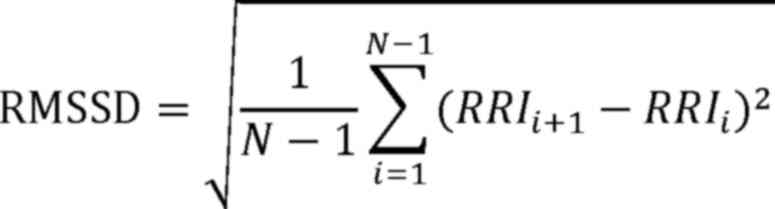Introduction
Endometrial cancer (EC) is one of the most common
malignant tumors of the female reproductive system (1), and its incidence is increasing
worldwide (2). In total,
>410,000 new cases and >90,000 associated deaths were
estimated to occur globally in 2020 (1). Previous studies have confirmed that
patients with malignant tumors are 7–10 fold more likely to exhibit
abnormalities in coagulation and/or fibrinolysis than are patients
in the general population (3–5). For
patients with EC, changes in the levels of coagulation biomarkers,
including fibrinogen (FIB) and D-dimer, may indicate the occurrence
of coagulation dysfunction (6,7). Some
treatments administered to patients with EC, such as chemotherapy
and radiotherapy can cause changes in the physiological status of
the patient; for example, changes in coagulation status or
autonomic nervous system (ANS) function may occur as side effects
of chemotherapy (8,9). However, the specific physiological
mechanism of coagulation in patients with EC is not fully
understood, and further in-depth research is needed.
The ANS plays a vital role in the maintenance of
homeostasis in the human body. Activation of the sympathetic
nervous system via the intravenous injection of adrenaline has been
reported to accelerate blood clotting (10). Specifically, the activities of blood
FIB, coagulation factor VIII, von Willebrand factor, platelets and
other substances associated with clotting are increased, indicating
that the ANS contributes to the regulation of blood coagulation
(11). Dysfunction of the ANS due
to the presence of malignant tumors can lead to cytokine release
and the occurrence of inflammatory responses (12,13).
This may induce tumor coagulation, such as extravascular
coagulation in the tumor (12–14).
However, the induction of coagulation disorders by tumor-induced
inflammatory reactions and the cytokines released from tumors may
also require participation by the ANS (14,15).
Heart rate variability (HRV) analysis is commonly used to evaluate
the status of the ANS and has been widely used to assess ANS
activity in patients with various types of cancers (16). Previous studies have confirmed that
the ANS participates in regulation of the tumor microenvironment
and mediates the occurrence of inflammation in patients with
cancer, and that these physiological processes are associated with
specific HRV parameters (17,18).
The interaction between tumor coagulation and inflammation in
patients with malignant tumors may be the basis for the regulatory
role of the ANS. In a study of gastric cancer and HRV, Hu et
al (19) reported that
downregulated HRV indices in patients with gastric cancer may be
associated with an unbalanced inflammatory response. This
inflammation, caused by an immune response, may lead to a
coagulation disorder (20). In
addition, Wang et al (21)
reported an association between HRV and coagulation parameters in
patients with breast cancer, indicating that it is feasible to use
HRV to assess the association between ANS function and tumor
coagulation. However, there is a paucity of research on the
relationship between HRV and coagulation function in patients with
EC. Therefore, in the present study, the aim was to investigate the
association between the ANS and coagulation function in patients
with EC via HRV analysis.
Materials and methods
Patients
A total of 122 patients (age range, 34–80 years)
with EC who were treated at the Department of Gynecological
Oncology of The First Affiliated Hospital of Bengbu Medical
University (Bengbu, China) from December 2021 to March 2023 were
enrolled in the study. The inclusion criterion was EC confirmed by
postoperative pathology as pathological type adenocarcinoma. The
exclusion criteria were as follows: i) Complicated with at least
one other malignant tumor; ii) cerebral embolism; iii) abnormal or
poor electrocardiogram quality; iv) atrial fibrillation; and v)
ectopic heartbeats comprising >5% of total heartbeats. It was
identified that 22 patients did not meet the exclusion and
inclusion criteria, and 100 patients were eventually included. The
present study was approved by the Institutional Review Committee of
the First Affiliated Hospital of Bengbu Medical University
(approval no. 2021KY010), and conducted in strict accordance with
the principles of the Declaration of Helsinki. All patients signed
informed consent forms.
Data collection
An electrocardiogram recorder (HeaLink-R211B;
HeaLink Ltd.) was used to collect 5-min single-lead
electrocardiogram data from the patients before surgery for HRV
analysis. The electrocardiograph sampling rate was 400 Hz, and a V6
lead was used. During electrocardiogram collection, the subjects
were required to remain quiet and were tested in a supine position
at 25±2°C. Ligation was performed by ligation of the bilateral
fallopian tubes in patients with EC. The staging of this study
adopted the International Federation of Gynecology and Obstetrics
guidelines 2023 (22).
Venous blood was collected from all patients in the
fasting state prior to surgery. To prevent coagulation, the
anticoagulant sodium citrate was combined with the blood samples at
a ratio of 1:9 by volume, respectively. The collected blood samples
were analyzed using an automatic coagulation analyzer (Sysmex
CS51000; Sysmex Corporation). Five coagulation biomarkers were
measured, namely prothrombin time (PT), international normalized
ratio of PT (PT-INR), prothrombin activity (PTA), activated partial
thromboplastin time (APTT) and FIB using test reagents supplied by
the instrument manufacturer.
HRV analysis
Kubios HRV software (version 3.1.0; https://www.kubios.com; Kubios Oy) was used for HRV
analysis of the time and frequency domains.
The time domain parameters included the standard
deviation of the normal-normal intervals (SDNN) and the root mean
square of successive interval differences (RMSSD). The specific
time-domain calculation formulae are as follows (23):
In these formulae, RRI¯ is the mean of the R-to-R
intervals (RRIs).
The frequency domain parameters included
low-frequency power (0.04–0.15 Hz) and high-frequency power (HF;
0.15–0.4 Hz). Prior to frequency domain analysis, the RRI time
series was resampled using cubic spline interpolation (24). Fast Fourier transform based on the
Welch periodogram method (with a 150-sec window width and 50%
overlapping window) was used to estimate the power spectral density
of the RRI time series (25). These
operations equalized the spectrum of the overlapping segments to
reduce the variance of the -spectrum.
Statistical analysis
Before data analysis, the Shapiro-Wilk test was used
to evaluate the normality of continuous data. Since the
hematological and HRV parameters were found to be non-normally
distributed, bivariate Spearman correlation analysis was used to
explore the correlations between the hematological and HRV
parameters. The HRV parameters with significant correlations were
subsequently incorporated into a separate model for multiple linear
regression analysis to test the relationships between the
hematological indicators and HRV parameters. In each model,
hematological indicators were used as the dependent variable, and
HRV indicators as the independent variable. In addition, the model
was adjusted for confounding factors, namely age, body mass index
(BMI), menopause, ligation, diabetes, hypertension, adjuvant
chemotherapy and mean heart rate (HR). P<0.05 was considered to
indicate a statistically significant result. Statistical analysis
was performed using SPSS 26.0 software (IBM Corp.).
Results
The general demographic characteristics, coagulation
biomarkers and HRV parameters of the patients with EC are
summarized in Table I.
 | Table I.Basic clinical data of the patients
with endometrial cancer. |
Table I.
Basic clinical data of the patients
with endometrial cancer.
| Variables | Values |
|---|
| Age, years | 55.2±7.3 |
| BMI,
kg/m2 | 26.4±4.3 |
| Mean HR, bpm | 72.2±10.9 |
| Menopausal, n |
|
|
Yes | 59 |
| No | 41 |
| Ligation, n |
|
|
Yes | 58 |
| No | 42 |
| Diabetes, n |
|
|
Yes | 15 |
| No | 85 |
| Hypertension,
n |
|
|
Yes | 25 |
| No | 75 |
| Adjuvant
chemotherapy, n |
|
|
Yes | 2 |
| No | 98 |
| FIGO, n |
|
| I | 83 |
| II | 11 |
|
III | 5 |
| IV | 1 |
| LF | 85 (44, 144) |
| HF | 79 (34, 228) |
| SDNN, msec | 14.7 (11.1,
21.4) |
| RMSSD, msec | 15.6 (9.6,
26.7) |
| PT, sec | 10.7 (10.4,
11.2) |
| PT-INR | 0.9 (0.9, 1.0) |
| PTA, % | 113.8 (104.5,
121.0) |
| APTT, sec | 24.9±2.0 |
| FIB, g/l | 2.5 (2.2, 3.0) |
Bivariate Spearman correlation analyses (Table II) revealed that PT, PT-INR and
APTT were significantly positively correlated with SDNN and RMSSD
(P<0.05). In addition, PTA was significantly negatively
correlated with RMSSD, while PT and PT-INR were significantly
positively correlated with HF (all P<0.05).
 | Table II.Correlation analysis of HRV
parameters and coagulation markers. |
Table II.
Correlation analysis of HRV
parameters and coagulation markers.
|
| HRV parameters |
|---|
|
|
|
|---|
|
| SDNN | RMSSD | LF | HF |
|---|
|
|
|
|
|
|
|---|
| Coagulation
markers | ρ | P-value | ρ | P-value | ρ | P-value | ρ | P-value |
|---|
| PT | 0.265 | 0.008 | 0.282 | 0.004 | 0.195 | 0.052 | 0.269 | 0.007 |
| PT-INR | 0.259 | 0.009 | 0.276 | 0.005 | 0.189 | 0.059 | 0.262 | 0.008 |
| PTA | −0.187 | 0.063 | −0.208 | 0.038 | −0.154 | 0.126 | −0.192 | 0.055 |
| APTT | 0.223 | 0.025 | 0.239 | 0.017 | 0.255 | 0.255 | 0.177 | 0.078 |
| FIB | −0.136 | 0.177 | −0.121 | 0.229 | −0.085 | 0.401 | −0.153 | 0.128 |
To evaluate the independent associations of SDNN,
RMSSD and HF with coagulation biomarkers in patients with EC, a
linear regression analysis was conducted, excluding confounding
factors such as age, BMI, menopause and ligation. The results
revealed that SDNN, RMSSD and HF still significantly correlated
with PT and PT-INR (P<0.05; Table
IIIA). Specifically, when the SDNN, RMSSD and HF decreased by 1
standard deviation, PT decreased by 0.320, 0.307 and 0.298 sec,
respectively, and PT-INR decreased by 0.322, 0.309 and 0.303,
respectively. The changes of 1 standard deviation were calculated
by the β value.
 | Table III.Linear regression analysis of HRV and
coagulation markers. |
Table III.
Linear regression analysis of HRV and
coagulation markers.
| A, Excluding main
confounding factors |
|---|
|
|---|
| HRV parameters | PT | PT-INR | PTA | APTT |
|---|
| SDNN |
|
|
|
|
| B | 0.016 | 0.001 | - | −0.008 |
| SE | 0.007 | 0.001 | - | 0.021 |
|
β | 0.253 | 0.263 | - | −0.043 |
|
P-value | 0.034 | 0.027 | - | 0.709 |
| RMSSD |
|
|
|
|
| B | 0.010 | 0.001 | −0.038 | −0.005 |
| SE | 0.005 | 0.000 | 0.121 | 0.014 |
|
β | 0.238 | 0.247 | −0.082 | −0.043 |
|
P-value | 0.043 | 0.036 | 0.495 | 0.707 |
| HF |
|
|
|
|
| B | 0.000 | 0.000 | - | - |
| SE | 0.000 | 0.000 | - | - |
| β | 0.232 | 0.243 | - | - |
|
P-value | 0.031 | 0.024 | - | - |
|
| B, Excluding
main confounding factors and HR |
|
| HRV
parameters | PT | PT-INR | PTA | APTT |
|
| SDNN |
|
|
|
|
| B | 0.019 | 0.002 | - | 0.028 |
| SE | 0.006 | 0.001 | - | 0.020 |
| β | 0.303 | 0.309 | - | 0.154 |
|
P-value | 0.004 | 0.003 | - | 0.150 |
| RMSSD |
|
|
|
|
| B | 0.012 | 0.001 | −0.100 | 0.019 |
| SE | 0.004 | 0.000 | 0.104 | 0.013 |
| β | 0.290 | 0.294 | −0.099 | 0.156 |
|
P-value | 0.005 | 0.004 | 0.340 | 0.136 |
| HF |
|
|
|
|
| B | 0.001 | 0.000 | - | - |
| SE | 0.000 | 0.000 | - | - |
| β | 0.280 | 0.288 | - | - |
|
P-value | 0.007 | 0.005 | - | - |
After further incorporating mean HR into the
confounding factors, the SDNN, RMSSD and HF remained significantly
correlated with PT and PT-INR (P<0.05; Table IIIB). When the SDNN, RMSSD and HF
decreased by 1 standard deviation, PT decreased by 0.256, 0.240 and
0.237 sec, respectively, and PT-INR decreased by 0.265, 0.247 and
0.246, respectively.
Correlation scatter plots for each pair of outcomes,
namely PT and PT-INR, and the HRV parameters SDNN, RMSSD and HF,
with adjustment for age, BMI, menopause, ligation, diabetes,
hypertension, adjuvant chemotherapy and mean HR are presented in
Fig. 1.
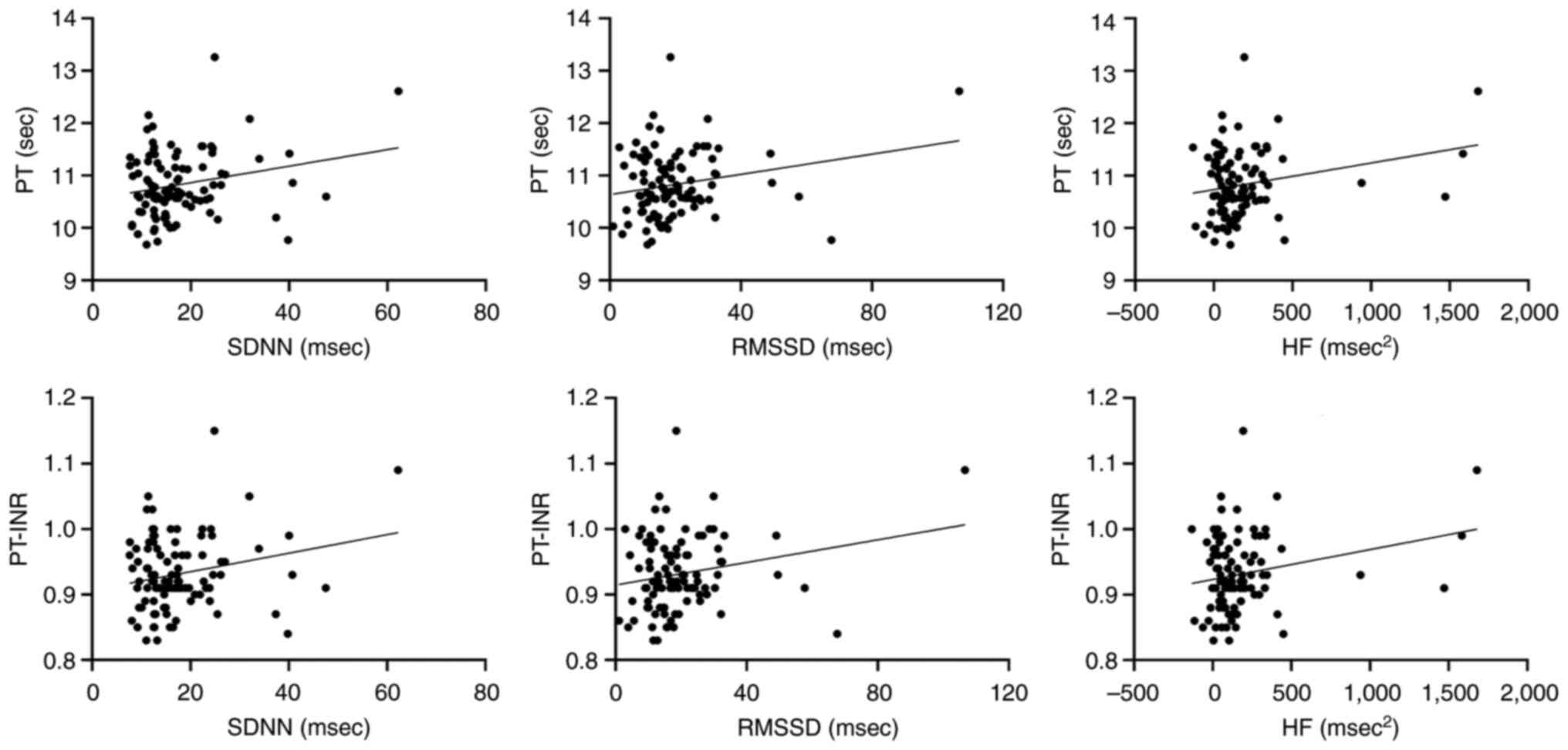 | Figure 1.Partial correlation plots for heart
rate variability parameters and blood biomarkers, following the
exclusion of the confounding factors age, body mass index,
menopause, ligation, diabetes, hypertension, adjuvant chemotherapy
and mean heart rate. PT, prothrombin time; PT-INR, international
normalized ratio of PT; SDNN, standard deviation of the
normal-normal intervals; RMSSD, root mean square of successive
interval differences; HF, high-frequency power. |
Discussion
In the present study, the relationships of
coagulation biomarkers with HRV time and frequency domain
parameters in patients with EC were explored. The results revealed
that SDNN, RMSSD and HF were significantly positively correlated
with PT and PT-INR, independent of the confounding factors age,
BMI, menopausal status, ligation, diabetes, hypertension, adjuvant
chemotherapy and mean HR.
Previous explanations of the mechanisms underlying
tumor coagulation disorders have focused mainly on the coagulation
cascade and the pathophysiological role of platelets. The
upregulation of coagulation factors in the tumor stroma and
vascular endothelial cells promotes the formation of
cancer-associated thrombosis (26,27).
During tumor progression, the upregulation of certain fibroin
degradation inhibitors, such as plasminogen activator inhibitor-1,
leads to the inhibition of fibrinolysis and the occurrence of
abnormal coagulation (28,29). Furthermore, specific changes in the
microenvironment of malignant tumors increase the release of
platelets, thereby directly or indirectly affecting thrombosis and
affecting coagulation processes in the body (20,30).
Coagulation biomarkers are considered reliable indicators of the
coagulation status of the body (7,31,32).
The present study confirmed correlations between coagulation
biomarkers and HRV parameters, implying an association between the
ANS and tumor coagulation abnormalities.
The ANS is a vital system comprising sympathetic and
parasympathetic nervous systems which regulate various human
physiological functions (33).
Interactions between the sympathetic and parasympathetic nervous
systems can affect the occurrence, development, metastasis and
prognosis of cancer (34–36). Previous studies have shown that the
stress reflex system, which is regulated by the central nervous
system, affects tumor angiogenesis via β2 adrenergic
receptors (12,14). Abnormal cell proliferation in tumors
has been indicated to lead to the upregulation of vascular
endothelial growth factor expression and downregulation of
thrombospondin-1 expression, which in turn cause extracellular
coagulation (13,37). In addition to the aforementioned
pathways, tumor cells can also increase thrombin levels by directly
activating the coagulation system (38) or promoting the synthesis and
expression of multiple coagulation factors through various
physicochemical pathways (15,39).
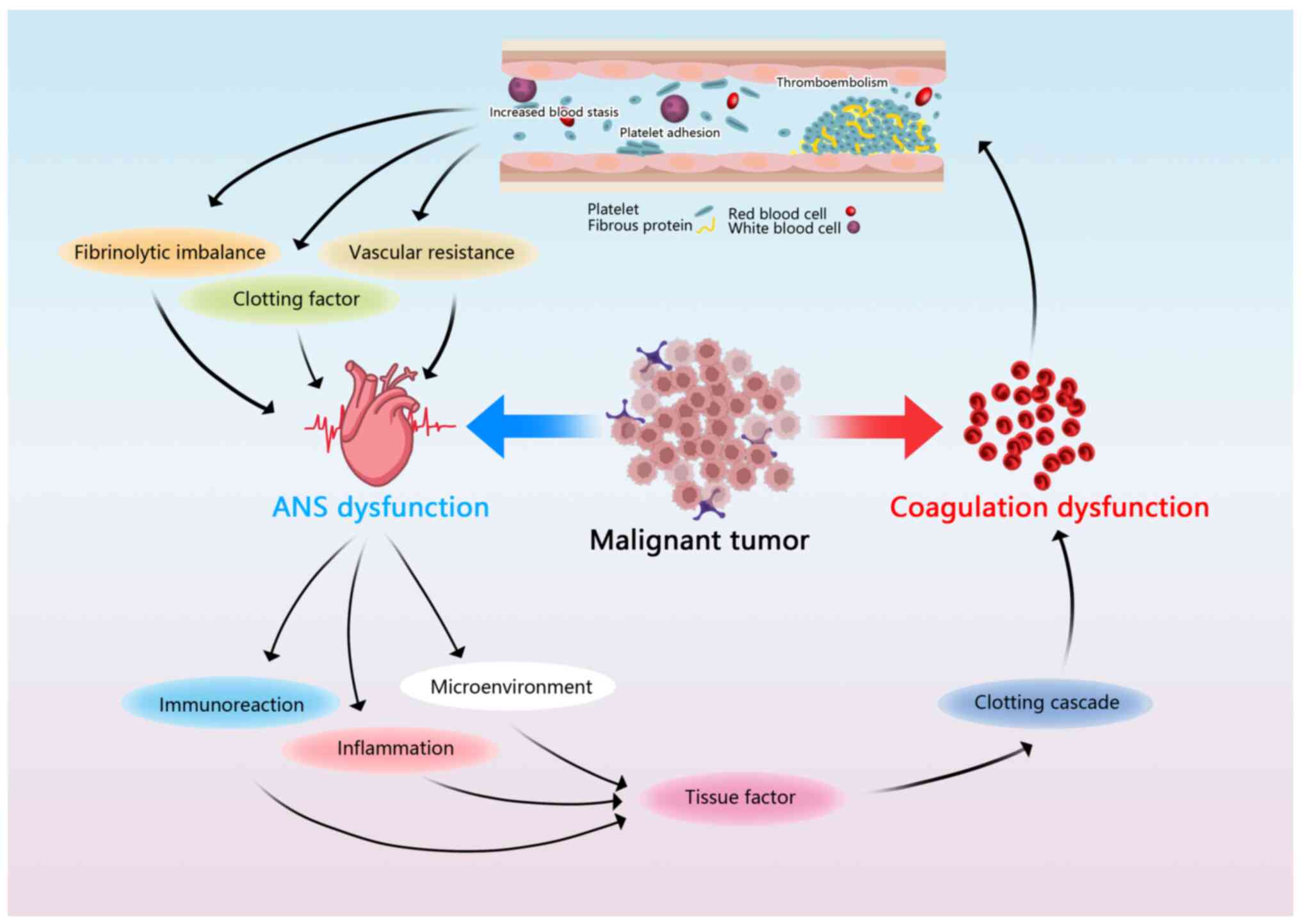
These pathways leading to coagulation may involve the participation
of the ANS (Fig. 2).
As aforementioned, the ANS comprises sympathetic and
parasympathetic components, and the vagus nerve is an important
parasympathetic nerve. The regulatory effects of the nerves from
both types of ANS on body physiology are nonlinear and complex
(40). In HRV analysis, SDNN
represents overall autonomous regulation, whereas RMSSD and HF
reflect changes in vagal nerve tension (41,42).
The present study revealed that coagulation parameters PT and
PT-INR were positively correlated with SDNN, RMSSD and HF, which
suggests that changes in the coagulation function of patients with
EC may be influenced by both the sympathetic and parasympathetic
nervous systems. PT and its derivative PT-INR are currently the
most commonly used coagulation biomarkers for assessing the status
of human coagulation function (43,44).
Malignant tumors can adversely affect the coagulation function of
patients with cancer, leading to thromboembolism, increased blood
clotting and changes in hemodynamics, such as increased vascular
resistance, the activation of coagulation factors and imbalance of
the fibrinolytic system (45). The
associated changes in blood flow may increase baroreceptor activity
and reflexivity, leading to the activation of sympathetic nerve
activity and vagal nerve excitation, which can manifest as
increases in the SDNN and RMSSD (45). In a study on vagus nerve activity
and breast cancer, Ricon-Becker et al (46) reported a positive correlation of
SDNN with proinflammatory transcription factor activity and serum
cytokine levels, which are involved in physiological processes that
require baroreceptor mediation. Vagus nerve excitation caused by
coagulation dysfunction increases the levels of certain cytokines,
such as tissue factor, leading to an inflammatory response
(13). Notably, ANS function and
tumor coagulation interact with and influence each other. Van den
Berg et al (47) reported
that the tumor-induced inflammatory response and tumor-driven
cytokine expression can be influenced by the involvement or
mediating effects of the ANS. When the ANS is disturbed or
dysfunctional, the vagus-mediated immune and inflammatory responses
are affected, which leads to changes in coagulation function
(8,48). In addition, Wang et al
(21) observed that PT increased as
RMSSD increased in patients with breast cancer, which provides
evidence for involvement of the ANS in the regulation of
coagulation function. However, these studies did not elucidate
precise physiological mechanisms to support their conclusions,
which may be a potential research direction in the future.
There are several limitations to the present study.
First, although the type of cancer was restricted to endometrial
adenocarcinoma, a stratified analysis based on clinical stage was
not performed due to the limited sample size, which may affect the
experimental results to some extent. Second, the collected data
were measured at a single time point, and changes in the HRV and
coagulation parameters of patients after treatment were not
considered. However, the collection of more treatment data is
planned in the future. Factors such as diet and drug therapy may
have affected the HRV parameters and coagulation function of the
experimental subjects, and subsequent studies should control for
these factors. Finally, this was a single-center study, and no data
were included from healthy individuals for comparison with the
study cohort. In the future, multicenter and multigroup data will
be included to support the findings of the present study.
In summary, the present study revealed a correlation
between coagulation biomarkers and HRV indicators in EC patients,
with lower SDNN, RMSSD and HF values being indicative of lower PT
and PT-INR values. Given that HRV is a quantitative indicator of
the ANS, the results suggest an association between the ANS and
coagulation function in patients with EC. Further exploration of
the physiological mechanism of abnormal coagulation in patients
with malignant tumors may aid in the identification of novel
strategies for clinical treatment. The use of wearable HRV devices
may enable the functional ANS status of patients with cancer to be
monitored more effectively and noninvasively.
Acknowledgements
Not applicable.
Funding
This research was funded by the Natural Science Research Project
of the Anhui Educational Committee (grant nos. KJ2021A0803 and
2022AH051471).
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
YQW, WZG, YFZ, YLW, BS, JL and SZ contributed to
study conception and design. Material preparation, data collection
and analysis were performed by WZG, YFZ and YLW. The first draft of
the manuscript was written by YQW and all authors commented on
previous versions of the manuscript. YFZ and YLW confirm the
authenticity of all the raw data. All authors read and approved the
final version of the manuscript.
Ethical approval and consent to
participate
This study was approved by the Institutional Review
Committee of the First Affiliated Hospital of Bengbu Medical
College (No. 2021KY010) and was conducted in strict accordance with
the principles of the Declaration of Helsinki. Written informed
consent was obtained from all individual participants included in
the study.
Patient consent for publication
Not applicable.
Competing interests
A direct family member of BS owns stock in HeaLink
Ltd. The other authors declare that they have no competing
interests.
References
|
1
|
Sung H, Ferlay J, Siegel RL, Laversanne M,
Soerjomataram I, Jemal A and Bray F: Global cancer statistics 2020:
GLOBOCAN estimates of incidence and mortality worldwide for 36
cancers in 185 countries. CA Cancer J Clin. 71:209–249. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Wang X, Glubb DM and O'Mara TA: 10 years
of GWAS discovery in endometrial cancer: Aetiology, function and
translation. EBioMedicine. 77:1038952022. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Dhami SPS, Patmore S and O'Sullivan JM:
Advances in the management of cancer-associated thrombosis. Semin
Thromb Hemost. 47:139–149. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Thomas S and Krishnan A: Platelet
heterogeneity in myeloproliferative neoplasms. Arterioscler Thromb
Vasc Biol. 41:2661–2670. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Falanga A, Marchetti M and Vignoli A:
Coagulation and cancer: Biological and clinical aspects. J Thromb
Haemost. 11:223–233. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Li Q, Cong R, Kong F, Ma J, Wu Q and Ma X:
Fibrinogen is a coagulation marker associated with the prognosis of
endometrial cancer. Onco Targets Ther. 12:9947–9956. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ge L, Liu G, Hu K, Huang K, Zhang M, Zhou
J, Teng F, Cao J, Dai C and Jia X: A new risk index combining
d-Dimer, fibrinogen, HE4, and CA199 differentiates suspecting
endometrial cancer from patients with abnormal vaginal bleeding or
discharge. Technol Cancer Res Treat. 19:15330338199011172020.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kvolik S, Jukic M, Matijevic M, Marjanovic
K and Glavas-Obrovac L: An overview of coagulation disorders in
cancer patients. Surg Oncol. 19:e33–e46. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Adams SC, Schondorf R, Benoit J and
Kilgour RD: Impact of cancer and chemotherapy on autonomic nervous
system function and cardiovascular reactivity in young adults with
cancer: A case-controlled feasibility study. BMC Cancer.
15:4142015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
von Känel R and Dimsdale JE: Effects of
sympathetic activation by adrenergic infusions on hemostasis in
vivo. Eur J Haematol. 65:357–369. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Majerova K, Zvarik M, Ricon-Becker I,
Hanalis-Miller T, Mikolaskova I, Bella V, Mravec B and Hunakova L:
Increased sympathetic modulation in breast cancer survivors
determined by measurement of heart rate variability. Sci Rep.
12:146662022. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhou J, Liu Z, Zhang L, Hu X, Wang Z, Ni
H, Wang Y and Qin J: Activation of β2-adrenergic receptor promotes
growth and angiogenesis in breast cancer by down-regulating PPARγ.
Cancer Res Treat. 52:830–847. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wojtukiewicz MZ, Sierko E, Klement P and
Rak J: The hemostatic system and angiogenesis in malignancy.
Neoplasia. 3:371–384. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Thaker PH, Han LY, Kamat AA, Arevalo JM,
Takahashi R, Lu C, Jennings NB, Armaiz-Pena G, Bankson JA, Ravoori
M, et al: Chronic stress promotes tumor growth and angiogenesis in
a mouse model of ovarian carcinoma. Nat Med. 12:939–944. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Strous MTA, Daniels AM, Zimmermann FM, van
Erning FN, Gidron Y and Vogelaar FJ: Is pre-operative heart rate
variability a prognostic indicator for overall survival and cancer
recurrence in patients with primary colorectal cancer? PLoS One.
15:e02372442020. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhou X, Ma Z, Zhang L, Zhou S, Wang J,
Wang B and Fu W: Heart rate variability in the prediction of
survival in patients with cancer: A systematic review and
meta-analysis. J Psychosom Res. 89:20–25. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Cole SW, Nagaraja AS, Lutgendorf SK, Green
PA and Sood AK: Sympathetic nervous system regulation of the tumour
microenvironment. Nat Rev Cancer. 15:563–572. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Aeschbacher S, Schoen T, Dörig L,
Kreuzmann R, Neuhauser C, Schmidt-Trucksäss A, Probst-Hensch NM,
Risch M, Risch L and Conen D: Heart rate, heart rate variability
and inflammatory biomarkers among young and healthy adults. Ann
Med. 49:32–41. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Hu S, Lou J, Zhang Y and Chen P: Low heart
rate variability relates to the progression of gastric cancer.
World J Surg Oncol. 16:492018. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Bauer AT, Gorzelanny C, Gebhardt C, Pantel
K and Schneider SW: Interplay between coagulation and inflammation
in cancer: Limitations and therapeutic opportunities. Cancer Treat
Rev. 102:1023222022. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wang L, Wang J, Li P, Wang X, Wu S and Shi
B: Association between short-term heart rate variability and blood
coagulation in patients with breast cancer. Sci Rep. 11:154142021.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Berek JS, Matias-Guiu X, Creutzberg C,
Fotopoulou C, Gaffney D, Kehoe S, Lindemann K, Mutch D and Concin
N; Endometrial Cancer Staging Subcommittee and FIGO Women's Cancer
Committee, : FIGO staging of endometrial cancer: 2023. Int J
Gynaecol Obstet. 162:383–394. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wierig M, Mandtler LP, Rottmann P, Stroh
V, Müller U, Büscher W and Plümer L: Recording heart rate
variability of dairy cows to the cloud-why smartphones provide
smart solutions. Sensors (Basel). 18:25412018. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Singh D, Vinod K and Saxena SC: Sampling
frequency of the RR interval time series for spectral analysis of
heart rate variability. J Med Eng Technol. 28:263–272. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Welch P: The use of fast Fourier transform
for the estimation of power spectra: A method based on time
averaging over short, modified periodograms. IEEE Transactions on
Audio and Electroacoustics. 15:70–73. 1967. View Article : Google Scholar
|
|
26
|
Van Es N, Hisada Y, Di Nisio M, Cesarman
G, Kleinjan A, Mahé I, Otten HM, Kamphuisen PW, Berckmans RJ,
Büller HR, et al: Extracellular vesicles exposing tissue factor for
the prediction of venous thromboembolism in patients with cancer: A
prospective cohort study. Thromb Res. 166:54–59. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Rondon AMR, Kroone C, Kapteijn MY,
Versteeg HH and Buijs JT: Role of tissue factor in tumor
progression and cancer-associated thrombosis. Semin Thromb Hemost.
45:396–412. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Frischmuth T, Hindberg K, Aukrust P,
Ueland T, Braekkan SK, Hansen JB and Morelli VM: Elevated plasma
levels of plasminogen activator inhibitor-1 are associated with
risk of future incident venous thromboembolism. J Thromb Haemost.
20:1618–1626. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Hisada Y and Mackman N: Mechanisms of
cancer-associated thrombosis. Res Pract Thromb Haemost.
7:1001232023. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Braun A, Anders HJ, Gudermann T and
Mammadova-Bach E: Platelet-cancer interplay: Molecular mechanisms
and new therapeutic avenues. Front Oncol. 11:6655342021. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Moik F and Ay C: Hemostasis and cancer:
Impact of haemostatic biomarkers for the prediction of clinical
outcomes in patients with cancer. J Thromb Haemost. 20:2733–2745.
2022. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Bian J, Sun X, Li B and Ming L: Clinical
significance of serum HE4, CA125, CA724, and CA19-9 in patients
with endometrial cancer. Technol Cancer Res Treat. 16:435–439.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Lauer MS: Autonomic function and
prognosis. Cleve Clin J Med. 76 (Suppl 2):S18–S22. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Cheng Y, Sun F, D'Souza A, Dhakal B,
Pisano M, Chhabra S, Stolley M, Hari P and Janz S: Autonomic
nervous system control of multiple myeloma. Blood Rev.
46:1007412021. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Sloan EK, Priceman SJ, Cox BF, Yu S,
Pimentel MA, Tangkanangnukul V, Arevalo JM, Morizono K, Karanikolas
BD, Wu L, et al: The sympathetic nervous system induces a
metastatic switch in primary breast cancer. Cancer Res.
70:7042–7052. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Chang A, Sloan EK, Antoni MH, Knight JM,
Telles R and Lutgendorf SK: Biobehavioral pathways and cancer
progression: Insights for improving well-being and cancer outcomes.
Integr Cancer Ther. 21:153473542210960812022. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Rak J, Yu JL, Klement G and Kerbel RS:
Oncogenes and angiogenesis: Signaling three-dimensional tumor
growth. J Investig Dermatol Symp Proc. 5:24–33. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Heissig B, Eiamboonsert S, Salama Y,
Shimazu H, Dhahri D, Munakata S, Tashiro Y and Hattori K: Cancer
therapy targeting the fibrinolytic system. Adv Drug Deliv Rev.
99:172–179. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Szewczyk G, Maciejewski TM and Szukiewicz
D: Current progress in the inflammatory background of angiogenesis
in gynecological cancers. Inflamm Res. 68:247–260. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Tiwari R, Kumar R, Malik S, Raj T and
Kumar P: Analysis of heart rate variability and implication of
different factors on heart rate variability. Curr Cardiol Rev.
17:e1607211897702021. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Lee DY, Lee MY, Cho JH, Kwon H, Rhee EJ,
Park CY, Oh KW, Lee WY, Park SW, Ryu S and Park SE: Decreased vagal
activity and deviation in sympathetic activity precedes development
of diabetes. Diabetes Care. 43:1336–1343. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Katayama M, Kubo T, Yamakawa T, Fujiwara
K, Nomoto K, Ikeda K, Mogi K, Nagasawa M and Kikusui T: Emotional
contagion from humans to dogs is facilitated by duration of
ownership. Front Psychol. 10:16782019. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Dorgalaleh A, Favaloro EJ, Bahraini M and
Rad F: Standardization of prothrombin time/international normalized
ratio (PT/INR). Int J Lab Hematol. 43:21–28. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Tripodi A, Caldwell SH, Hoffman M, Trotter
JF and Sanyal AJ: Review article: The prothrombin time test as a
measure of bleeding risk and prognosis in liver disease. Aliment
Pharmacol Ther. 26:141–148. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Dirix LY, Oeyen S, Buys A, Liégois V,
Prové A, Van De Mooter T, Van Laere S and Vermeulen PB:
Coagulation/fibrinolysis and circulating tumor cells in patients
with advanced breast cancer. Breast Cancer Res Treat. 192:583–591.
2022. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Ricon-Becker I, Fogel E, Cole SW, Haldar
R, Lev-Ari S and Gidron Y: Tone it down: Vagal nerve activity is
associated with pro-inflammatory and anti-viral factors in breast
cancer-An exploratory study. Compr Psychoneuroendocrinol.
7:1000572021. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Van den Berg YW, Osanto S, Reitsma PH and
Versteeg HH: The relationship between tissue factor and cancer
progression: Insights from bench and bedside. Blood. 119:924–932.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Sloan RP and Cole SW: Parasympathetic
neural activity and the reciprocal regulation of innate antiviral
and inflammatory genes in the human immune system. Brain Behav
Immun. 98:251–256. 2021. View Article : Google Scholar : PubMed/NCBI
|