Introduction
Esophageal cancer (ESCA) is a type of common
malignant tumor of the digestive tract, with the incidence ranking
seventh and the mortality ranking sixth globally among all cancer
types (1). Esophageal squamous cell
carcinoma (ESCC) is the main pathological type of ESCA, accounting
for ~90% of cases (2). Despite the
emergence of immunotherapy and the combination of several treatment
methods such as surgery, radiotherapy and chemotherapy, the
prognosis of advanced ESCC is not favorable with a 5-year survival
rate of <20%. This is largely attributed to the characteristics
of ESCC, including the insidious early symptoms and the lack of
specific markers for diagnosis and evaluating prognosis (3–5).
Therefore, it is essential to identify more efficient markers which
can be used for the diagnosis, prediction of prognosis and
treatment of patients with ESCC.
According to the invasion depth described in Tumor
(T)-Node (N)-Metastasis (M) staging system (6), ESCC can be divided into T1-4 stages.
Moreover, a previous study reported that the proportions of 1,033
postoperative patients with ESCC at stages T1-4 were 19.2, 23, 55.7
and 2.1%, with 5-year survival rates of 74.6, 47.3, 32.8 and 15.6%,
respectively (7). These data
suggest that, in postoperative ESCC, stage T2-3 ESCC accounts for
the vast majority (~80%) of cases and the prognosis markedly
deteriorates from stage T2 onwards. Therefore, it is of great
clinical significance to perform comprehensive research on stage
T2-3 ESCC to assess the potential prognostic markers and
therapeutic targets.
Tumor necrosis factor (TNF) receptor (TNFR)2, also
known as TNF receptor superfamily member 1b, is a member of the
TNFR family and includes membrane-binding TNFR2 and soluble
(s)TNFR2 (8). The role of TNFR2 in
cancer has attracted increasing attention. Babic et al
(9) reported that high sTNFR2 in
the blood was associated with a poor prognosis in patients with
colorectal cancer. Furthermore, Torrey et al (10) reported that targeting TNFR2 with
antagonistic antibodies inhibited the proliferation of ovarian
cancer cells and tumor-associated regulatory T cells. However, in
the current era of advocating precision therapy, the roles of TNFR2
in different subgroups of tumors need more detailed research as the
clinical significance of TNFR2 in patients with stage T2-3 ESCC
remains unclear.
The current study retrieved the mRNA expression data
of TNFR2 from online databases and detected the expression of TNFR2
in esophageal tissues from 404 patients with stages T2-3 ESCC and
40 healthy patients using immunohistochemistry (IHC) staining. The
association between TNFR2 with the clinical parameters and overall
survival (OS) of patients with stage T2-3 ESCC was then assessed.
Further stratified analysis based on age and invasion depth was
also performed to analyze the clinical significance of TNFR2 more
deeply. The results of the present study will help clinicians to
have a more accurate understanding of the role of TNFR2 in
different subgroups of patients with ESCC, providing a basis for a
more precise use of TNFR2 as a prognostic marker and therapeutic
target.
Materials and methods
Database analysis of the expression of
TNFR2 mRNA in human cancers
The Tumor IMmune Estimation Resource (TIMER;
http://timer.cistrome.org/) and The
Cancer Genome Atlas (TCGA; http://cancergenome.nih.gov/) databases were used to
compare the expression of TNFR2 mRNA in tumor tissues of patients
with ESCA and adjacent normal tissues from some of the ESCA cases.
P<0.05 was considered to indicate a statistically significant
difference.
Collection of tissue samples
The present study was performed in accordance with
the principles of The Declaration of Helsinki and approved by the
Ethics Committee of the Affiliated Hospital of Jining Medical
University (Jining, China; approval no. 2017-Research-01). Tumor
tissues from a total of 404 patients with stage T2-3 ESCC between
January 2008 and December 2014 diagnosed by pathologists were used
in the present study for IHC staining. The inclusion criteria were
as follows: i) Radical resection of ESCC, diagnosed by
pathologists; ii) stage T2 or 3 confirmed according to the TNM
staging of esophageal cancer of the 7th edition of the American
Joint Commission on Cancer (T2, tumor invading intrinsic
muscularis; and T3, tumor exceeding muscularis and invading the
esophageal fibrous membrane) (6,11); and
iii) no administration of chemotherapy, radiotherapy or
immunotherapy before surgery. Normal esophageal tissues from 40
healthy outpatients obtained by gastroscopy were used as
controls.
IHC staining and scoring
The tissue specimens from patients with ESCC were
fixed in 10% formalin for 6–72 h at room temperature, followed by
dehydration and paraffin embedding. ESCC tissue was cut into 4-µm
thick paraffin sections, then deparaffinized in xylene and
rehydrated in graded ethanol. Antigen retrieval was performed by
microwaving in 10 mmol/l citrate buffer (pH 6.0) for 20 min. After
treatment with 0.3% Triton X-100 for 30 min at room temperature,
sections were immersed in 3% H2O2 for 10 min
to block endogenous peroxidase and in goat serum (ready-to-use;
AR0009; Wuhan Boster Biological Technology, Ltd.) for 15 min at
room temperature to block nonspecific antigens. After incubation at
room temperature for 2 h with the primary antibody of TNFR2 (1:400;
28746-1-AP; Proteintech Group, Inc.), sections were washed with
phosphate-buffered saline and incubated in horseradish peroxidase
goat antirabbit/mouse IgG polymer (ready-to-use; KIT5010; Fuzhou
Maixin Biotechnology Development Co., Ltd.) at room temperature for
30 min. Finally, sections were stained with 3,3′-diaminobenzidine
for 30 sec at room temperature and counterstained with hematoxylin
for 3 sec at room temperature. The proportion score (0, 1, 2 or 3)
represented the estimated fraction of positive staining tumor cells
(0, 0–25%; 1, 26–50%; 2, 51–75%; and 3, >75% cell staining). The
intensity score (0, 1, 2 and 3) represented the estimated average
staining intensity of positive tumor cells (0, negative; 1, weak;
2, moderate; and 3, strong). The expression of TNFR2 was evaluated
using the product of the proportion score and the intensity score
in five random fields at ×400 magnification under a light
microscope (DM2500; Leica Microsystems GmbH), and the mean value
was obtained (≤4, low expression; and >4, high expression).
Statistical analysis
The association between TNFR2 and clinical
parameters was analyzed using the χ2 test. TNFR2
expression detected by IHC and the numbers of metastatic lymph
nodes were compared between two groups using the Mann-Whitney U
test. Factors which were associated with lymph node metastasis were
identified using logistic regression analysis. Factors which were
associated with OS were identified using Cox regression analysis.
The aforementioned statistical analyses were performed using SPSS
29.0 software (IBM Corp.). TNFR2 expression was compared between
tumor tissue and normal tissue in TIMER and TCGA databases using
the Wilcoxon rank sum test in R 4.2.1 software (The R Foundation).
Survival analysis was performed using a log-rank test in R 4.2.1
software. The correlation between two factors was analyzed using
Spearman's correlation analysis in R 4.2.1 software. P<0.05 was
considered to indicate a statistically significant difference.
Results
TNFR2 is associated with clinical
stage, invasion depth, metastatic lymph node and poor OS in
patients with stage T2-3 ESCC
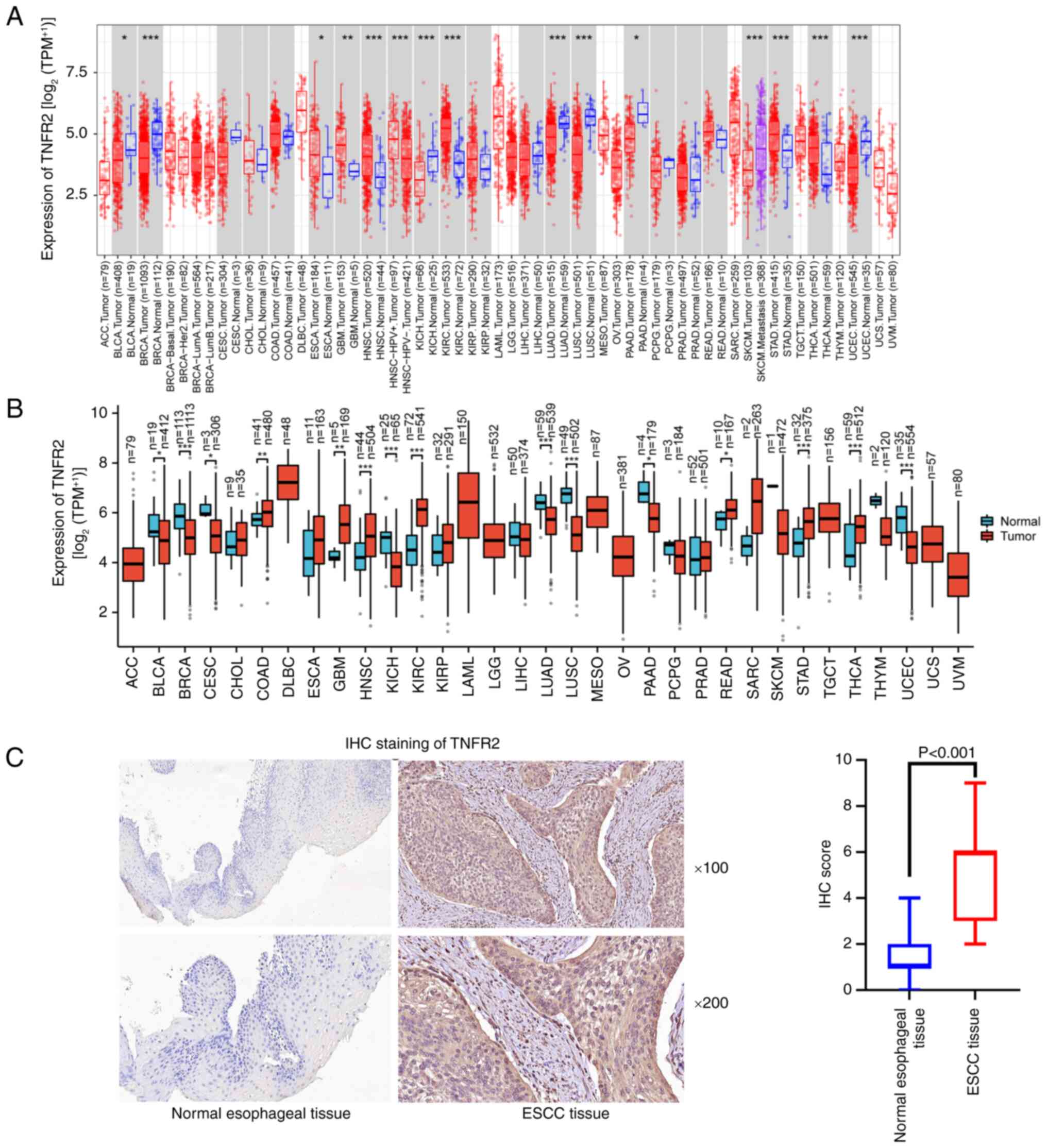
Compared with those in normal tissues, the TIMER
database revealed that the TNFR2 mRNA levels were significantly
higher in ESCA, glioblastoma multiforme (GBM), head and neck
squamous cell carcinoma (HNSC), kidney renal clear cell carcinoma
(KIRC), stomach adenocarcinoma (STAD) and thyroid carcinoma (THCA)
(Fig. 1A). TCGA revealed that TNFR2
mRNA levels were significantly higher in colon adenocarcinoma, GBM,
HNSC, KIRC, rectum adenocarcinoma, STAD and THCA tumor tissues
compared with normal tissues (Fig.
1B). In addition, TCGA revealed that TNFR2 mRNA levels in ESCA
tissues were also higher than those in normal tissue, although the
difference was not statistically significant (Fig. 1B). Furthermore, strong IHC staining
of TNFR2 was detected in the cytoplasm and membrane of ESCC
tissues, which was significantly higher than the weak staining
observed in the normal esophageal tissues (P<0.001; Fig. 1C). All 404 specimens from patients
with stage T2-3 ESCC were divided into two groups according to the
expression level of TNFR2 stained by IHC (Table I). Out of 223 cases with high
expression of TNFR2, 180 were at stage III–IV, compared with
106/181 cases for the group with low expression of TNFR2
(P<0.001). A total of 165/223 cases in the high TNFR2 expression
group had a stage T3 invasion depth, compared with 105/181 cases in
the low TNFR2 expression group (P<0.001). Moreover, 163/223
cases in the high TNFR2 expression group had metastatic lymph
nodes, compared with 107/181 cases in the group with low expression
of TNFR2 (P=0.008). There was no significant difference in sex,
age, differentiation and tumor diameter between the two groups.
 | Table I.Association of tumor necrosis factor
receptor 2 expression with the clinical parameters of patients with
tumor stage 2–3 esophageal squamous cell carcinoma. |
Table I.
Association of tumor necrosis factor
receptor 2 expression with the clinical parameters of patients with
tumor stage 2–3 esophageal squamous cell carcinoma.
|
| TNFR2 expression |
|
|---|
|
|
|
|
|---|
| Clinical
parameter | Low (n=181) | High (n=223) | P-value |
|---|
| Sex |
|
| 0.588 |
| Male | 146 (80.66) | 175 (78.48) |
|
|
Female | 35 (19.34) | 48 (21.52) |
|
| Age |
|
| 0.537 |
| ≤60
years | 90 (49.72) | 104 (46.63) |
|
| >60
years | 91 (50.28) | 119 (53.37) |
|
| Clinical stage |
|
|
<0.001a |
|
I–II | 75 (41.44) | 43 (19.28) |
|
|
III–IV | 106 (58.56) | 180 (80.72) |
|
| Invasion depth |
|
|
<0.001a |
| T2 | 76 (41.98) | 58 (26.01) |
|
| T3 | 105 (58.02) | 165 (73.99) |
|
| Metastatic lymph
node |
|
| 0.008a |
| No | 74 (40.88) | 63 (28.25) |
|
|
Yes | 107 (59.12) | 163 (71.75) |
|
|
Differentiation |
|
| 0.368 |
|
Low | 86 (47.51) | 116 (52.02) |
|
|
Moderate/high | 95 (52.49) | 107 (47.98) |
|
| Tumor diameter |
|
| 0.328 |
| ≤4
cm | 107 (59.12) | 121 (54.26) |
|
| >4
cm | 74 (40.88) | 102 (45.74) |
|
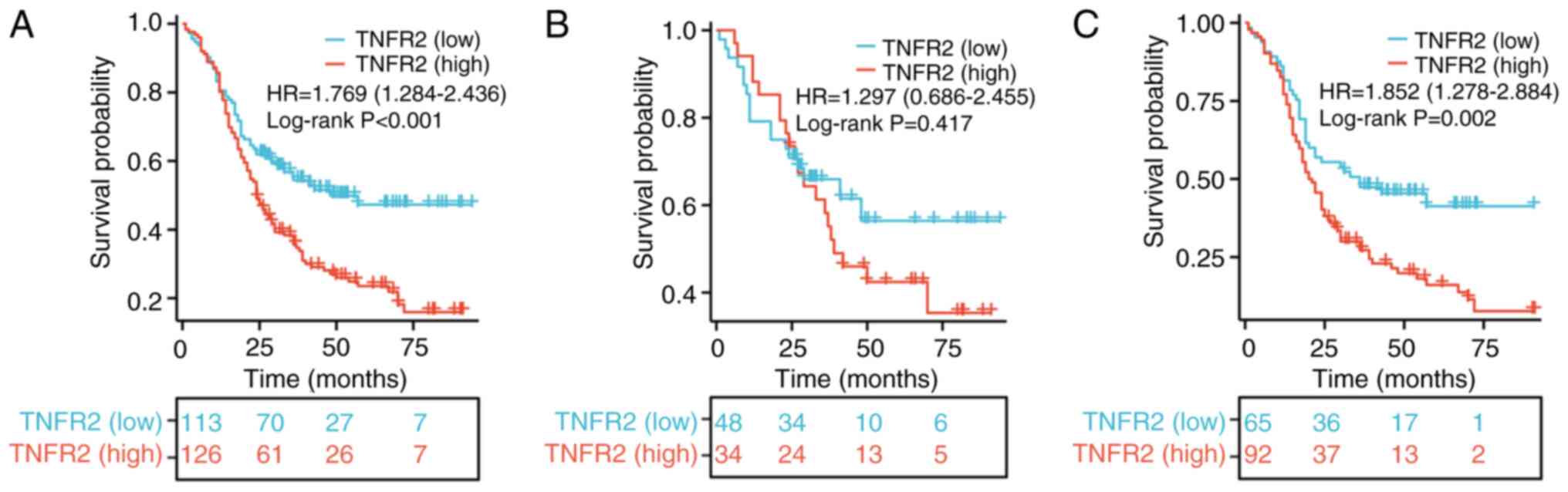
To evaluate the role of TNFR2 in predicting
prognosis, survival curves were drawn and compared using the
log-rank test. For patients with stage T2-3 ESCC, the OS rate in
the group with high TNFR2 expression was much significantly worse
than that in the group with low TNFR2 expression, beginning from
~12 months after surgery [hazard ratio (HR), 1.769; 95% confidence
interval (CI), 1.284–2.436; P<0.001; Fig. 2A]. For patients with stage T2 ESCC
only, the difference in OS between the two groups was not
statistically significant; however, the OS rate of the group with
high TNFR2 expression was notably improved compared with the low
expression group within 25 months after surgery (HR, 1.297; 95% CI,
0.686–2.455; P=0.417; Fig. 2B). For
patients with stage T3 ESCC only, the OS rate in the high TNFR2
expression group was significantly worse than that in the group
with low expression of TNFR2 (HR, 1.852; 95% CI, 1.278–2.684;
P=0.002; Fig. 2C), and the
difference began earlier than in patients with stage T2-3 ESCC.
Analysis of factors associated with
lymph node metastasis in patients with stage T2-3 ESCC and survival
analysis
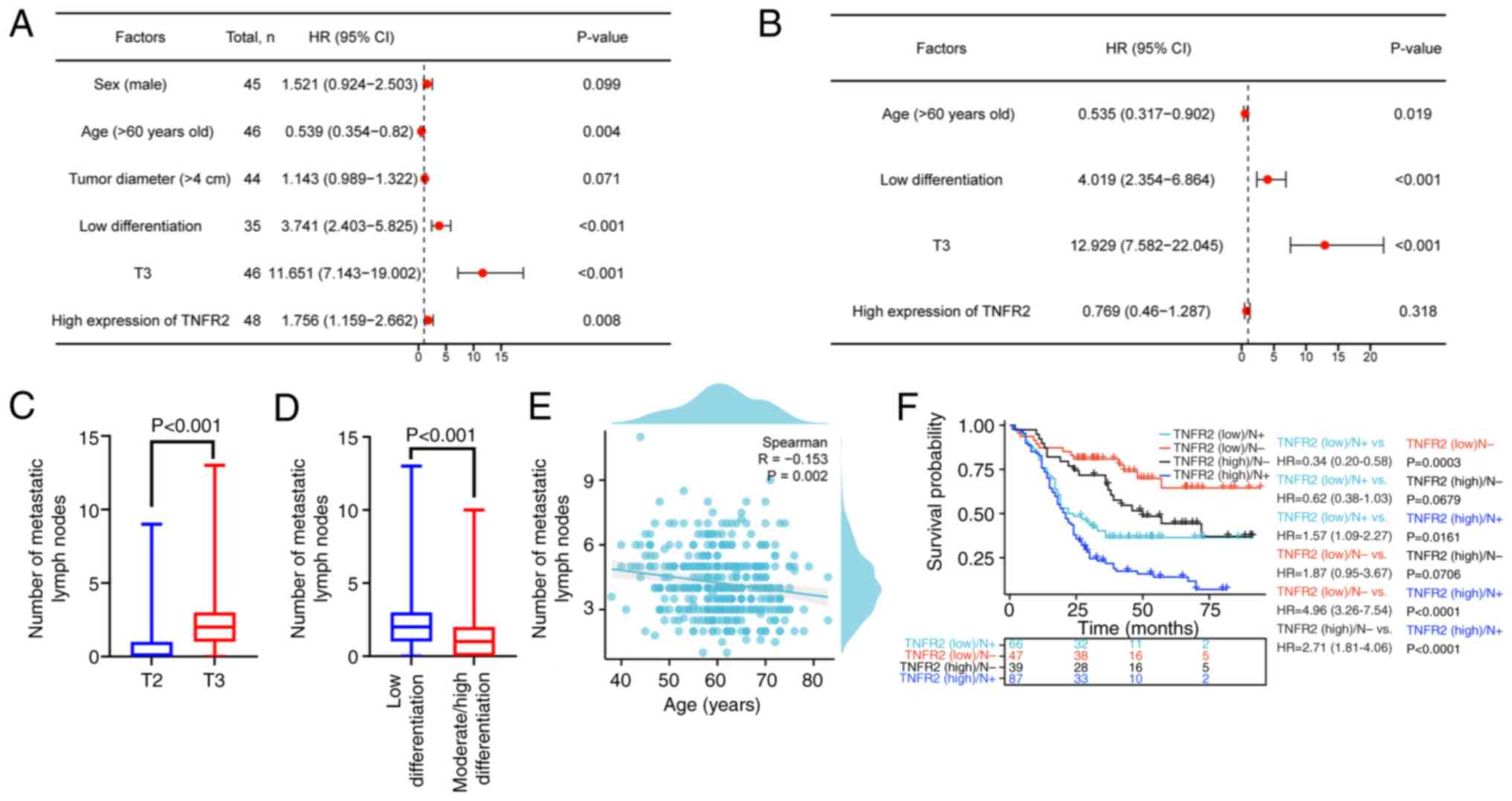
To further assess the factors affecting lymph node
metastasis, logistic regression analysis was performed. Univariate
logistic regression analysis revealed that an age of >60 years,
low differentiation, an invasion depth of T3 and high expression of
TNFR2 were significantly associated with lymph node metastasis
(P=0.004, P<0.001, P<0.001 and P=0.008, respectively;
Fig. 3A). Further multivariate
logistic regression analysis demonstrated that low differentiation
and an invasion depth of T3 significantly increased the risk of
lymph node metastasis by 3.019-fold and 11.929-fold, respectively
(P<0.001 and P<0.001, respectively; Fig. 3B); however, an age of >60 years
significantly reduced the risk of lymph node metastasis by 46.5%
(P=0.019; Fig. 3B). Moreover, TNFR2
expression was not significantly associated with lymph node
metastasis (P=0.318; Fig. 3B). To
further assess the relationship between age, differentiation and
invasion depth with lymph node metastasis, the association of these
three factors with the number of metastatic lymph nodes was
evaluated. The results revealed that the median number of
metastatic lymph nodes in the group with T3 invasion depth was 2
(range, 1–3), which was significantly higher than in the group with
T2 invasion depth (median, 0; range, 0–1; P<0.001; Fig. 3C). Moreover, the median number of
metastatic lymph nodes in the group with low differentiation was 2
(range, 1–3), which was significantly higher than in the group with
moderate/high differentiation (median, 1; range, 0–2; P<0.001;
Fig. 3D). Furthermore, the
Spearman's analysis demonstrated that age was significantly
negatively correlated with the number of metastatic lymph nodes
(R=−0.153; P=0.002; Fig. 3E).
Survival analysis of TNFR2 and lymph node metastasis
was performed and the results revealed that the group with low
expression of TNFR2 and lymph node metastasis had a markedly worse
OS compared with that in the group with high expression of TNFR2
and no lymph node metastasis, although the difference was not
statistically significant (HR, 0.62; 95% CI, 0.38–1.03; P=0.0679;
Fig. 3F). With the exception of the
comparison between the aforementioned two groups, groups with a
high expression of TNFR2 demonstrated worse OS than the groups with
low expression of TNFR2: The low TNFR2 expression/lymph node
metastasis group vs. the high TNFR2 expression/lymph node
metastasis group (HR, 1.57; 95% CI, 1.09–2.27; P=0.0161), the low
TNFR2 expression/no lymph node metastasis group vs. the high TNFR2
expression/no lymph node metastasis group (HR, 1.87; 95% CI,
0.95–3.67; P=0.0706) and the low TNFR2 expression/no lymph node
metastasis group vs. the high TNFR2 expression/lymph node
metastasis group (HR, 4.96; 95% CI, 3.26–7.54; P<0.0001;
Fig. 3F). This revealed the
association between TNFR2 expression combined with lymph node
metastasis and prognosis of patients with ESCC.
TNFR2 is associated with clinical
stage, lymph node metastasis and poor OS in patients with stage
T2-3 ESCC aged ≤60 years
A total of 194 patients with stage T2-3 ESCC aged
≤60 years were divided into a low TNFR2 expression group (n=90) and
a high TNFR2 expression group (n=104) using IHC staining. In the
group with high TNFR2 expression, 85/104 cases had a clinical stage
of III–IV, which was significantly greater than the 56/90 cases in
the group with low TNFR2 expression (P=0.002). Moreover, 85/104
cases in the high TNFR2 expression group had metastatic lymph
nodes, which was significantly higher than the 57/90 cases in the
group with low expression of TNFR2 (P=0.004). However, there were
no significant differences demonstrated for sex, invasion depth,
differentiation and tumor diameter between the two groups (Table II).
 | Table II.Association of tumor necrosis factor
receptor 2 expression with the clinical parameters of patients with
tumor stage 2–3 esophageal squamous cell carcinoma aged ≤60
years. |
Table II.
Association of tumor necrosis factor
receptor 2 expression with the clinical parameters of patients with
tumor stage 2–3 esophageal squamous cell carcinoma aged ≤60
years.
|
| TNFR2
expression |
|
|---|
|
|
|
|
|---|
| Clinical
parameter | Low (n=90) | High (n=104) | P-value |
|---|
| Sex |
|
| 0.791 |
|
Male | 74 (82.22) | 87 (83.65) |
|
|
Female | 16 (17.78) | 17 (16.35) |
|
| Clinical stage |
|
| 0.002a |
|
I–II | 34 (37.78) | 19 (18.27) |
|
|
III–IV | 56 (62.22) | 85 (81.73) |
|
| Invasion depth |
|
| 0.055 |
| T2 | 34 (37.78) | 26 (25.00) |
|
| T3 | 56 (62.22) | 78 (75.00) |
|
| Metastatic lymph
node |
|
| 0.004a |
| No | 33 (36.67) | 19 (18.27) |
|
|
Yes | 57 (63.33) | 85 (81.73) |
|
|
Differentiation |
|
| 0.596 |
|
Low | 57 (63.33) | 62 (59.62) |
|
|
Moderate/high | 33 (36.67) | 42 (40.38) |
|
| Tumor diameter |
|
| 0.773 |
| ≤4
cm | 46 (51.11) | 51 (49.04) |
|
| >4
cm | 44 (48.89) | 53 (50.96) |
|
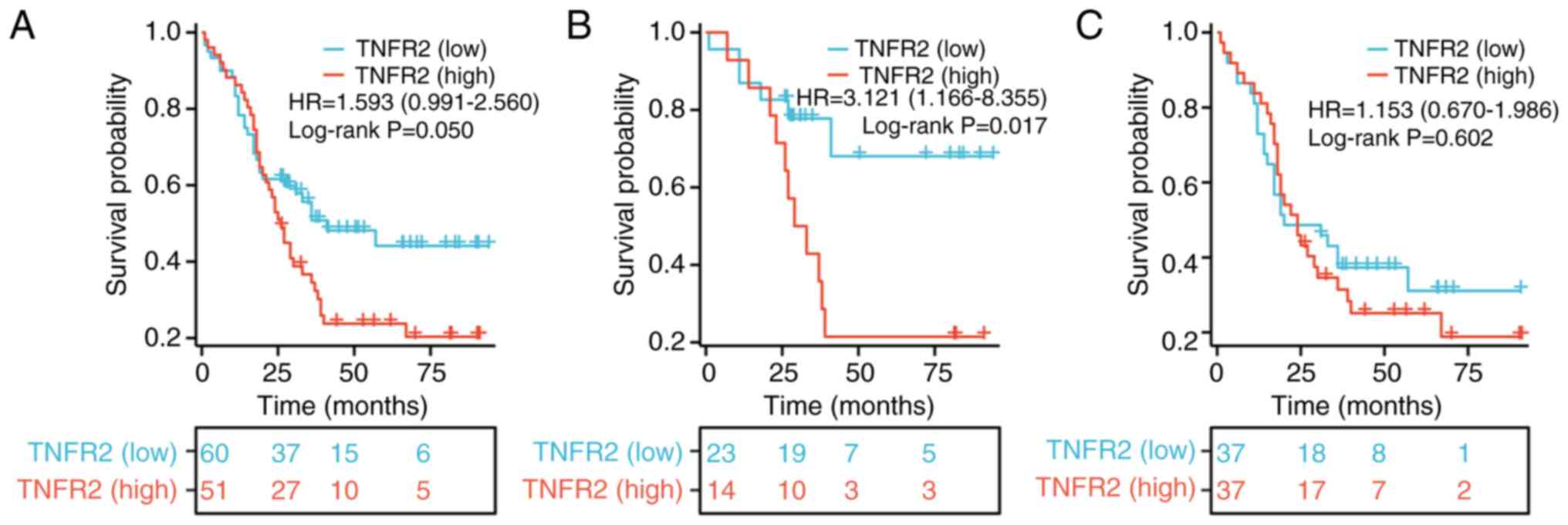
To evaluate the role of TNFR2 in predicting the
prognosis of patients with stage T2-3 ESCC aged ≤60 years, a
survival analysis was performed. The results revealed that the OS
of the group with high TNFR2 expression was markedly worse than
that of the group with low TNFR2 expression, beginning ~20 months
after surgery; however, the difference was not statistically
significant (HR, 1.593; 95% CI, 0.991–2.56; P=0.05; Fig. 4A). For patients aged ≤60 years with
stage T2 ESCC only, the OS rate of the high TNFR2 expression group
was significantly worse than that of the group with low TNFR2
expression (HR, 3.121; 95% CI, 1.166–8.355; P=0.017; Fig. 4B). However, for patients aged ≤60
years with stage T3 ESCC only, the OS rate in the high TNFR2
expression group was notably poorer than that in the group with low
TNFR2 expression, although the difference was not statistically
significant (HR, 1.153; 95% CI, 0.67–1.986; P=0.602; Fig. 4C).
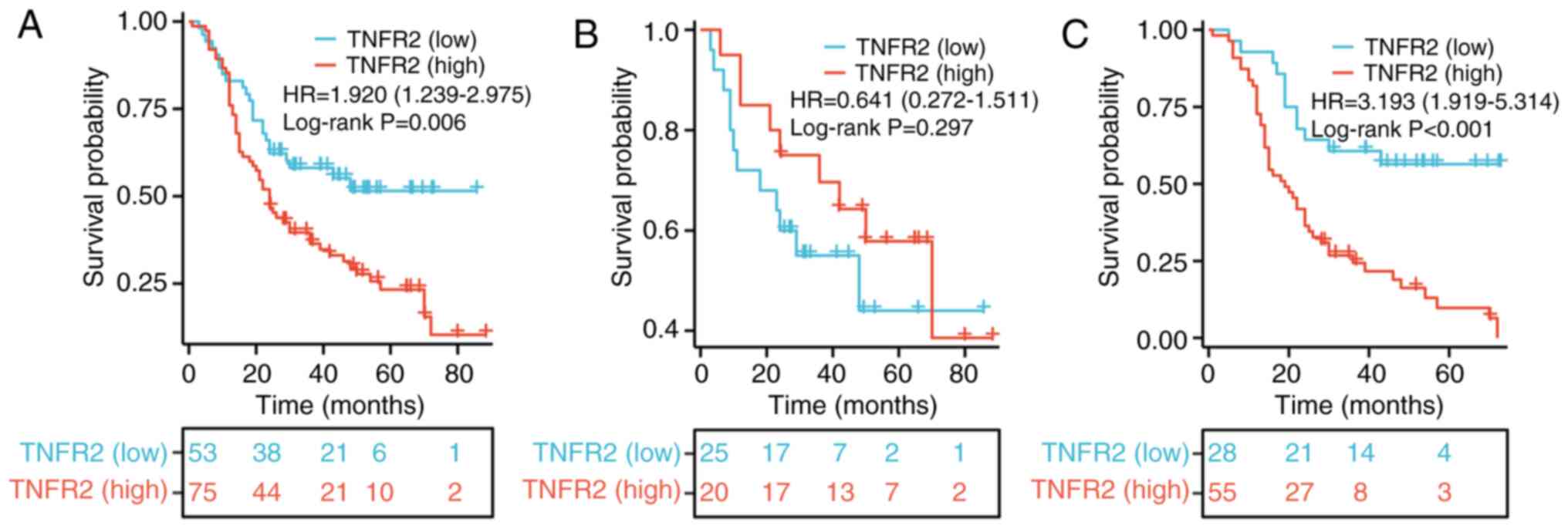
TNFR2 is associated with clinical
stage, lymph node metastasis and poor OS in patients with stage
T2-3 ESCC aged >60 years
A total of 210 patients with stage T2-3 ESCC aged
>60 years old were divided into a low TNFR2 expression group
(n=91) and a high TNFR2 expression group (n=119) using IHC
staining. In the group with high expression of TNFR2, 87/119 cases
had a T3 invasion depth, which was significantly higher than the
49/91 cases in the low TNFR2 expression group (P=0.004). However,
there was no significant difference in sex, clinical stage,
metastatic lymph node, differentiation and tumor diameter between
the two groups (Table III).
 | Table III.Association of tumor necrosis factor
receptor 2 expression with the clinical parameters of patients with
tumor stage 2–3 esophageal squamous cell carcinoma aged >60
years. |
Table III.
Association of tumor necrosis factor
receptor 2 expression with the clinical parameters of patients with
tumor stage 2–3 esophageal squamous cell carcinoma aged >60
years.
|
| TNFR2
expression |
|
|---|
|
|
|
|
|---|
| Clinical
parameter | Low (n=91) | High (n=119) | P-value |
|---|
| Sex |
|
| 0.383 |
|
Male | 72 (79.12) | 88 (73.95) |
|
|
Female | 19 (20.88) | 31 (26.05) |
|
| Clinical stage |
|
| 0.237 |
|
I–II | 41 (45.05) | 44 (36.97) |
|
|
III–IV | 50 (54.95) | 75 (63.03) |
|
| Invasion depth |
|
| 0.004a |
| T2 | 42 (46.15) | 32 (26.89) |
|
| T3 | 49 (53.85) | 87 (73.11) |
|
| Metastatic lymph
node |
|
| 0.237 |
| No | 41 (45.05) | 44 (36.97) |
|
|
Yes | 50 (54.95) | 75 (63.03) |
|
|
Differentiation |
|
| 0.494 |
|
Low | 37 (40.66) | 54 (45.38) |
|
|
Moderate/High | 54 (59.34) | 65 (54.62) |
|
| Tumor diameter |
|
| 0.224 |
| ≤4
cm | 61 (67.03) | 70 (58.82) |
|
| >4
cm | 30 (32.97) | 49 (41.18) |
|
To evaluate the role of TNFR2 in predicting the
prognosis of patients with stage T2-3 ESCC aged >60 years, a
survival analysis was performed. The results demonstrated that the
OS rate of the high TNFR2 expression group was significantly worse
than that of the group with low expression of TNFR2, beginning ~10
months after surgery (HR, 1.92; 95% CI, 1.239–2.975; P=0.006;
Fig. 5A). However, for patients
with stage T2 ESCC aged >60 years, the log-rank test revealed no
significant difference in OS between the group with high expression
of TNFR2 and the group with low expression of TNFR2 (HR, 0.641; CI,
0.272–1.511; P=0.297; Fig. 5B).
Moreover, for patients aged >60 years with stage T3 ESCC only,
the OS rate of the group with high expression of TNFR2 was
significantly worse than that in the group with low expression of
TNFR2, beginning ~3 months after surgery (HR, 3.193; 95% CI,
1.919–5.314; P<0.001; Fig.
5C).
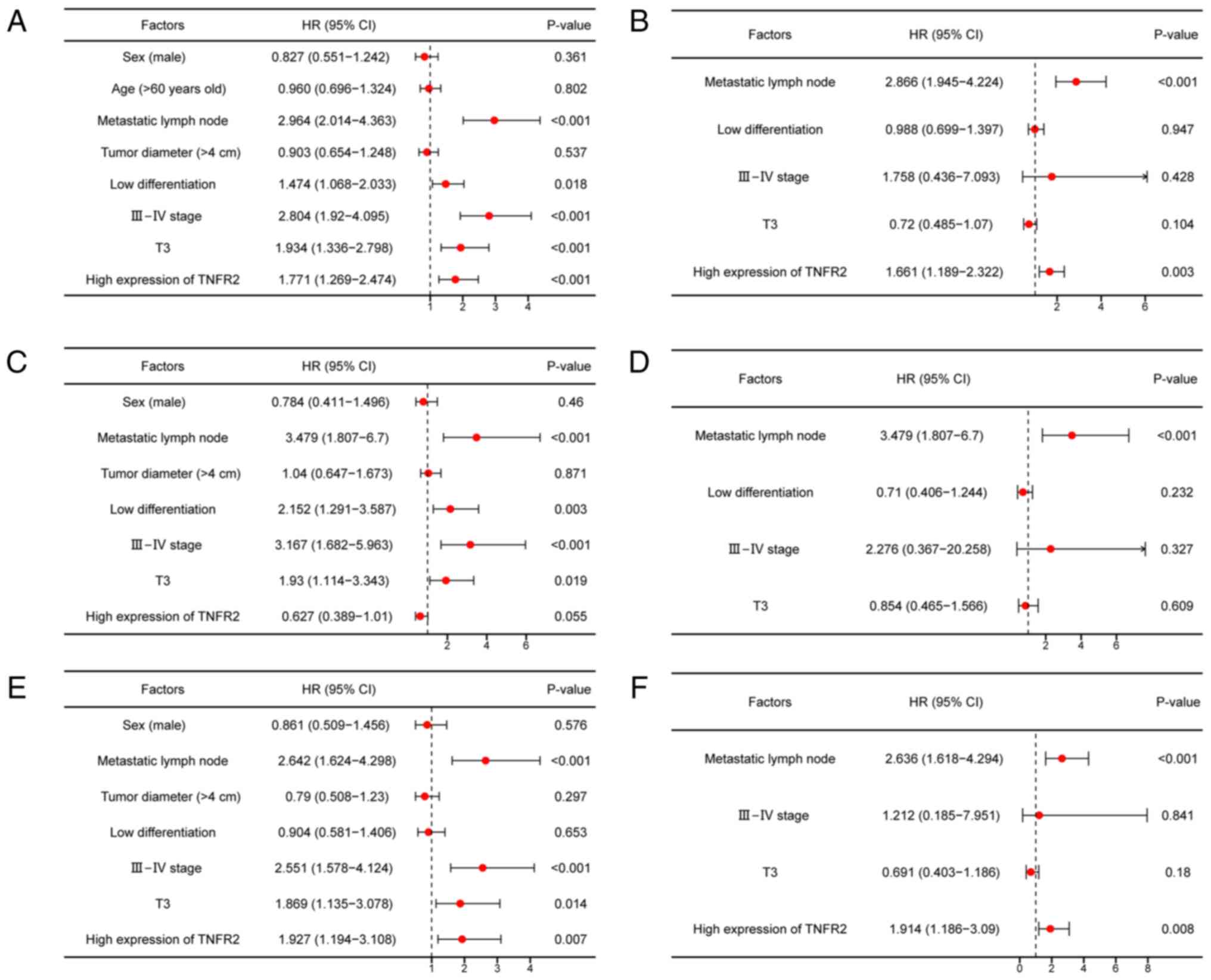
Cox regression analysis of potential
factors affecting the OS of patients with stage T2-3 ESCC
To assess the potential factors which may affect the
OS of patients with stage T2-3 ESCC, univariate and multivariate
Cox regression analyses were performed. For patients with stage
T2-3 ESCC, univariate Cox regression analysis revealed that
metastatic lymph node, low differentiation, clinical stage III–IV,
invasion depth of T3 and high expression of TNFR2 were
significantly associated with a poor OS (P<0.001, P=0.018,
P<0.001, P<0.001 and P<0.001, respectively; Fig. 6A). Moreover, multivariate Cox
regression analysis demonstrated that metastatic lymph node and
high expression of TNFR2 significantly increased the risk of death
by 1.866- and 0.661-fold, respectively (P<0.001 and P=0.003
respectively; Fig. 6B). For
patients with stage T2-3 ESCC aged ≤60 years, univariate Cox
regression analysis revealed that metastatic lymph node, low
differentiation, clinical stage III–IV and invasion depth of T3
were significantly associated with a poor OS (P<0.001, P=0.003,
P<0.001 and P=0.019, respectively; Fig. 6C). Furthermore, multivariate Cox
regression analysis demonstrated that only metastatic lymph node
significantly increased the risk of death by 2.479-fold
(P<0.001; Fig. 6D). For patients
with stage T2-3 ESCC aged >60 years, univariate Cox regression
analysis revealed that metastatic lymph node, clinical stage
III–IV, invasion depth of T3 and high expression of TNFR2 were
significantly associated with a poor OS (P<0.001, P<0.001,
P=0.014 and P=0.007, respectively; Fig.
6E). Moreover, multivariate Cox regression analysis
demonstrated that metastatic lymph node and high expression of
TNFR2 significantly increased the risk of death by 1.636- and
0.914-fold, respectively (P<0.001 and P=0.008, respectively;
Fig. 6F).
Discussion
TNFR2 is a promising factor in terms of predicting
prognosis and finding therapeutic targets of ESCC, as it is highly
expressed in several types of tumor cells and normal cells such as
interstitial fibroblasts, endothelial cells, immune cells and
hematopoietic cells (12–14). In recent years, several studies
reported the role of TNFR2 in tumor occurrence and development in
different manners: Gao et al (15) reported that TNFR2 can promote the
proliferation, migration and invasion of pancreatic cancer cells
via the NF-κB signaling pathway; Wang et al (16) reported that TNFR2 can promote the
switch from fibroblasts to cancer-associated fibroblasts in the
microenvironment of colorectal cancer, which facilitates cancer
metastasis; and Qu et al (17) reported that activation of the
TNF-α/TNFR2 pathway promotes the immunosuppressive phenotype and
function of Tregs in gastric cancer, resulting in cancer
progression. Tumor heterogeneity is an important reason for poor
treatment outcomes, which may exist among different types of
tumors, different patients with the same type of tumor or even
different parts of the same tumor (18). Although TNFR2 exhibits significant
protumor effects, it is unclear whether its role in different
subgroup patients is also the same. Therefore, the present study
focused on assessing the role of TNFR2 in stage T2-3 ESCC and
further stratified subgroup patients to provide more accurate data
on the role of TNFR2 in ESCC.
The present study demonstrated a high expression of
TNFR2 both at the mRNA and protein level and revealed that high
expression of TNFR2 was positively associated with advanced
clinical stage, invasion depth and lymph node metastasis, which is
in line with the role of TNFR2 in tumors reported by the
aforementioned studies (15–17).
Moreover, age is an important factor in the occurrence and
development of malignant tumors. Previous research has reported
that the incidence of malignant tumors increases with age but,
compared with young patients, the tumors of older patients tend to
exhibit inert phenotypes and different patterns of management
(19). Patel et al (19) reported that older patients with
colon cancer had a decreased rate of distant metastasis and lymph
node metastasis compared with younger patients. Recently, Lin et
al (20) reported that 60 years
was the optimal cut-off age for differences in OS and
progression-free survival (PFS) in a study including 568 patients
with ESCC. Moreover, several studies on ESCC used 60 years as the
cutoff for age grouping and reported marked differences in
variables, such as gene expression, biological behavior and
prognosis (21–25). Therefore, the present study
performed a subgroup analysis based on the age of 60 years to
further evaluate the clinical significance of TNFR2 in patients
with stage T2-3 ESCC. Notably, different to patients aged ≤60 years
old, for patients aged >60 years old, high expression of TNFR2
was only associated with invasion depth, but not advanced clinical
stage or lymph node metastasis. This demonstrates the heterogeneity
among subgroups and may be explained by the inert characteristics
of malignant tumors in elderly patients reported previously
(19). In addition, no association
between TNFR2 and tumor size was demonstrated in total T2-3 cases
or stratified subgroups split by age (60 years old). This result is
not consistent with the promoting effect of TNFR2 in pancreatic
cancer reported by Gao et al (15), and the difference may be explained
by the heterogeneity derived from different tumor types. Further
detailed cell experiments are required for validation. Meanwhile,
the present study confirmed that an age of >60 years reduced the
risk of lymph node metastasis, and age was associated with the
number of metastatic lymph nodes. This again reflects the inert
characteristics of ESCC in elderly patients, in line with the
report by Patel et al (19).
The association between TNFR2 with prognosis has
been reported in several types of tumors but at present it remains
controversial. In 2021, Silva Raju et al (26) reported that patients in Malaysia
with a high level of TNFR2 expression in ovarian cancer tissue
exhibited no significant difference in PFS interval compared with
patients with a low level of TNFR2. In 2019, Zhang et al
(27) reported that TNFR2 was
expressed in non-small cell lung cancer tissues and was related to
the poor prognosis of patients in China. The present study
demonstrated that a high expression of TNFR2 is associated with the
poor prognosis of patients with stage T2-3 ESCC, but this was not
associated with the presence of lymph nodes metastasis. This
finding is in line with the report of Zhang et al (27) but inconsistent with the results of
Silva Raju et al (26),
which may be explained by differences in tumor origin or ethnicity.
Further stratified analysis revealed there was no significant
effect of TNFR2 on OS of patients with stage T2 ESCC, patients with
stage T2-3 ESCC aged ≤60 years old, patients with stage T3 ESCC
aged ≤60 years old, or patients with stage T2 ESCC aged >60
years old. The aforementioned results indicate differences in the
role of TNFR2 in different subgroups of patients with stage T2-3
ESCC, suggesting that TNFR2 may not be suitable as a potential
prognostic marker for these four stratified subgroup patients.
The occurrence and development of tumors is a
complex process and prognosis is influenced by a combination of
different factors. Therefore, the independent prognostic factors
among different subgroups may be different. For all patients with
stage T2-3 ESCC, metastatic lymph nodes and a high expression of
TNFR2 were independent prognostic factors. This was also
demonstrated for patients with stage T2-3 ESCC age >60 years.
However, for patients with stage T2-3 ESCC aged ≤60 years, only the
presence of metastatic lymph nodes was an independent prognostic
factor, whilst TNFR2 expression did not exhibit a significant
effect on prognosis even in univariate Cox regression analysis.
These results further confirm the unsuitability of TNFR2 as a
potential prognostic marker for patients with T2-3 ESCC aged ≤60
years. Moreover, these different results may be related to
age-based biological differences of tumors; however, the impact of
a limited number of cases on the result is also unavoidable,
especially the limited number of cases in subgroups.
In addition to the limited number of cases
considered, there are other limitations in the present study:
Nearly all cases were from one area, so the data were regional and
the universality of the results is limited. A larger number of
cases from multiple centers will provide more reliable and
universal results. In addition, the present study was retrospective
and the results could have easily been influenced by bias and
confounding effects; therefore, further validation in prospective
studies is needed.
In conclusion, the results of the present study
demonstrated the association of TNFR2 expression with progression
and poor prognosis in patients with stage T2-3 ESCC and different
stratified subgroups. These findings will enrich the current
knowledge of the roles of TNFR2 in tumors. Moreover, they will help
clinicians have a more accurate understanding of the clinical
significance of TNFR2 in different subgroups of patients with ESCC,
providing a basis for more precise utilization of TNFR2 as a
prognostic marker and therapeutic target.
Acknowledgements
Not applicable.
Funding
The present study was supported by the Postdoctoral Program of
the Affiliated Hospital of Jining Medical University (grant no.
JYFY321205), the Postdoctoral Program of Jining No.1 People's
Hospital (grant no. 2023-BSH-003) and the Jining Research and
Development Program (grant no. 2023YXNS046).
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
DY and SQ were responsible for conception, design,
definition of intellectual content and final approval of the
version to be published. MR and SJ were responsible for
immunohistochemical staining of esophageal squamous cell carcinoma
tissue and data analysis. ZL was responsible for
immunohistochemical staining of esophageal squamous cell carcinoma
tissue, data analysis, and drafting the article and revising it
critically for important intellectual content. DY and ZL confirmed
the authenticity of all the raw data. All authors have read and
approved the final manuscript.
Ethics approval and consent to
participate
The present study was performed in accordance with
the principles of the Declaration of Helsinki and approved by the
Ethics Committee of the Affiliated Hospital of Jining Medical
University (Jining, China; approval no. 2017-Research-01). Written
informed consent was provided by the patients or their
guardians.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Morgan E, Soerjomataram I, Rumgay H,
Coleman HG, Thrift AP, Vignat J, Ferlay J and Arnold M: The global
landscape of esophageal squamous cell carcinoma and esophageal
adenocarcinoma incidence and mortality in 2020 and projections to
2040: new estimates from GLOBOCAN 2020. Gastroenterology.
163:649–658.e2. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Codipilly DC and Wang KK: Squamous cell
carcinoma of the esophagus. Gastroenterol Clin North Am.
51:457–484. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Voron T, Julio C and Pardo E: Esophageal
carcinoma: Novelties and challenges in surgery. Bull Cancer.
110:533–539. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Xu J, Kato K, Raymond E, Hubner RA, Shu Y,
Pan Y, Park RS, Ping L, Jiang Y, Zhang J, et al: Tislelizumab plus
chemotherapy versus placebo plus chemotherapy as first-line
treatment for advanced or metastatic oesophageal squamous cell
carcinoma (RATIONALE-306): A global, randomised,
placebo-controlled, phase 3 study. Lancet Oncol. 24:483–495. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Waters JK and Reznik SI: Update on
management of squamous cell esophageal cancer. Curr Oncol Rep.
24:375–385. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Edge SB and Compton CC: The American joint
committee on cancer: The 7th edition of the AJCC cancer
staging manual and the future of TNM. Ann Surg Oncol. 17:1471–1474.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wang J, Wu N, Zheng QF, Yan S, Lv C, Li SL
and Yang Y: Evaluation of the 7th edition of the TNM
classification in patients with resected esophageal squamous cell
carcinoma. World J Gastroenterol. 20:18397–18403. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Mensink M, Tran TNM, Zaal EA, Schrama E,
Berkers CR, Borst J and Kivit SD: TNFR2 costimulation
differentially impacts regulatory and conventional CD4(+) T-Cell
Metabolism. Front Immunol. 13:8811662022. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Babic A, Shah SM, Song M, Wu K, Meyerhardt
JA, Ogino S, Yuan C, Giovannucci EL, Chan AT, Stampfer MJ, et al:
Soluble tumour necrosis factor receptor type II and survival in
colorectal cancer. Br J Cancer. 114:995–1002. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Torrey H, Butterworth J, Mera T, Okubo Y,
Wang L, Baum D, Defusco A, Plager S, Warden S, Huang D, et al:
Targeting TNFR2 with antagonistic antibodies inhibits proliferation
of ovarian cancer cells and tumor-associated Tregs. Sci Signal.
10:2017. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wei MX, Song X, Zhao XK, Han WL, Bao Q,
Han XN, Xu RH, Li XM, Fan ZM, Wang R, et al: Clinicopathological
characteristics and postoperative prognosis of patients with
nuclear pedigree of esophageal squamous cell carcinoma. Front
Oncol. 13:11904572023. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhang H and Xiao W: TNFR1 and TNFR2
differentially mediate TNF-α-induced inflammatory responses in
rheumatoid arthritis fibroblast-like synoviocytes. Cell Biol Int.
41:415–422. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Bai J, Ding B and Li H: Targeting TNFR2 in
Cancer: All roads lead to rome. Front Immunol. 13:8449312022.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
He T, Zhao Y, Zhao P, Zhao L, Zakaria J
and Wang K: Signaling pathway(s) of TNFR2 required for the
immunoregulatory effect of CD4(+)Foxp3(+) regulatory T cells. Int
Immunopharmacol. 108:1088232022. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Gao Z, Zhang Q, Chen H, Chen J, Kang J, Yu
H, Song Y and Zhang X: TNFR2 promotes pancreatic cancer
proliferation, migration, and invasion via the NF-κB signaling
pathway. Aging. 15:8013–8025. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wang L, Yang D, Tian J, Gao A, Shen Y, Ren
X, Li X, Jiang G and Dong T: Tumor necrosis factor receptor 2/AKT
and ERK signaling pathways contribute to the switch from
fibroblasts to CAFs by progranulin in microenvironment of
colorectal cancer. Oncotarget. 8:26323–26333. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Qu Y, Wang X, Bai S, Niu L, Zhao G, Yao Y,
Li B and Li H: The effects of TNF-α/TNFR2 in regulatory T cells on
the microenvironment and progression of gastric cancer. Int J
Cancer. 150:1373–1391. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kashyap A, Rapsomaniki MA, Barros V,
Fomitcheva-Khartchenko A, Martinelli AL, Rodriguez AF, Gabrani M,
Rosen-Zvi M and Kaigala G: Quantification of tumor heterogeneity:
From data acquisition to metric generation. Trends Biotechnol.
40:647–676. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Patel SS, Nelson R, Sanchez J, Lee W,
Uyeno L, Garcia-Aguilar J, Hurria A and Kim J: Elderly patients
with colon cancer have unique tumor characteristics and poor
survival. Cancer. 119:739–7347. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Lin Y, Ye Y, Huang Q, Zheng B, Yang Y,
Chen Y, Li W, Ke H, Lin C, Zhang Y, et al: Influence of age as a
continuous variable on survival outcomes and treatment options in
patients with upper thoracic esophageal carcinoma. J Cancer.
14:1039–1048. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhang P, Wang S, Wu JZ and Song Q:
Clinical and prognostic significance of perioperative change in red
cell distribution width in patients with esophageal squamous cell
carcinoma. BMC Cancer. 23:3192023. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Feng RB, Zhou QZ, Cheng R, Li P, Zhu ST,
Min L and Zhang ST: Expression and significance of N-myc downstream
regulated gene 2 in the process of esophageal squamous cell
carcinogenesis. Bioengineered. 13:3275–3283. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Luo QY, Di T, Qiu MZ, Xia ZF, Du Y, Lin
RD, Yang LQ, Sun YT, Yang DJ, Sun J and Zhang L: High AKAP8L
expression predicts poor prognosis in esophageal squamous cell
carcinoma. Cancer Cell Int. 22:902022. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Xie C, Chen Z, Xu J, Meng Z, Huang Z and
Lin J: Influence of lymphangio vascular (V) and perineural (N)
invasion on survival of patients with resected esophageal squamous
cell carcinoma (ESCC): A single-center retrospective study. Peer J.
10:e129742022. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wang N, Li Y, Zhou RM, Wang GY, Wang CM,
Chen ZF and Liu W: Hsa-miR-196a2 functional SNP is associated with
the risk of ESCC in individuals under 60 years old. Biomarkers.
19:43–48. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Silva Raju J, Abd Aziz NH, Atallah GA,
Teik CK, Shafiee MN, Mohd Saleh MF, Jeganathan R, Md Zin RR and
Kampan NC: Prognostic value of TNFR2 and STAT3 among high-grade
serous ovarian cancer survivors according to platinum sensitivity.
Diagnostics (Basel). 11:5262021. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zhang YW, Chen QQ, Cao J, Xu LQ, Tang X,
Wang J, Zhang J and Dong LX: Expression of tumor necrosis factor
receptor 2 in human non-small cell lung cancer and its role as a
potential prognostic biomarker. Thoracic Cancer. 10:437–444. 2019.
View Article : Google Scholar : PubMed/NCBI
|