Introduction
Invasive breast cancer originating in the nipple is
very rare, occurring in 0.25% of breast cancers (1). Sanders et al (2) was the first to term this rare
condition as nipple-invasive primary carcinoma. Nipple-invasive
primary carcinoma has previously been described in two conditions:
i) Secondary to Paget's disease, where tumor cells of the epidermis
invade directly into the dermis; and ii) invasive carcinoma arising
from the internal ducts or lobules of the papilla (2). The present case belonged to the
latter. Previous cases have reported that this rare condition may
have multiple clinical manifestations (2–11).
Only 6 out of 23 (26%) cases presented with a nipple mass, 12 (48%)
had pagetoid changes such as eczema, pruritus or erosion, and 6
presented with only an edematous thickened nipple (Table I) (2–11).
Imaging examinations occasionally show no significant
abnormalities, and only 11 of the 21 cases with imaging findings
reported abnormal changes, such as masses or microcalcifications in
the nipple (2–11). Atypical clinical manifestations and
imaging findings are considered to lead to missed diagnoses despite
the absence of definitive statistical studies. Axillary lymph node
metastases have been identified in up to 8 (40%) of the previous 20
cases in which axillary surgery was performed, and it can be
speculated that this may be associated with delayed diagnosis
(2–11). Therefore, the present report aims to
increase attention of this condition.
 | Table I.Characteristics of reported cases. |
Table I.
Characteristics of reported cases.
| First author/s,
year | Age, years | Clinical
presentation | Imaging | Histological
type | ER | PR | HER-2 | Other focus | Paget's disease | Surgical method | Lymph nodes
metastasis | Other therapy | (Refs.) |
|---|
| Kasuga et al,
1993 | 38 | Eczematoid changes
and pruritus | No | Scirrhous
carcinoma | NR | NR | NR | No | No | Modified radical
mastectomy | No | Tamoxifen and
5-fluorouracil for 2 years | (4) |
| Ohsumi et al,
1996 | 71 | Eczematoid change
and | Yes (MGR: mass and
US: mass) a mass | IDC and
solid-tubular | NR | NR | NR | No | No | Modified radical
mastectomy | Yes | Tamoxifen | (5) |
| Ahmed and Basit,
2011 | 47 | Eczematous
change | No | carcinoma IDC | + | NR | - | No | No | Central segmentectomy
and sentinel node biopsy | Yes (1/18) | Radiation therapy,
chemotherapy and hormonal treatment | (6) |
| Erben et al,
2012 | 65 | Asymmetrically
enlarged nipple | No | ILC | + | + | - | No | No | Central lumpectomy
and sentinel node biopsy | Yes | Radiation therapy and
anastrozole | (7) |
| Moennich et
al, 2015 | 47 | Erosion and bloody
discharge | No | IDC | NR | NR | + | No | No | Lumpectomy with
nipple removal and sentinel node biopsy | No | Radiation
therapy | (8) |
| Pasquali et
al, 2016 | 65 | Yellowish tinge and
appeared infiltrate | Yes (US:
pseudonodular and hypoechoic aspect) | ILC | + | + | - | No | No | NR | Yes | NR | (9) |
| Moliere et al,
2018 | 45 | Swollen nipple | Yes (MGR: accentuated
density and MRI: mass) | IDC | + | + | - | No | No | Excision of the
nipple-areolar complex associated with sentinel lymph node
dissection | No | Radiation therapy and
tamoxifen | (3) |
| Sanders et
al, | 67 | Nipple mass | NR | IDC | + | - | - | NR | No | NR | No | NR | (2) |
| 2018 | 51 | Erythema and
exudative crust | No | IDC | + | - | - | NR | No | NR | No | NR |
|
|
| 37 | Nipple mass | No | IDC | + | + | - | NR | Yes | NR | No | NR |
|
|
| 77 | Pruritus | No | IDC | + | + | + | NR | No | NR | Yes | NR |
|
|
| 62 | Skin changes | No | IDC | + | + | - | NR | No | NR | No | NR |
|
|
| 44 | Nipple
thickening | Yes (MGR:
calcifications) | IDC | + | + | + | NR | No | NR | Yes | NR |
|
|
| 86 | Nipple
thickening | Yes (MGR: Nipple
thickening) | IDC | + | - | - | NR | No | NR | NR | NR |
|
|
| 55 | Erythema and
exudative crust | No | IDC and ILC | + | + | - | NR | Yes | NR | Yes (1) | NR |
|
|
| 68 | Erythema | Yes (MRI: Nipple
thickening) | IDC and ILC | + | + | - | NR | No | NR | Not sampled | NR |
|
|
| 60 | Pruritus and
exudative crust | No | IDC and ILC | + | - | - | NR | Yes | NR | No | NR |
|
|
| 58 | Nipple mass | Yes (US: mass and
MRI: mass) | IDC and ILC | + | + | - | NR | No | NR | No | NR |
|
|
| 80 | Nipple
thickening | NR | ILC | + | NR | - | NR | No | NR | Not sampled | NR |
|
|
| 61 | Nipple mass | Yes (US: mass) | ILC | + | + | - | NR | No | NR | No | NR |
|
|
| 85 | Nipple
retraction | Yes (US: mass and
MGR: Distortion) | ILC | + | + | - | NR | No | NR | Not sampled | NR |
|
| Tan and Mihir,
2019 | 69 | Nipple mass | Yes (MGR: mass; US:
mass; and MRI: non-mass) enhancement | IDC within the
nipple and DCIS within the breast | - | - | + | Yes | No | Mastectomy and
sentinel lymph node biopsy | No | Adjuvant
chemotherapy, targeted therapy and radiation therapy | (10) |
| Hamzah et
al, 2019 | 62 | Bloody discharge
and pagetoid changes of nipple | Yes (MGR:
pleomorphic microcalcifications in nipple and US: distended
retroareolar ducts and a small indeterminate echogenic focus) | IDC within the
nipple and DCIS within the breast | + | - | + | Yes | Yes | Mastectomy and
axillary clearance | Yes (1/29) | Adjuvant
chemotherapy, targeted therapy, radiotherapy and hormonal
therapy | (11) |
Case report
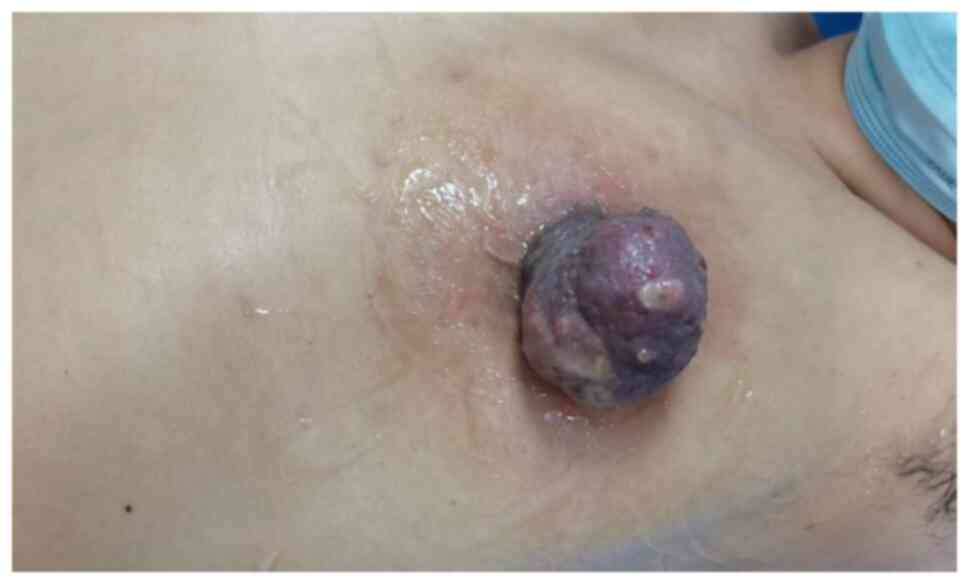
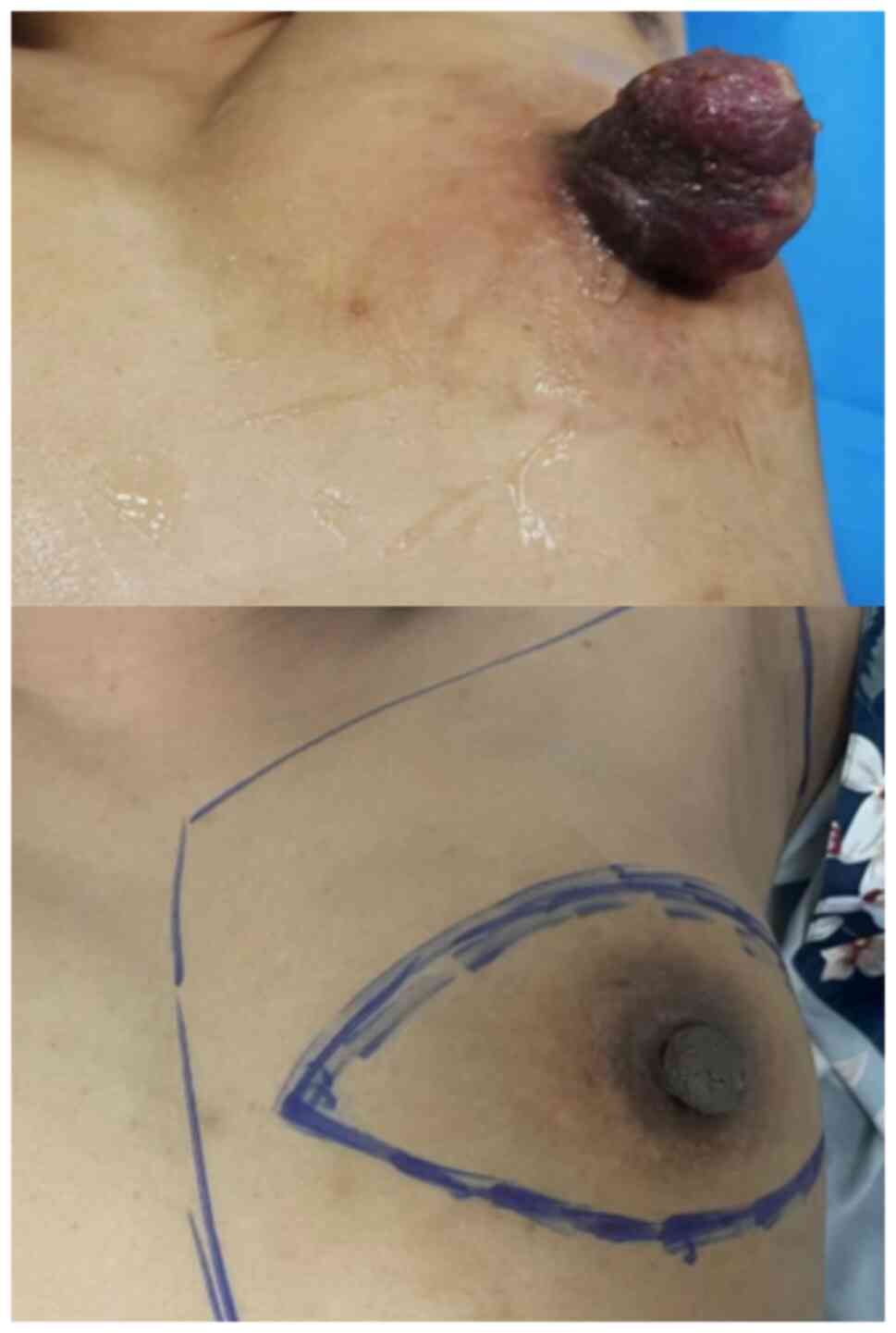
A 40-year-old female patient was admitted to the
Second Hospital of Jilin University (Changchun, China) on 16 August
2022 due to an enlarged firm mass on the left nipple with an
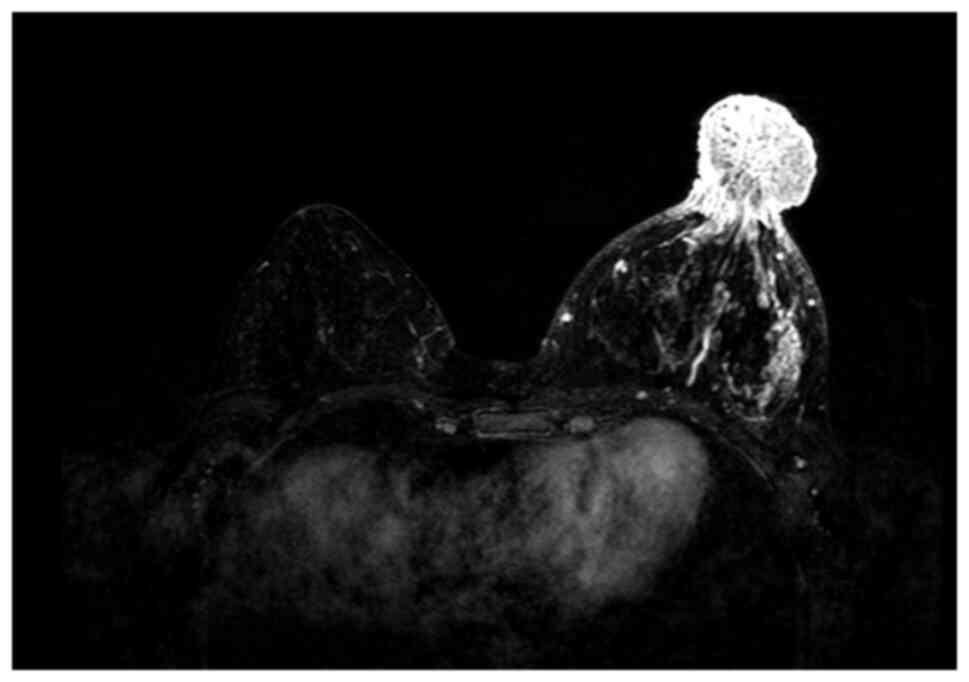
ulcerated surface and exudation of clear fluid (Fig. 1). X-ray examination revealed an
enlargement and increased density of the left nipple, measuring ~
3.5×3.2 cm, and MRI demonstrated a mass-like abnormal signal shadow
in the left papillary region and axillary lymphadenopathy (Fig. 2).
A total of three months prior, the patient had
noticed left nipple enlargement and then underwent pathological
biopsy of the nipple skin at The First Hospital of Jilin University
(Changchun, China), which showed spongiform dermatitis of the
nipple skin with lymphedema. However, the mass continued to enlarge
in the following 3 months and showed enlarged axillary lymph nodes,
therefore a second pathological biopsy was performed. A core needle
biopsy was performed on the nipple mass and axillary lymph nodes,
and the results revealed invasive ductal carcinoma of the nipple
(World Health Organization classification of breast tumors) and
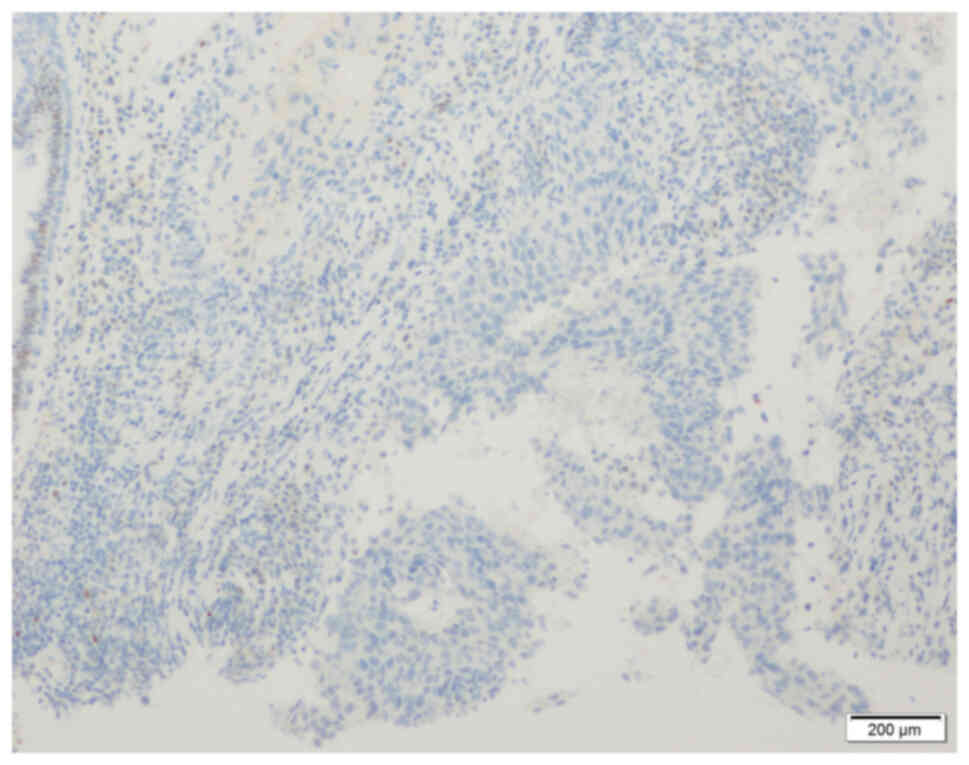
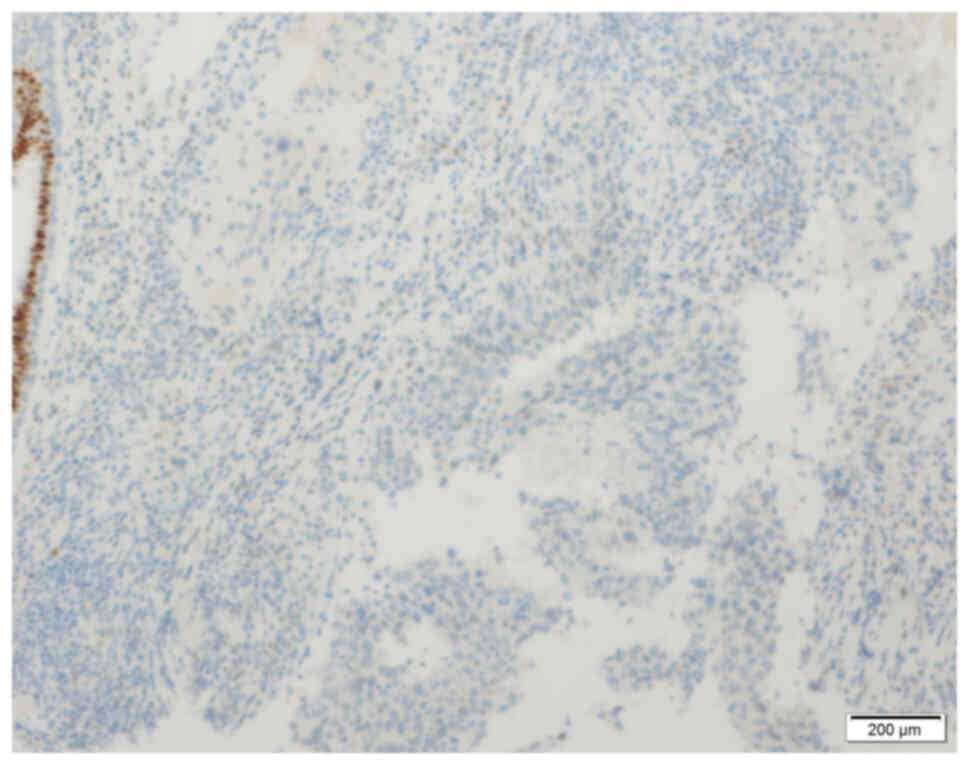
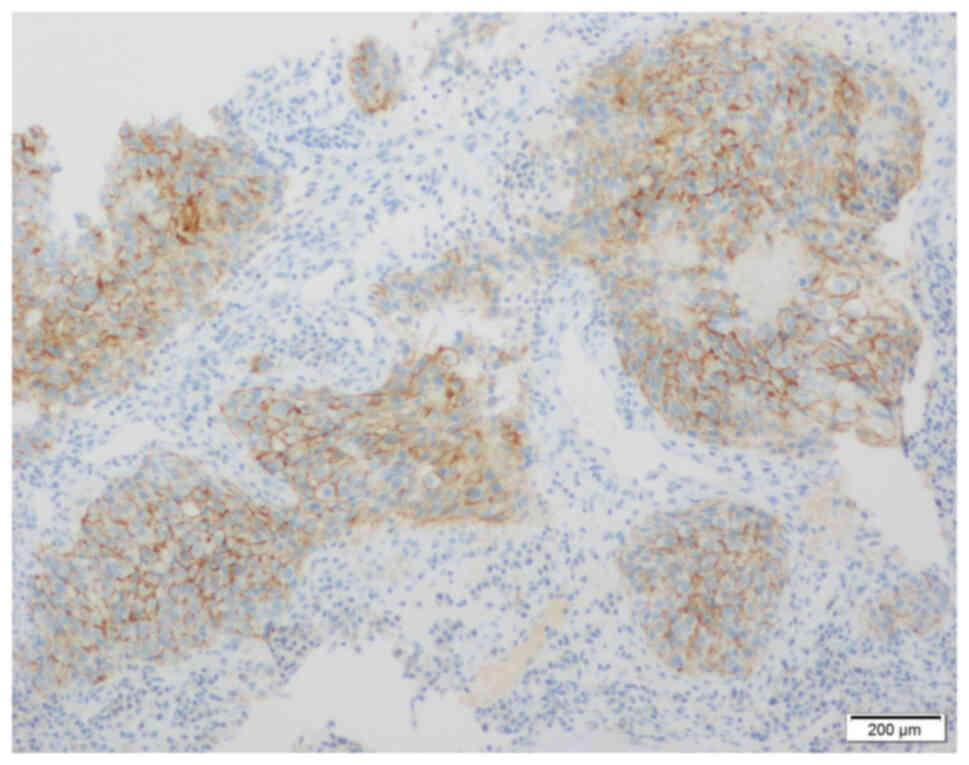
axillary lymph node metastasis (12). The immunohistochemistry (IHC)
results were negative for estrogen receptor (ER; Fig. 3), progesterone receptor (PR;
Fig. 4) and human epidermal growth
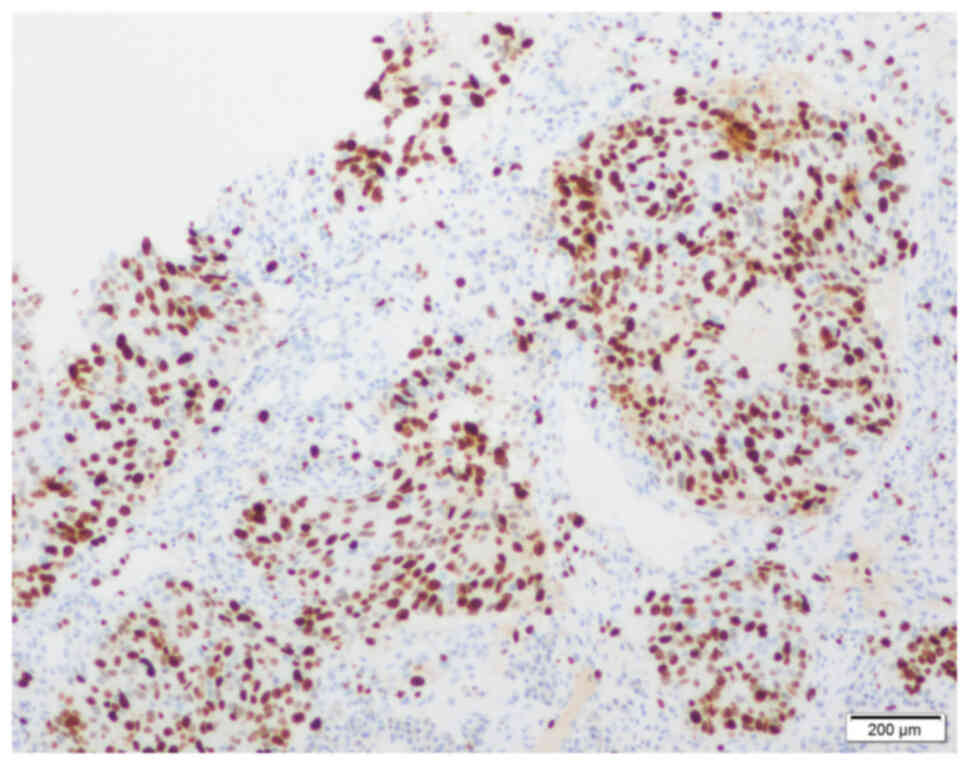
factor receptor-2 (HER-2; Fig. 5).
Ki-67 index was measured manually as 60% (Fig. 6). IHC staining was performed using
paraffin-embedded breast cancer tissue sections of 3 µm thickness
soaked in 10% neutral formaldehyde for 6–12 h at room temperature.
Antigen retrieval was performed by soaking the sample in sodium
citrate buffer (pH 6.0; 0.01 M), heating the water barrier to
92–98°C for 15–20 min, and then cooling at room temperature for
20–30 min followed by rinsing with distilled water and PBS buffer.
Permeabilization was performed in 0.1–0.3% TritonX-100 at room
temperature for 25 min, and rinsed with PBS three times for 5 min
each. Blocking was performed with 2–5% bovine serum albumin at room
temperature for 10–30 min. Sections were soaked in 3% hydrogen
peroxide for 20 min to block endogenous peroxidase activity.
Primary antibodies used for ER, PR, HER2 and Ki-67 were EP1
(Origene Technologies, Inc.; cat. no. ZA-0102; 5 µg/ml), EP2
(Origene Technologies, Inc.; cat. no. ZA-0255; 5 µg/ml), UMAB36
(Origene Technologies, Inc.; cat. no. ZM-0065; 1:50) and UMAB107
(Origene Technologies, Inc.; cat. no. ZM-0166; 1:300),
respectively, and incubated with the tissues at 37°C for 32 min.
Secondary antibodies were provided by the ultraView Universal DAB
Detection Kit (Roche Tissue Diagnostics; cat. no. 760-500) and
incubated for 8 min at 37°C. The chromogen detection reagent was
DAB. Counterstaining was performed with hematoxylin for 10 sec at
room temperature. The OLYMPUS BX51 Fluorescence Microscope (Olympus
Corporation) was used for observation. Staging examinations
included bilateral supraclavicular ultrasound, liver ultrasound
lung CT, and whole-body bone scintigraphy, which revealed no
distant metastasis (The American Joint Committee on Cancer)
(13).
Considering the locally advanced disease, the
patient was administered 6 cycles of neoadjuvant chemotherapy with
nab-paclitaxel [intravenously guttae (i.v.gtt); 260
mg/m2], epirubicin (i.v.gtt; 75 mg/m2) and
cyclophosphamide (i.v.gtt; 500 mg/m2) -, and each cycle
was 21 days (Fig. 7). The first
administration of chemotherapy was performed in August 2022.
Subsequently, the patient underwent a modified radical mastectomy
in December 2022 and achieved a pathological complete response
(pCR), which included the axillary lymph nodes. A total of 25
cycles of adjuvant radiotherapy was administered postoperatively.
The patient was last reviewed in April 2024 and showed no signs of
recurrence or metastasis.
Discussion
Table I presents the
previously reported cases of nipple-invasive primary carcinoma (not
secondary to Paget's disease). Previous cases have reported that
this invasive carcinoma arising in the nipple has several
manifestations, such as nipple mass, eczematous changes, edematous
thickened nipples or nipple bleeding (2–11).
Notably, eczematoid lesions do not equate to epidermal invasion,
and there are certain cases in which Paget's disease-like changes
are present but there are no Paget's cells present in the
epidermis; therefore, Paget's disease is excluded (2,4–9).
Imaging studies can sometimes detect abnormal changes, such as
masses or microcalcifications in the nipple, but there are also
cases in which eczematous changes in the nipple are only present,
whilst imaging studies do not show any abnormalities, suggesting
that a pathological biopsy can actively be performed (2,4,6–8).
Core needle biopsy can be performed for nipple
masses and suspicious lymph nodes, whilst open freehand biopsy can
be performed for eczematous changes. Notably, certain cases have no
epidermal infiltration, so epidermal scraping alone may lead to a
missed diagnosis 4. The patient in the present case presented to
another healthcare facility with nipple enlargement 3 months prior
to diagnosis. Punch biopsy of the nipple skin and core needle
biopsy of the axillary lymph nodes revealed spongiform dermatitis
of the nipple skin with dermal lymphedema, and no carcinomatous
infiltration of the axillary lymph nodes. This could have been due
to small lesions or inadequate biopsy sampling at that time.
Core needle biopsy pathology confirmed the present
case as triple-negative breast cancer (TNBC), that is, lack of
expression of ER, PR, and HER-2; however, most previous reported
cases were hormone receptor-positive (95%) (2,3,6,7,9,11).
Compared with other molecular subtypes, TNBC has a more unfavorable
prognosis, but is more sensitive to chemotherapy, and pCR is more
likely to be achieved after neoadjuvant chemotherapy (14). According to the National
Comprehensive Cancer Network guidelines for breast cancer,
neoadjuvant chemotherapy is recommended for TNBC that is >2 cm
in diameter or TNBC with positive lymph nodes (15). In the present case, Ki-67
proliferation index was relatively high at 60%. Ki-67 index is used
as an indicator of proliferative activity and also as a predictor
of response to treatment (16).
Many previous studies have reported that patients with TNBC with
high Ki-67 protein levels are more likely to achieve pCR after
receiving neoadjuvant chemotherapy (17). Pathologic response to neoadjuvant
chemotherapy is associated with long-term prognosis, with prolonged
survival in patients achieving pCR than in those with residual
tumor, and this association has been reported to be strongest in
TNBC (14).
To the best of our knowledge, the present case is
the first case of neoadjuvant chemotherapy for invasive breast
cancer originating in the nipple. Following the Chinese Society of
Clinical Oncology guidelines for breast cancer, the present case
administered a TEC regimen and the patient achieved pCR (including
axillary lymph nodes) after 6 cycles of chemotherapy (18). Other regimens have also demonstrated
efficacy in neoadjuvant chemotherapy for TNBC, but anthracycline-
and taxane-based regimens remain the first choice (19). Furthermore, a retrospective study by
Liedtke et al (14) reported
that anthracycline combined with taxane had the highest pCR rate
compared with anthracycline alone, taxane alone, or other regimens.
It was concluded that anthracycline + taxane was the most effective
regimen for TNBC.
In the choice of the surgical approach, total
mastectomy is performed in most cases, and the surgical principles
of the axilla are identical to those of invasive breast cancer
(4,5,10,11).
However, Moliere et al (3)
performed central breast-conserving surgery with negative margins
followed by postoperative radiotherapy in the absence of other
suspicious lesions on MRI, suggesting that, although nipple breast
cancer may occur with other breast lesions, central
breast-conserving surgery is also an option under the premise of
adequate imaging assessment.
To summarize, the present paper reports a rare case
of primary invasive carcinoma of the nipple. For abnormal changes
in the nipple, clinicians should be aware of the possibility of
breast cancer in the nipple and timely select the appropriate way
for pathological biopsy to avoid delay in diagnosis and treatment.
The present case also suggests that for primary breast cancer of
the nipple suitable for neoadjuvant therapy, the choice of
treatment can also refer to common breast cancer.
Acknowledgements
Not applicable.
Funding
Funding was received from the Natural Science Foundation of
Jilin Province of China (grant no. YDZJ202401165ZYTS).
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
KS proposed the main conceptual ideas, developed the
structure, wrote and edited the manuscript. MZ obtained medical
images, collected data, assisted with the preparation of tables and
figures and polished the language. GC performed biopsy procedures,
managed the patient during diagnosis and treatment, revised the
manuscript and provided guidance and supervision throughout the
writing process. BL made treatment decisions, advised on
neoadjuvant chemotherapy, performed surgical treatment and provide
financial support for this project. All authors have read and
approved the final manuscript. KS and BL confirm the authenticity
of all the raw data.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
The verbal informed consent was obtained from the
patient for the publication of their anonymous information in the
present article. Written informed consent was not obtained due to
the personal circumstances of the patient.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Congdon GH and Dockerty MB: Malignant
lesions of the nipple exclusive of Paget's disease. Surg Gynecol
Obstet. 103:185–192. 1956.PubMed/NCBI
|
|
2
|
Sanders MA, Brock JE, Harrison BT,
Wieczorek TJ, Hong X, Guidi AJ, Dillon DA, Max L and Lester SC:
Nipple invasive primary carcinomas: Clinical, imaging, and
pathologic features of breast carcinomas originating in the nipple.
Arch Pathol Lab Med. 142:598–605. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Moliere S, Lodi M, Roedlich MN and
Mathelin C: Invasive ductal carcinoma limited to the nipple. Breast
J. 24:10832018. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kasuga Y, Oohashi T, Nagai N, Tsuchiya S
and Sugenoya A: Primary scirrhous carcinoma of the nipple with
symptoms simulating Paget's disease: Report of a case. Surg Today.
23:356–359. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ohsumi S, Nozaki II, Takashima S and
Mandai K: Invasive ductal carcinoma of the nipple: A case report.
Breast Cancer. 3:215–218. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ahmed M and Basit A: Isolated
adenocarcinoma of the nipple. BMJ Case Rep. 2011:bcr02201138252011.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Erben Y, Ghosh K, Nassar A, Gimenez E and
Jakub JW: Invasive lobular carcinoma of the nipple. Breast J.
18:280–281. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Moennich J, Ort R, High W and Brown M:
Breast carcinoma masquerading as basal cell carcinoma of the
nipple. JAAD Case Rep. 1:361–363. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Pasquali P, Freites-Martinez A, Camacho E
and Fortuno A: A painful nipple: A rare presentation for an
infiltrating lobular carcinoma. Breast J. 22:117–118. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Tan QT and Mihir AG: A case of invasive
ductal carcinoma presenting as an exophytic nipple mass. Breast J.
25:1000–1001. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Hamzah JL, Ong KW and Tan BY: Isolated
invasive ductal carcinoma of the nipple-areolar complex: A rare
occurrence yet to be reported in current literature. Breast J.
25:706–708. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Tan PH, Ellis I, Allison K, Brogi E, Fox
SB, Lakhani S, Lazar AJ, Morris EA, Sahin A, Salgado R, et al: The
2019 world health organization classification of tumours of the
breast. Histopathology. 77:181–185. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kalli S, Semine A, Cohen S, Naber SP,
Makim SS and Bahl M: American joint committee on cancer's staging
system for breast cancer, eighth edition: What the radiologist
needs to know. Radiographics. 38:1921–1933. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Liedtke C, Mazouni C, Hess KR, Andre F,
Tordai A, Mejia JA, Symmans WF, Gonzalez-Angulo AM, Hennessy B,
Green M, et al: Response to neoadjuvant therapy and long-term
survival in patients with triple-negative breast cancer. J Clin
Oncol. 41:1809–1815. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Gradishar WJ, Moran MS, Abraham J, Aft R,
Agnese D, Allison KH, Anderson B, Burstein HJ, Chew H, Dang C, et
al: Breast cancer, version 3.2022, NCCN clinical practice
guidelines in oncology. J Natl Compr Canc Netw. 20:691–722. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Denkert C, Budczies J, von Minckwitz G,
Wienert S, Loibl S and Klauschen F: Strategies for developing Ki67
as a useful biomarker in breast cancer. Breast. 24 (Suppl
2):S67–S72. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
van den Ende NS, Nguyen AH, Jager A, Kok
M, Debets R and van Deurzen CHM: Triple-negative breast cancer and
predictive markers of response to neoadjuvant chemotherapy: A
systematic review. Int J Mol Sci. 24:29692023. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Jiang Z, Li J, Chen J, Liu Y, Wang K, Nie
J, Wang X, Hao C, Yin Y, Wang S, et al: Chinese society of clinical
oncology (CSCO) breast cancer guidelines 2022. Transl Breast Cancer
Res. 3:132022. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Yagata H, Kajiura Y and Yamauchi H:
Current strategy for triple-negative breast cancer: Appropriate
combination of surgery, radiation, and chemotherapy. Breast Cancer.
18:165–173. 2011. View Article : Google Scholar : PubMed/NCBI
|