Introduction
Mesonephric adenocarcinomas (MAs) are rare malignant
human papillomavirus (HPV)-independent cervical tumours that arise
from vestiges of the embryological female reproductive system
(Wolffian/mesonephric duct) remnants located deep in the cervical
wall (1,2). MAs constitute <1% of cervical
adenocarcinomas (3). While these
tumours can arise across a wide age range, they are rarely
diagnosed in patients <30 years old (2). Clinically, this type of tumour
typically manifests as abnormal vaginal bleeding or is identified
as a cervical mass during a pelvic examination (4). MAs display diverse morphologic
architectural patterns, including tubular, glandular, papillary,
cribriform, retiform, spindle cell and solid structures (2). Morphologically, the tumour is
characterized by mesonephric remnants, mesonephric hyperplasia and
eosinophilic luminal secretions (5). MAs are characterized by recurrent
KRAS mutations (3).
Molecularly, 75–100% of patients with MAs exhibit KRAS
mutations (3,6,7).
Patients with MAs have a worse prognosis compared
with those with cervical squamous cell carcinoma and other types of
adenocarcinomas (8,9). Spindle cell components are observable
in MAs; however, they have been minimally explored in studies
concerning their biological behaviour and prognosis (3). A total of 3 cases of cervical MAs that
featured prominent spindle cell components are reported in the
present study, and a comprehensive literature review was carried
out to determine the potential associations between spindle
morphology and, unique clinicopathological and molecular
characteristics.
Materials and methods
Samples and clinical data
Female patients diagnosed with primary uterine
cervical MA with prominent spindle cell components at West China
Second University Hospital, Sichuan University (Chengdu, China)
between January 2020 and December 2023 were included in the present
study. All procedures performed in studies involving human
participants adhered to ethical standards. The staging system used
for cervical MAs with spindle cell components was the 2018 revision
by the International Federation of Gynaecology and Obstetrics
(FIGO) (10). The
clinicopathological information of the patients, including age,
clinical presentations, procedures, tumour size, follow-up
information and FIGO stage, were extracted from the electronic
medical records of the patients. Two gynaecological pathologists
reviewed all haematoxylin and eosin (H&E) sections, and the
immunohistochemical findings of the included cases. A total of 5
cases were excluded due to the lack of complete clinicopathological
information or the absence of a spindle cell component. Ultimately,
3 cases were included in the present study.
Immunohistochemistry
The tissue was fixed using a 10% neutral buffered
formalin solution at room temperature for 24 h. Immunohistochemical
staining was performed on 4-µm-thick formalin-fixed
paraffin-embedded (FFPE) tumour samples with the automated staining
system Bond III (Leica Biosystems) based on the EnVision method.
FFPE sections (4-µm-thick) were immersed in xylene, and 100, 95, 85
and 75% ethanol for dewaxing and hydration. Antigen retrieval was
performed by heating the sections to 95°C in citrate buffer (pH
6.0) for 20 min. For intracellular antigens or membrane proteins
with an internal epitope, 0.1% Triton X-100 solution was used for
permeabilization at room temperature for 10 min. The BOND polymer
Refine Detection kit (cat. no. DS9800) from Leica Biosystems, using
Bond III, including 3–4% hydrogen peroxide as the peroxide block,
was used for 5 min at room temperature. For blocking of
non-specific binding, the sections were incubated with BOND™
Primary Antibody Diluent (Leica Biosystems), which includes 1–3%
BSA, at room temperature for 10 min. The sections were incubated
with anti-rabbit poly-HRP IgG (<25 µg/ml; from the DS9800 kit)
for 15 min at room temperature. All immunohistochemically stained
tumour samples were evaluated using appropriate internal (including
liver, kidney, tonsil, renal, fallopian tube and thyroid tissues)
and external (including lymphocytes, mesothelial cells and normal
cervical epithelial cells) controls. The chromogen used to
visualize the staining was 3,3′-diaminobenzidine (included in the
BOND Polymer Refine Detection kit). The following antibodies were
used: Cytokeratin (CK) pan (cat. no. RAB-0050; 1:500; Fuzhou Maixin
Biotechnology Development Co., Ltd.), epithelial membrane antigen
(EMA; cat. no. Kit-0011; 1:100; Fuzhou Maixin Biotechnology
Development Co., Ltd.), paired box 8 (PAX8; cat. no. RMA-1024;
1:200; Fuzhou Maixin Biotechnology Development Co., Ltd.),
oestrogen receptor (ER; cat. no. Kit-0012; 1:100; Fuzhou Maixin
Biotechnology Development Co., Ltd.), progesterone receptor (PR;
cat. no. Kit-0013; 1:100; Fuzhou Maixin Biotechnology Development
Co., Ltd.), p16 (cat. no. MAB-0673; 1:1,000; Fuzhou Maixin
Biotechnology Development Co., Ltd.), GATA3 (cat. no. MAB-0695;
1:100; Fuzhou Maixin Biotechnology Development Co., Ltd.), CD10
(cat. no. MAB-0668; 1:400; Fuzhou Maixin Biotechnology Development
Co., Ltd.), transcription termination factor 1 (TTF1; cat. no.
MAB-0266; 1:100; Fuzhou Maixin Biotechnology Development Co.,
Ltd.), p53 (cat. no. MAB-0674; 1:1,000; Fuzhou Maixin Biotechnology
Development Co., Ltd.), vimentin (Vim; cat. no. MAB-0735; 1:600;
Fuzhou Maixin Biotechnology Development Co., Ltd.), hepatocyte
nuclear factor-1β (HNF1β; cat. no. ZA-0129; ready-to-use; OriGene
Technologies, Inc.) and Ki67 (cat. no. MAB-0672; 1:300; Fuzhou
Maixin Biotechnology Development Co., Ltd.). Sections were
incubated with primary antibodies at room temperature for 15 min.
The immunohistochemical staining was analyzed using an Olympus BX43
light microscope (magnification, ×100; Olympus Corporation).
Targeted next-generation sequencing
(NGS)
The genomic alteration profiling test was conducted
by Precision Scientific, Inc. using targeted NGS technology. The
targeted NGS panel assessed 107 genes (Table SI). DNA was extracted from
unstained FFPE tumour samples from all 3 patients after selecting a
region with >20% tumour cells. DNA was prepared for sequencing
using the QIAamp DNA FFPE Tissue Kit (cat. no. 56404; Qiagen, Inc.)
according to the manufacturer's protocol. Quality control was
completed using a Qubit (Thermo Fisher Scientific, Inc.), and
agarose gel electrophoresis was carried out to assess the extracted
genomic DNA. Library construction and capture were performed using
the KAPA HyperPlus Kit (cat. no. KK8514; Roche Sequencing), with a
standard DNA starting quantity of ≥200 ng and an output library
concentration of ≥0.5 ng/µl. Sequencing was conducted on the
Illumina NovaSeq 6000 platform (Illumina, Inc.), using the NovaSeq
6000 S4 Reagent Kit (300 cycles; cat. no. 20028312; Illumina,
Inc.), with an average sequencing depth of ≥100× for control
samples and ≥500× for tumour tissue samples. The sequencing type
was paired-end with a read length of 150 bp. The final library
loading concentration was 300 pM, and was measured using the Qubit
3.0 Fluorometer (Thermo Fisher Scientific, Inc.). Post-sequencing,
internally developed bioinformatics analysis was carried out, where
the proportion of sites in the capture region with a depth >0.2×
had an average depth of ≥90%, the sequence alignment rate was ≥90%,
and the sequencing data Q30 were ≥80%. Variant detection included
single nucleotide variations, small fragment insertions/deletions,
gene copy number variations and gene fusions within the capture
range with breakpoints. Data analysis was conducted using the
DRAGEN Bio-IT Platform (version 3.8.4; Illumina, Inc.), and the
results were interpreted using the software's variant calling
pipeline (Illumina DRAGEN variant caller; http://www.illumina.com/products/by-type/informatics-products/dragen-bio-it-platform.html).
Results
Clinical features
The clinicopathological features of the patients are
summarized in Table I. The present
study included 3 postmenopausal female patients aged 51–60 years
(mean age, 56 years) with primary uterine cervical MA with
prominent spindle cell components. The 3 patients presented with
different clinical symptoms, including cervical ThinPrep cytologic
test results indicating abnormal (11), abdominal distension and pain, and
postmenopausal vaginal bleeding. In case 1, colposcopy demonstrated
a 1×0.5 cm ulcerated area on the cervix at the 11 o'clock
position.
 | Table I.Clinicopathological features. |
Table I.
Clinicopathological features.
| Parameters | Case 1 | Case 2 | Case 3 |
|---|
| Age, years | 51 | 60 | 57 |
| Clinical
presentation | None | Abdominal
distension and pain for 1 month | Postmenopausal
vaginal bleeding for 5 months |
| Surgical
procedure | TAHBSO + LND | TAHBSO + LND +
CRT | TAHBSO + LND +
CT |
| Tumour size,
cm | 1 | 7.3 | 3.5 |
| FIGO stage | IB | IIB | IIB |
| Gross
appearance | Ulceration | Cauliflower-like
mass | Cauliflower-like
mass |
| Outcomes | DFS | DFS | DFS |
| Follow-up,
months | 9 | 11 | 16 |
| Initial
diagnosis | Synovial
sarcoma | Mesenchymal
tumour | Clear cell
carcinoma |
| Imaging
findings | Enlarged cervix
with heterogeneous enhancement | A solid-cystic
mass | A low-density
mass |
| Serum tumour
markers | CA125 and CA19-9
levels elevated | CA125, CA19-9 and
CEA levels elevated | CA125 level
elevated |
Imaging examinations indicated cervical masses in 2
cases. In case 2, a contrast-enhanced computed tomography scan
demonstrated a solid-cystic mass on the left side of the pelvic
cavity, measuring 7.3×6.4×5.4 cm, and unclear boundaries with the
left adnexa and the posterior wall of the uterus were.
Additionally, computed tomography imaging also showed a low-density
uterine cervical mass in case 3, measuring 3.5×2.8×2.7 cm.
The 3 cases showed slightly elevated serum tumour
markers, including serum CA125, CA19-9 and CEA. Furthermore, case 1
and 2 both had a history of surgery for pulmonary adenocarcinoma.
The family histories of all patients were unremarkable.
Treatment and follow-up
All 3 patients underwent total abdominal
hysterectomy and bilateral salpingo-oophorectomy (TAHBSO) with
pelvic lymph node dissection (LND). Of the included patients, 2
patients were classified as FIGO stage IIB, while the remaining
patient was classified as FIGO stage IB. Patients with FIGO stage
IIB received postoperative adjuvant chemotherapy (CT) using
carboplatin and paclitaxel for 6 cycles, while case 2 additionally
underwent adjuvant radiation therapy at a dose of 6 Gy/fraction
(total of 28 times).
Follow-up information was obtained for all 3
patients. The latest prognostic data showed no recurrences or
deaths among the 3 patients, who were followed up for 9, 11 and 16
months.
Pathological features
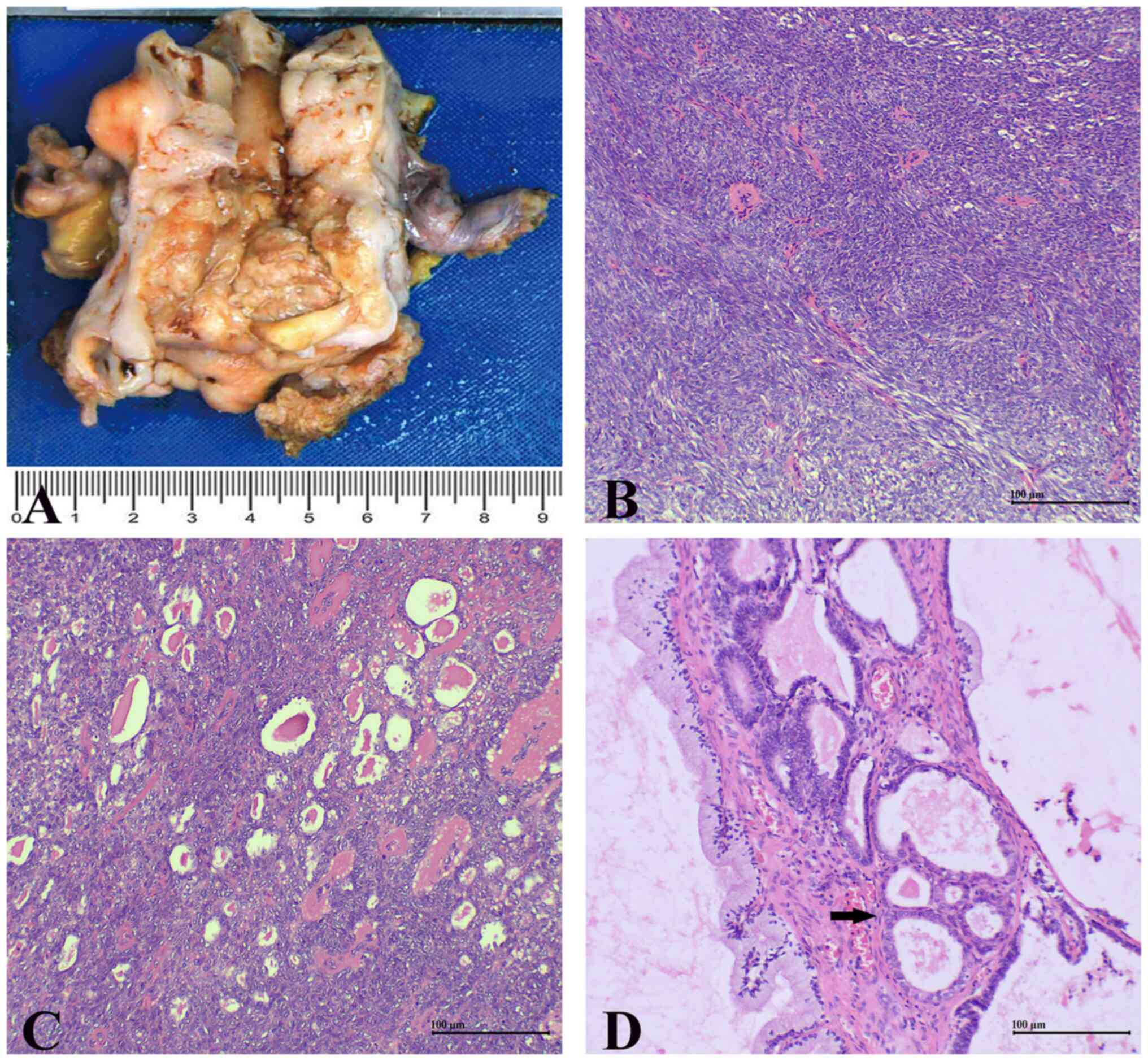
On gross examination, two tumours presented as
cauliflower-like masses in the cervix (Fig. 1A). A total of two biopsy specimens,
one frozen and three surgical specimens were reviewed from all 3
cases.
Histologically, all surgical specimens from the 3
cases exhibited biphasic tumours characterized by the coexistence
of epithelioid and spindle cell areas. There was a transition from
the epithelioid areas to the spindle cell areas. In the spindle
cell areas, tumour cells exhibited an invasive growth pattern,
arranged in fascicular and storiform patterns (Fig. 1B). These spindle cells had small
amounts of indistinct cytoplasm, oval to fusiform nuclei,
non-prominent nucleoli and moderate nuclear atypia. Mitotic figures
were identified. Heterologous sarcomatous components, highly
heterotypic tumour cells and definite necrosis were not
present.
In the adjacent area to the spindle cell areas,
glandular, papillary, cribriform and back-to-back tubular
structures were observed, lined by cuboidal tumour cells with
moderate-to-marked nuclear atypia, mitotic figures and nuclear
grooves. The cuboidal epithelioid cells formed glandular tubular
structures with luminal eosinophilic hyaline secretions (Fig. 1C). Additionally, benign mesonephric
remnants and hyperplasia surrounding the tumour were visible
(Fig. 1D).
In the biopsy specimens of case 1, the tumour was
primarily composed of spindle cells with occasional glandular
components, when examined microscopically. The initial diagnosis,
at the local hospital, had been synovial sarcoma. In another biopsy
specimen of case 3, no spindle cell component was observed, leading
to an initial diagnosis of clear cell carcinoma. Prominent spindle
cell components were also observed in the frozen specimen. In the
frozen sections, only diffuse spindle tumour cells were observed,
with no evidence of epithelioid areas. Based on the aforementioned
morphological features, the initial diagnosis of the intraoperative
frozen sample was spindle cell tumour, tending towards mesenchymal
tumour.
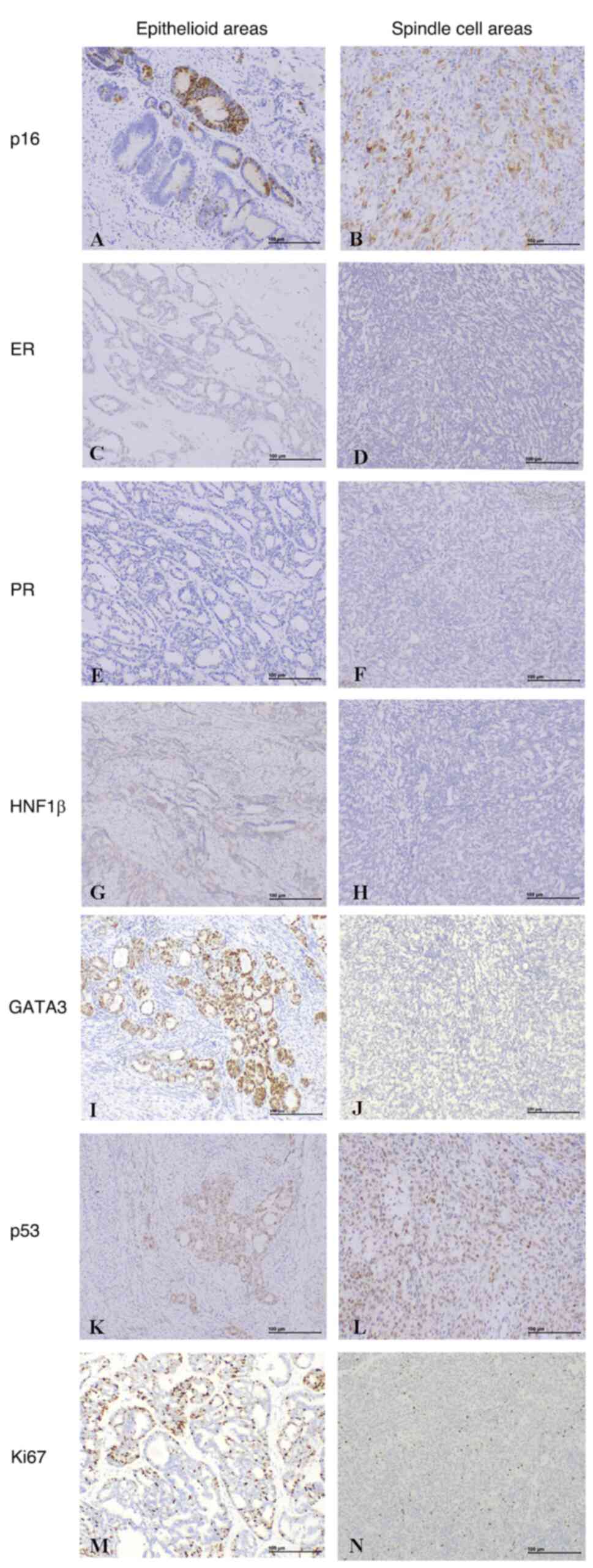
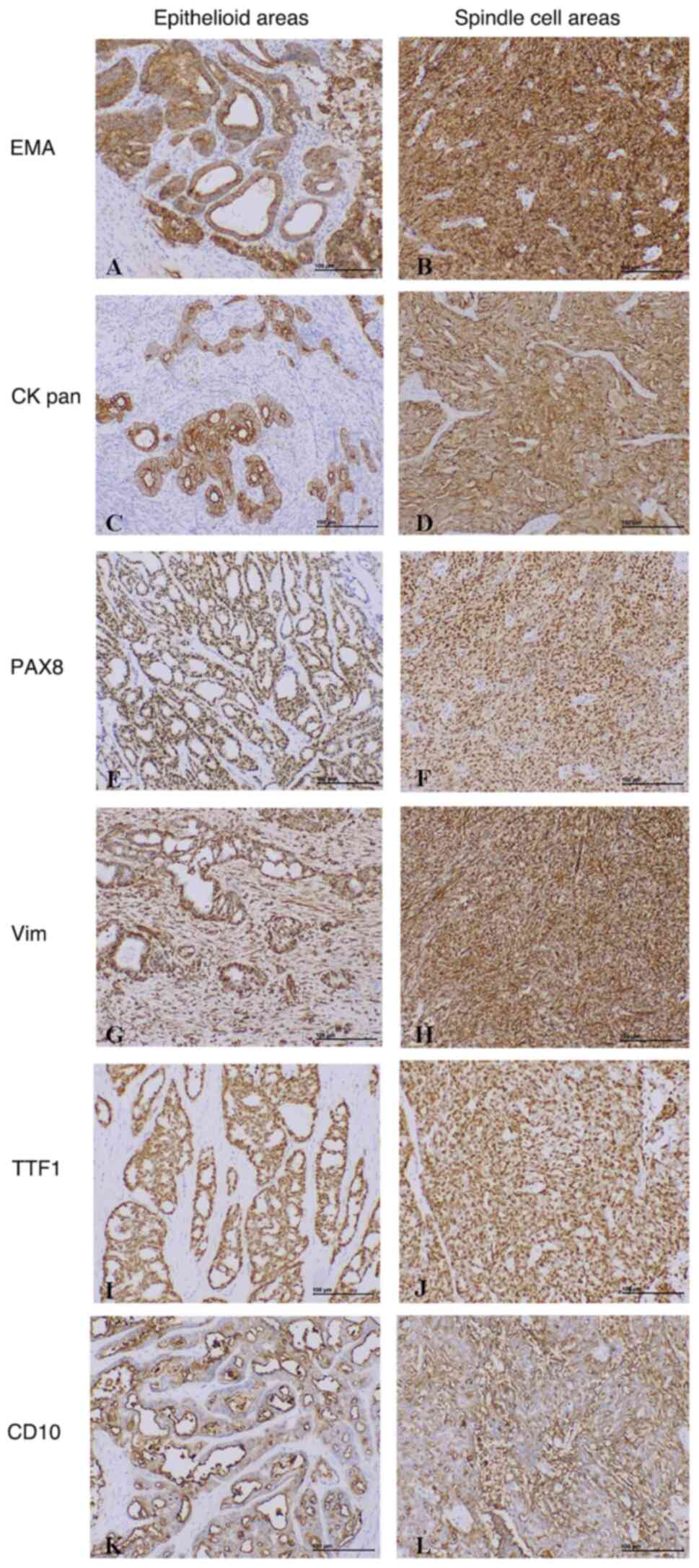
The immunohistochemistry results are shown in
Table II. Immunohistochemically,
tumour cells stained positive for epithelial markers, EMA and CK
pan (Fig. 2A-D), PAX8 (Fig. 2E and F) and Vim (Fig. 2G and H) both in spindle cell
components and in epithelioid areas. Staining for TTF1 (Fig. 2I and J) and CD10 (Fig. 2K and L) were positive in 1 case and
in 2 cases, respectively. Staining for p16 (Fig. 3A and B) was focal or patchy positive
in tumour cells. Tumour cells were negative or focal positive for
ER (Fig. 3C and D) and PR (Fig. 3E and F) staining. Tumour cells were
negative for HNF1β staining (Fig. 3G
and H) in 1 case. In case 1 and 2, staining for GATA3 was
positive both in spindle cell components and epithelioid areas,
while in case 3, staining for GATA3 was negative in spindle cell
components and positive in epithelioid areas (Fig. 3I and J). p53 was expressed at normal
levels in all 3 cases (Fig. 3K and
L). The range of the Ki67 proliferative index was 5–40%
(Fig. 3M and N).
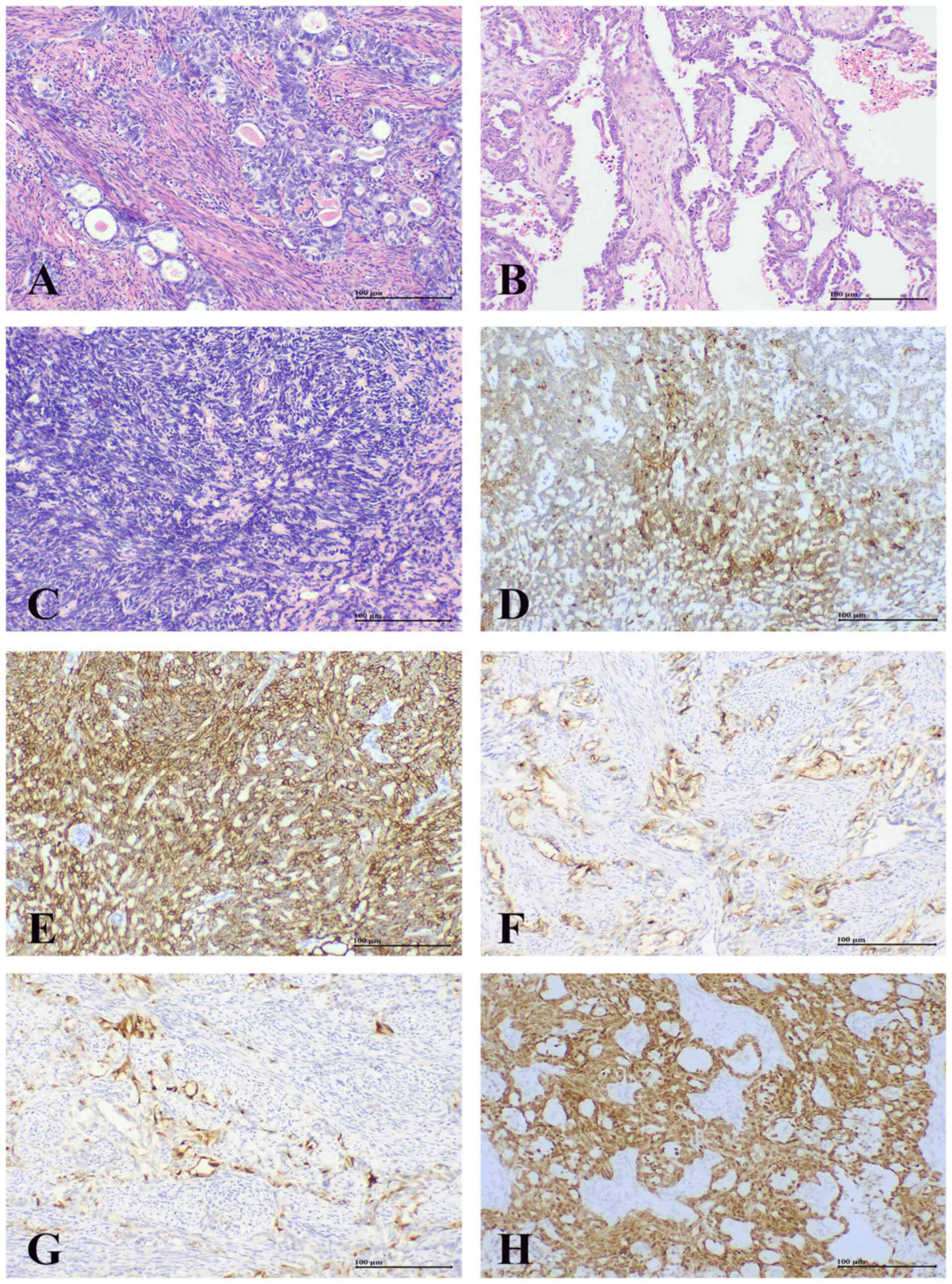
 | Figure 2.Representative images of
immunohistochemical results from the included cases. The tumour
cells showed positive expression of (A and B) EMA, (C and D) CK
pan, (E and F) PAX8, (G and H) Vim and (I and J) TTF1 in both
epithelioid and spindle cell areas (magnification, ×100). (K) CD10
had luminal positive expression in epithelioid areas and (L)
positive expression in spindle cell areas (magnification, ×100).
CK, cytokeratin; EMA, epithelial membrane antigen; PAX8, paired box
8; Vim, vimentin; TTF1, transcription termination factor 1. |
 | Table II.Immunohistochemical findings. |
Table II.
Immunohistochemical findings.
|
| Case 1 | Case 2 | Case 3 |
|---|
|
|
|
|
|
|---|
| Parameters | Epithelioid
areas | Spindle cell
areas | Epithelioid
areas | Spindle cell
areas | Epithelioid
areas | Spindle cell
areas |
|---|
| EMA | + | + | + | + | + | + |
| CK pan | + | + | NA | NA | + | + |
| PAX8 | + | + | + | + | + | + |
| GATA3 | + | + | + | + | + | - |
| CD10 | - | - | + | + | + | + (focal) |
| TTF1 | - | - | + | + | - | - |
| ER | + (focal) | + (focal) | - | - | - | - |
| PR | + (focal) | + (focal) | - | - | - | - |
| p16 | + (focal) | + (focal) | + (patchy) | + (patchy) | + (patchy) | - |
| Vim | + | + | NA | NA | + | + |
| HNF1β | NA | NA | NA | NA | - | - |
| p53 | Normal | Normal | Normal | Normal | Normal | Normal |
| Ki67, % | 10 | 20 | 20 | 10 | 40 | 5 |
Molecular features
NGS was performed in all 3 cases. Microsatellite
instability was not identified in any of the included cases. In
case 1, only one KRAS mutation (p.Q61K) was identified. In
case 2, one KRAS mutation (p.G12D) and one checkpoint kinase
2 mutation (c.909-1G>A), along with amplifications of KRAS,
MDM4 and neurotrophic receptor tyrosine kinase 1 (NTRK1)
were identified. A PIK3CA mutation (p.E726K) and
amplification of NTRK1 were identified in case 3. The
histopathological and immunohistochemical findings of case 3 are
shown in Fig. 4. Microscopically,
the tumour consisted of two components: An epithelioid area with
glandular (Fig. 4A) and papillary
(Fig. 4B) patterns, and a solid
spindle cell area (Fig. 4C).
Immunohistochemically, both EMA (Fig.
4D) and CK pan (Fig. 4E) showed
positive expression. In the epithelioid area, CD10 luminal staining
was positive (Fig. 4F), and p16
staining showed patchy positive expression (Fig. 4G). PAX8 was positively expressed in
both the epithelioid and spindle cell areas (Fig. 4H).
Discussion
Primary cervical MAs are rare malignant tumours,
that represent only 1% of all cervical malignancies (2). Currently, cervical MAs have only been
reported as case reports or small series (3,4,6,9,12,13).
The largest study on cervical MAs to date is the multicentre study
conducted by Pors et al (9),
which included 30 cases. In the published literature, most MA
reports did not clearly describe the presence of spindle cell
components, and a few MAs with spindle cell components were
diagnosed as mesonephric carcinosarcomas, previously referred to as
mesonephric mixed tumours. To the best of our knowledge, only 11
cases of cervical MAs with spindle cell components have been
reported, which includes the cases described in the present study
(Table III).
 | Table III.Mesonephric adenocarcinomas with
spindle cell components in the literature and the present
cases. |
Table III.
Mesonephric adenocarcinomas with
spindle cell components in the literature and the present
cases.
| First author (s),
year | Case no. | Age, years | Tumour size,
cm | FIGO stage | Spindle cell
component, % | Outcome | Follow-up,
months | KRAS/NRAS
mutation | (Refs.) |
|---|
| Mirkovic et
al, | 1 | 76 | 2.2 | IIB | 5 | NA | NA | Yes | (3) |
| 2015 | 2 | 47 | NA | IIB | 80 | NA | NA | Yes |
|
|
| 3 | 38 | NA | IIIB | 15 | LWD | 10 | Yes |
|
|
| 4 | 64 | 4.5 | IB | 60 | DFS | 36 | Yes |
|
|
| 5 | 67 | 1 | NA | 40 | NA | NA | Yes |
|
|
| 6 | 54 | 12 | NA | 90 | NA | NA | Yes |
|
|
| 7 | 48 | NA | NA | 5 | NA | NA | No |
|
|
| 8 | 37 | NA | NA | 30 | NA | NA | No |
|
| Present study | 9 | 51 | 1 | IB | 80 | DFS | 9 | Yes | - |
|
| 10 | 60 | 7.3 | IIB | 50 | DFS | 11 | Yes |
|
|
| 11 | 57 | 3.5 | IIB | 60 | DFS | 16 | No |
|
The median age at diagnosis of cervical MAs with
spindle cell components was 54 years (range, 37–76 years). The age
at diagnosis was similar to that previously reported in cases of
MAs without spindle cell components (52–59 years) and mesonephric
carcinosarcomas (54 years) (8,9,14). The
median tumour size of cervical MAs with spindle cell components was
4.5 cm (range, 1–12 cm), which was larger compared with that of
mesonephric carcinosarcomas (3.5 cm) (14). Among the 7 patients with MAs and
spindle cell components, 5 (71%) were diagnosed at FIGO stage
II–IV. A previous review reported that only 30% of patients with
MAs without spindle cell components are diagnosed at FIGO stage
II–IV (8). Contrary to the
aforementioned review, the multicentre study by Pors et al
(9) showed that a higher proportion
of patients (60%) with MAs and without spindle cell components were
diagnosed at an advanced stage (FIGO stage II–IV). Moreover, ~40%
of patients with mesonephric carcinosarcomas were diagnosed at FIGO
stage II–IV (14,15). These reports indicate that MAs with
spindle cell components are more likely to be diagnosed at an
advanced stage compared with MAs without spindle cell components
and mesonephric carcinosarcomas. In the current study, spindle cell
components displayed moderate nuclear atypia with readily
identified mitotic figures. The advanced stage and malignant
morphology suggest that spindle cell components may contribute to
disease progression and aggressive biological behaviour.
Prognostic information was available for only 5
cases of MAs with spindle cell components, which includes the 3
cases presented in the present study. The mean duration of the
follow-up was 16.4 months (range, 9–36 months). Only 1 patient
(20%) lived with disease at FIGO stage IIIB and no death was
observed. The sites of recurrence and metastasis included the
abdomen, pelvis and liver (3). A
previous literature review of 31 patients reported that ~30% of
patients with MAs lacking spindle cell components experienced
recurrence and 23% died from the disease, irrespective of the
disease stage (8). The recent study
by Pors et al (9) which
included 30 cases reported that patients with MAs lacking spindle
cell components had a worse prognosis, with a 5-year overall
survival rate of 74% and progression-free survival rate of 60% in
cervical MAs, compared with the findings of previous literature
reviews. However, the composition of spindle cell components is not
clearly described in the literature, which potentially makes
prognostic evaluations challenging and unreliable when compared
between MAs with and without these components. Patients with MAs
and spindle cells components appear to have an improved prognosis
compared with those patients without these components. Fregnani
et al (16) reported that
among the 35 cases of cervical adenocarcinomas, the recurrence rate
was 16%, which was lower compared with that of MAs with spindle
cells components. In addition, in a literature review containing 9
mesonephric carcinosarcomas, the recurrence rate was 22%, which was
slightly higher compared with that of MAs with spindle cells
components (8,15). Despite limited data, MAs with
spindle cell components still show a poor prognosis.
In the present study, 2 of the 3 cases had a history
of surgery for pulmonary adenocarcinoma. Histologically, both cases
diagnosed with MAs showed glandular tubular structures with luminal
eosinophilic hyaline secretions, and benign mesonephric remnants
and hyperplasia. The TTF1 expression in MAs overlapped with that in
pulmonary adenocarcinoma. Therefore, additional immunohistochemical
markers were used to differentiate the cases. PAX8, a specific
marker for differential diagnosis, was positively expressed in both
cases in the present study. PAX8 primarily contributes to the
organogenesis of the thyroid gland, kidney and Müllerian system.
PAX8 typically shows negative expression in primary and metastatic
lung cancers, and positive expression in MAs (2,17,18).
The luminal staining pattern of CD10 is useful for confirming
primary cervical MAs, as it is absent in pulmonary adenocarcinoma
(19). Histological and
immunohistochemical characteristics aided in ruling out metastatic
pulmonary adenocarcinoma of the uterine cervix.
Histologically, MAs typically exhibit a mixture of
architectural patterns and overlap with other tumours (2). Spindle cell components in MAs pose
diagnostic challenges and complicate accurate diagnosis. In the 2
cases reported in the present study, the initial diagnoses were
initially considered to be mesenchymal tumours. Therefore, MAs
should be included in the differential diagnosis when obvious
spindle cell components are present morphologically, particularly
in biopsy specimens. The correct diagnostic rate is only 10% in
initial biopsy specimens (9).
Histological and immunohistochemical features serve an important
role in diagnostic work. Diagnostic clues include luminal
eosinophilic hyaline secretions, nuclear grooves, mesonephric
remnants, mesonephric hyperplasia, HPV independence and the absence
of heterologous sarcomatous components. Recommended
immunohistochemical panels include the epithelial markers EMA
and/or CK pan, and PAX8, CD10, GATA3, TTF1, ER, PR, HNF1β and
p16.
MAs typically exhibit positive epithelial marker
expression (EMA and/or CK pan) in both epithelial and spindle cell
areas, PAX8 positivity, luminal CD10 staining, negative or focal
positive ER and PR, positive GATA3 and/or TTF1, negative HNF1β and
non-diffuse positive p16 (18). In
the present study, there were no differences in the
immunohistochemical characteristics between MAs with and without
spindle cell components. Among the aforementioned
immunohistochemical markers, GATA3 is a highly sensitive and
specific marker for MAs (20,21).
In case 3, GATA3 showed negative staining in the spindle cell areas
and positive staining in the adenoid areas. The staining intensity
of GATA3 may decrease in the solid areas, which is consistent with
previous studies (20,21). Therefore, it is noteworthy that if a
spindle cell component is recognized with GATA3 expression as
negative or weakly positive in the biopsy specimen, it may also be
suspected of being MA. TTF1 may be useful in diagnosing cases where
GATA3 is negatively expressed, due to the inverse staining pattern
between GATA3 and TTF1 (21).
The overlapping morphological features of MAs with
prominent spindle cell components make for a broader range of
differential diagnoses. The main differential diagnoses include
endocervical adenocarcinoma, clear cell carcinoma and endometrioid
adenocarcinoma (2). Endocervical
adenocarcinoma, often associated with HPV, exhibits mucin
production or ciliation (2,4). p16 shows diffuse staining in
HPV-related endocervical adenocarcinomas but shows patchy staining
pattern in MAs (2,4). In cases with overlapping morphological
features that are challenging to diagnose, a combination of
immunohistochemical markers such as CEA, p16, GATA3 and CD10 can be
used (2). Clear cell carcinoma,
HPV-independent adenocarcinoma, is characterized by clear,
eosinophilic and hobnailed tumour cells (22). HNF1β, a marker commonly used in the
diagnosis of clear cell carcinoma, is also positively expressed in
a subset of MAs (18). Squamous and
mucinous differentiation can be used for the diagnosis of
endometrioid adenocarcinoma (2).
Immunohistochemical staining for ER and PR is usually positive in
endometrioid adenocarcinoma (23).
Moreover, the most challenging differential
diagnosis is that of mesonephric carcinosarcomas. Mesonephric
carcinosarcomas, which are biphasic tumours, consist of
distinguishable malignant epithelial and spindle cell components
(6). In these two subtypes tumours,
mesonephric hyperplasia and a transition from the malignant
epithelioid components to the spindle cell components were observed
(3,24–26),
which supports both tumours originating from mesonephric duct
remnants. Both MAs and mesonephric carcinosarcomas may contain a
population of morphologically spindle cells, and there are no clear
criteria for distinguishing between these two subtypes of tumours.
The present study demonstrated that there were differences in
histological and immunohistochemical features between these two
subtypes tumours. In MAs, the spindle cells are typically
cytologically more bland (27);
however, in mesonephric carcinosarcomas, spindle cells usually show
more marked atypia and heterologous sarcomatous components can also
be observed (15,26). Mirkovic et al (27) recommended that MAs with heterologous
mesenchymal elements should be diagnosed as mesonephric
carcinosarcomas. At present, reported heterologous sarcomatous
components in the literature have included osteosarcoma,
chondrosarcoma and rhabdomyosarcoma, while the homologous component
has been limited to endometrial stromal sarcoma (15). Immunohistochemically, Vim is
positively expressed in 70–100% of MAs, ranging from focal positive
to diffuse strong positive expression (18,26).
The present study showed that Vim was positively expressed in both
malignant epithelioid and spindle cell areas, which showed no
difference compared with mesonephric carcinosarcomas (24,25).
The spindle cell components have diffuse positive expression for
epithelial markers such as EMA and CK pan in MAs, while the
expression of patterns is reversed in mesonephric carcinosarcomas
(15,24,25).
In addition, Mirkovic et al (3) reported that there was no association
between molecular features and spindle cell composition in MAs.
Therefore, it was suggested that the spindle cell components in MAs
may be part of the morphologic spectrum of the tumours, which was
consistent with previous studies (27,28).
Although a transition from the malignant epithelioid components to
the spindle cell components was also observed in mesonephric
carcinosarcomas, in contrast to MAs, the sarcomatoid spindle cell
components of mesonephric carcinosarcomas may originate from the
malignant epithelioid components. Similar to uterine carcinosarcoma
(29), the development of
sarcomatoid spindle cell components in mesonephric carcinosarcomas
may be caused by epithelial-to-mesenchymal transition. In addition,
the co-expression of both cytokeratin and Vim was observed in
malignant epithelial areas of MAs, likely enhancing the progression
of epithelial-to-mesenchymal transition (30). These may explain the lack of
expression of epithelial immunohistochemical markers in spindle
cell components of mesonephric carcinosarcomas and the presentation
of sarcomatoid features on the histology. Therefore, epithelial
immunohistochemical markers and histological features, including
heterologous sarcomatous components and frankly malignant spindle
cell components, are useful for distinguishing between these
entities.
Consistent with previous studies (3), no specific molecular events were found
to differentiate MAs with spindle cell components from those
without spindle cell components. The molecular alterations of MAs
without spindle cell components mainly included KRAS/NRAS
mutations, microsatellite stability and gains of chromosome 1q
(3,7). KRAS/NRAS mutations are the most
common molecular alterations in MAs and are mutually exclusive.
KRAS mutations are more recurrent than NRAS
mutations, and it has been reported that the majority of patients
with MAs (range, 75–100%) harboured KRAS mutations, which
mainly affected the hotspot codons 12 and 13 (3,6,7). The
mutation range of KRAS in cervical adenocarcinoma is
13.9–17.5%, regardless of histological type (31,32).
Among the reported MAs with spindle components, including those in
the present study, 72.7% exhibited KRAS/NRAS mutations, a
rate similar to that of MAs without spindle components and markedly
higher compared with that in cervical adenocarcinomas. Meanwhile,
mesonephric hyperplasia lacks KRAS/NRAS mutations (33). KRAS/NRAS mutations may
contribute to the development of MAs (3,6). Other
molecular changes have also been reported, including chromatin
remodelling of ARID1A/B and SMARCA4 (3).
In the female reproductive system, PIK3CA
mutations are more common in endometrial and other types of
cervical adenocarcinomas arising from the Mullerian ducts (32,34–36).
The PIK3CA mutation rate range is 25–32.2% in cervical
adenocarcinoma, and 52% in HPV-independent cervical cancers
(31,32,37).
In the study by Mirkovic et al (3), PIK3CA mutations were not
identified from 13 cervical MAs. Subsequently, da Silva et
al (6) reported that 2 cervical
patients with MAs harboured simultaneous KRAS and
PIK3CA mutations. To the best of our knowledge, there have
been no previously reported cases of PIK3CA mutations
without KRAS or NRAS mutations in MAs or
mesonephric-like carcinomas and the present study is the first to
describe a case of a cervical MA with a PIK3CA mutation
only, without KRAS or NRAS mutations. Additionally,
β-catenin (CTNNB1) mutations are commonly found in
carcinomas originating from the Mullerian ducts, and a recent study
described an MA with mutations in both CTNNB1 and
KRAS (4). These observations
suggest that there are shared molecular alterations between MAs and
carcinomas that arise from the Mullerian ducts.
The present study demonstrated that MAs with spindle
cell components can harbour KRAS and PIK3CA mutations
independently. Activation of the mitogen-activated protein kinase
(MAPK) pathway leads to abnormal activation of the RAS-MAPK
pathway, promoting cellular proliferation, differentiation and
survival (38). PIK3CA
encodes the p110α catalytic subunit of the class IA PI3Ks (39). Mutations in PIK3CA can result
in abnormally increased catalytic activity of PI3Ks, thus promoting
cell carcinogenesis (39,40). According to a recent study in
HPV-independent cervical cancers, aberrant activation of the
PI3K-AKT pathway due to overexpression of ERBB4 and FGFR1/4 and
deletion of PTEN may drive oncogenesis, affecting cell
proliferation, survival and glycolysis (37). This suggests that the oncogenic
drivers of these tumours may involve the RAS-MAPK and PI3K/AKT
pathways, either individually or in combination. This finding
provides new insights into the pathogenesis of MAs. However, the
present study only included a small number of these tumour
subtypes; and it remains uncertain whether this is a unique
molecular alteration in MAs with spindle components. To validate
the findings of the current study, NGS and whole exome sequencing
on MAs without spindle components, mesonephric carcinosarcomas and
sufficient MAs with spindle components are needed in future
studies, which could enhance the understanding of the molecular
changes in these tumours.
NTRK genes encode tropomyosin receptor
kinases (Trk) (41). Fusion of
NTRK1/2/3 genes is the most common mechanism of Trk
activation in various cancers (41). In malignant melanoma, the
amplification of the NTRK1 gene is associated with poor
clinical outcomes (42).
NTRK1 amplification was detected in both cases of MAs with
FIGO stage IIB that reported in the current study. NTRK1
amplification may promote tumour cell proliferation in MAs leading
to an advanced stage.
All 3 patients included underwent TAHBSO and pelvic
LND. Of these, 2 patients received adjuvant CT with carboplatin and
paclitaxel, and 1 patient also underwent adjuvant radiation therapy
following primary surgery. Currently, there is no standardized
treatment for this rare tumour. Treatments depend on the stage of
MA, and the treatment principles are consistent with other types of
cervical adenocarcinomas (12). The
majority of patients with early-stage MAs underwent TAHBSO, while a
small proportion of patients also received adjuvant radiotherapy
and CT (8). While adjuvant CT with
carboplatin and paclitaxel is commonly used as first-line
treatment, its role in early-stage MAs is currently unclear
(43). Similarly, the efficacy of
adjuvant radiotherapy is also unclear. In the MAs lacking spindle
cell components, patients receiving adjuvant therapy experienced
higher recurrence and mortality rates compared with those who did
not (8). Adjuvant therapy is
reported to improve prognosis in patients with mesonephric
carcinosarcomas that contain spindle cell components, evidenced by
lower recurrence and mortality rates compared with those not
receiving therapy (15). As
aforementioned, MAs with spindle cell components often present at
advanced stages and have concerning recurrence rates. Therefore, it
is hypothesized that adjuvant therapy is crucial for these patients
as it may improve prognosis. However, larger studies are needed to
clarify the clinical effectiveness of adjuvant therapy in MAs with
spindle cell components.
The RAS-MAPK and PI3K-AKT pathways may be targets
for therapy in MAs with spindle cell components. Common KRAS
mutation sites include G12C, G12D and G12V (6,7). The
KRAS G12C inhibitor adagrasib has received approval by the
United States Food and Drug Administration (44). The KRAS G12D inhibitor has
also shown promising efficacy in preclinical studies (45,46).
The PI3Kα inhibitor alpelisib demonstrated therapeutic effects in
cervical solid tumours (47).
Additionally, in animal models, the PI3Kα inhibitor exhibited
improved antitumor effects in HPV-independent cervical cancers
(37). KRAS and
PIK3CA mutations in MAs with prominent spindle cell
components suggest that KRAS inhibitors and PI3K inhibitors
may be used as potential targeted therapies to improve prognosis.
However, KRAS and PI3Kα inhibitors have potential limitations and
challenges as targeted therapies for MAs with spindle cell
components. At present, there is no valid evidence of preclinical
data on the efficacy of KRAS and PI3Kα inhibitors for the treatment
of these rare tumours. In addition, MAs with spindle cell
components are histologically biphasic tumours, and the expression
of immunohistochemical markers, such as GATA3 as aforementioned,
may vary between different components. Thus, it is challenging to
determine the effect of the different histological components, as
well as immunohistochemical expression, on the therapeutic efficacy
of KRAS and PI3Kα inhibitors.
Spindle cell components in MAs have been largely
overlooked in previous studies. The present study found that MAs
with prominent spindle cell components were at an advanced stage
and exhibited unique PIK3CA mutations, which distinguished
them from MAs without such components. Further research is needed
to gather more clinicopathological and molecular data from MAs with
spindle cell components and other mesonephric tumours, to enhance
the understanding of the role of spindle cell components in
biological behaviour and molecular alteration. Prospective studies
with larger cohorts are necessary to validate the targeted
treatment with KRAS and PIK3CA inhibitors. If feasible, whole
genome sequencing could provide a comprehensive genetic analysis of
this rare tumour.
The present study had several limitations. Firstly,
it is retrospective and spindle cell components of MAs may not be
well documented in previous literature, which potentially leads to
selection bias in the literature review and data collection.
Secondly, the small sample size and lack of long-term follow-up
limit the representativeness of the findings regarding prognosis,
and molecular and clinicopathological features. Lastly, due to
limited resources, the targeted NGS panel only assessed a number of
gene mutations.
In conclusion, MAs exhibit morphologic diversity,
with those containing spindle cell components associated with
advanced stages and aggressive behaviour. Prominent spindle cell
components in MAs are diagnostic pitfalls, especially in cervical
biopsy specimens. Using a panel of immunohistochemical stains may
aid in differential diagnosis. Despite KRAS being the most
frequently observed molecular alteration, PIK3CA mutations
may also be identified independently in MAs.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
The present study was supported by the Sichuan Science and
Technology Program (grant no. 2022NSFSC0708).
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author. The NGS data generated in
the present study may be found in the BioProject database under the
accession number PRJNA1132741 or at the following URL: https://www.ncbi.nlm.nih.gov/sra/PRJNA1132741.
Authors' contributions
YF was responsible for the conceptualization, data
curation and writing of the manuscript. YH and LS collected and
analyzed the clinicopathologic data. TL contributed to the
immunohistochemical materials preparation. YS contributed to the
conceptualization, manuscript writing, review and editing,
supervision and funding acquisition of the present study. YF and YS
confirm the authenticity of all the raw data. All authors read and
approved the final manuscript.
Ethics approval and consent to
participate
The institutional ethics committee of West China
Second University Hospital, Sichuan University approved this study
(approval no. 2023125; Chengdu, China). Consent from patients was
obtained to perform further scientific research
(immunohistochemical staining and NGS) using their samples.
Patient consent for publication
Written informed consent was obtained from each
patient for the publication of this article and the accompanying
images.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Sajjad Y: Development of the genital ducts
and external genitalia in the early human embryo. J Obstet Gynaecol
Res. 36:929–937. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Howitt BE and Nucci MR: Mesonephric
proliferations of the female genital tract. Pathology. 50:141–150.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Mirkovic J, Sholl LM, Garcia E, Lindeman
N, MacConaill L, Hirsch M, Dal Cin P, Gorman M, Barletta JA, Nucci
MR, et al: Targeted genomic profiling reveals recurrent KRAS
mutations and gain of chromosome 1q in mesonephric carcinomas of
the female genital tract. Mod Pathol. 28:1504–1514. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Montalvo N, Redroban L and Galarza D:
Mesonephric adenocarcinoma of the cervix: A case report with a
three-year follow-up, lung metastases, and next-generation
sequencing analysis. Diagn Pathol. 14:712019. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Menon S, Kathuria K, Deodhar K and Kerkar
R: Mesonephric adenocarcinoma (endometrioid type) of endocervix
with diffuse mesonephric hyperplasia involving cervical wall and
myometrium: An unusual case report. Indian J Pathol Microbiol.
56:51–53. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
da Silva EM, Fix DJ, Sebastiao APM,
Selenica P, Ferrando L, Kim SH, Stylianou A, Da Cruz Paula A,
Pareja F, Smith ES, et al: Mesonephric and mesonephric-like
carcinomas of the female genital tract: Molecular characterization
including cases with mixed histology and matched metastases. Mod
Pathol. 34:1570–1587. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Mirkovic J, McFarland M, Garcia E, Sholl
LM, Lindeman N, MacConaill L, Dong F, Hirsch M, Nucci MR, Quick CM,
et al: Targeted genomic profiling reveals recurrent kras mutations
in mesonephric-like adenocarcinomas of the female genital tract. Am
J Surg Pathol. 42:227–233. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Dierickx A, Goker M, Braems G, Tummers P
and Van den Broecke R: Mesonephric adenocarcinoma of the cervix:
Case report and literature review. Gynecol Oncol Rep. 17:7–11.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Pors J, Segura S, Chiu DS, Almadani N, Ren
H, Fix DJ, Howitt BE, Kolin D, McCluggage WG, Mirkovic J, et al:
Clinicopathologic characteristics of mesonephric adenocarcinomas
and mesonephric-like adenocarcinomas in the gynecologic tract: A
multi-institutional study. Am J Surg Pathol. 45:498–506. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Bhatla N, Berek JS, Fredes MC, Denny LA,
Grenman S, Karunaratne K, Kehoe ST, Konishi I, Olawaiye AB, Prat J,
et al: Revised FIGO staging for carcinoma of the cervix uteri. Int
J Gynaecol Obstet. 145:129–135. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Klinkhamer PJ, Meerding WJ, Rosier PF and
Hanselaar AG: Liquid-based cervical cytology. Cancer. 99:263–271.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Devarashetty S, Chennapragada SS and
Mansour R: Not your typical adenocarcinoma: A case of mesonephric
adenocarcinoma of the cervix with fibroblast growth factor receptor
2 (FGFR2) mutation. Cureus. 14:e250982022.PubMed/NCBI
|
|
13
|
Jiang LL, Tong DM, Feng ZY and Liu KR:
Mesonephric adenocarcinoma of the uterine cervix with rare lung
metastases: A case report and review of the literature. World J
Clin Cases. 8:1735–1744. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Tseng CE, Chen CH, Chen SJ and Chi CL:
Tumor rupture as an initial manifestation of malignant mesonephric
mixed tumor: A case report and review of the literature. Int J Clin
Exp Pathol. 7:1212–1217. 2014.PubMed/NCBI
|
|
15
|
Ribeiro B, Silva R, Dias R and Patrício V:
Carcinosarcoma of the uterine cervix: A rare pathological finding
originating from mesonephric remnants. BMJ Case Rep.
12:e2270502019. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Fregnani JH, Soares FA, Novik PR, Lopes A
and Latorre MR: Comparison of biological behavior between
early-stage adenocarcinoma and squamous cell carcinoma of the
uterine cervix. Eur J Obstet Gynecol Reprod Biol. 136:215–223.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Jeong JH, Kim NY and Pyo JS: Analysis of
PAX8 immunohistochemistry in lung cancers: A meta-analysis. J
Pathol Transl Med. 54:300–309. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kenny SL, McBride HA, Jamison J and
McCluggage WG: Mesonephric adenocarcinomas of the uterine cervix
and corpus: HPV-negative neoplasms that are commonly PAX8, CA125,
and HMGA2 positive and that may be immunoreactive with TTF1 and
hepatocyte nuclear factor 1-β. Am J Surg Pathol. 36:799–807. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kadota K, Buitrago D, Lee MC,
Villena-Vargas J, Sima CS, Jones DR, Travis WD and Adusumilli PS:
Tumoral CD10 expression correlates with high-grade histology and
increases risk of recurrence in patients with stage I lung
adenocarcinoma. Lung Cancer. 89:329–336. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Howitt BE, Emori MM, Drapkin R, Gaspar C,
Barletta JA, Nucci MR, McCluggage WG, Oliva E and Hirsch MS: GATA3
is a sensitive and specific marker of benign and malignant
mesonephric lesions in the lower female genital tract. Am J Surg
Pathol. 39:1411–1419. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Pors J, Cheng A, Leo JM, Kinloch MA, Gilks
B and Hoang L: A comparison of GATA3, TTF1, CD10, and calretinin in
identifying mesonephric and mesonephric-like carcinomas of the
gynecologic tract. Am J Surg Pathol. 42:1596–1606. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Lee Y, Bae H and Kim HS: Endocervical
adenocarcinoma: Comprehensive histological review and
re-classification of 123 consecutive cases according to the updated
world health organization classification of female genital tumors.
Anticancer Res. 42:4627–4639. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Masjeed NMA, Khandeparkar SGS, Joshi AR,
Kulkarni MM and Pandya N: Immunohistochemical study of ER, PR, Ki67
and p53 in endometrial hyperplasias and endometrial carcinomas. J
Clin Diagn Res. 11:EC31–EC34. 2017.PubMed/NCBI
|
|
24
|
Meguro S, Yasuda M, Shimizu M, Kurosaki A
and Fujiwara K: Mesonephric adenocarcinoma with a sarcomatous
component, a notable subtype of cervical carcinosarcoma: A case
report and review of the literature. Diagn Pathol. 8:742013.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Bague S, Rodriguez IM and Prat J:
Malignant mesonephric tumors of the female genital tract: A
clinicopathologic study of 9 cases. Am J Surg Pathol. 28:601–607.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Silver SA, Devouassoux-Shisheboran M,
Mezzetti TP and Tavassoli FA: Mesonephric adenocarcinomas of the
uterine cervix: A study of 11 cases with immunohistochemical
findings. Am J Surg Pathol. 25:379–387. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Mirkovic J, Olkhov-Mitsel E, Amemiya Y,
Al-Hussaini M, Nofech-Mozes S, Djordjevic B, Kupets R, Seth A and
McCluggage WG: Mesonephric-like adenocarcinoma of the female
genital tract: Novel observations and detailed molecular
characterisation of mixed tumours and mesonephric-like
carcinosarcomas. Histopathology. 82:978–990. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
McCluggage WG: Mesonephric-like
Adenocarcinoma of the female genital tract: From Morphologic
observations to a well-characterized carcinoma with aggressive
clinical behavior. Adv Anat Pathol. 29:208–216. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Matsuzaki S, Klar M, Matsuzaki S, Roman
LD, Sood AK and Matsuo K: Uterine carcinosarcoma: Contemporary
clinical summary, molecular updates, and future research
opportunity. Gynecol Oncol. 160:586–601. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Kuburich NA, den Hollander P, Pietz JT and
Mani SA: Vimentin and cytokeratin: Good alone, bad together. Semin
Cancer Biol. 86:816–826. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Wright AA, Howitt BE, Myers AP, Dahlberg
SE, Palescandolo E, Van Hummelen P, MacConaill LE, Shoni M, Wagle
N, Jones RT, et al: Oncogenic mutations in cervical cancer: Genomic
differences between adenocarcinomas and squamous cell carcinomas of
the cervix. Cancer. 119:3776–3783. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Xi Q, Kage H, Ogawa M, Matsunaga A,
Nishijima A, Sone K, Kawana K and Oda K: Genomic landscape of
endometrial, ovarian, and cervical cancers in Japan from the
database in the center for cancer genomics and advanced
therapeutics. Cancers (Basel). 16:1362023. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Mirkovic J, Schoolmeester JK, Campbell F,
Miron A, Nucci MR and Howitt BE: Cervical mesonephric hyperplasia
lacks KRAS/NRAS mutations. Histopathology. 71:1003–1005. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Cancer Genome Atlas Research Network, .
Kandoth C, Schultz N, Cherniack AD, Akbani R, Liu Y, Shen H,
Robertson AG, Pashtan I, Shen R, et al: Integrated genomic
characterization of endometrial carcinoma. Nature. 497:67–73. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Mjos S, Werner HMJ, Birkeland E, Holst F,
Berg A, Halle MK, Tangen IL, Kusonmano K, Mauland KK, Oyan AM, et
al: PIK3CA exon9 mutations associate with reduced survival, and are
highly concordant between matching primary tumors and metastases in
endometrial cancer. Sci Rep. 7:102402017. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
McIntyre JB, Wu JS, Craighead PS, Phan T,
Köbel M, Lees-Miller SP, Ghatage P, Magliocco AM and Doll CM:
PIK3CA mutational status and overall survival in patients with
cervical cancer treated with radical chemoradiotherapy. Gynecol
Oncol. 128:409–414. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Wang Y, He M, He T, Ouyang X, Shen X, Shi
W, Huang S, Xiang L, Zou D, Jiang W and Yang H: Integrated genomic
and transcriptomic analysis reveals the activation of PI3K
signaling pathway in HPV-independent cervical cancers. Br J Cancer.
130:987–1000. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Olson JM and Hallahan AR: p38 MAP kinase:
A convergence point in cancer therapy. Trends Mol Med. 10:125–129.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Samuels Y, Diaz LA Jr, Schmidt-Kittler O,
Cummins JM, Delong L, Cheong I, Rago C, Huso DL, Lengauer C,
Kinzler KW, et al: Mutant PIK3CA promotes cell growth and invasion
of human cancer cells. Cancer Cell. 7:561–573. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Engelman JA: Targeting PI3K signalling in
cancer: Opportunities, challenges and limitations. Nat Rev Cancer.
9:550–562. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Cocco E, Scaltriti M and Drilon A: NTRK
fusion-positive cancers and TRK inhibitor therapy. Nat Rev Clin
Oncol. 15:731–747. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Pasini L, Re A, Tebaldi T, Ricci G, Boi S,
Adami V, Barbareschi M and Quattrone A: TrkA is amplified in
malignant melanoma patients and induces an anti-proliferative
response in cell lines. BMC Cancer. 15:7772015. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Xie C, Chen Q and Shen Y: Mesonephric
adenocarcinomas in female genital tract: A case series. Medicine
(Baltimore). 100:e271742021. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Janne PA, Riely GJ, Gadgeel SM, Heist RS,
Ou SI, Pacheco JM, Johnson ML, Sabari JK, Leventakos K, Yau E, et
al: Adagrasib in non-small-cell lung cancer harboring a
KRASG12C mutation. N Engl J Med. 387:120–131. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Wang X, Allen S, Blake JF, Bowcut V,
Briere DM, Calinisan A, Dahlke JR, Fell JB, Fischer JP, Gunn RJ, et
al: Identification of MRTX1133, a noncovalent, potent, and
selective KRASG12D inhibitor. J Med Chem. 65:3123–3133.
2022. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Hallin J, Bowcut V, Calinisan A, Briere
DM, Hargis L, Engstrom LD, Laguer J, Medwid J, Vanderpool D, Lifset
E, et al: Anti-tumor efficacy of a potent and selective
non-covalent KRASG12D inhibitor. Nat Med. 28:2171–2182.
2022. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Juric D, Rodon J, Tabernero J, Janku F,
Burris HA, Schellens JHM, Middleton MR, Berlin J, Schuler M,
Gil-Martin M, et al: Phosphatidylinositol 3-kinase α-selective
inhibition with alpelisib (BYL719) in PIK3CA-altered solid tumors:
Results from the first-in-human study. J Clin Oncol. 36:1291–1299.
2018. View Article : Google Scholar : PubMed/NCBI
|