Introduction
Breast cancer is recognized for its considerable
heterogeneity and is influenced by genetic, environmental,
age-related, lifestyle and dietary factors. As reported by global
cancer data analysis in 2022, cancer of the breast ranks fourth
globally in terms of cancer-related deaths. Furthermore, it has the
highest incidence and fatality rates (23.8 and 15.4%, respectively)
among women (1). American cancer
statistics for 2024 identified breast carcinoma as the most
prevalent malignant tumor among American females and the second
leading cause of cancer mortality, trailing only lung cancer
(2). For patients eligible for
adjuvant chemotherapy, regimens based on taxane drugs are
considered among the optimal treatments (3).
Paclitaxel (PTX), a plant alkaloid chemotherapeutic
agent, is widely used to treat several cancers, including breast
cancer. It inhibits tumor growth primarily by disrupting the
mitotic activity of cancer cells. This action is mediated by its
binding to tubulin, which impedes the dynamic stability of
microtubules, leading to abnormal aggregation and disassembly
during mitosis and ultimately causing the apoptosis of tumor cells
(4). Owing to its limited
solubility in water, solvent-based (sb)-PTX requires the use of
solvents for administration. In contrast, nanoparticle
albumin-bound (nab)-PTX represents an innovative formulation that
encapsulates the drug in albumin, increasing its solubility and
stability. This formulation permits shorter infusion times (20 min
compared with 120 min for the sb-PTX form), does not require
premedication or specialized infusion equipment, and reduces the
risk of toxicity and allergic reactions (5).
Previous evidence indicates that nab-PTX is markedly
more effective than its sb-PTX counterpart (6). A major international phase III trial
reported that ABI-007, a novel nab-PTX nanoparticle, had notable
effectiveness compared with traditional Taxol in managing advanced
breast cancers, offering reduced toxicity (7). Moreover, a meta-analysis further
corroborated that nab-PTX markedly extends OS compared with sb-PTX
taxanes in patients with metastatic breast carcinoma and improves
overall response and disease control rates, with toxicity and
discontinuation rates similar to those of traditional formulations
(8). For elderly patients with
advanced mammary breast cancer, weekly administration of nab-PTX
has been reported to be safer and more productive than the
triweekly sb-PTX regimen (9). With
promising efficacy and excellent tolerability, nab-PTX is now
approved in the USA for the management of breast cancer in
individuals for whom combination treatment for advanced cancer has
failed or for those who have relapsed within 6 months of adjuvant
therapy (10). Furthermore, the
GeparSepto-GBG69 phase III trial reported that, compared with
sb-PTX, nab-PTX notably increased the number of patients who
achieved a pathological complete response following anthracycline
therapy in neoadjuvant treatments for initial-stage breast
carcinoma (11). In addition, the
2024 Chinese Society of Clinical Oncology Breast Cancer Treatment
Guidelines supported nab-PTX as an initial-line neoadjuvant
chemotherapy treatment and highlighted its potential benefits for
certain patients with metastatic mammary cancer after progression
following first-line taxane treatments (12).
Although the advantages of nab-PTX over sb-PTX for
the neoadjuvant treatment of breast cancer and the treatment of
patients with advanced breast cancer have been confirmed, the
evidence for the use of nab-PTX in postoperative adjunctive
chemotherapy remains limited due to the long-term nature of the
clinical results, such as disease-free survival (DFS) and overall
survival (OS) rates. Considering the convenience and manageable
adverse effects of nab-PTX, there are questions as to whether
nab-PTX could be more widely used. Consequently, the present study
aimed to analyze the efficacy and adverse reactions of nab-PTX
compared with sb-PTX in postoperative adjuvant chemotherapy for
patients with mammary cancer, and to identify predictors of risk
for postoperative recurrence and metastasis.
Materials and methods
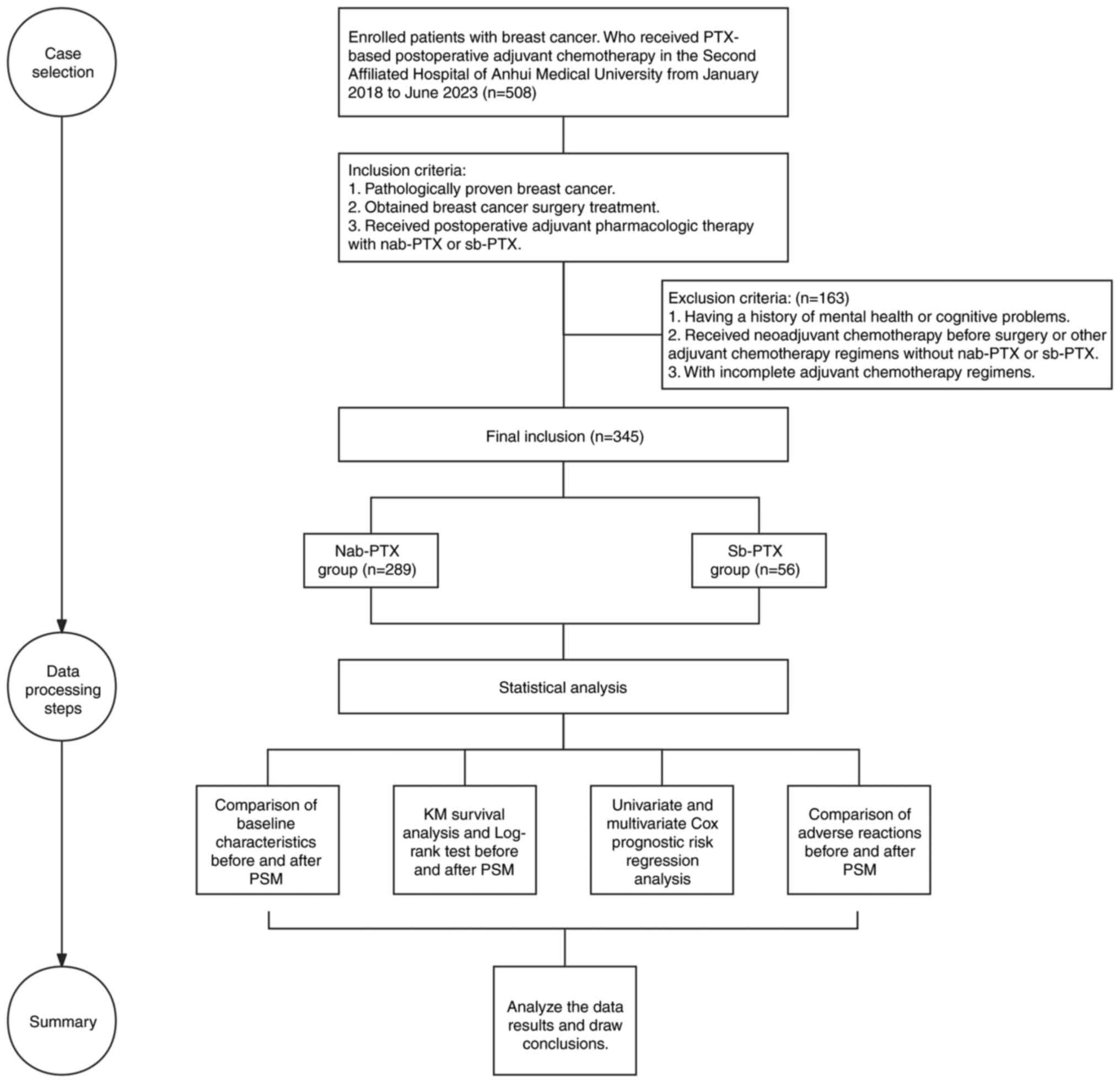
Ethical approval and study flow
chart
The present single-center retrospective study
followed the guidelines of the Declaration of Helsinki, and the
Ethics Committee of the Second Affiliated Hospital of Anhui Medical
University (Hefei, China) approved the study [approval no.
YX2023-203(F1)]. Fig. 1 presents a
flow chart of the study design.
Inclusion and exclusion criteria
The inclusion criteria for the present study were as
follows: i) Pathologically-confirmed breast cancer; ii) surgical
treatment for breast carcinoma; and iii) postoperative adjuvant
chemical treatment with either nab-PTX or sb-PTX. The exclusion
criteria were as follows: i) Previous instances of psychiatric or
cognitive issues; ii) neoadjuvant chemotherapy prior to surgery or
other adjuvant chemotherapy regimens without nab-PTX or sb-PTX; and
iii) incomplete adjuvant chemotherapy regimens.
Patient selection and grouping
The patient hospital records were first accessed in
February 2024 for the present study. The retrospective analysis of
cohorts included individuals who completed surgical treatment for
breast cancer and were administered taxane-based adjuvant
chemotherapy between January 2018 and June 2023 at the Second
Affiliated Hospital of Anhui Medical University. The collected
clinical and pathological data of patients included age, underlying
disease, expression levels of immunohistochemical markers [hormone
receptor, human epidermal growth factor receptor (HER)-2 and
Ki-67], breast and axillary operative treatment, tumor volume,
lymph gland condition, blood vessel invasion, World Health
Organization (WHO) tumor grade (13) and postoperative pathology.
Information was also collected on patient outcomes, specifically
recurrence or metastasis, alongside adverse reactions to
chemotherapy, including hematologic toxicity, allergic reactions,
musculoskeletal pain and neuropathy, until February 2024. The
participants were divided into two groups according to the type of
PTX used: Nab-PTX and sb-PTX.
Treatment regimen and follow-up
Under the National Comprehensive Cancer Network
guidelines, patients with postoperative mammary cancer should
receive a PTX-based adjuvant regimen, including the following
regiments: docetaxel and cyclophosphamide; doxorubicin and
cyclophosphamide followed by docetaxel; epirubicin and
cyclophosphamide (EC); and PTX with carboplatin. When medically
indicated, nab-PTX should be substituted for docetaxel or standard
PTX, with a weekly dose not exceeding 125 mg/m2.
Postoperative radiation should be applied to high-risk breast
tissues. Furthermore, hormone receptor-positive and HER-2-positive
individuals should receive corresponding endocrine and targeted
treatments (14). Hormone receptor
positivity is defined as ≥1% expression according to the American
Society of Clinical Oncology/College of American Pathologists
guidelines (15). Initial
assessment of tumor HER-2 status is assigned as HER2-positive when
scored as 3+ using immunohistochemistry or amplified by
fluorescence in situ hybridization (16). Post-treatment follow-up should
consist of a periodic history/physical examination every 4–6 months
for the initial 5 years after the first treatment and annually
thereafter. Mammography should be performed yearly (14). DFS is the primary endpoint.
Laboratory and imaging tests are used to screen for the recurrence
or metastasis of breast cancer. The Common Terminology Criteria for
Adverse Events, version 5.0 from the National Cancer Institute
(17), was used to evaluate and
classify adverse reactions.
Statistical analysis
R V4.3.3 (The R Foundation) and SPSS V26.0 (IBM
Corp.) were used for data analysis. The Mann-Whitney U test was
used for the analysis of ordinal data, and the Pearson
χ2 test or Fisher's exact test were used for the
assessment of other categorical variables. A propensity score
matching (PSM) method based on logistic regression was used to
adjust for confounding factors. A minimum sample size estimation
after PSM was performed using Pass 2021 (v.21.0.3; NCSS, LLC) based
on the non-inferiority study Cox risk regression analysis module.
The non-inferiority margin was set at 1.55 as assessed by
professional clinicians. A non-inferiority test with an overall
sample size of 108 subjects [of which 54 were in the control group
(sb-PTX) and 54 were in the treatment group (nab-PTX)] achieved 80%
power at a 0.05 significance level when the N1/N2 ratio was set to
1:1. Kaplan-Meier curves were used to analyze survival, and the
log-rank test was used to compare survival rates. Cox regression
analyses revealed the predictors. P<0.05 was considered to
indicate a statistically significant difference.
Results
Baseline characteristics before and
after PSM
The present retrospective study included 345
individuals (all female) with breast cancer at the Second
Affiliated Hospital of Anhui Medical University who received
postoperative taxane-based adjuvant chemotherapy between January
2018 and June 2023. In the nab-PTX group, 23.2% (67/289) were ≤45
years of age, and 76.8% (222/289) were >45 years of age. In the
sb-PTX group, 37.5% (21/56) were aged ≤45 years, whereas 62.5%
(35/56) were aged >45 years, with a statistically significant
age disparity (P=0.024). The presence of underlying disease was
noted in 20.1% (58/289) of the nab-PTX group compared with 8.9%
(5/56) of the sb-PTX group, with a significant difference between
the two groups (P=0.048). Lymph node involvement was demonstrated
in 41.2% (119/289) of the nab-PTX group and 91.1% (51/56) of the
sb-PTX group, and the difference was significant (P<0.001). For
axillary surgery, 45.7% (132/289) of patients in the nab-PTX group
underwent a sentinel lymph node biopsy only, whereas 7.1% (4/56) of
patients in the sb-PTX group underwent a sentinel lymph node biopsy
only. Furthermore, an axillary lymph node dissection occurred in
54.3% (157/289) of patients in the nab-PTX group, compared with
92.9% (52/56) of patients in the sb-PTX group. These differences
were significant (P<0.001). Immunohistochemical analysis
revealed that 30.8% (89/289) of the nab-PTX patients were HER-2
positive, whereas 14.3% (8/56) of the sb-PTX patients were HER-2
positive, with significant differences between the two groups
(P=0.012). No notable differences were demonstrated for hormone
receptor or Ki-67 expression, postoperative pathology, tumor size
or WHO tumor grade (all P>0.05). Moreover, PSM at a 1:2 ratio
was performed to adjust for age, underlying disease, tumor size,
lymph node status, vascular invasion, WHO tumor grading, surgical
methods, hormone receptor, HER-2 and Ki-67 levels, axillary surgery
and postoperative pathology. Following matching, all the baseline
characteristics revealed no significant differences between the two
groups (all P>0.05). Table I
presents information about the baseline characteristics.
 | Table I.Baseline information of the
participants before and after propensity score matching. |
Table I.
Baseline information of the
participants before and after propensity score matching.
|
| Before PSM | After PSM |
|---|
|
|
|
|
|---|
| Baseline
characteristic | Nab-PTX (n=289) | Sb-PTX (n=56) | P-value | Nab-PTX (n=90) | Sb-PTX (n=56) | P-value |
|---|
| Age |
|
|
|
|
|
|
| ≤45
years | 67 (23.2) | 21 (37.5) | 0.024 | 30 (33.3) | 21 (37.5) | 0.608 |
| >45
years | 222 (76.8) | 35 (62.5) |
| 60 (66.7) | 35 (62.5) |
|
| Underlying
disease |
|
|
|
|
|
|
| No | 231 (79.9) | 51 (91.1) | 0.048 | 79 (87.8) | 51 (91.1) | 0.536 |
| Yes | 58 (20.1) | 5 (8.9) |
| 11 (12.2) | 5 (8.9) |
|
| Tumor size |
|
|
|
|
|
|
| ≤2
cm | 112 (38.8) | 14 (25.0) | 0.050 | 25 (27.8) | 14 (25.0) | 0.712 |
| >2
cm | 177 (61.2) | 42 (75.0) |
| 65 (72.2) | 42 (75.0) |
|
| Lymph node
involvement |
|
|
|
|
|
|
|
Negative | 170 (58.8) | 5 (8.9) | <0.001 | 11 (12.2) | 5 (8.9) | 0.536 |
|
Positive | 119 (41.2) | 51 (91.1) |
| 79 (87.8) | 51 (91.1) |
|
| Vascular
invasion |
|
|
|
|
|
|
| No | 158 (54.7) | 22 (39.3) | 0.035 | 34 (37.8) | 22 (39.3) | 0.855 |
|
Yes | 131 (45.3) | 34 (60.7) |
| 56 (62.2) | 34 (60.7) |
|
| WHO grade |
|
|
|
|
|
|
| I | 6 (2.1) | 2 (3.6) | 0.770 | 1 (1.1) | 2 (3.6) | 0.703 |
| II | 205 (70.9) | 40 (71.4) |
| 66 (73.3) | 40 (71.4) |
|
|
III | 78 (27.0) | 14 (25.0) |
| 23 (25.6) | 14 (25.0) |
|
| Hormone
receptor |
|
|
|
|
|
|
|
Negative | 69 (23.9) | 11 (19.6) | 0.492 | 16 (17.8) | 11 (19.6) | 0.778 |
|
Positive | 220 (76.1) | 45 (80.4) |
| 74 (82.2) | 45 (80.4) |
|
| HER-2 |
|
|
|
|
|
|
|
Negative | 200 (69.2) | 48 (85.7) | 0.012 | 71 (78.9) | 48 (85.7) | 0.302 |
|
Positive | 89 (30.8) | 8 (14.3) |
| 19 (21.1) | 8 (14.3) |
|
| Ki-67 |
|
|
|
|
|
|
|
<14% | 22 (7.6) | 3 (5.4) | 0.779 | 4 (4.4) | 3 (5.4) | 1.000 |
|
≥14% | 267 (92.4) | 53 (94.6) |
| 86 (95.6) | 53 (94.6) |
|
| Breast surgery |
|
|
|
|
|
|
|
Mastectomy | 254 (87.9) | 47 (83.9) | 0.416 | 78 (86.7) | 47 (83.9) | 0.647 |
|
Lumpectomy | 35 (12.1) | 9 (16.1) |
| 12 (13.3) | 9 (16.1) |
|
| Axillary
surgery |
|
|
|
|
|
|
| SLNB
only | 132 (45.7) | 4 (7.1) | <0.001 | 10 (11.1) | 4 (7.1) | 0.428 |
|
ALND | 157 (54.3) | 52 (92.9) |
| 80 (88.9) | 52 (92.9) |
|
| Pathology |
|
|
|
|
|
|
|
Infiltrating | 278 (96.2) | 56 (100.0) | 0.223 | 89 (98.9) | 56 (100.0) | 1.000 |
|
Non-infiltrating | 11 (3.8) | 0 (0.0) |
| 1 (1.1) | 0 (0.0) |
|
DFS before and after PSM
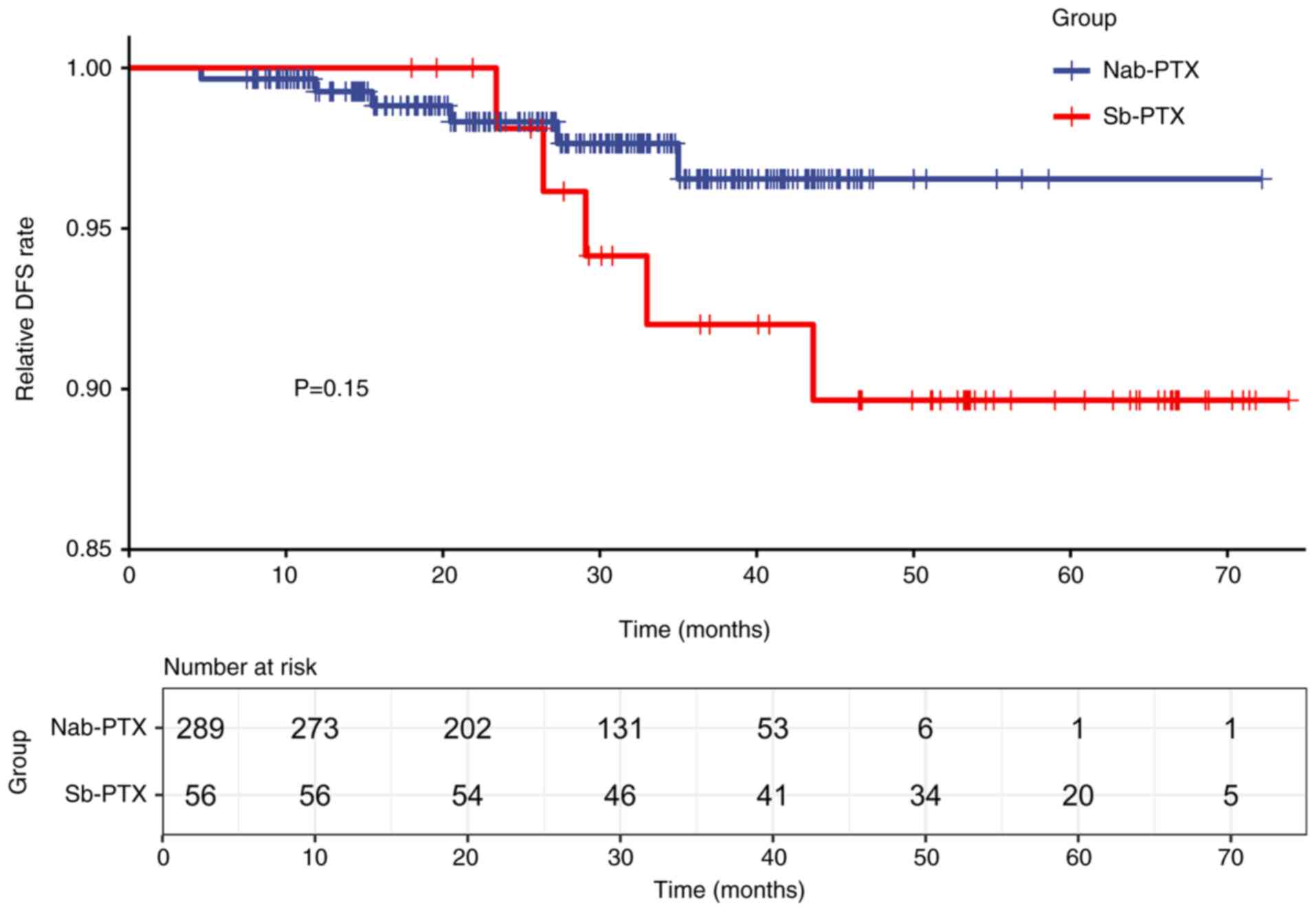
As of January 2024, after a median follow-up of 30.4
months, 6/289 patients in the nab-PTX group experienced recurrence
or metastasis, compared with 5/56 in the sb-PTX group. Therefore,
the recurrence rates of breast cancer in the sb-PTX and nab-PTX
groups before PSM were 9 and 2%, respectively. Kaplan-Meier
survival analysis revealed that the 73-month DFS rate was 91.1% for
the sb-PTX cohort and 97.9% for the nab-PTX cohort, demonstrating
no statistically significant differences in DFS rates between the
two groups (log-rank test, P=0.15; Fig.
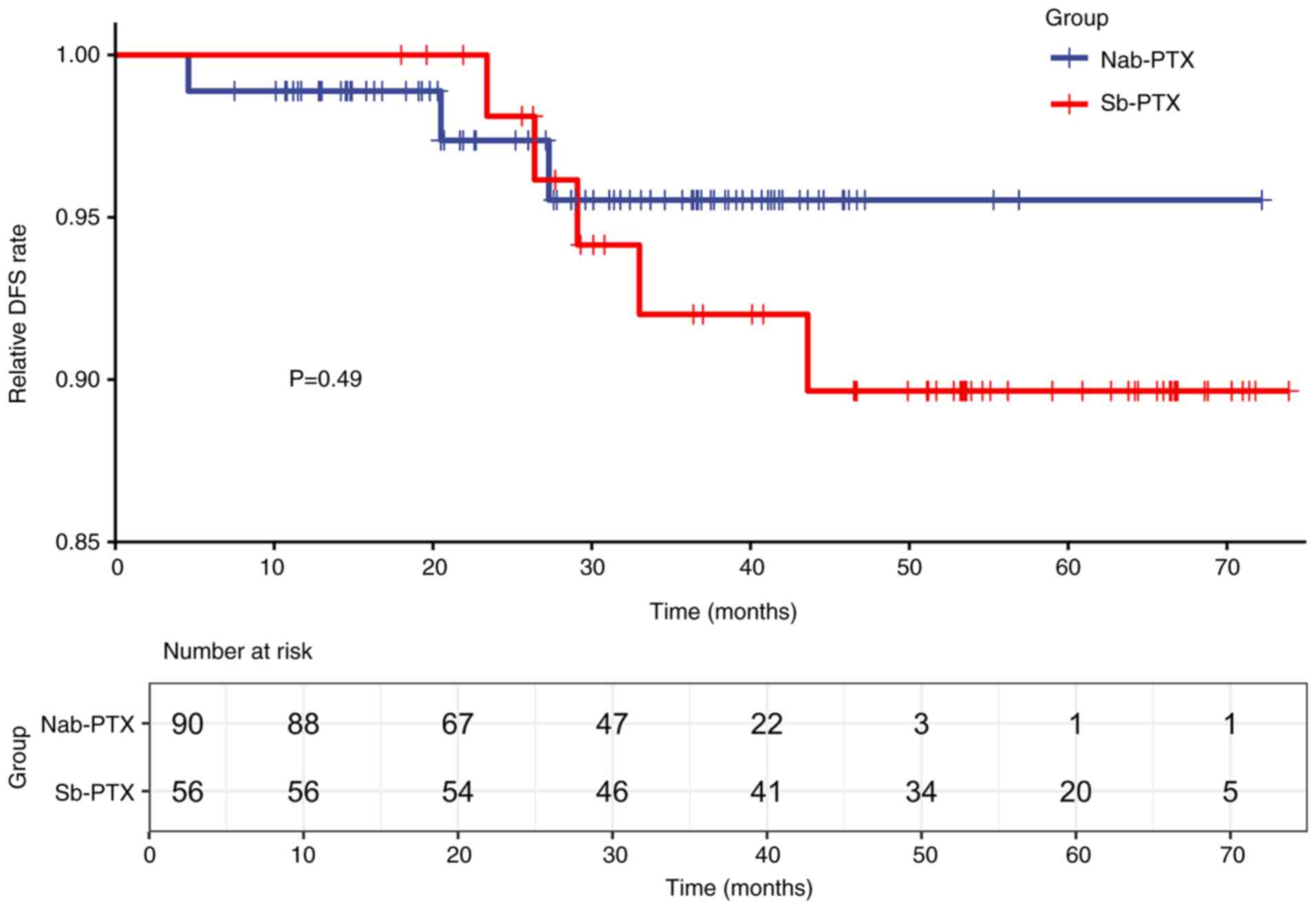
2). After PSM, 3/90 patients in the nab-PTX group experienced
recurrence, compared with 5/56 patients in the sb-PTX group.
Kaplan-Meier survival analysis revealed no significant differences
in DFS between the two cohorts (log-rank test, P=0.49; Fig. 3).
Predictor of postsurgical recurrence
for patients with breast cancer
In all patients with breast cancer treated with
sb-PTX and nab-PTX in postoperative adjuvant chemotherapy in the
present study, univariate and multivariate Cox regression analyses
revealed that underlying disease, lymph node metastasis and an age
of ≤45 years were significantly associated with increased risks of
postsurgical recurrence for patients with breast cancer. Notably,
patients aged >45 years had a significantly lower recurrence
risk than those aged ≤45 years [hazard ratio (HR), 0.197; 95%
confidence interval (CI), 0.052–0.753; P=0.018]. Patients with
underlying disease had a significantly greater risk of recurrence
than did those without underlying disease (HR, 5.352; 95% CI,
1.310–21.854; P=0.019). Moreover, lymph node involvement
significantly increased the risk of recurrence (HR, 8.930; 95% CI,
1.121–71.161; P=0.039). Other subgroup analyses revealed no
statistically significant differences between groups (all
P>0.05). In addition, there was a notable trend toward a lower
postoperative risk of breast cancer in the nab-PTX group compared
with the sb-PTX group, but no statistically significant difference
was observed (HR, 0.419; 95% CI, 0.124–1.418; P=0.162). Table II details the results of the Cox
regression analyses.
 | Table II.Cox analysis of postsurgical
recurrence in all patients. |
Table II.
Cox analysis of postsurgical
recurrence in all patients.
|
| Univariate
analysis | Multivariate
analysis |
|---|
|
|
|
|
|---|
| Variable | HR (95% CI) | P-value | HR (95% CI) | P-value |
|---|
| Group |
|
|
|
|
|
Sb-PTXa |
|
|
|
|
|
Nab-PTX | 0.419
(0.124–1.418) | 0.162 |
|
|
| Age |
|
|
|
|
| ≤45
yearsa |
|
|
|
|
| >45
years | 0.314
(0.096–1.028) | 0.056 | 0.197
(0.052–0.753) | 0.018 |
| Underlying
disease |
|
|
|
|
|
Noa |
|
|
|
|
|
Yes | 2.462
(0.717–8.450) | 0.152 | 5.352
(1.310–21.854) | 0.019 |
| Tumor size |
|
|
|
|
| ≤2
cma |
|
|
|
|
| >2
cm | 2.213
(0.477–10.260) | 0.310 |
|
|
| Lymph node
involvement |
|
|
|
|
|
Negativea |
|
|
|
|
|
Positive | 7.682
(0.979–60.287) | 0.052 | 8.930
(1.121–71.161) | 0.039 |
| Vascular
invasion |
|
|
|
|
|
Noa |
|
|
|
|
|
Yes | 2.954
(0.783–11.141) | 0.110 |
|
|
| WHO grade |
|
|
|
|
|
Ia |
|
|
|
|
| II | 8300.010
(0.000->1000.000) | 0.959 |
|
|
|
III | 8816.518
(0.000->1000.000) | 0.959 |
|
|
| Hormone
receptor |
|
|
|
|
|
Negativea |
|
|
|
|
|
Positive |
2.663(0.341–20.814) | 0.350 |
|
|
| HER-2 |
|
|
|
|
|
Negativea |
|
|
|
|
|
Positive | 0.992
(0.263–3.741) | 0.991 |
|
|
| Ki-67 |
|
|
|
|
|
<14%a |
|
|
|
|
|
≥14% | 22.599
(0.001->1000.000) | 0.542 |
|
|
| Breast surgery |
|
|
|
|
|
Mastectomya |
|
|
|
|
|
Lumpectomy | 1.688
(0.364–7.824) | 0.503 |
|
|
| Axillary
surgery |
|
|
|
|
| SLNB
onlya |
|
|
|
|
|
ALND | 4.718
(0.601–37.054) | 0.140 |
|
|
| Pathology |
|
|
|
|
|
Infiltratinga |
|
|
|
|
|
Non-infiltrating | 0.048
(0.000->1000.000) | 0.759 |
|
|
Adverse events before and after
PSM
Table III presents
the adverse effects associated with both cohorts before and after
PSM. The safety evaluation of hematologic toxicity revealed no
significant differences in the incidence of Grade I–II leukopenia
(32.5% vs. 35.7%) or Grade III–IV leukopenia (3.1% vs. 1.8%)
between the nab-PTX and the sb-PTX groups (P=0.851). Similarly,
there was no significant difference in the rate of neutropenia
between the two groups (P=0.193). However, significant differences
were demonstrated in the prevalence of anemia: 77.9% of the nab-PTX
group experienced grade I–II anemia, compared with 66.1% of the
sb-PTX group, and grade III–IV anemia was also more common in the
nab-PTX cohort (4.5% vs. 1.8%; P=0.011). The incidence of
thrombocytopenia did not differ significantly between the groups
(P=0.183). Furthermore, for nonhematologic adverse events, no Grade
III–IV events were present for anaphylaxis, musculoskeletal pain or
peripheral neuropathy. Notably, allergic reactions occurred in 3.6%
of the sb-PTX group but there were no cases in the nab-PTX group
(P=0.026). Peripheral neuropathy was significantly more prevalent
in the nab-PTX cohort than in the sb-PTX cohort (77.5% vs. 57.1%;
P=0.001). However, musculoskeletal pain was not significantly
different between the two groups (P=0.328). After PSM, analysis of
hematologic toxicity revealed significant differences in the
incidence of anemia between the groups (grades I–II: 76.7% in the
nab-PTX group vs. 66.1% in the sb-PTX group; grades III–IV: 5.6%
vs. 1.8%, respectively; P=0.030). Additionally, the proportion of
patients with peripheral neuropathy was significantly greater in
the nab-PTX group than the sb-PTX group (74.4% vs. 57.1%; P=0.030).
However, no significant differences in the incidences of
leukopenia, neutropenia, thrombocytopenia, allergic reactions or
musculoskeletal pain were observed after matching (all
P>0.05).
 | Table III.Adverse events associated with
nanoparticle albumin-bound paclitaxel and solvent-based paclitaxel
before and after propensity score matching. |
Table III.
Adverse events associated with
nanoparticle albumin-bound paclitaxel and solvent-based paclitaxel
before and after propensity score matching.
|
| Before PSM | After PSM |
|---|
|
|
|
|
|---|
| Adverse event | Nab-PTX
(n=289) | Sb-PTX (n=56) | P-value | Nab-PTX (n=90) | Sb-PTX (n=56) | P-value |
|---|
| Leukopenia |
|
|
|
|
|
|
| No | 186 (64.4) | 35 (62.5) | 0.851 | 62 (68.9) | 35 (62.5) | 0.483 |
|
I–II | 94 (32.5) | 20 (35.7) |
| 25 (27.8) | 20 (35.7) |
|
|
III–IV | 9 (3.1) | 1 (1.8) |
| 3 (3.3) | 1 (1.8) |
|
| Neutropenia |
|
|
|
|
|
|
| No | 191 (66.1) | 42 (75.0) | 0.193 | 64 (71.1) | 42 (75.0) | 0.556 |
|
I–II | 83 (28.7) | 12 (21.4) |
| 20 (22.2) | 12 (21.4) |
|
|
III–IV | 15 (5.2) | 2 (3.6) |
| 6 (6.7) | 2 (3.6) |
|
| Anemia |
|
|
|
|
|
|
| No | 51 (17.6) | 18 (32.1) | 0.011 | 16 (17.8) | 18 (32.1) | 0.030 |
|
I–II | 225 (77.9) | 37 (66.1) |
| 69 (76.7) | 37 (66.1) |
|
|
III–IV | 13 (4.5) | 1 (1.8) |
| 5 (5.6) | 1 (1.8) |
|
|
Thrombocytopenia |
|
|
|
|
|
|
| No | 222 (76.8) | 38 (67.9) | 0.183 | 73 (81.1) | 38 (67.9) | 0.077 |
|
I–II | 62 (21.5) | 18 (32.1) |
| 16 (17.8) | 18 (32.1) |
|
|
III–IV | 5 (1.7) | 0 (0.0) |
| 1 (1.1) | 0 (0.0) |
|
| Anaphylaxis |
|
|
|
|
|
|
| No | 289 (100.0) | 54 (96.4) | 0.026 | 90 (100.0) | 54 (96.4) | 0.145 |
|
Yes | 0 (0.0) | 2 (3.6) |
| 0 (0.0) | 2 (3.6) |
|
| Musculoskeletal
pain |
|
|
|
|
|
|
| No | 165 (57.1) | 28 (50.0) | 0.328 | 42 (46.7) | 28 (50.0) | 0.695 |
|
Yes | 124 (42.9) | 28 (50.0) |
| 48 (53.3) | 28 (50.0) |
|
| Peripheral
neuropathy |
|
|
|
|
|
|
| No | 65 (22.5) | 24 (42.9) | 0.001 | 23 (25.6) | 24 (42.9) | 0.030 |
|
Yes | 224 (77.5) | 32 (57.1) |
| 67 (74.4) | 32 (57.1) |
|
Discussion
Breast cancer is a significant oncological concern
within gynecology and has consistently emerged as the most common
cancer among women (5). Previous
data revealed that ~36% of women initially diagnosed with breast
malignancy presented with local or distant metastatic disease, yet
89.9% achieved a survival rate of ≥5 years post-diagnosis (18). For the majority of patients,
adjuvant chemotherapy is advised to improve their prognosis, except
for those with stage I or II hormone
receptor-positive/HER-2-negative cancers (19). In the present single-center cohort
study, 345 patients with postoperative breast cancer underwent
taxane-based chemotherapy; 289 received nab-PTX and 56 received
sb-PTX. Statistical analyses identified an age of ≤45 years,
underlying disease and lymph node positivity as predictors of
postoperative recurrence and revealed that a superiority or
inferiority of nab-PTX efficacy to sb-PTX was not found based on
the results of survival analysis. Compared with the sb-PTX group,
the nab-PTX group presented an increased incidence of anemia and
peripheral nerve damage; however, the nab-PTX group presented an
advantage in terms of allergic reactions. These statistical results
provide theoretical evidence for the use of nab-PTX in
postoperative adjuvant chemotherapy for breast cancer and emphasize
the importance of further research.
According to the Kaplan-Meier survival analysis, the
DFS rates at 73 months were 91.1% for the sb-PTX group and 97.9%
for the nab-PTX group. Although the sb-PTX cohort had lower DFS
rates than the nab-PTX cohort, there were no significant
differences in DFS. A study of nab-PTX and cyclophosphamide
combined with trastuzumab in patients with initial-stage breast
carcinoma reported that the combination of nab-PTX and
cyclophosphamide, regardless of the addition of trastuzumab, was
feasible and well accepted (20).
Additionally, a comprehensive study comparing nab-PTX and
conventional PTX across all stages of mammary cancer reported no
major variations in short-term or long-term efficacy across the
formulations (18,21). A study on adjuvant therapy for
high-risk initial-stage breast carcinoma reported that combining
nab-PTX with continuous anthracycline and cyclophosphamide
chemotherapy provide marked benefits, with 2- and 6-year DFS rates
of 93 and 82%, respectively (22).
Furthermore, the ICE II-GBG 52 trial reported that non-frail
elderly adults aged ≥65 years with early moderate-to high-risk
breast cancer could benefit from comprehensive taxane-based
chemotherapy. After ~23 months of follow-up, no notable difference
in OS was detected between individuals receiving nab-PTX and those
receiving EC or cyclophosphamide, methotrexate and fluorouracil
regimens (23). However, the
current research on the efficacy of nab-PTX for postoperative
adjuvant therapy in breast carcinoma is restricted by a lack of
robust evidence owing to the relatively limited number of clinical
cases and the potential for outdated treatment regimens. These
limitations highlight the necessity for further studies.
The analysis in the present study revealed that
among patients with breast cancer treated with sb-PTX and nab-PTX
in postoperative adjuvant chemotherapy, patients aged ≤45 years
with underlying disease or positive lymph nodes, are at greater
risk of postoperative recurring mammary cancer, which is in
agreement with the findings of previous studies. Research has
indicated that patients diagnosed before the age of 40 years, those
with estrogen receptor-positive tumors, those who are undergoing
breast-conserving surgery, those with ≥4 positive lymphedema cases,
and those with primary lesions ≥20 mm are more likely to experience
late recurrence (24). Furthermore,
a history of diabetes in women with breast cancer is associated
with worse outcomes (25,26), potentially due to dysregulation of
the mTOR/AKT signaling pathway, which fosters tumor development
under diabetic conditions (27).
Moreover, chronic conditions, including hypertension, diabetes and
dyslipidemia, adversely affect the treatment outcomes, prognosis
and survival in patients with cancer, particularly in patients with
diabetes with mammary cancer, increasing the chance of recurrence
and mortality (28).
The present study performed a systematic evaluation
of adverse reactions in two treatment groups, specifically
hematologic toxicity, allergic reactions, musculoskeletal pain and
peripheral neuropathy. The findings revealed that the nab-PTX group
had considerably greater rates of anemia and peripheral neuropathy
than the sb-PTX group, but it had fewer allergic reactions. These
differences reached statistical significance. A systematic review
of adverse events in solid organ tumors reported that nab-PTX is
related to a higher frequency of grade 3/4 anemia and a lower
incidence of anaphylaxis than its sb-PTX counterparts (29). In comparative studies of individuals
with several stages of breast carcinoma, nab-PTX was reported to be
associated with higher rates of fatigue, nausea, vomiting and
peripheral sensory neuropathy than conventional PTX formulations
(18,21). The most common severe hematologic
and nonhematologic side effects associated with nab-PTX have been
reported to be neutropenia and peripheral neuropathy (30). Furthermore, in the previous study,
post-neoadjuvant therapy based on the efficacy and safety analyses
of paclitaxel drugs revealed that the liposomal paclitaxel group
had a lower rate of severe leukopenia than the sb-PTX group did
(31). Nevertheless, no significant
differences in neutropenia-related adverse reactions were
demonstrated between the groups in the present study, potentially
due to the widespread use of prophylactic leukocyte-boosting
medications before the initiation of adjuvant chemotherapy.
The present study has several limitations. As a
single-center study, its findings may not adequately represent
broader demographics. Furthermore, despite spanning 73 months, the
widespread use of nab-PTX, owing to its tolerability and physician
prescribing preferences, resulted in a smaller sample size for the
sb-PTX group. Analysis of previous studies also found that lymph
node infiltration and underlying disease in patients with breast
cancer increased the risk of postoperative recurrence (32,33)
and the number of infiltrating lymph nodes is used as an adjuvant
therapeutic reference in breast cancer treatment guidelines
(14). The Cox multifactorial
regression analysis in the present study revealed that the 95% CI
of the HR for lymph node positivity and underlying disease factors
was wide. This may be caused by an insufficient sample size. During
the Cox regression analysis, a one-factor regression analysis
screening was performed before a multifactor regression analysis,
and the goodness of fit of the regression model fit showed that the
model modeling was successful. It was therefore concluded that
lymph node infiltration and underlying disease are risk factors for
postoperative recurrence in patients with breast cancer.
Additionally, the limited overall sample size restricted the
feasibility of performing a subgroup analysis of positive lymph
node counts. Finally, discrepancies were observed in the data
analysis results before and after PSM, likely due to the
quasi-experimental design of PSM, which is intended to minimize
selection bias by controlling for known confounding factors. The
matching process itself may alter the data distribution and
variability. In addition, the need to incorporate many matching
variables and exclude unmatched data during PSM typically results
in a reduced sample number, which may reduce the statistical power
of the analysis and increase the uncertainty of the results.
Despite its effectiveness in mitigating selection bias, PSM cannot
eliminate all confounding variables due to its nonrandomized
nature. Therefore, although PSM helps reduce selection bias, the
resulting statistical analysis still has inherent limitations.
In conclusion, the 73-month DFS rate was lower in
the sb-PTX cohort than in the nab-PTX cohort in patients receiving
adjuvant treatments for postoperative breast carcinoma. However, no
significant differences were demonstrated between the two groups,
and further ongoing surveillance is needed. The identified risk
factors for postoperative recurrence and prognosis in patients with
breast cancer include an age of ≤45 years, comorbid conditions and
lymph node positivity. Moreover, compared with the sb-PTX cohort,
the nab-PTX cohort had a greater incidence of post-chemotherapy
anemia and peripheral neuropathy but fewer allergic reactions.
Acknowledgements
Not applicable.
Funding
The present work was supported by the Clinical Medical Research
Transformation Project of Anhui (grant no. 202304295107020024).
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
MZ, HL and YH contributed to the research conception
and design. The data were gathered and assessed by YH and HL. HL
wrote the draft of the manuscript. For the data analysis, YZ, SL
and BL were major contributors. The manuscript was revised by MZ,
YH and SL. MZ, HL and YH confirm the authenticity of all the raw
data. All authors have read and approved the final manuscript.
Ethics approval and consent to
participate
The guidelines of the Declaration of Helsinki were
followed during the investigation. The present study was approved
by the Ethics Committee of the Second Affiliated Hospital of Anhui
Medical University [Hefei, China; approval no. YX2023-203(F1)].
Written informed consent was obtained from each patient or their
guardian.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Bray F, Laversanne M, Sung H, Ferlay J,
Siegel RL, Soerjomataram I and Jemal A: Global cancer statistics
2022: GLOBOCAN estimates of incidence and mortality worldwide for
36 cancers in 185 countries. CA Cancer J Clin. 74:229–263. 2024.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Siegel RL, Giaquinto AN and Jemal A:
Cancer statistics, 2024. CA Cancer J Clin. 74:12–49. 2024.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Loibl S, Poortmans P, Morrow M, Denkert C
and Curigliano G: Breast cancer. Lancet. 397:1750–1769. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Urbaniak A, Piña-Oviedo S, Yuan Y,
Huczyński A and Chambers TC: Limitations of an ex vivo breast
cancer model for studying the mechanism of action of the anticancer
drug paclitaxel. Eur J Pharmacol. 891:1737802021. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Dranitsaris G, King J, Kaura S, Yu B and
Zhang A: Nab-paclitaxel, docetaxel, or solvent-based paclitaxel in
metastatic breast cancer: A cost-utility analysis from a Chinese
health care perspective. Clinicoecon Outcomes Res. 7:249–256. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Gradishar WJ: Albumin-bound paclitaxel: A
next-generation taxane. Expert Opin Pharmacother. 7:1041–1053.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Gradishar WJ, Tjulandin S, Davidson N,
Shaw H, Desai N, Bhar P, Hawkins M and O'Shaughnessy J: Phase III
trial of nanoparticle albumin-bound paclitaxel compared with
polyethylated castor oil-based paclitaxel in women with breast
cancer. J Clin Oncol. 23:7794–7803. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lee H, Park S, Kang JE, Lee HM, Kim SA and
Rhie SJ: Efficacy and safety of nanoparticle-albumin-bound
paclitaxel compared with solvent-based taxanes for metastatic
breast cancer: A meta-analysis. Sci Rep. 10:5302020. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Aapro M, Tjulandin S, Bhar P and Gradishar
W: Weekly nab-paclitaxel is safe and effective in ≥65 years old
patients with metastatic breast cancer: A post-hoc analysis.
Breast. 20:468–474. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Megerdichian C, Olimpiadi Y and Hurvitz
SA: Nab-paclitaxel in combination with biologically targeted agents
for early and metastatic breast cancer. Cancer Treat Rev.
40:614–625. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Untch M, Jackisch C, Schneeweiss A, Conrad
B, Aktas B, Denkert C, Eidtmann H, Wiebringhaus H, Kümmel S,
Hilfrich J, et al: Nab-paclitaxel versus solvent-based paclitaxel
in neoadjuvant chemotherapy for early breast cancer (GeparSepto-GBG
69): A randomised, phase 3 trial. Lancet Oncol. 17:345–356. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Guidelines of Chinese Society of Clinical
Oncology(CSCO)-Breast Cancer2024, . 2024.www.csco.org.cn
|
|
13
|
Breast Tumours WHO Classification of
Tumours, . 2019.www.iarc.who.int
|
|
14
|
NCCN Clinical Practice Guidelines in
Oncology-Breast Cancer, . 2024.www.nccn.org/patients
|
|
15
|
Allison KH, Hammond MEH, Dowsett M,
McKernin SE, Carey LA, Fitzgibbons PL, Hayes DF, Lakhani SR,
Chavez-MacGregor M, Perlmutter J, et al: Estrogen and progesterone
receptor testing in breast cancer: American society of clinical
Oncology/College of American Pathologists Guideline update. Arch
Pathol Lab Med. 144:545–563. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wolff AC, Somerfield MR, Dowsett M,
Hammond MEH, Hayes DF, McShane LM, Saphner TJ, Spears PA and
Allison KH: Human epidermal growth factor receptor 2 testing in
breast cancer: ASCO-college of American Pathologists Guideline
update. J Clin Oncol. 41:3867–3872. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Common Terminology Criteria for Adverse
Events (CTCAE)v5.0, . 2017.ctep.cancer.gov
|
|
18
|
Li B, Chen X, Ding T, Liu Y, Ma T, Zhang G
and Wang X: Nanoparticle albumin-bound paclitaxel versus
solvent-based paclitaxel in breast cancer. Medicine.
100:e245142021. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Burstein1 HJ, Curigliano G, Loibl S,
Dubsky P, Gnant M, Poortmans P, Colleoni M, Denkert C,
Piccart-Gebhart M, Regan M, et al: Estimating the benefits of
therapy for early-stage breast cancer: The St. Gallen International
Consensus Guidelines for the primary therapy of early breast cancer
2019. Ann Oncol. 30:1541–1557. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Yardley D, Burris H, Peacock N, Raefsky E,
Melnik M, Inhorn R, Shipley D and Hainsworth J: A pilot study of
adjuvant nanoparticle albumin-bound (nab) paclitaxel and
cyclophosphamide, with trastuzumab in HER2-positive patients, in
the treatment of early-stage breast cancer. Breast Cancer Res
Treat. 123:471–475. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Li BX, Chen XJ, Ding TJ, Liu YH, Ma TT,
Zhang GL and Wang XM: Potentially overestimated efficacy of
nanoparticle Albumin-bound paclitaxel compared with Solvent-based
paclitaxel in breast cancer: A systemic review and Meta-analysis. J
Cancer. 12:5164–5172. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Cho E, Wu Q, Rubinstein L, Linden H,
Gralow J, Specht J, Gadi V and Ellis G: Adjuvant continuous
metronomic adriamycin + cyclophosphamide followed by weekly
nab-paclitaxel for high-risk early-stage breast cancer. Breast J.
24:610–614. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
von Minckwitz G, Conrad B, Reimer T,
Decker T, Eidtmann H, Eiermann W, Hackmann J, Möbus V, Marmé F,
Potenberg J, et al: A randomized phase 2 study comparing EC or CMF
versus nab-paclitaxel plus capecitabine as adjuvant chemotherapy
for nonfrail elderly patients with moderate to high-risk early
breast cancer (ICE II-GBG 52). Cancer. 121:3639–3648. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Pedersen RN, Esen BÖ, Mellemkjær L,
Christiansen P, Ejlertsen B, Lash TL, Nørgaard M and Cronin-Fenton
D: The incidence of breast cancer recurrence 10–32 years after
primary diagnosis. J Natl Cancer Inst. 114:391–399. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Schoen MW, Chen J, Tu Y, Mohammed KA,
Rodin MB and Hinyard LJ: Diabetes outcomes in patients with breast
cancer. J Clin Oncol. 36:e220782018. View Article : Google Scholar
|
|
26
|
Zhao XB and Ren GS: Diabetes mellitus and
prognosis in women with breast cancer: A systematic review and
meta-analysis. Medicine. 95:e56022016. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Chou PC, Choi HH, Huang Y, Fuentes-Mattei
E, Velazquez-Torres G, Zhang F, Phan L, Lee J, Shi Y, Bankson JA,
et al: Impact of diabetes on promoting the growth of breast cancer.
Cancer Commun (Lond). 41:414–431. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Calip GS, Elmore JG and Boudreau DM:
Characteristics associated with nonadherence to medications for
hypertension, diabetes, and dyslipidemia among breast cancer
survivors. Breast Cancer Res Treat. 161:161–172. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
He F, Liu J, Shen X, Wang Z, Li Q and Li
G: Adverse event profile for nanoparticle albumin-bound paclitaxel
compared with solvent-based taxanes in solid-organ tumors: A
systematic review and meta-analysis of randomized clinical trials.
Ann Pharmacother. 56:898–909. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Brufsky A: Nab-paclitaxel for the
treatment of breast cancer: An update across treatment settings.
Exp Hematol Oncol. 6:72017. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Bi Z, Chen P, Liu YB, Zhao T, Sun X, Song
XR and Wang YS: Efficacy and safety analysis of paclitaxel,
docetaxel and liposomal paclitaxel after neoadjuvant therapy in
breast cancer. Breast Cancer Res Treat. 184:397–405. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Elleson KM, Englander K, Gallagher J,
Chintapally N, Sun W, Whiting J, Mallory M, Kiluk J, Hoover S,
Khakpour N, et al: Factors predictive of positive lymph nodes for
breast cancer. Curr Oncol. 30:10351–10362. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Anwar SL, Cahyono R, Prabowo D, Avanti WS,
Choridah L, Dwianingsih EK, Harahap WA and Aryandono T: Metabolic
comorbidities and the association with risks of recurrent
metastatic disease in breast cancer survivors. BMC Cancer. 21:1–13.
2021. View Article : Google Scholar : PubMed/NCBI
|