Introduction
The lifetime risk of breast cancer (BC) for women
has increased over the last four decades, making it the most common
malignancy and the second leading cause of cancer-related mortality
in women (1). BC places an enormous
burden on the families of patients and national health systems
(2). During 2023, 297,790 cases of
BC were newly diagnosed in the United States, resulting in 43,170
deaths (1). Although the overall
death rate has declined, black American women are at a higher risk
of death compared with white American women (27.6 vs. 19.7 deaths
per 100,000 in 2016–2020), as are adult women >50 years (12.1
vs. 6.5 deaths per 100,000 in 2016–2020) (1). The risk of local and metastatic
recurrence is 5–60%, which can occur more than three decades after
the primary diagnosis. In total, >70% of patients with
metastatic BC (mBC) have hormone receptor positive (HR+)
and receptor tyrosine-protein kinase erbB-2-negative
(HER2−) disease (3).
Endocrine therapy is recommended for these patients to improve
their chances of survival, according to oncology guidelines
(4). However, there is currently a
lack of evidence on the most effective regimen for the maintenance
treatment of patients with HR+/HER2− mBC.
Historically, limited by less advanced medical
science, tamoxifen and medroxyprogesterone alone were standard
treatment options for patients with HR+/HER2−
mBC (5). The emergence of aromatase
inhibitors (AIs), including letrozole and fulvestrant, marked an
important milestone in endocrine therapy for mBC, due to their
powerful antitumor effect (6).
Fulvestrant was set as the standard treatment method in
authoritative guidelines, paving the way for setting endocrine
therapy as the principal remedy in these patients (7,8).
Further research, including the PALOMA-1 trial, reported a longer
progression-free survival (PFS) when a cyclin-dependent kinase
(CDK)4/6 inhibitor, namely palbociclib, was added to fulvestrant to
treat patients who had disease progression during endocrine
therapy, starting a new era in the treatment of
HR+/HER2− mBC (9). This led to the development of several
CDK4/6 inhibitors, including abemaciclib, dalpiciclib and
ribociclib, which have been used in various trials evaluating the
combined efficacy of CDK4/6 inhibitors plus endocrine therapy,
including fulvestrant or other AIs, with encouraging results
(10). Although clinicians are
fortunate to have a number of strategies in the treatment of
HR+/HER2− mBC, their decisions are based on
few head-to-head comparisons between regimens.
In recent years, there has been an increasing number
of Bayesian network meta-analyses (NMAs) on CDK4/6 inhibitors, with
non-conclusive evidence. In 2017, Chirila et al (11) reported that, compared with other
endocrine therapies in untreated patients with advanced/mBC, the
palbociclib plus AI regimen led to a significant increase in PFS.
Similarly, in 2020, Liu et al (12) showed that palbociclib plus
fulvestrant was the most effective treatment, but those results
were limited since the authors included some second-line drug
studies in the analysis, such as studies on everolimus. In terms of
data analytics, the authors only considered the covariates of
hazard ratio (HR) and inferred the result by surface under the
cumulative ranking curve (SUCRA), rendering the final results less
reliable.
For that reason, the present Bayesian NMA of
first-line randomized controlled trials (RCT) included direct and
indirect comparisons among regimens to identify the most effective
maintenance treatment for patients with
HR+/HER2− mBC that, in turn, could improve
clinical oncology.
Materials and methods
The present Bayesian NMA was guided by the Preferred
Reporting Items for Systematic Reviews and Meta-analysis guidelines
(13).
Search strategy
Google Scholar (https://scholar.google.hk), Cochrane Library (Cochrane
Central Register of Controlled Trials; CENTRAL (https://www.cochranelibrary.com), PubMed
(https://pubmed.ncbi.nlm.nih.gov), Scopus
(https://www.scopus.com) and Embase (https://www.embase.com) were searched from inception
to August, 2023, with the following MeSH terms: (‘Breast Cancer’ OR
‘Breast Carcinoma’ OR ‘Breast Neoplasm’ OR ‘Breast Tumor’ OR
‘Breast Malignant Tumor’) AND (‘Metastatic’ OR ‘Advanced’) AND
(‘First-Line’ OR ‘First Line’ OR ‘Initial’) AND (‘Hormone Receptor
Positive’ OR ‘Endocrine Receptor Positive’ OR ‘Endocrine
Sensitivity’) AND (‘HER-2 Negative’ OR ‘Human Epidermal Factor
Receptor 2 Negative’ OR ‘ErbB-2 Receptor Negative’ OR ‘C-erbB-2
Negative’) AND (‘Randomized’ OR ‘Allocation Random’ OR
‘Randomization’).
Selection criteria
The study inclusion criteria were as follows: i) The
study subjects had been diagnosed with
HR+/HER2− mBC; ii) the study was an RCT
regarding first-line endocrine therapy; and iii) sufficient
information was provided on the PFS and/or overall survival (OS).
The exclusion criteria were as follows: i) The data required for
analysis was not reported; ii) articles were observational studies,
letters or reviews; and iii) the articles were not written in
English.
Data extraction and quality
assessment
In total, two investigators independently searched
and assessed the eligibility of each study by reading the title and
abstract or the full text when necessary. Data were also
independently extracted by the investigators. Any discrepancy was
arbitrated by the senior investigator. Additionally, the risk of
bias for each included RCT was assessed by the Cochrane Risk of
Bias tool (Revman 5.2; http://methods.cochrane.org/bias/risk-bias-tool).
The following data were collected from the studies: Name of the
first author, publication year, country, number of patients,
condition, therapeutic drugs, treatment dosage, and the HRs and
confidence intervals (CIs) associated with the PFS and OS.
Subsequently, the data regarding PFS and OS at 3, 6, 12, 18, 24, 30
and 36 months were collected from Kaplan-Meier curves using GetData
2.26 (https://getdata.sourceforge.net/download.).
Research endpoint
Due to the incompleteness of data for OS, the
primary endpoint was PFS rate at each time node generated by the
Bayesian NMA.
Data analysis
For the PFS and OS rates at each time node, odds
ratios (ORs) were generated by NMA using STATA 17.0 MP (https://www.stata.com/statamp/), to make pairwise
comparisons among regimens. Statistical significance was determined
if the lower limit of the 95% CI was >1 or the upper limit was
<1. Similarly, for the absolute PFS and OS values, the standard
mean difference (SMD) was generated. Surface under the SUCRA was
also formulated, where a higher SUCRA indicated a higher
probability of being the superior treatment. However, whether the
effect size between any pair with the corresponding SUCRAs reached
the level of significance was determined by net-league table, also
termed a matrix in algebra. Additionally, inconsistency and
consistency tests were conducted to examine the existence of any
inconsistency. Publication bias was also assessed by funnel
plot.
For HRs of OS and PFS in each study, Napierian
Logarithm HR (lnHR) and standard error of lnHR (selnHR) were
calculated using STATA 17.0 MP. Subsequently these data (lnHR and
selnHR for OS and PFS) were input into Rstudio 4.2.2 (https://cran.rstudio.com/bin/windows/base) using the
‘gemtc’ package to conduct a Bayesian NMA to generate pairwise HRs,
SUCRA and matrix. Markov-chain Monte Carlo (MCMC) was used to
obtain posterior distributions, with 2,000 burn-ins and 100,000
iterations of 4 for each chain and a thinning interval of 10 for
each outcome. Brooks-Gelman-Rubin diagnostics and Trace and density
plots were used to evaluate and visualize the convergence of the
model over iterations. The random-effect model was used when
conducting the NMA. However, to ensure the reliability of the data
and minimize the impact of heterogeneity on the final results, a
fixed-effects model was used when comparing direct and indirect
comparison results (14).
Results
Characteristics of the included
studies
A detailed description of the included studies can
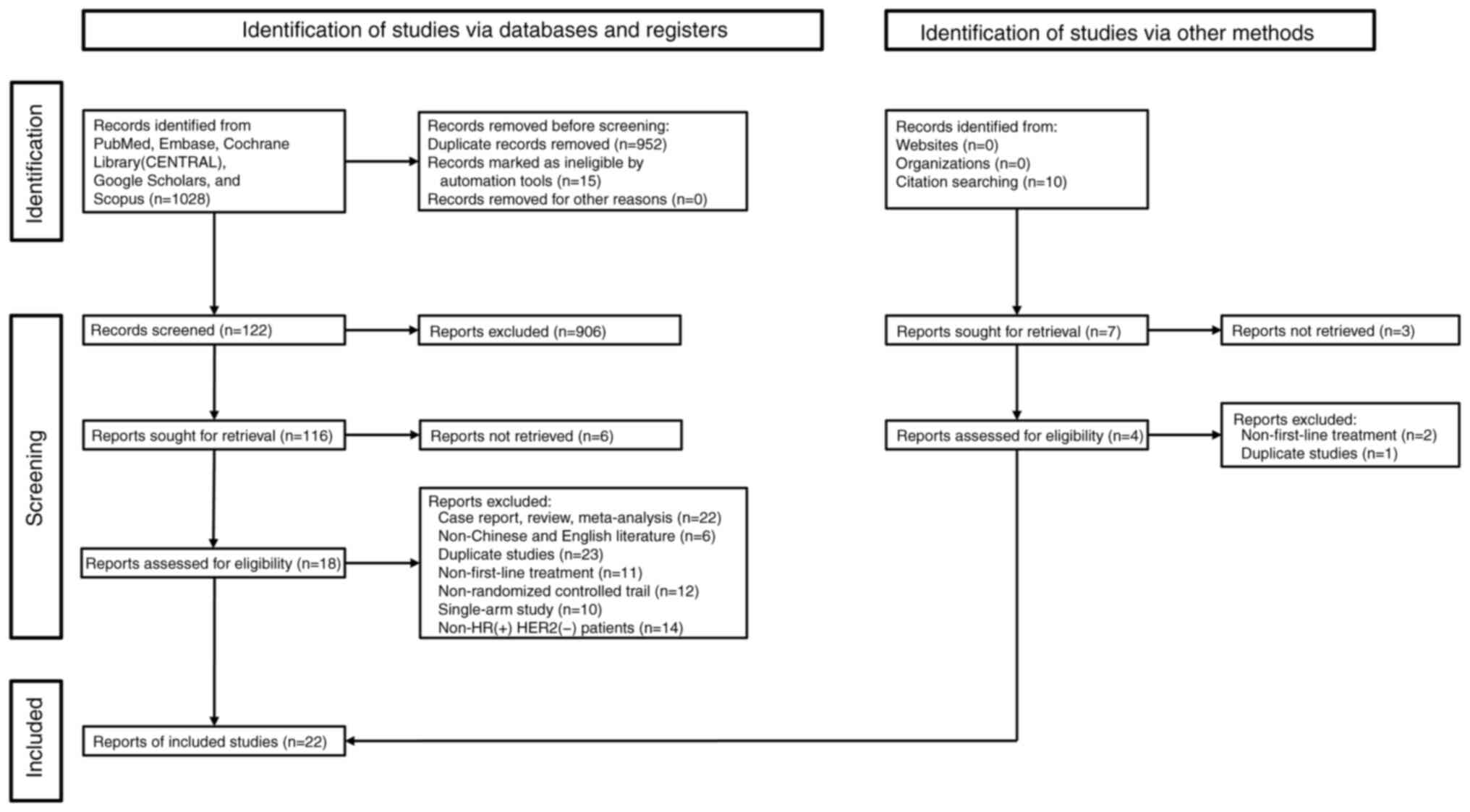
be found in Table I. Initially,
1,028 articles from all five databases were retrieved after
meticulous screening; however, only 16 RCTs reporting 7,174
patients were eligible for the present study (reporting 1,292
patients with only bone metastasis, 3,294 patients with visceral
metastasis, 2,416 patients with non-visceral metastasis, 2,512
patients with 1 or 2 metastasis sites, 1,596 patients with >2
metastasis sites and 1,109 patients were de novo; some
patients belonged to several categories) (Fig. 1) (7,15–35).
At total of 11 different maintenance regimens were evaluated within
the included studies, namely, AI alone, abemaciclib plus AI
(AbeAI), abemaciclib plus fulvestrant (AbeFul), dalpiciclib plus AI
(DalpAI), fulvestrant (Ful), fulvestrant plus AI, lapatinib plus
AI, palbociclib plus AI (PalboAI), palbociclib plus fulvestrant
(PalboFul), ribociclib plus AI (RiboAI) and ribociclib plus
fulvestrant (RiboFul). The included patients were from Asia,
Europe, South/North America and Africa. As shown in Fig. S1, the risk of bias assessment
results showed that there was no high risk of bias in the included
studies.
 | Table I.Characteristics of first-line
systemic therapy for hormone receptor-positive and receptor
tyrosine-protein kinase erbB-2-negative metastatic breast cancer
studies included in the Bayesian network meta-analysis. |
Table I.
Characteristics of first-line
systemic therapy for hormone receptor-positive and receptor
tyrosine-protein kinase erbB-2-negative metastatic breast cancer
studies included in the Bayesian network meta-analysis.
| Trial acronym | First author,
year | Country | Treatment | Sample size | Dosage | Outcomes | (Refs.) |
|---|
| - | Wang et al,
2021 | China | Fulvestrant/AI | 77/67 | Fulvestrant 500 mg
on days 0, 14 and 28 and every 28±3 days thereafter/Exemestane 25
mg daily. | PFS | (15) |
| DAWNA-2 | Zhang et al,
2023 | China | Dalpiciclib +
AI/AI | 303/153 | Dalpiciclib (150 mg
daily for 3 weeks, followed by 1 week off + Letrozole 2.5 mg or
Anastrozole 1 mg daily/Letrozole 2.5 mg or Anastrozole 1 mg
daily. | PFS | (16) |
| FALCON | Robertson et
al, 2016 | UK | Fulvestrant/AI | 230/232 | Fulvestrant 500 mg
on days 0, 14 and 28 and every 28 days thereafter/Anastrozole 1 mg
daily. | PFS, OS | (17) |
| FIRST | Ellis et al,
2015 | USA | Fulvestrant/AI | 102/103 | Fulvestrant 500 mg
on days 0, 14 and 28 and every | OS | (7) |
|
| Robertson et
al, 2012 | UK |
|
| 28 days
thereafter/Anastrozole 1 mg daily. | PFS | (18) |
| - | Llombart et
al, 2021 | Spain | Fulvestrant +
Palbociclib/AI + Palbociclib | 243/243 | Palbociclib 125 mg
daily (in cycles of 3 weeks of treatment followed by 1 week off) +
Fulvestrant 500 mg on days 1, 15, 29 and once monthly
thereafter/Palbociclib 125 mg daily (in cycles of 3 weeks of
treatment followed by 1 week off) + Letrozole 2.5 mg daily. | PFS, OS | (19) |
| - | Johnston et
al, 2009 | UK | Lapatinib +
AI/AI | 478/474 | Letrozole 2.5 mg +
Lapatinib 1,500 mg daily/Letrozole 2.5 mg daily. | PFS, OS | (20) |
| MONARCH-3 | Goetz et al,
2022 | USA | Abemaciclib + | 328/165 | Abemaciclib 150 mg
twice daily continuous | OS | (21) |
|
| Goetz et al,
2017 | USA | AI/AI |
| schedule +
Anastrozole 1 mg or Letrozole 2.5 mg daily/Anastrozole 1 mg or
Letrozole 2.5 mg daily. | PFS | (22) |
| FLIPPER | Albanell et
al, 2021 | Spain | Palbociclib +
Fulvestrant/Fulvestrant | 94/95 | Palbociclib 125 mg
daily (in cycles of 3 weeks of treatment followed by 1 week off) +
Fulvestrant 500 mg on days 1, 15, 29 and once monthly
thereafter/Fulvestrant 500 mg on days 1, 15, 29 and once monthly
thereafter. | PFS | (28) |
| MONARCH-2 | Neven et al,
2021 | Belgium | Abemaciclib +
Fulvestrant/Fulvestrant | 265/133 | Abemaciclib 150 mg
twice daily + Fulvestrant 500 mg on days 0, 14 and 28 and every 28
days thereafter/Fulvestrant 500 mg on days 0, 14 and 28 and every
28 days thereafter. | PFS, OS | (23) |
| PALOMA-1 | Finn et al,
2015 | USA | Palbociclib +
AI/AI | 84/81 | Palbociclib 125 mg
daily (3/1 schedule) + Letrozole | PFS | (24) |
|
| Finn et al,
2020 | USA |
|
| 2.5 mg daily,
continuous/Letrozole 2.5 mg daily, continuous. | OS | (25) |
| MONALEESA-3 | Slamon et
al, 2021 | USA | Ribociclib +
Fulvestrant/Fulvestrant | 237/128 | Ribociclib 600 mg
(once daily for 21 days, followed by 7 days off, 28 days for a
cycle) + Fulvestrant 500 mg on days 0, 14 and 28 and every 28 days
thereafter/Fulvestrant 500 mg on days 0, 14 and 28 and every 28
days thereafter. | PFS, OS | (30) |
| MONALEESA-2 | Hortobagy et
al, 2022 | USA | Ribociclib +
AI/AI | 334/334 | Ribociclib (600 mg,
once daily for 21 days, | OS | (26) |
|
| Hortobagyi et
al, 2018 | USA |
|
| followed by 7 days
off, 28 days for a cycle) + Letrozole 2.5 mg daily/Letrozole 2.5 mg
daily. | PFS | (31) |
| PALOMA-4 | Xu et al,
2022 | China | Palbociclib +
AI/AI | 168/171 | Palbociclib 125 mg
daily (3 weeks on, 1 week off) + Letrozole 2.5 mg daily,
continuously/Letrozole 2.5 mg daily, continuously. | PFS | (27) |
| MONALEESA-7 | Tripathy et
al, 2018 | USA | Ribociclib +
AI/AI | 335/337 | Ribociclib 600 mg
(once daily for 21 days, followed | PFS | (29) |
|
| Lu et al,
2021 | China |
|
| by 7 days off, 28
days for a cycle) + Letrozole 2.5 mg or Anastrozole 1 mg,
daily/Letrozole 2.5 mg or Anastrozole 1 mg, daily. | OS | (34) |
| PALOMA-2 | Finn et al,
2022 | USA | Palbociclib + | 444/222 | Palbociclib (125
mg/day; 3 weeks on, 1 week off) + | OS | (32) |
|
| Rugo et al,
2019 | USA | AI/AI |
| Letrozole 2.5 mg
daily, continuously/Letrozole 2.5 mg daily, continuously. | PFS | (33) |
| FACT | Bergh et al,
2012 | Sweden | Fulvestrant +
AI/AI | 258/256 | Fulvestrant 500 mg
on days 0, 14 and 28 and every 28 days thereafter/Anastrozole 1 mg
orally daily. | PFS, OS | (35) |
Primary analysis of PFS
PFS at each time node
Fig. 2A shows the
network graphs from the pairwise comparison of regimens at each PFS
time point. On the 3rd month, compared with AI, AbeAI (OR=4.92; 95%
CI, 1.28–18.90) and PalboAI (OR=2.22; 95% CI, 1.10–4.47)
significantly increased the 3-month PFS rate. Compared with the top
SUCRA-ranked intervention AbeAI, PalboAI did not exhibit a
significant advantage (OR=0.45; 95% CI, 0.10–2.05) (Table SI).
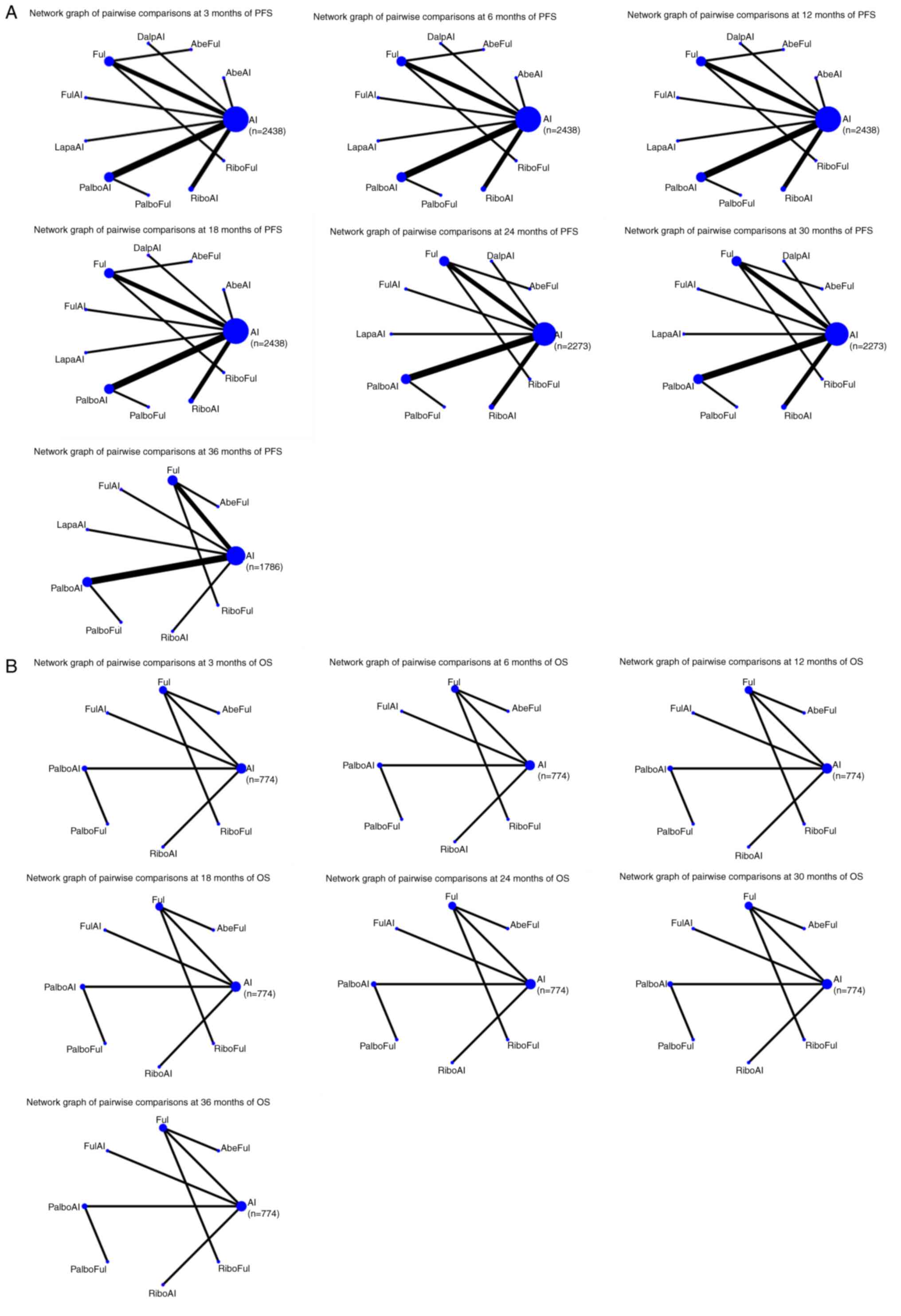 | Figure 2.(A) Network graphs of the pairwise
comparisons of regimens at each time point of the PFS curve. (B)
Network graphs of the pairwise comparison of regimens at each time
point of the OS curve. PFS, progression free survival; OS, overall
survival; AI, aromatase inhibitor; AbeAI, abemaciclib plus AI;
AbeFul, abemaciclib plus fulvestrant; DalpAI, dalpiciclib plus AI;
Ful, fulvestrant; FulAI, fulvestrant plus AI; LapaAI, lapatinib
plus AI; PalboAI, palbociclib plus AI; PalboFul, palbociclib plus
fulvestrant; RiboAI, ribociclib plus AI; RiboFul, ribociclib plus
fulvestrant. |
On the 6th month, compared with AI, only PalboAI
(OR=2.39; 95% CI, 1.21–4.69), significantly increased the 6-month
PFS rate (Table SII).
On the 12th month, compared with AI, the treatments
performed as follows: AbeAI (OR=2.02; 95% CI, 1.11–3.70), RiboFul
(OR=3.07; 95% CI, 1.22–7.71), PalboAI (OR=1.94; 95% CI, 1.34–2.79),
RiboAI (OR=1.93; 95% CI, 1.21–3.07), AbeFul (OR=2.61; 95% CI,
1.25–5.44) and DalpAI (OR=2.07; 95% CI, 1.13–3.79). AbeAI, PalboAI,
RiboAI, AbeFul and DalpAI did not exhibit a significant advantage
when compared with the top SUCRA-ranked intervention RiboFul
(Table SIII).
On the 18th month, compared with AI, the treatments
performed as follows: AbeAI (OR=2.10; 95% CI, 1.18–3.73), RiboFul
(OR=2.66; 95% CI, 1.19–5.96), PalboAI (OR=2.38; 95% CI, 1.65–3.44),
RiboAI (OR=1.85; 95% CI, 1.20–2.85), AbeFul (OR=2.64; 95% CI,
1.25–5.59), DalpAI (OR=2.11; 95% CI, 1.18–3.76) and PalboFul
(OR=2.13; 95% CI, 1.08–4.18). AbeAI, RiboFul, PalboAI, RiboAI,
DalpAI and PalboFul did not show a significant advantage when
compared with the top SUCRA-ranked intervention AbeFul (Table SIV).
On the 24th month, compared with AI, the treatments
performed as follows: RiboFul (OR=2.12; 95% CI, 1.03–4.38), PalboAI
(OR=2.39; 95% CI, 1.67–3.43), RiboAI (OR=1.84; 95% CI, 1.27–2.68),
AbeFul (OR=4.01; 95% CI, 1.93–8.31), DalpAI (OR=2.36; 95% CI,
1.39–4.03), PalboFul (OR=2.16; 95% CI, 1.16–4.03) and Ful (OR=1.68;
95% CI, 1.11–2.54). RiboFul, PalboAI, RiboAI, DalpAI, PalboFul and
Ful did not exhibit a significant advantage when compared with the
top SUCRA-ranked intervention AbeFul (Table SV).
On the 30th month, compared with AI, the treatments
performed as follows: RiboFul (OR=2.35; 95% CI, 1.34–4.10), PalboAI
(OR=2.10; 95% CI, 1.62–2.74), RiboAI (OR=1.60; 95% CI, 1.24–2.06),
AbeFul (OR=4.94; 95% CI, 2.62–9.33), DalpAI (OR=3.97; 95% CI,
2.59–6.08), PalboFul (OR=1.78; 95% CI, 1.15–2.78) and Ful (OR=1.62;
95% CI, 1.15–2.27). RiboFul, PalboAI, RiboAI, DalpAI, PalboFul and
Ful did not exhibit a significant advantage when compared with the
top SUCRA-ranked intervention AbeFul (Table SVI).
On the 36th month, compared with AI, the treatments
performed as follows: PalboAI (OR=2.66; 95% CI, 1.37–5.18) and
AbeFul (OR=6.21; 95% CI, 1.71–22.57). Compared with the top
SUCRA-ranked intervention AbeFul, PalboAI did not exhibit a
significant advantage (OR=0.43; 95% CI, 0.10–1.82) (Table SVII).
As such, the regimen with the most significant
effect on PFS between 3–36 months compared with AI was PalboAI
(Table II).
 | Table II.Progression free survival for
interventions that were significant compared with AI. |
Table II.
Progression free survival for
interventions that were significant compared with AI.
| Method | Control group | AbeAI, HR (CI) | RiboFul, HR
(CI) | PalboAI, HR
(CI) | RiboAI, HR
(CI) | AbeFul, HR
(CI) | DalpAI, HR
(CI) | LapaAI, HR
(CI) | PalboFul, HR
(CI) | FulAI, HR (CI) | Ful, HR (CI) |
|---|
| 3M | AI | 4.92 | x | 2.22 | x | x | x | x | x | x | x |
|
|
| (1.28–18.90) |
| (1.10–4.47) |
|
|
|
|
|
|
|
| 6M | AI | x | x | 2.39 | x | x | x | x | x | x | x |
|
|
|
|
| (1.21–4.69) |
|
|
|
|
|
|
|
| 12M | AI | 2.02 | 3.07 | 1.94 | 1.93 | 2.61 | 2.07 | x | x | x | x |
|
|
| (1.11–3.70) | (1.22–7.71) | (1.34–2.79) | (1.21–3.07) | (1.25–5.44) | (1.13–3.79) |
|
|
|
|
| 18M | AI | 2.10 | 2.66 | 2.38 | 1.85 | 2.64 | 2.11 | x | 2.13 | x | x |
|
|
| (1.18–3.73) | (1.19–5.96) | (1.65–3.44) | (1.20–2.85) | (1.25–5.59) | (1.18–3.76) |
| (1.08–4.18) |
|
|
| 24M | AI | NR | 2.12 | 2.39 | 1.84 | 4.01 | 2.36 | x | 2.16 | x | 1.68 |
|
|
|
| (1.03–4.38) | (1.67–3.43) | (1.27–2.68) | (1.93–8.31) | (1.39–4.03) |
| (1.16–4.03) |
| (1.11–2.54) |
| 30M | AI | NR | 2.35 | 2.10 | 1.60 | 4.94 | 3.97 | x | 1.78 | x | 1.62 |
|
|
|
| (1.34–4.10) | (1.62–2.74) | (1.24–2.06) | (2.62–9.33) | (2.59–6.08) |
| (1.15–2.78) |
| (1.15–2.27) |
| 36M | AI | NR | x | 2.66 | x | 6.21 | NR | x | x | x | x |
|
|
|
|
| (1.37–5.18) |
| (1.71–22.57) |
|
|
|
|
|
| HR | AI | 1.96 | 2.23 | 1.7 | 1.76 | 2.45 | 1.96 | x | 2.93 | x | 1.4 |
|
|
| (1.25–3.09) | (1.4–3.65) | (1.36–2.16) | (1.35–2.3) | (1.59–3.93) | (1.29–2.98) |
| (1.68–5.21) |
| (1.11–1.82) |
| Absolute value | AI | NR | NR | 0.37 | 0.62 | NR | NR | NR | NR | NR | NR |
|
|
|
|
| (0.25–0.49) | (0.28–0.96) |
|
|
|
|
|
|
HR of PFS
A total of 16/22 articles reported outcomes
associated with the HRs of PFS. The 11 included interventions were
compared directly and indirectly. The corresponding network graph
is shown in Fig. 3A, and detailed
results are shown in Table SVIII.
The interventions that exhibited significant differences compared
with AI were AbeAI [HR=1.96; 95% credible interval (Crl),
1.25–3.09], AbeFul (HR=2.45; 95% Crl, 1.59–3.93), DalpAI (HR=1.96;
95% Crl, 1.29–2.98), Ful (HR=1.4; 95% Crl, 1.11–1.82), PalboAI
(HR=1.7; 95% Crl, 1.36–2.16), PalboFul (HR=2.93; 95% Crl,
1.68–5.21), RiboAI (HR=1.76; 95% Crl, 1.35–2.3) and RiboFul
(HR=2.23; 95% Crl, 1.4–3.65). Compared with the top SUCRA-ranked
intervention, PalboFul, 7 treatments (PalboFul, AbeFul, DalpAI,
PalboAI, PalboFul, RiboAI and RiboFul) were included in the first
echelon; fulvestrant was included in the second echelon.
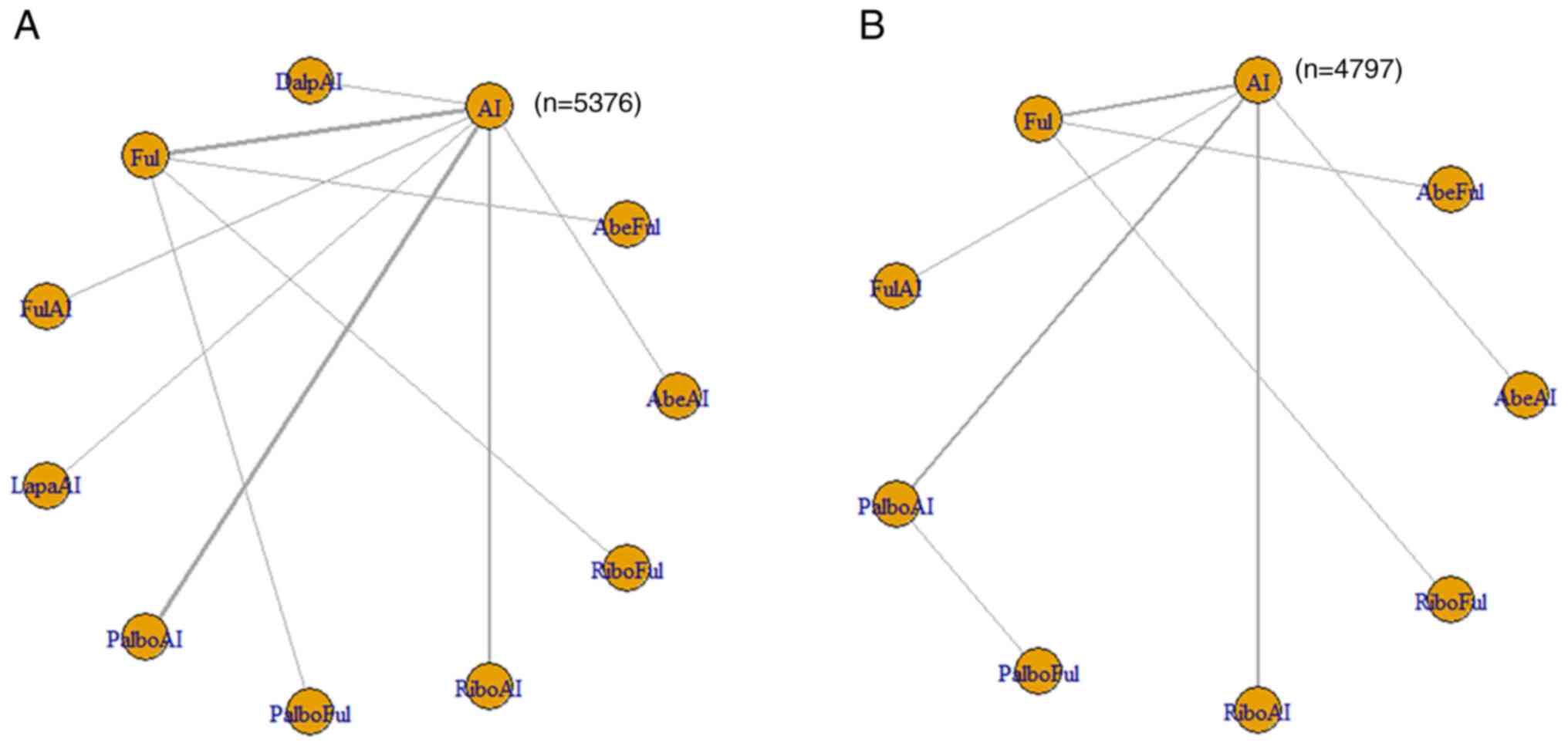 | Figure 3.(A) Network meta-analysis plots for
HR of progression free survival. (B) Network meta-analysis plots
for HR of overall survival. HR, hazard ratio; AI, aromatase
inhibitor; AbeAI, abemaciclib plus AI; AbeFul, abemaciclib plus
fulvestrant; DalpAI, dalpiciclib plus AI; Ful, fulvestrant; FulAI,
fulvestrant plus AI; LapaAI, lapatinib plus AI; PalboAI,
palbociclib plus AI; PalboFul, palbociclib plus fulvestrant;
RiboAI, ribociclib plus AI; RiboFul, ribociclib plus
fulvestrant. |
For the HR of PFS, the Brooks-Gelman Rubin
convergence diagnostic revealed that the inferential iterations for
each MCMC were stable and reproducible. The history feature was
also used to confirm the convergence of the model in all outcomes.
Detailed results are presented in Figs. S2 and S3.
Absolute PFS value
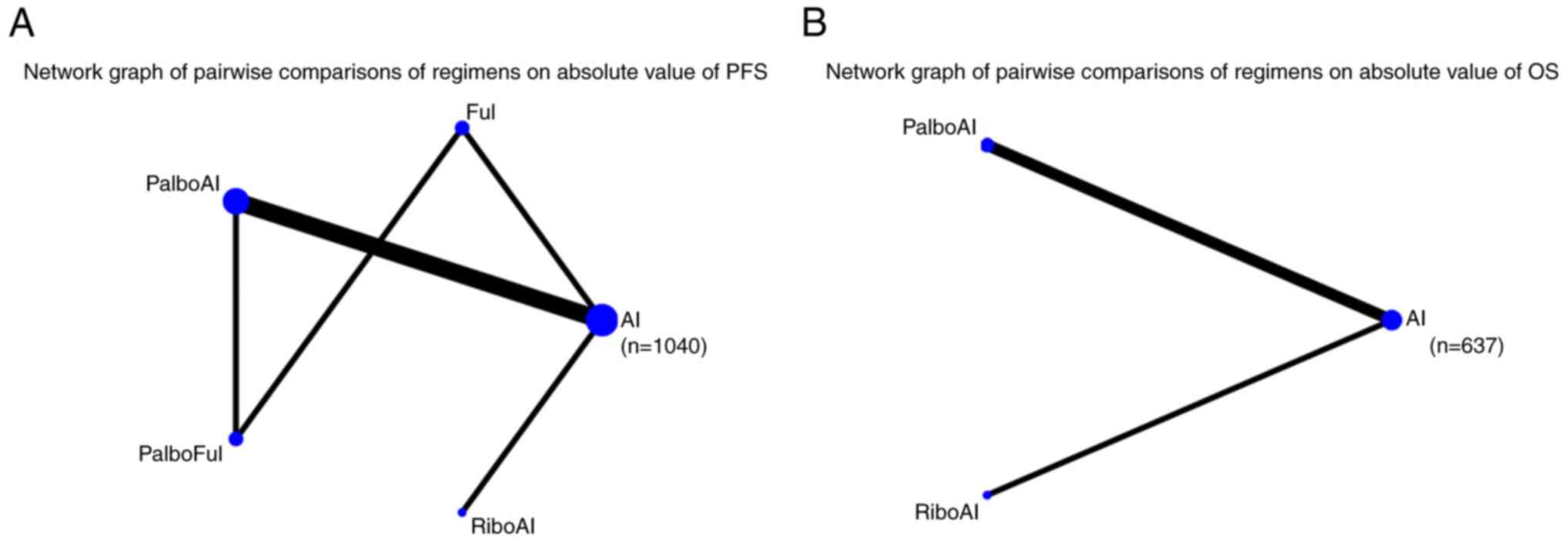
In total, 7 articles reported outcomes on the
absolute PFS value. The SMD was used to compare the 5 eligible
studies directly and indirectly (the treatments reported in 2
articles failed to connect in the network comparison). The network
graph is shown in Fig. 4A and
detailed results are shown in Table
SIX. The interventions that exhibited significant differences
compared with AI were PalboAI (SMD=0.37; 95% Crl, 0.25–0.49) and
RiboAI (SMD=0.62; 95% Crl, 0.28–0.96).
Primary analysis of OS
OS at each time node
Compared with AI, only RiboFul (OR=2.66; 95% Cl,
1.37–5.18) exhibited a significant advantage at 18 months. The
network graphs of pairwise comparisons among regimens on each time
point of the OS curve are shown in Fig.
2B, and detailed results are listed in Table SX, Table SXI, Table SXII, Table SXIII, Table SXIV, Table SXV, Table SXVI.
HR of OS
Of the 22 articles, 12 reported outcomes related to
the HR of OS. The 11 included interventions were compared directly
and indirectly. The only intervention that exhibited a significant
difference compared with AI was RiboFul (HR=1.99; 95% Crl,
1.14–3.47; Table SXVII). The
network graph is shown in Fig. 3B,
and detailed results are listed in Table SV. For the HR of OS, the
Brooks-Gelman Rubin convergence diagnostic revealed that the
inferential iterations for each MCMC were stable and reproducible.
The history feature was also used to confirm the convergence of the
model in all outcomes. Detailed results are presented in Figs. S4 and S5.
Absolute value of OS
Only 3 articles reported outcomes related to the
absolute OS value. The SMD was used to compare the three included
interventions directly and indirectly. The intervention measure
with a significant difference compared with AI was RiboAI
(SMD=0.17; 95% Crl, 0.02–0.32). The network graph is shown in
Fig. 4B and detailed results are
shown in Fig. S6.
Inconsistency tests, heterogeneity
analysis and small sample effect tests
No inconsistency and heterogeneity were observed
between studies included in the present Bayesian NMA. The small
sample effect was explored by a network funnel plot, and the small
sample effect was not observed. P<0.05 was considered to
indicate a statistically significant difference (Fig. S7, Fig.
S8, Fig. S9).
Discussion
Current status of breast cancer
treatment
Due to the heterogeneity of tumor cells, different
differentiation cycles have distinct characteristics. Different
antitumor drugs target different cell cycles, and thus the
comprehensive treatment of tumors is complicated (36). For example, certain drugs act on
nucleic acid replication and certain endocrine drugs act on
different targets. During the differentiation process of tumor
cells, resistance to a certain pharmacological mechanism may occur,
which means a single antitumor treatment is ineffective (37).
In recent years, several new surgical and medicinal
technologies and drugs have emerged for the treatment of BC, which
have brought great benefits to patients. Even small tumors that are
non-palpable can be accurately removed through ultrasound combined
with marker positioning technology to reduce damage while ensuring
the margins (38,39). Patients with axillary lymph
node-positive tumors typically require further dissection, and the
most common complication is seroma, which makes patients feel
uncomfortable and can cause further infection. A new hemostatic
device (Thunderbeat) can effectively reduce the occurrence of serum
swelling (40). There are also
several treatments for mBC. The aim of the present study was to
discuss the best options for patients with
ER+/HER2− mBC.
To the best of our knowledge, the present study
reports the first Bayesian NMA comparing relative efficacy of all
current available maintenance therapies for
HR+/HER2− mBC. The findings were as
follows:
Core findings
Transverse comparisons
Compared with AI, the interventions that exhibited
significantly different effects on PFS were PalboAI and AbeAI on
the 3rd month of follow-up. Similarly, PalboAI and AbeFul exhibited
significantly different effects on the 36th month, and only PalboAI
exhibited significantly different effects on the 6th month.
Riboful, PalboAI, RiboAI, AbeAI and DalpAI exhibited significantly
different effects between months 12 and 30. PalboAI demonstrated
benefits at each time node between months 3 and 36. Based on the
Bayesian NMA of HRs and the absolute value time as a covariate of
PFS, PalboAI also exhibited benefits.
Longitudinal comparisons
With regards to the PFS up to 36 months of
follow-up, and compared with AI therapy, AbeAI exhibited a similar
efficacy to that of PalboAI on the 3rd month. DalpAI also had
improved results compared with AI, but due to the lack of data, it
was not possible to analyze whether the significance persisted
until the 36th month. AbeFul had improved results compared with AI
between the 12th and 36th months, but it did not demonstrate a
significant advantage between the 3rd and 6th months. Similarly,
RiboAI and RiboFul revealed a significant advantage between the
12th and 30th months. PalboAI was the only regimen that
demonstrated significant efficacy between the 3rd and 36th months
of follow-up.
Regarding OS up to the 36th month of follow-up, it
was found that most of the studies did not report this endpoint.
Due to the lack of data, the OS results were not considered in the
present study.
Clinicopathological correlation
CDK4/6 inhibitors efficiently and accurately inhibit
the activity of CDK4/6 kinases in BC cells to block the
phosphorylation of retinoblastoma protein, thus blocking the
progression of the cell cycle from the G1 to the S phase, in turn
inhibiting the proliferation of tumor cells. CDK4/6 inhibitors also
inhibit the expression of the upstream estrogen receptor signaling
pathway, and there is a synergistic effect of CDK4/6 inhibitors
combined with endocrine therapy to delay and reverse endocrine drug
resistance (41). The different
CDK4/6 inhibitors have comparable molecular weights and share the
same core group. The substituent of pabociclib is relatively large
and shows high kinase selectivity (42). Pabociclib has a significant
pharmacokinetic profile [volume of distribution of 2583 L
(ribociclib 1090 L, abemaciclib 690 L), bioavailability of 46%
(ribociclib not reported, abemaciclib 45%) protein binding ratio of
85% (ribociclib 70%, abemaciclib 93%) and half-life of 29±5 h
(ribociclib 30–55 h, abemaciclib 18.3 h] (43). These properties may explain the
early benefits and sustained effects at each point of the PFS time
nodes observed in this analysis.
Feasibility analysis
A common adverse effect of CDK4/6 inhibitors is
neutropenia. In contrast to the mechanism of chemotherapy-induced
myelosuppression, CDK4/6 inhibitors do not cause neutrophil
precursor death, reducing the risk of febrile neutropenia (44). However, close clinical monitoring
and management is recommended. The reported incidence of
hematological adverse events associated with CDK4/6 inhibitors is
low and the severity is milder: i) Neutropenia, palbociclib 79.5%,
abemaciclib 80% and dalpiciclib 99%; ii) leucopenia, palbociclib
39%, abemaciclib 76.1% and dalpiciclib 98.3%; iii) anemia,
palbociclib 24.1%, abemaciclib 62% and dalpiciclib 66.9%; and iv)
thrombocytopenia, palbociclib 15.5%, abemaciclib 44.4% and
dalpiciclib 53.3% (16,45,46).
Diarrhea is the most common gastrointestinal adverse
event associated with all available CDK4/6 inhibitors, with the
highest incidence observed in patients treated with abemaciclib
(80%); therefore, in patients with poor gastrointestinal function,
it is recommended to prioritize other CDK4/6 inhibitors with less
probability of this adverse event, such as ribociclib (20%),
palbociclib (10.7%) or dalpiciclib (10.4%) (16,45–47).
There are no reports on the metabolic safety of pabociclib in
diabetic patients. However, it has been reported that abemaciclib
causes severe hypoglycemia and ribociclib causes lactic acidosis
(48–50).
Of all the available CDK4/6 inhibitors, palbociclib
has a relatively low incidence of overall adverse events and, since
all palbociclib combination regimens are oral formulations, this
therapy is medically accessible and suitable for all patient
groups. In particular, all palbociclib regimens have been included
in China's national health insurance catalogue, which reduces the
financial burden for patients.
Limitations
First, the sample sizes of certain included studies
were inadequate, resulting in the small sample effect and potential
bias. Second, in the analyses of absolute OS/PFS values, certain
studies had not reported mean/median OS/PFS and 95% confidence
intervals or the interquartile range. These studies had to be
excluded, which potentially shrank the sample size by another
means, eventually increasing random error. Third, the limited
resolution of survival curve images in certain studies was
compromised. Finally, the quality of some of the studies was low,
bringing potential interference.
Perspectives
We hope that the design of future clinical trials
will be more precise and the final OS data will be reported.
Adverse events should be evaluated after long-term follow-ups and
could be compared at each time node. When making clinical
decisions, adverse effects should be considered. FDA Adverse Event
Reporting System database can provide some information on these
adverse events.
In the present study, significant heterogeneity was
not observed when analyzing the heterogeneity of the computational
model, indicating that the baseline differences among patients
included in the NMA were not sufficient to affect the final
results. The patients included in the present study all had
metastatic disease and accepted first-line treatments; therefore,
the results are applicable to these first-line patients with
metastases. In conclusion, considering the benefit of treatment on
PFS emerged earlier and over a long period of time, PalboAI should
be recommended as the optimal therapy in
HR+/HER2− mBC. However, it is necessary to
design more RCTs to confirm this result.
Supplementary Material
Supporting Data
Supporting Data
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
YCJ and JD confirm the authenticity of all the raw
data. YCJ, JD and RLDW designed the study and supervised the
overall project; JJY and HTZ participated in collecting data; RZ,
SR and LATR participated in the interpretation of the results. YCJ
and JD provided the statistical analysis. YCJ, JD, SR, LATR and
RLDW wrote the draft of the manuscript. All authors read and
approved the final version of the manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Siegel RL, Miller KD, Wagle NS and Jemal
A: Cancer statistics, 2023. CA Cancer J Clin. 2:7317–48. 2023.
|
|
2
|
Trogdon JG, Baggett CD, Gogate A,
Reeder-Hayes KE, Rotter J, Zhou X, Ekwueme DU, Fairley TL and
Wheeler SB: Medical costs associated with metastatic breast cancer
in younger, midlife, and older women. Breast Cancer Res Treat.
181:653–665. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Moy B and Goss PE: Estrogen receptor
pathway: Resistance to endocrine therapy and new therapeutic
approaches. Clin Cancer Res. 12:4790–4793. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Gradishar WJ, Moran MS, Abraham J,
Abramson V, Aft R, Agnese D, Allison KH, Anderson B, Burstein HJ
and Chew H: NCCN Guidelines® Insights: Breast cancer,
version 4.2023. J Natl Compr Canc Netw. 21:594–608. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Atkins CD: Tamoxifen versus
medroxyprogesterone acetate for metastatic breast cancer. J Clin
Oncol. 12:2515–2516. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Paridaens RJ, Dirix LY, Beex LV, Nooij M,
Cameron DA, Cufer T, Piccart MJ, Bogaerts J and Therasse P: Phase
III study comparing exemestane with tamoxifen as first-line
hormonal treatment of metastatic breast cancer in postmenopausal
women: The European Organisation for research and treatment of
cancer breast cancer cooperative group. J Clin Oncol. 26:4883–4890.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ellis MJ, Llombart-Cussac A, Feltl D,
Dewar JA, Jasiówka M, Hewson N, Rukazenkov Y and Robertson JF:
Fulvestrant 500 mg Versus Anastrozole 1 mg for the First-Line
treatment of advanced breast cancer: Overall survival analysis from
the phase II FIRST study. J Clin Oncol. 33:3781–3787. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Untch M, Augustin D, Ettl J, Haidinger R,
Harbeck N, Lück HJ, Lüftner D, Marmé F, Müller L, Overkamp F, et
al: ABC3 consensus commented from the perspective of the German
guidelines: Third international consensus conference for advanced
breast cancer (ABC3), Lisbon, 07. 11. 2015. Geburtshilfe
Frauenheilkd. 76:156–163. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Turner NC, Ro J, André F, Loi S, Verma S,
Iwata H, Harbeck N, Loibl S, Huang Bartlett C, Zhang K, et al:
Palbociclib in Hormone-receptor-positive advanced breast cancer. N
Engl J Med. 373:209–219. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Spring LM, Wander SA, Zangardi M and
Bardia A: CDK 4/6 inhibitors in breast cancer: Current
controversies and future directions. Curr Oncol Rep. 21:252019.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Chirila C, Mitra D, Colosia A, Ling C,
Odom D, Iyer S and Kaye JA: Comparison of palbociclib in
combination with letrozole or fulvestrant with endocrine therapies
for advanced/metastatic breast cancer: Network meta-analysis. Curr
Med Res Opin. 33:1457–1466. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Liu S, Sun X, Xu X and Lin F: Comparison
of endocrine therapies in hormone Receptor-Positive and human
epidermal growth factor receptor 2-Negative locally advanced or
metastatic breast cancer: A network Meta-Analysis. J Breast Cancer.
23:460–483. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Page MJ, McKenzie JE, Bossuyt PM, Boutron
I, Hoffmann TC, Mulrow CD, Shamseer L, Tetzlaff JM, Akl EA, Brennan
SE, et al: The PRISMA 2020 statement: An updated guideline for
reporting systematic reviews. Syst Rev. 10:892021. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Sbidian E, Chaimani A, Garcia-Doval I, Do
G, Hua C, Mazaud C, Droitcourt C, Hughes C, Ingram JR, Naldi L, et
al: Systemic pharmacological treatments for chronic plaque
psoriasis: A network meta-analysis. Cochrane Database Syst Rev.
1:CD011535. 2020.
|
|
15
|
Wang J, Xu B, Cai L, Song Y, Kang L, Sun
T, Teng Y, Tong Z, Li H, Ouyang Q, et al: 235P Efficacy and safety
of first-line therapy with fulvestrant or exemestane for
postmenopausal ER+/HER2-advanced breast cancer patients after
adjuvant nonsteroidal aromatase inhibitor treatment: A randomized,
open-label, multicenter study. Ann Oncol. 32 (Suppl 5):S461–S462.
2021. View Article : Google Scholar
|
|
16
|
Zhang P, Zhang Q, Tong Z, Sun T, Li W,
Ouyang Q, Hu X, Cheng Y, Yan M, Pan Y, et al: Dalpiciclib plus
letrozole or anastrozole versus placebo plus letrozole or
anastrozole as first-line treatment in patients with hormone
receptor-positive, HER2-negative advanced breast cancer (DAWNA-2):
A multicentre, randomised, double-blind, placebo-controlled, phase
3 trial. Lancet Oncol. 24:646–657. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Robertson JFR, Bondarenko IM, Trishkina E,
Dvorkin M, Panasci L, Manikhas A, Shparyk Y, Cardona-Huerta S,
Cheung KL, Philco-Salas MJ, et al: Fulvestrant 500 mg versus
anastrozole 1 mg for hormone receptor-positive advanced breast
cancer (FALCON): An international, randomised, double-blind, phase
3 trial. Lancet. 388:2997–3005. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Robertson JF, Lindemann JP,
Llombart-Cussac A, Rolski J, Feltl D, Dewar J, Emerson L, Dean A
and Ellis MJ: Fulvestrant 500 mg versus anastrozole 1 mg for the
first-line treatment of advanced breast cancer: Follow-up analysis
from the randomized ‘FIRST’ study. Breast Cancer Res Treat.
136:503–511. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Llombart-Cussac A, Pérez-García JM, Bellet
M, Dalenc F, Gil-Gil M, Ruíz-Borrego M, Gavilá J, Sampayo-Cordero
M, Aguirre E, Schmid P, et al: Fulvestrant-Palbociclib vs
Letrozole-Palbociclib as initial therapy for Endocrine-sensitive,
hormone Receptor-positive, ERBB2-negative advanced breast cancer: A
randomized clinical trial. JAMA Oncol. 7:1791–1799. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Johnston S, Pippen J Jr, Pivot X,
Lichinitser M, Sadeghi S, Dieras V, Gomez HL, Romieu G, Manikhas A,
Kennedy MJ, et al: Lapatinib combined with letrozole versus
letrozole and placebo as first-line therapy for postmenopausal
hormone receptor-positive metastatic breast cancer. J Clin Oncol.
27:5538–5546. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Goetz MP, Toi M, Huober J, Sohn J, Tredan
O, Park H, Campone M, Chen SC, Sanchez LM, Shahir A, et al: LBA15
MONARCH 3: Interim overall survival (OS) results of abemaciclib
plus a nonsteroidal aromatase inhibitor (NSAI) in patients (pts)
with HR+, HER2-advanced breast cancer (ABC). Ann Oncol. 33 (Suppl
7):S13842022. View Article : Google Scholar
|
|
22
|
Goetz MP, Toi M, Campone M, Sohn J,
Paluch-Shimon S, Huober J, Park IH, Trédan O, Chen SC, Manso L, et
al: MONARCH 3: Abemaciclib as initial therapy for advanced breast
cancer. J Clin Oncol. 35:3638–3646. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Neven P, Johnston SRD, Toi M, Sohn J,
Inoue K, Pivot X, Burdaeva O, Okera M, Masuda N, Kaufman PA, et al:
MONARCH 2: Subgroup analysis of patients receiving abemaciclib plus
fulvestrant as first-line and second-line therapy for HR+,
HER2-advanced breast cancer. Clin Cancer Res. 27:5801–5809. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Finn RS, Crown JP, Lang I, Boer K,
Bondarenko IM, Kulyk SO, Ettl J, Patel R, Pinter T, Schmidt M, et
al: The cyclin-dependent kinase 4/6 inhibitor palbociclib in
combination with letrozole versus letrozole alone as first-line
treatment of oestrogen receptor-positive, HER2-negative, advanced
breast cancer (PALOMA-1/TRIO-18): A randomised phase 2 study.
Lancet Oncol. 16:25–35. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Finn RS, Boer K, Bondarenko I, Patel R,
Pinter T, Schmidt M, Shparyk YV, Thummala A, Voitko N, Bananis E,
et al: Overall survival results from the randomized phase 2 study
of palbociclib in combination with letrozole versus letrozole alone
for first-line treatment of ER+/HER2-advanced breast cancer
(PALOMA-1, TRIO-18). Breast Cancer Res Treat. 183:419–428. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Hortobagyi GN, Stemmer SM, Burris HA, Yap
YS, Sonke GS, Hart L, Campone M, Petrakova K, Winer EP, Janni W, et
al: Overall survival with ribociclib plus letrozole in advanced
breast cancer. N Engl J Med. 386:942–950. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Xu B, Hu X, Li W, Sun T, Shen K, Wang S,
Cheng Y, Zhang Q, Cui S, Tong Z, et al: Palbociclib plus letrozole
versus placebo plus letrozole in Asian postmenopausal women with
oestrogen receptor-positive/human epidermal growth factor receptor
2-negative advanced breast cancer: Primary results from PALOMA-4.
Eur J Cancer. 175:236–245. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Albanell J, Martínez MT, Ramos M, O'Connor
M, de la Cruz-Merino L, Santaballa A, Martínez-Jañez N, Moreno F,
Fernández I, Alarcón J, et al: Randomized phase II study of
fulvestrant plus palbociclib or placebo in endocrine-sensitive,
hormone receptor-positive/HER2-advanced breast cancer:
GEICAM/2014-12 (FLIPPER). Eur J Cancer. 161:26–37. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Tripathy D, Im SA, Colleoni M, Franke F,
Bardia A, Harbeck N, Hurvitz SA, Chow L, Sohn J, Lee KS, et al:
Ribociclib plus endocrine therapy for premenopausal women with
hormone-receptor-positive, advanced breast cancer (MONALEESA-7): A
randomised phase 3 trial. Lancet Oncol. 19:904–915. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Slamon DJ, Neven P, Chia S, Jerusalem G,
De Laurentiis M, Im S, Petrakova K, Valeria Bianchi G, Martín M,
Nusch A, et al: Ribociclib plus fulvestrant for postmenopausal
women with hormone receptor-positive, human epidermal growth factor
receptor 2-negative advanced breast cancer in the phase III
randomized MONALEESA-3 trial: Updated overall survival. Ann Oncol.
32:1015–1024. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Hortobagyi GN, Stemmer SM, Burris HA, Yap
YS, Sonke GS, Paluch-Shimon S, Campone M, Petrakova K, Blackwell
KL, Winer EP, et al: Updated results from MONALEESA-2, a phase III
trial of first-line ribociclib plus letrozole versus placebo plus
letrozole in hormone receptor-positive, HER2–negative advanced
breast cancer. Ann Oncol. 29:1541–1547. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Finn RS, Rugo HS, Dieras VC, Harbeck N, Im
SA, Gelmon KA, Walshe JM, Martin M, Mac Gregor MC, Bananis E, et
al: Overall survival (OS) with first-line palbociclib plus
letrozole (PAL+ LET) versus placebo plus letrozole (PBO+ LET) in
women with estrogen receptor-positive/human epidermal growth factor
receptor 2-negative advanced breast cancer (ER+/HER2-ABC): Analyses
from PALOMA-2. Am Soc Clin Oncol. 402022.
|
|
33
|
Rugo HS, Finn RS, Diéras V, Ettl J,
Lipatov O, Joy AA, Harbeck N, Castrellon A, Iyer S, Lu DR, et al:
Palbociclib plus letrozole as first-line therapy in estrogen
receptor-positive/human epidermal growth factor receptor 2-negative
advanced breast cancer with extended follow-up. Breast Cancer Res
Treat. 174:719–729. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Lu YS, Im SA, Colleoni M, Franke F, Bardia
A, Cardoso F, Harbeck N, Hurvitz S, Chow L, Sohn J, et al: Updated
overall survival of ribociclib plus endocrine therapy versus
endocrine therapy alone in Pre- and Perimenopausal patients with
HR+/HER2-advanced breast cancer in MONALEESA-7: A phase III
randomized clinical trial. Clin Cancer Res. 28:851–859. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Bergh J, Jönsson PE, Lidbrink EK, Trudeau
M, Eiermann W, Brattström D, Lindemann JP, Wiklund F and Henriksson
R: FACT: An open-label randomized phase III study of fulvestrant
and anastrozole in combination compared with anastrozole alone as
first-line therapy for patients with receptor-positive
postmenopausal breast cancer. J Clin Oncol. 30:1919–1925. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Skibinski A and Kuperwasser C: The origin
of breast tumor heterogeneity. Oncogene. 34:5309–5316. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Lainetti PF, Leis-Filho AF, Laufer-Amorim
R, Battazza A and Fonseca-Alves CE: Mechanisms of resistance to
chemotherapy in breast cancer and possible targets in drug delivery
systems. Pharmaceutics. 12:11932020. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Parisi S, Ruggiero R, Gualtieri G, Volpe
ML, Rinaldi S, Nesta G, Bogdanovich L, Lucido FS, Tolone S,
Parmeggiani D, et al: Combined LOCalizer™ and intraoperative
ultrasound localization: First experience in localization of
Non-palpable breast cancer. In Vivo. 35:1669–1676. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Parisi S, Gambardella C, Conzo G, Ruggiero
R, Tolone S, Lucido FS, Iovino F, Fisone F, Brusciano L,
Parmeggiani D and Docimo L: Advanced localization technique for
Non-Palpable breast cancer: Radiofrequency alone VS combined
technique with ultrasound. J Clin Med. 12:50762023. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Gambardella C, Clarizia G, Patrone R, Offi
C, Mauriello C, Romano R, Filardo M, Conzo A, Sanguinetti A,
Polistena A, et al: Advanced hemostasis in axillary lymph node
dissection for locally advanced breast cancer: New technology
devices compared in the prevention of seroma formation. BMC Surg.
18 (Suppl 1):S1252019. View Article : Google Scholar
|
|
41
|
Spring LM, Wander SA, Andre F, Moy B,
Turner NC and Bardia A: Cyclin-dependent kinase 4 and 6 inhibitors
for hormone receptor-positive breast cancer: Past, present, and
future. Lancet. 395:817–827. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Chen P, Lee NV, Hu W, Xu M, Ferre RA, Lam
H, Bergqvist S, Solowiej J, Diehl W, He YA, et al: Spectrum and
degree of CDK drug interactions predicts clinical performance. Mol
Cancer Ther. 15:2273–2281. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Marra A and Curigliano G: Are all
cyclin-dependent kinases 4/6 inhibitors created equal? NPJ Breast
Cancer. 5:272019. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Spring LM, Zangardi ML, Moy B and Bardia
A: Clinical management of potential toxicities and drug
interactions related to Cyclin-dependent kinase 4/6 inhibitors in
breast cancer: Practical considerations and recommendations.
Oncologist. 22:1039–1048. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Finn RS, Martin M, Rugo HS, Jones S, Im
SA, Gelmon K, Harbeck N, Lipatov ON, Walshe JM, Moulder S, et al:
Palbociclib and letrozole in advanced breast cancer. N Engl J Med.
375:1925–1936. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Zhang QY, Sun T, Yin YM, Li HP, Yan M,
Tong ZS, Oppermann CP, Liu YP, Costa R, Li M, et al: MONARCH plus:
Abemaciclib plus endocrine therapy in women with HR+/HER2-advanced
breast cancer: The multinational randomized phase III study. Ther
Adv Med Oncol. 12:17588359209639252020. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Hortobagyi GN, Stemmer SM, Burris HA, Yap
YS, Sonke GS, Paluch-Shimon S, Campone M, Blackwell KL, André F,
Winer EP, et al: Ribociclib as first-line therapy for HR-positive,
advanced breast cancer. N Engl J Med. 375:1738–1748. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Roncato R, Angelini J, Pani A, Cecchin E,
Sartore-Bianchi A, Siena S, De Mattia E, Scaglione F and Toffoli G:
CDK4/6 inhibitors in breast cancer treatment: Potential
interactions with drug, gene, and pathophysiological conditions.
Int J Mol Sci. 21:63502020. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Horie T, Kijima T, Yamaguchi M, Honda S,
Horie M, Ishitobi K, Yamagata S, Sakano S and Kurokohchi K: Severe
hypoglycaemia under abemaciclib administration in a patient with
breast cancer: A case report. Mol Clin Oncol. 14:612021. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Lagampan C, Poovorawan N and
Parinyanitikul N: Lactic acidosis, a potential toxicity from
drug-drug interaction related to concomitant ribociclib and
metformin in preexisting renal insufficiency: A case report. Cancer
Rep (Hoboken). 5:e15752022. View Article : Google Scholar : PubMed/NCBI
|