Introduction
Lung cancer (LC) is one of the leading causes of
cancer-related morbidity and mortality worldwide (1,2). In
2020, ~2.2 million new cases and 1.8 million deaths associated with
LC were reported, accounting for ~11% of all cancer diagnoses and
18% of cancer deaths (3). In
addition, data from 2017 indicate that LC was responsible for 40
million disability-adjusted life years (4). It has a notable prevalence among the
elderly population, with an average age of diagnosis of ~70 years
(5).
With recent advances in diagnostic techniques,
treatment modalities and personalized medicine, there are more
efforts to better understand several factors that may impact the
course and progression of LC (6–8).
Numerous studies are focusing on the interplay between systemic
health conditions, such as renal dysfunction, and cancer outcomes.
Renal dysfunction, which may range from a mild decline in
glomerular filtration rate (GFR) to end-stage renal disease
(9), affects millions of
individuals globally (10). Its
intricate relationship with cancer outcomes has emerged as an area
of growing interest in oncology (9–11).
Kidneys can potentially influence cancer progression and treatment
response through mechanisms that may include altered drug
metabolism, immune system modulation, inflammation and disruption
of hormonal pathways (11–13). Renal insufficiency (RI) is
particularly prevalent in elderly patients, where there is an
annual decline of ~1% in the average creatinine clearance (14).
In several cancer types, the diagnosis of chronic
kidney disease (CKD) as a comorbidity has been associated with
unfavorable prognoses (15).
However, the exact impact of CKD on the prognosis of patients with
LC remains a subject of debate. Whilst certain studies have
reported no significant association between CKD and overall
survival (OS) and/or disease-free survival (DFS) in patients with
LC, others report different outcomes (16–18).
These discrepancies could be attributed to differences in study
populations, methodologies and outcome measures. To date, no
systematic reviews and meta-analyses on the influence of RI and/or
CKD on LC outcomes have been performed, to the best of our
knowledge. Therefore, the present study aimed to systematically
assess and quantify the association between renal dysfunction
and/or CKD and the survival outcomes (OS and DFS) of LC.
Materials and methods
Search for relevant literature
An extensive systematic literature search of the
PubMed (https://pubmed.ncbi.nlm.nih.gov/), Embase (https://www.embase.com/landing?status=grey) and Scopus
(https://www.scopus.com/home.uri)
databases was performed. The search method of the present study
incorporated relevant keywords, synonyms and Medical Subject
Headings (MeSH) terms. Furthermore, search terms that allowed
keyword placement specification were used. In PubMed, the (tiab)
tag was used to search for key words in titles and abstracts. The
search strategies used in the PubMed, Embase and Scopus databases
are presented in Table SI.
Notably, the term ‘renal injury’ was not included in the search
strategies as it tends to refer to acute conditions, such as acute
kidney injury, which are different from chronic conditions, such as
CKD, that the present study intended to focus on. Moreover, the
present study aimed to assess the intersection of CKD and LC,
specifically looking at long-term clinical outcomes, and using
specific search terms ensured that the studies relevant to chronic
conditions were retrieved, rather than acute episodes of kidney
damage, which have different etiologies, treatment approaches and
outcomes.
The searches were limited to human studies and
articles published in the English language. Furthermore, the search
scope was confined to studies published from the inception of the
databases up to July 31st, 2023. Reference lists and relevant
review articles were also manually reviewed to complement the
electronic search and ensure the inclusion of any potentially
overlooked studies.
The study inclusion criteria were as follows: i)
Observational studies (cohort and case-control) and clinical trials
that evaluated the association between RI/CKD and OS and/or DFS in
patients with LC; ii) studies in adult human populations with
confirmed LC diagnoses; iii) studies with the criteria for RI or
CKD specified; iv) studies with a comparator group (normal renal
function); and v) studies reporting relevant outcomes, such as OS,
DFS and progression-free survival. The study exclusion criteria
were as follows: i) Studies with overlapping or insufficient data;
ii) studies that used inappropriate comparators i.e., comparison
group does not comprise subjects with LC and normal kidney
function, or the study did not have any comparison group; and iii)
review articles, conference abstracts and case reports.
Screening, selection and data
extraction
After performing the search strategy across the
three databases and assembling the initial pool of studies,
duplicate entries were removed. After an initial assessment of
titles and abstracts, two independent study authors performed a
comprehensive review of full-text articles, evaluating the
eligibility of each study based on the predetermined criteria. In
the event of differences in judgment, disagreements were resolved
through discussion, and if necessary, the perspective of a third
author was sought.
To enhance reliability throughout the article
selection process, study authors involved in the screening and
selecting articles underwent training sessions that included
detailed guidelines and examples illustrating the application of
inclusion and exclusion criteria. Calibration exercises were
performed to ensure consistency in decision-making. Inter-rater
reliability statistic, namely, Cohen's κ coefficient, was
calculated to assess agreement between study authors (κ ranged from
0.88–0.97). Meetings were held regularly to discuss challenging
cases and clarify ambiguous situations, ensuring all decisions were
well-founded and transparently documented.
The present research adhered to the Preferred
Reporting Items for Systematic reviews and Meta-Analyses guidelines
to ensure the transparency and rigor of the methodology (19). The study protocol was formally
registered in the International Prospective Register of Systematic
Reviews (registration no. CRD42023455318). A total of two authors
independently extracted data into a standardized form. The
Newcastle-Ottawa Scale (NOS) assessed the quality of the included
studies (20).
Statistical analysis
Statistical analyses were performed using STATA
version 15.0 (StataCorp LP). As the outcomes were categorical and
observed across extended follow-up periods, the combined effect
size is presented as the hazard ratio (HR) and 95% confidence
interval (CI). A random-effects model was used for all the analyses
in the present study to account for variances in the baseline
characteristics among the studies included. Group analyses were
based on the stage and histological type of LC, the chosen
treatment approach and the criteria for assessing RI/CKD. To
evaluate potential publication bias, Egger's test and funnel plots
were used (21). P<0.05 was
considered to indicate a statistically significant difference.
Results
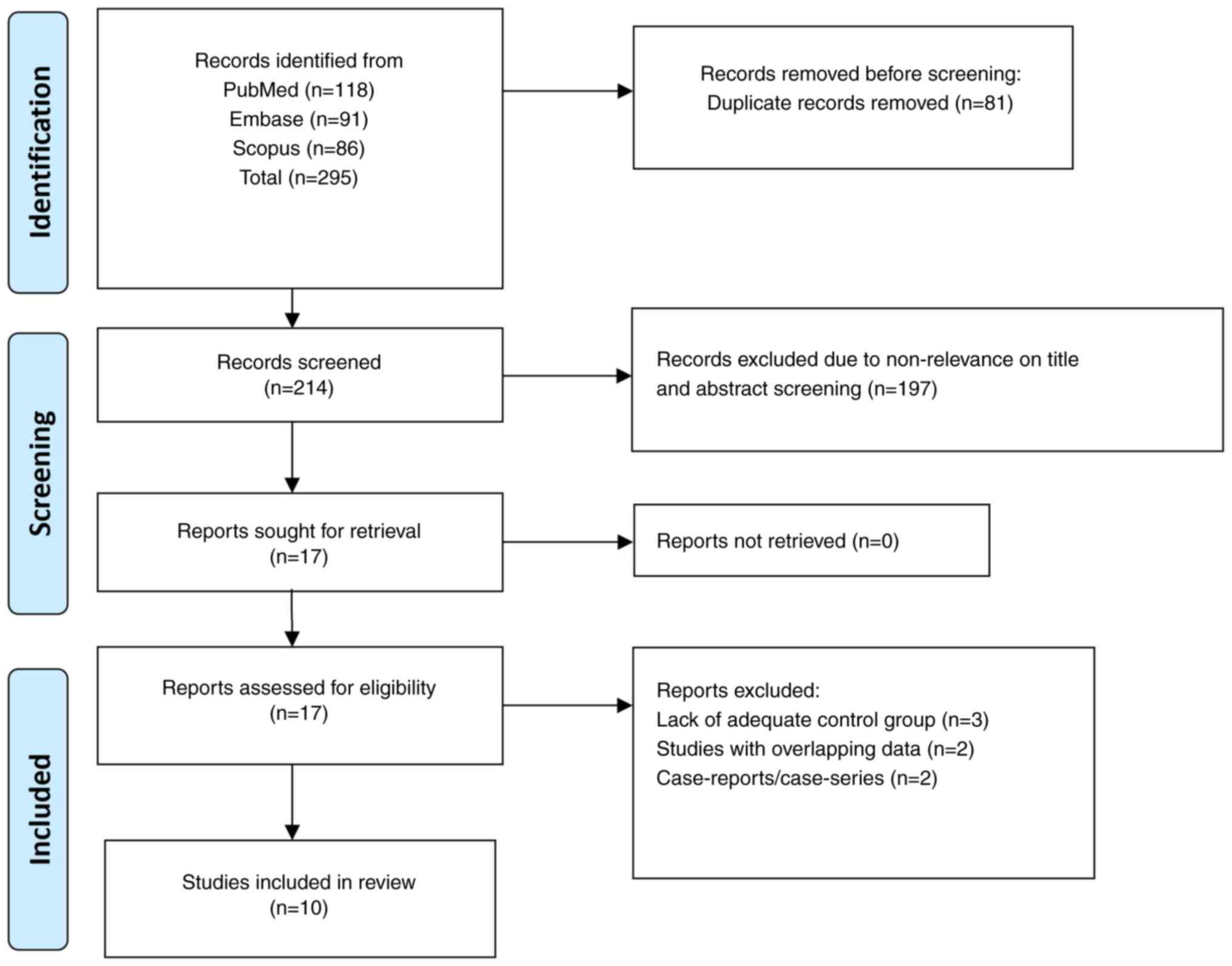
Flow of study selection
The search approach identified a total of 295
studies. After removing 81 duplicates, the remaining 214 studies
underwent initial screening based on their titles and abstracts,
and an additional 197 reports were removed. These reports were
excluded because, based on the title and the abstract, the articles
were found to be non-relevant to the scientific question being
addressed. Full-text examination of the remaining 17 studies
resulted in the exclusion of an additional seven studies. Out of
these seven excluded studies, three studies did not have an
appropriate control group, two studies had data that overlapped
with studies already included for the meta-analysis and two were
case reports. Ultimately, the meta-analysis included 10 studies
(16–18,22–28).
Fig. 1 illustrates the selection
process of the studies in the present review.
Characteristics of the included
studies
Table I provides
details of the included studies. All studies were observational in
design and used retrospective data. Studies were performed in Japan
(n=2), the United States (n=2), China (n=2), Taiwan (n=2), the
Republic of Korea (n=1) and France (n=1). The duration of follow-up
was 12 months to 5 years. In seven studies, most of the patients
had non-small cell (NSC) LC. In four studies, most patients had
stage I or II LC, and in five, patients had stage III or IV tumors.
A total of four studies reported predominantly surgical management
of LC, whilst in another 4 studies, medical management (such as
chemotherapy, radiotherapy or targeted therapy) was used. A total
of two studies did not report on the management modality. The
diagnosis of RI or CKD was based on different criteria in the
included studies: A total of three studies used International
Statistical Classification of Diseases (ICD)-9-based diagnoses
(29), and another three studies
used the CKD-Epidemiology Collaboration (CKD-EPI) equation
(30) and considered an estimated
(e)GFR of <60 ml/min/1.73 m2 to denote the presence
of RI/CKD (31). The remaining
studies used definitions of RI/CKD based on serum creatinine, the
Cockcroft-Gault formula and the Modification of Diet in Renal
Disease equation (32,33). A total of five studies reported
marked baseline differences in the prevalence of comorbidities
among patients with and without RI/CKD. Patients with RI/CKD had a
notably higher incidence of diabetes, hypertension, cardiovascular
diseases and anemia. All included studies reported adjusted effect
sizes using multivariable regression analysis. Factors such as age,
sex, comorbidities and other relevant parameters differing between
groups, were accounted for in these analyses. The total number of
included patients in all 10 studies was 35,249 (4,302 patients with
RI and 30,947 patients without RI). The mean quality assessment
score of all studies was 7.1 (acceptable quality). A total of seven
studies had a NOS score of 7 (with 9 being the maximum), two had a
score of 8, and one study had a score of 6 (Table I).
 | Table I.Study details. |
Table I.
Study details.
| First author/s,
year | Study design | Location | Age | Sex | Tumor type and
stage | Mode of
management | Follow-up
duration | Definitions
used | Sample size | Newcastle-Ottawa
quality score | Adjustment/matching
variables | (Refs.) |
|---|
| Lu et al,
2023 | Retrospective
observational | Taiwan | Median age of ~68
years | Male (64%) | NSCLC (78%); stage
III–IV (70%) | Medical treatment
(chemotherapy and/or radiotherapy or targeted therapy) in 40% | Not reported | End-stage renal
disease defined as per ICD-9 code 585 | CKD, n=133 and no
CKD, n=532 | 7 | Matching performed
for age, sex, cancer histological type, cancer stage and treatment
type | (16) |
| Saito et al,
2022 | Retrospective
observational | Japan | Median age of 69
years | Male (~60%) | Subjects had NSCLC
with stage I–II | Surgery | ~5 years | RI defined as serum
creatinine ≥1.5 mg/dl | RI, n=113 and no
RI, n=16,169 | 7 | Age, sex, renal
function status, smoking, comorbidities, pathological stage,
histological type, grade and surgical procedure | (17) |
| Cho et al,
2021 | Retrospective
observational | Republic of
Korea | Mean age of ~64
years | Male (~70%) | Subjects had NSCLC
with stage III–IV | Surgery with or
without chemotherapy (52%) | Mean follow up: 2.6
years | RI defined as eGFR
<60 ml/min/1.73 m2; eGFR calculated based on CKD-EPI
equation | RI, n=1,419 and no
RI, n=1,783 | 7 | Age, sex, BMI,
smoking, initial eGFR, anemia, hyponatremia and pathological
subtype | (22) |
| Jia et al,
2020 | Retrospective
observational | China | 54% aged >60
years | Male (68%) | Subjects had NSCLC;
stage I–II (86%) | Radical
surgery | Not reported | CKD defined as eGFR
<60 ml/min/1.73 m2; eGFR calculated based on CKD-EPI
equation | CKD, n=63 and no
CKD, n=77 | 7 | Age, sex, smoking,
drinking, TNM stage, tumor histology/differentiation/maximum tumor
diameter, eGFR, adjuvant or neoadjuvant treatment, and anemia | (23) |
| Magali et
al, 2020 | Retrospective
observational | France | Mean age of ~60
years | Male (~60%) | Subjects had NSCLC
with stage III–IV | Medical treatment
(chemotherapy) | 12 months | RI defined as eGFR
60–89 ml/min/1.73 m2; eGFR calculated using the MDRD
formula | RI, n=25 and no RI,
n=87 | 7 | Age, sex, GFR at
diagnosis, cardiovascular disease, diabetes, malnutrition, and
cumulated dose of cisplatin and pemetrexed | (24) |
| Yamamoto et
al, 2019 | Retrospective
observational | Japan | Mean age of ~70
years | Male (58%) | Subjects had NSCLC;
Majority with stage I–II | Surgical
(lobectomy) | Median follow-up of
~50 months | CKD defined as eGFR
<60 ml/min/1.73 m2; eGFR calculated based on Clinical
Practice Guideline book for the diagnosis and treatment of CKD
(2012) published by Japanese Society of Nephrology | CKD, n=55 and no
CKD, n=616 | 8 | Age, sex, smoking,
hypertension, diabetes, COPD, histology, pathological stage,
post-operative complication and surgical approach (VATS or
thoracotomy) | (25) |
| Wei et al,
2018 | Retrospective
observational | Taiwan | Mean age of ~75
years early stage and | 73% males | Early stage;
histologic type of tumor not reported | ~80% received
non-surgical treatment | ~5 years | Diagnosis of CKD
was determined using specific ICD codes and one of the following
criteria: i) ≥3 outpatient visits within a 6-month period, each
with a diagnosis of CKD; or ii) inpatients with diagnosis of CKD on
admission | CKD, n=2,269 and no
CKD, n=9,076 | 7 | Age, type of
surgery and multiple comorbidities (hypertension, diabetes mellitus
and COPD, congestive heart failure, cholelithiasis and
hyperlipidemia) | (18) |
| Yang et al,
2016 | Retrospective
observational | China | Mean age of 53
years | Male (59%) | Histologic type of
tumor not reported; Majority with stage III–IV | Details on mode of
management not provided | Median follow-up of
40 months | RI defined as eGFR
<60 ml/min/1.73 m2; eGFR calculated based on CKD-EPI
equation | RI, n=56 and no
CKD, n=1,323 | 8 | Age, sex, eGFR,
tumor stage, hypertension, diabetes, CVD, smoking and presence of
proteinuria | (26) |
| Kutluk Cenik et
al, 2013 | Retrospective
observational | United States | Mean age of ~58
years | Male (57%) | Subjects had NSCLC
with stage III–IV | Medical treatment
(chemotherapy) | Not provided | CKD defined as eGFR
<60 ml/min/1.73 m2; eGFR calculated using the CKD-CG
formula | CKD, n=101 and no
CKD, n=197 | 7 | Age, sex and
ethnicity | (27) |
| Tammemagi et
al, 2003 | Retrospective
observational | United States | Not reported | Male (59%) | Not reported | Not reported | Median follow up of
819 days | End-stage renal
disease defined as per ICD-9 | CKD, n=68 and no
CKD, n=1,087 | 6 | Age, sex, smoking
status, histology and stage | (28) |
Findings for the outcomes of
interest
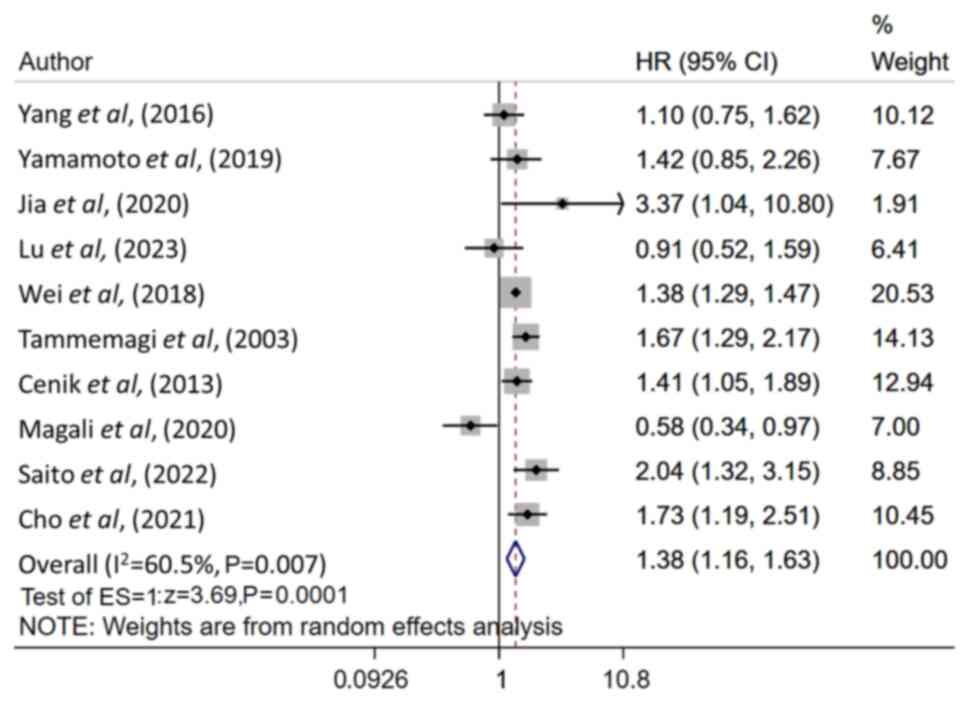
Patients with RI/CKD had a notably worse OS at
follow-up (HR, 1.38; 95% CI, 1.16–1.63; n=10; I2=60.5%)
compared with patients without RI/CKD (Fig. 2). There was no apparent publication
bias demonstrated by Egger's test (P=0.98) and the funnel plot
(Fig. S1). Subgroup analysis
demonstrated a marked association between poor OS and RI/CKD in
patients with stage I/II LC (HR, 1.76; 95% CI, 1.30–2.37; n=5;
I2=58.6%), but not in patients with stage III/IV LC (HR,
1.18; 95% CI, 0.91, 1.54; n=6; I2=64.6%; Table II). Furthermore, when subgroup
analysis was done based on methods to diagnose CKD, studies that
used an ICD-9-based diagnosis reported a notable association
between presence of RI/CKD and poor OS (HR, 1.40; 95% CI,
1.14–1.71; n=3; I2=52.0%), when compared to those with
LC and no associated RI/CKD. No such association was detected in
studies that used an CKD-EPI-based diagnosis (HR, 1.55; 95% CI,
0.97–2.47; n=3, I2=58.6%; Table II). Similarly, we conducted
subgroup analysis based on the histologic type of LC. In this
analysis, when only studies with patients with NSCLC were analyzed,
a marked association between RI/CKD and poor OS was demonstrated
(HR, 1.54; 95% CI, 1.22–1.94; n=7; I2=33.8%), compared
to those with LC and no associated RI/CKD. Irrespective of the
treatment modality, RI/CKD had a notable association with poor OS
at follow-up (surgical management: HR, 1.78; 95% CI, 1.40–2.27;
n=4; I2=0.0% and medical management: HR, 1.37; 95% CI,
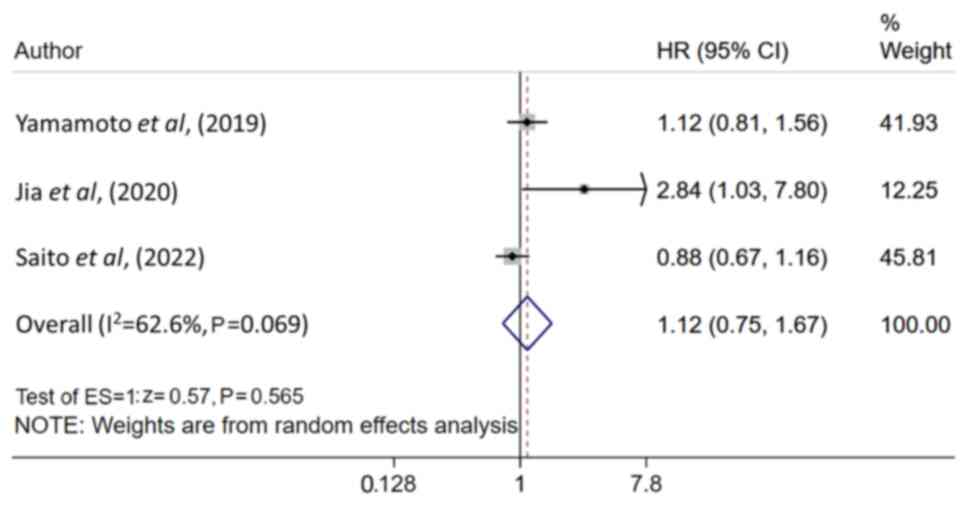
1.25–1.50; n=4; I2=6.3%; Table II). Patients with LC with RI/CKD
had statistically similar DFS, compared to patients with LC and no
associated RI/CKD (HR, 1.12; 95% CI, 0.75–1.67; n=3;
I2=62.6%; Fig. 3), with
no apparent publication bias demonstrated using Egger's test
(P=0.204) and the funnel plot (Fig.
S2).
 | Table II.Overall survival subgroup
analysis. |
Table II.
Overall survival subgroup
analysis.
| Variable | n | HR (95% CI) | I2,
% |
|---|
| Stage |
|
|
|
|
I/II | 5 | 1.76
(1.30–2.37)a | 58.6 |
|
III/IV | 6 | 1.18
(0.91–1.54) | 64.6 |
| Criteria for
calculating eGFR |
|
|
|
|
CKD-EPI | 3 | 1.55
(0.97–2.47) | 58.6 |
|
ICD-9 | 3 | 1.40
(1.14–1.71)a | 52.0 |
| Tumor type
(non-small cell lung cancer) | 7 | 1.54
(1.22–1.94)a | 33.8 |
| Primary
management |
|
|
|
|
Surgery | 4 | 1.78
(1.40–2.27)a | 0.0 |
| Medical
(chemotherapy/radiotherapy/targeted therapy) | 4 | 1.37
(1.25–1.50)a | 6.3 |
Discussion
The results of the present study indicate that
RI/CKD is associated with a poor OS of patients with LC,
particularly in patients with stage I/II cancer. This finding
underscores the importance of identifying renal function status
early in the diagnostic process and considering it as a crucial
factor in treatment decision-making. The finding related to reduced
OS remained consistent regardless of the treatment modality
(surgical intervention or medical management). The consistency of
the association across different treatment modalities suggests that
the influence of RI/CKD on OS is not limited to a specific
therapeutic approach. Therefore, renal function should be
considered a key factor affecting prognosis and treatment planning
regardless of the proposed treatment method. The observed lack of a
significant difference in DFS between patients with and without
RI/CKD suggests that renal function may have a more pronounced
impact on long-term survival rather than on the occurrence of new
disease. This result highlights the need for further research into
the potential mechanisms behind this association.
Factors such as altered drug metabolism, impaired
immune function and increased vulnerability to treatment-related
toxicities due to renal dysfunction could contribute to the
observed effect on OS (11–13,34,35).
Patients with RI or CKD often face challenges in receiving optimal
chemotherapy and other treatments due to compromised kidney
function (36). Additionally, the
usual presence of multiple comorbidities such as cardiovascular
disease and diabetes in these patients may exacerbate overall
health status and treatment tolerance (37,38).
Moreover, at times, delays in cancer diagnosis and treatment
initiation may occur due to presence of co-morbidities, including
renal dysfunction, potentially allowing the disease to progress to
more advanced stages (39,40). Recent studies suggest several
potential molecular mechanisms underlying these associations. CKD
induces systemic inflammation and immune dysfunction, creating a
microenvironment that promotes tumor growth (41,42).
Furthermore, impaired renal function can alter the metabolism and
pharmacokinetics of anticancer drugs, leading to suboptimal drug
levels or increased toxicity, which may affect treatment efficacy
(43,44). Additionally, renal dysfunction is
associated with increased oxidative stress and DNA damage,
processes that contribute to cancer progression and resistance to
therapy (45,46).
The observed disparity in findings between studies
that used an ICD-9-based diagnosis of CKD and those employing other
objective criteria, such as the CKD-EPI, raises questions regarding
the influence of diagnostic criteria on the reported associations
with survival outcomes. ICD-9 codes are primarily used for
administrative and billing purposes and may not capture subtle
variations in kidney function that could be relevant for predicting
survival outcomes (47).
Additionally, ICD-9-based diagnoses may capture more severe cases
of CKD, as these codes are often assigned when the condition has
progressed significantly enough to necessitate medical attention or
intervention. Conversely, the objective criteria of the CKD-EPI
equation consider several factors such as age, sex and serum
creatinine levels, and may, therefore, be more sensitive and
specific in assessing kidney function (31–33).
As a result, studies relying on these objective criteria may
provide a more accurate representation of kidney function
status.
The association observed between RI/CKD and poor OS
in studies that specifically focused on NSCLC could have important
implications for understanding the relationship between renal
function and survival outcomes in this particular cancer subtype.
When narrowing the analysis to studies that focused on NSCLC, the
sample size may become more homogenous regarding cancer type,
treatment modalities and patient demographics. This homogeneity
(I2≤40% in the present study) may reduce the confounding
effects of these variables, making it easier to detect the
influence of RI/CKD on OS. In heterogeneous patient groups that
include several cancer types, the effects of renal function may be
masked by other factors that differ among the included studies.
The results of the present study may have important
clinical implications. RI/CKD is a common comorbidity, especially
among the elderly who have associated lung cancer (48). Close monitoring of renal function
and potential adjustment of treatment plans to account for altered
drug metabolism and potential treatment-related toxicities may help
to optimize patient outcomes. However, despite the valuable
insights provided by the present meta-analysis, certain limitations
should be acknowledged: The included studies were retrospective in
design, and the unadjusted confounders may have impacted the
results. Heterogeneity across included studies, differences in
sample sizes, treatment protocols, tumor grades, histologic types,
and variations in the definition of RI/CKD may also have influenced
the results. Another limitation is that the medical management of
RI/CKD and its potential impact on the association with LC outcomes
could not be assessed. Upon reviewing the included studies, it was
demonstrated that they primarily focused on reporting LC
characteristics and treatments rather than detailed information on
the management of RI/CKD itself. Consequently, limited data are
available within the reviewed studies to perform a comprehensive
analysis of how specific medical treatments for RI/CKD may
influence the outcomes of patients with LC. It is of critical
importance to investigate the role of RI/CKD management in
influencing LC outcomes. Future studies specifically designed to
assess the impact of renal treatments, including nephroprotective
strategies during cancer therapy and optimization of renal
function, would provide valuable insights into improving outcomes
for patients with LC with pre-existing renal dysfunction.
In conclusion, the results of the present
meta-analysis shed light on the association between RI/CKD and poor
OS in patients with LC. The findings underscore the need for a
holistic approach to patient care that includes a thorough
assessment of renal function early in the diagnostic process and
its incorporation into treatment decisions. However, further
studies are needed to clarify the mechanisms of this association
and to develop approaches that can optimize outcomes for patients
with LC with compromised renal function
Supplementary Material
Supporting Data
Supporting Data
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
HQ and SL conceived and designed the study. HQ, SL
and ZH were involved in acquisition of data, analysis, and
interpretation of data. HQ and SL were involved in the writing of
the manuscript and ZH was involved in revising it critically for
important intellectual content. All authors have read and approved
the final manuscript. HQ, SL and ZH confirm the authenticity of all
the raw data.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Barta JA, Powell CA and Wisnivesky JP:
Global epidemiology of lung cancer. Ann Glob Health. 85:82019.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Thandra KC and Barsouk A, Saginala K,
Aluru JS and Barsouk A: Epidemiology of lung cancer. Contemp Oncol
(Pozn). 25:45–52. 2021.PubMed/NCBI
|
|
3
|
Sung H, Ferlay J, Siegel RL, Laversanne M,
Soerjomataram I, Jemal A and Bray F: Global cancer statistics 2020:
GLOBOCAN estimates of incidence and mortality worldwide for 36
cancers in 185 countries. CA Cancer J Clin. 71:209–249. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
GBD 2021 Diseases and Injuries
Collaborators, . Global incidence, prevalence, years lived with
disability (YLDs), disability-adjusted life-years (DALYs), and
healthy life expectancy (HALE) for 371 diseases and injuries in 204
countries and territories and 811 subnational locations, 1990–2021:
A systematic analysis for the Global Burden of Disease Study 2021.
Lancet. 403:2133–2161. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Dela Cruz CS, Tanoue LT and Matthay RA:
Lung cancer: Epidemiology, etiology, and prevention. Clin Chest
Med. 32:605–644. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Garinet S, Wang P, Mansuet-Lupo A, Fournel
L, Wislez M and Blons H: Updated prognostic factors in localized
NSCLC. Cancers (Basel). 14:14002022. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Puderecki M, Szumiło J and Marzec-Kotarska
B: Novel prognostic molecular markers in lung cancer. Oncol Lett.
20:9–18. 2020.PubMed/NCBI
|
|
8
|
Mahar AL, Compton C, McShane LM, Halabi S,
Asamura H, Rami-Porta R and Groome PA; Molecular Modellers Working
Group of American Joint Committee on Cancer, : Refining prognosis
in lung cancer: A report on the quality and relevance of clinical
prognostic tools. J Thorac Oncol. 10:1576–1589. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Dimopoulos MA and Terpos E: Renal
insufficiency and failure. Hematology Am Soc Hematol Educ Program.
2010:431–436. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kovesdy CP: Epidemiology of chronic kidney
disease: An update 2022. Kidney Int Suppl (2011). 12:7–11. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Stengel B: Chronic kidney disease and
cancer: A troubling connection. J Nephrol. 23:253–262.
2010.PubMed/NCBI
|
|
12
|
Malyszko J, Tesarova P, Capasso G and
Capasso A: The link between kidney disease and cancer:
Complications and treatment. Lancet. 396:277–287. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Hu M, Wang Q, Liu B, Ma Q, Zhang T, Huang
T, Lv Z and Wang R: Chronic Kidney disease and cancer:
Inter-relationships and mechanisms. Front Cell Dev Biol.
10:8687152022. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Shlipak MG, Katz R, Kestenbaum B, Fried
LF, Newman AB, Siscovick DS, Stevens L and Sarnak MJ: Rate of
kidney function decline in older adults: A comparison using
creatinine and cystatin C. Am J Nephrol. 30:171–178. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lees JS, Elyan BMP, Herrmann SM, Lang NN,
Jones RJ and Mark PB: The ‘other’ big complication: How chronic
kidney disease impacts on cancer risks and outcomes. Nephrol Dial
Transplant. 38:1071–1079. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lu MS, Chen MF, Yang YH, Lee CP, Lin CC,
Tseng YH and Tsai YH: Appraisal of lung cancer survival in patients
with end-stage renal disease. Arch Med Sci. 19:86–93.
2023.PubMed/NCBI
|
|
17
|
Saito T, Murakawa T, Shintani Y, Okami J,
Miyaoka E, Yoshino I and Date H; Japanese Joint Committee of Lung
Cancer Registry, : Preoperative renal dysfunction and long-term
survival after surgery for non-small cell lung cancer. J Thorac
Cardiovasc Surg. 164:227–239.e6. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wei YF, Chen JY, Lee HS, Wu JT, Hsu CK and
Hsu YC: Association of chronic kidney disease with mortality risk
in patients with lung cancer: A nationwide Taiwan population-based
cohort study. BMJ Open. 8:e0196612018. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Page MJ, McKenzie JE, Bossuyt PM, Boutron
I, Hoffmann TC, Mulrow CD, Shamseer L, Tetzlaff JM, Akl EA, Brennan
SE, et al: PRISMA. Transparent reporting of systematic reviews and
meta-analyses. BMJ. 372:n712021. View
Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wells G, Shea B, O'Connell D, Robertson J,
Peterson J, Losos M and Tugwell P: The Newcastle-Ottawa Scale (NOS)
for Assessing the Quality of Nonrandomized Studies in
Meta-analysis. Jan;2024.
|
|
21
|
Egger M, Davey Smith G, Schneider M and
Minder C: Bias in meta-analysis detected by a simple, graphical
test. BMJ. 315:629–634. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Cho S, Kang E, Kim JE, Kang U, Kang HG,
Park M, Kim K, Kim DK, Joo KW, Kim YS, et al: Clinical significance
of acute kidney injury in lung cancer patients. Cancer Res Treat.
53:1015–1023. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Jia W, Wang C and Cheng Y: Decreased
preoperative estimated glomerular filtration rate was related with
poor prognosis of NSCLC patients. Technol Cancer Res Treat.
19:15330338209523552020. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Magali L, Pascal F, Serge A, Mathieu B,
Ayoube Z, Claire T and Christiane M: Better survival in impaired
renal function patients with metastatic non-small cell lung cancer
treated by cisplatin-pemetrexed. Eur J Clin Pharmacol.
76:1573–1580. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Yamamoto Y, Kanzaki R, Kanou T, Ose N,
Funaki S, Minami M and Shintani Y: Long-term outcomes of pulmonary
resection for lung cancer patients with chronic kidney disease.
World J Surg. 43:3249–3258. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Yang Y, Li H-Y, Zhou Q, Peng ZW, An X, Li
W, Xiong LP, Yu XQ, Jiang WQ and Mao HP: Renal function and
all-cause mortality risk among cancer patients. Medicine
(Baltimore). 95:e37282016. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kutluk Cenik B, Sun H and Gerber DE:
Impact of renal function on treatment options and outcomes in
advanced non-small cell lung cancer. Lung Cancer. 80:326–332. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Tammemagi CM, Neslund-Dudas C, Simoff M
and Kvale P: Impact of comorbidity on lung cancer survival. Int J
Cancer. 103:792–802. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
ICD-9-CM official guidelines for coding
and reporting. Aug 23–2011.
|
|
30
|
Levey AS, Stevens LA, Schmid CH, Zhang YL,
Castro AF III, Feldman HI, Kusek JW, Eggers P, Van Lente F, Greene
T, et al: A new equation to estimate glomerular filtration rate.
Ann Intern Med. 150:604–612. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Earley A, Miskulin D, Lamb EJ, Levey AS
and Uhlig K: Estimating equations for glomerular filtration rate in
the era of creatinine standardization: A systematic review. Ann
Intern Med. 156:785–795. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Michels WM, Grootendorst DC, Verduijn M,
Elliott EG, Dekker FW and Krediet RT: Performance of the
Cockcroft-gault, MDRD, and new CKD-EPI formulas in relation to GFR,
age, and body size. Clin J Am Soc Nephrol. 5:1003–1009. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Stevens LA, Schmid CH, Greene T, Zhang YL,
Beck GJ, Froissart M, Hamm LL, Lewis JB, Mauer M, Navis GJ, et al:
Comparative performance of the CKD Epidemiology Collaboration
(CKD-EPI) and the modification of diet in renal disease (MDRD)
study equations for estimating GFR levels above 60 mL/min/1.73 m2.
Am J Kidney Dis. 56:486–495. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Launay-Vacher V, Oudard S, Janus N,
Gligorov J, Pourrat X, Rixe O, Morere JF, Beuzeboc P and Deray G;
Renal Insufficiency and Cancer Medications (IRMA) Study Group, :
Prevalence of Renal Insufficiency in cancer patients and
implications for anticancer drug management: The renal
insufficiency and anticancer medications (IRMA) study. Cancer.
110:1376–1384. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Kato S, Chmielewski M, Honda H,
Pecoits-Filho R, Matsuo S, Yuzawa Y, Tranaeus A, Stenvinkel P and
Lindholm B: Aspects of immune dysfunction in end-stage renal
disease. Clin J Am Soc Nephrol. 3:1526–1533. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Launay-Vacher V, Janus N and Deray G:
Renal insufficiency and cancer treatments. ESMO Open.
1:e0000912016. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Swamy S, Noor SM and Mathew RO:
Cardiovascular disease in diabetes and chronic kidney disease. J
Clin Med. 12:69842023. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Kim S, Kim G and Kim JH: Additive
interaction of diabetes mellitus and chronic kidney disease in
cancer patient mortality risk. Sci Rep. 12:199572022. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Khorana AA, Tullio K, Elson P, Pennell NA,
Grobmyer SR, Kalady MF, Raymond D, Abraham J, Klein EA, Walsh RM,
et al: Time to initial cancer treatment in the United States and
association with survival over time: An observational study. PLoS
One. 14:e02132092019. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Cone EB, Marchese M, Paciotti M, Nguyen
DD, Nabi J, Cole AP, Molina G, Molina RL, Minami CA, Mucci LA, et
al: Assessment of Time-to-Treatment initiation and survival in a
cohort of patients with common cancers. JAMA Netw Open.
3:e20300722020. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Tinti F, Lai S, Noce A, Rotondi S, Marrone
G, Mazzaferro S, Di Daniele N and Mitterhofer AP: Chronic kidney
disease as a systemic inflammatory syndrome: Update on mechanisms
involved and potential treatment. Life (Basel).
11:4192021.PubMed/NCBI
|
|
42
|
Espi M, Koppe L, Fouque D and Thaunat O:
Chronic kidney disease-associated immune dysfunctions: Impact of
protein-bound uremic retention solutes on immune cells. Toxins
(Basel). 12:3002020. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Sprangers B, Perazella MA, Lichtman SM,
Rosner MH and Jhaveri KD: Improving cancer care for patients with
CKD: The need for changes in clinical trials. Kidney Int Rep.
7:1939–1950. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Shirali AC and Sprangers B: Cancer drug
dosing in chronic kidney disease and dialysis. Adv Chronic Kidney
Dis. 29:208–216.e1. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Verma S, Singh P, Khurana S, Ganguly NK,
Kukreti R, Saso L, Rana DS, Taneja V and Bhargava V: Implications
of oxidative stress in chronic kidney disease: A review on current
concepts and therapies. Kidney Res Clin Pract. 40:183–193. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Oberacker T, Fritz P, Schanz M, Alscher
MD, Ketteler M and Schricker S: Enhanced oxidative DNA-damage in
peritoneal dialysis patients via the TXNIP/TRX axis. Antioxidants
(Basel). 11:11242022. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Cartwright DJ: ICD-9-CM to ICD-10-CM
codes: What? Why? How? Adv Wound Care (New Rochelle). 2:588–592.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Sorino C, Scichilone N, Pedone C, Negri S,
Visca D and Spanevello A: When kidneys and lungs suffer together. J
Nephrol. 32:699–707. 2019. View Article : Google Scholar : PubMed/NCBI
|