Introduction
Histiocytic sarcoma (HS) is a rare non-Langerhans
histiocyte disorder that presents as unifocal or multifocal
extranodal tumors. The diagnosis of HS can be difficult due to its
rarity and histologic similarity to a number of other tumors
(1). Diagnosis depends on the
identification of morphological features and the appropriate use of
immunohistochemistry (IHC) markers to validate the histiocytic
lineage and exclude others (2). HS
may be sporadic or clonally related to hematological malignancies,
including follicular lymphoma or acute lymphoblastic leukemia
(3). In addition, HS has been
reported in patients with mediastinal germ cell tumors, such as
malignant teratomas, suggesting a possible derivation of such
tumors from pluripotent germ cells (4–6).
However, only a few hundred such cases have been reported in the
literature (7).
Crizotinib is a kinase inhibitor approved by the
Food and Drug Administration for the treatment of anaplastic
lymphoma kinase (ALK)-positive or c-ros oncogene 1 receptor
kinase-positive patients with metastatic non-small cell lung cancer
(NSCLC) (8). Since the first case
of an inflammatory myofibroblastic tumor (IMT) with ALK
rearrangement demonstrated a favorable response to crizotinib, it
gradually became an off-label treatment for inoperable sarcomas
with ALK fusions (4,5). However, to the best of our knowledge,
whether single-agent therapy using crizotinib is optimal and
whether the specific partner protein in ALK fusion variants affects
the response of the tumor to crizotinib remains unknown (6). The present case report describes the
response to ALK inhibitors of an aggressive ALK-positive soft
tissue sarcoma with intracardiac metastases and severe
leukocytosis.
Case report
Case report
A 27-year-old woman presenting with shortness of
breath, palpitations and fatigue was admitted to the emergency
department (July 2022; Cukurova University Medical Faculty Balcalı
Hospital, Adana, Turkey). The patient had a fever of 38°C and a
pulse rate of 140 beats/min, their electrocardiogram demonstrated
sinus tachycardia, and the blood gas test indicated hypoxia and
hypocapnia. The white blood cell (WBC) count and hemoglobin levels
were 55,000/µl and 10 g/dl, respectively (reference range,
48,000-108,000/µl and 14–18 g/dl, respectively). The patient also
exhibited a high C-reactive protein level of 236 mg/l and
hypoalbuminemia was detected in blood tests (32.79 g/l) (reference
range, 0–5 mg/l and 34–54 g/dl, respectively). Flow cytometry and
ELISA methods were used and these data were obtained from medical
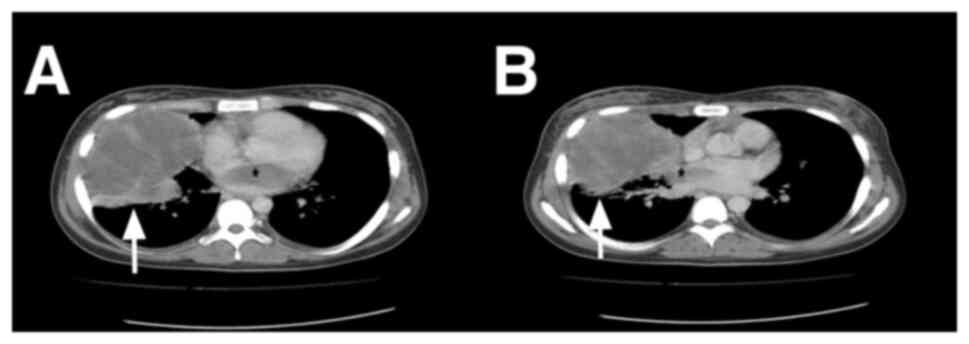
records. Thoracic computed tomography revealed a mass ~11 cm in
size located in the right hemithorax, causing a tumor thrombus that
extended to the left atrium through the pulmonary veins (Figs. 1 and 2A), and these findings indicated lung and
cardiac metastasis. Sputum culture and rapid antigen tests for
specific infectious agents yielded negative results. A peripheral
blood smear identified neutrophilic leukocytosis and a bone marrow
biopsy indicated myeloid hyperplasia. The standard diagnostic
procedure of the hospital includes JAK2 V617F mutation assessment
(Allele-Specific PCR; data were obtained from medical records) when
myeloproliferative disease is suspected. Since the JAK2 V617F
mutation result was negative, which suggested that another ailment
may exhibit comparable symptoms or that the disease had another
etiology, further testing was conducted.
A mediastinal biopsy demonstrated spindle cells with
orthochromatic and indistinct nucleoli. Neoplastic cells were
arranged in fascicles, nests and sheets in the inflammatory and
vascular backgrounds. IHC demonstrated no staining for HMB45, CD34,
smooth muscle actin (SMA), S100, CD117, chromogranin,
synaptophysin, CD56, transcription factor binding to IGHM enhancer
3 (TFE3) or mucin-4 (MUC-4).
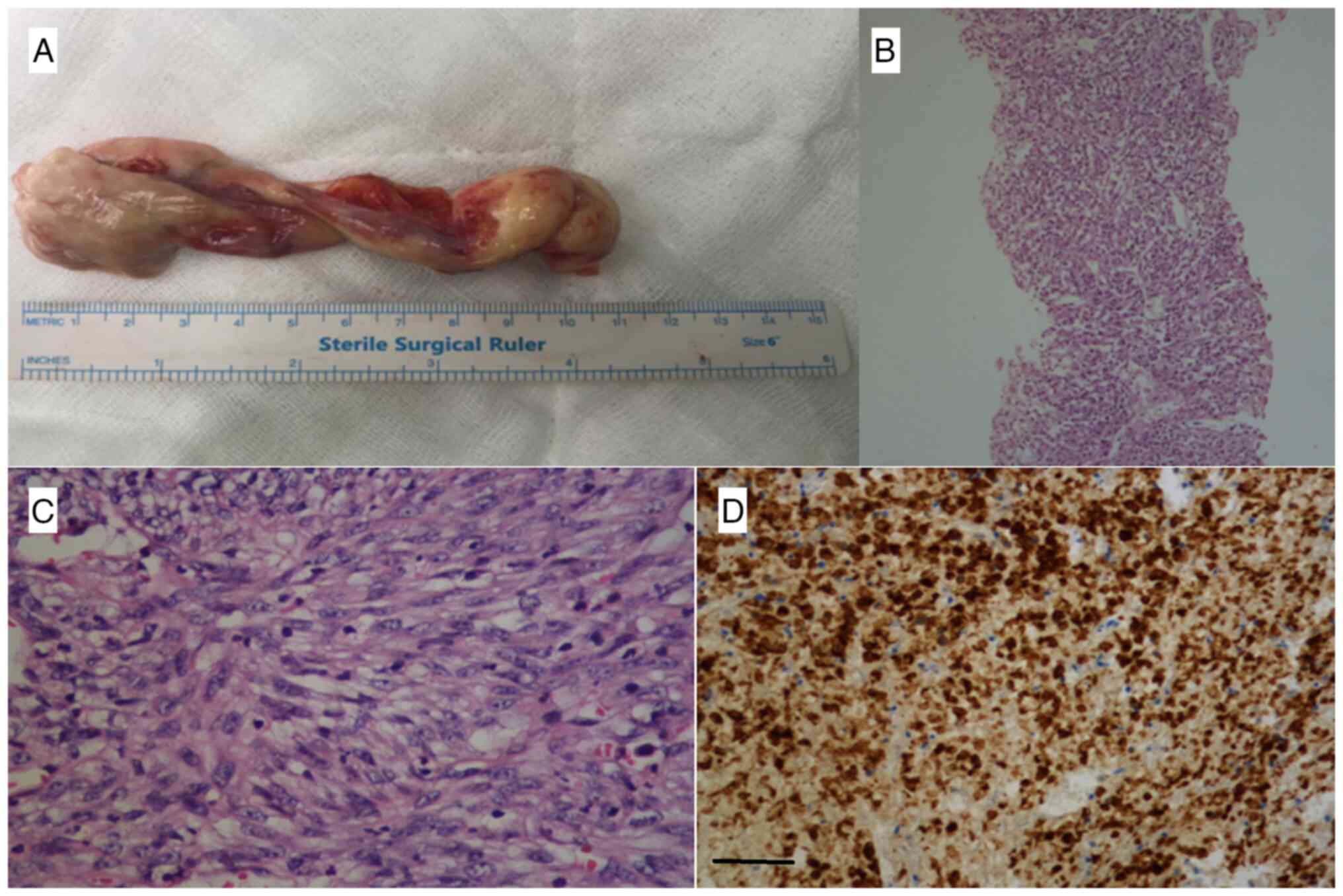
Subsequently, the patient underwent a right middle
lobectomy, and it was demonstrated that the tumor completely
infiltrated the entire tissue sample obtained. Microscopic
assessment revealed a spindled tumor mixed with inflammatory cells,
and H&E initial biopsy indicated monotonous spindle cells with
mild atypia accompanied by inflammatory cells. The H&E
resection specimen exhibited oval round vesicular nuclei and
abundant eosinophilic cytoplasm (Fig.
2B and C; data were obtained from medical records) and a barely
malignant tumor. The case exhibited areas of stag horn vessels.
Immunostaining was performed for CD163, lysozyme,
CD10, CD13, ALK, CD21, CD23, TFE3, Inhibin, CD4, STAT6, ETS
transcription factor ERG (ERG), cytokeratin (Pan) (PanCK),
epithelial membrane antigen (EMA), CD34, CD117 and myeloperoxidase
(MPO). IHC revealed positivity for CD163 (Fig. 2D), lysozyme, CD10, CD13 and ALK. IHC
for CD21, CD23, TFE3, Inhibin, CD4, STAT6, ERG, PanCK, EMA, CD34,
CD117 and MPO was negative.
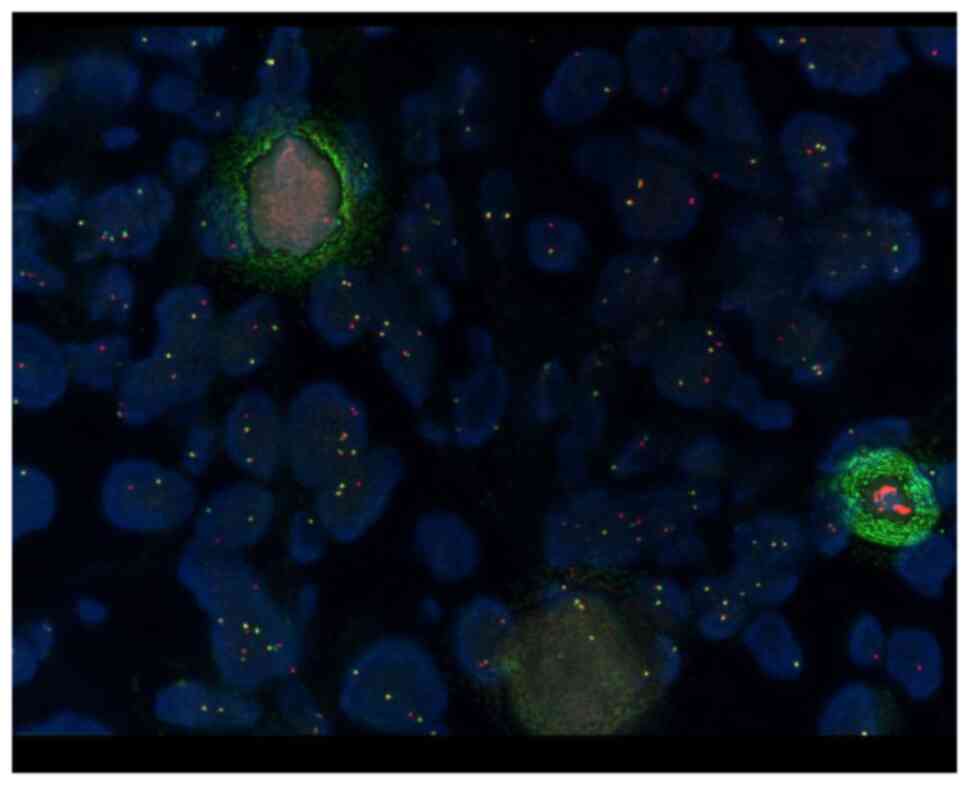
Translocation of EML4/ALK 2p23 was assessed using
the Vysis ALK break-apart fluorescence in situ hybridization
(FISH) probe kit (cat. no. 30-608916/R8; Abbott Laboratories)
(Fig. 3). The combination of
visceral crisis, dyspnea, high WBC count and the necessity for
major surgery indicated an aggressive hematologic malignancy.
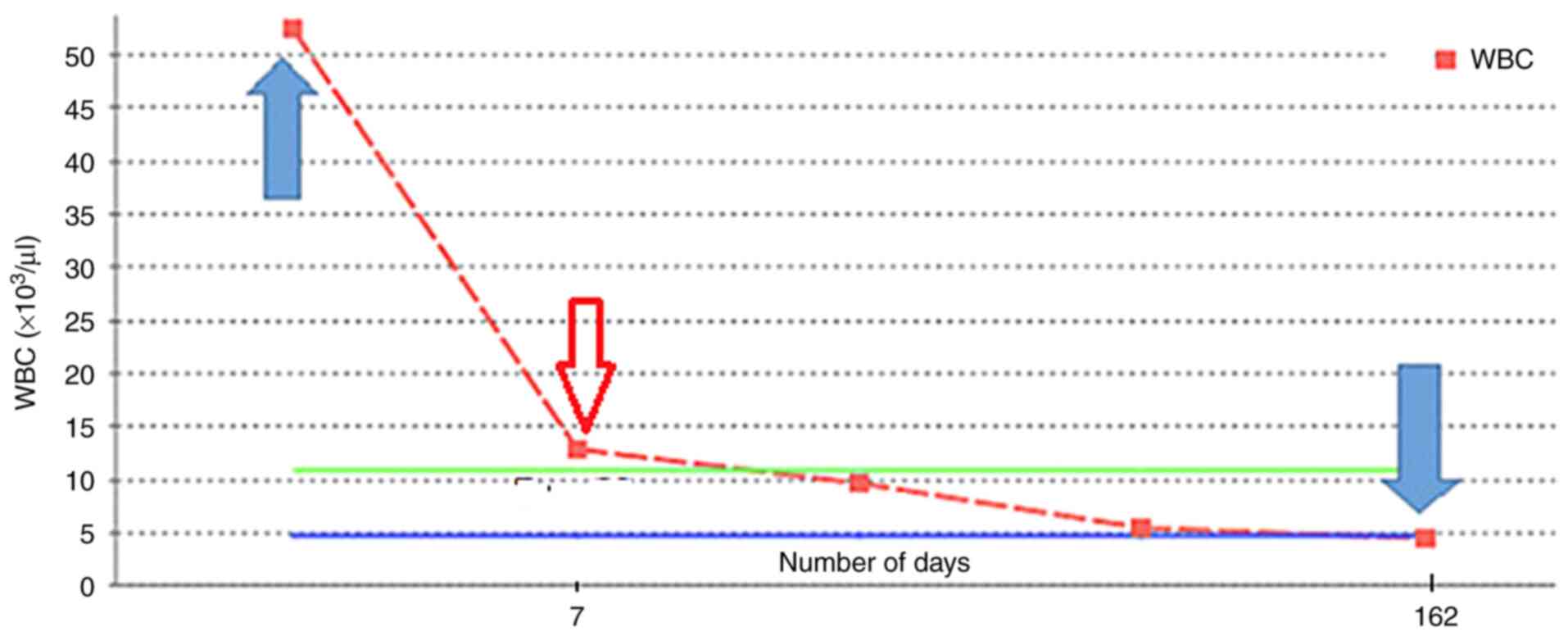
A positive clinical response was observed in the
patient after treatment with 250 mg crizotinib, administered
orally, twice a day. A decrease in the WBC count within 10 days of
crizotinib treatment was observed (Fig.
4). However, 6 months later, the patient began to experience
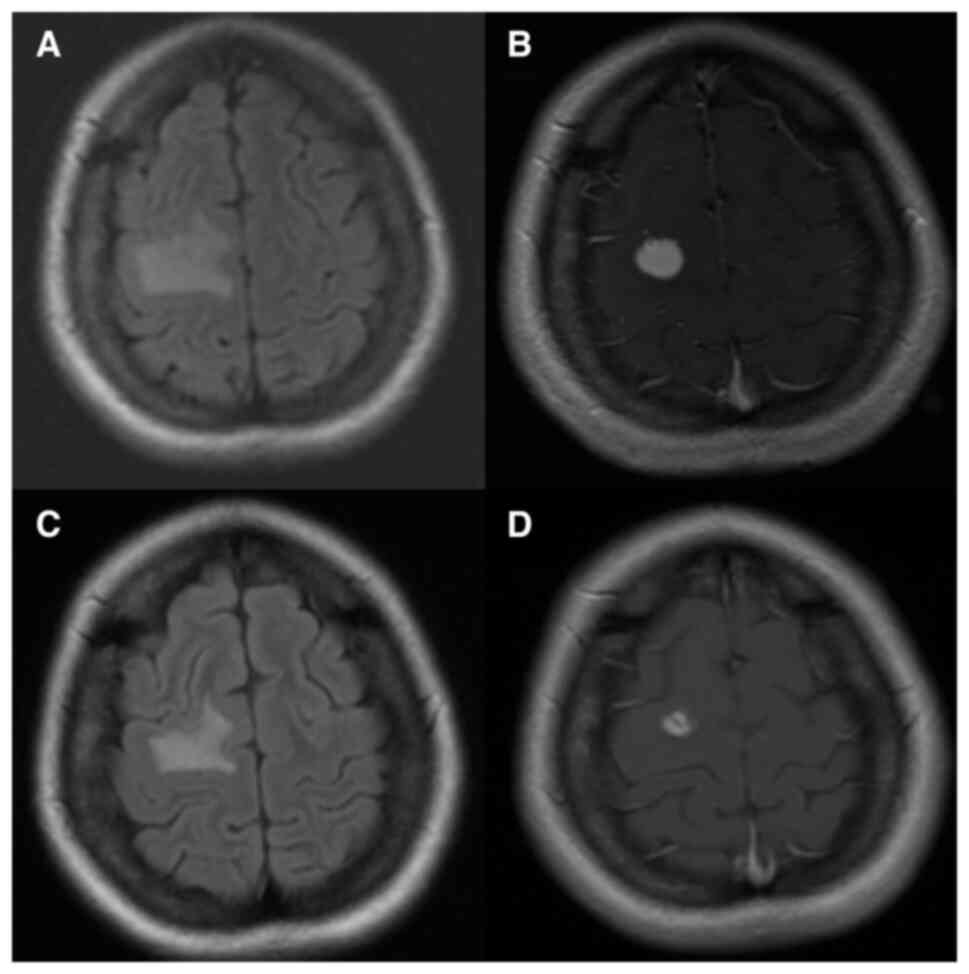
headaches and brain magnetic resonance imaging demonstrated a mass
in the right frontal lobe [central nervous system (CNS)
involvement; Fig. 5A and B]. After
progression, liquid biopsy and next-generation sequencing (NGS)
were performed, and the ALK p.I1171T mutation was detected. The
patient was positioned on the Gamma Knife couch, with the head
frame fixed in place to maintain the correct alignment. The Gamma
Knife device delivered focused gamma rays to the targeted lesions.
The treatment was typically performed in a single session, with
each lesion receiving a precise dose of radiation according to the
pre-established plan. Throughout the procedure, the patient was
closely monitored to ensure stability and comfort. The medical team
could communicate with the patient and make any necessary
adjustments. Furthermore, 600 mg alectinib, administered orally,
twice daily was prescribed because of an improved intracranial
response (9). Although the patient
tolerated alectinib for 2 years (Fig.
5C and D), the intra-abdominal lymph nodes progressed. NGS was
performed again and a ALK p.I1171N mutation was detected and
lorlatinib treatment (administered orally; 100 mg; once a day) was
started. After the treatment, the patient was followed up with a
haemogram and renal and liver tests every month, and no side
effects were observed. The patient was planned to be followed up
with PET CT after treatment and this will be done at 3-month
intervals.
FISH
FISH was performed using paraffin-embedded tissue
sections, which were fixed with 3% paraformaldehyde at room
temperature for 10–20 min. The samples were permeabilized with a
0.1% pepsin solution (prepared from pepsin powder from
Sigma-Aldrich; Merck KGaA) in 0.01 N HCl at 37°C for 10 min. The
sections were fixed with 3% paraformaldehyde at room temperature
for 10 min. Selected paraffin blocks were sectioned into 4-µm-thick
sections and mounted on positively charged slides. The sections
were incubated overnight at 56°C to ensure proper adhesion to the
slides. Slides were deparaffinized by immersion in three changes of
xylene for 10 min each. This was followed by dehydration in
absolute ethanol (two changes; 5 min each) and air-drying to remove
any residual solvent. Pre-treatment involved placing the slides in
a heat-resistant container with deparaffinization pre-wash solution
and incubating the slides at 80°C. Each slide box contained 15 cc
of distilled water and 150 µl of 1 mol HCl, and was incubated at
37°C. After this step, the slides were washed in 2X SSC solution
(two times; 3 min each) and passed through a graded ethanol series
(70, 85 and 100%) for 3 min each, followed by air-drying
(pre-hybridization). The hybridization buffer comprised 50%
formamide, 10% dextran sulfate and 2X SSC solution. Before the
probe was applied, the sections were incubated in hybridization
buffer at 37°C for 30 min. The probe (~1 ng/µl) was applied to the
sections, and coverslips were placed over the sections. The slides
were denatured at 73°C for 5 min using a ThermoBrite device. The
slides were then incubated at 37°C for 16 h to allow hybridization.
Post-hybridization, the slides were washed in a SSC solution at
73°C for 3 min to remove any non-specifically bound probes. This
was followed by an additional wash with SSC solution at room
temperature for 2 min. The sections were counterstained with 10 µl
DAPI at room temperature for 5–10 min and coverslipped. The slides
were stored at −20°C for 1 h before evaluation. The FISH slides
were evaluated using a fluorescence microscope (Olympus BX61;
Olympus Corporation). The slides were analyzed for the presence of
specific signals indicating genetic abnormalities. Positive cases
such as the present case were identified by the presence of
break-apart signals, where the distance between red and green
signals was at least twice the estimated signal diameter, or the
presence of a single red signal in >15% of the tumor cells.
IHC
IHC was performed as part of the diagnosis and some
specific experimental details were not available. Tissue slides
were produced from surgical pathology formalin-fixed,
paraffin-embedded neoplastic tissue samples. Paraffin-embedded
tissues were fixed with 10% formaldehyde at 60–62°C for 3 h.
Paraffin-embedded tissue samples were cut into sections (2–4 µm
thick) and mounted on positively charged slides. Tissue slides were
pretreated with high pH heat-induced epitope retrieval using Cell
Conditioning 1 solution, which is part of the VENTANA BenchMark
ULTRA automated staining platform kit (Roche Diagnostics), at
60–62°C for 88 min. Rehydration was performed using a descending
alcohol series. The slides were incubated with primary antibodies
against CD163 (10D6; 1:100; mouse monoclonal antibody; Cell Marque;
Merck KGaA), ALK (ALK1; 1:100; mouse monoclonal antibody; Dako;
Agilent Technologies, Inc.), lysosome (LAMP1; H4A3; 1:100; mouse
monoclonal antibody; Abcam), CD23 (1:100; Liquid Mouse Monoclonal
Antibody; Novocastra Laboratories Ltd.), CD4 (1:100; Novocastra
Liquid Mouse Monoclonal Antibody; Novocastra Laboratories Ltd.),
CD10 (1:100; Novocastra Liquid Mouse Monoclonal Antibody;
Novocastra Laboratories Ltd.), CD21 (2G9; 1:100; Cell Marque; Merck
KGaA), TFE3 (MRQ37; 1:100; rabbit monoclonal antibody; Cell Marque;
Merck KGaA), STAT6 (EP325; 1:100; rabbit monoclonal antibody; Cell
Marque; Merck KGaA), MPO (1:400; Cell Marque; polyclonal antibody;
Merck KGaA), CD45 (2B11 & PD7/26; 1:200; mouse monoclonal
antibody; Cell Marque; Merck KGaA), EMA (E29; 1:400; Cell Marque;
Merck KGaA), ERG (EPR3864; rabbit monoclonal antibody; Roche Tissue
Diagnostics; Roche Diagnostics, Ltd.), CD117 (YR145; 1:100; rabbit
monoclonal antibody; Cell Marque; Merck KGaA) and CD34 (1:100;
mouse monoclonal antibody; Novocastra Laboratories Ltd.) for 16 min
at room temperature. Staining was visualized using an ultraView
Universal DAB Detection Kit (Roche Diagnostics). IHC was performed
on the automated Benchmark XT platform (Roche Tissue Diagnostics;
Roche Diagnostics, Ltd.). The stained slides were assessed under an
Olympus BX46 light microscope (Olympus Corporation).
NGS
Peripheral blood samples (10 ml each) were collected
into biological specimen collection tubes. The circulating
cell-free DNA (ccfDNA) was isolated using the Qiagen QIAamp
Circulating Nucleic Acid Kit (cat. no. 55114; Qiagen, Inc.) with
the aid of the Qiagen QIAvac 24 Plus vacuum system (cat. no. 19413;
Qiagen, Inc.). After isolation, ccfDNA concentrations were measured
using the Qubit 3.0 Fluorometer (cat. no. Q33216; Thermo Fisher
Scientific, Inc.). The quality and integrity of the processed
ccfDNA samples were verified using the Qubit 3.0 Fluorometer, which
provides precise concentration measurements ensuring that samples
meet the required thresholds for further processing. For
sequencing, genomic DNA (gDNA) was extracted from formalin-fixed,
paraffin-embedded (FFPE) tissue samples using the Qiagen GeneReader
FFPE Kit (cat. no. 180134; Qiagen, Inc.) and the Qiagen QIAcube
isolation device (cat. no. 9001794; Qiagen, Inc.). The DNA quality
was confirmed using the Qubit 3.0 Fluorometer. Only samples with a
gDNA concentration of at least 1.5 ng/µl proceeded to sequencing.
The sequencing performed was NGS with paired-end reads of 150
nucleotides in length. The sequencing was carried out using the
Illumina TruSeq DNA PCR-Free Library Prep Kit (cat. no. 20015960;
Illumina, Inc.). The final library loading concentration was
determined using the Qubit 3.0 Fluorometer, ensuring accurate and
reliable measurements. The sequencing data were analyzed using the
Illumina BaseSpace Sequence Hub (version 5.0; Illumina, Inc.),
accessible at Illumina BaseSpace. This software provided
comprehensive tools for data analysis, including alignment, variant
calling and annotation, allowing for detailed insights into the
genomic data obtained from the samples.
Discussion
The first pathological diagnosis in the present case
was IMT. IMT is a rarely metastasizing tumor which is composed of
myofibroblastic and fibroblastic cells accompanied by an infiltrate
of plasma cells, lymphocytes and eosinophils (6). However, further assessment
demonstrated that the tumor was composed of spindle cells with
orthochromatic nuclei and indistinct nucleoli. Neoplastic cells
were arranged in fascicles, nests and sheets in the inflammatory
and vascular background. The neoplastic cells exhibited malignant
features with enlarged nuclei, frequent and atypical mitosis with
necrosis, which is different from IMT. Furthermore, the tissue was
demonstrated to be diffusely positive for CD163, which is not
observed in IMT (5). In IMT, one or
more patterns are often observed in a single tumor. The spindle
cells contain vesicular nuclei and small nucleoli. Necrosis is
uncommon and mitotic activity is generally low.
Immunohistochemically, the neoplastic cells display variable
staining for SMA, desmin and calponin, whereas ALK positivity is
observed in 50–60% of IMTs (7). In
the present case, a diagnosis of IMT was initially considered
because of the presence of low-grade spindle cells mixed with
inflammatory cells; however, malignancy was demonstrated by the
presence of enlarged nuclei, frequent and atypical mitosis, and
necrosis, distinguishing it from IMT. These features include
abnormal large nuclei, high mitotic activity with atypical figures,
and areas of cell death, which are indicative of aggressive tumor
behavior and differentiate it from IMT, and IMT was excluded.
Although HS is diagnosed at all ages, it is most
common among adults. The median patient age is 63 years (range,
18–96 years) according to the US Surveillance, Epidemiology, and
End Results database, which covers 159 cases of HS. These 159 cases
include 99 men and 60 women (10).
In the present case, the patient was 27 years old, and thus,
younger than the median patient age.
Histiocytic neoplasms are derived from macrophages,
dendritic cells or histiocytes. These neoplasms are rare and
account for <1% of the tumors present in the lymph nodes or soft
tissues. HS is commonly present at extranodal sites. The tumor
cells have oval-round vesicular nuclei and abundant eosinophilic
cytoplasm (11). In the present
case, reactive inflammatory cells were observed in the background,
mimicking inflammatory neoplasms such as IMTs. Other differential
diagnoses included solitary fibrous tumors, vascular neoplasms,
myeloid sarcomas, interdigitating dendritic cell sarcomas,
follicular dendritic cell sarcomas and inflammatory
pseudotumor-like follicular/fibroblastic dendritic cell sarcomas,
which were all excluded by negative immunostaining for STAT6, CD34,
ERG, MPO, S100, CD21 and CD23, respectively (12).
The pathogenesis of HS is unclear; it is not a true
sarcoma but is derived from cells of the monocyte/macrophage
system. The clinical presentation of HS varies, depending on the
organ involved. Solitary involvement of the lymph nodes is observed
in <20% of cases (13).
Furthermore, cytopenia is observed in one-third of cases and
hemophagocytosis is observed in a few cases (5–7). The
patient in the current case report presented with severe
neutrophilic leukocytosis. The WBC count rapidly decreased with
specific treatment. No patients with HS and leukocytosis were
identified in the literature. A biopsy of the tumor demonstrated an
infiltrative process with a diffuse growth pattern and effacement
of the normal architecture (7). IHC
demonstrated lysozyme and CD163 positivity and negative results for
T and B cell markers, myeloid cell markers CD1a and S100, and
epithelial markers. HS must be distinguished from other histiocytic
and dendritic cell disorders, metastatic solid or hematopoietic
neoplasms, primary familial lymphohistiocytic disorders and
acquired causes of hemophagocytic macrophage activation syndrome.
The present case was diagnosed using resected tissues and multiple
immunohistochemical stains, including CD163, lysozyme, CD10, CD13,
ALK, CD21, CD23, TFE3, Inhibin, CD4, STAT6, ERG, PanCK, EMA, CD34,
CD117 and MPO. Additionally, FISH analysis confirmed ALK p.I1171N
mutation positivity.
Severe leukocytosis was a prominent laboratory
finding in the present case, suggesting myeloproliferative
neoplasia (14). However, there was
no evidence of this entity, suggesting that leukocytosis was
associated with HS. The positive response to treatment with ALK
inhibitors suggests that leukocytosis was associated with HS
(15). Leukocytosis associated with
HS (total WBC count in the blood, 25.57×109/l) has, to
the best of our knowledge, only been reported in 1 case of a
84-year-old male patient (16).
ALK-positive sarcoma is predominantly observed in
pediatric or middle-aged patients. In a previous study of a cohort
of 33 cases, only 2 patients were aged >60 years. IMT and its
variant epithelioid IMTs comprised the majority of cases,
accounting for 78.8% (26/33) of cases (17). Although limited by sample size, a
pooled analysis reported a 86.7% efficacy of crizotinib in
ALK-positive sarcomas or sarcomatoid malignancies, with equal
effectiveness in both adult and pediatric patients (8). Furthermore, mutations in the ALK
fusion gene cause resistance to crizotinib in ~30% of cases
(18). Second-generation
ALK-tyrosine kinase inhibitors (TKIs), including ceritinib,
brigatinib and alectinib, are usually effective at later stages,
and the advantage of these drugs is intracranial tumor control
(9).
A previous case series reported the efficacy of
ALK-TKIs, including alectinib, a second-generation ALK-TKI, across
a number of tumor types and fusion partners in patients with
advanced ALK-rearranged, solid tumors other than NSCLC (10,11).
Previous studies assessing ALK-positive histiocytosis (Table I) (15), HS and parotid gland adenocarcinoma
have reported the efficacy of alectinib as a first- or second-line
treatment (12–14). The responses of cases varied, with
some patients experiencing stable or regressive disease for several
years. Treatments included ALK inhibitors such as alectinib,
lorlatinib and ceritinib, along with surgery and radiotherapy. A
total of 67 cases were submitted to the accessory cell and
histiocytic neoplasms session at the European Association of
Haematopathology/Society in Hematopathology workshop 2016. From
this, 12 cases had HS and 5 of these cases were reported to have
the BRAF V650E mutation, which is a targetable somatic genetic
change. However, no cases of ALK rearrangements were noted among
these cases (13). Additionally, 1
patient with disseminated histiocytosis was reported to be
ALK-positive. This patient showed partial recovery after treatment
with alectinib and a response duration of >7 months (15).
 | Table I.Histiocytosis cases with EML4-ALK
fusion or on alectinib therapy (15). |
Table I.
Histiocytosis cases with EML4-ALK
fusion or on alectinib therapy (15).
| First author/s,
year | Patient sex, age
(years) | Site of disease | Targetable
mutations | Treatment | Response | (Refs.) |
|---|
| Kemps et
al, | M, 17 | Lung | EML4-ALK | N/A | Lost to
follow-up | (15) |
| 2022 | F, 51 | CNS, bone, lung | EML4-ALK | Alectinib for 16
months, followed by lorlatinib for 14 months and then ceritinib for
7 weeks, followed by antalgic radiotherapy of two metastases and
chemotherapy | Alive with stable
disease (3 years) |
|
|
| F, 4 | CNS/PNS, bone, lung,
liver, lymph node, breast, pancreas | EML4-ALK | Alectinib | Alive with stable
bone lesions on MRI; other lesions regressed (2 years) |
|
|
| F, 19 | Soft tissue: A yellow
nodule in the left main bronchus | KIF5B-ALK | Surgery | Alive with no disease
(17 years) |
|
|
| F, 28 | CNS/PNS, bone | KIF5B-ALK | Alectinib | Alive with regressive
disease (9 months) |
|
| Present study | F, 27 | Lung, cardiac
metastasis, CNS | EML4-ALK | Alectinib | Near-complete
response for 2 years, with the exception of lymph node
metastasis. | - |
Solitary fibrous tumor is a fibroblastic tumor,
which is characterized by a prominent branching thin-walled dilated
vasculature (5). The present case
exhibited areas of stag horn vessels; however, STAT6 was negative
based on IHC. Vascular neoplasms, particularly epithelioid
hemangioma and epithelioid hemangioendothelioma, exhibit features
similar to the present case. They typically exhibit voluminous
cytoplasm and enlarged epithelioid cells (19). However, there are distinguishing
characteristics between the two. Epithelioid hemangioma exhibits
well-formed vascular channels, whereas epithelioid
hemangioendothelioma is typically associated with a myxochondroid
stroma (20,21). The differential diagnosis is made by
IHC, with negativity of vascular markers observed in the present
case.
Another differential diagnosis is myeloid sarcoma,
which is a malignant tumor composed of myeloblasts occurring at a
site other than the bone marrow. The myeloblasts mimic carcinoma,
lymphoma or sarcoma (22).
Differential diagnosis between myeloid sarcoma and the present case
was challenging. However, the CD56, CD34, CD117 and MPO negativity,
and CD163 and CD13 positivity were helpful. Differential diagnosis
between myeloid sarcoma and the present case was challenging due to
overlapping histological features. However, immunohistochemical
staining provided valuable insights. The negativity for CD56, CD34,
CD117 and MPO was particularly helpful in ruling out myeloid
sarcoma, as these markers are typically expressed in myeloid
lineage cells (23). On the other
hand, the positivity for CD163 and CD13 supported the diagnosis of
the present case, as these markers are more commonly associated
with histiocytic and monocytic lineage (2), aligning with the characteristics of
the tumor. Interdigitating dendritic cell sarcoma is another
differential diagnosis, and is composed of whorls of large,
pleomorphic cells with abundant pale cytoplasm with inflammatory
infiltrate (24). However, the IHC
results demonstrated that the present case was negative for S100,
CD45 and EMA. S100 is a marker commonly associated with neural and
melanocytic tumors, and thus, its negativity suggests that the
tumor was unlikely to originate from these lineages (24,25).
CD45, also known as leukocyte common antigen, is a marker for
hematopoietic cells, particularly lymphoid tissue. Its absence
indicates that the tumor was not of lymphoid origin (26). Furthermore, follicular dendritic
cell sarcoma is composed of spindled cells with dispersed
chromatin, small nucleoli and eosinophilic cytoplasm accompanied by
inflammatory cells. The differential diagnosis was made by IHC
(21), where CD21 and CD23 were
negative. Furthermore, no staining was detected for HMB45, CD34,
SMA, S100, CD117, chromogranin, synaptophysin, CD56, TFE3 and MUC-4
in the present case. In a more detailed IHC analysis, positive
results were obtained for CD163, lysozyme, CD10, CD13 and ALK.
However, negative results were obtained for CD21, CD23, TFE3,
Inhibin, CD4, STAT6, ERG, PanCK, EMA, CD34, CD117 and MPO.
Furthermore, EML4/ALK 2p23 translocation was detected. These
findings indicate that certain different diagnoses have been
excluded in the present case, confirming its association with ALK
translocation, which characterizes an aggressive form of HS.
Therefore, the aforementioned differential diagnoses were excluded
based on the negative immunostaining results and contributed to the
identification of HS in the case (18,27,28).
The present case illustrates three findings. First,
although both HS and leukocytosis are rare and complex conditions
that require separate treatments, they were successfully controlled
in the patient. The patient presented with severe neutrophilic
leukocytosis and responded to first-line crizotinib; however,
alectinib was administered because of progression in the CNS.
To the best of our knowledge, this is the first
reported case of ALK-rearranged p.I1171N-mutated HS with cardiac
and CNS involvement that rapidly responded to alectinib, an
ALK-TKI.
ALK-TKI resistance generally develops via
ALK-dominant second-step mutations and non-dominant mechanisms
(15). This mutation (p.I1171N) may
be responsible for resistance to first-line crizotinib treatment.
Frequent crizotinib resistance and low CNS efficacy in ALK-positive
patients are a challenge for clinicians (28,29).
Therefore, treatment with alectinib, a more effective
second-generation TKI that is also effective on the CNS (16), was initiated and continued as the
response persisted.
Resistance to first-line therapy is considered a
novel entity among ALK-positive non-Langerhans histiocytic diseases
(11). The present case report
offers a rational treatment analysis of a problematic case of HS
diagnosed in a young patient presenting with severe leukocytosis
with an aggressive course and important organ metastases
specifically involving the heart (cardiac metastases) and lungs
(lung metastases). The patient also had a limited response to
chemotherapy treatments and poor prognosis and is rare. The rarity
is highlighted by the young age, severe leukocytosis and aggressive
course of the disease with metastases to critical organs such as
the heart and lungs. Additionally, while ALK fusions or
rearrangements are reported in both solid and hematological tumors,
the specific presentation of HS with these characteristics is
uncommon in the literature. A number of notable cases of both solid
and hematological tumor types with ALK fusions or rearrangements
have been reported in the literature (3,14–17).
This emphasizes the necessity of a multidisciplinary approach for
evaluating ALK-positive histiocytosis with a solid component.
Overall, the diagnosis of such sarcomas can be complex, and
combination of histological, immunohistochemical and genetic
analyses is required.
In conclusion, the present case illustrates the
importance of the clinical integration of the molecular profile of
the tumor for patient-specific treatment selection. Treatment
selection based on the specific molecular characteristics of a
patient can improve treatment success. Therefore, broad molecular
profiling and clinical applications of these technologies can help
patients achieve improved outcomes in the future.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
EB contributed to the conception of the study, as
well as to the literature search for related studies. EB, UAP, KEE,
MT, HY and SP were involved in the writing of the manuscript, and
in the analysis and interpretation of the patient data. EB, UAP and
KEE contributed to the literature review, study design, revision of
the manuscript and the processing of the figures. EB and UAP
confirm the authenticity of all the raw data. All authors have read
and approved the final version of the manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
The patient provided written consent for the
publication of the present report.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Ansari J, Naqash AR, Munker R, El-Osta H,
Master S, Cotelingam JD, Griffiths E, Greer AH, Yin H, Peddi P and
Shackelford RE: Histiocytic sarcoma as a secondary malignancy:
Pathobiology, diagnosis, and treatment. Eur J Haematol. 97:9–16.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Hung YP and Qian X: Histiocytic sarcoma.
Arch Pathol Lab Med. 144:650–654. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Egan C, Lack J, Skarshaug S, Pham TA,
Abdullaev Z, Xi L, Pack S, Pittaluga S, Jaffe ES and Raffeld M: The
mutational landscape of histiocytic sarcoma associated with
lymphoid malignancy. Mod Pathol. 34:336–347. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Butrynski JE, D'Adamo DR, Hornick JL, Dal
Cin P, Antonescu CR, Jhanwar SC, Ladanyi M, Capelletti M, Rodig SJ,
Ramaiya N, et al: Crizotinib in ALK-rearranged inflammatory
myofibroblastic tumor. N Engl J Med. 363:1727–1733. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Gambacorti-Passerini C, Orlov S, Zhang L,
Braiteh F, Huang H, Esaki T, Horibe K, Ahn JS, Beck JT, Edenfield
WJ, et al: Long-term effects of crizotinib in ALK-positive tumors
(excluding NSCLC): A phase 1b open-label study. Am J Hematol.
93:607–614. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
International Agency for Research on
Cancer (IARC), . Soft tissue and bone tumours. Vol 3. IARC; Lyon,
France: 2020
|
|
7
|
Sethi B, Pai T, Allam A and Epari S:
Anaplastic lymphoma kinase-positive pulmonary inflammatory
myofibroblastic tumor with sarcomatous morphology and distant
metastases: An unusual histomorphology and behavior. Indian J
Pathol Microbiol. 58:509–512. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wang L and Wang W: Safety and efficacy of
anaplastic lymphoma kinase tyrosine kinase inhibitors in non-small
cell lung cancer (Review). Oncol Rep. 45:13–28. 2021.PubMed/NCBI
|
|
9
|
Zou Z, Xing P, Hao X, Wang Y, Song X, Shan
L, Zhang C, Liu Z, Ma K, Dong G and Li J: Intracranial efficacy of
alectinib in ALK-positive NSCLC patients with CNS metastases-a
multicenter retrospective study. BMC Med. 20:122022. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
WHO, . Classification of tumours editorial
board. Soft tissue and bone tumours. Lyon (France): International
agency for research on cancer. Journal. 3:1042020.
|
|
11
|
Go RS, Jacobsen E, Baiocchi R, Buhtoiarov
I, Butler EB, Campbell PK, Coulter DW, Diamond E, Flagg A, Goodman
AM, et al: Histiocytic neoplasms, version 2.2021, NCCN clinical
practice guidelines in oncology. J Natl Compr Cancer Netw.
19:1277–1303. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Swerdlow SH, Campo E, Harris NL, Jaffe ES,
Pileri SA, Stein H and Thiele J: WHO classification of tumours of
haematopoietic and lymphoid tissues. International agency for
research on cancer Lyon; 2008
|
|
13
|
Picarsic J and Jaffe R: Pathology of
histiocytic disorders and neoplasms and related disorders.
Histiocytic disorders. Abla O and Janka G: Springer International
Publishing; Cham: pp. 3–50. 2018, View Article : Google Scholar
|
|
14
|
Yogarajah M and Tefferi A: Leukemic
transformation in myeloproliferative neoplasms: A literature review
on risk, characteristics, and outcome. Mayo Clin Proc.
92:1118–1128. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kemps PG, Picarsic J, Durham BH,
Hélias-Rodzewicz Z, Hiemcke-Jiwa L, van den Bos C, van de Wetering
MD, van Noesel CJM, van Laar JAM, Verdijk RM, et al: ALK-positive
histiocytosis: A new clinicopathologic spectrum highlighting
neurologic involvement and responses to ALK inhibition. Blood.
139:256–280. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Reddivari AKR, Mehta P and Janapala US:
Rare presentation of a rare tumor: Histiocytic sarcoma. Cureus.
12:e77702020.PubMed/NCBI
|
|
17
|
Solomon BJ, Mok T, Kim DW, Wu YL, Nakagawa
K, Mekhail T, Felip E, Cappuzzo F, Paolini J, Usari T, et al:
First-line crizotinib versus chemotherapy in ALK-positive lung
cancer. N Engl J Med. 371:2167–2177. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Xu X, Li H, Peng K, Yu Y, Chen L, Fang Y,
Sun Y, Hou Y and Liu T: ALK-G1269A mutation in epithelioid
inflammatory myofibroblastic sarcoma after progression on
crizotinib: A case report. Oncol Lett. 17:2370–2376.
2019.PubMed/NCBI
|
|
19
|
Shon W and Billings SD: Epithelioid
vascular tumors: A review. Adv Anat Pathol. 26:186–197. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Luzar B and Calonje E: Update on cutaneous
epithelioid vascular tumours. Diagnostic Histopathology.
24:273–287. 2018. View Article : Google Scholar
|
|
21
|
Folpe AL: Vascular tumors of intermediate
malignancy: An update. Hum Pathol. 147:114–128. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Magdy M, Karim NA, Eldessouki I, Gaber O,
Rahouma M and Ghareeb M: Myeloid sarcoma. Oncol Res Treat.
42:224–229. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Thalhammer-Scherrer R, Mitterbauer G,
Simonitsch I, Jaeger U, Lechner K, Schneider B, Fonatsch C and
Schwarzinger I: The immunophenotype of 325 adult acute leukemias:
Relationship to morphologic and molecular classification and
proposal for a minimal screening program highly predictive for
lineage discrimination. Am J Clin Pathol. 117:380–389. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Xue T, Jiang XN, Wang WG, Zhou XY and Li
XQ: Interdigitating dendritic cell sarcoma: Clinicopathologic study
of 8 cases with review of the literature. Ann Diagn Pathol.
34:155–160. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Szumera-Ciećkiewicz A, Bosisio F, Teterycz
P, Antoranz A, Delogu F, Koljenović S, van de Wiel BA, Blokx W, van
Kempen LC, Rutkowski P, et al: SOX10 is as specific as S100 protein
in detecting metastases of melanoma in lymph nodes and is
recommended for sentinel lymph node assessment. Eur J Cancer.
137:175–182. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Tuffaha MSA, Guski H and Kristiansen G:
Markers and immunoprofile of lymphoid neoplasms.
Immunohistochemistry in tumor diagnostics. Springer International
Publishing; Cham: pp. 207–250. 2023, View Article : Google Scholar
|
|
27
|
Facchetti F, Simbeni M and Lorenzi L:
Follicular dendritic cell sarcoma. Pathologica. 113:316–329. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Russo A, Franchina T, Ricciardi GRR,
Ferraro G, Scimone A, Bronte G, Russo A, Rolfo C and Adamo V:
Central nervous system involvement in ALK-rearranged NSCLC:
promising strategies to overcome crizotinib resistance. Expert Rev
Anticancer Ther. 16:615–623. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Nelson TA and Wang N: Targeting lung
cancer brain metastases: A narrative review of emerging insights
for anaplastic lymphoma kinase (ALK)-positive disease. Transl Lung
Cancer Res. 12:379–392. 2023. View Article : Google Scholar : PubMed/NCBI
|