Introduction
Ocular melanoma constitutes ~5% of all melanoma
cases. Uveal melanoma is a rare and highly malignant intraocular
tumor that predominantly affects adults. This type of cancer
exhibits distinct differences in biological characteristics and
clinical manifestations compared with cutaneous melanoma (1). The risk of developing metastasis for
patients with uveal melanoma is much higher compared to patients
with a primary cutaneous melanoma and can be >50% in high-risk
tumors of the posterior uvea (2–4).
Research indicates that the incidence of both melanoma and
cataracts is associated with exposure to ultraviolet radiation
(5). The clinical symptoms of uveal
melanoma often depend on the location and volume of the tumor
(6), with early peripheral tumors
being asymptomatic and frequently discovered during routine
examinations. Most patients typically present with painless loss of
vision or changes in vision, such as visual distortion or visual
field defects. Fundoscopic examination may reveal the presence of
an orange-red pigment or extensive serous retinal detachment,
supporting the diagnosis of uveal melanoma (7). Uveal melanoma is known for its high
malignancy, substantial metastatic potential and poor overall
survival rates (8).
Uveal melanoma mainly occurs in the Caucasian
population, while its incidence rate in various regions of Asia is
0.2-0.6 per million. The age at presentation for the Asian
population is commonly 40–55 years, which is younger than that of
Caucasian individuals, who have a mean age of 58 years at
presentation (9). Uveal melanoma is
the most common intraocular malignant tumor in adults, with 90% of
cases of uveal melanoma occurring in the choroid, 6% in the ciliary
body and 4% in the iris (8). Of
note, the incidence of ocular melanoma is higher in males than in
females (10). As the tumor grows,
it may disrupt the nutrition or metabolism of the lens;
furthermore, expansion of the tumor can push the lens-iris
diaphragm forward, compressing the anterior chamber angle and the
trabecular meshwork. In addition, cells or pigments shed by the
tumor may enter the vitreous and aqueous humor and block the
anterior chamber angle (11),
leading to the development of complicated cataracts and secondary
glaucoma.
The present study describes a typical case where the
growth of the melanoma resulted in complicated cataracts and
secondary glaucoma. Notably, slit-lamp examination revealed a
reflection of the tumor surface. The present case report emphasizes
the importance of careful slit-lamp examination for the detection
of anterior segment tumors, and suggests that the presence of
intraocular lesions should be evaluated in patients presenting with
cataracts and glaucoma.
Case report
A 52-year-old Chinese man presented at the First
Affiliated Hospital of Harbin Medical University (Harbin, China) in
November 2023 with painless sharp vision loss in the right eye over
the previous year, accompanied by eye swelling and pain in the week
prior to seeking medical attention. The patient had only light
perception in the right eye but normal visual acuity in the left
eye. The left eye had an intraocular pressure of 15 mmHg, while the
right eye had an intraocular pressure of >60 mmHg. A slit-lamp
examination revealed extensive black pigmentation near the iris
root at 4–6 o'clock in the right eye and in other scattered areas
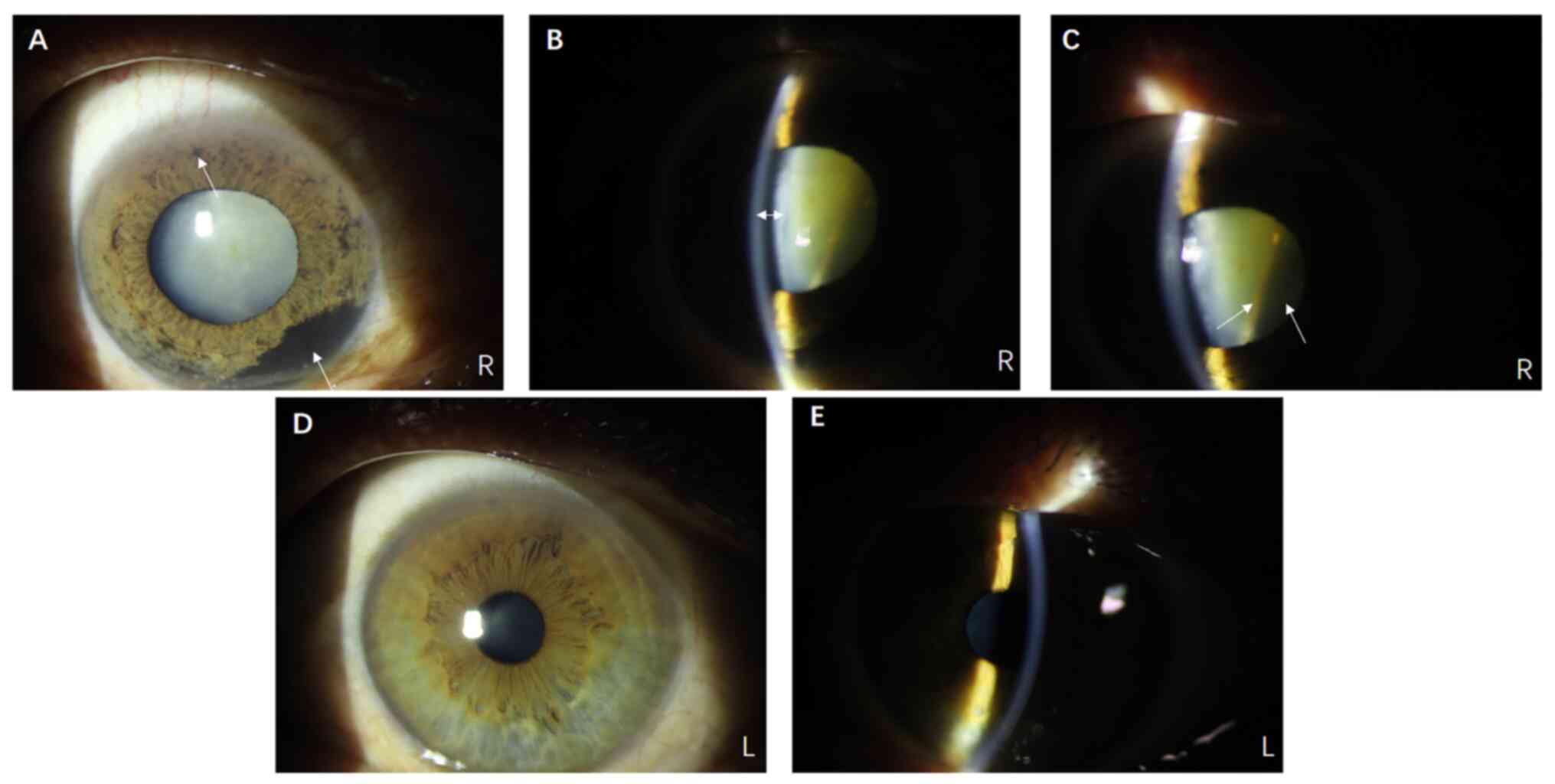
of the iris, with a shallow anterior chamber (Fig. 1A and B). In addition, the lens
clearly exhibited white opacity (Fig.
1A). Notably, a reflective band was faintly visible behind the
lens on the nasal side, suggesting the possibility of intraocular
occupancy (Fig. 1C). Mild partial
cataracts could be seen in the left eye, and no abnormal black
pigment was found on the surface of the iris (Fig. 1D and E).
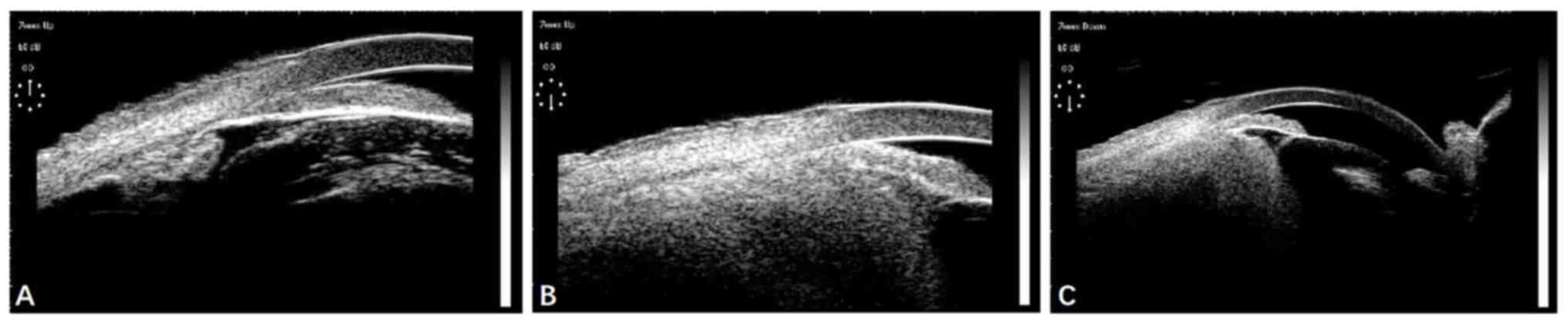
Ultrasound biomicroscopy of the right eye showed a
shallow anterior chamber, bulging of the iris and a closed anterior
chamber angle. The lens-iris diaphragm was displaced anteriorly
(Fig. 2A), and a tumor was
discovered in the ciliary body and choroid, which altered their
structure (Fig. 2B and C).
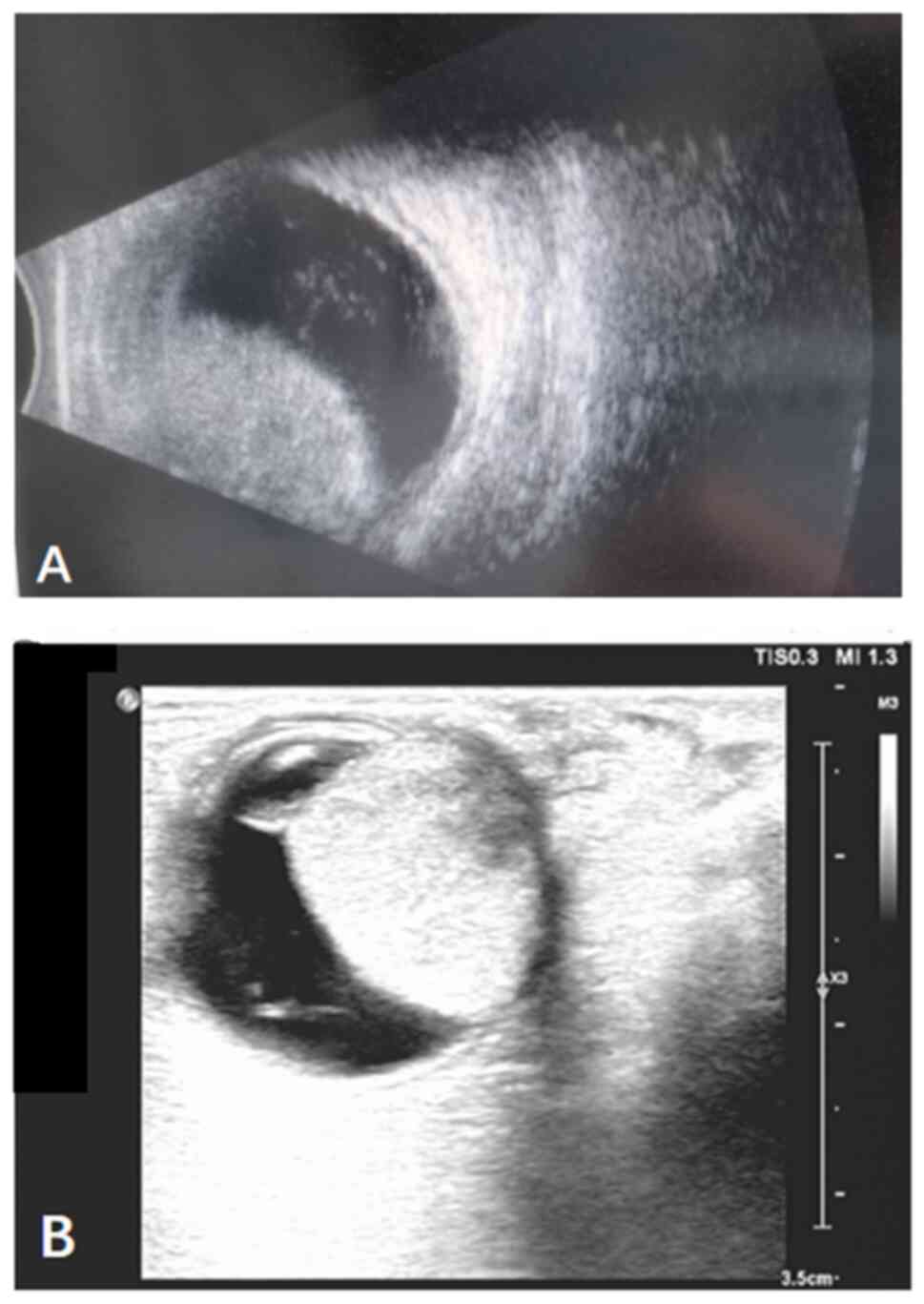
B-ultrasound and color Doppler ultrasound examinations revealed a
hemispherical solid mass contiguous with the eyeball wall, with
clear boundaries (Fig. 3). Orbital
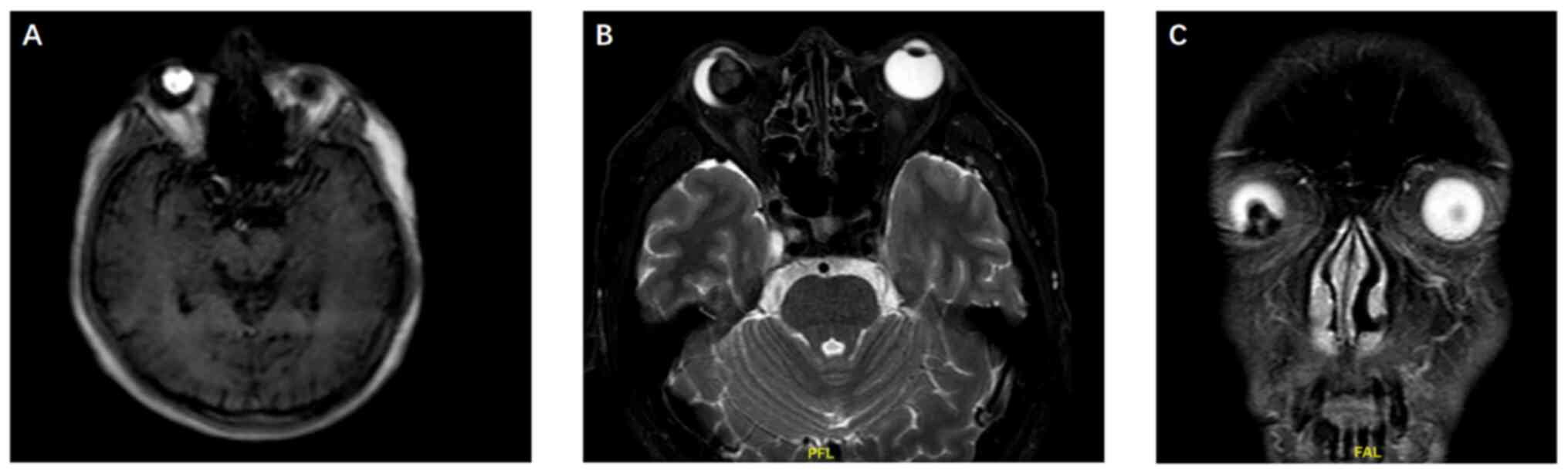
magnetic resonance imaging (MRI) demonstrated a T1 hyperintense, T2
hypointense lesion extending into the vitreous cavity from the
lower part of the right eye. Due to the paramagnetic nature of
melanin in tumors (12), MRI often
exhibits characteristic features, namely high signal on T1WI and
low signal on T2WI, which is different from most tumors that show a
low to medium signal on T1WI and a medium to high signal on T2WI
(13,14). These MRI features were strongly
suggestive of malignant melanoma (Fig.
4). The lesion measured 16×18×14 mm and was closely associated
with the posterior lens. Subsequent extensive systemic
examinations, including brain, chest and abdominal CT scans,
digestive and urological ultrasound scans, and positron emission
tomography/CT-MRI, revealed no primary tumors in other parts of the
body, which excluded the possibility of metastasis (Fig. S1, Fig.
S2, Fig. S3).
Eye enucleation surgery was performed, and the eye
was histopathologically examined (Fig.
5). The tumor tissue appeared black to the naked eye (Fig. 5A) and was large in size (apical
height, 16 mm; largest basal diameter, 24 mm). The tumor invaded
the choroid (Fig. 5B and C),
ciliary body and iris. The tumor tissue exhibited a high melanin
content and a clear boundary with the surrounding normal uvea
(Fig. 5D). Part of the adjacent
uvea was deformed by tumor compression (Fig. 5E). The tumor was in close contact
with the inner surface of the sclera (Fig. 5F) and the interior of the tumor was
uneven (Fig. 5G). At a higher
magnification, tumor cells and pigments were clearly visible in the
histological images (Fig. 5H). The
tumor cells were mainly poorly differentiated epithelioid cells
with a small number of spindle cells (Fig. 5I). Immunohistochemical staining
(supplementary materials of immunohistochemical methods)
demonstrated that the number of Ki67-positive cells in the tumor
tissue was markedly higher than that in normal tissue, although the
melanin within the tumor impeded clear identification of the
staining (Fig. 5J-L). BRAF gene
mutation testing gave a negative result, showing that wild-type
BRAF gene was present. In addition, a panel of 68 genes, named as
homologous recombination repair genes (supplementary materials of
genes), was analyzed by second-generation sequencing (BGI Genomics
Co., Ltd.). The results showed the absence of a somatic
BRCA-associated protein-1 (BAP1) mutation, whereas germline MutL
protein homolog 1 (MLH1) mutation c.283T>G, RAD54 like (RAD54L)
mutation c.1170-8T>C and SWI/SNF related, matrix associated,
actin dependent regulator of chromatin, subfamily a, member 4
(SMARCA4) mutation c.2123+20G>T were detected (Table I). The patient underwent enucleation
surgery and the incision gradually healed. At three weeks after
surgery, the conjunctiva was relatively smooth and no residual
black tissue was found by slit lamp microscopy observation, and no
distant metastasis was found during the examination. The patient
decided to not have any other treatments and planned to undergo
regular physical examinations to detect possible metastases.
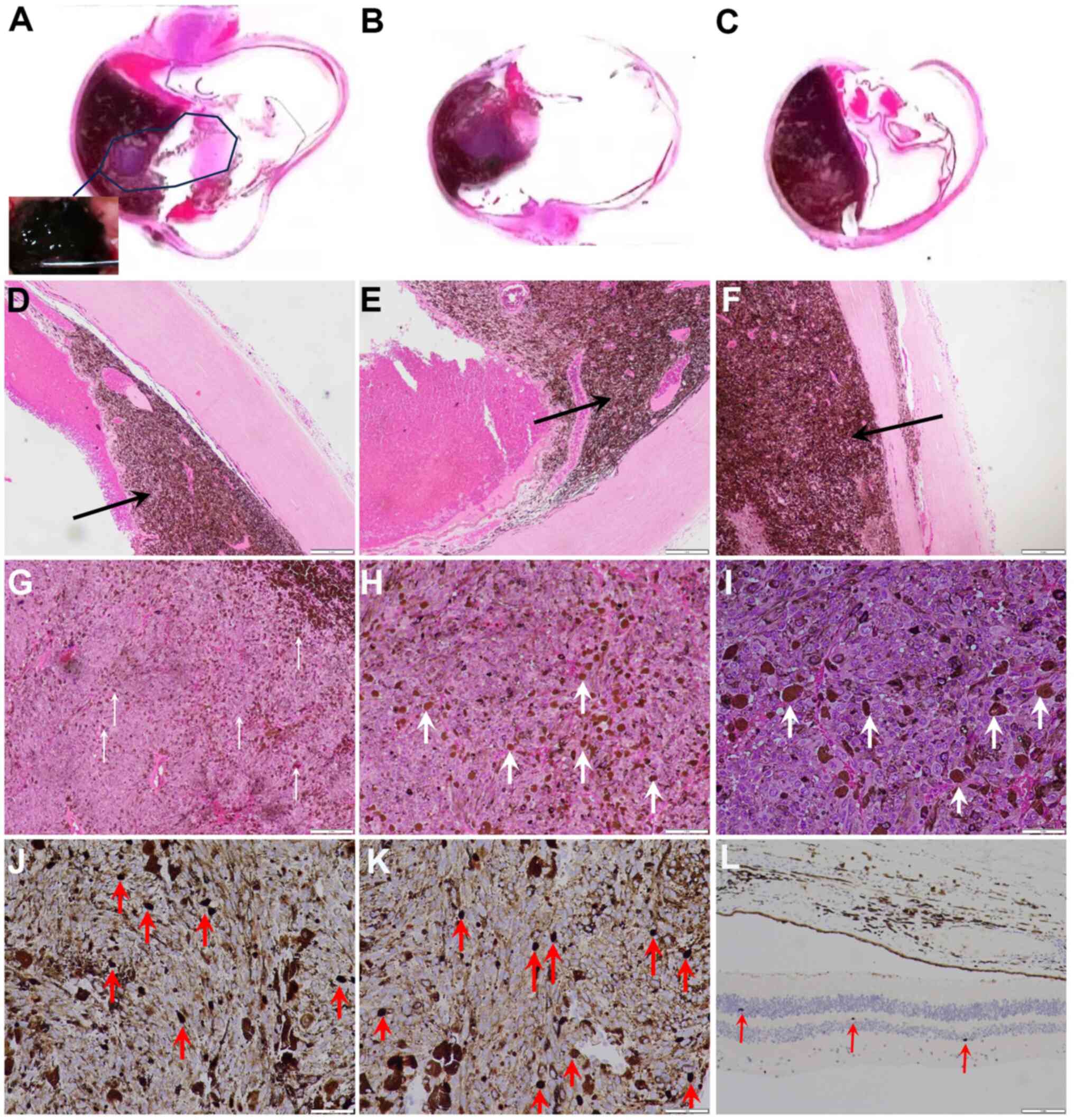 | Figure 5.Histological and pathological
examination results. (A) Extracted eyeball and tumor morphology.
The inset in A shows that the tumor in the eye is black
(magnification, ×5). (B) Section and tumor morphology of the
eyeball (magnification, ×5). (C) Another section of the eyeball and
tumor morphology (magnification, ×5). (D) Tumor invasion near the
normal uvea. (E) Tumor compresses the uvea. (F) Tumor tissue is
present on the inner surface of the sclera (magnification in D-F,
×40; scale bar, 0.25 mm; the tumor tissue is indicated by black
arrows). (G) Uveal melanoma tumor cell morphology. Pigmented tumor
cells are indicated by white arrows. Most of the tumor cells were
epithelioid cells (magnification, ×40; scale bar, 0.25 mm). (H)
Higher magnification image of G (magnification, ×100; scale bar,
0.1 mm) and (I) a further magnified image (×200; scale bar, 0.05
mm). Ki67 immunohistochemical results of (J and K) tumor tissue
(magnification, ×200; scale bar, 0.05 mm) and (L) normal tissue
(magnification, ×100; scale bar, 0.1 mm). There were markedly more
Ki67-positive cells in the tumor tissue than in normal tissue.
Ki67-positive cells are indicated by red arrows. |
 | Table I.Results of the detection of germline
variations in tumor susceptibility genes. |
Table I.
Results of the detection of germline
variations in tumor susceptibility genes.
| Gene name | NM number | Nucleotide
changes | Functional
changes | Mutation
typea |
|---|
| SMARCA4 | NM_001128849.1 |
c.2123+20G>T | Splice | Unknown
significance |
| RAD54L | NM_003579.3 | c.1170-8T>C | Splice | Unknown
significance |
| MLH1 | NM_000249.3 | c.283T>G | Missense | Unknown
significance |
Discussion
In the present case, the patient initially only had
painless vision loss, similar to that associated with cataracts,
with mild eye swelling and pain that presented ~1 week before the
patient sought medical attention. This indicates that patients may
notice the condition at a late stage and then seek medical care.
The tumor size was large according to the Collaborative Ocular
Melanoma Study (COMS) classification (15), as it exceeded the dimensions of a
medium tumor in this classification standard, defined as an apical
height of 2.5–10 mm and largest basal diameter of ≤16 mm. A study
by Liu et al (16) found
that medium-sized tumors are most commonly detected, comprising 78%
of cases, with large-sized tumors being less common and small-sized
tumors being rare. Tumor size at the time of treatment has been
indicated to be the most important factor associated with patient
survival (17). According to the
modified Callender's classification of uveal melanoma (18), the tumor in the present case, with
its large proportion of epithelioid cells and small proportion of
spindle cells, was mixed-cell type. Studies performed in India
(19,20) and China (16) indicate that the incidence of spindle
type tumors is higher than that of mixed cell-type tumors. Tumors
with few epithelioid cells are generally associated with a slightly
improved prognosis than tumors with more abundant epithelioid
cells; however, one study found that tumors composed of 1–50%
epithelioid cells had the same prognosis as tumors composed
predominantly of epithelioid cells (18). A large volume and a large number of
epithelioid cells indicate the probability of a higher mortality
rate (17). The most notable
characteristic of the present case was the concurrent involvement
of the choroid, ciliary body and iris, with multi-regional
involvement of the iris. A large study from China previously
reported iris involvement in only 0.2% of uveal melanoma cases
(9). The COMS trials reported 5-
and 10-year cumulative metastasis rates of 25 and 34% respectively,
with 80% of the patients with metastasis dying within 1 year and
92% within 2 years after the diagnosis of metastases (21). In >90% of patients, metastases
involve the liver. Other sites of metastasis include bone (29%) and
the lungs (29%) (22). The largest
tumor basal diameter and ciliary body involvement have been shown
to be associated with metastasis and mortality (23). The average time from diagnosis to
metastasis in Asian patients is reported to be 35 months (9). Despite the lack of distant metastases
in the present case, lifelong follow-up is necessary.
Ocular ultrasound is valuable for the diagnosis of
uveal melanoma, with characteristic findings of a hemispherical or
mushroom-shaped solid mass contiguous with the eye wall (24). The tumor may appear hollow when
imaged, consistent with the ultrasound findings in the present
case. Additionally, the unique MRI characteristics of choroidal
melanoma, which include high-signal intensity on T1-weighted
imaging and low-signal intensity on T2-weighted imaging, contribute
to significant contrast on the corresponding weighted images
(25), aligning with the findings
in the current case.
Tumor compression of the lens, invasion of the lens
capsule or local circulatory disturbances due to tumor-derived
products can lead to nutritional or metabolic disorders in the
lens, causing cataracts. It has been suggested that tumor cells can
express high levels of transforming growth factor-β and other
cytokines, thereby promoting the development of cataracts (26). In addition, infiltration of the
tumor into the anterior chamber angle can disrupt normal
circulation of the aqueous humor, subsequently hindering aqueous
outflow and causing a sustained increase in intraocular pressure
(27), leading to secondary
glaucoma. In the present case, a slit-lamp examination not only
confirmed the presence of cataracts and a closed anterior chamber
angle, but also, even in the presence of a visibly opaque lens,
allowed a faint reflection of the tumor surface to be observed
through the lens.
Primary uveal melanoma can be classified into two
subgroups based on gene expression profiling: Class I, which is
associated with a low metastatic risk, and class II, which is
associated with a high metastatic risk (28). BAP1 has been shown to be mutated in
~40% of patients with uveal melanoma (29). Of note, in metastatic uveal
melanoma, BAP1 mutations are detected in up to 80% of cases, which
suggests that BAP1 inactivation is an important contributor to
disease progression (30,31). BAP1 modulates chromatin-associated
processes, including gene expression, DNA replication and DNA
repair, and contributes to the activation of regulatory immune
cells; therefore, its loss is associated with the suppression of
immune responses and increased tumor immune evasion (32). In the present case, neither somatic
nor germline BAP1 mutations were identified. However, three
homologous recombination repair gene mutations affecting other
genes, namely MLH1, RAD54L and SMARCA4, were detected. SMARCA4
deficiency has been shown to be a synthetic lethal factor when
combined with CDK4/6 inhibition (33), and high levels of SMARCA4 expression
are associated with poor prognosis in numerous types of tumors,
including liver hepatocellular carcinoma, and kidney renal clear
cell carcinoma (34). The nonrandom
deletion of RAD54L is associated with significant heterogeneity in
the malignant progression of tumors such as melanoma (35). In the present case, a missense
mutation in the MLH1 gene, a mismatch repair (MMR) gene was
observed. Notably, the detection of high-frequency microsatellite
instability/MMR deficiency is increasingly being included in the
routine tumor treatment of patients with various types of advanced
solid tumors (36). This is driven
by several key reasons: i) The microsatellite instability (MSI)/MMR
status can significantly influence treatment decisions; ii) major
oncology societies, including the National Comprehensive Cancer
Network (NCCN) and the European Society for Medical Oncology
(ESMO), now recommend MSI/MMR testing as part of routine assessment
in specific types of solid tumors; iii) beyond guiding treatment
choices, the MSI/MMR status serves as a prognostic indicator.
However, whether these three gene mutations have a role in the
pathogenesis of uveal melanoma requires further study.
Although ~99% of patients with ocular melanoma
exhibit no evidence of systemic metastatic disease at the initial
diagnosis, patients may develop metastases at any time thereafter,
with the liver being the most common site (37). Therefore, regular monitoring is
crucial in the follow-up of patients with ocular melanoma. The
treatment choices for uveal melanoma vary according to tumor size,
and the most frequently used modalities are enucleation and focal
radiotherapy, particularly plaque therapy (38–42).
With regard to programmed cell death protein 1 (PD-1) and
programmed death ligand 1 (PD-L1) expression, uveal melanoma most
frequently has PD-1−/PD-L1− or
PD-1+/PD-L1− status, which indicates
immunological tolerance, with the absence or functional suppression
of tumor-infiltrating lymphocytes in the tumor microenvironment,
respectively (43). This may
explain why uveal melanoma exhibits a poor response to anti-PD-1
therapy (44). Uveal melanoma is
also associated with high expression of glycoprotein 100 (gp100),
melanoma-associated antigen, melanoma antigen recognized by T cells
and tyrosinase-related protein-1, which are known to be immunogenic
cancer antigens (45–47). Therefore, these may represent
targets for uveal melanoma therapy. For instance, tebentafusp, also
known as IMCgp100, a bispecific fusion protein directed against
gp100, has been approved by the FDA for unresectable or metastatic
uveal melanoma.
In conclusion, ocular melanoma is the most common
primary intraocular malignant tumor in adults, and cataracts and
glaucoma can be secondary manifestations of intraocular primary
lesions. Slit-lamp examination may reveal the presence of tumor
cells as localized areas of black pigmentation in the iris, and may
also show reflections and shadows of the tumor. Therefore,
slit-lamp examination is essential for the preliminary diagnosis of
ocular tumors.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
This study was supported by grants from the National Natural
Science Foundation of China (grant no. 81800811) and the
Outstanding Young Medical Talent Training Funding Project of the
First Affiliated Hospital of Harbin Medical University (grant no.
2021J13).
Availability of data and materials
The high-throughput sequencing data generated in the
present study may be found in the China National Center for
Bioinformation under accession number HRA007562 or at the following
URL: (https://ngdc.cncb.ac.cn/search/specific?db=hra&q=HRA007562).
The other data generated in the present study may be requested from
the corresponding author.
Authors' contributions
YW conceived the study and wrote the manuscript.
QSun and ZL designed the study and assisted with the drafting of
the manuscript. XH and QSu collected data from imaging examinations
and participated in histological and morphological detection. SS
revised the manuscript and made substantial contributions to the
design of the study. FL designed the gene tests, analyzed the
mutation results and participated in revision of the manuscript. SS
and YW confirm the authenticity of all the raw data. All authors
read and approved the final version of the manuscript.
Ethics approval and consent to
participate
The present study was conducted according to the
guidelines of the Declaration of Helsinki. Written informed consent
was obtained from the patient.
Patient consent for publication
Written informed consent was obtained from the
patient for publication of the data and images in this case
report.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Bol KF, Donia M, Heegaard S, Kiilgaard JF
and Svane IM: Genetic biomarkers in melanoma of the ocular region:
What the medical oncologist should know. Int J Mol Sci.
21:52312020. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Singh AD, Turell ME and Topham AK: Uveal
melanoma: Trends in incidence, treatment, and survival.
Ophthalmology. 118:1881–1885. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kujala E, Mäkitie T and Kivelä T: Very
long-term prognosis of patients with malignant uveal melanoma.
Invest Ophthalmol Vis Sci. 44:4651–4659. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Jensen OA: Malignant melanomas of the
human uvea: 25-year follow-up of cases in Denmark, 1943--1952. Acta
Ophthalmol (Copenh). 60:161–182. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Varssano D, Friedman M, Goldstein M,
Bar-Sela S, Sella T, Shalev V and Chodick G: Association between
cataract and keratinocytic skin cancers or melanoma: Speculating on
the common role of sun and ultraviolet radiation exposures.
Ophthalmic Epidemiol. 24:336–340. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Fish GE, Jost BF, Snyder WI, Fuller DG and
Birch DG: Cataract extraction after brachytherapy for malignant
melanoma of the choroid. Ophthalmology. 98:619–622. 1991.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Factors predictive of growth and treatment
of small choroidal melanoma, . COMS Report No. 5. The Collaborative
Ocular Melanoma Study Group. Arch Ophthalmol. 115:1537–1544.
1997.PubMed/NCBI
|
|
8
|
Shields CL, Furuta M, Thangappan A, Nagori
S, Mashayekhi A, Lally DR, Kelly CC, Rudich DS, Nagori AV, Wakade
OA, et al: Metastasis of uveal melanoma millimeter-by-millimeter in
8033 consecutive eyes. Arch Ophthalmol. 127:989–998. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Manchegowda P, Singh AD, Shields C, Kaliki
S, Shah P, Gopal L and Rishi P: Uveal melanoma in Asians: A review.
Ocul Oncol Pathol. 7:159–167. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
McLaughlin CC, Wu XC, Jemal A, Martin HJ,
Roche LM and Chen VW: Incidence of noncutaneous melanomas in the
U.S. Cancer. 103:1000–1007. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Weinreb RN, Aung T and Medeiros FA: The
pathophysiology and treatment of glaucoma: A review. JAMA.
311:1901–1911. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Adam G, Brab M, Bohndorf K and Günther RW:
Gadolinium-DTPA-enhanced MRI of intraocular tumors. Magn Reson
Imaging. 8:683–689. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Gomori JM, Grossman RI, Shields JA,
Augsburger JJ, Joseph PM and DeSimeone D: Choroidal melanomas:
Correlation of NMR spectroscopy and MR imaging. Radiology.
158:443–445. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Peyster RG, Augsburger JJ, Shields JA,
Hershey BL, Eagle R Jr and Haskin ME: Intraocular tumors:
Evaluation with MR imaging. Radiology. 168:773–779. 1988.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Design and methods of a clinical trial for
a rare condition, . The collaborative ocular melanoma study. COMS
report no. 3. Control Clin Trials. 14:362–391. 1993.PubMed/NCBI
|
|
16
|
Liu YM, Li Y, Wei WB, Xu X and Jonas JB:
Clinical characteristics of 582 patients with uveal melanoma in
China. PLoS One. 10:e01445622015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Diener-West M, Hawkins BS, Markowitz JA
and Schachat AP: A review of mortality from choroidal melanoma. II.
A meta-analysis of 5-year mortality rates following enucleation,
1966 through 1988. Arch Ophthalmol. 110:245–250. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
McLean IW, Foster WD, Zimmerman LE and
Gamel JW: Modifications of callender's classification of uveal
melanoma at the armed forces institute of pathology. Am J
Ophthalmol. 96:502–550. 1983. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kashyap S, Venkatesh P, Sen S, Khanduja S,
Shrey D, Tinwala S and Garg S: Clinicopathologic characteristics of
choroidal melanoma in a north Indian population: Analysis of
10-year data. Int Ophthalmol. 34:235–239. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Meeralakshmi P, Shah PK and Narendran V:
Experiences of two different modalities in the management of
choroidal melanoma in the Asian Indian population. South Asian J
Cancer. 6:134–136. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Diener-West M, Reynolds SM, Agugliaro DJ,
Caldwell R, Cumming K, Earle JD, Hawkins BS, Hayman JA, Jaiyesimi
I, Jampol LM, et al: Development of metastatic disease after
enrollment in the COMS trials for treatment of choroidal melanoma:
Collaborative ocular melanoma study group report no. 26. Arch
Ophthalmol. 123:1639–1643. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Steckler AM, Francis JH, Shoushtari AN,
Abramson DH and Barker CA: Uveal melanoma metastatic at initial
diagnosis: A case series. Melanoma Res. 32:120–123. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zhou N, Zhang R, Liu Y and Wei W: Clinical
characteristics of UM and association of metastasis of uveal
melanoma with congenital oculocutaneous melanosis in Asian
patients: Analysis of 1151 consecutive eyes. Ophthalmol Retina.
5:1164–1172. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Jacobsen BH, Ricks C and Harrie RP: Ocular
ultrasound versus MRI in the detection of extrascleral extension in
a patient with choroidal melanoma. BMC Ophthalmol. 18:3202018.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Neupane R, Gaudana R and Boddu SHS:
Imaging techniques in the diagnosis and management of ocular
tumors: Prospects and challenges. AAPS J. 20:972018. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kase S, Parikh JG, Youssef PN, Murphree AL
and Rao NA: Transforming growth factor beta in
retinoblastoma-related cataract. Arch Ophthalmol. 126:1539–1542.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Camp DA, Yadav P, Dalvin LA and Shields
CL: Glaucoma secondary to intraocular tumors: Mechanisms and
management. Curr Opin Ophthalmol. 30:71–81. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Onken MD, Worley LA, Ehlers JP and Harbour
JW: Gene expression profiling in uveal melanoma reveals two
molecular classes and predicts metastatic death. Cancer Res.
64:7205–7209. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Field MG, Durante MA, Anbunathan H, Cai
LZ, Decatur CL, Bowcock AM, Kurtenbach S and Harbour JW: Punctuated
evolution of canonical genomic aberrations in uveal melanoma. Nat
Commun. 9:1162018. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Harbour JW, Onken MD, Roberson ED, Duan S,
Cao L, Worley LA, Council ML, Matatall KA, Helms C and Bowcock AM:
Frequent mutation of BAP1 in metastasizing uveal melanomas.
Science. 330:1410–1413. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Karlsson J, Nilsson LM, Mitra S, Alsen S,
Shelke GV, Sah VR, Forsberg EMV, Stierner U, All-Eriksson C,
Einarsdottir B, et al: Molecular profiling of driver events in
metastatic uveal melanoma. Nat Commun. 11:18942020. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Figueiredo CR, Kalirai H, Sacco JJ,
Azevedo RA, Duckworth A, Slupsky JR, Coulson JM and Coupland SE:
Loss of BAP1 expression is associated with an immunosuppressive
microenvironment in uveal melanoma, with implications for
immunotherapy development. J Pathol. 250:420–439. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Xue Y, Meehan B, Fu Z, Wang XQD, Fiset PO,
Rieker R, Levins C, Kong T, Zhu X, Morin G, et al: SMARCA4 loss is
synthetic lethal with CDK4/6 inhibition in non-small cell lung
cancer. Nat Commun. 10:5572019. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Guerrero-Martínez JA and Reyes JC: High
expression of SMARCA4 or SMARCA2 is frequently associated with an
opposite prognosis in cancer. Sci Rep. 8:20432018. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Ryu B, Kim DS, Deluca AM and Alani RM:
Comprehensive expression profiling of tumor cell lines identifies
molecular signatures of melanoma progression. PLoS One. 2:e5942007.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Latham A, Srinivasan P, Kemel Y, Shia J,
Bandlamudi C, Mandelker D, Middha S, Hechtman J, Zehir A,
Dubard-Gault M, et al: Microsatellite instability is associated
with the presence of lynch syndrome pan-cancer. J Clin Oncol.
37:286–295. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Balasubramanya R, Selvarajan SK, Cox M,
Joshi G, Deshmukh S, Mitchell DG and O'Kane P: Imaging of ocular
melanoma metastasis. Br J Radiol. 89:201600922016. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Davidorf FH, Pajka JT, Makley TA Jr and
Kartha MK: Radiotherapy for choroidal melanoma. An 18-year
experience with radon. Arch Ophthalmol. 105:352–355. 1987.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Lommatzsch PK: Results after
beta-irradiation (106Ru/106Rh) of choroidal melanomas. Twenty
years' experience. Am J Clin Oncol. 10:146–151. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Gass JD: Comparison of prognosis after
enucleation vs cobalt 60 irradiation of melanomas. Arch Ophthalmol.
103:916–923. 1985. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Augsburger JJ, Gamel JW, Sardi VF,
Greenberg RA, Shields JA and Brady LW: Enucleation vs cobalt plaque
radiotherapy for malignant melanomas of the choroid and ciliary
body. Arch Ophthalmol. 104:655–661. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Augsburger JJ, Gamel JW, Lauritzen K and
Brady LW: Cobalt-60 plaque radiotherapy vs enucleation for
posterior uveal melanoma. Am J Ophthalmol. 109:585–592. 1990.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Rossi E, Schinzari G, Zizzari IG, Maiorano
BA, Pagliara MM, Sammarco MG, Fiorentino V, Petrone G, Cassano A,
Rindi G, et al: Immunological backbone of uveal melanoma: Is there
a rationale for immunotherapy? Cancers (Basel). 11:10552019.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Javed A, Arguello D, Johnston C, Gatalica
Z, Terai M, Weight RM, Orloff M, Mastrangelo MJ and Sato T: PD-L1
expression in tumor metastasis is different between uveal melanoma
and cutaneous melanoma. Immunotherapy. 9:1323–1330. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
de Vries TJ, Trancikova D, Ruiter DJ and
van Muijen GN: High expression of immunotherapy candidate proteins
gp100, MART-I, tyrosinase and TRP-I in uveal melanoma. Br J Cancer.
78:1156–1161. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
de Vries TJ, Fourkour A, Wobbes T,
Verkroost G, Ruiter DJ and van Muijen GN: Heterogeneous expression
of immunotherapy candidate proteins gp100, MART-1, and tyrosinase
in human melanoma cell lines and in human melanocytic lesions.
Cancer Res. 57:3223–3229. 1997.PubMed/NCBI
|
|
47
|
Luyten GP, van der Spek CW, Brand I,
Sintnicolaas K, de Waard-Siebinga I, Jager MJ, de Jong PT, Schrier
PI and Luider TM: Expression of MAGE, gp100 and tyrosinase genes in
uveal melanoma cell lines. Melanoma Res. 8:11–16. 1998. View Article : Google Scholar : PubMed/NCBI
|