Breast cancer is the most prevalent cancer in the
world and the fifth primary cause of cancer-related deaths
(1). Triple-negative breast cancer
(TNBC) accounts for 15–20% of all cases of breast cancer in women
(2). TNBC is highly invasive, prone
to metastasis and can easily recur, which leads to a poor clinical
prognosis (3,4). Cytotoxic chemotherapy is the primary
treatment option for TNBC (5).
However, a number of patients with TNBC are resistant to
chemotherapy or poly(ADP-ribose) polymerase inhibitor therapy
(6). The remaining tumor and
metastases following chemotherapy can often lead to tumor
recurrence (7,8). Therefore, it is imperative to explore
the metabolic characteristics of TNBC to identify novel therapeutic
targets.
Unlike healthy cells, tumor cells exhibit notably
altered metabolic patterns to obtain increased energy and resources
for cell proliferation (9,10). Additionally, the cellular metabolism
of tumor cells varies considerably among breast cancer subtypes,
due to their highly heterogeneous nature (5). Differences in lipid metabolism among
breast cancer subtypes may also be attributed to estrogen receptor
status (11). A number of
malignancies impact amino acid metabolism and particularly their
transport system, which indicates that targeting these pathways
could be a potential approach for treating certain types of cancers
(12). Normal and cancer cells
exhibit distinct amino acid compositions (12). Certain types of cancers rely on
specific amino acids for growth. For instance, leucine and cystine
are crucial in melanoma (13) and
von Hippel-Lindau (VHL)-deficient renal cell carcinoma,
respectively (14,15). The association between amino acid
metabolism and breast cancer was first evaluated through the
investigation of glutamine metabolism. Targeted inhibition of
glutamine may represent a possible therapeutic strategy for TNBC,
as it demonstrated the potential to increase the antitumor
lymphocyte activity specific to TNBC (16). Furthermore, cystine/cysteine serves
a crucial role in tumor cells. The involvement of cystine/cysteine
metabolism in cancer was first evaluated in chronic lymphocytic
leukemia (15,17). Cystine deficiency has been shown to
promote the development of ferroptosis in numerous cancer cells,
indicating that it may be a treatment target in breast cancer
(18). The present study reviews
the current research relating to the relationship between TNBC and
cystine/cysteine metabolism.
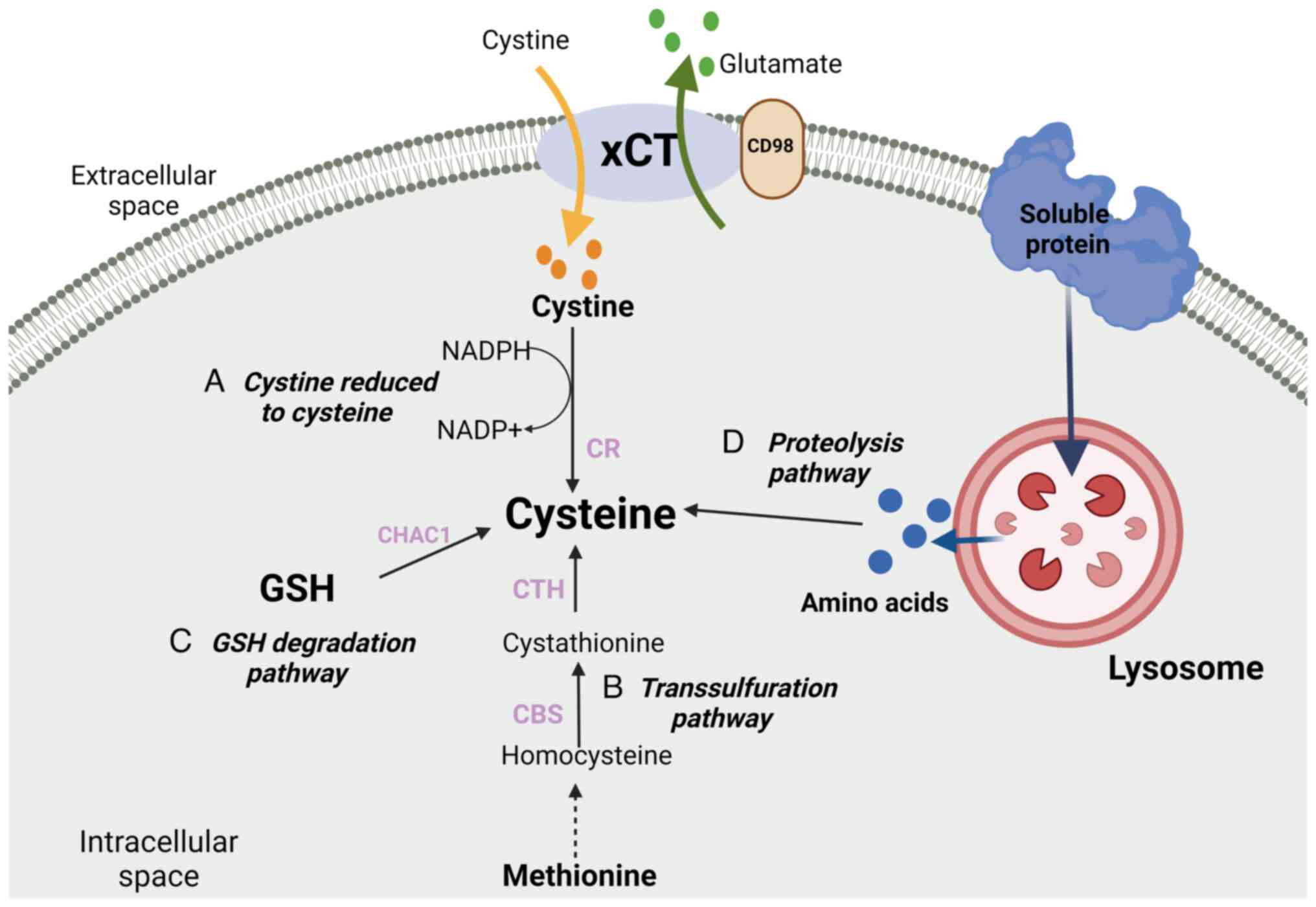
Cysteine and cystine are two interconvertible forms
of a sulfur-containing amino acid. Although cysteine is a
non-essential amino acid, its significance becomes pronounced
during periods of high nutritional demand (18). Typically, cysteine is detected in
vitro as cystine with disulfide bonds, owing to its
susceptibility to redox changes (19). Upon transportation into cells,
cystine can be converted into cysteine by the action of reducing
agents such as glutathione (GSH) or specific enzymes like
thioredoxin. Within cells, cysteine participates in cell
metabolism, whereas upon cell exit, cysteine is oxidized into
cystine and rejoins the circulatory system. In addition,
intracellular cystine can be generated from homocysteine via the
transsulfuration pathway, glutathione catabolism or through
recycling from protein degradation (Fig. 1) (19–22).
Homocysteine is a product of methionine demethylation. When
cysteine is in short supply, homocysteine can enter the
transsulfuration pathway by combining with serine, thereby
providing cysteine (19). For
example, the liver produces cysteine via glutathione catabolism or
homocysteine through the transsulfuration pathway, following which
it can be recycled through protein degradation (18). PIK3-catalytic subunit α mutant
breast cancer cell lines also synthesize cysteine via the
transsulfuration pathway (23).
However, this process of amino acid interchange does not provide
adequate cysteine for the rapid growth of malignant cells, thus
necessitating the import of exogenous cystine into cells (24).
Exogenous cystine facilitates the survival and
proliferation of numerous types of cancers. Basal-like breast
cancer (BLBC) accounts for 70–80% of TNBC cases (25,26). A
previous study demonstrated that cystine deprivation induces rapid
necrosis in BLBC cells (27). It
was demonstrated that cystine deprivation can alter the phenotype
of TNBC cells, as cystine deprivation instigated the development of
necroptosis and ferroptosis in TNBC cells, and triggered
mitochondrial fragmentation and reactive oxygen species (ROS)
production (28). By contrast,
luminal-type breast cancer cells, which are commonly found in
patients with breast cancer with hormone receptor-positive status,
are cystine-independent and therefore less affected by cystine
deprivation (27,28). The role of cystine in TNBC may be
associated with the epithelial-mesenchymal transition (EMT) of
cancer cells (7). EMT endows TNBC
cells with characteristics of cancer stem cells (CSC), such as
heightened metastatic potential and increased chemotherapy
resistance (29). Furthermore, the
mechanisms and behavioral traits of cell death mediated by cystine
deletion have been shown to be similar to those in renal cell
carcinoma cells with VHL deletion (13). Further research is warranted to
elucidate the molecular mechanisms by which cystine deprivation
affects TNBC cells.
Cysteine serves a significant role in tumor
progression, growth and the development of resistance to treatment
(21). Both clinical and animal
studies have indicated that cysteine might impede cancer
development by enhancing cellular detoxification from carcinogens
(23,30). Clinical investigations have
established associations between cysteine levels and certain types
of cancers, such as esophageal and gastric cancers (30). In a prospective case-control study,
increased plasma cysteine levels were positively correlated with an
increased risk of breast cancer. The TNBC patient subgroup did not
exhibit a significant elevation in cysteine levels compared with
the estrogen and progesterone-receptor positive breast cancer cases
(30). Furthermore, decreased
folate levels, which potentially resulted in the accumulation of
homocysteine and its subsequent conversion to cysteine, were
reported to increase the positive correlation between plasma
cysteine levels and the risk of breast cancer. Consequently, the
buildup of homocysteine due to folate deficiency may intensify its
detrimental effects on breast cancer development (30).
Cysteine uptake primarily relies on heterodimeric
amino acid transporters (HATs) located on the cell membrane. HATs
consist of the heavy chain solute carrier family (SLC) 3 and light
chain SLC7 (48). The heavy chain
SLC3 is essential for plasma membrane localization and light chain
stabilization, and comprises of two subunits, SLC3A1 and SLC3A2
(49). Most light chain SLC7s
interact with SLC3A2, while SLC3A1 forms heterodimers with SLC7A9,
which are associated with cystinuria (50). A large proportion of cancer cells
experience high levels of oxidative stress (51,52),
which indicates that increasing cysteine through de novo
biosynthesis or protein metabolism cannot satisfy the high demand
for antioxidant defense in cancer cells. Instead, cancer cells
typically acquire cystine from the extracellular environment
primarily through the aforementioned cystine transporter, system
xc−, which is then converted to cysteine
(37). However, certain types of
cancer cells, including chronic lymphocytic leukemia cells, can
preferentially obtain extracellular cysteine via cysteine
transporters (17). SLC3A1 has been
indicated to promote the proliferation of stem cells in
hepatocellular carcinoma cells (53) and was evidenced to be an effective
therapeutic target in metastatic colorectal cancer through the
recognition of metabolic signatures specific to metastatic cell
lines (54). In addition, SLC3A1
may be used to assess the prognosis of renal clear cell carcinoma
and is a prognostic indicator (55). Furthermore, SLC3A1 upregulation has
been demonstrated to promote breast cancer growth through cysteine
uptake (49).
Previous research has shown that SLC3A1 is highly
upregulated in various breast cancer cell lines compared with the
upregulation of the light chains SLC7A5, SLC7A7 and SLC7A9
(49). Furthermore, the expression
levels of SLC3A1 correlates with the clinical stage of breast
cancer (49). These findings
indicate that intracellular cysteine in breast cancer cells may
also be acquired from extracellular uptake via cysteine transporter
proteins. However, it is unclear whether the expression of SCL3A1
in TNBC is distinct from other subtypes.
Glutamate serves a crucial role in the growth and
development of malignant breast cancer cells, with its levels
primarily regulated by amino acid transporters, notably SLC1A5 and
SLC7A5 (56). SLC1A5 serves as a
standalone prognostic marker in breast cancer and is implicated in
drug resistance and breast cancer growth through numerous pathways
(57–59). Similarly, SLC7A11 participates in
the growth of TNBC (45,46). Although most breast cancer cells are
resistant to glutamine deprivation, occasionally TNBC cells are
sensitive to glutamine deprivation effects, necessitating the
influx of cysteine via SLC7A11 (45).
The survival of breast cancer cells, particularly
TNBC cells, is cystine-dependent. Therefore, inhibition of cystine
uptake can rapidly induce breast cancer cell death, particularly
affecting TNBC cells, which inhibits tumor progression and growth
(28). SLC3A1 is highly upregulated
in breast cancers. Patients with breast cancer with high expression
levels of SLC3A1 expression tend to have worse prognoses across all
histological grades compared with those with low SLC3A1 expression
levels (49). In addition,
overexpression of SLC3A1 was indicated to accelerate breast cancer
growth in an in vitro study (49). Furthermore, inhibition of SLC3A1 was
shown to suppress the malignancy-promoting effects of NAC in an
animal model of TNBC (49).
Dietary supplementation with the antioxidant
n-acetylcysteine (NAC) significantly accelerated tumor growth and
reduced the survival of an animal model of lung carcinoma (60).
With the exception of BLBC, metastasis of all
subtypes of breast cancer predominantly occurs in the bone
(61). However, BLBC has notably
decreased rates of liver and bone metastasis compared with brain,
lung and lymph node metastasis (61). TNBC is classified as a subtype of
BLBC based on gene expression profiling, that demonstrates an
overlap of 60–90% between TNBC and BLBC, compared with 11.5% for
non-TNBC and BLBC (62). SLC7A11
contributes to the distant metastasis of breast cancer,
particularly in cases of TNBC (44). Moreover, RNA sequencing analysis has
demonstrated that SLC7A11 expression levels were significantly
increased in mouse models of breast cancer with brain metastases
(63,64). In addition, increased SLC7A11
expression levels in breast cancer cells have been associated with
lung metastasis (65). The basal
breast cancer marker CD44 binds to SLC7A11, which stabilizes its
expression levels and consequently increases cystine intake. CD44
has also been also associated with increased lung metastasis in
patients with TNBC (66). The
aberrant upregulation of the transmembrane glycoprotein MUC1 has
been demonstrated in TNBC; MUC1 directly binds to the intracellular
domain of CD44, which further stabilizes the expression levels of
SLC7A11 (7,46). Further characterization of these
genes related to distant metastasis in breast cancer could provide
a foundation for the development of novel therapeutic strategies to
improve the prognosis of patients with cancer.
In previous years, the concept of metabolic
reprogramming has garnered attention in the field of cancer
therapy. In addition to glucose metabolism in cancer cells, amino
acid metabolism has also become a research hotspot, notably
treatments related to the restriction of amino acid metabolism that
can selectively target highly proliferative cancer cells (Fig. 2; Table
I).
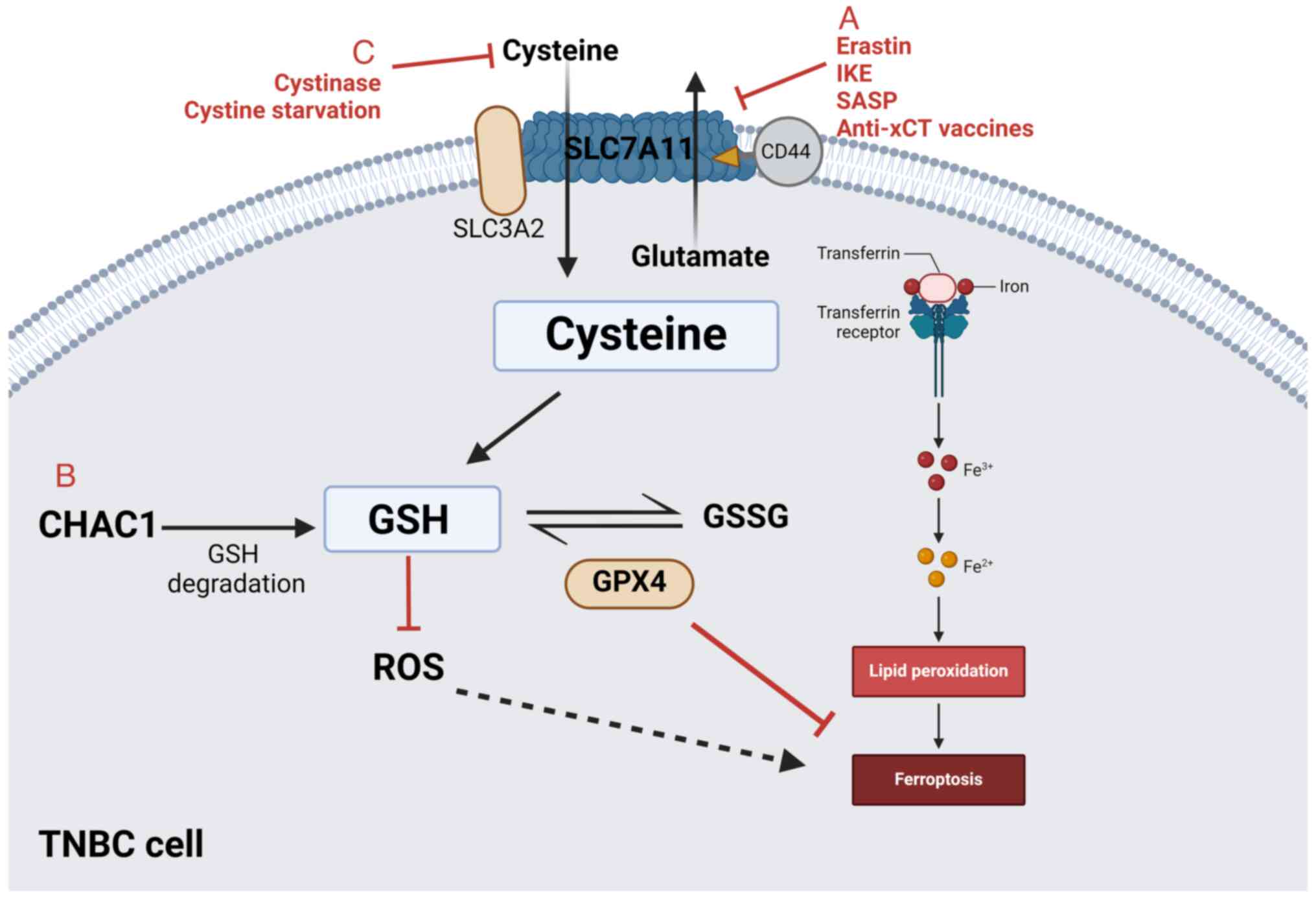
xCT and other amino acid transporters, such as
L-type amino acid transporter 1 (LAT1) and LAT2, are potential
therapeutic targets due to their availability and pharmacological
properties. Particularly, xCT is essential for cell survival and
the maintenance of glutathione (GSH) homeostasis and has thus been
identified as key target for anticancer therapy. Increased
expression levels of xCT signify that cancer cells rely on
extracellular cystine, as it is upregulated in CSCs in several
solid tumors, including breast cancer (43). Moreover, high xCT expression levels
are associated with poor prognosis of patients with breast cancer
(44,45). CSCs are a subset of cancer cells
with the ability to self-renew, differentiate and possess unlimited
self-renewal potential. Furthermore, the presence of EMT markers in
CSCs confers a high metastatic potential in breast cancer (67). CSCs exhibit a resistance to
radiation and chemotherapy due to the upregulation of numerous
detoxifying enzymes, such as superoxide dismutase 2, glutathione
peroxidases and heme oxygenase 1, that increase drug efflux and DNA
repair capabilities (44,67,68).
The development of therapies that effectively reduce tumor size
through the eradication of CSCs is challenging. The identification
of optimal CSC-associated targets is difficult as CSCs can switch
between a more quiescent state and a proliferative state (69). Conventional anticancer methods
primarily target developed tumors but are ineffective against CSCs.
However, therapies targeting xCT, such as xCT inhibitors and
targeted xCT vaccines, have been utilized in the treatment of
exogenous cystine-dependent cancer cells (65).
SASP is an anti-inflammatory drug that is commonly
used to treat ulcerative colitis and rheumatoid arthritis (74). Additionally, SASP inhibits the amino
acid transporter xCT, which reduced intracellular GSH levels,
diminishes cellular antioxidant defense and induces ferroptosis in
cancer cells (74). In addition,
SASP can inhibit TNBC growth through the suppression of the
expression levels of inflammation-related genes such as NF-κB, TNF,
RELA and IL-6 and MMP-related genes such as MMP1, MMP2 and MMP9
(75). SASP also demonstrates
significant inhibitory activity against lymphoma, small cell lung
cancer and prostate cancer (76–78).
Clinical trials have explored the use of SASP alone or in
combination with chemotherapy such as cisplatin for the treatment
of gastric cancer (79,80) and lung cancer (81). SAS monotherapy reduced the number of
CD44v+ CSCs, while combination therapy significantly
improved progression-free survival of patients with breast cancer
(64). The National Cancer
Institute conducted chemoinformatics analysis of 60 cell lines and
showed a negative correlation between xCT expression levels and
sensitivity to compounds associated with GSH-mediated resistance,
which indicated that xCT expression induces chemoresistance via
GSH-mediated ROS detoxification activity (82). Several studies have demonstrated
that the combination of xCT inhibition with chemotherapy can
effectively counteract this chemoresistance. For example, SASP
reduces GSH expression levels at the cellular level, which induces
growth arrest in breast cancer cells and increase the efficacy of
anticancer drugs, such as doxorubicin (83), similar to preclinical investigations
in vitro and in vivo (84).
Recent preclinical mouse models have shown that
immune-targeting the xCT antigen can enhance the activity of a
viral vectors-based vaccine against HER2, and reduces the growth of
HER2+ breast cancer, frequency of CSCs and metastatic
events (85). Furthermore, SASP can
inhibit xCT activity and TNBC growth (45,75).
Notably, TNBC cell lines are more sensitive to SASP treatment
compared with other breast cancer cell types (28). Also, SASP induces a more pronounced
growth-inhibiting phenotype in TNBC cells compared to other breast
cancer cell types (86). The
combination of xCT immune-targeting treatments with traditional or
novel medicines could activate the immune response and target
differentiated cancer cells or CSCs. For example, the glioma-toxic
impact of temozolomide (TMZ) can be potentiated by xCT inhibitors,
such as erastin, thus enhancing the efficacy of TMZ (87). Furthermore, high vitamin E doses
combined with SASP have synergistic antitumor effects on breast
cancer cells (88).
Nevertheless, SASP can increase mortality in mice
and also cause adverse effects such as weight loss and hypothermia,
regardless of the impact of intact SASP or its metabolites on the
system (89). However, clinical
trials have shown that xCT inhibitors are ineffective in patients
with glioma and have numerous SASP-related side effects, which
highlights the need for further clinical trials in patients with
high tumor burdens (90).
Therefore, future clinical trials should ascertain whether SASP can
be used in the treatment of breast cancer and particularly TNBC.
Also, further studies should assess whether SASP may be used for
the prevention or the treatment of metastatic breast cancer.
Several vaccines have been developed using plasmid
DNA, virus-like particles (VLP) and viral vectors. A DNA vaccine
targeting SLC7A11 has been developed to prevent breast cancer
metastasis in mice. This vaccine induces a humoral immune response
and delays the growth of initial tumors (43,91).
The injection of a DNA vaccine expressing xCT proteins and immune
targeting of xCT antigens on the cell surface effectively inhibited
subcutaneous tumor growth and lung metastasis in mice and increased
the chemosensitivity of breast CSCs to doxorubicin (44,83,92).
Although numerous clinical trials are underway with promising
results, DNA vaccines have not been formally utilized in patients
with cancer (49,93). A novel VLP-based immunotherapy that
targets xCT could significantly decrease lung metastases in a
treatment model of aggressive TNBC (49,94,95).
Furthermore, the bovine herpesvirus 4 vector that
expresses the full-length murine xCT protein could induce
T-lymphocyte activation and generation of anti-xCT antibodies in
mice, through the production of antibody-dependent cytotoxicity.
Preclinical models of TNBC and HER2+ breast cancer have
shown that this immune response can suppresses the development and
spread of the malignancy (91).
These findings suggest that xCT immunotargeting could inhibit
cancer growth and reduce the formation of metastases, although it
may not represent a curative treatment method for the disease.
Anti-xCT immunization may be utilized as an adjuvant therapy in
patients with breast cancer who are resistant to conventional
therapies.
In addition to the ongoing clinical trials for the
DNA targeting of SLC7A11, two anti-xCT vaccines are in the
preclinical testing phase, at the time of writing (44,96,97).
The development of effective vaccines for treating TNBC is
anticipated to improve disease management. Future research to
discover additional vaccines that target xCT and its associated
pathways is warranted in order to broaden the spectrum of available
treatment options and the clinical utility of such vaccines for
patients with breast cancer. Furthermore, clinical studies are
warranted to evaluate whether the anti-xCT vaccine could serve as
an adjuvant therapy for patients with breast cancer who have
developed resistance to standard therapy.
Ferroptosis is a form of cell death independent of
apoptosis, which relies on iron ions and ROS to induce lipid
peroxidation (70). Numerous
pathways, including the GTP cyclohydrolase
1/tetrahydrobiopterin-phospholipid axis (98), the cystine/cysteine-GSH-peroxidase 4
(GPX4) axis (99,100), the ferroptosis suppressor protein
1-coenzyme Q (CoQ) 10 axis on the plasma membrane (101,102) and the mitochondrial dihydroorotate
dehydrogenase/CoQ system, serve to counteract ferroptosis and
maintain basal lipid peroxidation (103). As a result, ferroptosis is mainly
induced trough the disruption of the aforementioned endogenous
ferroptosis inhibitory pathways. Notably, the
cystine/cysteine-involved ferroptosis pathway has been extensively
studied. Depletion of cystine/cysteine reduces intracellular GSH
levels and GPX4 activity within the cystine/cysteine-GSH-GPX4 axis,
thus causing ferroptosis (18,99).
Furthermore, cysteine depletion triggers extensive ferroptosis
reactions compared with GSH deletion (104). Additionally, limiting cystine
availability effectively induces ferroptosis in pancreatic cancer
and head and neck cancer (105,106). Previous studies have demonstrated
that it may be a promising strategy for treating certain types of
tumors, including TNBC (107,108).
While TNBC may exhibit increased susceptibility to
ferroptosis compared with other breast cancer subtypes, the precise
mechanisms are unknown (28). A
recent study demonstrated that cystine deprivation can reduce the
expression of GPX4 by preventing mTORC1/eukaryotic translation
initiation factor 4E-binding protein 1-mediated protein repression
(109). Chemical and genetic
inhibition of mTORC1 signaling induced ferroptosis in cancer cells
under cystine starvation conditions (109). Furthermore, it was reported that
the combination of mTORC1 inhibitors with IKE exhibits synergistic
tumor suppression in lung cancer models (109). Moreover, SLC7A11 overexpression
inhibited ROS-induced ferroptosis and counteracted p53-mediated
tumor growth inhibition (110).
Previous research has demonstrated that xCT serves a
crucial role in ferroptosis in certain cell types such as F98,
143B, BjeHLT, BJeLR, Calu-1 and HT-1080 cells (87,111).
The aforementioned small molecule inhibitors targeting xCT could
induce ferroptosis in tumor cells through inhibition of xCT
activity. Moreover, several US Food and Drug
Administration-approved clinical medications, including sorafenib
and artesunate, demonstrated the ability to induce ferroptosis in
numerous types of cancer cells, such as renal cell carcinoma, head
and neck cancer and TNBC, which indicates that ferroptosis may be
used in both preclinical and clinical settings (111,112). Lei et al (113) reported that the proteasomal
chaperone gankyrin inhibits ferroptosis through the activation of
the p53/SLC7A11/GPX4 signaling axis in TNBC cells. These novel
mechanisms provide valuable insights to guide further research and
develop new treatments for TNBC. Decreased cystine absorption and
increased expression levels of ferroptosis-inhibiting molecules,
such as SLC7A11 and GPX4, inhibited the occurrence of ferroptosis
in TNBC cells (109). The
aforementioned studies have identified a novel mechanism by which
these molecules influence the survival of cancer cells through
ferroptosis-induced cell death. In summary, triggering ferroptosis
is an effective therapeutic strategy for TNBC.
The dietary control of non-essential amino acids
(NEAA) has garnered considerable attention in recent years, owing
to the increased demand of NEAA reported in cancer cells (21). Certain types of cancers demonstrate
an increased ability for amino acid synthesis, occasionally
necessitating de novo NEAA synthesis to support their growth
and viability (114). In
vivo studies have demonstrated that dietary deprivation of
methionine and cystine decreases the growth of glioma cells in mice
(115). Furthermore, inhibition of
asparagine production or elimination of asparagine from the diet
could significantly inhibit the metastasis of breast cancer
(116). Cystine is indispensable
in TNBC growth and progression (27). An alternative treatment to xCT
inhibition involves the depletion of its substrate, cystine.
Cysteine deprivation or restriction of xCT by erastin or SASP could
limit GSH synthesis, increase the levels of lipid peroxidative
stress and ultimately induce ferroptosis in cysteine-dependent
tumor cells (70). Cystine
starvation has been shown to impede TNBC growth, which affects stem
cell properties and chemotherapy resistance in TNBC (27), thereby promoting TNBC cell
ferroptosis through activation of the general control
nonderepessible 2-eukaryotic translation initiation factor 2
subunit 1-activating transcription factor 4-CHAC1 pathway through
the specific cytosolic GSH degradation enzyme CHAC1 (28).
Cysteine deprivation may induce anticancer effects
through reducing the ability of cancer cells to remove ROS. Cancer
cells typically exhibit elevated levels of ROS (50). While excessive levels of ROS can
cause apoptosis in cancer cells, moderate ROS levels promote tumor
development and progression (49).
Cancer cells mitigate these ROS levels through GSH, thereby
inhibiting tumor cell apoptosis. Limiting dietary intake of
cysteine lowers plasma cysteine levels, and consequently increases
ROS levels in cancer cells through the reduction of GSH
biosynthesis (117). However,
cysteine starvation may protect tumor cells by disruption of the
polyamine pathway, which is used by cancer cells to defend
themselves against ROS (118).
Cysteine starvation may diminish the capacity of the
immune system to destroy cancer cells as cysteine is necessary for
T cell activation and function (119). However, a previous study has shown
that cysteine starvation could increase the anticancer immune
response of T cells (120).
Consequently, the precise impact of cysteine starvation on the
efficacy of the immune system in combating cancer remains currently
unclear. Cramer et al (121) reported that cystinase could
eliminate cystine from the body. Cyst(e)inase prevented breast
tumor growth, prolonged the survival of mice with chronic
lymphocytic leukemia and slowed the growth of prostate tumors. In
contrast to SASP toxicity, long-term treatment with cysteine
enzymes did not produce toxic side effects in mice.
Despite the promising outcomes associated with
cystine-restricted approaches, numerous challenges remain. Research
has suggested that increased activity of the endogenous
transsulfuration pathway could improve cancer cell survival in
environments with diminished extracellular cysteine levels
(22). Future investigations should
confirm the efficacy of reducing amino acid intake in patients with
cancer. Another challenge is that only a subset of TNBCs exhibiting
EMT, which represents ~50% of TNBC cases, are vulnerable to
eradication through cysteine depletion therapy (27). This suggests that a large portion of
TNBC and luminal breast carcinoma cells are not reliant on cysteine
and may demonstrate a limited response to cysteine deficiency.
However, a significant number of luminal breast cancers and TNBCs
with epithelial characteristics are also independent of cysteine
and exhibit resistance to cysteine deficiency. Research suggests
that histone deacetylase (HDAC) 6 inhibitors, such as tubacin,
could improve the synthetic lethality of cysteine deprivation and
overcome resistance in non-mesenchymal TNBCs (122).
Overall, cysteine starvation therapy aims to
diminish the availability of cystine/cysteine or enhance its
elimination. The impact of cysteine deprivation on TNBC encompasses
various aspects, including proliferation, resistance to
chemotherapy and antitumor properties. Therefore, additional
research is needed to validate the impact of a reduced amino acid
intake in patients with cancer.
In addition to the aforementioned prospective
treatments outlined for TNBC, ongoing efforts are concentrated on
developing novel therapeutic approaches. A promising avenue
involves the utilization of cysteine-rich protein-based tactics
targeting cysteine-rich angiogenic inducer 61 (CCN1/CYR61)
(123), which could potentially
mitigate the biological aggressiveness of TNBC or BLBC. CCN1 is a
small secreted cysteine-rich protein which mainly supports cell
adhesion, migration and survival. Another focus of current research
is the development of customized protein nanoparticles tailored for
TNBC treatment. This involves injecting whey protein nanoparticles
modified to carry chemotherapy drugs like doxorubicin into mice
induced with breast cancer and observing their tumor-killing
effects. The use of cysteine modification presents a novel and
potentially efficacious nanoparticulate strategy for the treatment
of TNBC. Overall, the group with cysteine-modified nanoparticle
treatment exhibited the greatest shrinkage and damage to breast
cancer cells, indicating that cysteine-modified nanoparticles have
excellent anticancer and targeting capabilities (107).
TNBC is a subtype of breast cancer that currently
lacks effective therapeutic targets. The metabolism of
cystine/cysteine metabolism serves an important role in the onset
and progression of TNBC and may be closely associated with patient
responses to therapy. Hence, targeting the cystine/cysteine
metabolic system presents a viable therapeutic approach for the
treatment of TNBC, as it leverages the metabolic characteristics,
biomarkers and associated signaling pathways of the disease.
However, further comprehensive research into the regulatory
mechanisms governing cystine/cysteine metabolism in TNBC is
warranted, given the limited number of recognized regulatory
pathways. The exploration of additional mechanisms may identify
vital targets for the development of efficacious treatments for
TNBC. Similarly, the metabolic heterogeneity and adaptive responses
in TNBC poses numerous challenges to the clinical application of
metabolic therapy. The metabolic heterogeneity of cancer cells
arises from a diverse range of factors, such as genetic mutations
and the tumor microenvironment (5,7).
Therefore, future studies could combine metabolic therapy with
radiotherapy, chemotherapy, targeted therapy or immunotherapy to
counteract tumor heterogeneity and improve the prognosis of
patients with TNBC (119).
Another notable challenge is the cellular dependence
on cysteine, as demonstrated by mesenchymal TNBC cells, whereas
non-mesenchymal TNBC cells frequently display resistance to
cysteine deprivation (27). The
development of supplementary inhibitors may significantly improve
the efficacy of targeted therapies aimed at exploiting cysteine
dependence to treat certain subtypes of breast cancer. In the
future, the identification of specific genes or pathways could
serve as direct targets, in conjunction with cysteine deprivation
for cancer therapy. The search for comprehensive and combination
therapies for cancer, particularly strategies that target cysteine
or cysteine metabolism, has shown promising results. The
cooperation observed with HDAC6 inhibitors and erastin as
treatments for TNBC highlights the need for further investigation
into their cellular and molecular pathways. Therefore, the
identification of novel cellular molecules or pathways could
potentially serve as valuable direct targets for combination
treatments of TNBC.
Not applicable.
This work was supported by the Medical Science and Technology
Project of Zhejiang Province (grant no. 2023KY1284) and Jinhua
Science and Technology Bureau (grant no. 2021-3-084).
WX and CX were responsible for the conception and
design of study. WX wrote the first draft of the manuscript. WX and
CX worked on further versions of the manuscript. Both authors read
and approved the final version of the manuscript. Data
authentication is not applicable.
Not applicable.
Not applicable.
Not applicable.
The authors declare that they have no competing
interests.
|
1
|
Sung H, Ferlay J, Siegel RL, Laversanne M,
Soerjomataram I, Jemal A and Bray F: Global cancer statistics 2020:
GLOBOCAN estimates of incidence and mortality worldwide for 36
cancers in 185 countries. CA Cancer J Clin. 71:209–249. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Morris GJ, Naidu S, Topham AK, Guiles F,
Xu Y, McCue P, Schwartz GF, Park PK, Rosenberg AL, Brill K and
Mitchell EP: Differences in breast carcinoma characteristics in
newly diagnosed African-American and Caucasian patients: A
single-institution compilation compared with the National Cancer
Institute's Surveillance, Epidemiology, and End Results database.
Cancer. 110:876–884. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Singh DD and Yadav DK: TNBC: Potential
targeting of multiple receptors for a therapeutic breakthrough,
nanomedicine, and immunotherapy. Biomedicines. 9:8762021.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Dietze EC, Sistrunk C, Miranda-Carboni G,
O'Regan R and Seewaldt VL: Triple-negative breast cancer in
African-American women: Disparities versus biology. Nat Rev Cancer.
15:248–254. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Gandhi N and Das GM: Metabolic
reprogramming in breast cancer and its therapeutic implications.
Cells. 8:892019. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Litton JK, Rugo HS, Ettl J, Hurvitz SA,
Gonçalves A, Lee KH, Fehrenbacher L, Yerushalmi R, Mina LA, Martin
M, et al: Talazoparib in patients with advanced breast cancer and a
germline BRCA mutation. N Engl J Med. 379:753–763. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wang Z, Jiang Q and Dong C: Metabolic
reprogramming in triple-negative breast cancer. Cancer Biol Med.
17:44–59. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Nedeljković M and Damjanović A: Mechanisms
of chemotherapy resistance in triple-negative breast cancer-how we
can rise to the challenge. Cells. 8:9572019. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Pavlova NN, Zhu J and Thompson CB: The
hallmarks of cancer metabolism: Still emerging. Cell Metab.
34:355–377. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
DeBerardinis RJ, Lum JJ, Hatzivassiliou G
and Thompson CB: The biology of cancer: Metabolic reprogramming
fuels cell growth and proliferation. Cell Metab. 7:11–20. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Tang X, Lin CC, Spasojevic I, Iversen ES,
Chi JT and Marks JR: A joint analysis of metabolomics and genetics
of breast cancer. Breast Cancer Res. 16:4152014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wei Z, Liu X, Cheng C, Yu W and Yi P:
Metabolism of amino acids in cancer. Front Cell Dev Biol.
8:6038372021. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Sheen JH, Zoncu R, Kim D and Sabatini DM:
Defective regulation of autophagy upon leucine deprivation reveals
a targetable liability of human melanoma cells in vitro and in
vivo. Cancer Cell. 19:613–628. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Tang X, Wu J, Ding CK, Lu M, Keenan MM,
Lin CC, Lin CA, Wang CC, George D, Hsu DS and Chi JT: Cystine
deprivation triggers programmed necrosis in VHL-Deficient renal
cell carcinomas. Cancer Res. 76:1892–1903. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Iglehart J, York RM, Modest AP, Lazarus H
and Livingston D: Cystine requirement of continuous human lymphoid
cell lines of normal and leukemic origin. J Biol Chem.
252:7184–7191. 1977. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Edwards DN, Ngwa VM, Raybuck AL, Wang S,
Hwang Y, Kim LC, Cho SH, Paik Y, Wang Q, Zhang S, et al: Selective
glutamine metabolism inhibition in tumor cells improves antitumor T
lymphocyte activity in triple-negative breast cancer. J Clin
Invest. 131:e1401002021. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zhang W, Trachootham D, Liu J, Chen G,
Pelicano H, Garcia-Prieto C, Lu W, Burger JA, Croce CM, Plunkett W,
et al: Stromal control of cystine metabolism promotes cancer cell
survival in chronic lymphocytic leukaemia. Nat Cell Biol.
14:276–286. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Daher B, Vučetić M and Pouysségur J:
Cysteine depletion, a key action to challenge cancer cells to
ferroptotic cell death. Front Oncol. 10:7232020. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Stipanuk MH: Sulfur amino acid metabolism:
Pathways for production and removal of homocysteine and cysteine.
Annu Rev Nutr. 24:539–577. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zhang HF, Klein Geltink RI, Parker SJ and
Sorensen PH: Transsulfuration, minor player or crucial for cysteine
homeostasis in cancer. Trends Cell Biol. 32:800–814. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Combs JA and DeNicola GM: The
non-essential amino acid cysteine becomes essential for tumor
proliferation and survival. Cancers (Basel). 11:6782019. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zhu J, Berisa M, Schwörer S, Qin W, Cross
JR and Thompson CB: Transsulfuration activity can support cell
growth upon extracellular cysteine limitation. Cell Metab.
30:865–876.e5. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Lien EC, Ghisolfi L, Geck RC, Asara JM and
Toker A: Oncogenic PI3K promotes methionine dependency in breast
cancer cells through the cystine-glutamate antiporter xCT. Sci
Signal. 10:eaao66042017. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Pajares MA and Perez-Sala D: Mammalian
sulfur amino acid metabolism: A nexus between redox regulation,
nutrition, epigenetics, and detoxification. Antioxid Redox Signal.
29:408–452. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Yin L, Duan JJ, Bian XW and Yu SC:
Triple-negative breast cancer molecular subtyping and treatment
progress. Breast Cancer Res. 22:612020. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Prat A, Parker JS, Karginova O, Fan C,
Livasy C, Herschkowitz JI, He X and Perou CM: Phenotypic and
molecular characterization of the claudin-low intrinsic subtype of
breast cancer. Breast Cancer Res. 12:R682010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Tang X, Ding CK, Wu J, Sjol J, Wardell S,
Spasojevic I, George D, McDonnell DP, Hsu DS, Chang JT and Chi JT:
Cystine addiction of triple-negative breast cancer associated with
EMT augmented death signaling. Oncogene. 36:4235–4242. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Chen MS, Wang SF, Hsu CY, Yin PH, Yeh TS,
Lee HC and Tseng LM: CHAC1 degradation of glutathione enhances
cystine-starvation-induced necroptosis and ferroptosis in human
triple negative breast cancer cells via the GCN2-eIF2α-ATF4
pathway. Oncotarget. 8:114588–114602. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Shibue T and Weinberg RA: EMT, CSCs, and
drug resistance: The mechanistic link and clinical implications.
Nat Rev Clin Oncol. 14:611–629. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Lin J, Lee IM, Song Y, Cook NR, Selhub J,
Manson JE, Buring JE and Zhang SM: Plasma homocysteine and cysteine
and risk of breast cancer in women. Cancer Res. 70:2397–2405. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Wu LL and Wu JT: Hyperhomocysteinemia is a
risk factor for cancer and a new potential tumor marker. Clin Chim
Acta. 322:21–28. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Sun CF, Haven TR, Wu TL, Tsao KC and Wu
JT: Serum total homocysteine increases with the rapid proliferation
rate of tumor cells and decline upon cell death: A potential new
tumor marker. Clin Chim Acta. 321:55–62. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Xue F and Michels KB: Diabetes, metabolic
syndrome, and breast cancer: A review of the current evidence. Am J
Clin Nutr. 86:S823–S835. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
La Vecchia C, Giordano SH, Hortobagyi GN
and Chabner B: Overweight, obesity, diabetes, and risk of breast
cancer: Interlocking pieces of the puzzle. Oncologist. 16:726–729.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Lewerenz J, Hewett SJ, Huang Y, Lambros M,
Gout PW, Kalivas PW, Massie A, Smolders I, Methner A, Pergande M,
et al: The Cystine/Glutamate Antiporter System xc-in Health and
disease: From molecular mechanisms to novel therapeutic
opportunities. Antioxid Redox Signa. 18:522–555. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Sato H, Tamba M, Ishii T and Bannai S:
Cloning and expression of a plasma membrane cystine/glutamate
exchange transporter composed of two distinct proteins. J Biol
Chem. 274:11455–11458. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Koppula P, Zhuang L and Gan B: Cystine
transporter SLC7A11/xCT in cancer: Ferroptosis, nutrient
dependency, and cancer therapy. Protein Cell. 12:599–620. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Kandasamy P, Gyimesi G, Kanai Y and
Hediger MA: Amino acid transporters revisited: New views in health
and disease. Trends Biochem Sci. 43:752–789. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Miyazaki I, Murakami S, Torigoe N,
Kitamura Y and Asanuma M: Neuroprotective effects of levetiracetam
target xCT in astrocytes in parkinsonian mice. J Neurochem.
136:194–204. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Sugano K, Maeda K, Ohtani H, Nagahara H,
Shibutani M and Hirakawa K: Expression of xCT as a predictor of
disease recurrence in patients with colorectal cancer. Anticancer
Res. 35:677–682. 2015.PubMed/NCBI
|
|
41
|
Robert SM, Buckingham SC, Campbell SL,
Robel S, Holt KT, Ogunrinu-Babarinde T, Warren PP, White DM, Reid
MA, Eschbacher JM, et al: SLC7A11 expression is associated with
seizures and predicts poor survival in patients with malignant
glioma. Sci Transl Med. 7:289ra862015. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Ji X, Qian J, Rahman SMJ, Siska PJ, Zou Y,
Harris BK, Hoeksema MD, Trenary IA, Heidi C, Eisenberg R, et al:
xCT (SLC7A11)-mediated metabolic reprogramming promotes non-small
cell lung cancer progression. Oncogene. 37:5007–5019. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Ruiu R, Rolih V, Bolli E, Barutello G,
Riccardo F, Quaglino E, Merighi IF, Pericle F, Donofrio G, Cavallo
F and Conti L: Fighting breast cancer stem cells through the
immune-targeting of the xCT cystine-glutamate antiporter. Cancer
Immunol Immunother. 68:131–141. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Lanzardo S, Conti L, Rooke R, Ruiu R,
Accart N, Bolli E, Arigoni M, Macagno M, Barrera G, Pizzimenti S,
et al: Immunotargeting of antigen xCT attenuates Stem-like cell
behavior and metastatic progression in breast cancer. Cancer Res.
76:62–72. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Timmerman LA, Holton T, Yuneva M, Louie
RJ, Padró M, Daemen A, Hu M, Chan DA, Ethier SP, van't Veer LJ, et
al: Glutamine sensitivity analysis identifies the xCT antiporter as
a common triple-negative breast tumor therapeutic target. Cancer
Cell. 24:450–465. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Hasegawa M, Takahashi H, Rajabi H, Alam M,
Suzuki Y, Yin L, Tagde A, Maeda T, Hiraki M, Sukhatme VP, et al:
Functional interactions of the cystine/glutamate antiporter, CD44v
and MUC1-C oncoprotein in triple-negative breast cancer cells.
Oncotarget. 7:11756–11769. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Ishimoto T, Nagano O, Yae T, Tamada M,
Motohara T, Oshima H, Oshima M, Ikeda T, Asaba R, Yagi H, et al:
CD44 variant regulates redox status in cancer cells by stabilizing
the xCT subunit of system xc- and thereby promotes tumor growth.
Cancer Cell. 19:387–400. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Fotiadis D, Kanai Y and Palacín M: The
SLC3 and SLC7 families of amino acid transporters. Mol Aspects Med.
34:139–158. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Jiang Y, Cao Y, Wang Y, Li W, Liu X, Lv Y,
Li X and Mi J: Cysteine transporter SLC3A1 promotes breast cancer
tumorigenesis. Theranostics. 7:1036–1046. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Jeong JY, Oh KJ, Sohn JS, Jun DY, Shin JI,
Lee KH and Lee JY: Clinical course and mutational analysis of
patients with cystine stone: A Single-Center experience.
Biomedicines. 11:27472023. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Trachootham D, Alexandre J and Huang P:
Targeting cancer cells by ROS-mediated mechanisms: A radical
therapeutic approach? Nat Rev Drug Discov. 8:579–591. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Chio C II and Tuveson DA: ROS in cancer:
The burning question. Trends Mol Med. 23:411–429. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Haraguchi N, Inoue H, Tanaka F, Mimori K,
Utsunomiya T, Sasaki A and Mori M: Cancer stem cells in human
gastrointestinal cancers. Hum Cell. 19:24–29. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Tarragó-Celada J, Foguet C,
Tarrado-Castellarnau M, Marin S, Hernández-Alias X, Perarnau J,
Morrish F, Hockenbery D, Gomis RR, Ruppin E, et al: Cysteine and
folate metabolism are targetable vulnerabilities of metastatic
colorectal cancer. Cancers (Basel). 13:4252021. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Bao WEI, Han Q, Guan X, Wang Z and Gu MIN:
Solute carrier-related signature for assessing prognosis and
immunity in patients with clear-cell renal cell carcinoma. Oncol
Res. 31:181–192. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Cha YJ, Kim ES and Koo JS: Amino acid
transporters and glutamine metabolism in breast cancer. Int J Mol
Sci. 19:9072018. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Todorova VK, Kaufmann Y, Luo S and
Klimberg VS: Tamoxifen and raloxifene suppress the proliferation of
estrogen receptor-negative cells through inhibition of glutamine
uptake. Cancer Chemother Pharmacol. 67:285–291. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Bernhardt S, Bayerlová M, Vetter M,
Wachter A, Mitra D, Hanf V, Lantzsch T, Uleer C, Peschel S, John J,
et al: Proteomic profiling of breast cancer metabolism identifies
SHMT2 and ASCT2 as prognostic factors. Breast Cancer Res.
19:1122017. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Jeon YJ, Khelifa S, Ratnikov B, Scott DA,
Feng Y, Parisi F, Ruller C, Lau E, Kim H, Brill LM, et al:
Regulation of glutamine carrier proteins by RNF5 determines breast
cancer response to ER Stress-Inducing chemotherapies. Cancer Cell.
27:354–369. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Sayin VI, Ibrahim MX, Larsson E, Nilsson
JA, Lindahl P and Bergo MO: Antioxidants accelerate lung cancer
progression in mice. Sci Transl Med. 6:221ra152014. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Kennecke H, Yerushalmi R, Woods R, Cheang
MCU, Voduc D, Speers CH, Nielsen TO and Gelmon K: Metastatic
behavior of breast cancer subtypes. J Clin Oncol. 28:3271–3277.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Weigelt B and Reis-Filho JS: Histological
and molecular types of breast cancer: Is there a unifying taxonomy?
Nat Rev Clin Oncol. 6:718–730. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Sato R, Nakano T, Hosonaga M, Sampetrean
O, Harigai R, Sasaki T, Koya I, Okano H, Kudoh J, Saya H and Arima
Y: RNA sequencing analysis reveals interactions between breast
cancer or melanoma cells and the tissue microenvironment during
brain metastasis. Biomed Res Int. 2017:80329102017. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Hosonaga M, Saya H and Arima Y: Molecular
and cellular mechanisms underlying brain metastasis of breast
cancer. Cancer Metastasis Rev. 39:711–720. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Ruiu R, Cossu C, Iacoviello A, Conti L,
Bolli E, Ponzone L, Magri J, Rumandla A, Calautti E and Cavallo F:
Cystine/glutamate antiporter xCT deficiency reduces metastasis
without impairing immune system function in breast cancer mouse
models. J Exp Clin Cancer Res. 42:2542023. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Hu J, Li G, Zhang P, Zhuang X and Hu G: A
CD44v+ subpopulation of breast cancer stem-like cells with enhanced
lung metastasis capacity. Cell Death Dis. 8:e26792017. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Lollini PL, Cavallo F, Giovanni CD and
Nanni P: Preclinical vaccines against mammary carcinoma. Expert Rev
Vaccines. 12:1449–1463. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Nagano O, Okazaki S and Saya H: Redox
regulation in stem-like cancer cells by CD44 variant isoforms.
Oncogene. 32:5191–5198. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Kinoshita H, Okabe H, Beppu T, Chikamoto
A, Hayashi H, Imai K, Mima K, Nakagawa S, Ishimoto T, Miyake K, et
al: Cystine/glutamic acid transporter is a novel marker for
predicting poor survival in patients with hepatocellular carcinoma.
Oncol Rep. 29:685–689. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Dixon SJ, Lemberg KM, Lamprecht MR, Skouta
R, Zaitsev EM, Gleason CE, Patel DN, Bauer AJ, Cantley AM, Yang WS,
et al: Ferroptosis: An Iron-dependent form of nonapoptotic cell
death. Cell. 149:1060–1072. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Hassannia B, Vandenabeele P and Vanden
Berghe T: Targeting ferroptosis to iron out cancer. Cancer Cell.
35:830–849. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Xu T, Ding W, Ji X, Ao X, Liu Y, Yu W and
Wang J: Molecular mechanisms of ferroptosis and its role in cancer
therapy. J Cell Mol Med. 23:4900–4912. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Zhang Y, Tan H, Daniels JD, Zandkarimi F,
Liu H, Brown LM, Uchida K, O'Connor OA and Stockwell BR: Imidazole
ketone erastin induces ferroptosis and slows tumor growth in a
mouse lymphoma model. Cell Chem Biol. 26:623–633.e9. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Zheng YW, Miao XY, Xiong L, Chen B, Kong
FH, Zhou JJ, Liu ZT, Wen Y, Zhang ZJ and Zou H: Sulfasalazine
sensitizes polyhematoporphyrin-mediated photodynamic therapy in
cholangiocarcinoma by targeting xCT. Front Pharmacol.
12:7234882021. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Yu H, Hu K, Zhang T and Ren H:
Identification of target genes related to sulfasalazine in
triple-negative breast cancer through Network pharmacology. Med Sci
Monit. 26:e9265502020. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Gout P, Buckley A, Simms C and Bruchovsky
N: Sulfasalazine, a potent suppressor of lymphoma growth by
inhibition of the x(c)-cystine transporter: A new action for an old
drug. Leukemia. 15:1633–1640. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Guan J, Lo M, Dockery P, Mahon S, Karp CM,
Buckley AR, Lam S, Gout PW and Wang YZ: The × c-cystine/glutamate
antiporter as a potential therapeutic target for small-cell lung
cancer: Use of sulfasalazine. Cancer Chemother Pharmacol.
64:463–472. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Doxsee DW, Gout PW, Kurita T, Lo M,
Buckley AR, Wang Y, Xue H, Karp CM, Cutz JC, Cunha GR and Wang YZ:
Sulfasalazine-induced cystine starvation: Potential use for
prostate cancer therapy. Prostate. 67:162–171. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Shitara K, Doi T, Nagano O, Imamura CK,
Ozeki T, Ishii Y, Tsuchihashi K, Takahashi S, Nakajima TE, Hironaka
S, et al: Dose-escalation study for the targeting of CD44v+ cancer
stem cells by sulfasalazine in patients with advanced gastric
cancer (EPOC1205). Gastric Cancer. 20:341–349. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Shitara K, Doi T, Nagano O, Fukutani M,
Hasegawa H, Nomura S, Sato A, Kuwata T, Asai K, Einaga Y, et al:
Phase 1 study of sulfasalazine and cisplatin for patients with
CD44v-positive gastric cancer refractory to cisplatin (EPOC1407).
Gastric Cancer. 20:1004–1009. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Otsubo K, Nosaki K, Imamura CK, Ogata H,
Fujita A, Sakata S, Hirai F, Toyokawa G, Iwama E, Harada T, et al:
Phase I study of salazosulfapyridine in combination with cisplatin
and pemetrexed for advanced non-small-cell lung cancer. Cancer Sci.
108:1843–189. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Dai Z, Huang Y, Sadee W and Blower P:
Chemoinformatics analysis identifies cytotoxic compounds
susceptible to chemoresistance mediated by glutathione and
cystine/glutamate transport system xc-. J Med Chem. 50:1896–1906.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Narang VS, Pauletti GM, Gout PW, Buckley
DJ and Buckley AR: Sulfasalazine-induced reduction of glutathione
levels in breast cancer cells: Enhancement of growth-inhibitory
activity of doxorubicin. Chemotherapy. 53:210–217. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Barutello G, Di Lorenzo A, Gasparetto A,
Galiazzi C, Bolli E, Conti L and Cavallo F: Immunotherapy against
the Cystine/Glutamate Antiporter xCT improves the efficacy of
APR-246 in preclinical breast cancer models. Biomedicines.
10:28432022. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Conti L, Bolli E, Di Lorenzo A, Franceschi
V, Macchi F, Riccardo F, Ruiu R, Russo L, Quaglino E, Donofrio G
and Cavallo F: Immunotargeting of the xCT Cystine/Glutamate
antiporter potentiates the efficacy of HER2-targeted
immunotherapies in breast cancer. Cancer Immunol Res. 8:1039–53.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Yu H, Yang C, Jian L, Guo S, Chen R, Li K,
Qu F, Tao K, Fu Y, Luo F and Liu S: Sulfasalazine-induced
ferroptosis in breast cancer cells is reduced by the inhibitory
effect of estrogen receptor on the transferrin receptor. Oncol Rep.
42:826–838. 2019.PubMed/NCBI
|
|
87
|
Sehm T, Rauh M, Wiendieck K, Buchfelder M,
Eyüpoglu IY and Savaskan NE: Temozolomide toxicity operates in a
xCT/SLC7a11 dependent manner and is fostered by ferroptosis.
Oncotarget. 7:746302016. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Wei CW, Yu YL, Lu JY, Hung YT, Liu HC and
Yiang GT: Anti-cancer effects of sulfasalazine and Vitamin E
succinate in MDA-MB 231 Triple-negative breast cancer cells. Int J
Med Sci. 16:494–500. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Verbruggen L, Sprimont L, Bentea E,
Janssen P, Gharib A, Deneyer L, De Pauw L, Lara O, Sato H, Nicaise
C and Massie A: Chronic sulfasalazine treatment in mice induces
system xc−-Independent adverse effects. Front
Pharmacol. 12:6256992021. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Robe PA, Martin DH, Nguyen-Khac MT, Artesi
M, Deprez M, Albert A, Vanbelle S, Califice S, Bredel M and Bours
V: Early termination of ISRCTN45828668, a phase 1/2 prospective,
randomized study of sulfasalazine for the treatment of progressing
malignant gliomas in adults. BMC Cancer. 9:3722009. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Donofrio G, Tebaldi G, Lanzardo S, Ruiu R,
Bolli E, Ballatore A, Rolih V, Macchi F, Conti L and Cavallo F:
Bovine herpesvirus 4-based vector delivering the full length xCT
DNA efficiently protects mice from mammary cancer metastases by
targeting cancer stem cells. Oncoimmunology. 7:e14941082018.
View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Wang F and Yang Y: Suppression of the
xCT-CD44v antiporter system sensitizes triple-negative breast
cancer cells to doxorubicin. Breast Cancer Res Treat. 147:203–210.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Marin-Acevedo JA, Soyano AE, Dholaria B,
Knutson KL and Lou Y: Cancer immunotherapy beyond immune checkpoint
inhibitors. J Hematol Oncol. 11:82018. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Ruzzi F, Semprini MS, Scalambra L,
Palladini A, Angelicola S, Cappello C, Pittino OM, Nanni P and
Lollini PL: Virus-like particle (VLP) vaccines for cancer
immunotherapy. Int J Mol Sci. 24:129632023. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Rolih V, Caldeira J, Bolli E, Salameh A,
Conti L, Barutello G, Riccardo F, Magri J, Lamolinara A, Parra K,
et al: Development of a VLP-based vaccine displaying an xCT
extracellular domain for the treatment of metastatic breast cancer.
Cancers. 12:14922020. View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Bolli E, O'Rourke JP, Conti L, Lanzardo S,
Rolih V, Christen JM, Barutello G, Forni M, Pericle F and Cavallo
F: A Virus-Like-Particle immunotherapy targeting Epitope-specific
anti-xCT expressed on cancer stem cell inhibits the progression of
metastatic cancer in vivo. Oncoimmunology. 7:e14087462018.
View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Lopes A, Vandermeulen G and Preat V:
Cancer DNA vaccines: Current preclinical and clinical developments
and future perspectives. J Exp Clin Cancer Res. 38:1462019.
View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Kraft VAN, Bezjian CT, Pfeiffer S,
Ringelstetter L, Müller C, Zandkarimi F, Merl-Pham J, Bao X,
Anastasov N, Kössl J, et al: GTP cyclohydrolase
1/Tetrahydrobiopterin counteract ferroptosis through lipid
remodeling. ACS Cent Sci. 6:41–53. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Yang WS, SriRamaratnam R, Welsch ME,
Shimada K, Skouta R, Viswanathan VS, Cheah JH, Clemons PA, Shamji
AF, Clish CB, et al: Regulation of ferroptotic cancer cell death by
GPX4. Cell. 156:317–331. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Imai H, Matsuoka M, Kumagai T, Sakamoto T
and Koumura T: Lipid Peroxidation-dependent cell death regulated by
GPx4 and ferroptosis. Curr Top Microbiol Immunol. 403:143–170.
2017.PubMed/NCBI
|
|
101
|
Lv Y, Liang C, Sun Q, Zhu J, Xu H, Li X,
Li X, Li YY, Wang Q, Yuan H, et al: Structural insights into FSP1
catalysis and ferroptosis inhibition. Nat Commun. 14:59332023.
View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Bersuker K, Hendricks JM, Li Z, Magtanong
L, Ford B, Tang PH, Roberts MA, Tong B, Maimone TJ, Zoncu R, et al:
The CoQ oxidoreductase FSP1 acts parallel to GPX4 to inhibit
ferroptosis. Nature. 575:688–692. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Mao C, Liu X, Zhang Y, Lei G, Yan Y, Lee
H, Koppula P, Wu S, Zhuang L, Fang B, et al: DHODH-mediated
ferroptosis defence is a targetable vulnerability in cancer.
Nature. 593:586–590. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Harris IS, Endress JE, Coloff JL, Selfors
LM, McBrayer SK, Rosenbluth JM, Takahashi N, Dhakal S, Koduri V,
Oser MG, et al: Deubiquitinases maintain protein homeostasis and
survival of cancer cells upon glutathione depletion. Cell Metab.
29:1166–1181.e6. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
105
|
Shin D, Lee J, You JH, Kim D and Roh JL:
Dihydrolipoamide dehydrogenase regulates cystine
deprivation-induced ferroptosis in head and neck cancer. Redox
Biol. 30:1014182020. View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Ensink EJT, Medeiros HCD, Thurston G,
Pardal A, Yu L and Lunt SY: Pyruvate kinase activity regulates
cystine starvation induced ferroptosis through malic enzyme 1 in
pancreatic cancer cells. bioRxiv. 2023.doi:
10.1101/2023.09.15.557984. PubMed/NCBI
|
|
107
|
Singh S, Maurya P, Rani S, Mishra N, Nisha
R, Singh P and Saraf SA: Development of doxorubicin
hydrochloride-loaded whey protein nanoparticles and its surface
modification with N-acetyl cysteine for triple-negative breast
cancer. Drug Deliv Transl Res. 12:3047–3062. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
108
|
Hangauer MJ, Viswanathan VS, Ryan MJ, Bole
D, Eaton JK, Matov A, Galeas J, Dhruv HD, Berens ME, Schreiber SL,
et al: Drug-tolerant persister cancer cells are vulnerable to GPX4
inhibition. Nature. 551:247–250. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
109
|
Zhang Y, Swanda RV, Nie L, Liu X, Wang C,
Lee H, Lei G, Mao C, Koppula P, Cheng W, et al: mTORC1 couples
cyst(e)ine availability with GPX4 protein synthesis and ferroptosis
regulation. Nat Commun. 12:15892021. View Article : Google Scholar : PubMed/NCBI
|
|
110
|
Jiang L, Kon N, Li T, Wang SJ, Su T,
Hibshoosh H, Baer R and Gu W: Ferroptosis as a p53-mediated
activity during tumour suppression. Nature. 520:57–62. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
111
|
Dixon SJ, Patel DN, Welsch M, Skouta R,
Lee ED, Hayano M, Thomas AG, Gleason CE, Tatonetti NP, Slusher BS
and Stockwell BR: Pharmacological inhibition of cystine-glutamate
exchange induces endoplasmic reticulum stress and ferroptosis.
Elife. 3:e025232014. View Article : Google Scholar : PubMed/NCBI
|
|
112
|
Lu B, Chen XB, Ying MD, He QJ, Cao J and
Yang B: The role of ferroptosis in cancer development and treatment
response. Front Pharmacol. 8:9922018. View Article : Google Scholar : PubMed/NCBI
|
|
113
|
Lei M, Zhang YL, Huang FY, Chen HY, Chen
MH, Wu RH, Dai SZ, He GS, Tan GH and Zheng WP: Gankyrin inhibits
ferroptosis through the p53/SLC7A11/GPX4 axis in triple-negative
breast cancer cells. Sci Rep. 13:219162023. View Article : Google Scholar : PubMed/NCBI
|
|
114
|
Tajan M and Vousden KH: Dietary approaches
to cancer therapy. Cancer Cell. 37:767–785. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
115
|
Liu H, Zhang W, Wang K, Wang X, Yin F, Li
C, Wang C, Zhao B, Zhong C, Zhang J, et al: Methionine and cystine
double deprivation stress suppresses glioma proliferation via
inducing ROS/autophagy. Toxicol Lett. 232:349–355. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
116
|
Knott SRV, Wagenblast E, Khan S, Kim SY,
Soto M, Wagner M, Turgeon MO, Fish L, Erard N, Gable AL, et al:
Asparagine bioavailability governs metastasis in a model of breast
cancer. Nature. 554:378–381. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
117
|
Jimenez-Alonso JJ and Lopez-Lazaro M:
Dietary manipulation of amino acids for cancer therapy. Nutrients.
15:28792023. View Article : Google Scholar : PubMed/NCBI
|
|
118
|
Zhang T, Bauer C, Newman AC, Uribe AH,
Athineos D, Blyth K and Maddocks ODK: Polyamine pathway activity
promotes cysteine essentiality in cancer cells. Nat Metab.
2:1062–1076. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
119
|
Srivastava MK, Sinha P, Clements VK,
Rodriguez P and Ostrand-Rosenberg S: Myeloid-derived suppressor
cells inhibit T-cell activation by depleting cystine and cysteine.
Cancer Res. 70:68–77. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
120
|
Wang W, Green M, Choi JE, Gijón M, Kennedy
PD, Johnson JK, Liao P, Lang X, Kryczek I, Sell A, et al:
CD8+ T cells regulate tumour ferroptosis during cancer
immunotherapy. Nature. 569:270–274. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
121
|
Cramer SL, Saha A, Liu J, Tadi S, Tiziani
S, Yan W, Triplett K, Lamb C, Alters SE, Rowlinson S, et al:
Systemic depletion of L-cyst(e)ine with cyst(e)inase increases
reactive oxygen species and suppresses tumor growth. Nat Med.
23:120–127. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
122
|
Alothaim T, Charbonneau M and Tang X:
HDAC6 inhibitors sensitize non-mesenchymal triple-negative breast
cancer cells to cysteine deprivation. Sci Rep. 11:109562021.
View Article : Google Scholar : PubMed/NCBI
|
|
123
|
Espinoza IKC, Park CH, Vander Steen T,
Kleer CG, Wiley E, Rademaker A, Cuyàs E, Verdura S, Buxó M,
Reynolds C, et al: Depletion of CCN1/CYR61 reduces
triple-negative/basal-like breast cancer aggressiveness. Am J
Cancer Res. 12:839–851. 2022.PubMed/NCBI
|