Introduction
Endometriosis is a common gynecological
proliferative disease that occurs in 5–15% of reproductive women
globally (1). It refers to abnormal
growth of endometrial tissues (glands and stroma) outside the
uterus, mainly in the ovaries and extra-ovarian sites, including
the sigmoid colon, rectum, bladder and abdominal wall, which causes
a series of clinical symptoms, including pelvic pain,
dysmenorrhoea, non-menstrual pelvic pain and infertility (2).
Although endometriosis is not a premalignant
disease, it may have malignant potential and become a malignancy
(3). The incidence of malignant
transformation of endometriosis ranges between 0.7 and 1%, with
~75% of cases involving the ovary and the remaining 25% developing
from extra-ovarian endometriosis (4,5). The
pathological criteria for the diagnosis of malignant transformation
of endometriosis include benign endometrial tissues coexisting in
the tumor, the demonstration of the neoplasm arising from
endometriosis and not elsewhere, as well as the demonstration of
the histological transition between benign and malignant
endometriosis (6,7). However, with the overgrowth of cancer
in some cases, the benign endometriotic foci are obliterated by
malignant components (8). Thus, it
is challenging to diagnose endometriosis-associated cancers due to
a lack of histological evidence of endometriosis and malignant
transformation. A pooled analysis of case-control studies has
demonstrated that endometrioid carcinoma and clear cell carcinoma
are the most common pathological types of endometriosis-related
malignant tumors, which mostly develop from ovarian endometriosis
(4). The mechanism of
endometriosis-associated cancers is unclear, and studies of the
molecular mechanism have confirmed these are related to mutations
in the CTNNB1, PIK3CA, ERBB2, KRAS, ARID1A and PTEN
genes, and microsatellite instability (9,10).
Extra-ovarian endometriosis-associated cancer
involving the rectum is rare, making the diagnosis of the disease
challenging. The present report describes 2 female patients with
rectal lesions that were initially diagnosed as high-grade
intraepithelial neoplasia of the rectum by endoscopic biopsy.
Radical resection of the lesions with pathology and further
mutation analysis in case 1 confirmed the diagnosis of endometrioid
carcinoma of the rectum associated with endometriosis.
Case report
Case presentation
Case 1, a 49-year-old postmenopausal female patient,
was admitted to Hubei Cancer Hospital, Tongji Medical College,
Huazhong University of Science and Technology (Wuhan, China) in
September 2021 due to a pelvic mass on examination. Laboratory
examination showed that the serum cancer antigen 125 level was
elevated (247.300 U/ml; normal, 0–35 U/ml), while CA19-9 (0.64
U/ml; normal, <30 U/ml), CEA (1.895 ng/ml; normal, <5 ng/ml)
and AFP (3.99 ng/ml; normal, <7 ng/ml) levels were within the
normal range. A colonoscopy revealed an ulcerated fleshy neoplasm
12 cm from the anal margin, and it was possible to pass the
endoscope beyond the lesion. The surface of the lesion was
irregular and the surrounding mucosa was slightly rough. Pelvic MRI
revealed a 4.5×3.7-cm mass in the anterior rectal wall, which
severely adhered to the uterine wall (Fig. 1A). Since the endoscopic biopsy
specimens indicated high-grade intraepithelial neoplasia (highly
suspected adenocarcinoma), and the lesion was in the rectum and
adhered to the uterine wall, partial rectal resection with total
hysterectomy and bilateral salpingo-oophorectomy was performed.
There was no evidence of macroscopic tumor present after the
surgery.
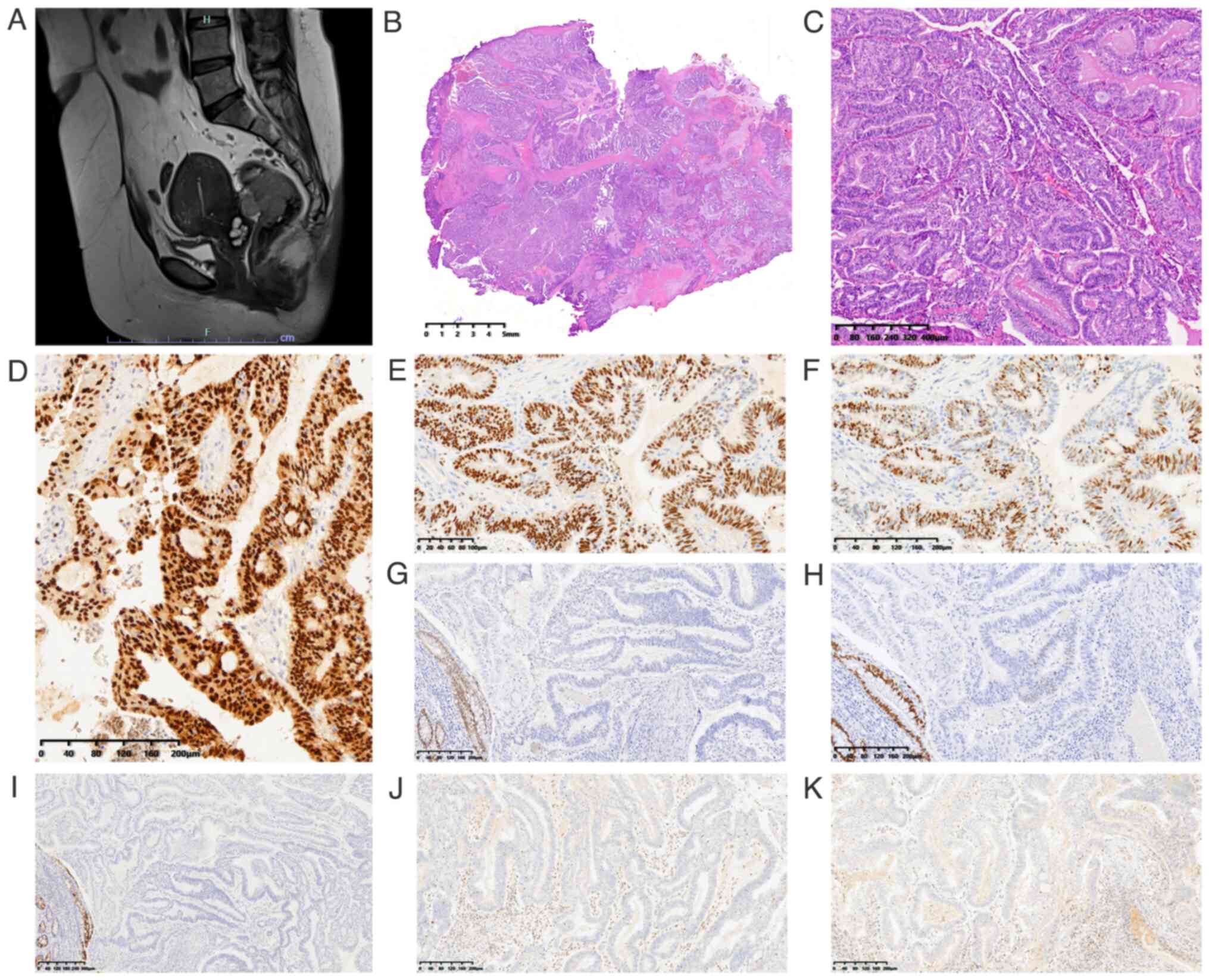 | Figure 1.MRI and pathological features of case
1. (A) Pelvic MRI revealed a mass in the rectum that severely
adhered to the uterine wall. Histopathologic features of the
adenocarcinoma infiltrating the rectum: (B) Moderately
differentiated glandular adenocarcinoma diffusely extending
throughout all layers of the intestinal wall (scale bar, 5 mm). (C)
Tumor cells were columnar with pseudostratified nuclei (scale bar,
400 µm). Immunohistochemical staining of the tumor revealed that
(D) paired box gene 8 staining was positive (scale bar, 200 µm),
(E) estrogen receptor staining was positive (scale bar, 100 µm),
(F) progesterone receptor staining was positive (scale bar, 200
µm), (G) caudal type homeobox 2 staining was negative (scale bar,
200 µm), (H) special AT-rich sequence-binding protein 2 staining
was negative (scale bar, 200 µm), (I) cytokeratin 20 was negative
(scale bar, 300 µm), and (J) mutL homolog 1 (scale bar, 200 µm) and
(K) PMS2 were lost (scale bar, 200 µm). |
Case 2, a 38-year-old reproductive female patient
admitted to Hubei Cancer Hospital, Tongji Medical College, Huazhong
University of Science and Technology (Wuhan, China) in November
2023, presented with hematochezia during the past 2 weeks. The
laboratory examination showed that the serum CA19-9 level was
elevated (49.33 U/ml; normal, <30 U/ml), while the CEA (1.520
ng/ml; normal, <5 ng/ml) level was within the normal range.
Pelvic MRI revealed eccentric rectal wall thickening with a rough
serosa (Fig. 2A). The uterus and
uterine endometrium were normal, and no abnormal signals were found
in the pelvic wall. The endoscopic biopsy specimens at Hubei
Provincial Hospital of Integrated Chinese and Western Medicine
(Wuhan, China) indicated high-grade intraepithelial neoplasia, and
a partial resection of the rectum was performed.
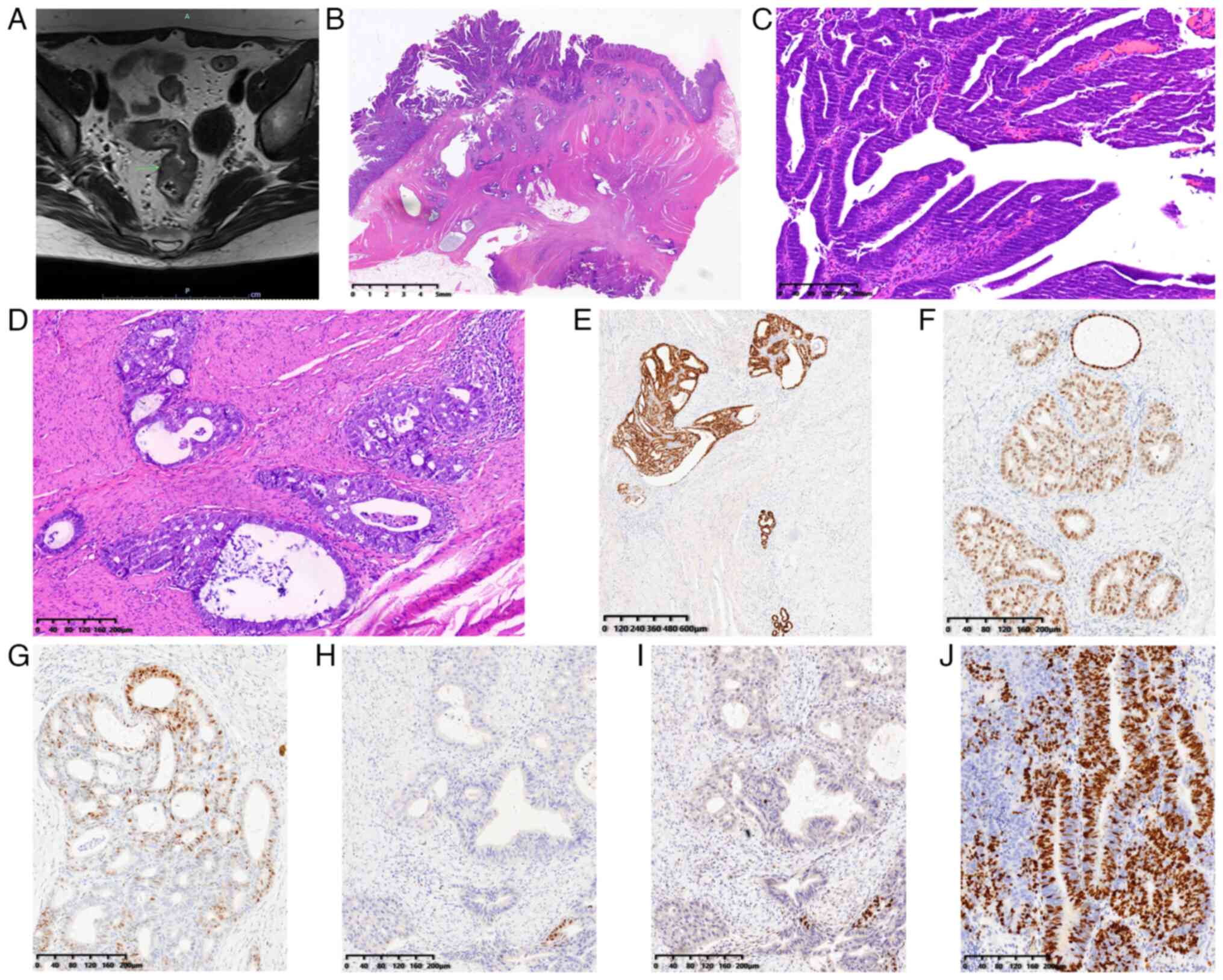 | Figure 2.MRI and pathological features of case
2. (A) Pelvic MRI revealed eccentric rectal wall thickening with a
rough serosa (green arrow, thickening rectal wall). (B)
Well-differentiated endometrioid carcinoma scatteredly infiltrated
all layers of the intestinal wall (scale bar, 5 mm). (C) Tumor
cells displayed villo-glandular architectures in the mucosa (scale
bar, 200 µm). (D) Tumor cells displayed cribriform patterns in the
muscularis propria to subserosa, and endometriosis foci as well as
atypical glands were seen adjacent to the neoplastic cells (scale
bar, 200 µm). Immunohistochemical staining showed that the tumor
cells were positive for (E) paired box gene 8 (scale bar, 600 µm)
and (F) estrogen receptor (scale bar, 200 µm), (G) variably
positive for progesterone receptor (scale bar, 200 µm), and focally
positive for (H) caudal type homeobox 2 (scale bar, 200 µm) and (I)
special AT-rich sequence-binding protein 2 (scale bar, 200 µm). (J)
The Ki67 index was 70% (scale bar, 200 µm). |
Pathology and mutation analysis
In case 1, a 4.5×3.7-cm hard mass involving the
rectum and adherent to the posterior myometrium of the uterus was
found. No other primary tumors were found in the endometrium,
cervix or bilateral adnexa. Examination of the histopathological
staining with hematoxylin and eosin according to a standard
protocol indicated that moderately differentiated glandular
adenocarcinoma diffusely extended throughout all layers of the
intestinal wall (Fig. 1B).
Intraluminal necrosis and segmental destruction of glands were not
observed. The tumor cells were columnar with pseudostratified
nuclei (Fig. 1C). Adenomyosis was
found in the uterine body adherent to the rectum. A total of 26
regional lymph nodes (LNs) were assessed and showed reactive
hyperplasia. Immunohistochemical (IHC) staining was performed as
described in our previous study (11). The results showed that the tumor
cells were positive for paired box gene 8 (PAX8; Fig. 1D), estrogen receptor (ER; Fig. 1E) and progesterone receptor (PR;
Fig. 1F), and negative for caudal
type homeobox 2 (CDX2; Fig. 1G),
special AT-rich sequence-binding protein 2 (SATB2; Fig. 1H) and cytokeratin 20 (Fig. 1I). The mismatch repair proteins were
detected by IHC staining, and mutL homolog 1 (Fig. 1J) and PMS2 (Fig. 1K) were lost. Next-generation
sequencing (NGS) was then performed as described previously
(12) using the formalin-fixed (4%
neutral formaldehyde solution; room temperature; 12–24 h) and
paraffin-embedded tumor tissue to elucidate mutation profiles for
41 genes in a panel that was designed to detect colorectal
cancer-associated genes, including BRCA1, BRCA2, ERBB2, BRAF,
KRAS, NRAS, POLE, TP53, PIK3CA, PTEN, MSH2, MSH6, MLH1 and
PMS2. The key mutations in the gene panel are listed in
Table SI. Mutations of the
BRCA1 (c.329dup), KRAS (c.35G>T), PIK3CA
(c.3140A>G) and PTEN (c.750_751del) genes were
identified, and microsatellite instability (MSI)-NGS was detected
to be high. The final diagnosis of rectal endometriosis-associated
endometroid carcinoma was made. The patient received adjuvant
chemotherapy with paclitaxel (175 mg/m2) followed by
AUC5 carboplatin on day 1 intravenously, every 21 days for 6
consecutive courses. The patient underwent tumor biomarker
detection nearly every 3 months (Table
SII), and the tumor biomarkers were within the normal range.
The patient also received MRI or CT scans nearly every year, and
there was no apparent recurrence or metastasis in December 2022
(Fig. S1), December 2023 (Fig. S2) and March 2024 (Fig. S3).
In case 2, a segment of the large bowel measuring 15
cm in length was examined, and a 1.7×1.5-cm ulcerated fleshy mass
with greyish, solid and friable cut surface could be seen.
Microscopically, well-differentiated endometrioid carcinoma was
seen scatteredly infiltrating all layers of the intestinal wall
(Fig. 2B), and benign endometriosis
foci contiguous with endometroid adenocarcinoma, and atypical
hyperplasia between the benign and malignant endometrial tissues
could be observed (Fig. 2C). The
tumor cells exhibited villo-glandular or confluent glandular
architectures with focal squamous differentiation in the mucosa
(Fig. 2D) and cribriform patterns
in the muscularis propria to subserosa. Dusty blue secretion was
observed in some benign endometrial glands. Tumor metastasis was
observed in 5 of the 22 local regional LNs. No other primary tumor
sites were found. IHC staining was performed in the same manner as
IHC staining for case 1, and the details for the antibodies are
listed in Table SIII. The results
showed that the tumor cells were positive for PAX8 (Fig. 2E), ER (Fig. 2F), and variably positive for PR
(Fig. 2G), whereas CDX2 (Fig. 2H) and SATB2 (Fig. 2I) were focally positive. The Ki67
index was 70% (Fig. 2J). The final
diagnosis was endometrioid carcinoma derived from rectal
endometriosis (5,6). The patient did not receive adjuvant
chemotherapy, and was followed-up by detecting tumor biomarkers
nearly every 3 months (Table SIV),
and the tumor biomarkers were within the normal range. The patient
also underwent CT examinations every half a year after resection,
and there was no apparent recurrence or metastasis in July 2024
(Fig. S4).
Discussion
Endometriosis is a common gynecological disease with
malignant potential and local aggressive biology (3). In 1925, Sampson (6) first reported the malignant
transformation of endometriosis. Subsequently, Scott (7) summarized and proposed four essential
criteria for the diagnosis of malignant transformation of
endometriosis: i) Both malignant and benign endometrial tissues
coexisted in the tumor; ii) histology of the neoplasm consistent
with an endometrial origin; iii) no other primary tumor sites can
be found; and iv) demonstration of the histological transition
between benign and malignant endometriosis. In the present case 2,
benign endometriosis foci contiguous with endometroid
adenocarcinoma invading the intestinal wall, and atypical
hyperplasia between the benign and malignant endometrial tissues
could be observed, and no other primary tumor sites were found,
which satisfied the criteria (7)
for the diagnosis of malignant transformation of the rectal
endometriosis.
However, not all cases meet the aforementioned four
criteria. In the present case 1, moderately differentiated
glandular adenocarcinoma diffusely extended throughout all layers
of the intestinal wall, while no evidence of endometriosis was
detected microscopically. We hypothesized that with the overgrowth
of cancer, the benign endometriotic foci are obliterated by
malignant components. Therefore, it is challenging to diagnose
endometriosis-associated cancers due to a lack of histological
evidence of endometriosis and malignant transformation (8).
Since the biological behavior, therapeutic
management and prognosis of endometriosis-associated rectal
adenocarcinoma are different from those of colorectal
adenocarcinoma (13,14), it is important to distinguish the
two. Colorectal adenocarcinoma usually originates from the mucosal
epithelium and gradually extends through the intestinal wall with
the growth of the tumor. Endometriosis-associated adenocarcinomas
invade the bowel wall from the outside, involving the serosa and
subserosa, and occasionally the muscular propria and mucosa
(13,14). Furthermore, cases of
endometriosis-associated adenocarcinomas involve the mucosa,
mimicking the intraepithelial neoplasia of the colorectal
carcinoma, as shown in the endoscopic biopsy specimens of the
present 2 patients. Therefore, it cannot be judged entirely from
the growth pattern whether or not it is a primary tumor when the
tumor extends through all layers of the intestinal wall. Secondly,
the histological features of the tumors are helpful for
differential diagnosis. Although both well-differentiated
colorectal adenocarcinoma and well-differentiated endometrioid
carcinoma could display glandular or cribriform growth patterns,
marked cytologic atypia and a high mitotic index are more often
observed in colorectal adenocarcinoma (13,14).
Abundant intraluminal ‘dirty’ necrosis and segmental destruction of
glands are characteristics of colorectal carcinoma, while squamous
differentiation is a characteristic feature of endometrioid
carcinoma (14). IHC staining may
be essential to confirm the diagnosis of colorectal endometrioid
carcinoma. PAX8 is a highly sensitive and specific marker of
Müllerian epithelial tumors, and a highly sensitive epithelial
marker for extragenital endometriosis (15). Furthermore, ER and PR are expressed
in most uterine endometrial carcinomas (16). CDX2 and SATB2 are sensitive and
specific markers of colorectal carcinoma (17,18).
Therefore, PAX8, ER, PR, CDX2 and SATB2 may be useful in
distinguishing colorectal adenocarcinoma from endometrioid
carcinoma. In the present report, both patients exhibited positive
IHC staining for PAX8, ER and PR, and negative or focal staining
for CDX2 and SATB2, compatible with a diagnosis of endometroid
carcinoma.
Studies have demonstrated that gene mutations, such
as CTNNB1, PIK3CA, ERBB2, KRAS, ARID1A and PTEN
mutations, contribute to the malignant transformation of
endometriosis (7,8). In case 1, diffuse endometrioid-like
adenocarcinoma was observed throughout the intestinal wall. Since
no other primary tumors were found in the endometrium, cervix or
bilateral adnexa, and adenomyosis was found in the uterine body
adherent to the rectum, we hypothesized that this case was caused
by endometriosis of the rectum. Further NGS analysis detected
BRCA1, KRAS, PIK3CA and PTEN gene mutations, and
MSI-high in the formalin-fixed paraffin-embedded tumor tissue,
which further confirmed the diagnosis of rectal
endometriosis-associated endometroid carcinoma.
Since rectal endometriosis-associated carcinoma is
rare, the clinicopathological features and treatments remain to be
elucidated. A total of 10 full-text articles published in English
in PubMed (https://pubmed.ncbi.nlm.nih.gov/) between 1978 and
2022 that described 11 patients with endometriosis-associated
rectal malignancies were identified (19–28),
and the characteristics of the 11 patients reported in the
literature as well as the 2 patients in the present report are
listed in Table I. The median age
of the patients age was 52 years (range, 38–75 years), and the main
symptoms were abdominal pain and rectal or vaginal bleeding. To the
best of our knowledge, the etiology of endometriosis-associated
rectal malignancies remains uncertain. Among the 13 cases, 8
(61.5%) patients had undergone previous pelvic surgery, including a
hysterectomy for endometriosis (19,20,22,23,25–27),
myomatosis (23) or myomectomy
(26,28) years ago. Therefore, pelvic surgery
could increase the risk of dissemination of endometriotic tissues
and malignant transformation of endometriosis in the rectum. The
other 5 patients had no medical history, including 1 patient
(28) with endometriosis foci
deposited in the rectovaginal septum and 1 patient (case 1) with
adenomyosis in the surgical specimen. We hypothesized that the
possible mechanism is the deposition and growth of endometrial
tissues in the peritoneal cavity via retrograde menstruation.
 | Table I.Characteristics of patients with
endometriosis-associated recto-sigmoid adenocarcinoma. |
Table I.
Characteristics of patients with
endometriosis-associated recto-sigmoid adenocarcinoma.
| First author/s,
year | Age, years | Signs/symptoms | Medical history | Histology | Treatment | LN metastasis | Follow-up | (Refs.) |
|---|
| Present case 1 | 49 | Pelvic mass on | None | Endometrioid | TH + BSO + RR +
CT | None | NR after | - |
|
|
| examination |
| carcinoma |
|
| 27 months |
|
| Present case 2 | 38 | Hematochezia | None | Endometrioid | RR | Metastasis of | NR after | - |
|
|
|
|
| carcinoma |
| 5 out of 22 | 3 months |
|
|
|
|
|
|
|
| LNs |
|
|
| Lott et al,
1978 | 53 | Rectal
bleeding | TH + BSO for
PE | Endometrioid | RRS | None | NR after | (19) |
|
|
|
| 15 years ago | carcinoma |
|
| 48 months |
|
| Jones et al,
2002 | 52 | Rectal
bleeding | TH + BSO for | Endometrioid | RRS | None | NR after | (20) |
|
|
|
| rectovaginal
DIE | carcinoma |
|
| 9 months |
|
|
|
|
| 12 years ago |
|
|
|
|
|
| Takeuchi et
al, | 67 | Backache and | None | Endometrioid | RH + pelvic and
para-aortic | Para-aortic | ND | (21) |
| 2004 |
| abdominal pain |
| carcinoma | LND + RRS + CT | LN metastasis |
|
|
| Kobayashi et
al, | 45 | Hematochezia
with | Estrogen therapy
+ | Endometrioid | Resection of
bilateral ovarian | Metastasis of | ND | (22) |
| 2010 |
| abdominal pain | RO 17 years
ago | carcinoma | tumors + RRS +
appendix + CT | 6 out of 8 LNs |
|
|
| García-Marín et
al, | 57 | Rectorrhagia | TH + BSO for
PE | Poorly | Neoadjuvant CT/RT
+ | ND | NR after | (23) |
| 2015 |
|
| and myomatosis | differentiated | RR+ CT |
| 84 months |
|
|
|
|
| 15 years ago | adenocarcinoma |
|
|
|
|
| Palla et al,
2017 | 75 | Abdominal pain | None | Endometrioid | RS | ND | NR after | (24) |
|
|
| and
enterorrhagia |
| carcinoma |
|
| 6 months |
|
| Li et al,
2018 | 55 | Rectorrhagia
and | Excision of | High-grade | TH + BSO + RR +
CT | Metastasis of | RE 22 months | (25) |
|
|
|
|
| serous |
|
| later |
|
|
|
| abdominal pain | bilateral
ovarian | carcinoma |
| 8 out of | 30 LNs |
|
|
|
|
| chocolate
cysts |
|
|
|
|
|
|
|
|
| 25 years ago |
|
|
|
|
|
| Yang et al,
2019 | 57 | Vaginal
bleeding | Caesarean
section | Endometrioid | RH + BSO + pelvic
LND + RR + | None | NR after | (26) |
|
|
| and abdominal | and myomectomy | carcinoma | pelvic
peritonectomy + |
| 12 months |
|
|
|
| pain | >20 years
ago |
| omentectomy +
appendectomy + CT |
|
|
|
| Li et al,
2022 | 49 | Hypogastralgia | RSO +
myomectomy | Endometrioid | RH + LSO + pelvic
and para- | None | NR after | (27) |
|
|
| and diarrhea | for right
ovarian | carcinoma | aortic LND + RR +
omentectomy + |
| 10 months |
|
|
|
|
| endometriosis
cyst |
| appendectomy +
CT |
|
|
|
|
|
|
| and uterine
fibroid |
|
|
|
|
|
| Ulrich et
al, | 41 | Abdominal | None | Endometrioid | RH + BSO + pelvic
LND + | None | NR after 24 | (28) |
| 2005 (case 1) |
| discomfort |
| carcinoma | RR + RT |
| months |
|
| Ulrich et
al, | 51 | Vaginal
bleeding | TH for myomas | Endometrioid | Resection of
proximal vagina + | None | RE 24 months | (28) |
| 2005 (case 2) |
|
| 6 years | carcinoma | RR + BSO + pelvic
LND + RT |
| later |
|
There is no consensus on the therapeutic approach
for endometriosis-associated colorectal malignancies, since most of
the literature reported are case reports (19–28),
and the main treatment is radical resection and primary
cytoreductive surgery of all macroscopic detectable lesions, with
adjuvant chemotherapy and radiotherapy. However, the
chemotherapeutic regimens have been individualized, and the overall
effectiveness of chemotherapy or radiotherapy is unknown. Among the
13 cases, all patients underwent rectal or rectosigmoid colon
resection, 8 underwent total hysterectomy and salpingo-oophorectomy
(19,20,22,23,25–28),
and 5 underwent para-aortic and pelvic LN dissections. A total of 4
patients exhibited LN metastasis, including 1 patient with
para-aortic LN metastasis and 3 patients with local LN metastasis.
Furthermore, 1 patient was offered neoadjuvant chemotherapy and
radiotherapy before surgery. A total of 7 patients received
chemotherapy as adjuvant therapy, and 2 patients received
radiotherapy as adjuvant therapy. Therefore, chemotherapy may be
the first-line adjuvant treatment and, similar to treatments for
endometrial cancer, the chemotherapeutic regimens typically consist
of platinum and taxane (29).
Furthermore, 1 of the 2 patients who received adjuvant radiotherapy
(28) suffered local pelvic
recurrence 24 months later, and the patient could only receive
chemotherapy for recurrence. Thus, radiation therapy may be
performed after surgery or for treatment of local recurrence after
the primary surgery and chemotherapy. Among 9 patients reported in
the literature and the 2 patients in the present report, the median
follow-up duration of rectal endometriosis-associated carcinoma was
22 months (range, 3–84 months), and 2 patients exhibited recurrence
after 22 and 24 months, respectively.
In conclusion, endometriosis-associated carcinoma of
the rectum is a rare malignant tumor that should be distinguished
from colorectal cancer so that the optimal treatment is
implemented. For patients who have previously received surgery for
endometriosis who present with abdominal pain and rectal bleeding,
the clinical suspicion of endometriosis-associated cancer of the
rectum is suggested. Surgery and pathologic examination with IHC
staining, even with molecular analysis, are essential for the final
diagnosis. Since there is currently no consensus on the standard
therapeutic approach for rectal endometriosis-associated
malignancies, primary cytoreductive surgery with resection of all
macroscopic detectable lesions should be performed whenever
possible. Although surgery combined with chemotherapy was performed
in most patients reported in the literature, the chemotherapeutic
regimens were individualized, and the exact therapeutic value of
chemotherapy is unclear. Therefore, more prospective, multicenter,
large-scale trials are required for the treatment of rectal
endometriosis-associated cancer.
Supplementary Material
Supporting Data
Supporting Data
Acknowledgements
Not applicable.
Funding
The study was supported by the Gynecological Cancer Research
Fund, Beijing Kanghua Foundation for Development of Traditional
Chinese and Western Medicine (grant no. KH-2021-LLZX-070).
Availability of data and materials
The data generated in the present study are not
publicly available due to protection of patient privacy but may be
requested from the corresponding author.
Authors' contributions
KZ and YH were involved in the conception and design
of the study. MH acquired and analyzed the data. XL and RY
performed the research and assessed the results. KZ drafted the
manuscript and MH revised the manuscript. KZ and YH confirm the
authenticity of all the raw data. All authors read and approved the
final version of the manuscript.
Ethics approval and consent to
participate
The study was approved by the Medical Ethics
Committee of Hubei Cancer Hospital, Tongji Medical College of
Huazhong University of Science and Technology (approval no.
LLHBCH2024YN-016; Wuhan, China), and written informed consent to
participate in the study was obtained from each patient.
Patient consent for publication
Written informed consent was obtained from each
patient to publish their details and images.
Competing interests
The authors declare that they have no competing
interests.
Use of artificial intelligence tools
During the preparation of this work, artificial
intelligence tools were used to improve the readability and
language of the manuscript, and subsequently, the authors revised
and edited the content produced by the artificial intelligence
tools as necessary, taking full responsibility for the ultimate
content of the present manuscript.
References
|
1
|
Simoens S, Dunselman G, Dirksen C,
Hummelshoj L, Bokor A, Brandes I, Brodszky V, Canis M, Colombo GL,
DeLeire T, et al: The burden of endometriosis: Costs and quality of
life of women with endometriosis and treated in referral centres.
Hum Reprod. 27:1292–1299. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Taylor HS, Kotlyar AM and Flores VA:
Endometriosis is a chronic systemic disease: Clinical challenges
and novel innovations. Lancet. 397:839–852. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Stern RC, Dash R, Bentley RC, Snyder MJ,
Haney AF and Robboy SJ: Malignancy in endometriosis: Frequency and
comparison of ovarian and extraovarian types. Int J Gynecol Pathol.
20:133–139. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Pearce CL, Templeman C, Rossing MA, Lee A,
Near AM, Webb PM, Nagle CM, Doherty JA, Cushing-Haugen KL, Wicklund
KG, et al: Association between endometriosis and risk of
histological subtypes of ovarian cancer: A pooled analysis of
case-control studies. Lancet Oncol. 13:385–394. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Benoit L, Arnould L, Cheynel N, Diane B,
Causeret S, Machado A, Collin F, Fraisse J and Cuisenier J:
Malignant extraovarian endometriosis: A review. Eur J Surg Oncol.
32:6–11. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Sampson JA: Endometrial carcinoma of the
ovary, arising in endometrial tissue of that organ. Arch Surg.
10:1–72. 1925. View Article : Google Scholar
|
|
7
|
Scott RB: Malignant changes in
endometriosis. Obstet Gynecol. 2:283–289. 1953.PubMed/NCBI
|
|
8
|
Gadducci A and Zannoni GF:
Endometriosis-associated extraovarian malignancies: A challenging
question for the clinician and the pathologist. Anticancer Res.
40:2429–2438. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Thomas EJ and Campbell IG: Molecular
genetic defects in endometriosis. Gynecol Obstet Invest. 50 (Suppl
1):S44–S50. 2000. View Article : Google Scholar
|
|
10
|
Sapalidis K, Machairiotis N, Zarogoulidis
P, Vasilakaki S, Sardeli C, Koimtzis G, Pavlidis E, Katsaounis A,
Giannakidis D, Michalopoulos N, et al: Genes' interactions: A major
contributor to the malignant transformation of endometriosis. Int J
Mol Sci. 20:18422019. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhao K, Zhao T, Yang R, Liu J and Hu M:
Peroxiredoxin 2 as a potential prognostic biomarker associated with
angiogenesis in cervical squamous cell cancer. Oncol Lett.
28:3282024. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Del Vecchio F, Mastroiaco V, Di Marco A,
Compagnoni C, Capece D, Zazzeroni F, Capalbo C, Alesse E and
Tessitore A: Next-generation sequencing: Recent applications to the
analysis of colorectal cancer. J Transl Med. 15:2462017. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ponz de Leon M and Di Gregorio C:
Pathology of colorectal cancer. Dig Liver Dis. 33:372–388. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kir G, Gurbuz A, Karateke A and Kir M:
Clinicopathologic and immunohistochemical profile of ovarian
metastases from colorectal carcinoma. World J Gastrointest Surg.
2:109–116. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Arakawa T, Fukuda S, Hirata T, Neriishi K,
Wang Y, Takeuchi A, Saeki A, Harada M, Hirota Y, Matsumoto T, et
al: PAX8: A highly sensitive marker for the glands in extragenital
endometriosis. Reprod Sci. 27:1580–1586. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Smith D, Stewart CJR, Clarke EM, Lose F,
Davies C, Armes J, Obermair A, Brennan D, Webb PM, Nagle CM and
Spurdle AB: ER and PR expression and survival after endometrial
cancer. Gynecol Oncol. 148:258–266. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Werling RW, Yaziji H, Bacchi CE and Gown
AM: CDX2, a highly sensitive and specific marker of adenocarcinomas
of intestinal origin: An immunohistochemical survey of 476 primary
and metastatic carcinomas. Am J Surg Pathol. 27:303–310. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Magnusson K, de Wit M, Brennan DJ, Johnson
LB, McGee SF, Lundberg E, Naicker K, Klinger R, Kampf C, Asplund A,
et al: SATB2 in combination with cytokeratin 20 identifies over 95%
of all colorectal carcinomas. Am J Surg Pathol. 35:937–948. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Lott JV, Rubin RJ, Salvati EP and Salazar
GH: Endometrioid carcinoma of the rectum arising in endometriosis:
Report of a case. Dis Colon Rectum. 21:56–60. 1978. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Jones KD, Owen E, Berresford A and Sutton
C: Endometrial adenocarcinoma arising from endometriosis of the
rectosigmoid colon. Gynecol Oncol. 86:220–222. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Takeuchi K, Yamanaka Y, Hamana S, Ohara N
and Maruo T: Invasive adenocarcinoma arising from uterine
adenomyosis involving the rectosigmoid colon. Int J Gynecol Cancer.
14:1004–1006. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kobayashi S, Sasaki M, Goto T, Asakage N,
Sekine M, Suzuki T, Tsukada K, Yamasaki S and Ukawa S: Endometrioid
adenocarcinoma arising from endometriosis of the rectosigmoid. Dig
Endos. 22:59–63. 2010. View Article : Google Scholar
|
|
23
|
García-Marín JA, Pellicer-Franco EM,
Soria-Aledo V, Mengual-Ballester M, Valero-Navarro G and
Aguayo-Albasini JL: Malignant degeneration of rectal endometriosis.
Rev Esp Enferm Dig. 107:761–763. 2015.PubMed/NCBI
|
|
24
|
Palla VV, Karaolanis G, Bliona T,
Katafigiotis I, Anastasiou I and Hassiakos D: Endometrioid
adenocarcinoma arising from colon endometriosis. SAGE Open Med Case
Rep. 5:2050313X177452042017.PubMed/NCBI
|
|
25
|
Li N, Zhou W, Zhao L and Zhou J:
Endometriosis-associated recto-sigmoid cancer: A case report. BMC
Cancer. 18:9052018. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Yang H, Gu JJ, Qi Y, Zhao W and Wang XL:
Endometrioid adenocarcinoma of the rectovaginal septum with
invasion of the rectum: A case report and review of literature.
World J Surg Oncol. 17:2062019. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Li B, Wang Y, Wang Y, Li S and Liu K: Deep
infiltrating endometriosis malignant invasion of cervical wall and
rectal wall with lynch syndrome: A rare case report and review of
literature. Front Oncol. 12:8322282022. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Ulrich U, Rhiem K, Kaminski M, Wardelmann
E, Trog D, Valter M and Richter ON: Parametrial and rectovaginal
adenocarcinoma arising from endometriosis. Int J Gynecol Cancer.
15:1206–1209. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Knisely A, Huang Y, Li Y, Prabhu VS and
Wright JD: Adjuvant and first line chemotherapy use for endometrial
cancer. Gynecol Oncol Rep. 41:1010022022. View Article : Google Scholar : PubMed/NCBI
|
















