Introduction
Ovarian serous carcinoma is a significant cause of
cancer-related deaths among females worldwide, primarily due to
late-stage diagnosis and high mortality rates (1). It is the most lethal gynecological
malignancy with the poorest prognosis compared to other
gynecological cancers (2). Most
patients with ovarian serous carcinoma are diagnosed when the
cancer is in an advanced stage (III or IV) due to the absence of
specific symptoms and reliable early-stage biomarkers (3). The typical treatment protocol includes
maximal debulking surgery followed by chemotherapy with a
combination of platinum and taxane agents (3). The National Comprehensive Cancer
Network Guidelines recommend the use of paclitaxel or carboplatin
as the first-line treatment for epithelial ovarian carcinoma
(4). Even though there is a strong
initial effectiveness of platinum-based chemotherapy, many patients
eventually develop resistance to these first-line treatments
(1,3,5). The
effectiveness of resuming platinum-based chemotherapy upon
recurrence is determined by the interval without platinum, which is
the duration between the last dose of platinum-based chemotherapy
and the recurrence of cancer. A recurrence within 6 months
classifies the cancer as ‘platinum-resistant’, whereas a recurrence
after 6 months classifies it as ‘platinum-sensitive’. (1,5–7). This
classification is a critical prognostic factor for overall and
progression-free survival (8,9).
Patients who develop platinum resistance have a significantly
poorer prognosis, with a median survival of <16 months (10). While germline BRCA1/2 mutations and
homologous recombination mutations are predictive of better overall
survival and platinum sensitivity (11–13),
these biomarkers have not yet provided definitive guidance for
treatment. There is an urgent need for noninvasive pretreatment
methods to identify patients unlikely to benefit from
platinum-based therapies, enabling the selection of alternative
treatments.
The Src family of kinases (SFKs) play a vital role
in controlling numerous cellular functions, such as migration,
proliferation, invasion, survival, angiogenesis, differentiation,
and motility across various cancer types. This is achieved via
phosphorylating tyrosine residues on target proteins that are part
of multiple signaling pathways (14,15).
One member of the SFK family of proteins, Fyn, is a non-receptor
tyrosine kinase with a molecular weight of 59 kDa and is encoded by
a gene on chromosome 6q21 (14,15).
In cancer, Fyn contributes to cancer development and progression by
inhibiting apoptosis and promoting proliferation, invasion,
migration, and metastasis. Overexpression of Fyn enhances the
anti-apoptotic activity of Akt through phosphorylation of focal
adhesion kinase (FAK) and activation of the PI3K/AKT pathway
(14–16). Thus, Fyn is considered a significant
molecule in conferring resistance to anti-cancer agents that induce
apoptosis, which can be relevant in platinum-based chemotherapy
drugs for ovarian carcinoma.
In the present study, the relationship between Fyn
expression and platinum sensitivity in patients with advanced-stage
high-grade serous carcinoma was assessed. A novel biomarker that
could be used to predict platinum sensitivity was identified, and
may have potential for improving the prognosis of patients with
ovarian serous carcinoma.
Materials and methods
Patients
This retrospective analysis encompassed 64 cases of
ovarian high-grade serous carcinoma at FIGO stages III or IV, all
of which were histologically confirmed as high-grade serous
carcinoma. The patients underwent primary debulking surgery
followed by chemotherapy (175 mg/m2 of paclitaxel
combined with AUC6 of carboplatin administered every 3 weeks)
between January 1, 2005, and December 31, 2014, at Osaka City
University Hospital. The patients were divided into two groups:
Platinum-sensitive group, did not relapse within 6 months after the
last platinum administration, and platinum-resistant group,
relapsed within 6 months. The characteristics of the patients,
including age, serum CA125 tumor marker levels, FIGO stage, and the
size of the residual tumor post-surgery were compared between the
two groups. This study received approval from the Institutional
Review Board of Osaka Metropolitan University Hospital (approval
no. 2022-108; Osaka, Japan). All patients provided written informed
consent for participation.
Immunohistochemistry
Fyn expression was evaluated using
immunohistochemical analysis on paraffin-embedded tissue sections
containing ovarian cancer tissues obtained via surgery.
Four-micrometer-thick sections were first deparaffinized and
rehydrated, followed by immersion for 10 min in 3% hydrogen
peroxide at room temperature to inhibit the activity of endogenous
peroxidase. Antigen retrieval was conducted by placing the sections
in 10 mM citrate buffer (pH 9.0; cat. no. S2367; Agilent
Technologies, Inc.) and heating them in an autoclave at 121°C for
20 min. The sections were incubated overnight at 4°C with a rabbit
polyclonal anti-Fyn antibody (cat. no. ab184276; Abcam; 1:250). To
visualize antibody binding, sections were treated with Dako REAL
EnVision Detection System Peroxidase/DAB+, Rabbit/Mouse (cat. no.
K5007; Agilent Technologies, Inc.) for 3 min at room temperature.
Then, we incubated them with a streptavidin-peroxidase complex,
using 3,3′-diaminobenzidine as the chromogen.
As the final step, the sections were counterstained
at room temperature with hematoxylin for 1 min. The scoring of Fyn
expression was determined by the weighted score method described by
Sinicrope et al (17).
Briefly, the proportion of positive cells was categorized as: 0,
<5%; 1, 5–25%; 2, 5–50%; 3, 50–75%; and 4, >75%. The staining
intensity was evaluated as follows: 0, no staining; 1, weak
staining; 2, moderate staining; or 3, strong staining. The final
Fyn expression score, ranging from 0–12, was obtained by
multiplying the score for percentage positivity by the score for
staining intensity.
Cell culture
The OVSAHO human ovarian serous carcinoma cell line
(cat. no. JCRB1046; National Institutes of Biomedical Innovation,
Health and Nutrition, Osaka, Japan) was grown in RPMI-1640 medium
(Gibco; Thermo Fisher Scientific, Inc.) supplemented with 10% FBS
(Gibco; Thermo Fisher Scientific, Inc.), 100 units/ml penicillin,
and 100 units/ml streptomycin. The cells were maintained at 37°C in
a humidified incubator supplied with 5% CO2. The culture
medium was replaced every two days to maintain optimal cell growth
and viability.
Cell viability assay and siRNA
transfection
OVSAHO cells were plated into 96-well plates at a
concentration of 1×104 cells/well. The cells were
allocated into two groups, a control group transfected with control
siRNA (cat. no. SIC001_10NMOL; MilliporeSigma) and a siFyn group
transfected with Fyn-specific siRNA (cat. no. sc-29321; Santa Cruz
Biotechnology, Inc.) The sequence of the siFyn construct was:
Sense, 5′-CAUCGAGCGCAUGAAUUAU-3′ and antisense
5′-AUAAUUCAUGCGCUCGAUG-3′. The manufacturer did not disclose the
sequence of the control siRNA. After the cells had adhered, the
cells in the siFyn group were cultured in fresh medium with Fyn
siRNA transfection complexes, while the control group was cultured
in fresh medium with control siRNA. Both groups were incubated at
37°C for 24 h. Following transfection, the cells were cultured in
medium containing varying concentrations of carboplatin (25, 50,
100, or 200 µM) and incubated for an additional 24 h at 37°C. Cell
viability was assessed using a Cell Counting Kit-8 assay (CCK-8;
Dojindo Molecular Technologies, Inc.). A 10 µl aliquot of the CCK-8
solution was added to each well, and the cells were incubated at
37°C for 1 h. Absorbance was measured at 450 nm using a microplate
reader (Corona Electric Co., Ltd.).
Reverse transcription-quantitative PCR
(RT-qPCR)
RT-qPCR was conducted to verify the knockdown of Fyn
at the mRNA level. Total RNA was extracted from the cells using the
RNeasy Mini kit (Qiagen GmbH). Subsequently, cDNA was synthesized
from the extracted RNA using the High-Capacity cDNA Reverse
Transcription kit (Thermo Fisher Scientific, Inc.). TaqMan
chemistry was employed in accordance with the manufacturer's
instructions, utilizing TaqMan primers and probes for Fyn (cat. no.
Hs00941613_m1) and hypoxanthine phosphoribosyl transferase 1
(HPRT1; cat. no. Hs02800695_m1; Thermo Fisher Scientific, Inc.) as
the internal control. RT-qPCR was performed with TaqMan Fast
Universal PCR Master Mix (Thermo Fisher Scientific, Inc.). The
RT-qPCR was performed using the following thermocycling cycling
conditions: Initial denaturation, 95°C for 20 sec; followed by 40
cycles of 95°C for 3 sec and 60°C for 30 sec. All steps were
performed following the manufacturer's protocols. Gene expression
changes were quantified relative to the control by employing the
2−ΔΔCq method (18).
Statistical analysis
Data are presented as the median (range).
Associations between categorical variables in the two groups were
assessed using a Fisher's exact test, while comparisons of median
values and mean values between groups were performed using a
Mann-Whitney U test or an unpaired Student's t-test, respectively.
A Receiver Operating Characteristic (ROC) curve was plotted to
determine the optimal Fyn score cutoff for predicting platinum
sensitivity. Survival analysis between the groups was performed
using the Kaplan-Meier method with log-rank tests. To identify
independent factors for platinum sensitivity, a multivariate
logistic regression analysis was used. RT-qPCR experiments were
replicated five times, and cell viability assays were performed
with 10 replicates. P<0.05 was considered to indicate a
statistically significant differences. All statistical analyses
were performed using GraphPad Prism Version 9 (GraphPad Software,
Inc.).
Results
Patient characteristics
Table I presents the
patients' characteristics. The platinum-sensitive group consisted
of 35 patients, while the platinum-resistant group included 29
patients. The median age for the two groups was 63 and 62 years,
respectively, with no significant difference between them
(P=0.656). Similarly, the distribution of the FIGO stage showed no
significant statistical difference between the groups (P=0.368).
The median CA125 value was 929 U/ml for the platinum-sensitive
group and 1,422 U/ml for the platinum-resistant group, with no
significant difference (P=0.246). Regarding the size of
postoperative residual tumors, the distributions were as follows:
No residual tumor in 9 vs. 3 cases, residual tumor ≤1 cm in 13 vs.
3 cases, and residual tumor >1 cm in 13 vs. 23 cases between the
platinum-sensitive group and the platinum-resistant group,
respectively. This distribution showed a significant statistical
difference (P<0.01).
 | Table I.Patient characteristics. |
Table I.
Patient characteristics.
| Characteristic | Platinum-sensitive
group | Platinum-resistant
group | P-value |
|---|
| No. of
patients | 35 | 29 |
|
| Median age, years
(range) | 63 (36–81) | 62 (39–76) | 0.656a |
| FIGO stage, n |
|
| 0.368b |
|
IIIA | 2 | 0 |
|
|
IIIB | 3 | 1 |
|
|
IIIC | 27 | 21 |
|
|
IVA | 2 | 4 |
|
|
IVB | 1 | 3 |
|
| Median CA125, U/ml
(range) | 929
(25–30,075) | 1,422
(209–9,478) | 0.246a |
| Postoperative
residual tumor, n |
|
|
<0.01b |
|
None | 9 | 3 |
|
| ≤1
cm | 13 | 3 |
|
| >1
cm | 13 | 23 |
|
Weighted score of Fyn expression and
cutoff value to predict platinum sensitivity
The weighted score of Fyn expression and its cutoff
value to predict platinum sensitivity were next evaluated. Fyn
expression was primarily observed in the cytoplasm and at the cell
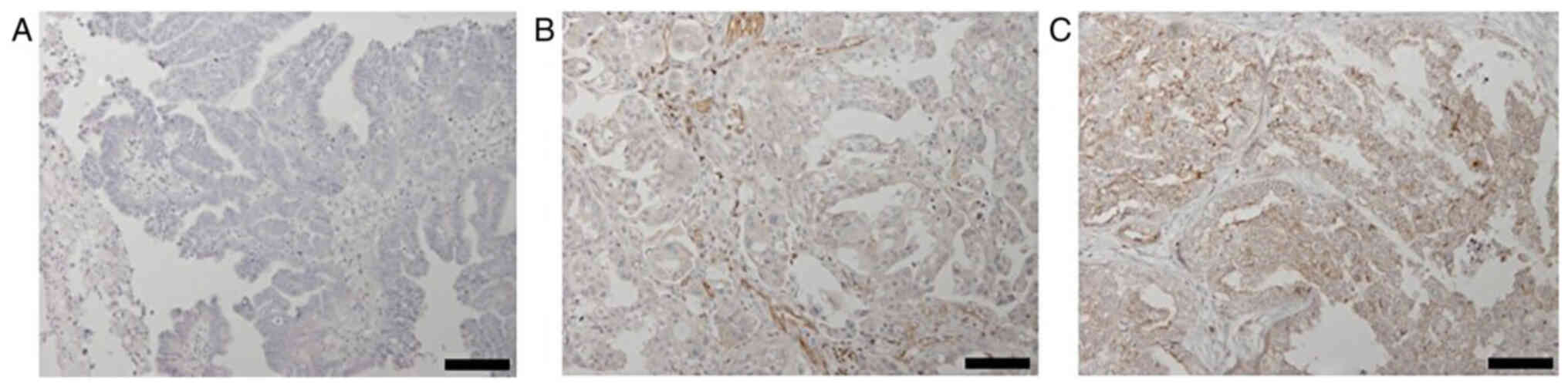
membrane (Fig. 1). The median
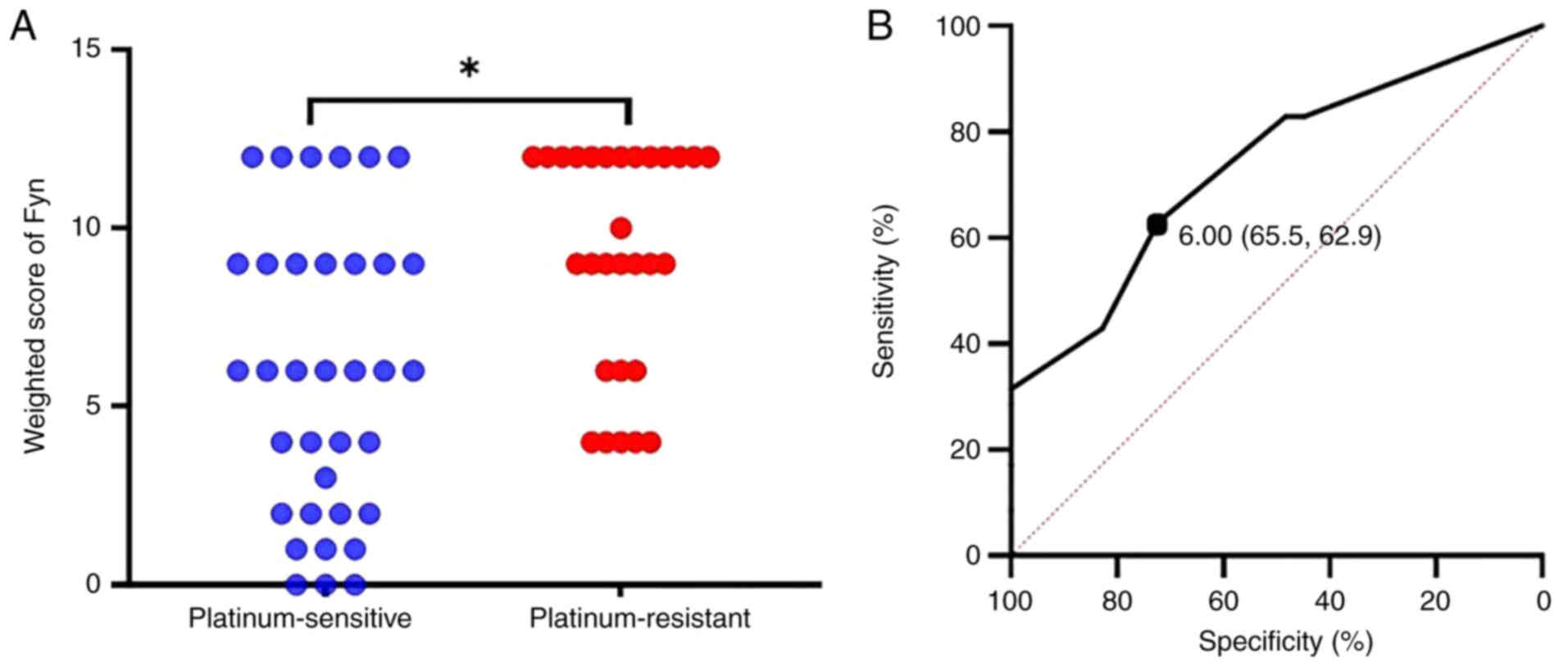
weighted score was 6 for the platinum sensitive group and 9 for the
platinum resistant group, with the latter being significantly
higher (P<0.01, Fig. 2A). To
determine the cutoff value of the Fyn weighted score for predicting
platinum sensitivity, a ROC curve was constructed. The analysis
revealed a cutoff value of 6, which predicted platinum sensitivity
with a specificity of 65.5%, a sensitivity of 62.9%, an AUC of
0.733, and a 95% confidence interval of 0.614-0.852 (Fig. 2B).
Association between platinum
sensitivity and overall survival based on Fyn expression
Using a cutoff value of 6 for the weighted score of
Fyn expression, the patients were divided into two groups, a low
expression group with a weighted score of Fyn ≤6 and a
high-expression group with a weighted score of Fyn ≥8. The
characteristics of the patients in both groups are described in
Table II. The low-expression group
consisted of 30 patients, while the high-expression group consisted
of 34 patients. The median ages of the patients were 60.5 and 63.0
years, respectively, with no significant difference (P=0.228).
Similarly, there was no significant difference in the distribution
of FIGO stages between the groups (P=0.142). The median CA125
values were 1,227.5 U/ml for the low expression group and 1,415.5
U/ml for the high-expression group, with no statistically
significant difference (P=0.360). Additionally, the distribution of
size of postoperative residual tumors showed no significant
difference between the groups (P=0.052). Platinum sensitivity was
compared between the two groups. In the low expression group, 73.3%
of the patients were platinum-sensitive, whereas in the
high-expression group, only 38.3% were platinum-sensitive. This
indicated that the platinum sensitivity rate was significantly
greater in the low-expression group than in the high-expression
group (P<0.01, Table III).
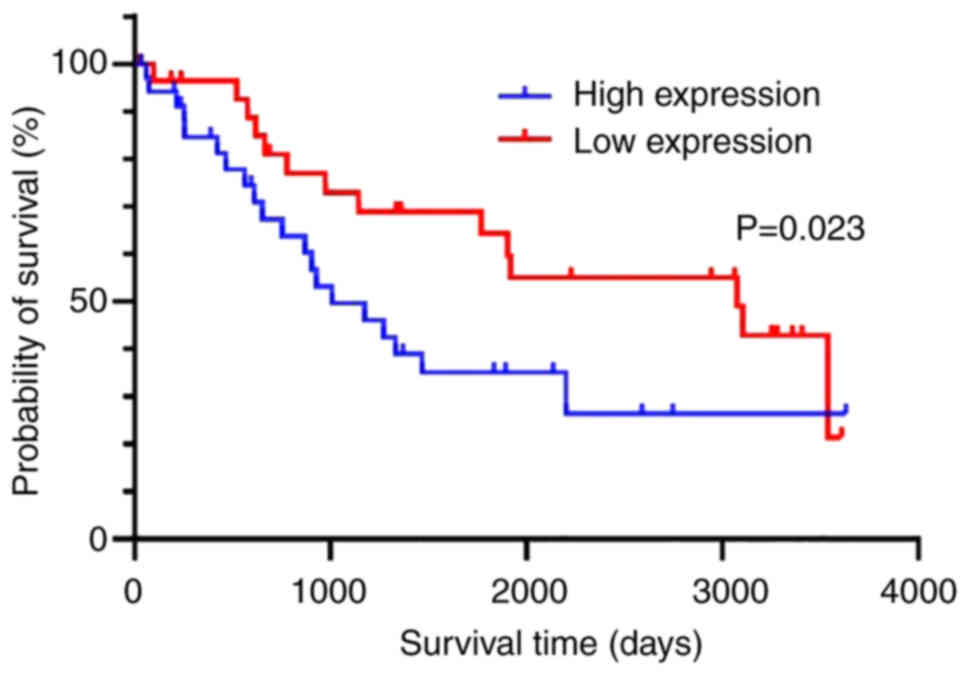
Additionally, overall survival (OS) was compared between the two
groups. The OS was significantly longer in the low-expression group
compared with the high-expression group (P=0.023, Fig. 3).
 | Table II.Characteristics of the patients based
on Fyn expression. |
Table II.
Characteristics of the patients based
on Fyn expression.
| Characteristic | Low expression
group | High expression
group | P-value |
|---|
| No. of
patients | 30 | 34 |
|
| Median age, years
(range) | 60.5 (36–79) | 63.0 (39–81) | 0.228a |
| FIGO stage, n |
|
| 0.142b |
|
IIIA | 2 | 0 |
|
|
IIIB | 2 | 2 |
|
|
IIIC | 23 | 25 |
|
|
IVA | 3 | 3 |
|
|
IVB | 0 | 4 |
|
| Median CA125, U/ml
(range) | 1,227.5
(25–30,075) | 1,415.5
(113–12,300) | 0.360a |
| Postoperative
residual tumor, n |
|
| 0.052b |
|
None | 8 | 4 |
|
| ≤1
cm | 10 | 6 |
|
| >1
cm | 12 | 24 |
|
 | Table III.Association between Fyn expression
and platinum sensitivity. |
Table III.
Association between Fyn expression
and platinum sensitivity.
| Fyn expression, n
(%) |
Platinum-sensitive |
Platinum-resistant | P-value |
|---|
| Low expression,
score ≤6 | 22 (73.3%) | 8 (26.7%) |
<0.01a |
| High expression,
score ≥8 | 13 (38.3%) | 21 (61.7%) |
|
Multivariate analysis of factors
independently related to platinum sensitivity
Multivariate analysis was performed to identify
factors independently related to platinum sensitivity, using
factors identified as significant in the univariate analysis (the
size of postoperative residual tumors and the Fyn weighted score).
Table IV demonstrates that both
Fyn expression and the size of postoperative residual tumors were
independently associated with platinum sensitivity. The odds ratio
for Fyn expression was 0.295 (95% CI: 0.096-0.906) with a P-value
of 0.033, while the odds ratio for the size of postoperative
residual tumors was 0.400 (95% CI: 0.181-0.882) with a P-value of
0.023.
 | Table IV.Multivariate analysis for detecting
independent factors of platinum sensitivity. |
Table IV.
Multivariate analysis for detecting
independent factors of platinum sensitivity.
|
| 95% confidence
interval |
|
|
|---|
|
|
|
|
|
|---|
| Variable | Odds ratio | Lower | Upper | P-value |
|---|
| Fyn expression,
low/high | 0.295 | 0.096 | 0.906 | 0.033a |
| Postoperative
residual tumors, 0 cm/<1 cm/≥1 cm | 0.400 | 0.181 | 0.882 | 0.023a |
Effect of Fyn knockdown on the
sensitivity of ovarian cancer cells to carboplatin
Ovarian cancer cells were allocated into two groups,
a control group transfected with control siRNA and a siFyn group
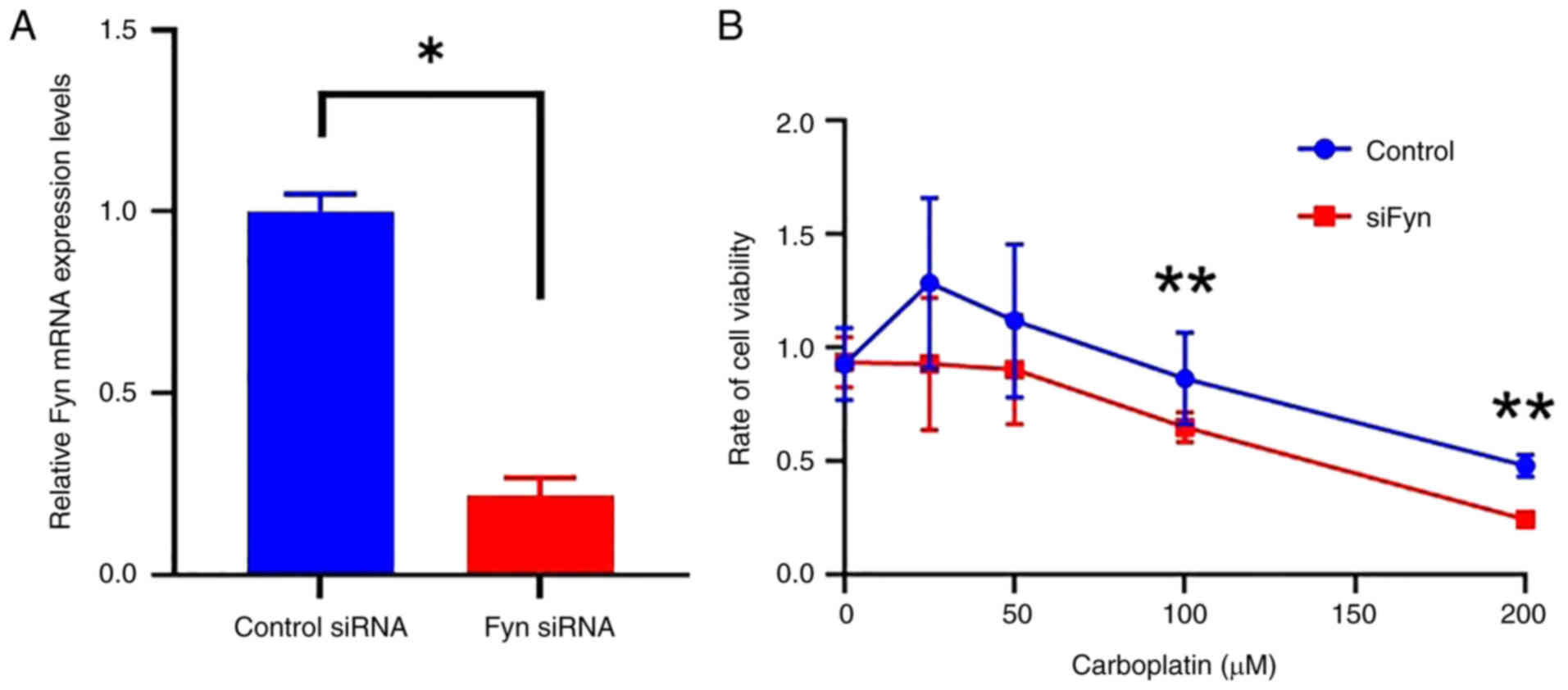
transfected with Fyn-specific siRNA. The successful knockdown of
Fyn expression using siRNA was confirmed by RT-qPCR. This
examination demonstrated a significant decrease in Fyn mRNA levels
in cells treated with Fyn-specific siRNA compared to those treated
with control siRNA (P<0.01, Fig
4A). Subsequently, the sensitivity of cells to carboplatin
between the control group and the siFyn group was compared by
assessing cell viability after administering various doses of
carboplatin. The results showed that at doses of ≥100 µM, cell
viability was significantly lower in the siFyn group compared to
the control group (P<0.05, Fig
4B). This indicates that reducing Fyn expression increases
ovarian cancer cells' sensitivity to carboplatin.
Discussion
Ovarian cancer remains a formidable disease with a
high mortality rate and poor prognosis, even with the introduction
of advanced therapeutic options such as PARP inhibitors and VEGF
inhibitors (19,20). Despite initial responsiveness to
chemotherapy, a large subset of patients eventually experience
recurrence and develop resistance to platinum-based treatments,
which are the cornerstone of ovarian cancer therapy (3,5).
Platinum resistance is a significant barrier in the effective
management of advanced ovarian carcinoma, leading to treatment
failure and disease progression (7,21).
Several theories have been proposed to explain platinum resistance,
including decreased cellular import and increased export of the
drug through transporters, enhanced DNA damage repair,
intracellular drug inactivation by detoxifying enzymes, and the
inactivation of cell death signaling pathways (7,22–24).
These multiple mechanisms may concurrently contribute to platinum
resistance (25–28).
Tyrosine kinases are classified into two primary
categories: Receptor tyrosine kinases and non-receptor tyrosine
kinases. Non-receptor tyrosine kinases comprise families such as
Src, Abl, Janus kinase, and FAK, whereas receptor tyrosine kinases
including the vascular endothelial growth factor receptor (VEGFR),
epidermal growth factor receptor (EGFR), and mesenchymal-epithelial
transition factor, respond to signals from soluble ligands
(29). When these tyrosine kinases
are dysregulated, they can cause cancer by disrupting cellular
function, growth, and morphology, which are key characteristics of
malignancy (29). The SFKs,
including Fyn, c-Src, Yes, Lck, Lyn, Fgr, Blk, and Hck, are among
these non-receptor kinases, with Fyn, c-Src, and Yes broadly
expressed across various tissues, unlike the others, which instead
have more limited expression patterns (29). Fyn, which is a non-receptor tyrosine
kinase, is involved in various biological processes by
phosphorylating tyrosine residues of the key molecules involved in
different signal pathways, such as signal transduction through T
cell receptors, signal transduction in neurons (impacting brain
function), and adhesion-mediated signaling in physiological
conditions (14,29). Moreover, Fyn is crucial in the onset
and advancement of multiple cancer types, regulating cell growth,
apoptosis, motility, migration, and morphogenic transformation
(14,29). Multiple malignancies such as glioma,
melanoma, breast cancer, prostate cancer, head and neck squamous
cell carcinoma, chronic myeloid leukemia, cholangiocarcinoma,
thyroid cancer, gastric cancer, and esophageal squamous cell
carcinoma exhibit Fyn involvement (30–35).
In cancer, Fyn is involved in receptor tyrosine kinase pathways,
including those involving VEGFR, EGFR, fibroblast growth factor,
and platelet-derived growth factor receptor. It conveys signals via
Ras-independent pathways (including PIK3/Akt, FAK, STAT3, VAV1,
β-catenin, paxillin, and/or SHC) and Ras-dependent pathways (via
Ras/MEK/ERK) (12,28). These pathways enable Fyn to mediate
anti-apoptotic effects of Akt/PKB mediated by growth factor
(16,36). Numerous studies have correlated Fyn
expression with response to anti-cancer drugs because of its role
in regulating apoptosis. For example, Fyn knockdown enhances
apoptosis induced by doxorubicin and boosts the sensitivity of
cells resistant to doxorubicin to this drug by inactivating MAPK
signaling (14,29). Furthermore, Fyn has been identified
as one of the hub genes of the interaction network in
cisplatin-resistant ovarian cancer cells (37). In pancreatic ductal adenocarcinoma,
increased Fyn expression decreases chemosensitivity to gemcitabine
via regulation of miR-125a-3p (38). Moreover, the effectiveness of PP2,
an SFK inhibitor, is significantly influenced by Fyn expression
levels, as PP2 induces apoptosis (39). Thus, Fyn is recognized as a critical
molecule in conferring resistance to anti-cancer agents, primarily
through its role in apoptosis induction (14,29).
Fyn expression was found to be associated with
platinum sensitivity and OS in patients with ovarian serous
carcinoma in the current study. Immunohistochemical evaluation
using a weighted scoring system revealed that Fyn expression was
significantly lower in the platinum-sensitive group compared to the
platinum-resistant group. Additionally, low Fyn expression
(weighted score ≤6) was significantly correlated with both
increased platinum sensitivity and a longer OS. Furthermore,
multivariate analysis demonstrated that Fyn expression was an
independent factor associated with platinum sensitivity,
demonstrating the highest odds ratio. Additionally, in vitro
experiments confirmed that Fyn knockdown using siRNA enhanced the
effectiveness of carboplatin against ovarian cancer cells, further
supporting the potential of Fyn as a therapeutic target to improve
treatment outcomes for patients with ovarian serous carcinoma.
However, there are several limitations to the
current study. Firstly, its retrospective nature inherently limits
the ability to establish causation. Additionally, the sample size
is relatively small, with only 64 cases, which can limit the
generalizability of the findings and reduce the statistical power
for the detection of significant differences or associations. The
study was conducted at a single institution, meaning the results
may not be representative of broader patient populations or other
ethnicities. Furthermore, the immunohistochemical evaluation and
the weighted scoring method for Fyn expression are subject to
variability and potential observer bias. The study also focused
solely on Fyn expression, without exploring other potential
biomarkers and molecular mechanisms influencing platinum
sensitivity and resistance. Lastly, while the study shows an
association between Fyn expression and platinum sensitivity, it
does not thoroughly investigate the underlying biological
mechanisms or pathways through which Fyn influences resistance to
chemotherapy. These limitations suggest that further research is
required to confirm the findings and expand on the underlying
mechanisms. Prospective studies with larger, multi-center cohorts
and comprehensive biomarker analyses including those biomarkers,
such as UCP2, PRMT1, and TBX2, which our research team has reported
as predictors of platinum sensitivity (25–27)
are necessary to validate and extend these results. While
immunohistochemistry may influence the reliability of the results
to some extent, it remains a straightforward and practical method
in clinical practice, making it a valuable technique for assessing
sensitivity to platinum-based chemotherapy. While the current study
lacked mechanistic insights, establishing a correlation between Fyn
expression and platinum sensitivity provides a foundational step
toward exploring the underlying mechanisms of platinum sensitivity
in ovarian cancer patients.
As far as we are aware, this study is the first to
establish a link between Fyn expression and platinum sensitivity in
patients with advanced ovarian serous carcinoma. Understanding the
mechanisms underpinning platinum sensitivity in advanced ovarian
serous carcinoma is crucial for developing therapeutic strategies
that improve prognosis. In conclusion, the results of this study
highlight the potential of using Fyn expression for predicting
sensitivity to platinum-based chemotherapy for ovarian serous
carcinoma.
Acknowledgements
The authors would like to thank Dr Yukimi Kira
(Research Support Platform, Osaka Metropolitan University Graduate
School of Medicine, Osaka, Japan) for their technical assistance
and expertise.
Funding
This study received financial support from the Osaka Medical
Research Foundation for Intractable Diseases (grant no.
29-1-46).
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
EU, TF and TSu conceptualized and designed the
study. EU, TSe, TN, YA, TW, RT, MY and TY performed the experiments
and collected the data. EU, TF and TSu analyzed and interpreted the
data. EU and TF drafted the manuscript. EU and TF confirm the
authenticity of all the raw data. All authors have read and
approved the final version of the manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of Osaka Metropolitan University Hospital (approval no.
2022-108). Written informed consent was obtained from all
participants.
Patient consent for publication
All participants provided written informed consent
for the publication of this article.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Falzone L, Scandurra G, Lombardo V,
Gattuso G, Lavoro A, Distefano AB, Scibilia G and Scollo P: A
multidisciplinary approach remains the best strategy to improve and
strengthen the management of ovarian cancer (Review). Int J Oncol.
59:532021. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Bray F, Laversanne M, Sung H, Ferlay J,
Siegel RL, Soerjomataram I and Jemal A: Global cancer statistics
2022: GLOBOCAN estimates of incidence and mortality worldwide for
36 cancers in 185 countries. CA Cancer J Clin. 74:229–263. 2024.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Stuart GC, Kitchener H, Bacon M, duBois A,
Friedlander M, Ledermann J, Marth C, Thigpen T and Trimble E;
participants of 4th Ovarian Cancer Consensus Conference (OCCC);
Gynecologic Cancer Intergroup, : 2010 gynecologic cancer InterGroup
(GCIG) consensus statement on clinical trials in ovarian cancer:
Report from the fourth ovarian cancer consensus conference. Int J
Gynecol Cancer. 21:750–755. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Daly MB, Pal T, Maxwell KN, Churpek J,
Kohlmann W, AlHilli Z, Arun B, Buys SS, Cheng H, Domchek SM, et al:
NCCN Guidelines® insights: Genetic/Familial high-risk
assessment: Breast, ovarian, and pancreatic, version 2.2024. J Natl
Compr Canc Netw. 21:1000–1010. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Friedlander M, Trimble E, Tinker A,
Alberts D, Avall-Lundqvist E, Brady M, Harter P, Pignata S,
Pujade-Lauraine E, Sehouli J, et al: Clinical trials in recurrent
ovarian cancer. Int J Gynecol Cancer. 21:771–775. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Markman M, Rothman R, Hakes T, Reichman B,
Hoskins W, Rubin S, Jones W, Almadrones L and Lewis JL Jr:
Second-line platinum therapy in patients with ovarian cancer
previously treated with cisplatin. J Clin Oncol. 9:389–393. 1991.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
St Laurent J and Liu JF: Treatment
approaches for platinum-resistant ovarian cancer. J Clin Oncol.
42:127–133. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kyrgiou M, Salanti G, Pavlidis N,
Paraskevaidis E and Ioannidis JP: Survival benefits with diverse
chemotherapy regimens for ovarian cancer: Meta-analysis of multiple
treatments. J Natl Cancer Inst. 98:1655–1663. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Havasi A, Cainap SS, Havasi AT and Cainap
C: Ovarian cancer-insights into platinum resistance and overcoming
it. Medicina (Kaunas). 59:5442023. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
McMullen M, Madariaga A and Lheureux S:
New approaches for targeting platinum-resistant ovarian cancer.
Semin Cancer Biol. 77:167–181. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Khan MA, Vikramdeo KS, Sudan SK, Singh S,
Wilhite A, Dasgupta S, Rocconi RP and Singh AP: Platinum-resistant
ovarian cancer: From drug resistance mechanisms to liquid
biopsy-based biomarkers for disease management. Semin Cancer Biol.
77:99–109. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Pennington KP, Walsh T, Harrell MI, Lee
MK, Pennil CC, Rendi MH, Thornton A, Norquist BM, Casadei S, Nord
AS, et al: Germline and somatic mutations in homologous
recombination genes predict platinum response and survival in
ovarian, fallopian tube, and peritoneal carcinomas. Clin Cancer
Res. 20:764–775. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Colombo PE, Taoum C, Fabbro M, Quesada S,
Rouanet P and Ray-Coquard I: Impact of molecular testing on the
surgical management of advanced epithelial ovarian cancer. Crit Rev
Oncol Hematol. 202:1044692024. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Saito YD, Jensen AR, Salgia R and Posadas
EM: Fyn: A novel molecular target in cancer. Cancer. 116:1629–1637.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Li S, Liu C and Tang Y: Role of Fyn in
hematological malignancies. J Cancer Res Clin Oncol. 149:6759–6767.
2023. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Nanno S, Fukuda T, Noda T, Uchikura E,
Awazu Y, Imai K, Yamauchi M, Yasui T and Sumi T: Fyn expression is
associated with the response of patients with locally advanced
uterine cervical squamous cell carcinoma to neoadjuvant
chemotherapy. Mol Clin Oncol. 17:1472022. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Sinicrope FA, Ruan SB, Cleary KR, Stephens
LC, Lee JJ and Levin B: bcl-2 and p53 oncoprotein expression during
colorectal tumorigenesis. Cancer Res. 55:237–241. 1995.PubMed/NCBI
|
|
18
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
O'Malley DM, Krivak TC, Kabil N, Munley J
and Moore KN: PARP inhibitors in ovarian cancer: A review. Target
Oncol. 18:471–503. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Fukuda T, Noda T, Uchikura E, Awazu Y,
Tasaka R, Imai K, Yamauchi M, Ichimura T, Yasui T and Sumi T:
Real-world efficacy and safety of bevacizumab for advanced or
recurrent müllerian cancer: A single-institutional experience.
Anticancer Res. 43:3097–3105. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Friedlander ML, Stockler MR, Butow P, King
MT, McAlpine J, Tinker A and Ledermann JA: Clinical trials of
palliative chemotherapy in platinum-resistant or -refractory
ovarian cancer: Time to think differently? J Clin Oncol.
31:23622013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Dasari S and Tchounwou PB: Cisplatin in
cancer therapy: Molecular mechanisms of action. Eur J Pharmacol.
740:364–378. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ghosh S: Cisplatin: The first metal based
anticancer drug. Bioorg Chem. 88:1029252019. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Amable L: Cisplatin resistance and
opportunities for precision medicine. Pharmacol Res. 106:27–36.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Matsubara H, Fukuda T, Awazu Y, Nanno S,
Shimomura M, Inoue Y, Yamauchi M, Yasui T and Sumi T: PRMT1
expression predicts sensitivity to platinum-based chemotherapy in
patients with ovarian serous carcinoma. Oncol Lett. 21:1622021.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kawanishi M, Fukuda T, Shimomura M, Inoue
Y, Wada T, Tasaka R, Yasui T and Sumi T: Expression of UCP2 is
associated with sensitivity to platinum-based chemotherapy for
ovarian serous carcinoma. Oncol Lett. 15:9923–9928. 2018.PubMed/NCBI
|
|
27
|
Tasaka R, Fukuda T, Shimomura M, Inoue Y,
Wada T, Kawanishi M, Yasui T and Sumi T: TBX2 expression is
associated with platinum-sensitivity of ovarian serous carcinoma.
Oncol Lett. 15:3085–3090. 2018.PubMed/NCBI
|
|
28
|
Fukuda T, Kawanishi M, Awazu Y, Nanno S,
Shimomura M, Inoue Y, Matsubara H, Yamauchi M, Kasai M, Hashiguchi
Y, et al: Neutrophil-to-lymphocyte ratio is associated with
sensitivity to platinum-based chemotherapy and prognosis in
patients with advanced serous ovarian carcinoma. Mol Clin Oncol.
15:2172021. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Peng S and Fu Y: FYN: Emerging biological
roles and potential therapeutic targets in cancer. J Transl Med.
21:842023. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Huang C, Zhou J, Nie Y, Guo G, Wang A and
Zhu X: A new finding in the key prognosis-related proto-oncogene
FYN in hepatocellular carcinoma based on the WGCNA hub-gene
screening trategy. BMC Cancer. 22:3802022. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Lyu SC, Han DD, Li XL, Ma J, Wu Q, Dong
HM, Bai C and He Q: Fyn knockdown inhibits migration and invasion
in cholangiocarcinoma through the activated AMPK/mTOR signaling
pathway. Oncol Lett. 15:2085–2090. 2018.PubMed/NCBI
|
|
32
|
Yu J, Zhou Z, Wei Z, Wu J, OuYang J, Huang
W, He Y and Zhang C: FYN promotes gastric cancer metastasis by
activating STAT3-mediated epithelial-mesenchymal transition. Transl
Oncol. 13:1008412020. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Zhang X, Huang Z, Guo Y, Xiao T, Tang L,
Zhao S, Wu L, Su J, Zeng W, Huang H, et al: The phosphorylation of
CD147 by Fyn plays a critical role for melanoma cells growth and
metastasis. Oncogene. 39:4183–4197. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Dong W, Sun SJ, Qin JJ and Liu GM: Fyn
stimulates the progression of pancreatic cancer via Fyn-GluN2b-AKT
axis. Eur Rev Med Pharmacol Sci. 24:109–121. 2020.PubMed/NCBI
|
|
35
|
Zhang J, Zhao D, Zhang L, Xiao Y, Wu Q,
Wang Y, Chen J and Zhan Q: Src heterodimerically activates Lyn or
Fyn to serve as targets for the diagnosis and treatment of
esophageal squamous cell carcinoma. Sci China Life Sci.
66:1245–1263. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Elias D and Ditzel HJ: Fyn is an important
molecule in cancer pathogenesis and drug resistance. Pharmacol Res.
100:250–254. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Sakhare SS, Rao GG, Mandape SN and Pratap
S: Transcriptome profile of OVCAR3 cisplatin-resistant ovarian
cancer cell line. BMC Bioinformatics. 15:212014. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Liu G, Ji L, Ke M, Ou Z, Tang N and Li Y:
miR-125a-3p is responsible for chemosensitivity in PDAC by
inhibiting epithelial-mesenchymal transition via Fyn. Biomed
Pharmacother. 106:523–531. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Noronha G, Barrett K, Boccia A, Brodhag T,
Cao J, Chow CP, Dneprovskaia E, Doukas J, Fine R, Gong X, et al:
Discovery of [7-(2,6-dichlorophenyl)-5-methylbenzo
[1,2,4]triazin-3-yl]-[4-(2-pyrrolidin-1-ylethoxy)phenyl]amine-a
potent, orally active Src kinase inhibitor with anti-tumor activity
in preclinical assays. Bioorg Med Chem Lett. 17:602–608. 2007.
View Article : Google Scholar : PubMed/NCBI
|