Introduction
Malignant melanoma is a highly aggressive and
metastatic skin cancer. Although meningeal metastasis is rare
(5–25%), it indicates a very poor prognosis (1). The symptoms of meningeal metastasis
are diverse, including headaches, nausea, vomiting, seizures and
neurological deficits (2), often
meaning that the diagnosis is confused with that of other central
nervous system diseases, thus increasing the diagnostic difficulty.
Meningeal metastasis with concurrent hemorrhagic CSF is even rarer
(0.9–4.7%) (3), further
complicating the clinical diagnosis and treatment.
The present study reports a rare case of malignant
melanoma meningeal metastasis (MMMM) with concurrent hemorrhagic
CSF. By detailing the patient's clinical manifestations, imaging
characteristics, diagnostic process and treatment plan, the study
aims to provide a reference for the clinical management of similar
cases.
Case report
In September 2022, a 51-year-old male patient
presented with the chief complaint of a headache for >10 days,
worsening for 1 day at People's Hospital of Leshan (Leshan, China).
The patient had originally reported a persistent dull headache at
the start of this period, with no specific localization,
accompanied by nausea, vomiting (no projectile vomiting), unsteady
gait, fever (37.5°C) and night sweats. Symptoms did not resolve
after rest and the patient was therefore admitted to Meishan City
People's Hospital (Meishan, China) after 5 days, at the end of
August 2022. Upon admission, the patient underwent a computed
tomography (CT) scan of the head and digital subtraction
angiography (data not shown), which showed no abnormalities.
However, a lumbar puncture showed hemorrhagic CSF (Table I). Therefore, the patient was
initially diagnosed with a subarachnoid hemorrhage and intracranial
infection. After treatment for pain relief, reduction of the
intracranial pressure and hemostasis, the patient was discharged
from the hospital 4 days later, with improvement of the symptoms.
However, only 1 day after discharge, the patient was admitted to
the People's Hospital of Leshan with a worsening headache.
 | Table I.Cerebrospinal fluid analyses. |
Table I.
Cerebrospinal fluid analyses.
| Date | Pressure,
mmH20 | Appearance | Complete blood
counts | Biochemical
indicators | Genetic sequencing
and cytological examination |
|---|
| August 2022 | Initial, 360; final,
70 | Red, turbid | RBC,
2.7×1010/l; WBC, 8.9×107/l | Glucose, 0.86 mmol/l;
protein, 1,958.5 mg/l; protein, 1,958.5 mg/l; chloride, 130
mmol/l | None |
| September 2022 | Initial, 300; final,
240 | Pale yellow, slightly
turbid | RBC,
4.0×109/l; WBC, 3.5×107/l | Glucose, 0.40 mmol/l;
protein 2,599.9, mg/l; chloride, 118 mmol/l | Second-generation
sequencing of pathogen genes (negative) |
| September 2022 | Initial, 60; final,
40 | Red, clear | RBC,
5.7×1010/l; WBC, 2.1×108/l | Glucose, 0.60 mmol/l;
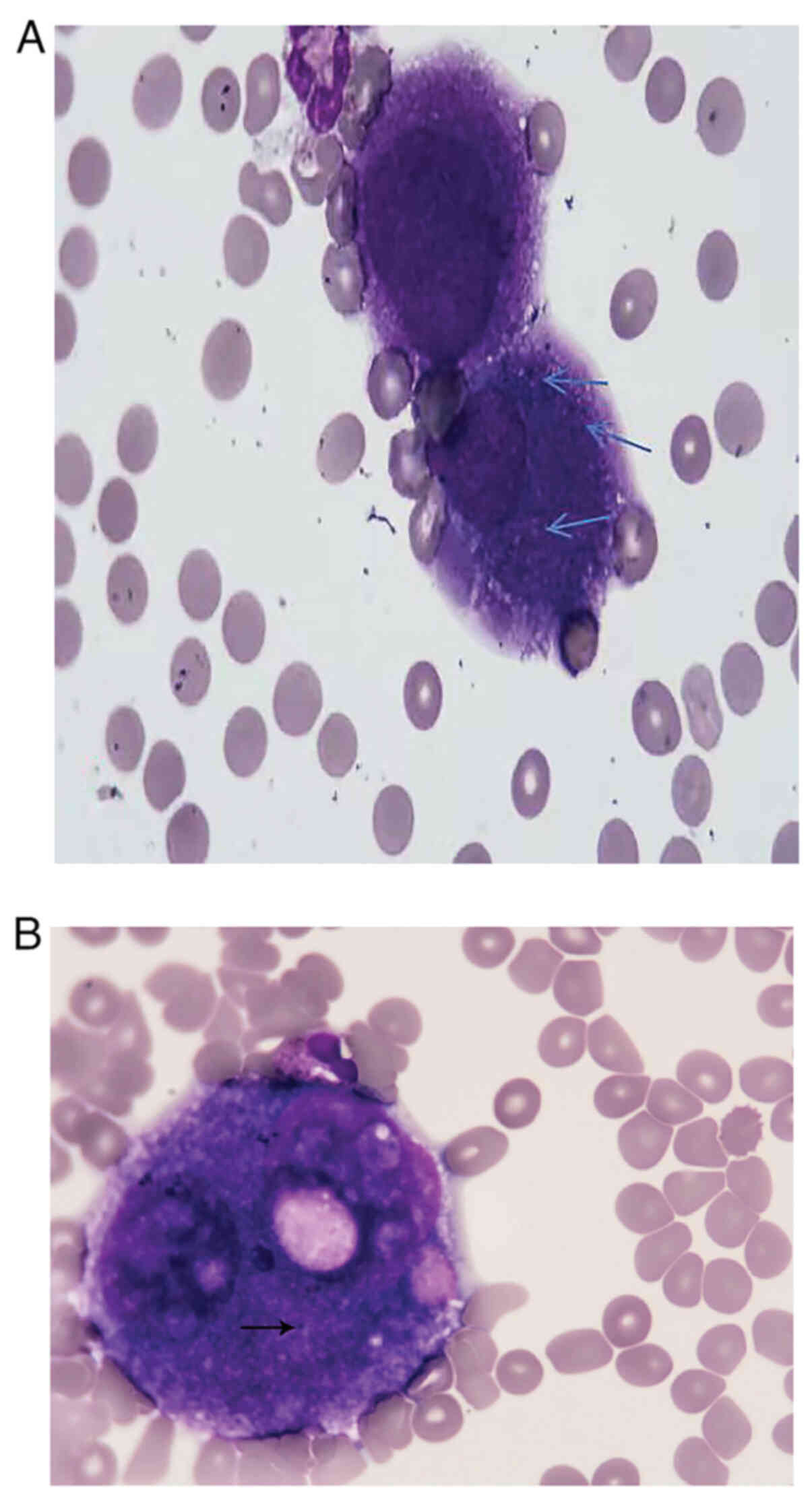
protein, 3,234.4 mg/l; chloride, 108.4 mmol/l | Cytology: Numerous
melanoma cells (Fig. 3) |
The patient's vital signs were stable, and there was
a lack of apparent abnormalities in physical examinations of the
heart, lungs and abdomen. Neurological analysis showed clear
consciousness, coherent speech, normal cranial nerves, typical
motor and sensory systems, and coordinated movement. The tests for
neck stiffness were positive, Kernig's sign was negative and
bilateral pathological signs were also negative.
There were no abnormalities observed in the
following blood tests: Complete blood counts, biochemical
indicators, coagulation function, pre-check for blood transfusion,
autoimmune panel, tumor markers, T-spot and G test. There were no
marked observations in the electroencephalogram or in the CT scans
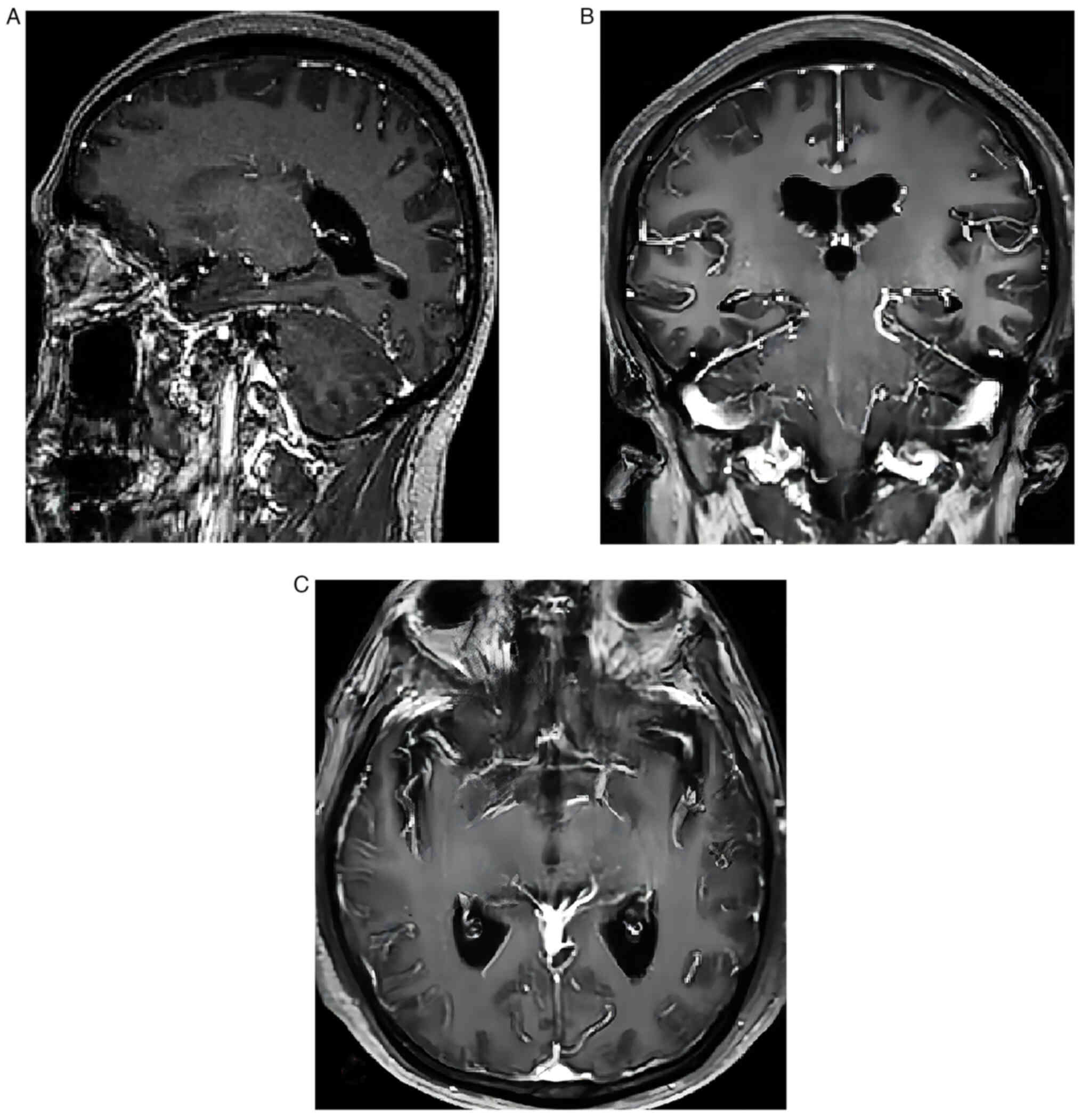
of the chest and abdomen. The results of magnetic resonance imaging
(MRI) and enhancement of brain sections revealed hyperintensity in
the cerebrovascular white matter, Fazekas grade 1 (4) (Fig.
1).
The patient underwent lumbar puncture examination in
Meishan People's Hospital, which showed cerebrospinal fluid
leukocytes: 89X10^6/l (normal value: 0–8X106/l), erythrocytes:
27,000X10^6/l (normal value: 0X106/l), protein level: 1,958.5 mg/l
(normal value: 150–450 mg/l), cerebrospinal fluid glucose: 0.65
mmol/l (normal range: 2.5–4.4 mmol/l) Targeted therapy
(ceftriaxone, 2 g I.V. fluids, once a day; tranexamic acid 1g ivgtt
qd and tramadol 50 mg qd analgesic therapy were administered, but
the headache symptoms did not improve. After being transferred to
Leshan People's Hospital, a repeat lumbar puncture performed on the
first day after admission showed a decrease in the number of
leukocytes and erythrocytes in the cerebrospinal fluid, suggesting
that an infection may have occurred. After transferring back to
People's Hospital of Leshan, a follow-up lumbar puncture
examination 1 week after the initial examination showed a decrease
in the number of white blood cells and red blood cells in the CSF
(4.0×109/l; WBC, 3.5×107/l, respectively), suggesting a
possible infection. Continuous treatment measures were
administered, This included intravenous mannitol 125 ml ivgtt q8h
to lower intracranial pressure, continued anti-infective treatment
with ceftriaxone 2 g ivgtt qd, and anti-spasmodic and other
treatments such as nimodipine 10 mg ivvp qd injection for one week.
Second-generation gene sequencing was performed at Chengdu Hemer
Yuning Medical Laboratory Center (Chengdu, Sichuan, China, and the
results were negative, ruling out the possibility of infectious
meningitis. However, the patient's headache symptoms still did not
improve. The patient underwent a repeat lumbar puncture 1 week
after admission to the hospital showing a significant increase in
red blood cells, white blood cells, and proteins in the
cerebrospinal fluid compared to the previous level (5.7×1010/l;
WBC, 2.1×108/l and protein, 3,234.4 mg/l) with no obvious
abnormalities on cranial CT and MRI. Further inquiry into the
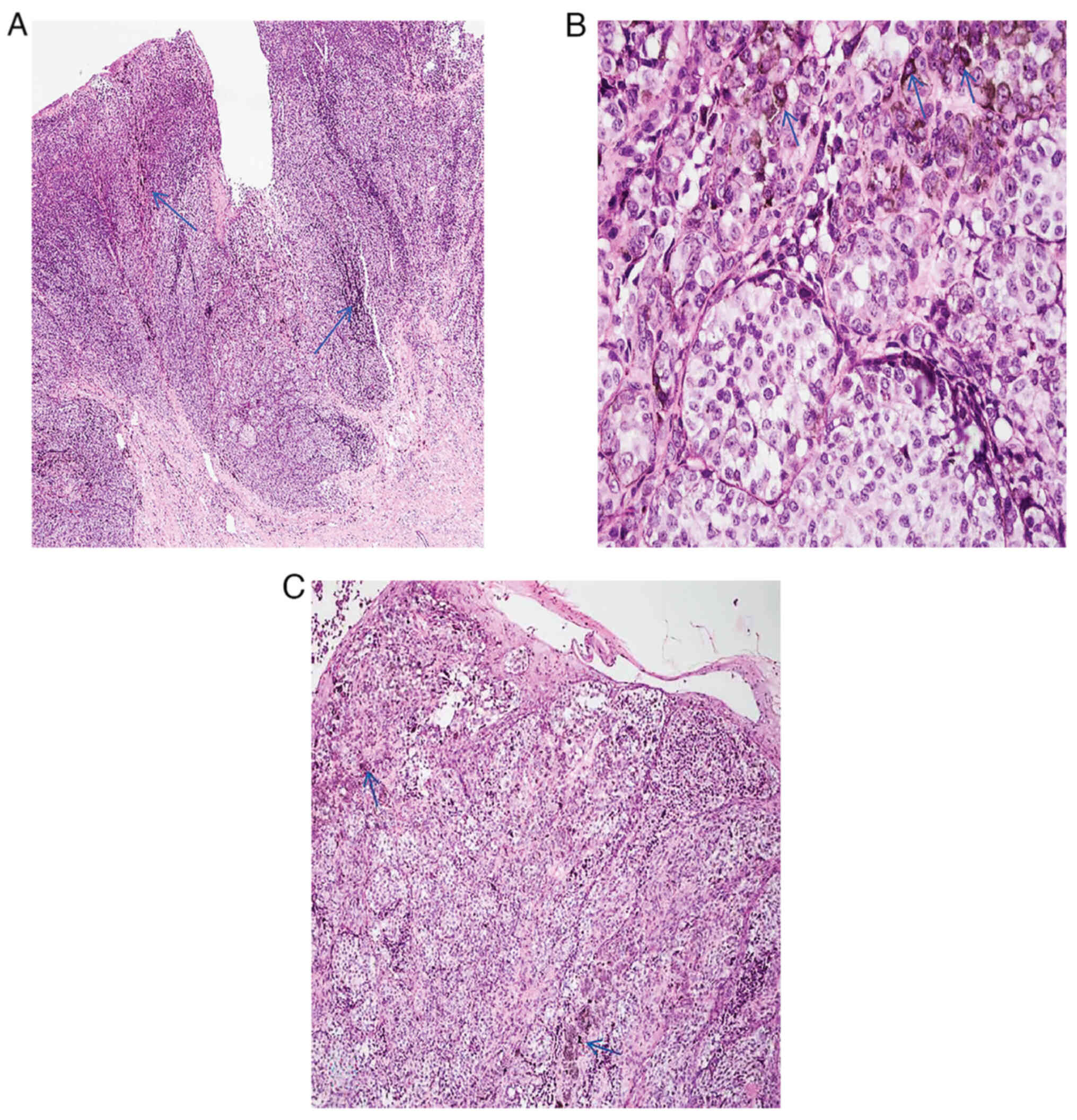
patient's medical history revealed that the patient had undergone
surgery to remove a malignant melanoma at the proximal joint of the
left thumb 3 months earlier. After surgery, tissue was fixed in 10%
neutral formaldehyde solution for 6–8 h at 20–25°C. After fixation,
the tissue was heated for 10 min and then stained with Eosin under
a Leica DM2000 light microscope at a temperature of 20–25°C for
5–10 min, and then examined at 40×, 100×, and 400× magnification,
and finally diagnosed as malignant melanoma. (Fig. 2). CSF cytology was performed after 1
week using an Olympus CKX53SFC fluorescence microscope under 100×
oil magnification, which revealed abnormal cells in the CSF. The
staining procedure consisted of fixation with 9 drops of Ragis
stain for 2 min at 20–25°C, followed by the addition of 3 drops of
PBS and staining for 13 min at a laboratory temperature of
approximately 25°C and humidity of approximately 45% (Fig. 3) that were labeled as malignant
melanoma cells, as they contained pigment granules. The cells were
different in morphology and size from those found in normal CSF.
Due to the insufficient number of cells, further confirmation using
other methods was not possible. A combination of the patient's past
and current medical history led to a diagnosis of MMMM and
carcinomatous meningitis. The patient was then automatically
discharged from hospital the day after the diagnosis and refused
any formal treatment. Subsequent to discharge, the patient
gradually developed bilateral hearing loss, a poor appetite,
multiple organ failure and other symptoms, and finally passed away
>1 month after discharge.
Discussion
Combining multiple lumbar punctures, the retained
sample was a homogenous and consistent CSF containing high protein
and white blood cell levels. Despite a decrease in all markers
early in the course of anti-infective therapy, the number of white
blood cells continued to rise as the course continued. Tests for
all types of pathogens were negative, and red blood cell
fluctuations were inconsistent with the patient's clinical
presentation. No metastatic lesions were detected, although the
patient underwent a comprehensive cranial CT and MRI on both the
previous admission and the current admission. CSF cytology was
suggestive of the presence of melanoma cells, indicating that the
patient's CSF hemorrhage was closely associated with meningeal
metastases (5).
Leptomeningeal metastasis (LM) is an advanced form
of distant metastasis from malignant tumors with a poor prognosis.
The overall survival time is 6–8 weeks in untreated patients and
can be extended to 3 to 9 months with intrathecal chemotherapy. The
incidence rate is 1–8% of all cancer cases (6). Current studies suggest that LM is due
to the activation of C3a receptors in the choroid plexus epithelium
by tumor cell-derived complement C3, which disrupts the blood-brain
barrier and increases endothelial permeability, thereby allowing
plasma components, such as deregulatory proteins and other
mitogens, to enter the CSF and promote tumor growth (7). Patients may present with brain
parenchyma involvement, symptoms of meningeal irritation (dizziness
and headache), cranial nerve involvement (vision/hearing loss) and
spinal nerve root compression (numbness and weakness of the limbs)
(8–10). Primary tumors include breast cancer,
lung cancer, melanoma and finally, primary central nervous system
cancer (11). Among the solid
tumors, meningeal metastases occur in up to 10–15% of patients with
advanced MM (12). Typical MRI
findings show multiple or single rounded lesions of abnormal
signal, and more commonly, homogeneous nodular or ring-like
enhancement images on image enhancement (13). In addition, it is estimated that
66–90% of patients with LM have positive CSF cytology. Non-specific
manifestations of CSF also include increased pressure, protein and
leukocytes, and decreased glucose (14). The present patient was exhibited the
aforementioned CSF features, and an increase in erythrocytes, which
is currently uncommon in LM. The present case was of a middle-aged
male with the sudden onset of a headache and a history of malignant
melanoma, with treatments such as radiotherapy after surgical
resection, although imaging did not show positive manifestations in
the past Su and Wei (15) analyzed
the characteristics of four cases of malignant melanoma and
suggested that there can be no abnormal imaging. The present study
is in line with the aforementioned report and to exclude the
infection, bleeding and other etiologies before LM diagnosis, which
is consistent with the diagnosis of LM. Although histopathological
biopsy of the brain can detect the presence of cancerous tissue in
the meninges, in recent years, the gold standard for the diagnosis
of LM has been the presence of tumor cells in the CSF (16). Further refinement of cerebrospinal
fluid cytology is required, leading to standardized anti-tumor
therapy.
In meningeal metastases, tumor cells are seen in the
CSF, which tends to be clear and transparent in appearance. Cases
of bloody CSF have rarely been reported (17,18).
In the present case, the CSF had a significantly elevated
erythrocyte count and a bloody appearance (puncture wounds had been
excluded), and so a subarachnoid hemorrhage was considered, but the
CT of the head in this patient did not show hemorrhagic changes in
the sulcus and cranium, and the vascular examination ruled out
aneurysms and arteriovenous malformations. The pathogenesis causing
the subarachnoid hemorrhage is currently unclear. In the clinic, a
small number of patients with herpes simplex virus encephalitis,
the CSF also showed homogeneous erythrocytes, the mechanism of
which may be related to the immune damage caused by the presence of
immune complexes formed by antibodies to herpes simplex virus IgM
in the cerebral vascular wall, which in turn causes necrosis of the
vascular wall and erythrocyte exudation (19). In the current case, the patient's
meningeal irritation sign was positive, which was considered to be
intracranial infectious disease, and therefore anti-infective
treatment was provided. Although the white blood cell count
decreased slightly in the early stage, the symptoms were not
relieved, and in the later stage, while still receiving
anti-infective treatment, the white blood cell count was
significantly elevated, but the genetic test for pathogens was
negative, so the infectious disease was excluded. Solid tumours
lead to meningeal metastases in about 1–8% of cases. It is not
uncommon for melanoma, as a type of solid tumour, to develop
meningeal metastases, up to 30%, and its metastases manifest as
elevated cerebrospinal fluid proteins and white blood cells
(16). There is currently no report
of blood-containing CSF in melanoma. Even if there is
blood-containing CSF in other solid tumor metastases, its imaging
examination is positive, and there is no negative imaging combined
with blood-containing CSF. Hematogenous CSF has not been reported
so far. The abnormal cells in the bloody CSF of the current patient
had the presence of pigment granules, and the cell morphology and
size did not belong to the cell morphology found in normal CSF.
However, the number of cells was not sufficient to be further
confirmed by other methods, and thus, in combination with the
patient's past and current medical history, we considered that a
diagnosis of CSF metastasis should be made. However, the etiology
of bloody CSF in patients with carcinomatous meningitis remains
unclear, and we hypothesize that it may be as follows: i)
Proliferation of cancer cells in CSF and invasion of the CSF
circulatory system may lead to the obstruction of CSF pathways or
damage to blood vessels, which may lead to hemorrhage; ii) invasion
of cancer cells into the meninges may lead to rupture of the
microvessels of the CSF or hemorrhage, which can lead to the
production of bloody CSF (20); or
iii) in carcinomatous meningitis, invasion of cancer cells can lead
to disruption of the blood-brain barrier, making it easier for
blood components (including erythrocytes) to enter the CSF, and the
exact mechanism needs to be studied. Therefore, when unexplained
bloody CSF is present, after excluding common bleeding disorders,
it is necessary to learn more about previous tumor history and
improve CSF exfoliative cytology to reduce misdiagnosis and
underdiagnosis.
Current LM treatments offer traditional cancer
treatment modalities, including surgery, radiotherapy, targeted
therapy and intrathecal drug injections (20). Patients with LM receiving
immunosuppressive agents show 1-year survival rates of 7 (MM),
16–24% (breast cancer) and 19% (lung cancer) (21,22).
However, most of the current treatments are palliative, so the
shortcoming of the present study lies in the fact that, due to the
patient refusal of all treatments, the therapeutic aspects of MMMM
with concomitant LM and the presence of bloody CSF are not yet
clear, and further validation is needed in the future.
In summary, when patients present with unexplained
symptoms of a headache and vomiting, there is no abnormality
detected by cranial MRI or CT examination, and treatment effect is
not satisfactory, doctors should be highly vigilant of the
possibility of LM and cancerous meningitis. Additionally, when CSF
shows bloody changes, in addition to considering diseases such as
aneurysmal subarachnoid hemorrhage, cerebral hemorrhage, viral
encephalitis and cerebral amyloid angiopathy-related inflammation,
doctors also need to be alert to the risk of cancerous meningitis.
Therefore, in cases of similar clinical manifestations but no
obvious abnormal results with a past history of tumor, CSF should
be performed and search for primary lesions to improve prognosis of
LM.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contribution
HH conceived the study and critically reviewed the
article. QM and BS conceptualized the study idea and drafted the
manuscript. KC and XS performed data collection. WW and WC made
recommendations for treatment and analyzed data. All authors read
and approved the final manuscript. HH and QM confirmed the
authenticity of all original data.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
The patient's family provided written informed
consent for publication of the study.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Krishnan K: Chapter 1 - Introduction to
Big Data. Data Warehousing in the Age of Big Data. Krishnan K:
Morgan Kaufmann; Boston, MA: pp. 3–14. 2013, View Article : Google Scholar
|
|
2
|
Bier G, Klumpp B, Roder C, Garbe C,
Preibsch H, Ernemann U and Hempel JM: Meningeal enhancement
depicted by magnetic resonance imaging in tumor patients:
Neoplastic meningitis or therapy-related enhancement?
Neuroradiology. 61:775–782. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Gavrilovic IT and Posner JB: Brain
metastases: Epidemiology and pathophysiology. J Neurooncol.
75:5–14. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Fazekas F, Chawluk JB, Alavi A, Hurtig HI
and Zimmerman RA: MR signal abnormalities at 1.5 T in Alzheimer's
dementia and normal aging. AJR Am J Roentgenol. 149:351–356. 1987.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Boire A, Zou Y, Shieh J, Macalinao DG,
Pentsova E and Massagué J: Complement component 3 adapts the
cerebrospinal fluid for leptomeningeal metastasis. Cell.
168:1101–1113.e3. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Nayak L, Fleisher M, Gonzalez-Espinoza R,
Lin O, Panageas K, Reiner A, Liu CM, Deangelis LM and Omuro A: Rare
cell capture technology for the diagnosis of leptomeningeal
metastasis in solid tumors. Neurology. 80:1598–1605; discussion
1603. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Nayar G, Ejikeme T, Chongsathidkiet P,
Elsamadicy AA, Blackwell KL, Clarke JM, Lad SP and Fecci PE:
Leptomeningeal disease: current diagnostic and therapeutic
strategies. Oncotarget. 8:73312–73328. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Le Rhun E, Taillibert S, Boulanger T,
Zairi F, Bonneterre J and Chamberlain MC: Prolonged response and
restoration of functional independence with bevacizumab plus
vinorelbine as third-line treatment for breast cancer-related
leptomeningeal metastases. Case Rep Oncol. 8:72–77. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Cacho-Díaz B, Lorenzana-Mendoza NA,
Chávez-Hernandez JD, González-Aguilar A, Reyes-Soto G and
Herrera-Gómez Á: Clinical manifestations and location of brain
metastases as prognostic markers. Curr Probl Cancer. 43:312–323.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Noh T and Walbert T: Brain metastasis:
clinical manifestations, symptom management, and palliative care.
Handb Clin Neurol. 149:75–88. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Nguyen A, Nguyen A, Dada OT, Desai PD,
Ricci JC, Godbole NB, Pierre K and Lucke-Wold B: Leptomeningeal
metastasis: A review of the pathophysiology, diagnostic
methodology, and therapeutic landscape. Curr Oncol. 30:5906–5931.
2023. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ferguson SD, Bindal S, Bassett RJ, Haydu
LE, Mccutcheon IE, Heimberger AB, Li J, O'Brien BJ, Guha-Thakurta
N, Tetzlaff MT, et al: Predictors of survival in metastatic
melanoma patients with leptomeningeal disease (LMD). J Neurooncol.
142:499–509. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Chatterjee S, Saini J, Kesavadas C,
Arvinda HR, Jolappara M and Gupta AK: Differentiation of tubercular
infection and metastasis presenting as ring enhancing lesion by
diffusion and perfusion magnetic resonance imaging. J Neuroradiol.
37:167–171. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Le Rhun E, Weller M, Brandsma D, Van den
Bent M, de Azambuja E, Henriksson R, Boulanger T, Peters S, Watts
C, Wick W, et al: EANO-ESMO clinical practice guidelines for
diagnosis, treatment and follow-up of patients with leptomeningeal
metastasis from solid tumours. Ann Oncol. 28 (Suppl_4):iv84–iv99.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Su X and Wei D: Four cases of meningeal
metastatic melanoma. Chin J Contemp Neurol Neurosurg. 8:485–486.
2008.(In Chinese).
|
|
16
|
Steininger J, Gellrich FF, Engellandt K,
Meinhardt M, Westphal D, Beissert S, Meier F and Glitza Oliva IC:
Leptomeningeal metastases in melanoma patients: An update on and
future perspectives for diagnosis and treatment. Int J Mol Sci.
24:114432023. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Clarke JL, Perez HR, Jacks LM, Panageas KS
and Deangelis LM: Leptomeningeal metastases in the MRI era.
Neurology. 74:1449–1454. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Gleissner B and Chamberlain MC: Neoplastic
meningitis. Lancet Neurol. 5:443–452. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Corovic A, Kelly S and Markus HS: Cerebral
amyloid angiopathy associated with inflammation: A systematic
review of clinical and imaging features and outcome. Int J Stroke.
13:257–267. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Piña Y, Yadugiri S, Yeboa DN, Ferguson SD,
Forsyth PA and Oliva I: Advances in diagnosis and treatment for
leptomeningeal disease in melanoma. Curr Oncol Rep. 24:43–54. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Lara-Medina F, Crismatt A,
Villarreal-Garza C, Alvarado-Miranda A, Flores-Hernández L,
González-Pinedo M, Gamboa-Vignolle C, Ruiz-González JD and Arrieta
O: Clinical features and prognostic factors in patients with
carcinomatous meningitis secondary to breast cancer. Breast J.
18:233–241. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Morris PG, Reiner AS, Szenberg OR, Clarke
JL, Panageas KS, Perez HR, Kris MG, Chan TA, Deangelis LM and Omuro
AM: Leptomeningeal metastasis from non-small cell lung cancer:
survival and the impact of whole brain radiotherapy. J Thorac
Oncol. 7:382–385. 2012. View Article : Google Scholar : PubMed/NCBI
|