Introduction
Rectal cancer is a severe malignancy that endangers
the lives of patients, which has an incidence rate ranking third in
the world and fifth in China (1,2).
Radiotherapy is recognized as an essential method in the treatment
of rectal cancer (3). It has been
demonstrated that the accuracy of the determined clinical target
volume (CTV) and organs at risk (OARs) when treating patients is
closely related to tumor control and radiation toxicity (4–6), even
beyond the impact of inadequate planning and relative significant
positioning errors (7,8). The specific process of manual
delineation (MD) is composed of the contouring by a junior
physician and the review and revision by a senior physician in the
same group. Therefore, manual contours can vary markedly due to the
differences in the knowledge level and clinical experience among
physicians and the anatomical structure among patients (9–13).
Meanwhile, it is time-consuming to perform MD for pelvic CTV and
OARs, which fills a large proportion of the preparation process
before radiotherapy (9,14).
With the development of computer technology,
automatic delineation (AD) of CTV and OARs for rectal cancer has
been increasingly applied in clinical practice (15–17).
The AD tool was developed with the aid of specific software that
was trained based on a large sample and can complete the AD of
contours for patients in a very short time (18). Meanwhile, the accuracy of AD is
increasing (19), and it is
independent of the body size, shape and age of patients (20). Among various AD tools, convolutional
neural network (CNN)-based tools have been increasingly applied in
clinical practice owing to their higher accuracy, and these
CNN-based tools have significantly improved working efficiency
while ensuring acceptable automatic contours (21–24).
Liu et al (25) evaluated a
deep neural network-based tool for automatic prostate segmentation
based on a large cohort of patient images. The authors made
comparisons between AD and MD using the Dice similarity coefficient
(DSC), Hausdorff distances (HD) and center of mass distances (CMD)
and found that the mean DSC and HD were >0.85 and >7.0,
respectively, and the mean CMD was within 5 mm. Based on these
results, it was concluded that the AD tool used achieved a high
level of accuracy in the contours of the prostate gland compared
with the consensus contours, thus exhibiting a promising
application prospect. Liu et al (26) introduced a CNN-based segmentation
model that could provide accurate AD in much less time compared
with manual contours. The authors also confirmed that the bladder,
bone marrow, left femoral head, right femoral head, rectum, small
intestine and spinal cord in 105 patients with cervical cancer were
delineated by the model. Compared with the corresponding manual
contours set as the reference values, the mean DSC was 0.924,
0.854, 0.906, 0.900, 0.791, 0.833 and 0.827 for the bladder, bone
marrow, femoral head left, femoral head right, rectum, small
intestine and spinal cord, respectively; the mean HD was 5.098,
1.993, 1.390, 1.435, 5.949, 5.281 and 3.269 for the aforementioned
OARs, respectively. The results corroborated that the AD contours
were highly acceptable in clinical practice. To et al
(27) and Breto et al
(28) implemented the CNN-based AD
on MR images for prostate and cervical regions, respectively, which
achieved desirable contours. Bi et al (29) revealed that the tool resulted in a
35% decrease in time spent in the comparison between AD and MD
(median, 9.59 vs. 14.81 min; P<0.001). Men et al
(20) suggested that the test time
for the CNN-based AD of the CTV, bladder, left and right femoral
heads, colon and intestine in rectal cancer was 45 sec per
patient.
The focus of most existing studies is placed on
different CNN-based tools used in prostate and cervical cancer
(25–28). Furthermore, the existing studies on
AD in rectal cancer either addressed one type of patient [such as
Sha et al (30) studied
preoperative radiotherapy patients and Song et al (15) analyzed postoperative patients] or
they do not discuss the efficiency of AD (31). For patients with rectal cancer that
did not require assessment for tumor staging or surgery type, the
accuracy and efficiency of CNN-based AD for CTV and OARs are rarely
analyzed. Therefore, the present study was conducted to identify
whether the commercial CNN-based tool could automatically delineate
both CTV and OARs in rectal cancer with high accuracy and
efficiency.
Materials and methods
Patient cohort
The planning CT images of 148 patients who were
diagnosed with rectal cancer without the distinguishment between
tumor stages and surgery were collected from March, 2021 to June,
2024. The present study was approved by the Ethics Committee of The
Second Affiliated Hospital of Zhengzhou University (Zhengzhou,
China; approval no. 20210302). Written informed consent was
obtained from the patients for the use of their anonymized data in
the present study. All methods were implemented following relevant
guidelines and regulations. The inclusion criteria were as follows:
i) Preoperative and postoperative patients with rectal cancer; ii)
patients who underwent pelvic CT for rectal cancer radiotherapy;
iii) patients who received the examination in a supine position;
iv) planning CT with or without intravenous contrast; and v)
patients who urinated and then drank 500 ml water 30 min before the
CT scan. Patients who had a radiotherapy history and received the
examination in a prone position were excluded from the present
study. The purpose of radiotherapy in the 105 preoperative patients
was to reduce the tumor stage and thus remove the lesions more
completely upon surgery; therefore, the radiotherapy administered
was not aggressive. The purpose of radiotherapy in the 43
postoperative patients was to irradiate subclinical lesions to
reduce the postoperative recurrence rate; therefore, the
radiotherapy administered was also not aggressive in this instance.
Of the 43 postoperative patients, anastomotic fistula occurred in 4
patients, bleeding occurred in 3, bowel obstruction occurred in 2,
urinary retention occurred in 3 and no other postoperative
complications were recorded. All patients were immobilized using a
radiotherapy-specific thermoplastic mold. A summary of the patient
characteristics is shown in Table
I.
 | Table I.Characteristics of the included 148
patients with rectal cancer. |
Table I.
Characteristics of the included 148
patients with rectal cancer.
|
Characteristics | Value |
|---|
| Median age, years
(range) | 61 (32–80) |
| Sex, n (%) |
|
|
Male | 80 (54.1) |
|
Female | 68 (45.9) |
| RT step, n (%) |
|
|
Preoperative RT | 105 (70.9) |
|
Postoperative adjuvant RT | 43 (29.1) |
| Tumor stage, n
(%) |
|
| II | 30 (20.3) |
|
III | 71 (48.0) |
| IV | 47 (31.7) |
| Pathological type,
n (%) |
|
|
Adenocarcinoma | 121 (81.8) |
|
Adenosquamous carcinoma | 10 (6.8) |
|
Undifferentiated
carcinoma | 17 (11.4) |
| Distance from the
anal verge, n (%) |
|
| <5
cm | 84 (56.8) |
| 5–10
cm | 34 (23.0) |
| >10
cm | 30 (20.2) |
The planning CT process was divided into 2 days. The
first day involved individualized customization of the
thermoplastic mold, which was left in place for 24 h to ensure
fixation and repeatability throughout the radiotherapy period. CT
images were acquired on the second day. All patients were scanned
using a Philips Brilliance Big Bore CT (Philips Healthcare). The
scan parameters were as follows: i) Scanned slice thickness, 5 mm;
ii) tube voltage, 120 kV; and iii) reconstructed with a 512×512
voxel matrix. The field of view was adapted to the size of the
patient.
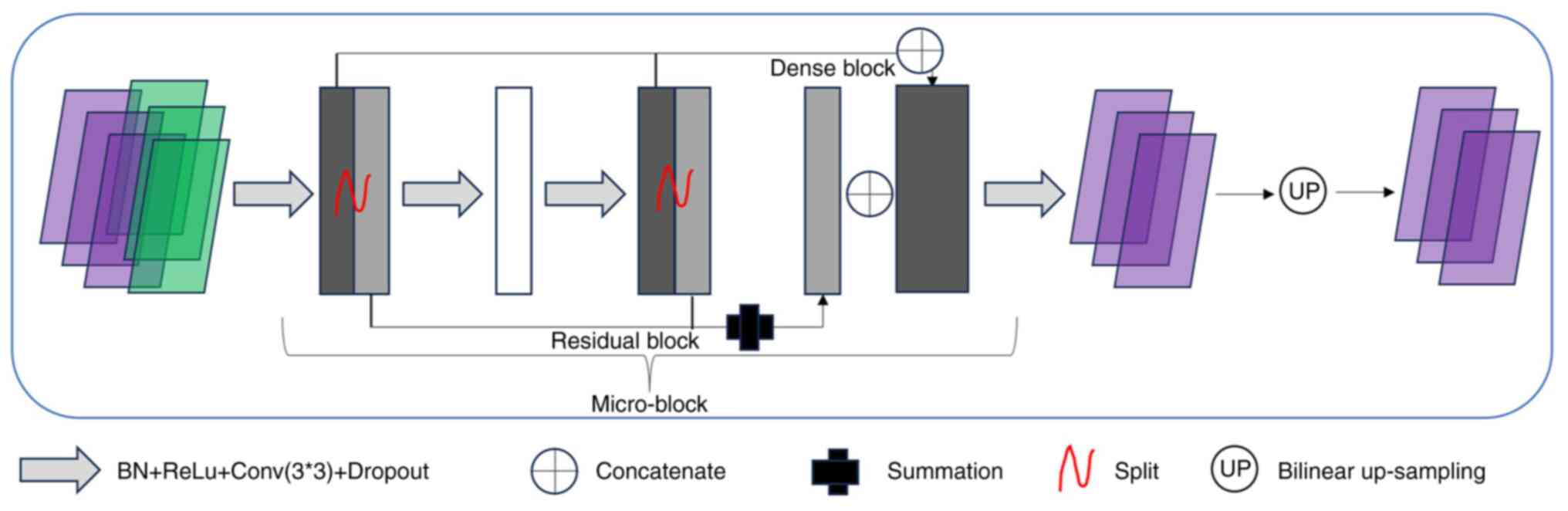
CNN architectures
The CNN architecture used was designed based on the
modified U-Net and termed ‘RT-Mind’ (version 1.1; MedMind
Technology Co., Ltd.) (http://www.medicalmind.cn/). The manufacturer's
description of the tool is as follows: In the basic U-Net, to learn
features from part to whole, the encoder aggregated semantic
information by reducing spatial information. Semantic information
was received by the decoder. Therefore, the feature extraction
ability of the encoder was very important. However, the simple
convolution layer of U-Net experienced difficulty in learning
complicated features efficiently. Therefore, the entire U-net
encoder was replaced by the dual path network (DPN) architecture. A
large number of advanced abstract features and parameters were
encoded into the input image by the DPN. The core of the DPN was
the micro-block, which combined the advantages of both the Residual
block and the Dense block into a dual-path architecture. Since the
Residual block enabled the reuse of features, while the Dense block
enabled the exploration of new features, this combination markedly
improved the representation learning ability. To achieve an
improved feature extraction capability, the whole DPN92
architecture (32) was used in the
U-net encoder. The micro-block was embedded in the decoder to
replace the standard convolution operation, thus enabling the
decoder to obtain the same performance in recovering abstract
features.
A CT slice can be considered a grey-scale image with
only one channel. This results in a 2D model that incorporates each
CT slice independently. The model used in the present study was
designed as a 2.5D architecture by assigning three adjacent slices
to three channels, to obtain the 3D information from the CT images.
The output was the center slice delineation. The output block can
also be modified so that a multi-class segmentation result can be
output. The modified U-Net was a combination of the ResNet block
and the DenseNet block, thus obtaining the ability to focus on a
larger receptive field of the image and to extract more high-level
semantic features for ambiguous boundary segmentation.
The learning, validation and test procedures were
previously completed by the tool manufacturer at Peking Union
Medical College Hospital (Beijing, China) (33,34).
Therefore, only the accuracy and efficiency of its clinical use
were assessed in the present study. Fig. 1 shows the main components of the CNN
architecture.
Contour delineation
Planning CT images were imported into the CNN-based
tool for AD. According to the delineation guidelines of Valentini
et al (35) and the
Radiation Therapy Oncology Group (36), the manual contours for all patients
were re-delineated by a junior physician and then reviewed and
modified together by three oncologists with >10 years collective
experience in radiotherapy for pelvic tumors; these contour were
set as the reference values. More specifically, in terms of the
CTV: i) The high-risk areas of the primary tumor included the tumor
or tumor bed, the rectal mesenteric and presacral areas and the
target area of low to medium rectal cancer, which included the
rectal sciatic fossa; and ii) the regional lymphatic drainage area
included the lymphatic drainage area of the common iliac vessels in
the true pelvis, the rectal mesenteric area, the lymphatic drainage
area of the internal iliac vessels and the closed lymph node area.
The MD OARs included the skin, bladder, left femoral head, right
femoral head, left kidney, right kidney, spinal cord and bowel bag.
The skin was considered the external contour of all CT images. The
delineation range for the bladder was defined inferiorly from its
base and superiorly to the dome. The delineation range for the left
and right femoral heads was defined as the proximal femur
inferiorly from the lowest level of the ischial tuberosities (right
or left) and superiorly to the top of the ball of the femur,
including the trochanters. The kidney was represented by the kidney
parenchyma. The bony inner edge of the spinal canal was defined as
the spinal cord, including all CT slices. A correctly delineated
bowel bag encompassed all the small bowel and colon contours, and
the upper bound was 4–5 CT slices further up in the uppermost layer
of the CTV (36).
AD accuracy
According to the comparative method proposed by
Yeghiazaryan and Voiculescu (37),
DSC, Jaccard coefficient (JAC) and HD were used to compare the
manual and automatic contours quantitatively. In the following
formulae, A and B represent the manual and automatic contour
volumes, respectively.
DSC represents the overlap degree between the
automatic and manual contours in terms of volume. The range of DSC
is 0–1. DSC=0 implies that the two contours do not overlap at all,
and DSC=1 implies that the two contours coincide entirely. The DSC
can be expressed as follows: DSC (A, B)= 2|A∩B||A|+|B|-
JAC represents the ratio of the intersection of the
automatic and manual contours to their combination, in which the
similarity and differences between the two sets of contours are
compared. The range of JAC is 0–1. JAC=0 indicates that the
automatic contours are entirely inconsistent with the manual
contours, and JAC=1 indicates that the automatic contours are
completely consistent with the manual contours. The JAC can be
expressed as follows: JAC A∩BA∪B-
HD can be used to quantify the maximum distance
between the surface of the automatic and manual contours. In
addition, this metric can be employed to quantify the maximum
distance between two contours by calculating the distance between
the closest points in both directions, from contour A to B and vice
versa. The HD can be expressed as follows: HD (A, B)=max [h (A, B),
h (B, A)], where h (A, B) represents the Euclidean distance between
voxels a and b belonging to contours A and B, which can be
expressed as follows, h (A,
B)=maxbεB(minaεA||a-b||).
It is necessary to analyze the variations in AD
accuracy in case of differences in the contour and CT slice numbers
of these patients. The effect of the CT slice number on the AD
accuracy was mainly analyzed based on correlation analyses, while
the effect of the contour number on the AD accuracy was mainly
achieved by comparing DSC, JAC and HD for two groups with different
numbers of contours. To analyze the effect of the contour number on
AD accuracy, the AD contours were divided into two groups. Contours
such as the CTV, spinal cord, left kidney, right kidney, bowel bag,
bladder, left femoral head, right femoral head, left femoral
head-neck and right femoral head-neck were assigned to Group C.
Contours such as the skin, CTV, bladder, left femoral head, right
femoral head, left femoral head-neck, and right femoral head-neck
were assigned to Group D. The accuracy of the identical contours
between the two groups was compared.
AD efficiency
To analyze the AD efficiency, the duration of the AD
and MD were recorded. The MD time included the delineation time of
the junior physicians and the review time of the senior physicians.
The contours included the skin, CTV, bladder, left femoral head,
right femoral head, left kidney, right kidney, spinal cord and
bowel bag. The editing time for the AD contours by the senior
physicians was also recorded.
To evaluate the influence of the CT slice number on
delineation efficiency, the AD time of all 148 patients was
counted, and the correlation between CT slice number and
delineation time was analyzed. Meanwhile, the influence of the
contour number on the AD time was also analyzed. The contour number
was divided based on Group C vs. Group D, as described in the AD
accuracy subsection.
Statistical analysis
Statistical analysis was performed using SPSS 26
(IBM Corp.). The Pearson correlation test was used to analyze the
effects of the CT slice number and contour number on the AD time,
as well as the effects of the CT slice number on the AD accuracy.
The paired t-test was used to compare between Group C to Group D.
The unpaired t-test was used to compare the MD time and the AD +
editing time. P<0.05 was considered to indicate a statistically
significant difference.
Results
Accuracy evaluation of the AD
contours
The DSC, JAC and HD between the manual (reference
values) and the automatic contours are shown in Table II. According to the results, for
CTV the DSC was 0.80±0.06, the JAC was 0.67±0.08 and the HD was
6.96±2.45 mm. For the OARs, the left kidney had the highest DSC
(0.93±0.04) and JAC (0.88±0.07), and the spinal cord had the lowest
HD (2.26±0.82 mm). By contract, the bowel bag had the worst
performance in terms of DSC (0.64±0.12), JAC (0.50±0.14) and HD
(12.84±4.70 mm). The other OARs had a DSC of >0.83±0.07 (left
and right femur), JAC of >0.71±0.10 (left femur) and HD
<3.86±1.66 mm (bladder). These results were also pictorially
shown in Fig. 2. Therefore, minor
modifications (only when needed) of the automatic contours were
required before clinical application, except for the bowel bag.
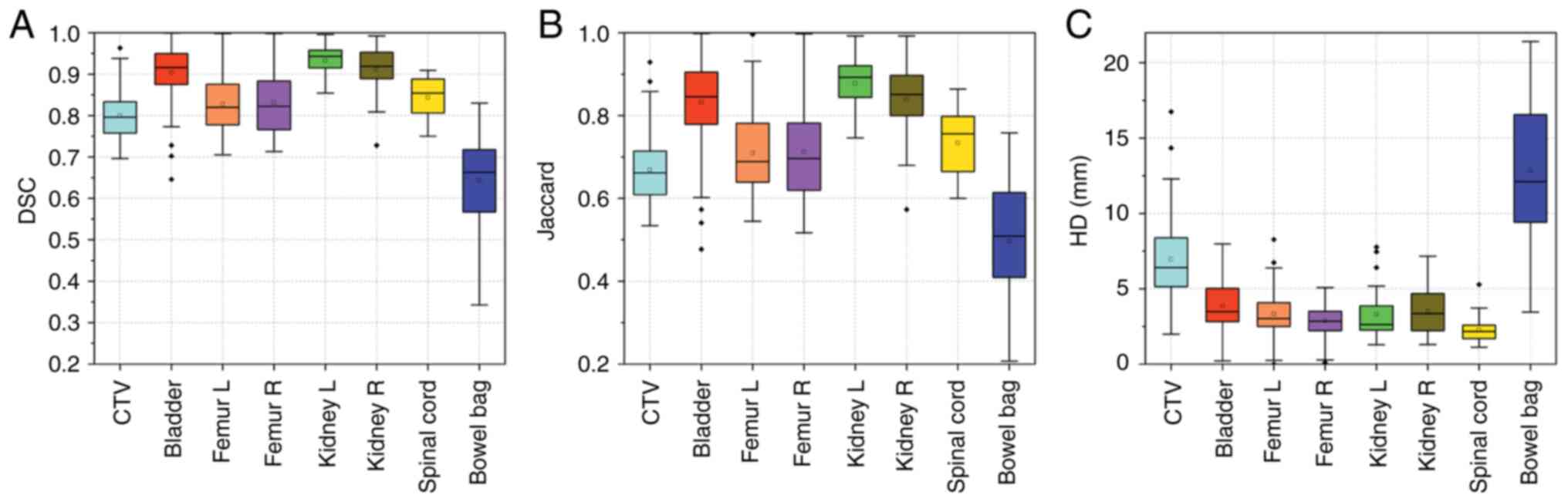 | Figure 2.Box plots of the (A) DSC, (B) JAC and
(C) HD for the CTV, bladder, femur L, femur R, kidney L, kidney R,
spinal cord and bowel bag. CTV, clinical target volume; Dice
similarity coefficient; JAC, Jaccard coefficient; HD, Hausdorff
distance; femur L/R, left/right femoral head. |
 | Table II.DSC, JAC and HD between the automatic
and manual contours and their correlation with the CT slice
number. |
Table II.
DSC, JAC and HD between the automatic
and manual contours and their correlation with the CT slice
number.
|
| CT slice number,
90.68±14.72 |
|---|
|
|
|
|---|
| Contour | Mean DSC ± SD | r, P-value | Mean JAC ± SD | r, P-value | Mean HD ± SD,
mm | r, P-value |
|---|
| CTV | 0.80±0.06 | 0.081, 0.614 | 0.67±0.08 | 0.074, 0.604 | 6.96±2.45 | −0.081, 0.688 |
| Bladder | 0.90±0.06 | −0.143, 0.435 | 0.83±0.10 | −0.147, 0.482 | 3.86±1.66 | 0.184, 0.253 |
| L femur | 0.83±0.07 | −0.265, 0.110 | 0.71±0.10 | −0.299, 0.077 | 3.32±1.32 | 0.174, 0.284 |
| R femur | 0.83±0.07 | −0.327, 0.056 | 0.71±0.11 | −0.284, 0.086 | 2.88±0.98 | 0.175, 0.356 |
| Left kidney | 0.93±0.04 | −0.053, 0.886 | 0.88±0.07 | −0.079, 0.882 | 3.29±1.65 | 0.031, 0.924 |
| Right kidney | 0.91±0.06 | 0.029, 0.952 | 0.84±0.09 | 0.014, 0.980 | 3.52±1.60 | −0.374, 0.255 |
| Spinal cord | 0.84±0.05 | 0.662,
0.027a | 0.73±0.08 | 0.514, 0.091 | 2.26±0.82 | −0.638,
0.019a |
| Bowel bag | 0.64±0.12 | 0.533, 0.087 | 0.50±0.14 | 0.486, 0.111 | 12.84±4.70 | −0.557, 0.185 |
Table II also shows
the correlation between the CT slice number and the AD accuracy
indices. Most AD accuracy indices were not statistically related to
the CT slice number, except for the DSC (r=0.662; P=0.027) and HD
(r=−0.638; P=0.019) of the spinal cord. The effect of the contour
number on the AD accuracy was also collected. The DSC and JAC of
all contours were 1.00 and 1.00, respectively, and the HD of all
contours ranged from 0.000 to 0.002 mm. Therefore, the contour
number had no impact on AD accuracy.
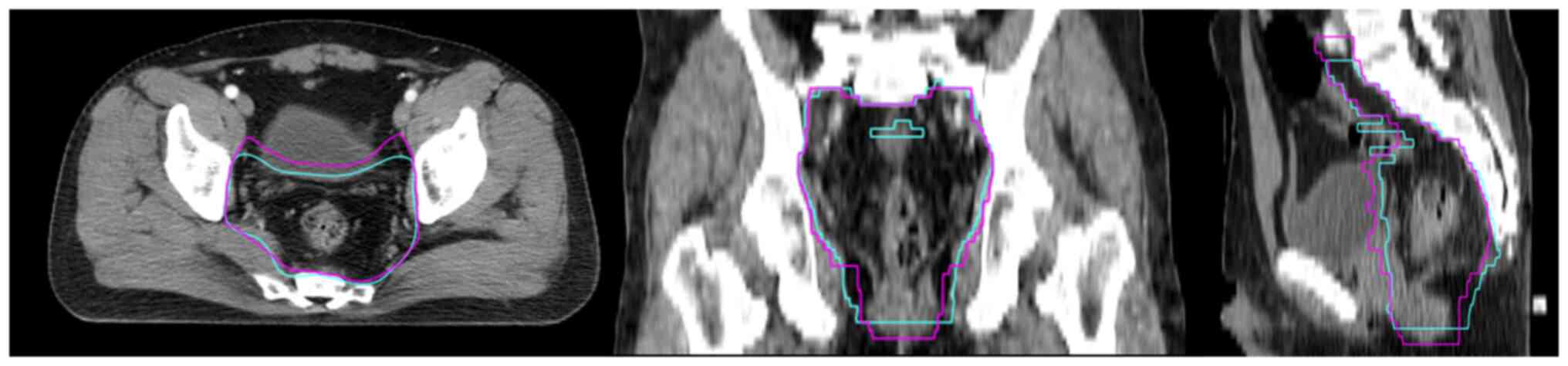
To present the accuracy of AD when determining the
CTV, an example was shown in Fig.
3. The automatic contours (cyan) of the CTV indicated a smaller
volume than the manual contours (pink) and insufficient layers in
the head and foot direction, expect for this, the AD of the CTV was
accurate. Fig. 4A, D and E
illustrate typical examples of the consistency between the
automatic and manual contours of the bladder, left and right
kidneys and spinal cord. A match for the femoral head and the
femoral head-neck is shown in Fig. 4B
and C. In the present study, both the femoral head and the
femoral head-neck were delineated by this AD tool. Hence, the
results showed a good match, regardless of the contour style
selected by physicians. Fig. 4F
shows an example of the bowel bag.
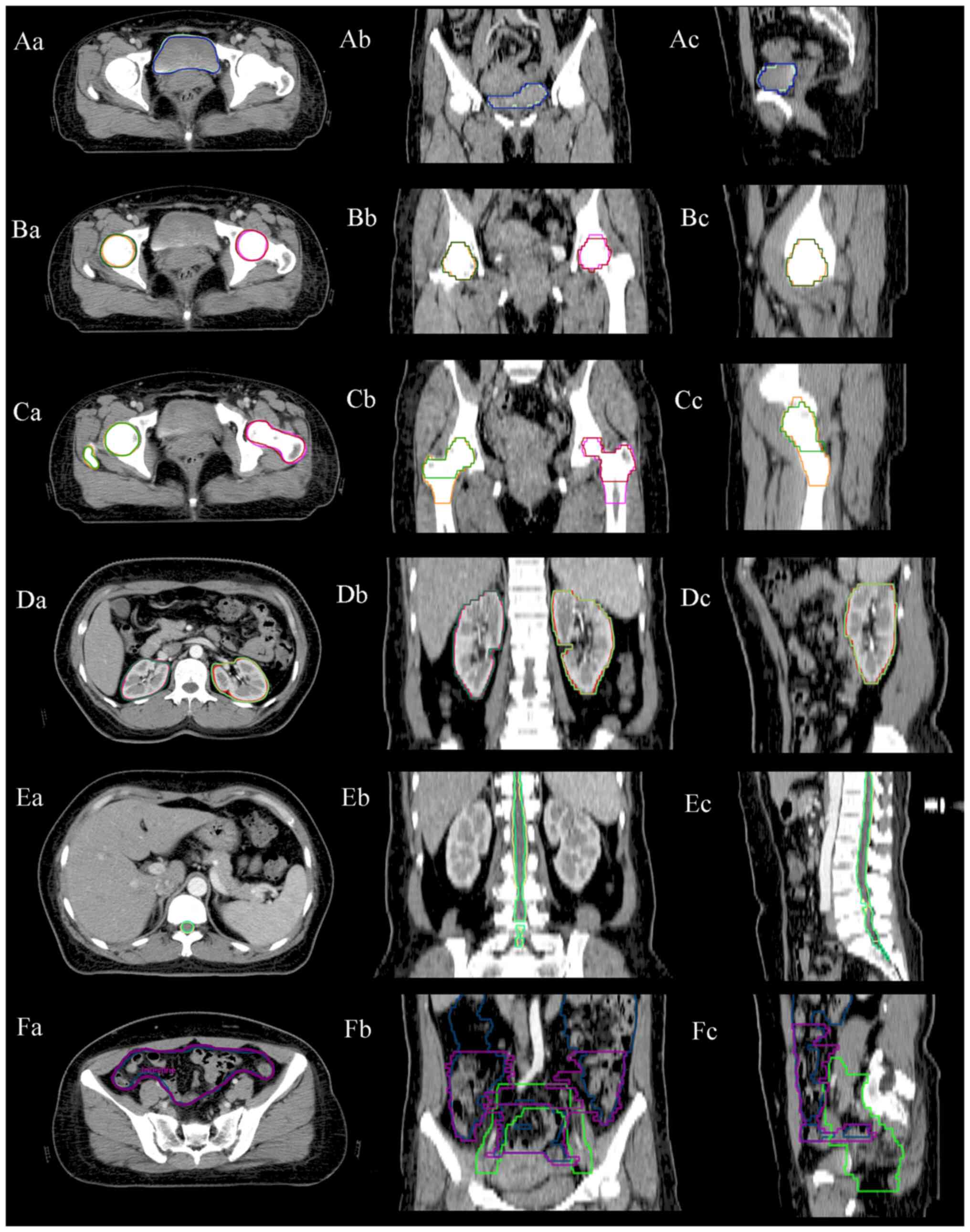 | Figure 4.Examples of AD contours vs. MD
contours for the (A) bladder (AD in green, MD in blue), (B) left
and right femoral heads (AD in yellow and pink, MD in green and
red), (C) femoral head-neck (AD in yellow and pink, MD in green and
red), (D) left and right kidneys (AD in magenta and red, MD in cyan
and green), (E) spinal cord (AD in green, MD in yellow) and (F)
bowel bag (AD in blue, MD in purple, clinical target volume in
green). MD, manual delineation; AD, automatic delineation. |
Evaluation of AD Efficiency
As shown in Table
III, there was a significant difference between the AD +
editing time (sum of delineation and review time) and the MD time
on the same contours in the same group of patients (P<0.001). To
obtain accurate contours, the AD + editing time was 662.97±195.57
sec, while the MD time was 3294.29±824.70 sec. In the AD + editing
process, the editing time had a predominant proportion with
584.57±193.79 sec. The AD efficiency increased by 5 times. The
contours with the most editing time were the bowel bag and CTV.
 | Table III.Duration of AD and MD on the same
contours in the same group of patients. |
Table III.
Duration of AD and MD on the same
contours in the same group of patients.
| Item | Mean ± SD
(range) | t-test | P-value |
|---|
| MD time, sec | 3294.29±824.70
(2100–4920) | 19.832a |
<0.001a |
| AD + editing time,
sec | 662.97±195.57
(246–1172) |
|
|
| AD
time, sec | 78.40±10.32
(65–101) |
|
|
| Editing
time for AD contours, sec | 584.57±193.79
(180–1080) |
|
|
Additionally, there was a significant positive
correlation between the CT slice number and the AD time (r=0.912;
P<0.001), as shown in Table IV.
The lower the CT slice number, the shorter the AD time. When these
contours were applied in Group C, the AD time was 80.83±9.88 sec in
an average CT slice number of 91. When these contours were applied
in Group D, the AD time was 57.96±7.90 sec in an average CT slice
number of 91. Given that Group D had a lower contour number than
Group C, it can therefore be considered that the lower the contour
number, the shorter the AD time. This difference was statistically
significant (P<0.001; Table
V).
 | Table IV.Correlation analysis of the CT slice
number and AD time. |
Table IV.
Correlation analysis of the CT slice
number and AD time.
| Item | Mean ± SD
(range) | r | P-value |
|---|
| CT slice number,
n | 90.68±14.72
(70–135) | 0.912 | <0.001 |
| AD time, sec | 80.83±9.88
(67–102) |
|
|
 | Table V.Comparison of the AD time for both
groups of contour numbers. |
Table V.
Comparison of the AD time for both
groups of contour numbers.
| Item | Mean ± SD
(range) | t | P-value |
|---|
| Group C | 80.83±9.88
(67–102) | 30.221 | <0.001 |
| Group D | 57.96±7.90
(48–89) |
|
|
Discussion
The delineation of the CTV and OARs is essential in
the radiotherapy of rectal cancer. The inaccurate delineation of
the CTV and OARs is one of the primary factors limiting the
feasibility and effectiveness of radiotherapy. At present, contour
delineation is still achieved manually, which may infer variable
accuracy between observers. Most notably, contour delineation may
be the most time-consuming step in radiotherapy (38,39).
As one of the technical solutions of deep learning,
CNN-based tools have been applied increasingly in medical image
analyses. Hence, it is essential to explore the accuracy of the
clinical implementation of these tools. In a previous study, AD and
MD were compared in cervical cancer (40). The results revealed that CNN-based
AD exhibited improved evaluation results. Moreover, the authors
argued that the higher accuracy of their model was attributed to
the large number of training cases, which may improve the accuracy
and robustness of AD in medical centers in developing countries.
Zabihollahy et al (41) also
evaluated the AD of the CTV in cervical cancer. It was found that
this tool achieved a DSC of 0.85±0.03 and a 95th percentile HD of
3.70±0.35 mm in the testing cases, which significantly outperformed
other novel tools (P<0.05). Meanwhile, the tool generated an
uncertainty map using Monte Carlo techniques to draw the attention
of physicians to highly uncertain regions where careful review and
manual editing may be required. Mohammadi et al (42) trained the ResU-Net model based on 72
patients with cervical cancer, which was further verified using 10
patients and tested using 30 patients. The DSC of the testing data
set was 95.7±3.7, 96.6±1.5 and 92.2±3.3% for the bladder, rectum
and sigmoid, respectively, and the HD was 4.05±5.17, 1.96±2.19 and
3.15±2.03 mm, respectively. The average symmetric surface distance
was 1.04±0.97, 0.45±0.09 and 0.79±0.25 mm, respectively, which
achieved a good agreement between the automatic and manual contours
and improved the robustness of AD. The aforementioned results
supported the conclusion that the CNN-based AD tool can provide
accurate contours.
Among the contours in the present study, except for
the bowel bag, the most significant difference was observed in the
CTV. The DSC (0.80±0.06), JAC (0.67±0.08) and HD (6.96±2.45 mm)
were somewhat inferior to those reported in other previous studies.
Ju et al (43) found that
the DSC, JAC and HD of automatic contours of the CTV in cervical
cancer were 0.82, 0.30 and 1.86 mm, respectively. Men et al
(20) obtained an average DSC of
0.877 in the automatic contours of the CTV in rectal cancer. Song
et al (15) concluded that
the DSC of the CTV was 0.88 in rectal cancer. As reported in
certain previous studies (15,20,43),
this evidence may be attributed to the perception and bias among
observers and the difficulty in differentiating the soft tissues of
CTV structures on CT images. It may also be related to the fact
that the AD tool used in the present study did not differentiate
between preoperative and postoperative rectal cancer options, and
the same delineation option was used for all patients. To achieve a
larger sample size and to assess the delineation efficiency of the
tool, both preoperative and postoperative patients were included in
the present study. Although the accuracy of the CTV was somewhat
compromised, it remained reasonably accurate. The tool also
provided criteria that were consistent with the CTV contours of
rectal cancer from leading experts in China (33,34),
which made the editing contours and the subsequent clinical work
more consistent. To visualize the accuracy of automatic CTV
determination, the automatic and manual contours of the CTV of a
single patient were also compared in the present study. The AD
volume was smaller than the MD volume, but the difference was not
notable. In general, necessary modifications are needed after the
completion of AD.
In the present study, except for the bowel bag, the
DSC of all OARs was >0.83±0.07 (left and right femur), with the
bladder reaching 0.90±0.06 and the left and right kidneys reaching
0.93±0.04 and 0.91±0.06, respectively. The JAC was >0.71±0.10
(left femur), particularly for the left kidney, which was
0.88±0.07. The maximum HD was 3.86±1.66 mm for the bladder. The
bladder, left and right kidneys and spinal cord had a higher
accuracy, exhibiting a larger DSC and JAC and a lower HD, which may
be related to there being little inconsistency in the boundary of
these OARs and these OARs exhibiting a notably different density
from the surrounding tissues. The contouring accuracy of physicians
may have also varied, which could be improved by AD tools (29,44,45).
For instance, whether the physicians chose to delineate the femoral
head or the femoral head-neck, the AD tool had a corresponding
contour to match it. The results of the OARs in the present study
were consistent with those reported in previous studies (26,40–42),
which can be regarded as notable support for the high accuracy of
this AD tool in rectal cancer.
In the present study, the effect of the CT slice
number on AD accuracy was also analyzed. It was found that only the
DSC and HD of the spinal cord were correlated with the CT slice
number, and the absolute values of the correlation coefficients
were both >0.6 with statistically significant correlations. This
indicated that the accuracy of the AD of the spinal cord was
strongly correlated with the CT slice number. Based on the fact
that the spinal cord is generally only evaluated for the maximum
dose, physicians delineate all the CT slices with the spinal cord.
This may be related to AD of the spinal cord only having a larger
error at the cauda equina division and a smaller error at other
parts. Thus, the larger the CT slice number, the smaller the
proportion of errors. This suggested that physicians should pay
attention to the spinal cord cauda equina junction in the
modification of the AD of the spinal cord. In the present study,
the AD accuracy was also compared with different contour numbers.
The results demonstrated that the contour number had no impact on
the AD accuracy, proving the robustness of the AD tool.
The most marked difference between the manual and
automatic contours was observed in the bowel bag. The AD of the
bowel bag had the smallest DSC and JAC, the largest HD and the
greatest dispersion of data. However, there were no marked
differences in the number of CT slices in the manual and automatic
contours of the bowel bag. In one case, when there were identical
CT slices between the AD and MD of the bowel bag, the DSC, JAC and
HD were 0.876, 0.780 and 6.764 mm, respectively, which was not
notably different from the accuracy of other AD contours. In our
department, due to the delineation efficiency and dose volume
limits of the bowel bag, physicians only delineated 4–5 CT slices
further up in the uppermost layer of the CTV, while the AD of the
bowel bag appeared in all CT slices where the bowel bag was
present. Hence, the difference in the contour volume naturally
resulted in poor accuracy of the bowel bag. When the dose-volume
parameters for the bowel bag, such as V50 Gy <10%,
were evaluated, the over-delineation of the bowel bag can lower the
evaluation accuracy and may ultimately lead to the emergence of
bowel bag toxicity (46). At
present, there is no option in the AD tool to decide the range of
the bowel bag, and it is suggested that the tool manufacturer
should arrange relevant options regarding the delineation range in
the future.
In the present study, the editing time for AD
contours including CTV and OARs was 584.57±193.79 sec. Although
relatively poor delineation accuracy may be obtained, physicians
can still modify existing contours to increase the delineation
efficiency (15,47). This conclusion was also validated in
the present study. To obtain accurate contours, the MD time of
contours including CTV and OARs was 3294.29±824.70 sec, while the
AD + editing time was 662.97±195.57 sec, and the efficiency
increased 5-fold. Owing to the time-saving advantage, AD may
increase clinical efficiency and reduce the waiting times for
initial treatment. In terms of the advantages of AD, Lustberg et
al (48) found a median time
reduction of 10 min for deep-learning AD. Ginn et al
(45) analyzed an AD tool for head,
neck and pelvic OAR delineation. The time of AD plus the
modification process was less than that of MD alone, with an
average time reduction of 43.4% or 11.8 min per patient. Hu et
al (49) analyzed the
efficiency of a cloud-based solution for AD. Based on the
difference between the average time for AD and MD, the average time
reduction was 291 sec for the male pelvic cavity and 210 sec for
the female pelvic cavity. In the present study, the mean AD time
was 78.40±10.32 sec when CTV, OARs (including skin, spinal cord,
left and right kidneys, duodenum, bowel bag, intestinal tube,
bladder, left and right femoral heads and left and right femoral
head-neck), and an average CT slice number of 91 were selected,
which was much shorter than the time (1,560-2,880 sec) for the MD
of the pelvic CTV (9) and the MD
time (3,294.29±824.70 sec) for delineating the CTV, bladder, left
and right femoral heads, left and right kidneys, spinal cord and
bowel bag in the present study. To explore the factors affecting
the MD efficiency, the CT slice number and the contour number were
further analyzed. The CT slice number displayed a significant
positive correlation with AD time (r=0.912; P<0.001), indicating
that as the CT slice number decreased, the AD time also decreased.
Different contour numbers also significantly affected the AD time
(P<0.001), indicating that as the contour number decreased, the
AD time also decreased.
However, there are several limitations to the
present study. First, the present study did not assess the
relationship between accurate delineation and treatment effect, and
this will be analyzed in our future work. Second, the present study
lacks data on recurrence and surgical cure rates in postoperative
patients. Third, there is a lack of localization training for the
AD tool, increasing the inaccuracy of the automatic contours
compared with manual contours.
In summary, the present study demonstrated through
quantitative analyses that the CNN-based AD tool could provide a
certain degree of clinically acceptable CTV and OARs for patients
diagnosed with rectal cancer in whom tumor stage and type of
surgery were not differentiated. Additionally, the AD tool may
provide a valuable starting point for manual editing, which will
significantly accelerate the contour delineation process. In the
present study, it was also found that only the AD accuracy of the
spinal cord had a positive correlation with the CT slice number,
and the AD accuracy of the bowel bag was not high due to the
absence of limits to the delineation range. Moreover, reducing the
CT slice number and contour number may improve AD efficiency. In
conclusion, it is suggested that the CNN-based AD tool should be
used in radiotherapy centers, particularly in those centers with
good economic conditions, to improve the accuracy and efficiency of
contouring.
Acknowledgements
Not applicable.
Funding
This work was supported by Henan Province Science and Technology
Development Project (grant no. 232102310091) and Henan Province
Medical Science and Technology Project (grant no.
LHGJ20210407).
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
ZL, YH and RS contributed to the conception and
design of the study. YH performed the majority of the experiments,
as well as the statistical analysis, and drafted the initial
manuscript. TQ and MY were responsible for data analysis. ZL
revised the final version of the manuscript. YH, RS, TQ, MY and ZL
confirm the authenticity of all the raw data. All authors have read
and approved the final manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of The Second Affiliated Hospital of Zhengzhou University
(Zhengzhou, China; approval no. 20210302). Written informed consent
was obtained from the patients for the use of their anonymized data
in the present study.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Siegel RL, Miller KD, Fuchs HE and Jemal
A: Cancer statistics, 2022. CA Cancer J Clin. 72:7–33. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Cao W, Chen HD, Yu YW, Li N and Chen WQ:
Changing profiles of cancer burden worldwide and in China: A
secondary analysis of the global cancer statistics 2020. Chin Med J
(Engl). 134:783–791. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Hanna CR, Slevin F, Appelt A, Beavon M,
Adams R, Arthur C, Beasley M, Duffton A, Gilbert A, Gollins S, et
al: Intensity-modulated radiotherapy for rectal cancer in the UK in
2020. Clin Oncol (R Coll Radiol). 33:214–223. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Chen AM, Chin R, Beron P, Yoshizaki T,
Mikaeilian AG and Cao M: Inadequate target volume delineation and
local-regional recurrence after intensity-modulated radiotherapy
for human papillomavirus-positive oropharynx cancer. Radiother
Oncol. 123:412–418. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Walker GV, Awan M, Tao R, Koay EJ,
Boehling NS, Grant JD, Sittig DF, Gunn GB, Garden AS, Phan J, et
al: Prospective randomized double-blind study of atlas-based
organ-at-risk autosegmentation-assisted radiation planning in head
and neck cancer. Radiother Oncol. 112:321–325. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Rezaeijo SM, Jafarpoor Nesheli S, Fatan
Serj M and Tahmasebi Birgani MJ: Segmentation of the prostate, its
zones, anterior fibromuscular stroma, and urethra on the MRIs and
multimodality image fusion using U-Net model. Quant Imaging Med
Surg. 12:4786–4804. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Voet PWJ, Dirkx MLP, Teguh DN, Hoogeman
MS, Levendag PC and Heijmen BJM: Does atlas-based autosegmentation
of neck levels require subsequent manual contour editing to avoid
risk of severe target underdosage? A dosimetric analysis. Radiother
Oncol. 98:373–377. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Daisne JF and Blumhofer A: Atlas-based
automatic segmentation of head and neck organs at risk and nodal
target volumes: A clinical validation. Radiat Oncol. 8:1542013.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ma CY, Zhou JY, Xu XT, Guo J, Han MF, Gao
YZ, Du H, Stahl JN and Maltz JS: Deep learning-based
auto-segmentation of clinical target volumes for radiotherapy
treatment of cervical cancer. J Appl Clin Med Phys. 23:e134702022.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Marin T, Zhuo Y, Lahoud RM, Tian F, Ma X,
Xing F, Moteabbed M, Liu X, Grogg K, Shusharina N, et al: Deep
learning-based GTV contouring modeling inter- and intra-observer
variability in sarcomas. Radiother Oncol. 167:269–276. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Casati M, Piffer S, Calusi S, Marrazzo L,
Simontacchi G, Di Cataldo V, Greto D, Desideri I, Vernaleone M,
Francolini G, et al: Clinical validation of an automatic
atlas-based segmentation tool for male pelvis CT images. J Appl
Clin Med Phys. 23:e135072022. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Vinod SK, Min M, Jameson MG and Holloway
LC: A review of interventions to reduce inter-observer variability
in volume delineation in radiation oncology. J Med Imaging Radiat
Oncol. 60:393–406. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wang Z, Chang Y, Peng Z, Lv Y, Shi W, Wang
F, Pei X and Xu XG: Evaluation of deep learning-based
auto-segmentation algorithms for delineating clinical target volume
and organs at risk involving data for 125 cervical cancer patients.
J Appl Clin Med Phys. 21:272–279. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Young AV, Wortham A, Wernick I, Evans A
and Ennis RD: Atlas-based segmentation improves consistency and
decreases time required for contouring postoperative endometrial
cancer nodal volumes. Int J Radiat Oncol Biol Phys. 79:943–947.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Song Y, Hu J, Wu Q, Xu F, Nie S, Zhao Y,
Bai S and Yi Z: Automatic delineation of the clinical target volume
and organs at risk by deep learning for rectal cancer postoperative
radiotherapy. Radiother Oncol. 145:186–192. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Chen W, Wang C, Zhan W, Jia Y, Ruan F, Qiu
L, Yang S and Li Y: A comparative study of auto-contouring
softwares in delineation of organs at risk in lung cancer and
rectal cancer. Sci Rep. 11:230022021. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Piqeur F, Hupkens BJP, Nordkamp S, Witte
MG, Meijnen P, Ceha HM, Berbee M, Dieters M, Heyman S, Valdman A,
et al: Development of a consensus-based delineation guideline for
locally recurrent rectal cancer. Radiother Oncol. 177:214–221.
2022. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Mackay K, Bernstein D, Glocker B,
Kamnitsas K and Taylor A: A review of the metrics used to assess
auto-contouring systems in radiotherapy. Clin Oncol (R Coll
Radiol). 35:354–369. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Li Y, Wu W, Sun Y, Yu D, Zhang Y, Wang L,
Wang Y, Zhang X and Lu Y: The clinical evaluation of atlas-based
auto-segmentation for automatic contouring during cervical cancer
radiotherapy. Front Oncol. 12:9450532022. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Men K, Dai J and Li Y: Automatic
segmentation of the clinical target volume and organs at risk in
the planning CT for rectal cancer using deep dilated convolutional
neural networks. Med Phys. 44:6377–6389. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ronneberger O, Fischer P and Brox T:
U-net: Convolutional networks for biomedical image segmentation:
Medical Image Computing and Computer-Assisted Intervention-MICCAI
2015: 18th International Conference, Munich, Germany, October 5–9,
2015, Proceedings Part III. Springer International Publishing;
Cham: pp. 234–241. 2015
|
|
22
|
Shen G, Jin X, Sun C and Li Q: Artificial
intelligence radiotherapy planning: Automatic segmentation of human
organs in CT images based on a modified convolutional neural
network. Front Public Health. 10:8131352022. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Luan S, Xue X, Ding Y, Wei W and Zhu B:
Adaptive attention convolutional neural network for liver tumor
segmentation. Front Oncol. 11:6808072021. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wu Y, Kang K, Han C, Wang S, Chen Q, Chen
Y, Zhang F and Liu Z: A blind randomized validated convolutional
neural network for auto-segmentation of clinical target volume in
rectal cancer patients receiving neoadjuvant radiotherapy. Cancer
Med. 11:166–175. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Liu C, Gardner SJ, Wen N, Elshaikh MA,
Siddiqui F, Movsas B and Chetty IJ: Automatic segmentation of the
prostate on CT images using deep neural networks (DNN). Int J
Radiat Oncol Biol Phys. 104:924–932. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Liu Z, Liu X, Xiao B, Wang S, Miao Z, Sun
Y and Zhang F: Segmentation of organs-at-risk in cervical cancer CT
images with a convolutional neural network. Phys Med. 69:184–191.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
To MNN, Vu DQ, Turkbey B, Choyke PL and
Kwak JT: Deep dense multi-path neural network for prostate
segmentation in magnetic resonance imaging. Int J Comput Assist
Radiol Surg. 13:1687–1696. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Breto A, Zavala-Romero O, Asher D,
Baikovitz J, Ford J, Stoyanova R and Portelance L: A deep learning
pipeline for per-fraction automatic segmentation of GTV and OAR in
cervical cancer. Int J Radiat Oncol Biol Phys. 105 (Suppl
1):S2022019. View Article : Google Scholar
|
|
29
|
Bi N, Wang J, Zhang T, Chen X, Xia W, Miao
J, Xu K, Wu L, Fan Q, Wang L, et al: Deep learning improved
clinical target volume contouring quality and efficiency for
postoperative radiation therapy in non-small cell lung cancer.
Front Oncol. 9:11922019. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Sha X, Wang H, Sha H, Xie L, Zhou Q, Zhang
W and Yin Y: Clinical target volume and organs at risk segmentation
for rectal cancer radiotherapy using the Flex U-Net network. Front
Oncol. 13:11724242023. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Li J, Song Y, Wu Y, Liang L, Li G and Bai
S: Clinical evaluation on automatic segmentation results of
convolutional neural networks in rectal cancer radiotherapy. Front
Oncol. 13:11583152023. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Chen Y, Li J, Xiao H, Jin X, Yan S and
Feng J: Dual path networks. Adv Neural Inf Process Syst.
30:2017.PubMed/NCBI
|
|
33
|
Liu Z, Liu X, Guan H, Zhen H, Sun Y, Chen
Q, Chen Y, Wang S and Qiu J: Development and validation of a deep
learning algorithm for auto-delineation of clinical target volume
and organs at risk in cervical cancer radiotherapy. Radiother
Oncol. 153:172–179. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Liu Z, Liu F, Chen W, Liu X, Hou X, Shen
J, Guan H, Zhen H, Wang S, Chen Q, et al: Automatic segmentation of
clinical target volumes for post-modified radical mastectomy
radiotherapy using convolutional neural networks. Front Oncol.
10:5813472021. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Valentini V, Gambacorta MA, Barbaro B,
Chiloiro G, Coco C, Das P, Fanfani F, Joye I, Kachnic L, Maingon P,
et al: International consensus guidelines on clinical target volume
delineation in rectal cancer. Radiother Oncol. 120:195–201. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Gay HA, Barthold HJ, O'Meara E, Bosch WR,
El Naqa I, Al-Lozi R, Rosenthal SA, Lawton C, Lee WR, Sandler H, et
al: Pelvic normal tissue contouring guidelines for radiation
therapy: A radiation therapy oncology group consensus panel atlas.
Int J Radiat Oncol Biol Phys. 83:e353–e362. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Yeghiazaryan V and Voiculescu I: Family of
boundary overlap metrics for the evaluation of medical image
segmentation. J Med Imaging (Bellingham). 5:0150062018.PubMed/NCBI
|
|
38
|
Geets X, Daisne JF, Arcangeli S, Coche E,
De Poel M, Duprez T, Nardella G and Grégoire V: Inter-observer
variability in the delineation of pharyngo-laryngeal tumor and
parotid glands and cervical spinal cord: Comparison between CT-scan
and MRI. Radiother Oncol. 77:25–31. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Brouwer CL, Steenbakkers RJHM, Bourhis J,
Budach W, Grau C, Grégoire V, Van Herk M, Lee A, Maingon P, Nutting
C, et al: CT-based delineation of organs at risk in the head and
neck region: DAHANCA, EORTC, GORTEC, HKNPCSG, NCIC CTG, NCRI, NRG
oncology and TROG consensus guidelines. Radiother Oncol. 117:83–90.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Rhee DJ, Jhingran A, Rigaud B, Netherton
T, Cardenas CE, Zhang L, Vedam S, Kry S, Brock KK, Shaw W, et al:
Automatic contouring system for cervical cancer using convolutional
neural networks. Med Phys. 47:5648–5658. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Zabihollahy F, Viswanathan AN, Schmidt EJ
and Lee J: Fully automated segmentation of clinical target volume
in cervical cancer from magnetic resonance imaging with
convolutional neural network. J Appl Clin Med Phys. 23:e137252022.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Mohammadi R, Shokatian I, Salehi M, Arabi
H, Shiri I and Zaidi H: Deep learning-based auto-segmentation of
organs at risk in high-dose rate brachytherapy of cervical cancer.
Radiother Oncol. 159:231–240. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Ju Z, Guo W, Gu S, Zhou J, Yang W, Cong X,
Dai X, Quan H, Liu J, Qu B and Liu G: CT based automatic clinical
target volume delineation using a dense-fully connected convolution
network for cervical Cancer radiation therapy. BMC Cancer.
21:2432021. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Chan JW, Kearney V, Haaf S, Wu S, Bogdanov
M, Reddick M, Dixit N, Sudhyadhom A, Chen J, Yom SS and Solberg TD:
A convolutional neural network algorithm for automatic segmentation
of head and neck organs at risk using deep lifelong learning. Med
Phys. 46:2204–2213. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Ginn JS, Gay HA, Hilliard J, Shah J,
Mistry N, Möhler C, Hugo GD and Hao Y: A clinical and time savings
evaluation of a deep learning automatic contouring algorithm. Med
Dosim. 48:55–60. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Åström LM, Behrens CP, Calmels L, Sjöström
D, Geertsen P, Mouritsen LS, Serup-Hansen E, Lindberg H and Sibolt
P: Online adaptive radiotherapy of urinary bladder cancer with full
re-optimization to the anatomy of the day: Initial experience and
dosimetric benefits. Radiother Oncol. 171:37–42. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
D'Aviero A, Re A, Catucci F, Piccari D,
Votta C, Piro D, Piras A, Di Dio C, Iezzi M, Preziosi F, et al:
Clinical validation of a deep-learning segmentation software in
head neck: An early analysis in a developing radiation oncology
center. Int J Environ Res Public Health. 19:90572022. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Lustberg T, van Soest J, Gooding M,
Peressutti D, Aljabar P, van der Stoep J, van Elmpt W and Dekker A:
Clinical evaluation of atlas and deep learning based automatic
contouring for lung cancer. Radiother Oncol. 126:312–317. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Hu Y, Nguyen H, Smith C, Chen T, Byrne M,
Archibald-Heeren B, Rijken J and Aland T: Clinical assessment of a
novel machine-learning automated contouring tool for radiotherapy
planning. J Appl Clin Med Phys. 24:e139492023. View Article : Google Scholar : PubMed/NCBI
|