Introduction
Globally, the burden of liver diseases results in 2
million deaths annually (1). These
diseases include liver cancer, viral liver disease, cirrhosis,
non-alcoholic fatty liver disease (NAFLD), AFLD and drug-induced
liver injury (DILI). Hepatocellular carcinoma (HCC), which accounts
for a significant proportion of liver diseases, is the sixth most
common and the fourth most lethal type of all cancers worldwide
(2). Despite advancements in modern
medicine, such as surgical resection, local ablation, percutaneous
intervention and liver transplantation, the highly invasive and
metastatic nature of HCC complicates complete surgical resection
(3,4). Simultaneously, the current first-line
chemotherapeutic drug, sorafenib, which is used for the treatment
of HCC, encounters challenges related to drug resistance (5), which significantly contributes to
recurrence following treatment (6,7).
Cirrhosis, a prevalent chronic liver disease that can lead to
death, can be caused by various factors, such as viral hepatitis
and fatty liver disease. Inflammatory stimulation causes the liver
to develop fibrotic lesions, which eventually deform and harden.
Viral infections, particularly hepatitis B and C, can result in
severe liver damage, cirrhosis and liver cancer. Hepatitis B virus
(HBV) and HCV infections are responsible for 75% of liver cancer
cases. Improvements in socioeconomic conditions and lifestyle
changes have increased the rates of obesity and alcohol
consumption, which contributes to the rising prevalence of fatty
liver disease (8,9). Fatty liver disease is characterised
into AFLD and NAFLD and is one of the most prevalent liver diseases
worldwide, which is accompanied by complex clinical symptoms, such
as certain metabolic-related syndromes (10). Therefore, effective biomarkers,
therapeutic targets, and screening strategies are necessary for the
early diagnosis, treatment and prognosis of liver diseases,
including cancer.
In previous years, the discovery of effective
biomarkers has significantly contributed to the early diagnosis and
treatment of liver diseases (11).
Among these biomarkers, microRNA (miRNA/miR)-22, which has been
proved by numerous studies to be associated with liver cancer and
affect the occurrence and development of liver cancer, has
attracted considerable attention (12–14).
miR-22 belongs to the miRNA class, comprising short non-coding RNAs
of 17–25 nucleotides that can serve an important regulatory role in
tumour development (15). They
inhibit target gene expression by targeting the 3′ untranslated
region (3′-UTR) of genes (16).
miR-22 exists in two functional forms, miR-22-3p (or miR-22) and
miR-22-5p (or miR-22β). miR-22-3p serves as a functional guide
strand and regulates its target by being complementary, whereas
miR-22-5p is generally regarded as a transient strand that is
easily degraded (17). miR-22-3p,
as a tumour suppressor, inhibits the development of various types
of cancers, including breast (18),
non-small-cell lung (19), gastric
(20) and colorectal cancers
(21). The role of miR-22 in the
liver has attracted research attention. A clinical study reported
lower miR-22 expression levels in patients with HCC compared with
healthy controls, which suggests a potential role as a tumour
suppressor in HCC (22).
Furthermore, miR-22 serves a unique role in fatty liver disease and
liver fibrosis (23,24). miR-22 can also reflect abnormal
glucose-lipid and alcohol metabolism in the liver, which may
contribute to future research on the detection and prevention of
liver disease. In addition, miR-22 has been linked to liver
lesions, such as liver cancer, fatty liver disease and liver
fibrosis (25–27). miR-22-3p has been shown to inhibit
the development of various liver diseases, while miR-22-5p acts as
an early diagnostic biomarker for acute myocardial infarction
(28,29).
However, reports on the role of miR-22 in liver
diseases are currently limited and the underlying molecular
mechanism of action has not been comprehensively elucidated.
Herein, the mechanism of action of miR-22 in the progression of
liver lesions was comprehensively and systematically reviewed in
the present manuscript and covered numerous stages of liver lesion
development such as fatty liver, liver fibrosis and liver
cancer.
miR-22 and liver cancer
MIR22HG, the host gene of human miR-22, belongs to
the long non-coding RNA (lncRNA) family located on chromosome
17p13.3 (30), and is involved in
regulating bio-signalling in multiple types of cancer cells;
therefore, MIR22HG can also act as a tumour suppressor gene that
inhibits the development of various types of cancer (31–34).
Conversely, MIR22HG can act as a tumour promoter in oesophageal
adenocarcinoma and glioblastoma (34), which highlights its complex
biological functions. With recent advancements in liver cancer
research, the role of miR-22 as an inhibitor of liver cancer has
received attention (35). In the
following sections, the molecular mechanisms of HCC development,
early diagnosis and prognosis will be reviewed to provide insights
into the role and effect of MIR22HG and related molecules in
HCC.
Mechanisms by which miR-22 regulates
HCC development
As a tumour suppressor, miR-22 regulates the
expression of tumour-related factors via multiple pathways, which
can inhibit liver cancer occurrence and development (36–38).
Conversely, miR-22 deletion promotes the occurrence and development
of tumours in vivo (39).
The expression levels of miR-22 are significantly downregulated in
HCC and show a gradual decrease with the continuous progression of
cancer stages (13,39,40).
Multiple mechanisms associated with miR-22 have been reported to be
involved in the occurrence and development of liver cancer
(40,41).
miR-22 inhibits the proliferation,
migration and invasion of HCC cells and promotes apoptosis
Zhang et al (35) reported that miR-22 overexpression in
HCC tissues significantly reduced histone deacetylase 4 (HDAC4)
expression, thereby inhibiting the proliferation of Hep3B and
SMMC7721 HCC cells. This inhibitory effect was confirmed both in
vivo and in vitro. The aforementioned study also
reported that miR-22-3p and MIR22HG were co-expressed and exhibited
a synergistic function. In HCC, MIR22HG functions as a competitive
endogenous RNA (ceRNA), which regulates miRNA-10a-5p/nuclear
receptor corepressor 2 to inhibit the Wnt/β-catenin signalling
pathway, thereby inhibiting the growth, invasion and migration of
HCC cells (42). Simultaneously,
miR-22-3p derived from MIR22HG interacts with human antigen R to
reduce β-catenin expression and target high mobility group box 1
(HMGB1) to inhibit HCC cell migration and invasion (43). Similarly, Luo et al (44) reported that miR-22 overexpression
reduced CD147 expression, inhibiting HCC migration and invasion.
Casitas B-lineage lymphoma (CBL) is a direct target of miR-22-3p
and the ubiquitin protein ligase (E3) of sprouty2 (SPRY2). By
inhibiting CBL expression, miR-22-3p can reduce SPRY2
ubiquitination and indirectly upregulate SPRY2 expression, thereby
inhibiting ERK signal transduction. This hindrance of the MAPK/ERK
signalling process inhibits the epithelial-mesenchymal transition,
migration and invasion of HCC cells, which contributes to cancer
inhibition (17,45).
However, the function of miR-22 in liver cancer is
negatively regulated by lncRNAs. lncRNAs comprise >200
nucleotides and are the most common type of non-protein-coding
transcripts (46). lncRNAs can act
as ceRNA, sequestering specific miRNAs from their target genes,
which reduces the abundance of their target miRNAs and inhibits
their stability and function. Multiple ceRNAs have a sponging
effect on miR-22 and influence its regulation of proliferation and
apoptosis in liver tumour cells, weakening or reversing the
tumour-inhibitory effect of miR-22 (47). Huang et al (37) reported that lncRNA DSCR8, a ceRNA of
miR-22-3p, prevented miR-22-3p from binding to the target
actin-related protein 2/3 complex subunit 5 (ARPC5) through
sponging, thereby failing to reduce the inhibitory effect of ARPC5
on tumour cell apoptosis, which ultimately promoted liver cancer
progression. Additionally, muskelin 1 antisense RNA (MKLN1-AS)
contributes to the growth and development of HCC cells (48). Considering these findings, Pan et
al (49) conducted RNA
immunoprecipitation (RIP) and reported that MKLN1-AS and miR-22-3p
were enriched in the anti-argonaute 2 group compared with those in
the anti-IgG group. miR-22-3p overexpression can directly
downregulate ETS proto-oncogene 1 (ETS1) in HuH7 and LM3 cells to
regulate protein levels related to cell proliferation, apoptosis
and migration. MKLN1-AS sponges miR-22-3p, indirectly upregulates
ETS1 expression, induces cell growth, angiogenesis, migration and
invasion and promotes the occurrence and development of HCC. ETS1
also regulates MKLN1-AS expression. ETS1 knockdown has been
reported to reduce the expression level of MKLN1-AS, whereas its
overexpression increases MKLN1-AS levels. ETS1 was reported to bind
to the MKLN1-AS promoter site 3.
The NCK adaptor protein 1 antisense RNA 1 (NCK1-AS1)
gene is typically found in the cytoplasm and serves a role in
processes that occur after genetic transcription (50). When NCK1-AS1 is suppressed, the
expression levels of miR-22-3p are elevated, which increases the
number of cells undergoing apoptosis; conversely, when miR-22-3p is
silenced, the increase in the number of apoptotic cells caused by
NCK1-AS1 knockdown is reversed and the proliferation, migration and
invasion abilities of certain cells are also affected (51). This suggests an inverse relationship
between NCK1-AS1 and miR-22-3p in liver cancer tissues (52). The presence of NCK1-AS1 in HCC
tissues is linked to increased levels of tyrosyl-tRNA synthetase
(YARS) expression, while miR-22-3p decreases the levels of YARS.
Therefore, NCK1-AS1 acts as a sponge by binding to miR-22-3p to
increase YARS expression levels, thus suppressing tumour cell
apoptosis and promoting cell growth and migration. Additionally,
suppressing YARS impedes the activation of key members of the
PI3K/AKT pathway (PI3K, AKT, ERK and mTOR), thereby hindering cell
proliferation (53). Therefore,
when positively regulating YARS through the miR-22-3p/YARS axis,
NCK1-AS1 can activate PI3K/AKT signalling to promote HCC
progression. However, silencing NCK1-AS1 or overexpressing
miR-22-3p can reverse this process and serve a role in liver cancer
inhibition (52).
Zhao et al (47) reported that myocardial
infarction-associated transcript (MIAT), an aging-related lncRNA
involved in HCC, was upregulated in human HCC and served a role in
tumour promotion. The expression level of MIAT decreases during
cell aging, whereas overexpression of MIAT can inhibit cell aging
and hinder tumour cell apoptosis. MIAT acts as a ceRNA by binding
specifically to miR-22-3p, inhibiting miR-22 expression and
increasing sirtuin 1 (SIRT1) expression, which is a direct target
of miR-22. This inhibits tumour suppressor pathways p53/p21 and
p16/pRb, which in turn, inhibit the production of
senescence-associated secretory phenotype and cell senescence,
promoting tumour cell proliferation and inhibiting apoptosis.
Overexpression of miR-22-3p significantly decreases SIRT1 levels.
Therefore, specific binding of MIAT and miR-22-3p can inhibit the
senescence phenotype and promote HCC progression by upregulating
SIRT1. Overexpression of miR-22-3p can reverse this phenomenon and
promote cell senescence in the human fibroblast cell lines 2BS,
IMR-90 and MRC-5. Conversely, miR-22-3p downregulation prevents the
progression of cell senescence and improves senescent cells.
miR-22 can not only be inhibited by the above
lncRNAs, but also induced by vitamin D3, bile acids and the
following exogenous substances. The positive regulation of butyrate
on miR-22 can inhibit the expression of SIRT1 and subsequently
promote the expression of PTEN and GSK-3, and promote the
accumulation of ROS, which not only reduces the expression of
phosphorylated (p)-AKT and β-catenin, but also releases cytochrome
C to promote cell apoptosis and serve an anti-cancer role (54). Catalpol is an exogenous substance
similar to butyrate. After being induced by catalpol, miR-22-3p is
negatively regulated by targeting metastasis associated 1 family
member 3 (MTA3), thus inhibiting the promotion of MTA3 on the
proliferation, migration and invasion of HCC cells (41). In addition, chenodeoxycholic acid
can effectively activate the bile acid receptor farnesol X receptor
(FXR) in Huh7 and HCT116 cells. FXR binds to the IR1 motif upstream
of miR-22 and induces the expression of miR-22 in Huh7 liver cells,
thus reducing the expression level of cyclin A2 (CCNA2) mRNA, and
the negative correlation of miR-22 and CCNA2 is similarly
demonstrated in the clinical data (36). Similarly, miR-22 mimics increased
the percentage of Huh7 and HCT116 cells in G0/G1 phase and
decreased the percentage of cells in S and G2 phases, which
indicated that miR-22 can inhibit the proliferation of tumour cells
by interfering with the cell cycle (36). In addition, waltonitone can be used
as an upstream regulatory substance to participate in the
FXR-miR-22-CCNA2 pathway to inhibit the occurrence and development
of liver cancer, and this potent correlation between waltonitone's
efficacy and the pathway-mediated inhibition of tumor proliferation
has been shown through analysis clinical tissue samples in the Gene
Expression Omnibus database (55).
In summary, miR-22 can inhibit cell growth or signal
transduction of cancer-promoting pathways by reducing the
expression level of proteins such as CD147, HDAC4, CBL and HMGB1 to
inhibit the proliferation, migration and invasion of HCC cells.
However, the expression levels of miR-22 can be inhibited by
various lncRNA such as DSCR8, NCK1-AS1, MKLN1-AS and MIAT, which
prevents miR-22 from exerting its anti-cancer effects. miR-22 can
also be induced by FXR, butyrate and catalpol, which can limit the
proliferation, migration and invasion of liver cancer cells by
affecting the normal progression of the cell cycle. Therefore,
miR-22 serves an indispensable role in the regulation of liver
cancer growth and has the potential to be used as an effective
target for future liver cancer treatment.
Immunomodulatory function of miR-22 in
HCC
The occurrence and development of tumours is closely
linked to immune regulation. Immune cells and related factors, such
as tumour-related macrophages, lymphocytes and mast cells, serve a
crucial role in initiating the body's anti-tumour response through
direct killing, antigen presentation and immune response
activation; immune cells also influence the metabolism of tumor
cells (56). This forms the basis
for regulating the body's immune system and maintaining the balance
of the tumour microenvironment (57). Dysregulation of T cells and their
effector lymphocytes can lead to immune escape by tumour cells
(58).
miR-22 is involved in the immunomodulatory effects
observed in HCC. T helper 17 (Th17) cells, a type of
CD4+ helper T cell that produce IL-17, are controlled by
the transcription factor retinoic acid receptor-related orphan
receptor γt (RORγT), an isomer of the RAR-related orphan receptor C
(RORC) (59). Zhang et al
(40) reported that when injected
into the subcutaneous tumours of mice with HCC, miR-22 expression
in T cells and tumour cells significantly increased, thereby
decreasing tumour size and weight. miR-22 overexpression inhibits
tumour growth by promoting the transformation of CD4+ T
cells into Th17 cells, while jumonji AT-rich interacting domain
containing 2 (JARID2) hinders the production of Th17 cells. miR-22
inhibits JARID2 expression in T cells by directly targeting the
JARID2 3′-UTR, which weakens JARID2-mediated inhibition. This aids
in maintaining the normal differentiation of Th17 cells and
regulating tumour cell apoptosis.
Hypoxic and hypoxia-inducible factor 1 alpha (HIF1α)
induces resistance in tumour cells to cytotoxic T cells (60). miR-22 silences HIF1α, which reduces
its signal transduction capacities and the resistance of tumour
cells and serves a tumour suppressive role. miR-22 also exerts an
anti-HCC effect by reducing the recruitment of HIF1α/RORγT/STAT3 to
the IL-17 promoter, which inhibits IL-17 signalling in T cells.
However, HIF1α reduction can also reduce the binding and expression
of RORC and IL-17 and HIF1α/RORγT recruitment to IL-17a.
Conversely, miR-22 inhibits the IL-23/IL-6/STAT3 signalling
pathway, reduces the recruitment of STAT3 to IL-17a and inhibits
the expression level of IL-17. Therefore, treating with miR-22 can
reduce the abundance of IL-17-producing T cells, inhibit the
IL-17-induced inflammatory response and activate cytotoxic T cells
to exert anti-HCC effects (13).
Additionally, regulatory T cells (Tregs) have immunosuppressive
functions in multiple cancers and miR-22 can inhibit tumour immune
evasion by limiting Treg expansion and activating anti-tumour
effector cells (61,62). In conclusion, miR-22 serves an
important role in the immune regulation of HCC and influences the
occurrence and development of HCC by regulating immune
processes.
In liver cancer, galectin-9 (Gal-9) induces
lymphocyte apoptosis and the immune escape of tumour cells, and
Tim-3 is an important inhibitory receptor in the tumour
microenvironment; Gal-9 induces apoptosis of HCC cells in the
absence of Tim-3 (63,64). A previous study reported the
involvement of Gal-9 in tumour immune escape by inducing
tumour-specific Tim3+ T cell death (65). Yang et al (66) reported that Gal-9 was significantly
elevated in human hepatoma cells, particularly in HepG2 cells, when
compared with normal hepatocytes. The aforementioned study also
used flow cytometry and a WST-1 assay to report that Gal-9 promoted
lymphocyte apoptosis and aided tumour cells in evading the immune
system. Additionally, HepG2 cells with higher Gal-9 expression
levels have higher proliferation capacity than negative control
cells. However, overexpression of miR-22 inhibited the expression
of Gal-9 and its interaction with Tim-3, thus reducing lymphocyte
apoptosis which partly restored the function of effector T cells
and regulated the immune response to the tumour, in turn,
decreasing tumour cell proliferation and immune evasion. Ubiquitin
ligase E4B (UBE4B), a novel E3 protein, belongs to the U-box family
of ubiquitin ligases. Shao et al (67) constructed a ceRNA network using
bioinformatics data analysis and showed that UBE4B was a
pro-tumourigenic protein crucial for HCC development. UBE4B acts
through the UBE4B-hsa-miR-22-3p-FGD5-AS1/LINC00858/SNHG16 axis to
regulate immune processes with a pro-carcinogenic role in HCC
development, which leads to poor prognosis and tumour immune
infiltration in HCC.
Role of miR-22 in hepatitis
virus-associated HCC
Viral hepatitis is primarily caused by viral
infections that lead to liver lesions, which is a major contributor
to liver disease progression. HBV and HCV infections significantly
increase the risk of HCC, and chronic viral infections can lead to
liver cirrhosis, which may ultimately progress to HCC (68). Research on miR-22 in hepatitis
virus-induced liver disease has focused mainly on the regulation of
HCC (69,70). Therefore, the difference between
changes in miR-22 expression levels in hepatitis virus-induced
liver cancer and other types liver cancer is of interest. Shi and
Xu (71) reported that in
HBV-associated HCC cells, such as HepG2.2.15, miR-22 expression
showed a more significant downward trend compared with that in
HepG2 cells, a trend also observed in miR-22 expression in clinical
specimens. Furthermore, CDK inhibitor 1A expression was
significantly reduced in HCC cells transfected with miR-22 compared
with that in control cells, which suggests that miR-22 serves an
inhibitory role by interfering with the normal tumour cycle.
Additionally, miR-22 transfection significantly inhibits hepatitis
B surface antigen (HBsAg) and hepatitis B e-antigen (HBeAg)
expression. Although experimental results suggest that miR-22 can
strongly inhibit HBV gene expression, the specific mechanism by
which this occurs warrants further investigation.
Ke et al (70) reported that heterogeneous nuclear
ribonucleoprotein A 1 (HNRNPA1) expression was significantly
elevated in HBV-positive HCC samples and correlated with a poor
prognosis in patients with HCC. HNRNPA1 expression was
significantly upregulated, while miR-22 expression was
significantly downregulated in HCC cells compared with that in
normal hepatocyte cell lines; however, and the difference between
HBV-positive HCC cells and normal cells was more obvious. miR-22
overexpression resulted in suppressed HNRNPA1 expression and EGFR
signalling pathway activity. However, HBV-negative HCC cells were
not used as a control group for this procedure; hence, the effects
of miR-22 on HBV-negative HCC cells warrant further study.
The FOXO3a protein can reduce the invasiveness of
HCC cells by blocking the WNT/β-catenin pathway and regulating
proteins associated with lymph node metastasis (72,73).
Chen et al (38) reported
that p-FOXO3a can be moved from the nucleus to the cytoplasm by
p-AKT, which reduces its activity through ubiquitination and
phosphorylation. Conversely, miR-22 can counteract the interference
of p-AKT on FOXO3a by inhibiting YWHAZ-mediated AKT
phosphorylation. This allows FOXO3a to maintain its
tumour-suppressing role in the nucleus.
In addition, using an anti-Ago2 RIP assay, Song
et al (14) reported that
translation regulatory lncRNA 1 (TRERNA1) induced by hepatitis B
virus-encoded X (HBx), acts as a sponge for miR-22-3p to regulate
NRAS proto-oncogene (NRAS) expression. During tumour formation,
TRERNA1 competes with miR-22-3p, thereby elevating NRAS expression
levels and HCC cell proliferation by eliminating the
NRAS/Raf/MEK/ERK pathway. Conversely, TRERNA1 knock down lowered
NRAS expression levels, which were restored following treatment
with an miR-22-3p inhibitor. This suggests that in the absence of
TRERNA1 sponging, miR-22-3p inhibits the activating effect of NRAS
on the NRAS/Raf/MEK/ERK pathway, which ultimately inhibits HCC
progression by hindering cell proliferation. Furthermore, the
upregulation of TRERNA1 by HBx contributes to sorafenib resistance
in HCC cells.
The miR-22/estrogen receptor (ER)α/IL-1α/IL-6
pathway is linked to liver tumours induced by HBV. IL-1α is
increased during liver cell death caused by ROS. This increase
stimulates Kupffer cells to increase the expression levels of IL-6,
which leads to the compensatory growth of damaged liver tissue and
the formation of tumours (74,75).
In HCC associated with HBV, oestrogen, in combination with ERα, may
inhibit IL-6 and IL-1α to protect the liver (76). Additionally, miR-22 can hinder the
production of ERα by directly targeting its 3′-UTR region, thereby
impeding its influence downstream (76,77);
Chronic hepatitis infections, especially with HBV, increases
oestrogen production in women, which suppresses the expression of
IL-1α in normal liver cells. Conversely, lower oestrogen levels and
higher miR-22 expression levels in men downregulate ERα, which
results in increased IL-1α expression and HCC development (76). Chen et al (78) treated HCC cells with IFN-γ to
replicate the environment of hepatitis virus-associated HCC and
reported that IFN-γ-induced Gal-9 expression in HCC cells was
positively correlated with enhancer of zeste homolog 2 (EZH2)
expression, which was significantly upregulated in both a
concentration- and time-dependent manner. Data set analysis,
quantitative PCR and western blotting showed that EZH2 inhibited
miR-22 transcription and promoted Gal-9 expression in a DNA
hypermethylation-independent manner. Consequently, EZH2′s effect on
GAL-9 is indirect, achieved through epigenetic repression of miR-22
(Fig. 1).
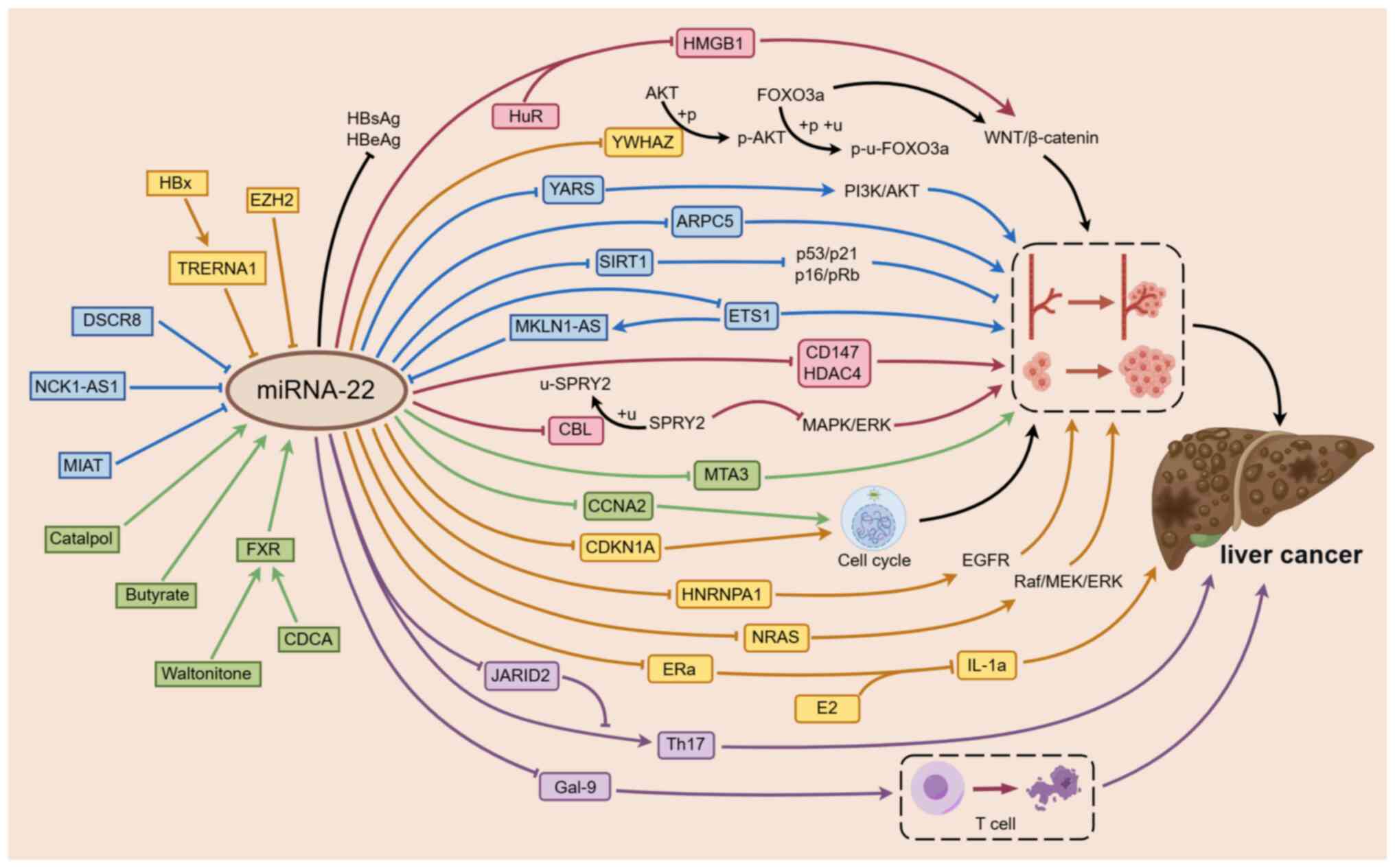 | Figure 1.Mechanism underlying miR-22
regulation of the occurrence and development of HCC. miR-22 is
involved in the regulation of the occurrence and development of
HCC. miR-22 mainly inhibits the proliferation, migration and
invasion of HCC by decreasing the expression levels of CD147 and
HDAC4 and participating in the immunomodulatory process by
regulating Gal-9 and Th17, which ultimately serves a role in cancer
inhibition. lncRNAs, such as DSCR8, NCK1-AS1 and MKLN1-AS, exhibit
sponging effects on miR-22, thereby weakening the inhibitory effect
on downstream cancer-promoting factors ARPC5, YARS and ETS1. In
hepatitis virus-associated liver cancer, the negative regulation of
miR-22 by TRERNA1 and EZH2 also weakens the inhibitory effect on
HNRNPA1 and NRAS, which promotes the occurrence and development of
liver cancer. Exogenous substances and metabolites, such as
catalpol, butyrate and FXR, induce the expression of miR-22, which
enhances the regulation of miR-22 on downstream factors MTA3 and
CCNA2 and inhibits the progression of HCC. An arrow-headed line
indicates promotion, whereas a bar-headed line signifies
inhibition. The rectangular box represents the upstream component
of miR-22, and the circular corner box represents the downstream
component regulated by miR-22. Yellow represents the mechanism of
hepatitis virus-associated liver cancer, blue represents the
mechanism related to lncRNA, green represents the mechanism related
to the induction of miR-22 by endogenous and exogenous substances,
purple represents the mechanism related to immunity and pink
represents the mechanism of miR-22 regulating the proliferation and
migration of liver cancer without the influence of other
substances. The dashed line box on the right shows the
proliferation and migration of liver cancer cells and the dashed
line box below shows the lysis and death of Tim3+ T cells. EZH2,
enhancer of zeste homolog 2; TRERNA1, translation regulatory lncRNA
1; HBx, HBV-encoded X; DSCR8, down syndrome critical region 8;
NCK1-AS1, NCK1 antisense RNA 1; MIAT, myocardial
infarction-associated transcript; FXR, farnesoid X receptor; CDCA,
chenodeoxycholic acid; HuR, human antigen R; HMGB1, high mobility
group box 1; YWHAZ, tyrosine 3-monooxygenase/tryptophan
5-monooxygenase activation protein zeta; YARS, tyrosyl-tRNA
synthetase; ARPC5, actin-related protein 2/3 complex subunit 5;
SIRT1, sirtuin 1; ROS, reactive oxygen species; MKLN1-AS, muskelin
1 antisense RNA; ETS1, ETS proto-oncogene 1; CD147; HDAC4, histone
deacetylase 4; CBL, casitas B-lineage lymphoma; MTA3, metastasis
associated 1 family member 3; CCNA2, cyclin A2; CDKN1A, CDK
inhibitor 1A; HNRNPA1, heterogeneous nuclear ribonucleoprotein A 1;
NRAS proto-oncogene; ERα, estrogen receptor α; E2, estradiol;
IL-1α, interleukin 1α; JARID2, jumonji AT rich interacting domain
containing 2; Th17, T helper cell 17; Gal-9, galectin-9. |
In conclusion, miR-22 may exhibit similar effects in
hepatitis virus-associated HCC and other forms of HCC. However, it
remains unclear why the level of miR-22 expression is much lower in
patients with hepatitis virus-associated HCC and the differences in
molecular mechanisms compared with non-hepatitis virus-induced HCC.
Further research on whether and how the hepatitis virus affects the
function of miR-22 in liver cancer is needed to explore these
specific regulatory mechanisms.
Role of miR-22 in enhancing the
therapeutic sensitivity of liver cancer cells
Due to the rise in resistance to the primary
chemotherapy drug sorafenib, many patients with HCC who depend on
sorafenib for their survival are unable to receive effective
treatment and face a poor prognosis (79). This necessitates further research
focussed on improving drug sensitivity in tumour cells and
identifying new anti-tumour targets.
Cheng et al (80) reported that the levels of reactive
oxygen species (ROS) and the redox state of sorafenib-resistant
cells are inhibited, which creates a protective state for tumour
cells, and SIRT1 can inhibit ROS production by regulating the
expression of cellular antioxidant genes, thereby reducing tumour
cell sensitivity to chemotherapeutic drugs. Lowering SIRT1
expression when treating HCC using chemotherapeutic drugs can help
induce tumour cell death (81).
Pant et al (54) reported
the impact of SIRT1 inhibition on ROS release using
2′,7′-dichlorofluorescein diacetate and showed that both butyrate
and miR-22 triggered ROS release and miR-22 suppressed SIRT1
expression. However, when cells were co-incubated with butyrate and
anti-miR-22, intracellular ROS production was significantly
reduced, which suggests that miR-22 may enhance drug sensitivity by
increasing tumour cell ROS levels, thereby promoting their death.
miR-22-5p can enhance the radiosensitivity of HCC by increasing
histone acetylation in the MIR22HG promoter region via radiolytic
inhibition of HDAC2 activity (82).
These findings suggest that miR-22 may increase the sensitivity of
liver tumour cells to chemotherapy and radiotherapy, and to some
extent, reverse their resistance to sorafenib and thus could
potentially serve as a target for anti-tumour treatments.
Macrocytic anaemia is common in HCC and a previous
animal study showed that lenvatinib, a first-line drug for liver
cancer, may exacerbate anaemia, whereas miR-22 does not have this
side effect (83). Instead, miR-22
increases white blood cell and platelet counts and could extend
survival time (13).
Notably, the expression of miR-22 is lower in liver
cancer induced by the hepatitis virus, and miR-22 can strongly
inhibit the expression of the HBsAg and HBeAg. In addition, the
regulation of miR-22 by HBx upregulating TRERNA1 expression
promotes the resistance of HCC cells to sorafenib (14). Therefore, the exploration of the
mechanism of action of miR-22 in viral hepatitis may be useful for
the study of liver cancer treatment and drug resistance. Combining
the diverse molecular mechanisms of miR-22 regulation in liver
cancer and the regulation of the miR-22 pathway at the clinical
level, in addition to actively exploring targets with similar
biological effects may counteract the side effects and drug
resistance observed with radiotherapy and chemotherapy treatments
at present, and provide reference for the treatment and remission
of liver cancer in the future.
Early diagnosis and prognosis of
miR-22 in liver cancer
Alpha fetoprotein (AFP) is an important marker in
diagnosing and predicting the outcome of HCC as it presents at high
levels in the serum of patients with liver cancer. However, AFP
levels can also be elevated in pregnant women and patients with
germ cell tumours (84). Therefore,
relying solely on AFP for early HCC diagnosis is not highly
specific or sensitive and is not recommended as a primary
diagnostic method. Combining ultrasound with AFP can improve the
rate of early liver cancer diagnosis (85), but some cases may still go
undetected, which may lead to clinical diagnostic issues (86). A previous study reported that the
expression of miR-22 was not only decreased in liver cancer
tissues, but that the difference in its serum expression levels
were also diagnostically significant (22). Zekri et al (87) demonstrated the detection methods
combining miRNA markers such as miR-22, miR-885-5p, miR-221 and
miR-122 in conjunction with AFP could be used to accurately
diagnose HCC in patients with liver cirrhosis. This combination is
particularly useful for the early diagnosis of HCC in patients with
liver cirrhosis. The expression levels of miR-199-3p and miR-22 are
significantly decreased in HCC and chronic hepatitis C infections.
The aforementioned miRNAs, in addition to AFP, could potentially be
used to assess the severity of chronic HCV infection and aid in
diagnosing HCC resulting from HCV development.
Additionally, miR-122, miR-22, miR-99a and miR-125b
expression levels are reported to be significantly higher in the
serum of HBV patients compared with those in healthy individuals.
The expression levels are also significantly linked to HBV DNA
levels (88). In chronic hepatitis
B (CHB) infection, miR-22, when combined with miR-210 and ALT, can
predict the virological and non-virological responses following
IFN-α treatment, which is an antiviral agent used to treat CHB.
This may be used to determine the efficacy of IFN-α treatment and
reduce its adverse effects and complications (89). Furthermore, baseline serum exosomal
miR-22-3p levels can forecast HBeAg seroconversion in patients with
CHB undergoing Peg-IFN treatment (90).
Zhang et al (35) reported a positive correlation
between miR-22 expression and the overall survival and disease-free
survival in patients with HCC using bioinformatics methods, such as
Kaplan-Meier analysis. Patients with HCC and normal or relatively
high miR-22 expression had a better prognosis, which was consistent
with the findings of another previous study (40). Chen et al (38) demonstrated that miR-22 was not only
a predictor of prognosis but could also be used as an independent
predictor of overall survival in patients with HCC. Therefore,
assessing miR-22 expression levels in HCC tissues could be used to
predict the prognosis of patients with HCC.
In summary, miR-22 could be used in the diagnosis
and prognostic assessment of liver cancer, particularly for
high-risk groups, such as patients with hepatitis virus infection
and liver cirrhosis. As miR-22 can be used as an independent
predictor of overall patient survival, a scientific detection and
diagnosis system and a prediction model of liver cancer prognosis
should be established to facilitate improved prognosis and survival
for patients with liver cancer.
miR-22 in fatty liver diseases
The liver serves a crucial role in lipid metabolism
and various metabolic disorders can arise if liver function is
disrupted (91). When the liver is
in a diseased state, it can lead to chronic metabolism-related
conditions, such as fatty liver disease, which includes AFLD and
NAFLD (92). miR-22 is involved in
the development of both types of fatty liver disease (93,94).
Alcoholic fatty liver diseases are closely linked to excessive
alcohol consumption, which disrupts hepatic lipid metabolism
pathways and leads to liver damage. miR-22 inhibitors can be used
to help improve alcohol-induced steatosis (95). NAFLD, is associated with certain
metabolic disorders, including obesity and diabetes mellitus, and
is characterized by the accumulation of fat and steatosis in the
liver (96). Additionally,
excessive glycogen accumulation has been identified as a key factor
in the development of liver malignant transformation, as reported
by Liu et al (97). The
progression from NAFLD to HCC is primarily caused by the excessive
accumulation of fat in the liver. This leads to abnormal signalling
pathways, which results in liver cell injury and chronic
inflammation. This process significantly increases the risk of
developing liver fibrosis and HCC (98). In the case of NAFLD, inhibitors of
miR-22 improve hepatic steatosis and reduce fat build-up in the
liver by regulating factors involved in fatty acid metabolism
(94). Therefore, miR-22 inhibitors
show promise as potential future therapeutic agents for managing
hepatic steatosis in fatty liver disease.
AFLD
Excessive alcohol consumption can lead to a
condition known as alcoholic liver disease (ALD), which causes
injury to the liver. In alcohol metabolism, by-products can have
toxic effects on the liver, which can ultimately result in ALD
(99). This initially presents as
alcoholic steatosis, which can progress to steatohepatitis, hepatic
fibrosis, cirrhosis and has the potential to develop into HCC. ALD
is closely linked to hepatic steatosis, with alcohol affecting
hepatic lipid metabolism by altering how the liver takes up lipids,
synthesizes lipids, oxidizes fatty acids, exports lipids, forms
lipid droplets and undergoes catabolism (100). A previous study reported a
positive relationship between β-catenin and miR-22 expression
levels. Inhibition of β-catenin activity decreases miR-22
expression (93). Chronic alcohol
intake activates β-catenin and increases the expression levels of
miR-22-3p, which in turn, inhibits tet methylcytosine dioxygenase 2
(TET2) and promotes HCC stemness and metastasis. In HCC, TET2
expression is reduced and alcohol exposure further increases
miR-22-3p expression levels, which leads to a decrease in TET2
expression. This promotes tumour growth and metastasis in HCC
cells. Therefore, the β-catenin/miR-22-3p/TET2 axis serves a role
in alcohol-induced HCC malignant progression.
The fibroblast growth factor 21 (FGF21) signalling
pathway is responsible for maintaining liver metabolic balance
(101) and utilizes FGF21 receptor
(FGFR1) as its receptor. FGFR1 deficiency diminishes FGF21
signalling in adipocytes, therefore FGF21 and FGFR1 are the primary
targets and regulators of certain metabolic diseases. Key
transcription factors for liver FGF21 are hepatic PPAR-activated
receptor-c coactivator-1α (PGC1α) and peroxisome
proliferator-activated receptor α (PPARα). In cases of fatty liver,
the expression levels of miR-22, FGF21, FGFR1 and PGC1α are
inversely correlated. Hu et al (95) reported that the expression levels of
FGFR1 and FGF21 decreased in Huh7 cells after treatment with
miR-22, which indicates the presence of a relationship between
miR-22 and FGF21 signal transduction. The study also reported that
miR-22 could directly target and reduce FGFR1. Additionally, miR-22
reduces the expression levels of FGF21 by reducing the regulation
of transcription factors PPARα and PGC1α, thereby limiting the
activation of ERK 1/2 and promoting fat accumulation. Inhibiting
miR-22 increases FGF21 and FGFR1 levels in the liver, which
strengthens the FGF21 signal transduction pathway in the liver
leading to the activation of AMPK and ERK1/2, thus promoting lipid
metabolism in alcoholic fatty liver and improving alcohol-induced
steatosis. Therefore, miR-22 inhibitors can be used to increase
FGF21 and FGFR1 levels and treat liver steatosis. In addition, the
miR-22 inhibitor was as effective as obeticholic acid in treating
steatosis and reducing the accumulation of liver fat. Combined
treatment with the two drugs significantly improves insulin
sensitivity, releases glucagon-like peptide 1 and reduces liver
triglycerides in obese mice.
Summarily, the efficacy of miR-22 in improving
alcohol-induced steatosis has been previously reported. Studies
have shown that mice injected with anti-miR-873-5p have relatively
high SIRT1 activity in their liver, which can delay the progress of
alcoholic liver disease by enhancing the activity of SIRT1
deacetylase (102). Iwagami et
al (103) reported that SIRT1
may be a key contributing factor for the actions of miR-34a to
reverse alcoholic fatty liver. In view of the complicated mechanism
of regulation of SIRT1 by miR-22 in liver cancer, investigating
whether miR-22 can improve AFLD by regulating SIRT1 is particularly
important. Although studies on miR-22 and alcoholic fatty liver are
currently scarce, the close relationship between miR-22 and
alcoholic fatty liver cannot be disregarded. In future, research in
this field will enable the understanding of the specific regulatory
mechanism of miR-22 in alcoholic fatty liver.
NAFLD
NAFLD is a clinicopathological syndrome
characterized by the accumulation of fat in the liver (104). Patients with NAFLD show liver
manifestations of metabolic syndrome, including fatty degeneration
of the liver observed using imaging techniques and histology
(105). Several experimental
studies have demonstrated the role of miR-22 in NAFLD (23,94,106).
Yang et al (94) investigated the genes involved in
regulating fat metabolism by miR-22. The authors induced obesity in
a mouse model using a high-fat diet (HFD) and treated a normal
human liver cell line with free fatty acids to stimulate fat
accumulation in liver cells. The study reported increased
expression levels of miR-22 in the obese mouse model and human
liver cells exposed to fatty acids and overexpressing miR-22
resulted in fat accumulation in the liver cells. PPARα and Sirt1
are involved in fatty acid metabolism and miR-22 interacts with
Sirt1 to participate in liver fat metabolism (25,94).
In the fatty acid-induced human hepatocyte line, L02, miR-22
expression levels were increased and Sirt1 expression levels were
decreased compared with the non-induced L02 cells. Sequence
analysis showed that miR-22 can directly interact with the 3′-UTR
of Sirt1 to regulate lipid metabolism and their expression levels
were negatively correlated. miR-22 analogues significantly reduced
the expression levels of PPARα and FOXO1, while miR-22 inhibitors
notably increased their expression levels. This suggests that
miR-22 serves a role in regulating a series of downstream genes
related to fatty acid metabolism. miR-22 inhibitors enhance the
expression of genes related to fatty acid metabolism, which reduces
lipid accumulation in the liver. Therefore, in the HFD-induced
mouse model, the upregulation of miR-22 expression is involved in
regulating lipid metabolism, energy balance and obesity. Decreasing
expression of the miR-22 gene can increase energy consumption and
disrupt lipid biosynthesis.
Thibonnier and Esau (107) reported that when the miR-22
antagonist APT-110 was introduced into human subcutaneous
preadipocytes, important factors for metabolism, such as
mitochondrial activity, uncoupling protein 1 expression and energy
expenditure, were increased. Additionally, after subcutaneous
injection of miR-22-3p antagonist APT-110 into the groin of mice
fed a HFD, a notable increase in metabolic and lipolysis rates,
accompanied by significant decreases in blood sugar, plasma insulin
and leptin levels were reported. Administration of APT-110 resulted
in a significant reduction in the overall weight gain and average
liver fat content in HFD mice compared with the control group
(saline injection). Inhibiting miR-22-3p led to a reduction in
genes related to the fatty acid biosynthesis pathway in the liver,
while generally increasing genes associated with fatty acid
metabolism in inguinal fat, thereby reducing liver fat accumulation
(108). These findings suggest
miR-22 inhibitors may be a promising new future approach for
controlling obesity and fatty degeneration of hepatocytes.
Panella et al (23) developed a mouse model with a miR-22
transgene controlled by Cre recombinase and reported that mice
carrying the miR-22 transgene gained weight quickly and showed high
levels of miR-22 in the liver tissue. The increased miR-22
expression levels in the liver induced fatty degeneration.
Additionally, the authors investigated the role of miR-22 in
metabolism by targeting mice with liver tissue-specific miR-22
knock-out. Compared with wild-type mice, the miR-22 knock-out mice
gained significantly less weight after 8 weeks on a HFD and
displayed reduced liver steatosis. Immunohistochemistry results
showed increased staining of the uncoupling protein 1 in miR-22
knock-out mice and these mice also exhibited white fat browning.
These findings indicate that knocking out miR-22 can decrease liver
cell fatty degeneration and obesity in mice under HFD
conditions.
Gjorgjieva et al (109) investigated the role of miR-22 in
liver lesions in mice fed with an HFD. The study used a model of
mice with a knocked-out miR-22 gene (miR-22KO) and fed them a HFD
for 12 weeks, which led to an increase in fat mass, hepatomegaly
and hepatic steatosis. These findings suggest the importance of
miR-22 in liver diseases. To further explore the link between obese
mice with miR-22 deficiency and the development of liver cancer,
Gjorgjieva et al (39) also
published a study using miR-22KO mice. Feeding the mice a HFD
resulted in the promotion of characteristic features of
nonalcoholic steatohepatitis (NASH), which included liver changes
resembling balloon-like structures. Diethylnitrosamine was
administered to miR-22KO and wild-type mice to induce liver cancer
and the animals were divided into two groups, where one group
received a standard diet and the other received an HFD. These
results indicated that the miR-22KO mice developed tumours earlier
and the HFD group had a high number of tumours with low
differentiation characteristics compared with the control group.
This suggests that NAFLD can worsen tumour development and
differentiation in mice with miR-22 deficiency. A comparison of the
research results from the aforementioned study and those reported
by Panella et al (23)
showed the complex metabolic regulatory role of miR-22 in the body,
which indicates a need for further research.
NAFLD is a systemic metabolic disease mainly caused
by obesity and type 2 diabetes mellitus (T2DM). T2DM can accelerate
the progression of NAFLD in liver disease. Worldwide, ~55.48% of
patients with T2DM have NAFLD and among those patients undergoing
liver biopsy, 37.33% have NASH and 17.02% have advanced fibrosis
(110), which highlights the close
relationship between diabetes and NAFLD. A previous study reported
that miRNAs serve a crucial role in insulin signalling
transduction, glucose metabolism regulation, HDL and LDL
homeostasis regulation and liver lipid metabolism (111). miR-22 is highly expressed in the
liver and regulates liver metabolism in disease states such as
diabetes. In mice with insulin resistance and T2DM, miR-22
expression levels are significantly increased and liver glucose
metabolism is regulated by targeting the transcription factor 7
(TCF7) in the Wnt pathway. Silencing miR-22 improves circulating
glucose and insulin levels and reduces fasting blood glucose levels
in mice (112). Additionally,
3,5-diiodine-L-thyronine (T2) serves a role in increasing the
resting metabolic rate as well as lipid and glucose metabolism
(113,114). miR-22 serves a prominent role in
T2 metabolism and is involved in the homeostasis of glucose
metabolism by T2. T2 downregulates miR-22 to upregulate its target
TCF7, impairs glucose production by inhibiting the expression of
glucose-producing enzyme and regulates glucose homeostasis
(115). The liver can also
regulate glucose homeostasis through various pathways that control
glucose metabolism. Mebhydrolin, a selective nuclear receptor FXR
antagonist, reduces miR-22-3p expression levels by antagonising
FXR. This inhibits hepatic gluconeogenesis through the
FXR/miR-22-3p/PI3K/AKT/FOXO1 pathway and promotes glycogen
synthesis via the FXR/miR-22-3p/PI3K/AKT/GSK-3β pathway to improve
blood glucose homeostasis in T2DM mice (116). Therefore, miR-22 may act as an
indicator to predict physiological and pathological changes in the
liver during T2DM.
Summarily, the expression levels of miR-22 increase
after high fat induction, and fatty degeneration of liver can be
induced by regulating fatty acid metabolism. The role of the miR-22
inhibitor in improving energy consumption and reducing liver fat
accumulation has been reported. In addition, in view of the close
relationship between glucose metabolism and NAFLD, as well as the
complex regulatory role and differential correlation of miR-22 in
NAFLD, further exploration of the regulatory mechanism of miR-22 is
necessary. Future research is expected to reveal additional new
targets of miR-22 regulating hepatic steatosis and provide
potential new strategies for the future treatment of fatty liver
diseases. In addition, the global prevalence of NAFLD is likely to
increase in the future and the role of miR-22 in NAFLD is expected
to become more prominent (10).
Improvements in miR-22-related detection methods and the
development of miR-22-related preparations will contribute to the
early detection and treatment of fatty liver disease in the future
(Fig. 2).
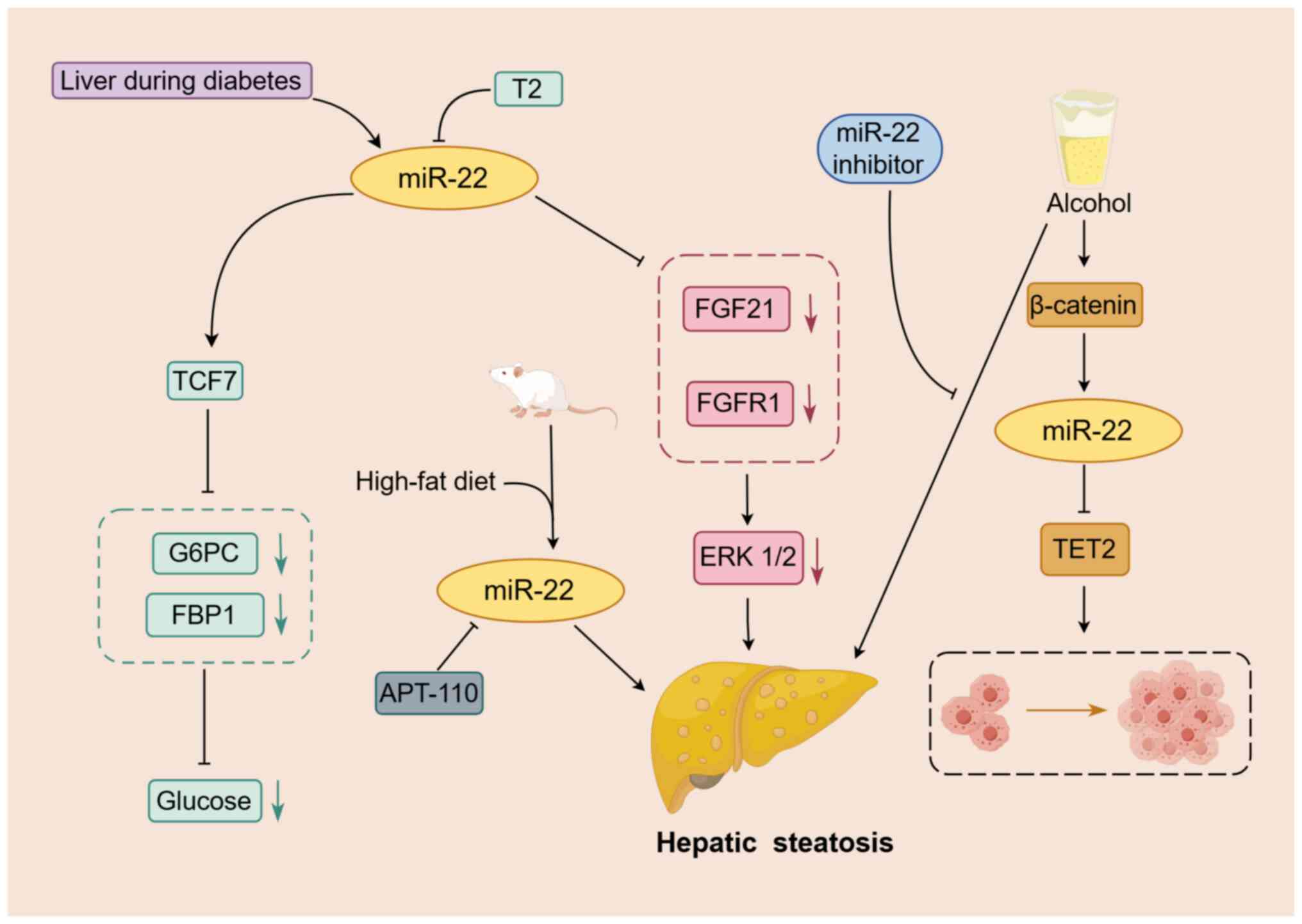 | Figure 2.Mechanism of action of miR-22 in
fatty liver disease. miR-22 is involved in regulating the
progression of fatty liver disease. Increased expression of miR-22
in liver tissue is observed with long-term alcohol intake, high-fat
diet and diabetes. miR-22 targets and inhibits FGFR1, FGF21 and
TET2, which contributes to hepatic steatosis and the progression of
HCC. Moreover, T2 upregulates TCF7 by downregulating miR-22, which
subsequently suppresses the expression of FBP1 and G6PC, and
regulates glucose homeostasis in the liver. An arrow-headed line
indicates promotion, whereas a bar-headed line signifies
inhibition. The yellow box indicates miR-22. Other boxes of the
same color represent important factors in the same pathway. The
green and red dotted boxes represent important factors in their
respective pathways. The black dotted box indicates HCC cell
proliferation. FBP1, fructose 1–6 bisphosphatase; G6PC, glucose
6-phosphatase; miR, microRNA; TCF7, transcription factor 7; T2,
3,5-diiodine-L-thyronine; TET2, tet methylcytosine dioxygenase 2;
FGF21, fibroblast growth factor 21; FGFR1, FGF21 receptor. |
Functional differences of miR-22 in
AFLD and NAFLD
miR-22 serves a central role in the pathogenesis of
both AFLD and NAFLD. Despite the common features between the two
diseases, there are obvious differences in the specific functional
manifestations and underlying molecular regulatory mechanisms.
miR-22 is upregulated in the fatty liver caused by two different
triggers, excessive alcohol consumption is the key to triggering
AFLD, while obesity, hyperlipidemia and type 2 diabetes are
important factors in NAFLD, and the overexpression of miR-22
aggravates the degree of hepatic steatosis (94,95).
In AFLD, the level of miR-22 is positively correlated with alcohol
consumption and inhibits the FGF21/FGFR1 signaling pathway to
promote the development of fatty liver by silencing PPARα (95). In NAFLD, miR-22 is associated with a
HFD and directly regulates Sirt1, PPARα and FOXO1 expression to
promote the formation of fatty liver (94). Therefore, PPARα serves a key role in
the regulation of miR-22. Additionally, miR-22 also participates in
the regulation of hepatic gluconeogenesis by regulating TCF7 in the
Wnt pathway, a process that may be inhibited by T2 (112,115), and maintains blood glucose
homeostasis through interacting with complex networks such as
FXR/miR-22-3p/PI3K/AKT (116),
which affects the development of NAFLD (Table I). Further, miR-22 inhibitors have
significant effects on improving both alcohol- and
non-alcohol-induced steatosis. In AFLD, miR-22 inhibitors activate
AMPK by upregulating the expression of FGF21 and FGFR1 to improve
alcohol-induced steatosis (95),
whereas in NAFLD, it also reduces steatosis and weight gain by
increasing total energy expenditure and improving insulin
sensitivity (107). This similar,
yet different, characteristic deepens the understanding of the
complex role of miR-22 in the process of hepatic steatosis and also
provides important perspectives which are useful for exploring
targeted therapeutic strategies.
 | Table I.Key roles of miR-22 in different
types of liver lesions. |
Table I.
Key roles of miR-22 in different
types of liver lesions.
| A, liver
cancer |
|---|
|
|---|
| Experimental
model | In vivo or
in vitro | Target | Function | (Refs.) |
|---|
| Human liver tissues
and Hep3B and SMMC7721 cells | In vivo and
in vitro | HDAC4 | miR-22 acts on
CD147, HDAC4, HMGB1, CBL and other targets to directly or
indirectly regulate the proliferation, migration, invasion and
apoptosis of HCC cells | (35) |
| Human liver tissues
and SK-Hep-1 and SMMC-7721 cells | In vivo and
in vitro | HMGB1 |
| (43) |
| Murine xenograft
model and MHCC-97H and SMMC-7721 | In vivo and
in vitro | CD147 |
| (44) |
| HepG2 and Huh7
cells | In vivo | CBL |
| (17) |
| Model of
subcutaneous tumors in nude mice (male BALB/c nude mice aged 4–5
weeks) and Hep3B and Huh7 cells | In vivo and
in vitro | ARPC5 | miR-22 is
negatively regulated by lncRNAs, and its inhibitory effect on
downstream targets is weakened, which results in the promotion of
growth and migration of liver cancer | (37) |
| Xenograft tumor
model (BALB/c male nude mice), human liver tissues and Huh7, Hep3B,
MHCC97-H and LM3 cells | In vivo and
in vitro | ETS1 |
| (49) |
| Human liver tissues
and Hep-3B, Huh7, HepG2, SK-Hep1 and L02 cells | In vivo and
in vitro | YARS |
| (52) |
| Subcutaneous tumor
model (female BALB/c mice aged 6–8 weeks) and HepG2, SMMC7721, Huh7
and SK-HEP-1 cells | In vivo and
in vitro | SIRT1 |
| (47) |
| In vivo
xenograft model of human HCC nude mice (male, aged 5 weeks) and
Huh7 and HCCLM3 cells | In vivo and
in vitro | MTA3 | After miR-22 is
induced by Catalpol and FXR, miR-22 restricts the proliferation,
migration and invasion of HCC cells by affecting the normal cell
cycle | (41) |
| Human liver
tissues, mouse model of liver cancer and Huh7 cells | In vivo and
in vitro | CCNA2 |
| (36) |
| Human liver tissues
and HepG2.2.15 cells | In vivo and
in vitro | CDKN1A | In the hepatitis
virus-related liver cancer model, miR-22 exerts an inhibitory
effect on downstream targets, which hinders the occurrence and
development of hepatitis virus-related liver cancer | (71) |
| Human liver tissues
and MHCC97H, Hep3B, HepG2.2.15, Huh7 and L02 cells | In vivo and
in vitro | HNRNPA1 |
| (70) |
| Human liver tissues
and L02, MHCC97L and HCCLM9 cells | In vivo and
in vitro | Tyrosine
3-monooxygenase/tryptophan 5-monooxygenase activation protein
zeta |
| (38) |
| Human liver tissues
and HepG2, HepG2.215, Hep3B and L02 cells | In vivo and
in vitro | NRAS |
| (14) |
| Human liver
tissues, immunoreconstituted mouse model (female C57BL/6 mice aged
8–10 weeks) and CD4+ T cells | In vivo and
in vitro | JARID2 and T
helper17cells | miR-22 hinders the
occurrence and progression of tumors by exerting immunomodulatory
effects, including inhibiting tumor growth and immune escape and
regulating the apoptosis of tumor cells. | (43) |
| Human liver tissues
and Lo2, HepG2 and SMMC7721 cells | In vivo and
in vitro | Galectin-9 |
| (66) |
|
| B, alcoholic
fatty liver disease |
|
| Experimental
model | In vivo
or in vitro | Target |
Function | (Refs.) |
|
| C57BL/6 wild-type
male and female mice and Huh7 cells | In vivo and
in vitro | FGF21 receptor | miR-22 reduces
FGF21 expression by reducing the regulation of FGF21 by the
transcriptional factors PPARα and hepatic PPAR-activated receptor-c
coactivator-1α, which limits AMPK and ERK1/2 activation, leading to
fat accumulation | (95) |
|
| C, nonalcoholic
fatty liver disease |
|
| Experimental
model | In vivo
or in vitro | Target |
Function | (Refs.) |
|
| Male C57BL/6 mice
and L02 cells | In vivo and
in vitro | Sirt1, PPARα and
FOXO1 | miR-22 regulates
the expression of Sirt1, PPARα and FOXO1 to regulate lipid
metabolism | (94) |
| Male diabetic
(C57BLKs-db/db) and non-diabetic (C57BLKs-db/+) mice and HepG2
cells | In vivo and
in vitro | TCF7 | miR-22 targets the
transcription factor TCF7 in the Wnt pathway to regulate hepatic
glucose gluconeogenesis | (112) |
| C57BL/6 mice, male
db/db mice and HEK293T cells | In vivo and
in vitro | FXR | miR-22 inhibits
hepatic gluconeogenesis and promotes glycogen synthesis to maintain
blood glucose homeostasis through the FXR/miR-22-3p/PI3K/AKT/FOXO1
and FXR/miR-22-3p/PI3K/AKT/GSK-3β pathways | (116) |
| Male Wistar
rats | In vivo | TCF7 |
3,5-diiodine-L-thyronine significantly
decreases the expression of miR-22, which results in a decrease in
the inhibitory effect of miR-22 on TCF7, thus impairing
gluconeogenesis | (115) |
|
| D, liver
fibrosis |
|
| Experimental
model | In vivo
or in vitro | Target |
Function | (Refs.) |
|
| Male C57BL/6J mice
and HSCs | In vivo and
in vitro | Cyth3 | Nuclear paraspeckle
assembly transcript 1 is highly expressed in mouse liver tissues
and acts as a competing endogenous RNA targeting miR-22,
upregulating Cyth3 which is involved in HSC activation and liver
fibrosis | (27) |
| Sprague-Dawley male
rats, NFs and LX-2 cells | In vivo and
in vitro | AKT3 | miR-22
significantly inhibits the proliferation and activation of LX-2
cells and alleviates liver fibrosis | (24) |
|
| E, drug-induced
liver injury |
|
| Experimental
model | In vivo
or in vitro | Target |
Function | (Refs.) |
|
| C57BL/6 mice | In vivo | NA | Mouse autoimmune
hepatitis is induced using concanavalin A and miR-22 expression is
downregulated | (136) |
| HepG2 cells | In
vitro | NA | The expression of
miR-22 in HepG2 cells is up-regulated under the influence of the
steatogenic drug cyclosporine A | (137) |
In conclusion, miR-22 serves a significant role in
the fatty liver. In the future, it is necessary to further explore
its interaction mechanism with target genes and its impact on
pathophysiology, accelerate the development of miR-22 inhibitors
and provide new strategies for treatment. Additionally, in view of
the differences in mechanisms between AFLD and NAFLD, treatment
should be personalized, multi-target combination therapy should be
consider and combined with lifestyle interventions. Research into
the mechanisms of action of miR-22 provide a new perspective for
the treatment of fatty liver and further in-depth research and drug
development is required to improve the efficacy of patient
treatment.
Role of miR-22 in liver fibrosis
Liver fibrosis can be caused by different factors,
such as viral hepatitis, alcoholic steatohepatitis, non-alcoholic
steatohepatitis and DILI (117).
These factors lead to the induction of the liver repair response,
which results in increased liver extracellular matrix (ECM) and the
formation of fibrous scars (118).
Currently, effective drug treatments for liver fibrosis are
lacking. It is an important step in the transition from chronic
liver disease to cirrhosis and is characterized by hepatic stellate
cell (HSC) activation and excessive ECM deposition (119). If not treated promptly and
effectively, liver fibrosis can progress to cirrhosis, liver
failure and potentially liver cancer. HSC activation significantly
influences the occurrence and development of liver fibrosis, with
various miRNAs having the ability to regulate liver fibrosis
signalling pathways and HSC activation (120). Each miRNA exerts distinct
regulatory effects. For example, miR-188-5p enhances HSC activation
and proliferation through the PTEN/PI3K/AKT pathway, thereby
promoting liver fibrosis (121).
In addition, miR-301a-3p promotes HSC activation and liver fibrosis
through the PTEN/PDGFR-β pathway (122). However, miR-22 can inhibit HSC
activation and the expression of related fibrotic mediators in
various ways, thereby mitigating the progression of liver fibrosis.
Collectively, miRNA has great research potential and value in the
treatment of liver fibrosis.
miR-22 is closely associated with HSC activation and
liver fibrosis. Huang et al (27) reported that the lncRNA nuclear
paraspeckle assembly transcript 1 (Neat1) functions as a ceRNA to
accelerate the progression of liver fibrosis in mice by targeting
miR-148a-3p and miR-22-3p, thereby upregulating cytohesin 3.
Conversely, downregulating Neat1 yielded contrasting results. The
inhibitors for miR-22-3p and miR-148a-3p stimulate the activation
of HSCs and the expression of collagen fibres, which can lead to
liver fibrosis.
AKT3, is a serine/threonine protein kinase, whose
expression level is regulated by the miRNA (123–125). AKT3 is a common target gene of
miR-22-3p and miR-29a-3p, promoting the proliferation, migration,
colony formation ability and the expression of fibrosis markers
collagen type I α 1 chain and α-smooth muscle actin in LX-2 cells
(24,123). Under the influence of miR-22-3p
and miR-29a-3p inhibitors, the expression of AKT3 increases,
thereby promoting the proliferation and activation of LX-2 cells.
In conclusion, the overexpression of miR-22-3p and miR-29a-3p
synergistically inhibits the proliferation and activation of LX-2
cells and alleviates the progression of liver fibrosis (24).
Silymarin (SIL)-loaded chitosan nanoparticles
combine chitosan nanoparticles with the hepatoprotective compound
SIL to enhance its therapeutic effect in liver diseases and improve
its anti-fibrotic efficacy in CCl rats. The mechanism of action
involves promoting the expression of protective factors miR-22,
miR-29c and miR-219a in the liver, which in turn inhibit the
expression of fibrosis mediators TGFβR1, TGFβR2 and collagen type
III α1 chain, thus slowing down the progression of liver fibrosis
(126). Additionally, Abdullah
et al (127) demonstrated
that SIL-gold nanoparticles serve a similar role in the process of
liver fibrosis. In summary, miR-22 acts as a protective factor in
the liver, inhibiting the expression of fibrotic mediators and
serving an anti-fibrotic role in liver fibrosis.
NAFLD speeds up the process of liver fibrosis caused
by carbon tetrachloride. In turn, liver fibrosis accelerates the
progression of liver cancer (128). NAFLD and liver fibrosis have a
significant impact on the occurrence and development of liver
cirrhosis and HCC. A study by Ji et al (129) reported that the expression levels
of miR-22 were negatively correlated with bone morphogenetic
protein 7 (BMP7) in the liver biopsies of 12 patients with liver
cirrhosis and this conclusion was verified in HepG2 cells.
Bioinformatics analysis of the target sequence of BMP7 shows that
miR-22 can target the 3′UTR of BMP7 mRNA and inhibit the expression
of BMP7, which leads to the occurrence of liver cirrhosis (129). miR-22 was delivered to the liver
through the common bile duct, thus effectively reducing the
potential interference of other tissues and organs on the
experimental results.
Although the role of miR-22 in the process of liver
fibrosis has been reported to a certain extent, numerous potential
mechanisms remain unclear, particularly in the study of drug
transformation. In addition, significant individual differences
exist in the clinical manifestations and severity of illness among
patients during the progression of liver fibrosis to cirrhosis and
whether the expression levels of miR-22 also differ has not yet
been determined. Therefore, it is crucial to identify and implement
therapeutic measures during the early stages of the disease. It is
also necessary to explore the underlying molecular mechanisms,
understand the formation and development of the disease and
identify appropriate therapeutic targets and treatment measures.
Simultaneously, a reasonable model for managing chronic liver
disease should be developed to effectively control its malignant
progression (Fig. 3).
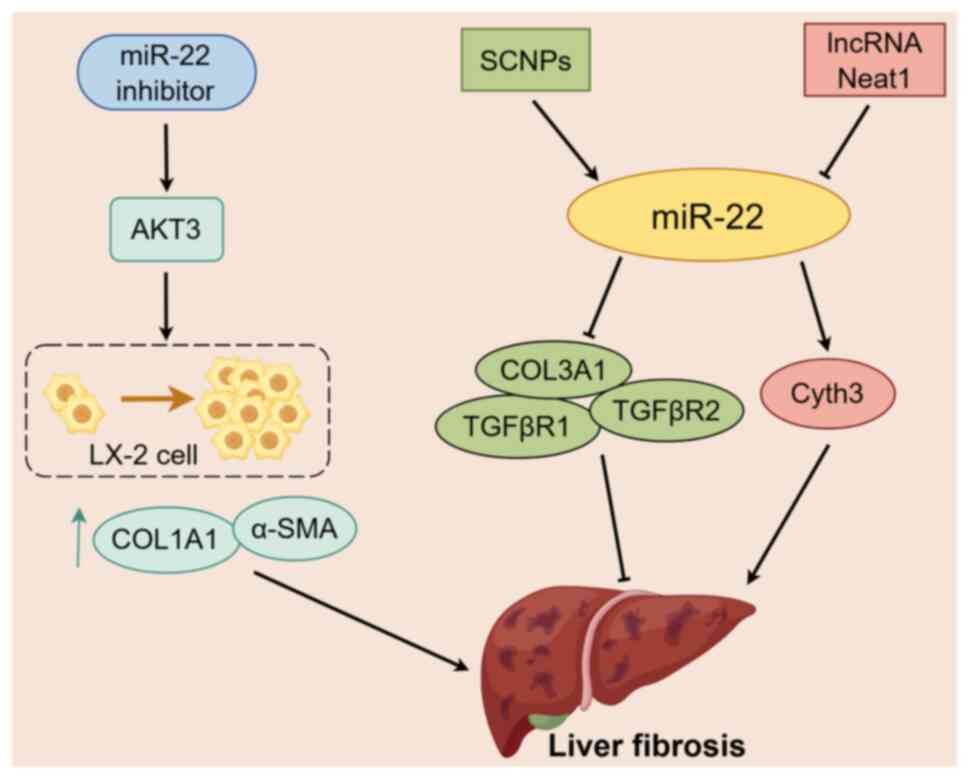 | Figure 3.Mechanism of miR-22 in liver
fibrosis. miR-22 is involved in regulating the progression of liver
fibrosis. The expression level of miR-22 is controlled by the
hepatoprotective complex SCNPs and lncRNA Neat1. miR-22 targets and
regulates the expression of fibrosis mediators TGFβR1, TGFβR2,
COL3A1 and Cyth3 to mitigate the advancement of liver fibrosis.
Inhibition of miR-22 can enhance the expression of AKT3 in the
liver, stimulate the proliferation of LX-2 cells and the expression
of fibrosis markers COL1A1 and α-SMA and accelerate liver fibrosis.
An arrow-headed line indicates promotion, whereas a bar-headed line
signifies inhibition. The yellow box indicates miR-22. Other boxes
of the same color represent important factors in the same pathway.
The black dotted box indicates the process of LX-2 cell
proliferation. miR, microRNA; COL1A1, collagen type I a1 chain;
a-SMA, a-smooth muscle actin; SCNPs, SIL-loaded chitosan
nanoparticles; lncRNA, long non-coding RNA; COL3A1, collagen type
III a1 chain; TGF-bR, TGF-b receptor; Cyth3, cytohesin 3; neat1,
nuclear paraspeckle assembly transcript 1. |
miR-22 and DILI
DILI is a rare but serious drug-induced adverse
reaction, which can lead to the early termination or withdrawal of
drug development studies (130).
DILI is responsible for a growing number of liver injuries in
previous years (131). The
pathology of DILI-induced liver injuries are varied and complex and
cases of DILI present as diverse histological types on liver
biopsies (132). Liver biopsies of
249 patients with suspected DILI showed predominantly acute and
chronic hepatitis (133). miRNAs
are closely associated with the regulation of biological behaviours
in various diseases. The role of miR-122 in DILI has been
previously reported and it shares similarities with that of miR-22
in liver-related diseases. Both miRNAs inhibit HCC progression,
facilitate NAFLD progression and detect hepatic fibrosis severity
(134,135). Previous studies have demonstrated
the main functions of miR-22 in the liver under drug induction
(136,137).
Pharmacological autoimmune hepatitis is a type of
liver injury induced by a drug or its metabolite, which triggers
the immune response against foreign substances. The role of miRNAs
in autoimmune hepatitis has been previously reported. Liu et
al (136) used concanavalin A
to induce autoimmune hepatitis in mice and the abnormal expression
of various miRNAs in autoimmune hepatitis was analysed using gene
microarray, enrichment analysis of Gene ontology (GO) and Kyoto
Encyclopedia of Genes and Genomes (KEGG) The expression level of
miR-22 was downregulated, which provided new insights into the role
of miR-22 in autoimmune hepatitis. This may potentially serve as a
predictor of autoimmune hepatitis pathogenesis and a future
therapeutic target for this disease.
Drug-induced hepatic steatosis is also a form of
DILI; however, it is usually a reversible form of chronic disease
(138). Obesity and NAFLD may
increase the risk of hepatotoxicity of certain drugs and
potentially exacerbate DILI (139). López-Riera et al (137) treated HepG2 cells with
steatosis-mimicking drugs, such as doxycycline and cyclosporine A,
and identified a group of miRNAs, including miR-22, that were
induced in human HepG2 cells. The expression levels of miR-22
increased inside the cells and was released outside the cells and
elevated levels of miR-22 were detected in the culture medium. The
production of related miRNAs, including miR-22, was also induced
when cells were exposed to prescription drugs, such as irbesartan
and fenofibrate, which are used for NAFLD treatment (137). In addition, these miRNA biomarkers
were detected in the sera of patients with NAFLD and their
expression was significantly increased. Therefore, miR-22 may
potentially be promising serum miRNA biomarker for drug-induced
steatosis for drug development and screening.
Although the specific molecular mechanism of miR-22
in DILI remains unclear, bioinformatics analysis of related studies
indicates its abnormal expression. Further research is needed to
elucidate the molecular biological mechanism of miR-22 in DILI. In
view of its abnormal expression in drug-induced liver injury, the
feasibility of miR-22 as a potential miRNA marker in serum needs to
be fully verified through in-depth research and clinical studies.
Therefore, miR-22 could act as an important biomarker for the early
diagnosis and prognosis evaluation of drug-induced liver injury in
clinical practice in the future.
Summary and outlook
As a member of the miRNA family, miR-22 can bind to
the 3′-UTR of target genes and regulate the expression of related
genes, serving certain biological functions in various types of
tumours. miR-22 predominantly acts as a tumour suppressor in
numerous types of cancer, but under specific circumstances, it can
act as a tumour promoter. The diverse molecular mechanisms of
miR-22 on the regulation of liver cancer from numerous perspectives
were comprehensively reviewed. Additionally, the role of miR-22 in
the regulation on cell proliferation and cell cycle, immune
regulation, sensitivity to treatment of liver cancer and evaluation
of prognosis were discussed. miR-22 serves an oncogenic role by
inhibiting the activities of liver cancer cells, such as
proliferation, migration and invasion, while promoting apoptosis
and participating in immunomodulation. miR-22 can be sponged and
inhibited by various lncRNAs and induced by certain exogenous
substances. Combined with the latest research of miR-22 in fatty
liver disease, liver fibrosis and drug-induced liver injury,
significant differences in the expression of miR-22 in different
stages of liver diseases were reported, as well as the complex and
subtle regulatory mechanisms underlying these differences.
Therefore, the regulatory role of miR-22 in the pathological and
physiological changes of the liver could be particularly important
and may provide a novel research target for the diagnosis,
treatment and prognosis prediction of liver diseases in the future.
The present manuscript highlighted the necessity for further
exploration of the detailed mechanisms of action of miR-22 in liver
diseases. However, comprehensive studies remain warranted to fully
understand the complex regulatory functions of miR-22 in different
types of tumours.
While the regulatory role of miR-22 in liver cancer
has been extensively studied, questions still remain regarding its
involvement in hepatitis virus-induced liver cancer. Furthermore,
the mechanisms underlying DILI and the specific regulatory
relationship between diabetes and fatty liver have yet to be fully
understood, in addition to the multifaceted regulatory functions of
miR-22 in various parts of the body. Further study into these areas
will facilitate the development of agonists, inhibitors or drug
combination therapies to utilize the complex regulatory functions
of miR-22 in liver lesions and of miR-22 as a screening indicator
or a prognostic model for liver lesions (Fig. S1).
Supplementary Material
Supporting Data
Acknowledgements
All figures in the article are drawn using Figdraw
(www.figdraw.com).
Funding
The Shandong Provincial Natural Science Foundation provided
financial support for this work (grant no. ZR2021QH151).
Availability of data and materials
Not applicable.
Authors' contributions
MW and XW consulted relevant research, searched the
literature and participated in the writing of manuscripts and
charts. YW, YG, JY and XX participated in the editing of the
article. XY provided constructive guidance and revised the article.
Data authentication is not applicable.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
HCC
|
hepatocellular carcinoma
|
|
miRNA
|
microRNA
|
|
UTR
|
untranslated region
|
|
HDAC4
|
histone deacetylase 4
|
|
HMGB1
|
high mobility group box 1
|
|
CBL
|
casitas B-lineage lymphoma
|
|
lncRNA
|
long non-coding RNA
|
|
SPRY2
|
sprouty2
|
|
DSCR8
|
down syndrome critical region 8
|
|
ARPC5
|
actin-related protein 2/3 complex
subunit 5
|
|
MKLN1-AS
|
muskelin 1 antisense RNA
|
|
ETS1
|
ETS proto-oncogene 1
|
|
NCK1-AS1
|
NCK1 antisense RNA 1
|
|
YARS
|
tyrosyl-tRNA synthetase
|
|
MIAT
|
myocardial infarction-associated
transcript
|
|
SIRT1
|
sirtuin 1
|
|
ROS
|
reactive oxygen species
|
|
AFP
|
alpha fetoprotein
|
|
CHB
|
chronic hepatitis B
|
|
MTA3
|
metastasis associated 1 family member
3
|
|
CCNA2
|
cyclin A2
|
|
FXR
|
farnesoid X receptor
|
|
JARID2
|
jumonji AT rich interacting domain
containing 2
|
|
HIF1α
|
hypoxia-inducible factor 1 alpha
|
|
Gal-9
|
galectin-9
|
|
UBE4B
|
ubiquitin ligase E4B
|
|
FGD5-AS1
|
FGD5 antisense RNA 1
|
|
LINC00858
|
long intergenic non-protein coding
RNA 858
|
|
SNHG16
|
small nucleolar RNA host gene 16
|
|
HBV
|
hepatitis B virus
|
|
HCV
|
hepatitis C virus
|
|
HNRNPA1
|
heterogeneous nuclear
ribonucleoprotein A 1
|
|
HBx
|
HBV-encoded X
|
|
YWHAZ
|
tyrosine 3-monooxygenase/tryptophan
5-monooxygenase activation protein zeta
|
|
TRERNA1
|
translation regulatory lncRNA 1
|
|
ERα
|
estrogen receptor α
|
|
NRAS
|
NRAS proto-oncogene
|
|
EZH2
|
enhancer of zeste homolog 2
|
|
NASH
|
nonalcoholic steatohepatitis
|
|
AFLD
|
alcoholic fatty liver disease
|
|
ALD
|
alcoholic liver disease
|
|
NAFLD
|
non-AFLD
|
|
FGF21
|
fibroblast growth factor 21
|
|
FGFR1
|
FGF21 receptor
|
|
PGC1α
|
hepatic PPAR-activated receptor-c
coactivator-1α
|
|
PPARα
|
peroxisome proliferator-activated
receptor α
|
|
HFD
|
high-fat diet
|
|
miR-22KO
|
miR-22 knock-out
|
|
T2DM
|
type 2 diabetes mellitus
|
|
T2
|
3,5-diiodine-L-thyronine
|
|
TCF7
|
transcription factor 7
|
|
HSC
|
hepatic stellate cell
|
|
ECM
|
extracellular matrix
|
|
Neat1
|
nuclear paraspeckle assembly
transcript 1
|
|
ceRNA
|
competing endogenous RNA
|
|
SIL
|
silymarin
|
|
DILI
|
drug-induced liver injury
|
References
|
1
|
Asrani SK, Devarbhavi H, Eaton J and
Kamath PS: Burden of liver diseases in the world. J Hepatol.
70:151–171. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Devarbhavi H, Asrani SK, Arab JP, Nartey
YA, Pose E and Kamath PS: Global burden of liver disease: 2023
Update. J Hepatol. 79:516–537. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Boldo E, Santafe A, Mayol A, Lozoya R,
Coret A, Escribano D, Fortea-Sanchis C, Muñoz A, Pastor JC, Perez
de Lucia G and Bosch N: Rare site hepatocellular carcinoma
metastasis. J Hepatocell Carcinoma. 7:39–44. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Singal AG, Kudo M and Bruix J:
Breakthroughs in hepatocellular carcinoma therapies. Clin
Gastroenterol Hepatol. 21:2135–2149. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Gao R, Kalathur RKR, Coto-Llerena M, Ercan
C, Buechel D, Shuang S, Piscuoglio S, Dill MT, Camargo FD,
Christofori G and Tang F: YAP/TAZ and ATF4 drive resistance to
sorafenib in hepatocellular carcinoma by preventing ferroptosis.
EMBO Mol Med. 13:e143512021. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Bodzin AS and Busuttil RW: Hepatocellular
carcinoma: Advances in diagnosis, management, and long term
outcome. World J Hepatol. 7:1157–1167. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zhang Y, Shi ZL, Yang X and Yin ZF:
Targeting of circulating hepatocellular carcinoma cells to prevent
postoperative recurrence and metastasis. World J Gastroenterol.
20:142–147. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zhu JZ, Zhou QY, Wang YM, Dai YN, Zhu J,
Yu CH and Li YM: Prevalence of fatty liver disease and the economy
in China: A systematic review. World J Gastroenterol. 21:5695–5706.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wu Y, Zheng Q, Zou B, Yeo YH, Li X, Li J,
Xie X, Feng Y, Stave CD, Zhu Q, et al: The epidemiology of NAFLD in
Mainland China with analysis by adjusted gross regional domestic
product: A meta-analysis. Hepatol Int. 14:259–269. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Younossi ZM, Golabi P, Paik JM, Henry A,
Van Dongen C and Henry L: The global epidemiology of nonalcoholic
fatty liver disease (NAFLD) and nonalcoholic steatohepatitis
(NASH): A systematic review. Hepatology. 77:1335–1347. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Greten TF, Villanueva A, Korangy F, Ruf B,
Yarchoan M, Ma L, Ruppin E and Wang XW: Biomarkers for
immunotherapy of hepatocellular carcinoma. Nat Rev Clin Oncol.
20:780–798. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhang Y, Wei C, Guo CC, Bi RX, Xie J, Guan
DH, Yang CH and Jiang YH: Prognostic value of microRNAs in
hepatocellular carcinoma: A meta-analysis. Oncotarget.
8:107237–107257. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Hu Y, Setayesh T, Vaziri F, Wu X, Hwang
ST, Chen X and Yvonne Wan YJ: miR-22 gene therapy treats HCC by
promoting anti-tumor immunity and enhancing metabolism. Mol Ther.
31:1829–1845. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Song W, Zheng C, Liu M, Xu Y, Qian Y,
Zhang Z, Su H, Li X, Wu H, Gong P, et al: TRERNA1 upregulation
mediated by HBx promotes sorafenib resistance and cell
proliferation in HCC via targeting NRAS by sponging miR-22-3p. Mol
Ther. 29:2601–2616. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Menon A, Abd-Aziz N, Khalid K, Poh CL and
Naidu R: miRNA: A promising therapeutic target in cancer. Int J Mol
Sci. 23:115022022. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Shukla GC, Singh J and Barik S: MicroRNAs:
Processing, maturation, target recognition and regulatory
functions. Mol Cell Pharmacol. 3:83–92. 2011.PubMed/NCBI
|
|
17
|
Cui S, Chen Y, Guo Y, Wang X and Chen D:
Hsa-miR-22-3p inhibits liver cancer cell EMT and cell
migration/invasion by indirectly regulating SPRY2. PLoS One.
18:e02815362023. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Fan T, Wang CQ, Li XT, Yang H, Zhou J and
Song YJ: MiR-22-3p suppresses cell migration and invasion by
targeting PLAGL2 in breast cancer. J Coll Physicians Surg Pak.
31:937–940. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wang X, Wang X, Jiang T, Zhang Z, Xie N
and Yang G: MiR-22-3p suppresses NSCLC cell migration and EMT via
targeting RAC1 expression. Funct Integr Genomics. 23:2812023.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Qiao H, Wang N, Guan QL, Xie P and Li XK:
miR-22-3p suppresses cell proliferation and migration of gastric
cancer by targeting ENO1. Altern Ther Health Med. 29:278–283.
2023.PubMed/NCBI
|
|
21
|
Liu Y, Chen X, Cheng R, Yang F, Yu M, Wang
C, Cui S, Hong Y, Liang H, Liu M, et al: The Jun/miR-22/HuR
regulatory axis contributes to tumourigenesis in colorectal cancer.
Mol Cancer. 17:112018. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zeng Z, Dong J, Li Y, Dong Z, Liu Z, Huang
J, Wang Y, Zhen Y and Lu Y: The expression level and diagnostic
value of microRNA-22 in HCC patients. Artif Cells Nanomed
Biotechnol. 48:683–686. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Panella R, Petri A, Desai BN, Fagoonee S,
Cotton CA, Nguyen PK, Lundin EM, Wagshal A, Wang DZ, Näär AM, et
al: MicroRNA-22 is a key regulator of lipid and metabolic
homeostasis. Int J Mol Sci. 24:128702023. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wang Y, Zhang R, Li J, Han X, Lu H, Su J,
Liu Y, Tian X, Wang M, Xiong Y, et al: MiR-22-3p and miR-29a-3p
synergistically inhibit hepatic stellate cell activation by
targeting AKT3. Exp Biol Med (Maywood). 247:1712–1731. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Azar S, Udi S, Drori A, Hadar R,
Nemirovski A, Vemuri KV, Miller M, Sherill-Rofe D, Arad Y,
Gur-Wahnon D, et al: Reversal of diet-induced hepatic steatosis by
peripheral CB1 receptor blockade in mice is
p53/miRNA-22/SIRT1/PPARα dependent. Mol Metab. 42:1010872020.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Chen J, Wu FX, Luo HL, Liu JJ, Luo T, Bai
T, Li LQ and Fan XH: Berberine upregulates miR-22-3p to suppress
hepatocellular carcinoma cell proliferation by targeting Sp1. Am J
Transl Res. 8:4932–4941. 2016.PubMed/NCBI
|
|
27
|
Huang W, Huang F, Zhang R and Luo H:
LncRNA Neat1 expedites the progression of liver fibrosis in mice
through targeting miR-148a-3p and miR-22-3p to upregulate Cyth3.
Cell Cycle. 20:490–507. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Li H, Zhang P and Li F, Yuan G, Wang X,
Zhang A and Li F: Plasma miR-22-5p, miR-132-5p, and miR-150-3p are
associated with acute myocardial infarction. Biomed Res Int.
2019:50126482019.PubMed/NCBI
|
|
29
|
Wang Y, Chang W, Zhang Y, Zhang L, Ding H,
Qi H, Xue S, Yu H, Hu L, Liu D, et al: Circulating miR-22-5p and
miR-122-5p are promising novel biomarkers for diagnosis of acute
myocardial infarction. J Cell Physiol. 234:4778–4786. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Peng WX, Koirala P and Mo YY:
LncRNA-mediated regulation of cell signaling in cancer. Oncogene.
36:5661–5667. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Xu J, Shao T, Song M, Xie Y, Zhou J, Yin
J, Ding N, Zou H, Li Y and Zhang J: MIR22HG acts as a tumor
suppressor via TGFbeta/SMAD signaling and facilitates immunotherapy
in colorectal cancer. Mol Cancer. 19:512020. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Zhang W, Shi C, Xu Q, Chen X, Zhu H and
Zheng B: Long non-coding RNA MIR22HG suppresses cell proliferation
and promotes apoptosis in prostate cancer cells by sponging
microRNA-9-3p. Bioengineered. 13:13108–13117. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Deng X, Ye D, Hua K, Song H, Luo Q,
Munankarmy A, Liu D, Zhou B, Zheng W, Zhou X, et al: MIR22HG
inhibits breast cancer progression by stabilizing LATS2 tumor
suppressor. Cell Death Dis. 12:8102021. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Zhang L, Li C and Su X: Emerging impact of
the long noncoding RNA MIR22HG on proliferation and apoptosis in
multiple human cancers. J Exp Clin Cancer Res. 39:2712020.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Zhang J, Yang Y, Yang T, Liu Y, Li A, Fu
S, Wu M, Pan Z and Zhou W: microRNA-22, downregulated in
hepatocellular carcinoma and correlated with prognosis, suppresses
cell proliferation and tumourigenicity. Br J Cancer. 103:1215–1220.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Yang F, Hu Y, Liu HX and Wan YJY:
MiR-22-silenced cyclin A expression in colon and liver cancer cells
is regulated by bile acid receptor. J Biol Chem. 290:6507–6515.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Huang JN, Zhang HM, Cai JD, Wang WL and
Wang P: Long noncoding RNA DSCR8 promotes the proliferation of
liver cancer cells and inhibits apoptosis via the miR-22-3p/ARPC5
axis. J Cancer. 14:35–49. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Chen M, Hu W, Xiong CL, Qu Z, Yin CQ, Wang
YH, Luo CL, Guan Q, Yuan CH and Wang FB: miR-22 targets YWHAZ to
inhibit metastasis of hepatocellular carcinoma and its
down-regulation predicts a poor survival. Oncotarget.
7:80751–80764. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Gjorgjieva M, Ay AS, Correia de Sousa M,
Delangre E, Dolicka D, Sobolewski C, Maeder C, Fournier M, Sempoux
C and Foti M: MiR-22 deficiency fosters hepatocellular carcinoma
development in fatty liver. Cells. 11:28602022. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Zhang L, Yang P, Wang J, Liu Q, Wang T,
Wang Y and Lin F: MiR-22 regulated T cell differentiation and
hepatocellular carcinoma growth by directly targeting Jarid2. Am J
Cancer Res. 11:2159–2173. 2021.PubMed/NCBI
|
|
41
|
Zhao L, Wang Y and Liu Q: Catalpol
inhibits cell proliferation, invasion and migration through
regulating miR-22-3p/MTA3 signalling in hepatocellular carcinoma.
Exp Mol Pathol. 109:51–60. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Wu Y, Zhou Y, Huan L, Xu L, Shen M, Huang
S and Liang L: LncRNA MIR22HG inhibits growth, migration and
invasion through regulating the miR-10a-5p/NCOR2 axis in
hepatocellular carcinoma cells. Cancer Sci. 110:973–984. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Zhang DY, Zou XJ, Cao CH, Zhang T, Lei L,
Qi XL, Liu L and Wu DH: Identification and functional
characterization of long non-coding RNA MIR22HG as a tumor
suppressor for hepatocellular carcinoma. Theranostics. 8:3751–3765.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Luo LJ, Zhang LP, Duan CY, Wang B, He NN,
Abulimiti P and Lin Y: The inhibition role of miR-22 in
hepatocellular carcinoma cell migration and invasion via targeting
CD147. Cancer Cell Int. 17:172017. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Yim DGR, Ghosh S, Guy GR and Virshup DM:
Casein kinase 1 regulates sprouty2 in FGF-ERK signaling. Oncogene.
34:474–484. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Quinn JJ and Chang HY: Unique features of
long non-coding RNA biogenesis and function. Nat Rev Genet.
17:47–62. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Zhao L, Hu K, Cao J, Wang P, Li J, Zeng K,
He X, Tu PF, Tong T and Han L: lncRNA miat functions as a ceRNA to
upregulate sirt1 by sponging miR-22-3p in HCC cellular senescence.
Aging (Albany NY). 11:7098–7122. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Guo C, Zhou S, Yi W, Yang P, Li O, Liu J
and Peng C: Long non-coding RNA muskelin 1 antisense RNA (MKLN1-AS)
is a potential diagnostic and prognostic biomarker and therapeutic
target for hepatocellular carcinoma. Exp Mol Pathol.
120:1046382021. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Pan G, Zhang J, You F, Cui T, Luo P, Wang
S, Li X and Yuan Q: ETS proto-oncogene 1-activated muskelin 1
antisense RNA drives the malignant progression of hepatocellular
carcinoma by targeting miR-22-3p to upregulate ETS proto-oncogene
1. Bioengineered. 13:1346–1358. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Guan B, Ma J, Yang Z, Yu F and Yao J:
LncRNA NCK1-AS1 exerts oncogenic property in gastric cancer by
targeting the miR-22-3p/BCL9 axis to activate the Wnt/β-catenin
signaling. Environ Toxicol. 36:1640–1653. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Wang B, Wang K, Jin T, Xu Q, He Y, Cui B
and Wang Y: NCK1-AS1 enhances glioma cell proliferation,
radioresistance and chemoresistance via miR-22-3p/IGF1R ceRNA
pathway. Biomed Pharmacother. 129:1103952020. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Zhou W, Wang J, Zhang J, Wang Y, Jiang L,
Guo T, Luo B, Xu Q and Huang Y: LncRNA NCK1-AS1 aggravates
hepatocellular carcinoma by the miR-22-3p/YARS axis to activate
PI3K/AKT signaling. J Gastrointestin Liver Dis. 31:48–59. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Zhang C, Lin X, Zhao Q, Wang Y, Jiang F,
Ji C, Li Y, Gao J, Li J and Shen L: YARS as an oncogenic protein
that promotes gastric cancer progression through activating
PI3K-Akt signaling. J Cancer Res Clin Oncol. 146:329–342. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Pant K, Yadav AK, Gupta P, Islam R, Saraya
A and Venugopal SK: Butyrate induces ROS-mediated apoptosis by
modulating miR-22/SIRT-1 pathway in hepatic cancer cells. Redox
Biol. 12:340–349. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Yang F, Gong J, Wang G, Chen P, Yang L and
Wang Z: Waltonitone inhibits proliferation of hepatoma cells and
tumorigenesis via FXR-miR-22-CCNA2 signaling pathway. Oncotarget.
7:75165–75175. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Xia L, Oyang L, Lin J, Tan S, Han Y, Wu N,
Yi P, Tang L, Pan Q, Rao S, et al: The cancer metabolic
reprogramming and immune response. Mol Cancer. 20:282021.
View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Donne R and Lujambio A: The liver cancer
immune microenvironment: Therapeutic implications for
hepatocellular carcinoma. Hepatology. 77:1773–1796. 2023.PubMed/NCBI
|
|
58
|
Jhunjhunwala S, Hammer C and Delamarre L:
Antigen presentation in cancer: Insights into tumour immunogenicity
and immune evasion. Nat Rev Cancer. 21:298–312. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Kumar R, Theiss AL and Venuprasad K:
RORgammat protein modifications and IL-17-mediated inflammation.
Trends Immunol. 42:1037–1050. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Lequeux A, Noman MZ, Xiao M, Van Moer K,
Hasmim M, Benoit A, Bosseler M, Viry E, Arakelian T, Berchem G, et
al: Targeting HIF-1 alpha transcriptional activity drives cytotoxic
immune effector cells into melanoma and improves combination
immunotherapy. Oncogene. 40:4725–4735. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Togashi Y, Shitara K and Nishikawa H:
Regulatory T cells in cancer immunosuppression-implications for
anticancer therapy. Nat Rev Clin Oncol. 16:356–371. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Kang JH and Zappasodi R: Modulating Treg
stability to improve cancer immunotherapy. Trends Cancer.
9:911–927. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Golden-Mason L and Rosen HR: Galectin-9:
Diverse roles in hepatic immune homeostasis and inflammation.
Hepatology. 66:271–279. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Sauer N, Janicka N, Szlasa W, Skinderowicz
B, Kołodzińska K, Dwernicka W, Oślizło M, Kulbacka J, Novickij V
and Karłowicz-Bodalska K: TIM-3 as a promising target for cancer
immunotherapy in a wide range of tumors. Cancer Immunol Immunother.
72:3405–3425. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Zhao L, Cheng S, Fan L, Zhang B and Xu S:
TIM-3: An update on immunotherapy. Int Immunopharmacol.
99:1079332021. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Yang Q, Jiang W, Zhuang C, Geng Z, Hou C,
Huang D, Hu L and Wang X: microRNA-22 downregulation of galectin-9
influences lymphocyte apoptosis and tumor cell proliferation in
liver cancer. Oncol Rep. 34:1771–1778. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Shao X, Zhu J, Shi Y, Fang H, Chen J,
Zhang Y, Wang J, Jian H, Lan S, Jiang F, et al: Upregulated UBE4B
expression correlates with poor prognosis and tumor immune
infiltration in hepatocellular carcinoma. Aging (Albany NY).
14:9632–9646. 2022.PubMed/NCBI
|
|
68
|
McGlynn KA, Petrick JL and El-Serag HB:
Epidemiology of hepatocellular carcinoma. Hepatology. 73 (Suppl
1):S4–S13. 2021. View Article : Google Scholar
|
|
69
|
Qiao DD, Yang J, Lei XF, Mi GL, Li SL, Li
K, Xu CQ and Yang HL: Expression of microRNA-122 and microRNA-22 in
HBV-related liver cancer and the correlation with clinical
features. Eur Rev Med Pharmacol Sci. 21:742–747. 2017.PubMed/NCBI
|
|
70
|
Ke RS, Zhang K, Lv LZ, Dong YP, Pan F,
Yang F, Cai QC and Jiang Y: Prognostic value and oncogene function
of heterogeneous nuclear ribonucleoprotein A1 overexpression in
HBV-related hepatocellular carcinoma. Int J Biol Macromol.
129:140–151. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Shi C and Xu X: MicroRNA-22 is
down-regulated in hepatitis B virus-related hepatocellular
carcinoma. Biomed Pharmacother. 67:375–380. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Qian C and Liu Q: FOXO3a inhibits
nephroblastoma cell proliferation, migration and invasion, and
induces apoptosis through downregulating the Wnt/β-catenin
signaling pathway. Mol Med Rep. 24:7962021. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Tian Y, Qi P and Hu X: Downregulated
FOXO3a associates with poor prognosis and promotes cell invasion
and migration via WNT/β-catenin signaling in cervical carcinoma.
Front Oncol. 10:9032020. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Naugler WE, Sakurai T, Kim S, Maeda S, Kim
K, Elsharkawy AM and Karin M: Gender disparity in liver cancer due
to sex differences in MyD88-dependent IL-6 production. Science.
317:121–124. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Sakurai T, He G, Matsuzawa A, Yu GY, Maeda
S, Hardiman G and Karin M: Hepatocyte necrosis induced by oxidative
stress and IL-1 alpha release mediate carcinogen-induced
compensatory proliferation and liver tumorigenesis. Cancer Cell.
14:156–165. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Jiang R, Deng L, Zhao L, Li X, Zhang F,
Xia Y, Gao Y, Wang X and Sun B: miR-22 promotes HBV-related
hepatocellular carcinoma development in males. Clin Cancer Res.
17:5593–5603. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Pandey DP and Picard D: miR-22 inhibits
estrogen signaling by directly targeting the estrogen receptor
alpha mRNA. Mol Cell Biol. 29:3783–3790. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Chen S, Pu J, Bai J, Yin Y, Wu K, Wang J,
Shuai X, Gao J, Tao K, Wang G and Li H: EZH2 promotes
hepatocellular carcinoma progression through modulating
miR-22/galectin-9 axis. J Exp Clin Cancer Res. 37:32018. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Guo L, Hu C, Yao M and Han G: Mechanism of
sorafenib resistance associated with ferroptosis in HCC. Front
Pharmacol. 14:12074962023. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Cheng Y, Takeuchi H, Sonobe Y, Jin S, Wang
Y, Horiuchi H, Parajuli B, Kawanokuchi J, Mizuno T and Suzumura A:
Sirtuin 1 attenuates oxidative stress via upregulation of
superoxide dismutase 2 and catalase in astrocytes. J Neuroimmunol.
269:38–43. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Portmann S, Fahrner R, Lechleiter A, Keogh
A, Overney S, Laemmle A, Mikami K, Montani M, Tschan MP, Candinas D
and Stroka D: Antitumor effect of SIRT1 inhibition in human HCC
tumor models in vitro and in vivo. Mol Cancer Ther. 12:499–508.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Jin Q, Hu H, Yan S, Jin L, Pan Y, Li X,
Peng Y and Cao P: lncRNA MIR22HG-derived miR-22-5p enhances the
radiosensitivity of hepatocellular carcinoma by increasing histone
acetylation through the inhibition of HDAC2 activity. Front Oncol.
11:5725852021. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Yang J, Yan B, Yang L, Li H, Fan Y, Zhu F,
Zheng J and Ma X: Macrocytic anemia is associated with the severity
of liver impairment in patients with hepatitis B virus-related
decompensated cirrhosis: A retrospective cross-sectional study. BMC
Gastroenterol. 18:1612018. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Adigun OO, Yarrarapu SNS, Zubair M and
Khetarpal S: Alpha-fetoprotein analysis. In: StatPearls [Internet].
Treasure Island (FL): StatPearls Publishing; 2024
|
|
85
|
Li CQ, Huang H, Ruan SM, Hu HT, Xian MF,
Xie XY, Lu MD, Kuang M, Wang Y and Chen LD: An assessment of liver
lesions using a combination of CEUS LI-RADS and AFP. Abdom Radiol
(NY). 47:1311–1320. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Tzartzeva K and Singal AG: Testing for AFP
in combination with ultrasound improves early liver cancer
detection. Expert Rev Gastroenterol Hepatol. 12:947–949. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Zekri ARN, Youssef ASED, El-Desouky ED,
Ahmed OS, Lotfy MM, Nassar AAM and Bahnassey AA: Serum microRNA
panels as potential biomarkers for early detection of
hepatocellular carcinoma on top of HCV infection. Tumour Biol.
37:12273–12286. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Xia L, Wang S, Zhang H, Yang Y, Wei J, Shi
Y, Zou C, Liu J, Luo M, Huang A and Wang D: The HBx and HBc of
hepatitis B virus can influence Id1 and Id3 by reducing their
transcription and stability. Virus Res. 284:1979732020. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Li J, Zhang X, Chen L, Zhang Z, Zhang J,
Wang W, Wu M, Shi B, Zhang X, Kozlowski M, et al: Circulating
miR-210 and miR-22 combined with ALT predict the virological
response to interferon-alpha therapy of CHB patients. Sci Rep.
7:156582017. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Hu Q, Wang Q, Zhang Y, Tao S, Zhang X, Liu
X, Li X, Jiang X, Huang C, Xu W, et al: Baseline serum
exosome-derived miRNAs predict HBeAg seroconversion in chronic
hepatitis B patients treated with peginterferon. J Med Virol.
93:4939–4948. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Badmus OO, Hillhouse SA, Anderson CD,
Hinds TD and Stec DE: Molecular mechanisms of metabolic associated
fatty liver disease (MAFLD): Functional analysis of lipid
metabolism pathways. Clin Sci (Lond). 136:1347–1366. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Zhang P, Wang W, Mao M, Gao R, Shi W, Li
D, Calderone R, Sui B, Tian X and Meng X: Similarities and
differences: A comparative review of the molecular mechanisms and
effectors of NAFLD and AFLD. Front Physiol. 12:7102852021.
View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Chen D, Yan Y, Wang X, Li S, Liu Y, Yu D,
He Y, Deng R, Liu Y, Xu M, et al: Chronic alcohol exposure promotes
HCC stemness and metastasis through β-catenin/miR-22-3p/TET2 axis.
Aging (Albany NY). 13:14433–14455. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Yang Z, Qin W, Huo J, Zhuo Q, Wang J and
Wang L: MiR-22 modulates the expression of lipogenesis-related
genes and promotes hepatic steatosis in vitro. FEBS Open Bio.
11:322–332. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Hu Y, Liu HX, Jena PK, Sheng L, Ali MR and
Wan YY: miR-22 inhibition reduces hepatic steatosis via FGF21 and
FGFR1 induction. JHEP Rep. 2:1000932020. View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Pouwels S, Sakran N, Graham Y, Leal A,
Pintar T, Yang W, Kassir R, Singhal R, Mahawar K and Ramnarain D:
Non-alcoholic fatty liver disease (NAFLD): A review of
pathophysiology, clinical management and effects of weight loss.
BMC Endocr Disord. 22:632022. View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Liu Q, Li J, Zhang W, Xiao C, Zhang S,
Nian C, Li J, Su D, Chen L, Zhao Q, et al: Glycogen accumulation
and phase separation drives liver tumor initiation. Cell.
184:5559–5576.e19. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Agosti P, Sabbà C and Mazzocca A: Emerging
metabolic risk factors in hepatocellular carcinoma and their
influence on the liver microenvironment. Biochim Biophys Acta Mol
Basis Dis. 1864:607–617. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Hyun J, Han J, Lee C, Yoon M and Jung Y:
Pathophysiological aspects of alcohol metabolism in the liver. Int
J Mol Sci. 22:57172021. View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Jeon S and Carr R: Alcohol effects on
hepatic lipid metabolism. J Lipid Res. 61:470–479. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Lu W, Li X and Luo Y: FGF21 in obesity and
cancer: New insights. Cancer Lett. 499:5–13. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Rodríguez-Agudo R, González-Recio I,
Serrano-Maciá M, Bravo M, Petrov P, Blaya D, Herranz JM,
Mercado-Gómez M, Rejano-Gordillo CM, Lachiondo-Ortega S, et al:
Anti-miR-873-5p improves alcohol-related liver disease by enhancing
hepatic deacetylation via SIRT1. JHEP Rep. 6:1009182023. View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Iwagami Y, Zou J, Zhang H, Cao K, Ji C,
Kim M and Huang CK: Alcohol-mediated miR-34a modulates hepatocyte
growth and apoptosis. J Cell Mol Med. 22:3987–3995. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Rinella ME, Neuschwander-Tetri BA,
Siddiqui MS, Abdelmalek MF, Caldwell S, Barb D, Kleiner DE and
Loomba R: AASLD practice guidance on the clinical assessment and
management of nonalcoholic fatty liver disease. Hepatology.
77:1797–1835. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
105
|
No authors listed. The diagnosis and
management of nonalcoholic fatty liver disease: Practice guidance
from the American association for the study of liver diseases. Clin
Liver Dis (Hoboken). 11:812018. View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Castano C, Novials A and Párrizas M:
Exosomes from short-term high-fat or high-sucrose fed mice induce
hepatic steatosis through different pathways. Cells. 12:1692022.
View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Thibonnier M and Esau C: Metabolic
benefits of MicroRNA-22 inhibition. Nucleic Acid Ther. 30:104–116.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
108
|
Thibonnier M, Esau C, Ghosh S, Wargent E
and Stocker C: Metabolic and energetic benefits of microRNA-22
inhibition. BMJ Open Diabetes Res Care. 8:e0014782020. View Article : Google Scholar : PubMed/NCBI
|
|
109
|
Gjorgjieva M, Sobolewski C, Ay AS, Abegg
D, Correia de Sousa M, Portius D, Berthou F, Fournier M, Maeder C,
Rantakari P, et al: Genetic ablation of MiR-22 fosters diet-induced
obesity and NAFLD development. J Pers Med. 10:1702020. View Article : Google Scholar : PubMed/NCBI
|
|
110
|
Younossi ZM, Golabi P, de Avila L, Paik
JM, Srishord M, Fukui N, Qiu Y, Burns L, Afendy A and Nader F: The
global epidemiology of NAFLD and NASH in patients with type 2
diabetes: A systematic review and meta-analysis. J Hepatol.
71:793–801. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
111
|
Agbu P and Carthew RW: MicroRNA-mediated
regulation of glucose and lipid metabolism. Nat Rev Mol Cell Biol.
22:425–438. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
112
|
Kaur K, Vig S, Srivastava R, Mishra A,
Singh VP, Srivastava AK and Datta M: Elevated hepatic miR-22-3p
expression impairs gluconeogenesis by silencing the Wnt-responsive
transcription factor Tcf7. Diabetes. 64:3659–3669. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
113
|
Petito G, Cioffi F, Silvestri E, De
Matteis R, Lattanzi D, de Lange P, Lombardi A, Moreno M, Goglia F,
Lanni A and Senese R: 3,5-Diiodo-L-thyronine (T2) administration
affects visceral adipose tissue inflammatory state in rats
receiving long-lasting high-fat diet. Front Endocrinol (Lausanne).
12:7031702021. View Article : Google Scholar : PubMed/NCBI
|
|
114
|
Silvestri E, Cioffi F, De Matteis R,
Senese R, de Lange P, Coppola M, Salzano AM, Scaloni A, Ceccarelli
M, Goglia F, et al: 3,5-Diiodo-L-thyronine affects structural and
metabolic features of skeletal muscle mitochondria in high-fat-diet
fed rats producing a co-adaptation to the glycolytic fiber
phenotype. Front Physiol. 9:1942018. View Article : Google Scholar : PubMed/NCBI
|
|
115
|
Senese R, Cioffi F, Petito G, de Lange P,
Russo A, Goglia F, Lanni A and Potenza N: miR-22-3p is involved in
gluconeogenic pathway modulated by 3,5-diiodo-L-thyronine (T2). Sci
Rep. 9:166452019. View Article : Google Scholar : PubMed/NCBI
|
|
116
|
Zhao T, Wang J, He A, Wang S, Chen Y, Lu
J, Lv J, Li S, Wang J, Qian M, et al: Mebhydrolin ameliorates
glucose homeostasis in type 2 diabetic mice by functioning as a
selective FXR antagonist. Metabolism. 119:1547712021. View Article : Google Scholar : PubMed/NCBI
|
|
117
|
Cohen-Naftaly M and Friedman SL: Current
status of novel antifibrotic therapies in patients with chronic
liver disease. Therap Adv Gastroenterol. 4:391–417. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
118
|
Elpek GÖ: Cellular and molecular
mechanisms in the pathogenesis of liver fibrosis: An update. World
J Gastroenterol. 20:7260–7276. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
119
|
Higashi T, Friedman SL and Hoshida Y:
Hepatic stellate cells as key target in liver fibrosis. Adv Drug
Deliv Rev. 121:27–42. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
120
|
Ezhilarasan D: MicroRNA interplay between
hepatic stellate cell quiescence and activation. Eur J Pharmacol.
885:1735072020. View Article : Google Scholar : PubMed/NCBI
|
|
121
|
Riaz F, Chen Q, Lu K, Osoro EK, Wu L, Feng
L, Zhao R, Yang L, Zhou Y, He Y, et al: Inhibition of miR-188-5p
alleviates hepatic fibrosis by significantly reducing the
activation and proliferation of HSCs through PTEN/PI3K/AKT pathway.
J Cell Mol Med. 25:4073–4087. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
122
|
Chen X, Zhu S, Chen SY, Wang JN, Sun LJ,
Tao SM, Li XF, Li HD, Sun YY, Xu CH, et al: miR-301a-3p promotes
hepatic stellate cells activation and liver fibrogenesis via
regulating PTEN/PDGFR-β. Int Immunopharmacol. 110:1090342022.
View Article : Google Scholar : PubMed/NCBI
|
|
123
|
Ju A, Shen Y and Yue A: Circ_0011232
contributes to hepatocellular carcinoma progression through
miR-503-5p/AKT3 axis. Hepatol Res. 52:532–545. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
124
|
Zheng Y, Cai B, Li X, Li D and Yin G:
MiR-125b-5p and miR-181b-5p inhibit keratinocyte proliferation in
skin by targeting Akt3. Eur J Pharmacol. 862:1726592019. View Article : Google Scholar : PubMed/NCBI
|
|
125
|
Zhang Y, Wang F, Chen G, He R and Yang L:
LncRNA MALAT1 promotes osteoarthritis by modulating miR-150-5p/AKT3
axis. Cell Biosci. 9:542019. View Article : Google Scholar : PubMed/NCBI
|
|
126
|
Abdullah AS, Sayed IETE, El-Torgoman AMA,
Kalam A, Wageh S and Kamel MA: Green synthesis of
silymarin-chitosan nanoparticles as a new nano formulation with
enhanced anti-fibrotic effects against liver fibrosis. Int J Mol
Sci. 23:54202022. View Article : Google Scholar : PubMed/NCBI
|
|
127
|
Abdullah AS, El Sayed IET, El-Torgoman
AMA, Alghamdi NA, Ullah S, Wageh S and Kamel MA: Preparation and
characterization of silymarin-conjugated gold nanoparticles with
enhanced anti-fibrotic therapeutic effects against hepatic fibrosis
in rats: Role of MicroRNAs as molecular targets. Biomedicines.
9:17672021. View Article : Google Scholar : PubMed/NCBI
|
|
128
|
Tsuchida T, Lee YA, Fujiwara N, Ybanez M,
Allen B, Martins S, Fiel MI, Goossens N, Chou HI, Hoshida Y and
Friedman SL: A simple diet- and chemical-induced murine NASH model
with rapid progression of steatohepatitis, fibrosis and liver
cancer. J Hepatol. 69:385–395. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
129
|
Ji D, Li B, Shao Q, Li F, Li Z and Chen G:
MiR-22 suppresses BMP7 in the development of cirrhosis. Cell
Physiol Biochem. 36:1026–1036. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
130
|
Onakpoya IJ, Heneghan CJ and Aronson JK:
Post-marketing withdrawal of 462 medicinal products because of
adverse drug reactions: A systematic review of the world
literature. BMC Med. 14:102016. View Article : Google Scholar : PubMed/NCBI
|
|
131
|
Li X, Tang J and Mao Y: Incidence and risk
factors of drug-induced liver injury. Liver Int. 42:1999–2014.
2022. View Article : Google Scholar : PubMed/NCBI
|
|
132
|
Kleiner DE: Drug-induced liver injury: The
hepatic pathologist's approach. Gastroenterol Clin North Am.
46:273–296. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
133
|
Kleiner DE, Chalasani NP, Lee WM, Fontana
RJ, Bonkovsky HL, Watkins PB, Hayashi PH, Davern TJ, Navarro V,
Reddy R, et al: Hepatic histological findings in suspected
drug-induced liver injury: Systematic evaluation and clinical
associations. Hepatology. 59:661–670. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
134
|
Yang Z, Wu W, Ou P, Wu M, Zeng F, Zhou B
and Wu S: MiR-122-5p knockdown protects against APAP-mediated liver
injury through up-regulating NDRG3. Mol Cell Biochem.
476:1257–1267. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
135
|
Vliegenthart ADB, Berends C, Potter CMJ,
Kersaudy-Kerhoas M and Dear JW: MicroRNA-122 can be measured in
capillary blood which facilitates point-of-care testing for
drug-induced liver injury. Br J Clin Pharmacol. 83:2027–2033. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
136
|
Liu Y, Chen H, Hao J, Li Z, Hou T and Hao
H: Characterization and functional prediction of the microRNAs
differentially expressed in a mouse model of concanavalin A-induced
autoimmune hepatitis. Int J Med Sci. 17:2312–2327. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
137
|
López-Riera M, Conde I, Tolosa L, Zaragoza
A, Castell JV, Gómez-Lechón MJ and Jover R: New microRNA biomarkers
for drug-induced steatosis and their potential to predict the
contribution of drugs to non-alcoholic fatty liver disease. Front
Pharmacol. 8:32017. View Article : Google Scholar : PubMed/NCBI
|
|
138
|
Amacher DE and Chalasani N: Drug-induced
hepatic steatosis. Semin Liver Dis. 34:205–214. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
139
|
Allard J, Le Guillou D, Begriche K and
Fromenty B: Drug-induced liver injury in obesity and nonalcoholic
fatty liver disease. Adv Pharmacol. 85:75–107. 2019. View Article : Google Scholar : PubMed/NCBI
|

















