Introduction
Papillary thyroid carcinoma (PTC) is the most common
pathological type of thyroid malignant tumor, with favorable
surgical outcomes and low mortality rates (1). However, the recurrence and persistence
of tumors in situ and in lymph nodes after PTC surgery is
not rare (2). An epidemiological
analysis indicated that the incidence of thyroid cancer in China
increased at an annual rate of ~20% from 2003 to 2011, and the rate
of increase ranks first among all malignant tumors (3). By 2022, thyroid cancer had become the
cancer with the third highest incidence in Chinese women, while the
incidence of that in men ranking seventh. (4). This may be due to a considerable
increase in accessibility of medical examination and medical
insurance in China. In a large number of cases, PTCs are small
tumors found by ultrasound examination; therefore, the mortality
rate of thyroid cancer in China has not increased markedly
(5). Studies have estimated that in
several countries, thyroid cancer is overdiagnosed in >80% of
female and ~70% of male patients (6–9),
making it essential to address the issue of overtreatment. For
example, thyroidectomy and lifelong hormone replacement therapy not
only lead to an unnecessary economic burden on individuals and
society, but also reduce the quality of life of patients (6). However, PTCs vary in their degree of
invasiveness, and if not effectively diagnosed and an appropriate
intervention applied, the tumor burden will continue to increase
and affect the quality of life of patients, even resulting in
death.
Multifocal PTC (MPTC) is the presence of two or more
anatomically independent PTC lesions in the same thyroid gland.
This multifocal phenomenon occurs frequently (10) and is considered to be a high-risk
factor for poor prognosis, requiring more active intervention and
treatment (11). Studies on the
clonal origins of MPTC tend to rely on the detection and analysis
of known key pathogenic genes. If multiple MPTC lesions are formed
due to the metastasis and dissemination of one cancer lesion in the
thyroid, the gene expression and status of the multiple cancer
lesions tend to be consistent. Conversely, when multiple cancerous
lesions in the thyroid gland arise from the cloning of progenitor
cells with independent origins, the cancer cells within these
lesions often display inconsistent or partially inconsistent gene
expression and states. This is known as multicenter independent
origin MPTC (12–15). A number of gene mutations are
frequently detected in PTC, including RAS, v-raf murine sarcoma
viral oncogene homolog B1 (BRAF), telomerase reverse transcriptase
(TERT) and rearranged during transfection (RET)/PTC mutations,
which are closely associated with the pathogenesis of PTC (16,17). A
mutation at the T1799A site in the BRAF gene continuously activates
the MAPK pathway, leading to dysregulation of the cell cycle, which
is significantly associated with PTC (18). The mutation rate of the BRAF gene in
PTC lesions is 23–83% overall, and >70% in Asian populations
(19). The TERT gene plays a role
in the maintenance of chromosome stability (20). While the mutation rate of the TERT
gene is relatively low at 10–13% in differentiated thyroid cancer,
it can reach as high as ~40% in undifferentiated and poorly
differentiated thyroid cancer (17). The presence of TERT gene mutations
in PTC often indicates a more aggressive tumor or rapid disease
progression (21). Thus, the
detection of these mutations is useful when exploring the clonal
origin of MPTC.
Numerous studies have been performed on the clonal
origin of MPTC, but studies exploring the differences in biological
behavioral between MPTCs with different clonal origins are lacking.
It would be beneficial to conduct in-depth research on this issue,
to enable the precise treatment of MPTC to be more evidence-based.
Therefore, the present study aimed to employ a combination of
dual-gene and dual-protein markers to analyze the genomic status
and expression patterns of clinical MPTC samples, and categorize
the tumors based on their clonal origin. Further analyses were
conducted to examine the differences in biological behavior between
cases with different clonal origins and provide evidence to guide
treatment timing and follow-up planning for different types of
MPTCs.
Materials and methods
General information
The case data of patients with MPTC in Ma'anshan
People's Hospital (Ma'anshan, China) from March 2020 to January
2024 were reviewed. Inclusion criteria included: i) Histologically
confirmed papillary thyroid cancer; ii) ≥2 cancer foci per case;
iii) a maximum tumor diameter of ≥1 mm to ensure that the accuracy
of the detection results was not compromised due to insufficient
tumor tissue; iv) absence of a history of neck radiation exposure;
and v) initial PTC cases. The study was conducted following
approval by the ethics committee of Ma'anshan People's Hospital
(approval no. 2022-077-007). A total of 52 patients were selected
using a random number method, and postoperative tumor tissue
specimens were obtained for testing. There were 33 females (63.46%)
aged between 19–75 years, and 19 males (36.54%) aged between 27–68
years. The clinicopathological data of these cases, including age
at diagnosis, sex, thyroid immunological indices, tumor size and
location were collected for analysis, along with data on tumor
invasive behavior, categorized as central lymph node metastasis,
lateral cervical lymph node metastasis and capsule invasion.
Detection methods
All MPTC cancer lesions were analyzed for the
presence of the BRAF gene (V600E) mutation using quantitative PCR
(qPCR) technology, and the TERT gene (C228T and C250T) mutations
were detected using Sanger sequencing technology.
Immunohistochemical staining was employed to assess the expression
levels of TERT and BRAF proteins in each cancer lesion, and the
gene mutations and protein expression patterns were recorded. The
genes and accession numbers analyzed in this study are as follows:
TERT, accession number NG_009265; BRAF, accession number
NG_007873.
Immunohistochemical staining
For each cancer lesion, 3 tissue sections with a
thickness of 4 µm were prepared. The primary antibodies used were
mouse anti-human BRAF monoclonal antibody (cat no. TA500431) and
rabbit anti-human TERT polyclonal antibody (cat. no. TA324097),
both from OriGene Technologies, Inc, a dilution of 1:200. The
secondary antibody kit adopted the ready-to-use rapid
immunohistochemical Max Vision 2 kit (cat. no. KIT-5920, MXB
Biotechnologies), which includes a secondary antibody for rodents
and rabbits and DAB staining solution. The blocking process used a
3% hydrogen peroxide solution at room temperature for 10 min. The
incubation conditions of primary antibody were set at 4°C for 12 h,
followed by rewarming at 37°C for 15 min. The positive staining of
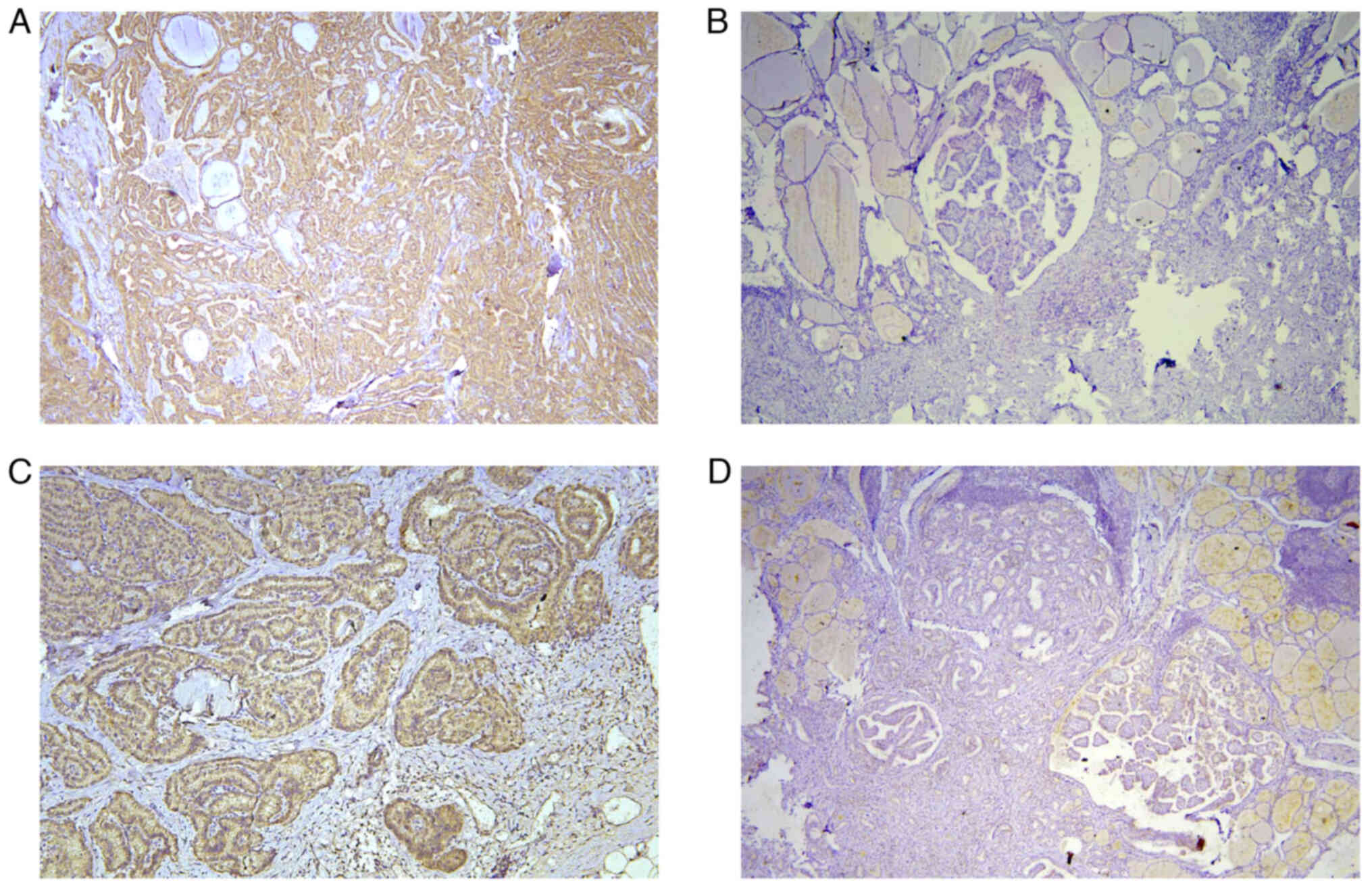
BRAF protein and TERT protein were yellow colored particles in
cytoplasm observed under light microscope. The staining results
were manually counted independently by two experienced
pathologists, and the positive cell rate and staining intensity of
each slice were graded and scored using the immune reactivity score
(IRS). The staining intensity score: negative, 0; Weak, 1; Medium,
2; Strong, 3. The positive cell rate score: 5 fields were randomly
selected under 400 magnification, <5%, 0; 5–25%, 1; 26–50%, 2;
51–75%, 3; >75%, 4. The positive intensity was determined by
calculating the product of these two indicators, and the mean of
the three sections was taken as the final staining result for each
case, and the score ≥5 was recorded as positive. Any inconsistent
results were assessed jointly by the two pathologists and discussed
until an agreed result is reached.
qPCR
A QIAmp DNA FFPE Tissue Kit (cat. no. 56404; Qiagen
AB) was used to extract nucleic acid from paraffin-embedded cancer
tissue. Quality control was performed using an SMA4000
spectrophotometer (Merinton Ltd.) to assess DNA concentration and
the ratio of optical density at 260 and 280 nm (1.6–2.0 was
considered to indicate adequate quality). For the detection of BRAF
mutations at specific sites [chr7:140453136, c.1799T>A,
p.Val600Glu (V600E)], PCR reaction systems and quality control
reaction mixtures were prepared separately. The PCR reaction
mixture (Human BRAF gene V600E mutation detection kit, Wuhan YZY
Biopharma Co., Ltd.) contained 22.8 µl PCR reaction mixture, 0.2 µl
enzyme mixture and 2 µl DNA template. PCR amplification was
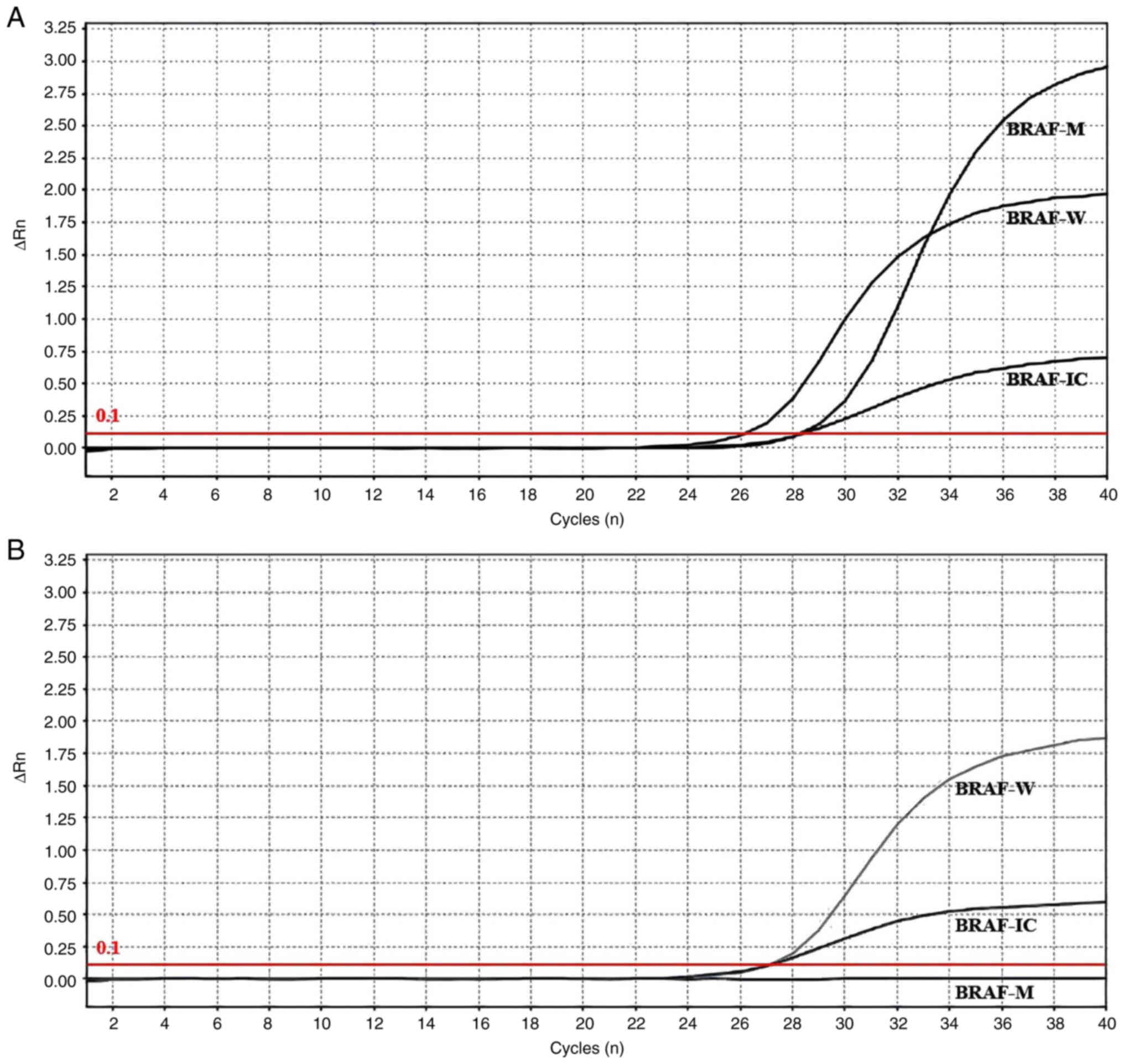
conducted using the Applied Biosystems 7,500 Real-Time PCR Systems
(Thermo Fisher Scientific, Inc.). The cycling condition was 95°C
for 5 min, followed by 40 cycles of 95°C for 15 sec and 60°C for 1
min. After amplification, a fluorescence threshold was established
based on the amplification curve, and Cq values were obtained for
different channels. These Cq values were used to calculate ΔCq
values. For mutation detection, the Cq value of the FAM channel was
observed. If the Cq value of the FAM channel was >38 or could
not be determined, the sample was considered negative or below the
detection limit of the kit. By contrast, if the Cq value of the FAM
channel was <38, the ΔCq value was calculated by subtracting the
Cq value of the external control signal (FAM) from the Cq value of
the mutation signal (FAM). If the calculated ΔCq value was <9,
the sample was determined to be mutation positive. Otherwise, it
was considered mutation negative or below the detection limit of
the kit. The primers and probes used in the amplification reaction
were as follows: BRAF upstream, 5′-CAACTGTTCAAACTGATGGGAC-3′; BRAF
downstream, 5′-AAAATAGGTGATTTTGGTCTAGCTACACA-3′; BRAF probe,
FAM-CATCGAGATTTCTGTG-MGB; internal control upstream,
5′-CTTCTTGCCTCTTGTCTCTTAGT-3′; internal control downstream,
5′-GCAACAATATCCACTTTACCAGA-3′; internal control probe,
CY5-TGACCAGGCGCCCAATACGA-BHQ3. Glyceraldehyde-3-phosphate
dehydrogenase was used as an endogenous control.
Sanger sequencing
QIAmp DNA FFPE Tissue Kit was used to extract
nucleic acid from paraffin-embedded cancer tissue samples.
Subsequently, the nucleic acids were quality-checked using SMA4000
UV–Vis Spectrophotometer to assess DNA concentration and the
OD260/280 ratio (1.6–2.0 was considered to indicate adequate
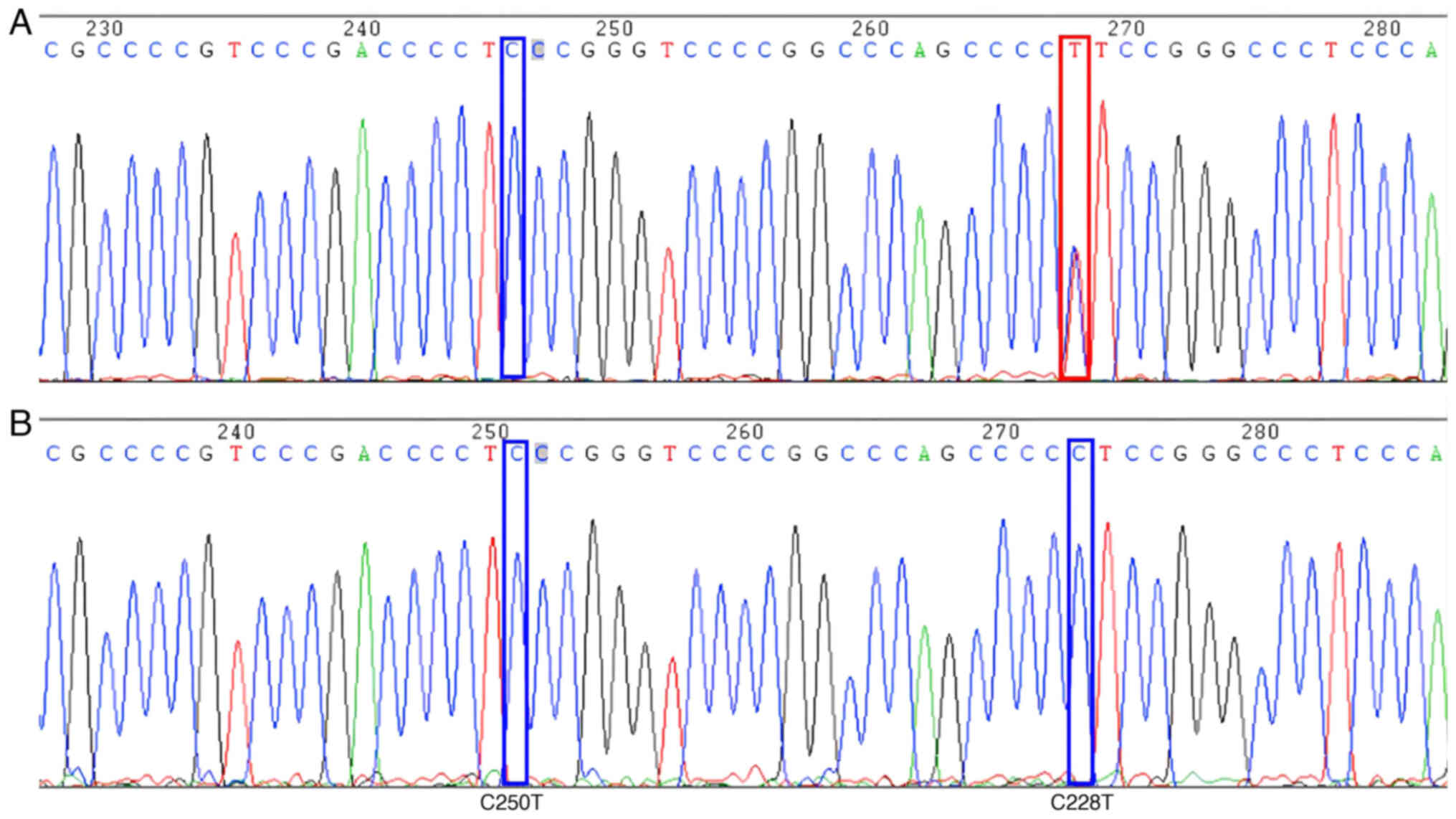
quality). For the detection of TERT sites (chr5:1295228, C228T;
chr5:1295250, C250T), primers and a PCR reaction mix were used. The
PCR reaction mixture comprised 2X PCR Mix (10 µl), PCR primers (10
µM; 1 µl), DNA template (2 µl) and double-distilled H2O
(7 µl). The PCR was performed under standard conditions and yielded
a PCR product with an expected size of 235 bp. Following PCR, 5 µl
of the product was analyzed by agarose gel electrophoresis. If a
clear and distinct target band was visible, the sample proceeded to
the next step. Sanger sequencing was then performed according to
standard operating procedures. Sequencing sample preparation was
carried out using the primers 5′-AGTGGATTCGCGGGCACAG-3′ (forward)
and 5′-CAGCGCTGCCTGAAACTCG-3′ (reverse). Sequencing was conducted
using the ABI 3730×l Genetic Analyzer (Thermo Fisher Scientific,
Inc.). The sequencing results were aligned with the reference
sequence using the SeqMan algorithm in Lasergene 17.3 software
(DNASTAR, Inc.). Alternatively, the sequencing results were
analyzed using ChromasPro 2.1.5 software (Technelysium Pty Ltd.) to
search for sequences before and after the target mutation site. The
base type at the target site and the presence of any significant
mutation peaks (A, green; T, red; C, blue; G, black) were observed.
If a significant difference in the target mutation base peak (T,
red) compared with the background peak was observed, it was
considered a mutation. Conversely, if only the target base peak (C,
blue) was present, there was deemed to be no mutation at that site.
In order to obtain consistent sequencing results, all PCR fragments
were sequenced at least twice. The TERT gene target mutation site
and its flanking nucleotide sequence were as follows:
CCGCCCCGTCCCGACCCCT[C250T]CCGGGTCCCCGGCCCAGCCCC[C228T]TCCGGGCCCTCCCA.
Statistical analysis
SPSS 22.0 software (IBM Corp.) was utilized for data
processing, employing the χ2 and Fisher's exact test as
appropriate to analyze protein expression, gene mutation and
clinical pathological data, including age, sex, tumor size and
location distribution, Hashimoto's thyroiditis, and tumor
invasiveness, in MPTCs with different clonal origins. P<0.05 was
considered to indicate a statistically significant difference.
Results
Detection of proteins and genes in
MPTC cases
A total of 128 PTC lesions were analyzed by
immunohistochemistry, of which 109 lesions tested positive for BRAF
protein expression (85.2%) and 69 lesions tested positive for TERT
protein expression (53.9%). The BRAF V600E gene mutation was
identified in 108 cancer foci (84.4%), and the TERT promoter
mutation was detected in 6 cancer foci (4.7%). Representative
detection results for BRAF and TERT proteins, BRAF gene and TERT
gene are presented in Fig. 1,
Fig. 2, Fig. 3, respectively.
Analysis of clonal origins in
MPTC
Through the detailed examination of protein
expression and gene mutation patterns across all cancerous foci,
the cases were categorized into two distinct groups: i)
Intraglandular metastasis lesion group; and ii) multicentric
independent origin lesion group. Cases where all cancerous foci
within the same patient exhibited identical gene mutations and
protein expression results were designated as having a common
clonal origin and assigned to the intraglandular metastatic lesion
group. Conversely, those displaying heterogeneous gene mutations or
protein expression results were considered to have distinct clonal
origins and were included in the multicentric independent origin
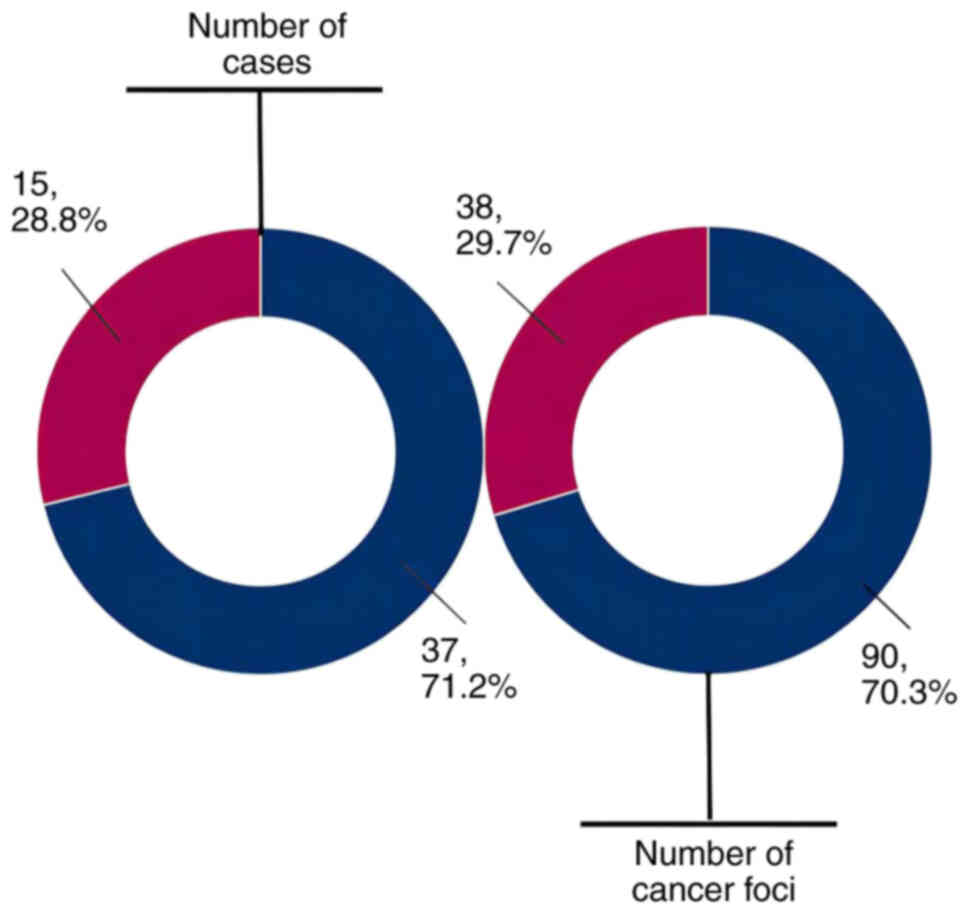
lesion group. Among the 52 cases examined, the analysis indicated
that foci in 37 MPTC cases (71.2%) originated from intraglandular
metastasis, while those in 15 MPTC cases (28.8%) had independent
multicentric origins. In addition, the intraglandular metastatic
lesion group encompassed 90 cancerous foci (70.3%), while the
multicentric independent origin lesion group comprised 38 foci
(29.7%; Fig. 4).
Genetic mutations and protein
expression patterns
The intraglandular metastatic lesion group exhibited
a significantly higher positive expression rate of BRAF protein
(91.1%) compared with the multicentric independent origin lesion
group (71.1%; P<0.05). However, no significant difference was
observed in the positive expression rate of TERT protein between
the two groups (58.9 vs. 42.1%; P>0.05). Additionally, the
intraglandular metastatic lesion group demonstrated a significantly
higher mutation rate of the BRAF V600E gene (88.9%) compared with
the multicentric independent origin lesion group (73.7%;
P<0.05). Conversely, no significant difference was observed in
the mutation rate of the TERT gene between the two groups (5.6 vs.
2.6; P>0.05; Table I).
 | Table I.Gene mutation and protein expression
in cases of multifocal papillary thyroid carcinoma with different
clonal origins. |
Table I.
Gene mutation and protein expression
in cases of multifocal papillary thyroid carcinoma with different
clonal origins.
| Test items | Intraglandular
metastatic group | Multicentric
carcinoma group | χ2 | P-value |
|---|
| BRAF protein |
|
| 8.505 | 0.004 |
|
Positive | 82 | 27 |
|
|
|
Negative | 8 | 11 |
|
|
| TERT protein |
|
| 3.029 | 0.082 |
|
Positive | 53 | 16 |
|
|
|
Negative | 37 | 22 |
|
|
| BRAF gene |
|
| 4.685 | 0.030 |
|
Mutated | 80 | 28 |
|
|
| Wild
type | 10 | 10 |
|
|
| TERT gene |
|
| - | 0.669 |
|
Mutated | 5 | 1 |
|
|
| Wild
type | 85 | 37 |
|
|
Clinical pathological data
The clinical characteristics of the MPTC cases were
compared between groups with different clonal origins. No
statistically significant difference in the clonal origin of PTC
multifocality was detected between men and women (P>0.05).
Notably, a significantly higher proportion of patients aged ≥50
years was observed in the intraglandular metastatic lesion group
(43.2%) compared with the multicentric independent origin lesion
group (13.3%). These results indicate that multiple cancerous foci
in older patients are more likely to be formed by metastasis within
the thyroid gland, while by contrast, the multiple cancerous foci
in younger patients are more likely to form independently from
multiple centers (P<0.05). In terms of central lymph node
metastasis, the intraglandular metastatic lesion group demonstrated
a slightly higher rate (75.7%) compared with the multicentric
independent origin lesion group (73.3%), but this difference was
not found to be statistically significant (P>0.05). However,
when considering lateral cervical lymph node metastasis, the
intraglandular metastatic lesion group exhibited a significantly
higher rate (29.7%) compared with the multicentric independent
origin lesion group (0%), indicating that the likelihood of lateral
neck lymph node metastasis is increased in multifocal cases with
intraglandular metastatic origin (P<0.05). Additionally, no
significant association was identified between Hashimoto's
thyroiditis and the clonal origin of multifocal carcinoma
(P>0.05). Furthermore, the tumor capsule invasion rate was
significantly higher in the intraglandular metastatic lesion group
(48.6%) compared with the multicentric independent origin lesion
group (13.3%), suggesting that multifocal tumors with
intraglandular metastatic origin have an increased propensity to
invade the thyroid capsule (P<0.05). Notably, the proportion of
tumors with a maximum diameter of >2 cm was significantly higher
in the multicentric independent origin lesion group (33.3%)
compared with the intraglandular metastatic lesion group (5.4%),
indicating that the tumor growth rate of MPTC with multicentric
independent origin may be faster than that of intraglandular
metastatic MPTC (P<0.05). However, no significant difference was
observed in the distribution of carcinoma foci between the two
groups (P>0.05; Table II).
 | Table II.Clinicopathological data of cases of
multifocal papillary thyroid carcinoma with different clonal
origins. |
Table II.
Clinicopathological data of cases of
multifocal papillary thyroid carcinoma with different clonal
origins.
| Variable | Intraglandular
metastatic group | Multicentric
carcinoma group | χ2 | P-value |
|---|
| Sex |
|
| 0.093 | 0.760 |
|
Male | 14 | 5 |
|
|
|
Female | 23 | 10 |
|
|
| Age, years |
|
| 4.219 | 0.040 |
|
≥50 | 16 | 2 |
|
|
|
<50 | 21 | 13 |
|
|
| Central lymph
nodes |
|
| - | 1.000 |
|
Metastasis | 28 | 11 |
|
|
| No
metastasis | 9 | 4 |
|
|
| Lateral cervical
lymph nodes |
|
| - | 0.022 |
|
Metastasis | 11 | 0 |
|
|
| No
metastasis | 26 | 15 |
|
|
| Hashimoto's
thyroiditis |
|
| - | 0.318 |
|
Yes | 9 | 6 |
|
|
| No | 28 | 9 |
|
|
| Capsule
invasion |
|
| 5.624 | 0.018 |
|
Yes | 18 | 2 |
|
|
| No | 19 | 13 |
|
|
| Maximum tumor
diameter, cm |
|
| - | 0.016 |
| ≤2 | 35 | 10 |
|
|
|
>2 | 2 | 5 |
|
|
| Tumor
distribution |
|
| 0.588 | 0.443 |
|
Unilateral | 14 | 4 |
|
|
|
Bilateral | 23 | 11 |
|
|
Discussion
Numerous studies have been carried out to compare
the pathological characteristics and clinical prognosis between
single-lesion PTC and MPTC (10,22–24).
Most of these studies indicate that MPTC is associated with a worse
patient prognosis compared with single-lesion PTC. For example, a
meta-analysis encompassing 23 studies with a total of 41,616
patients revealed that, compared with single-lesion PTC, MPTC has
an elevated risk of extrathyroidal invasion, lymphovascular
invasion, central and lateral neck lymph node metastasis, distant
metastasis, and postoperative recurrence (11). Another meta-analysis, which analyzed
26 studies involving 33,976 patients, and other experimental
studies found that MPTC was significantly associated with an
increased risk of tumor recurrence (25–28).
However, in terms of prognosis, no marked difference has been found
for mortality between MPTC and single-lesion PTC, as both have a
low total mortality rate (11,25).
Therefore, although MPTC is more aggressive, the overtreatment of
MPTC should be avoided. Furthermore, it is important to identify
the types of MPTC that might require more intensive treatment and
follow-up plans in order to improve the long-term quality-of-life
of patients and reduce socioeconomic costs. Therefore, the present
study subdivided MPTC cases according to the clonal origin of
multiple cancer foci and explored the biological differences
between MPTCs with different clonal origins.
There have been numerous studies on the clonal
origin of MPTC (29–32); however, there is no definitive
evidence indicating whether the cancer foci of MPTC are formed by
multicentric independent origin and/or by intraglandular
metastasis. The results of the present study indicate that the
multiple loci in MPTC are formed by intraglandular metastasis in
some cases and are of multicenter independent origin in others.
Analysis of the multi-molecular expression patterns within each
cancer lesion revealed that a notable 71.2% of cases had multiple
lesions with common molecular expression patterns. These data
strongly suggest that MPTC primarily develops from a monoclonal
origin, leading to the formation of multiple tumors via
intraglandular metastasis. This aligns with the findings of Park
et al (12) regarding MPTC
in the Korean population. By analyzing BRAF gene mutations in
cancerous lesions, the authors discovered that 39.3% of cases
exhibited heterogeneous mutations, suggesting multicentric clonal
origins. Conversely, the majority of cases exhibited intraglandular
metastases arising from monoclonal origins (12). Research by McCarthy et al
(14), based on the detection of X
chromosome inactivation, also indicated that the lesions of MPTC
more often have the same clonal origin, and suggested that
intraglandular metastasis plays an important role in the spread of
thyroid carcinoma. Lin et al (33) also found that most MPTC cases they
studied had a monoclonal origin, with a common mutation pattern, as
evidenced by small-fragment loss of heterozygosity and BRAF gene
mutation results. This led to the suggestion that the origin of
MPTC may be more important than tumor size in the prediction of
lymph node metastasis, invasion and prognosis (33). However, several studies offer
contrasting perspectives. Bansal et al (13) conducted a thorough analysis of BRAF,
neuroblastoma RAS viral oncogene homolog, Harvey rat sarcoma viral
oncogene homolog and Kirsten rat sarcoma viral oncogene homolog
point mutations along with RET/PTC1 and RET/PTC3 rearrangements in
each lesion. They found that ~60% of cases exhibited distinct gene
mutation patterns, supporting the hypothesis of a multicentric
origin (13). Lu et al
(34) reached similar conclusions
after applying next-generation sequencing technology to the genomic
detection of MPTC. Also, the results of X-chromosome inactivation
analysis in another study support the formation of multiple lesions
in MPTC as independent tumors (35).
In the present study, MPTC was categorized into two
distinct groups based on the type of clonal origin, and the
clinicopathological characteristics and biological behaviors
associated with different clonal origins were compared. The results
revealed that the incidence of MPTC was highest in women,
accounting for 63.5% of the study cohort, but sex was not found to
play a significant role in the clonal origin of MPTC. Age, an
independent adverse prognostic factor for PTC (36,37),
is incorporated in most staging or scoring systems, including the
following: Tumor-node-metastasis classification staging system;
age, metastases, extent, size scoring system; age, grade, extent,
size scoring system; and distant metastasis, patient age,
completeness of resection, local invasion, and tumor size scoring
system (38,39). The present study found that the
proportion of patients with intraglandular metastatic MPTC
significantly increased in patients aged ≥50 years. Since cases of
intraglandular metastasis are more aggressive than those of
multicenter independent origin, these findings suggest that older
patients are not only more prone to intraglandular metastasis but
also may experience faster local tumor progression compared with
younger patients, aligning with previous studies that highlight
advanced age as a risk factor for PTC (40,41).
When analyzing factors associated with tumor invasion, a noteworthy
observation was made. Specifically, the rate of lateral cervical
lymph node metastasis and incidence of tumor capsule invasion were
significantly elevated in intraglandular metastatic MPTC compared
with multicenter independent origin MPTC. However, no significant
difference was observed in the central lymph node metastasis rate
between the two groups. Previous studies on PTC have reported that
the risk of central lymph node metastasis is increased for MPTC
compared with unifocal PTC (42,43).
The findings of the present study indicate that MPTC of either
clonal origin exhibits a high probability of central lymph node
metastasis; regardless of the clonal origin of MPTC, there was no
significant difference in the high probability of central lymph
node metastasis. However, the risk of lateral cervical lymph node
metastasis and capsular invasion of the tumor for intraglandular
metastatic MPTC was significantly higher than that of multicentric
independent origin PTC, further confirming that MPTC formed by
intraglandular metastasis has higher invasiveness. These findings
are of great importance for in-depth understanding of tumor spread
mechanisms and for the development of individualized treatment
plans and follow-up strategies for different types of MPTC. Also,
these findings are consistent with the retrospective analysis of
2,095 patients with PTC by Kim et al (44). In the present study, using a tumor
diameter of 2 cm as the cutoff, the proportion of cases with
small-diameter tumors in the intraglandular metastatic MPTC group
significantly surpassed that in the multicentric independent origin
MPTC group. The finding is exemplified by a notable case of MPTC
reported by Korean researchers. Through morphological observation,
the authors found a 1.5-cm isthmus dominant tumor, with >30
smaller cancer foci distributed in the surrounding glandular lobes,
which decreased in density as the distance from the main focus in
the isthmus increased. BRAF gene mutations were found in all cancer
foci and metastatic lymph nodes, which supported the hypothesis of
intraglandular metastasis in the case (45). With regard to the distribution of
tumors, the present study did not find a significant difference in
unilateral and bilateral distribution between the two types of
MPTC, which may be due to the abundant lymph node drainage system
in the thyroid gland, which facilitates the metastasis of PTC
throughout the whole thyroid, rather than retaining it on one side
of the gland (46). Hashimoto's
thyroiditis has been suggested to elevate the prevalence of PTC,
including MPTC, and is associated with a heightened risk of distant
metastasis (47). However, the
present analysis did not detect significant differences in the
proportion of patients with Hashimoto's thyroiditis between the two
MPTC groups, indicating that Hashimoto's thyroiditis has limited
impact on the clonal origin of multifocal lesions.
The research methodology of the present study was
designed based on the insights and findings of previous studies,
with the aim of minimizing inaccuracies stemming from reliance on a
single gene mutation or protein expression. The prevalence of BRAF
gene mutations and protein expression was high in the thyroid
cancer foci, while the expression of TERT protein and the mutation
frequency of the TERT gene were notably lower. If only a single
gene or protein had been utilized in the analysis of clonal origin,
numerous cases may have been erroneously found to exhibit a common
molecular expression pattern, potentially leading researchers to an
inaccurate hypothesis. In the RAF family, the MEK kinase activity
of BRAF kinase is more prominent than those other isoforms, and the
activation and strong catalytic activity of BRAF can be achieved
through single-point mutation (48). Previous studies have demonstrated
that there is a close association between the BRAF gene V600E
mutation and the invasiveness and recurrence risk of thyroid cancer
(49,50). In the present study it was found
that the BRAF V600E mutation and BRAF protein expression in
intraglandular metastatic MPTC were significantly higher than those
in multicentric independent origin MPTC, which is consistent with
the aforementioned stronger tumor invasiveness in the
intraglandular metastatic cancer group. However, no significant
differences were found in the expression of TERT protein and the
incidence of TERT promoter mutations between the two groups, which
is consistent with the consensus that the overall survival
prognosis of MPTC is favorable, regardless of TERT status. However,
when TERT promoter mutations coexist with the BRAF V600E mutation,
they promote the progression of thyroid cancer by inducing cancer
cell dedifferentiation through ribosomal biogenesis, and induce the
formation of poorly differentiated thyroid cancer, indicating a
poor prognosis (51).
For MPTC, which tends to locally persist and has a
high risk of recurrence, the impact on long-term survival is low,
and the long-term quality of life of patients and the economic cost
are important factors to be considered. Given the risk of nerve and
parathyroid injuries in secondary thyroid surgeries, it is
recommended that the surgical approach for patients with MPTC
should favor more aggressive thyroidectomy and central compartment
lymph node dissection. In patients with intraglandular metastatic
MPTC, a meticulous assessment of cervical lymph node metastasis is
crucial, and a more proactive intervention in these nodes is
advisable to prevent long-term recurrence or persistence of the
tumor in the tissue and lymph nodes. When determining the
appropriate follow-up intensity for MPTC, it is important to
further analyze the clonal origin type by postoperative pathology
and genetic testing. This approach allows for a more active and
individualized follow-up strategy, including TSH inhibition
therapy, for the treatment of intraglandular metastatic MPTC.
In conclusion, intraglandular metastasis
predominates in MPTC, whereas cases where the MPTC is of
multicentric clonal origin are comparatively infrequent. In
comparison with MPTC of multicentric clonal origin, intraglandular
metastatic MPTC exhibits a more aggressive tumor phenotype, often
manifesting in lateral neck lymph node metastasis and capsular
invasion. This knowledge may be used to provide a more proactive
and individualized approach when determining the type of surgery,
use of TSH suppression treatment and follow-up intensity in cases
of intraglandular metastatic MPTC.
Acknowledgements
Not applicable.
Funding
This study was supported by the Zhejiang Provincial Health
Science and Technology Foundation (grant no. 2022KY054) and the
Ma'anshan City Science and Technology Foundation (grant no.
YL-2022-8).
Availability of data and materials
The data generated in the present study are
available from Ma'anshan People's Hospital Pathology Laboratory and
Zhejiang Dingjing Medical Laboratory Co., Ltd., China, but
restrictions apply to the availability of these data, which were
used under license for the current study, and so are not publicly
available. Data are however available from the authors upon
reasonable request and with permission of Ma'anshan People's
Hospital Pathology Laboratory and Zhejiang Dingjing Medical
Laboratory Co., Ltd., China.
Authors' contributions
WS and JW initiated the conception and design of the
study, participated in the interpretation of the data and wrote the
article. ZL and QZ were the main conductors of the experiments and
participated in the analysis of the data. QH participated in the
collection of experimental specimens, data acquisition and
analysis. WS and JW confirm the authenticity of all the raw data.
All authors read and approved the final version of the
manuscript.
Ethics approval and consent to
participate
The present retrospective study involve experimental
work on human tissue specimens collected after surgical procedures.
The Ethics Committee of Ma'anshan People's Hospital (Ma'anshan,
China) granted approval for the study following thorough ethical
scrutiny (approval no. 2022-077-007). Written informed consent was
obtained from each participating patient or their authorized
representative.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Choi WR, Roh JL, Gong G, Cho KJ, Choi SH,
Nam SY and Kim SY: Multifocality of papillary thyroid carcinoma as
a risk factor for disease recurrence. Oral Oncol. 94:106–110. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Zhang JW, Fei MJ, Hou YQ, Tang ZY, Zhan WW
and Zhou JQ: Long-term follow-up ultrasonography surveillance in a
large cohort of patients with papillary thyroid carcinoma.
Endocrine. 77:297–304. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Chen W, Zheng R, Baade PD, Zhang S, Zeng
H, Bray F, Jemal A, Yu XQ and He J: Cancer statistics in China,
2015. CA Cancer J Clin. 66:115–132. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Zheng RS, Chen R, Han BF, Wang SM, Li L,
Sun KX, Zeng HM, Wei WW and He J: Cancer incidence and mortality in
China, 2022. Zhonghua Zhong Liu Za Zhi. 46:221–231. 2024.(In
Chinese). PubMed/NCBI
|
|
5
|
Li M, Zheng R, Dal Maso L, Zhang S, Wei W
and Vaccarella S: Mapping overdiagnosis of thyroid cancer in China.
Lancet Diabetes Endocrinol. 9:330–332. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Li M, Dal Maso L and Vaccarella S: Global
trends in thyroid cancer incidence and the impact of overdiagnosis.
Lancet Diabetes Endocrinol. 8:468–470. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Vaccarella S, Franceschi S, Bray F, Wild
CP, Plummer M and Dal Maso L: Worldwide thyroid-cancer epidemic?
The increasing impact of overdiagnosis. N Engl J Med. 375:614–617.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Dal Maso L, Panato C, Franceschi S,
Serraino D, Buzzoni C, Busco S, Ferretti S, Torrisi A, Falcini F,
Zorzi M, et al: The impact of overdiagnosis on thyroid cancer
epidemic in Italy, 1998–2012. Eur J Cancer. 94:6–15. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Roman BR, Morris LG and Davies L: The
thyroid cancer epidemic, 2017 perspective. Curr Opin Endocrinol
Diabetes Obes. 24:332–336. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kiriakopoulos A, Petralias A and Linos D:
Multifocal versus solitary papillary thyroid carcinoma. World J
Surg. 40:2139–2143. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Cui L, Feng D, Zhu C, Li Q, Li W and Liu
B: Clinical outcomes of multifocal papillary thyroid cancer: A
systematic review and meta-analysis. Laryngoscope Investig
Otolaryngol. 7:1224–1234. 2022. View
Article : Google Scholar : PubMed/NCBI
|
|
12
|
Park SY, Park YJ, Lee YJ, Lee HS, Choi SH,
Choe G, Jang HC, Park SH, Park DJ and Cho BY: Analysis of
differential BRAF(V600E) mutational status in multifocal papillary
thyroid carcinoma: Evidence of independent clonal origin in
distinct tumor foci. Cancer. 107:1831–1838. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Bansal M, Gandhi M, Ferris RL, Nikiforova
MN, Yip L, Carty SE and Nikiforov YE: Molecular and histopathologic
characteristics of multifocal papillary thyroid carcinoma. Am J
Surg Pathol. 37:1586–1591. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
McCarthy RP, Wang M, Jones TD, Strate RW
and Cheng L: Molecular evidence for the same clonal origin of
multifocal papillary thyroid carcinomas. Clin Cancer Res.
12:2414–2418. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kuhn E, Teller L, Piana S, Rosai J and
Merino MJ: Different clonal origin of bilateral papillary thyroid
carcinoma, with a review of the literature. Endocr Pathol.
23:101–107. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Nikiforova MN and Nikiforov YE: Molecular
genetics of thyroid cancer: Implications for diagnosis, treatment
and prognosis. Expert Rev Mol Diagn. 8:83–95. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Liu R and Xing M: TERT promoter mutations
in thyroid cancer. Endocr Relat Cancer. 23:R143–R155. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Czarniecka A, Oczko-Wojciechowska M and
Barczyński M: BRAF V600E mutation in prognostication of papillary
thyroid cancer (PTC) recurrence. Gland Surg. 5:495–505. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Rashid FA, Munkhdelger J, Fukuoka J and
Bychkov A: Prevalence of BRAFV600E mutation in Asian
series of papillary thyroid carcinoma-a contemporary systematic
review. Gland Surg. 9:1878–1900. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Calado RT: Telomeres and marrow failure.
Hematology Am Soc Hematol Educ Program. 1:338–343. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Vuong HG, Altibi AMA, Duong UNP and
Hassell L: Prognostic implication of BRAF and TERT promoter
mutation combination in papillary thyroid carcinoma-a
meta-analysis. Clin Endocrinol (Oxf). 87:411–417. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Tam AA, Özdemir D, Çuhacı N, Başer H,
Aydın C, Yazgan AK, Ersoy R and Çakır B: Association of
multifocality, tumor number, and total tumor diameter with
clinicopathological features in papillary thyroid cancer.
Endocrine. 53:774–783. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Feng JW, Wu WX, Hu J, Hong LZ, Qin AC,
Jiang Y and Ye J: Influence of tumor number on clinicopathologic
features and outcomes of patients with papillary thyroid carcinoma.
Am J Clin Pathol. 154:848–858. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Qu N, Zhang L, Ji QH, Zhu YX, Wang ZY,
Shen Q, Wang Y and Li DS: Number of tumor foci predicts prognosis
in papillary thyroid cancer. BMC Cancer. 14:9142014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kim H, Kwon H and Moon BI: Association of
multifocality with prognosis of papillary thyroid carcinoma: A
systematic review and meta-analysis. JAMA Otolaryngol Head Neck
Surg. 147:847–854. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Feng JW, Qu Z, Qin AC, Pan H, Ye J and
Jiang Y: Significance of multifocality in papillary thyroid
carcinoma. Eur J Surg Oncol. 46:1820–1828. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Lin JD, Chao TC, Hsueh C and Kuo SF: High
recurrent rate of multicentric papillary thyroid carcinoma. Ann
Surg Oncol. 16:2609–2616. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Woo J, Kim H and Kwon H: Impact of
multifocality on the recurrence of papillary thyroid carcinoma. J
Clin Med. 10:51442021. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Muzza M: The clonal origin of multifocal
papillary thyroid cancer: Intrathyroidal spread or independent
tumors? Minerva Endocrinol (Torino). 46:35–44. 2021.PubMed/NCBI
|
|
30
|
Chen D, Qi W, Xie S, Feng L, Wang J, Wang
L and Guan H: Investigation of the clonal origin of multifocal
papillary thyroid carcinoma according to the X-chromosome
inactivation pattern. Oncol Lett. 17:4695–4700. 2019.PubMed/NCBI
|
|
31
|
Jovanovic L, Delahunt B, McIver B,
Eberhardt NL and Grebe SK: Most multifocal papillary thyroid
carcinomas acquire genetic and morphotype diversity through
subclonal evolution following the intra-glandular spread of the
initial neoplastic clone. J Pathol. 215:145–154. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Nakazawa T, Kondo T, Tahara I, Kasai K,
Inoue T, Oishi N, Mochizuki K, Kubota T and Katoh R: Multicentric
occurrence of multiple papillary thyroid carcinomas-HUMARA and BRAF
mutation analysis. Cancer Med. 4:1272–1280. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Lin X, Finkelstein SD, Zhu B and Silverman
JF: Molecular analysis of multifocal papillary thyroid carcinoma. J
Mol Endocrinol. 41:195–203. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Lu Z, Sheng J, Zhang Y, Deng J, Li Y, Lu
A, Zhang J, Yu H, Zhang M, Xiong Z, et al: Clonality analysis of
multifocal papillary thyroid carcinoma by using genetic profiles. J
Pathol. 239:72–83. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Shattuck TM, Westra WH, Ladenson PW and
Arnold A: Independent clonal origins of distinct tumor foci in
multifocal papillary thyroid carcinoma. N Engl J Med.
352:2406–2412. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Trimboli P, Piccardo A, Signore A,
Valabrega S, Barnabei A, Santolamazza G, Di Paolo A, Stati V,
Chiefari A, Vottari S, et al: Patient age is an independent risk
factor of relapse of differentiated thyroid carcinoma and improves
the performance of the American Thyroid Association stratification
system. Thyroid. 30:713–719. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Nixon IJ, Kuk D, Wreesmann V, Morris L,
Palmer FL, Ganly I, Patel SG, Singh B, Tuttle RM, Shaha AR, et al:
Defining a valid age cutoff in staging of welldifferentiated
thyroid cancer. Ann Surg Oncol. 23:410–415. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Mazzaferri EL and Kloos RT: Clinical
review 128: Current approaches to primary therapy for papillary and
follicular thyroid cancer. J Clin Endocrinol Metab. 86:1447–1463.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Voutilainen PE, Siironen P, Franssila KO,
Sivula A, Haapiainen RK and Haglund CH: AMES, MACIS and TNM
prognostic classifications in papillary thyroid carcinoma.
Anticancer Res. 23:4283–4288. 2003.PubMed/NCBI
|
|
40
|
Sun Y, Dai W, Liang Y and Xia N: Impact of
age on the prognosis of papillary thyroid carcinoma. Arch Iran Med.
23:169–174. 2020.PubMed/NCBI
|
|
41
|
Ito Y, Kudo T, Kobayashi K, Miya A,
Ichihara K and Miyauchi A: Prognostic factors for recurrence of
papillary thyroid carcinoma in the lymph nodes, lung, and bone:
Analysis of 5,768 patients with average 10-year follow-up. World J
Surg. 36:1274–1278. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Soylu L, Aydin OU, Ozbas S, Bilezikci B,
Ilgan S, Gursoy A and Kocak S: The impact of the multifocality and
subtypes of papillary thyroid carcinoma on central compartment
lymph node metastasis. Eur Rev Med Pharmacol Sci. 20:3972–3979.
2016.PubMed/NCBI
|
|
43
|
Genpeng L, Jianyong L, Jiaying Y, Ke J,
Zhihui L, Rixiang G, Lihan Z and Jingqiang Z: Independent
predictors and lymph node metastasis characteristics of multifocal
papillary thyroid cancer. Medicine (Baltimore). 97:e96192018.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Kim HJ, Sohn SY, Jang HW, Kim SW and Chung
JH: Multifocality, but not bilaterality, is a predictor of disease
recurrence/persistence of papillary thyroid carcinoma. World J
Surg. 37:376–384. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Pancer J, Mitmaker E, Ajise O, Tabah R and
How J: A thyroid gland with over 30 foci of papillary thyroid
carcinoma with activating BRAF V600E mutation. Endocrinol Diabetes
Metab Case Rep. 19:19–0006. 2019.PubMed/NCBI
|
|
46
|
Choi Y, Park KJ, Ryu S, Kim DH, Yun J,
Kang DK and Chun M: Papillary thyroid carcinoma involving cervical
neck lymph nodes: Correlations with lymphangiogenesis and
ultrasound features. Endocr J. 59:941–948. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Dong S, Xie XJ, Xia Q and Wu YJ:
Indicators of multifocality in papillary thyroid carcinoma
concurrent with Hashimoto's thyroiditis. Am J Cancer Res.
9:1786–1795. 2019.PubMed/NCBI
|
|
48
|
Zhao J and Luo Z: Discovery of Raf family
is a milestone in deciphering the Ras-mediated intracellular
signaling pathway. Int J Mol Sci. 23:51582022. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Russo M, Malandrino P, Nicolosi ML,
Manusia M, Marturano I, Trovato MA, Pellegriti G, Frasca F and
Vigneri R: The BRAF(V600E) mutation influences the short-and
medium-term outcomes of classic papillary thyroid cancer but is not
an independent predictor of unfavorable outcome. Thyroid.
24:1267–1274. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Jing C, Cao H, Ma R, Wu J and Wang Z:
Association between mutation profiles and clinicopathological
features in Chinese patients with thyroid cancer. Precision Medical
Sciences. 10:113–117. 2021. View Article : Google Scholar
|
|
51
|
Yu P, Qu N, Zhu R, Hu J, Han P, Wu J, Tan
L, Gan H, He C, Fang C, et al: TERT accelerates BRAF mutant-induced
thyroid cancer dedifferentiation and progression by regulating
ribosome biogenesis. Science Adv. 9:eadg71252023. View Article : Google Scholar : PubMed/NCBI
|