Introduction
Extraosseous osteosarcoma is an exceedingly rare
soft-tissue malignancy, constituting <1% of all primary renal
tumors (1,2). This form of primary renal osteosarcoma
is highly malignant, and effective treatment strategies remain
elusive. A review of historical case studies (3,4)
revealed that patients with primary renal osteosarcoma often lack
distinctive imaging and clinical features, which leads to many
being diagnosed at an advanced stage with consequently poor
treatment outcomes. Although a small subset of patients have been
observed to be free of recurrence or metastasis for up to 68 months
post-surgery, the majority are diagnosed at an advanced stage, with
an average survival time of ~15 months (5). The present report describes the
clinicopathological characteristics of a patient with primary renal
osteosarcoma, offering new insights and potential reference points
for the diagnosis and management of this rare condition.
Case report
Case introduction
The patient, a 46-year-old male, presented with a
2-month history of hematuria without an identifiable trigger, and
the symptoms had worsened over the month prior to admission. In
April 2024, a CT examination was performed at Yuxi People's
Hospital (Yuxi, China), revealing a mass-like tissue density in the
lower middle portion of the left kidney, measuring ~7.7×6.4 cm.
This finding was accompanied by evidence of cancerous thrombosis in
the left renal vein and the presence of multiple enlarged
retroperitoneal lymph nodes (data not shown). Consequently, the
patient was directed to Yunnan Cancer Hospital (Kunming, China) for
additional diagnostic procedures and treatment, with the referral
taking place in May 2024. The patient had a history of hypertension
and a smoking habit spanning >20 years. Furthermore, the patient
was diagnosed with renal failure in 2016, underwent a right kidney
transplant in 2017 and had been on long-term immunosuppressant
therapy and regular hemodialysis since the procedure. There were no
significant abnormalities identified in the family medical history.
A physical examination in Yunnan Cancer Hospital revealed a
palpable, fixed and large mass beneath the left rib cage.
Routine test results indicated moderate anemia, with
a hemoglobin level of 90 g/l (reference range, 130–175 g/l). The
patient had an abnormally elevated urinary leukocyte count of
429.5/µl (normal reference range, 0–25/µl) and a similarly abnormal
urinary erythrocyte count of 723.2/µl (normal reference range,
0–12/µl). Alkaline phosphatase levels were in the normal range (53
U/l; reference range, 45–125 U/l). Tumor marker tests revealed the
following: Carcinoembryonic antigen, 19.1 ng/ml (reference range,
<5 ng/ml); carbohydrate antigen (CA) 19–9, 478.7 U/ml (reference
range, 0–30 U/ml); and CA 242, 84.2 U/ml (reference range, 0–10
U/ml). The glomerular filtration rate for the left kidney was 5.15
ml/min (reference range, 90–120 ml/min), and for the right kidney
it was 6.43 ml/min, with all other indicators within the normal
range.
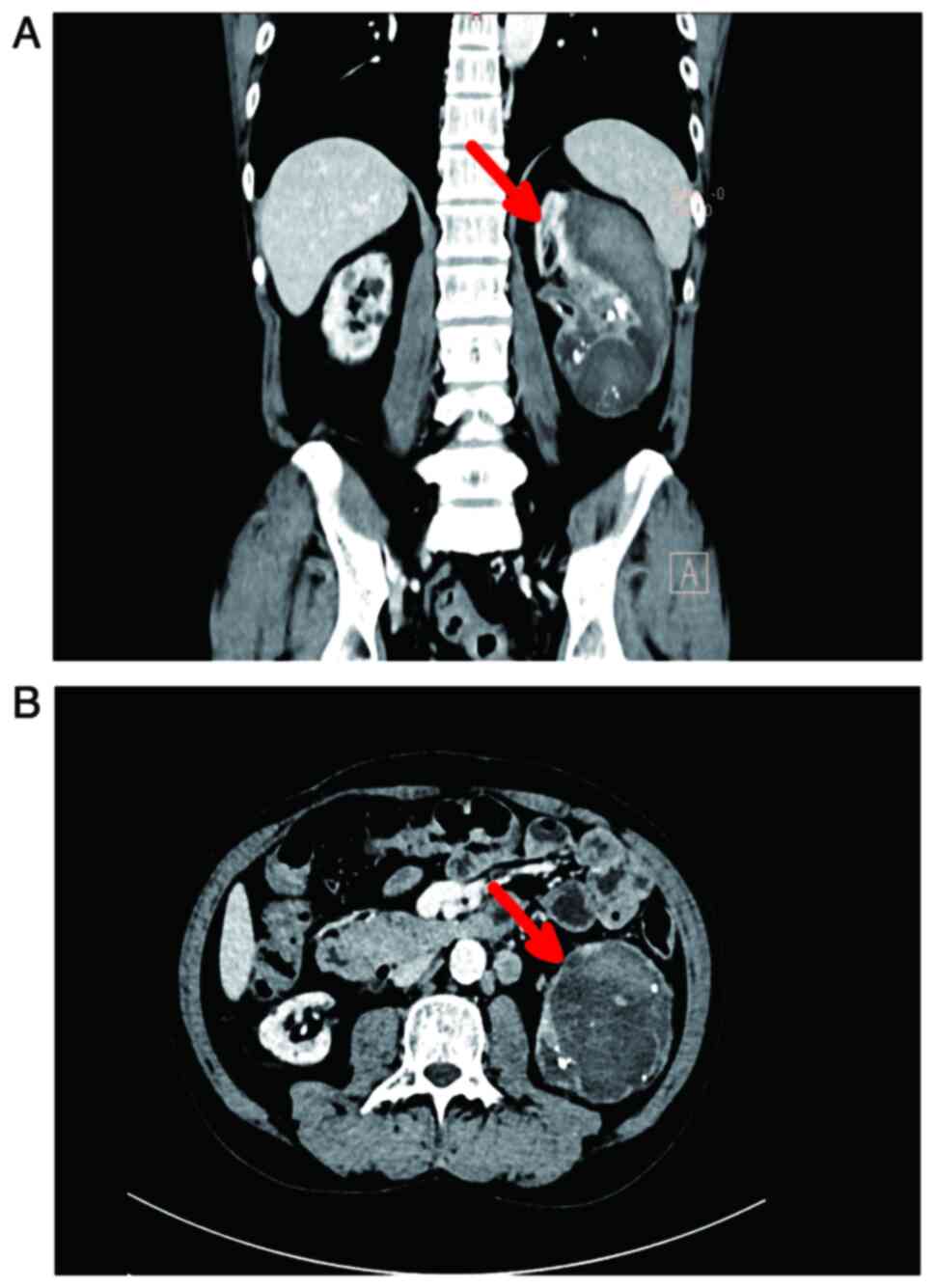
Further CT examination at Yunnan Cancer Hospital
revealed a mass-shaped cystic focus beneath the cortex of the left
kidney, measuring ~7.4×6.9×12.5 cm (Fig. 1). This focus showed scattered
internal pneumatization, a hypodense filling defect in the left
renal vein and multiple enlarged retroperitoneal lymph nodes. The
patient subsequently underwent surgical resection of the affected
kidney, which measured 14×10×8 cm. Upon incision along the renal
hilum, a grayish-red, solid mass measuring 8×6×5.5 cm was observed,
along with several hilar lymph nodes with diameters ranging from
1.5–4 cm.
Pathological examination, following hematoxylin and
eosin (H&E) staining, disclosed hyperplasia of short spindle
cells accompanied by necrosis (Fig. 2A
and B). Intravascular tumor thrombi tested positive, and
metastatic involvement was identified in the renal hilar lymph
nodes. However, no tumor invasion was detected in the renal pelvis
or ureter upon dissection.
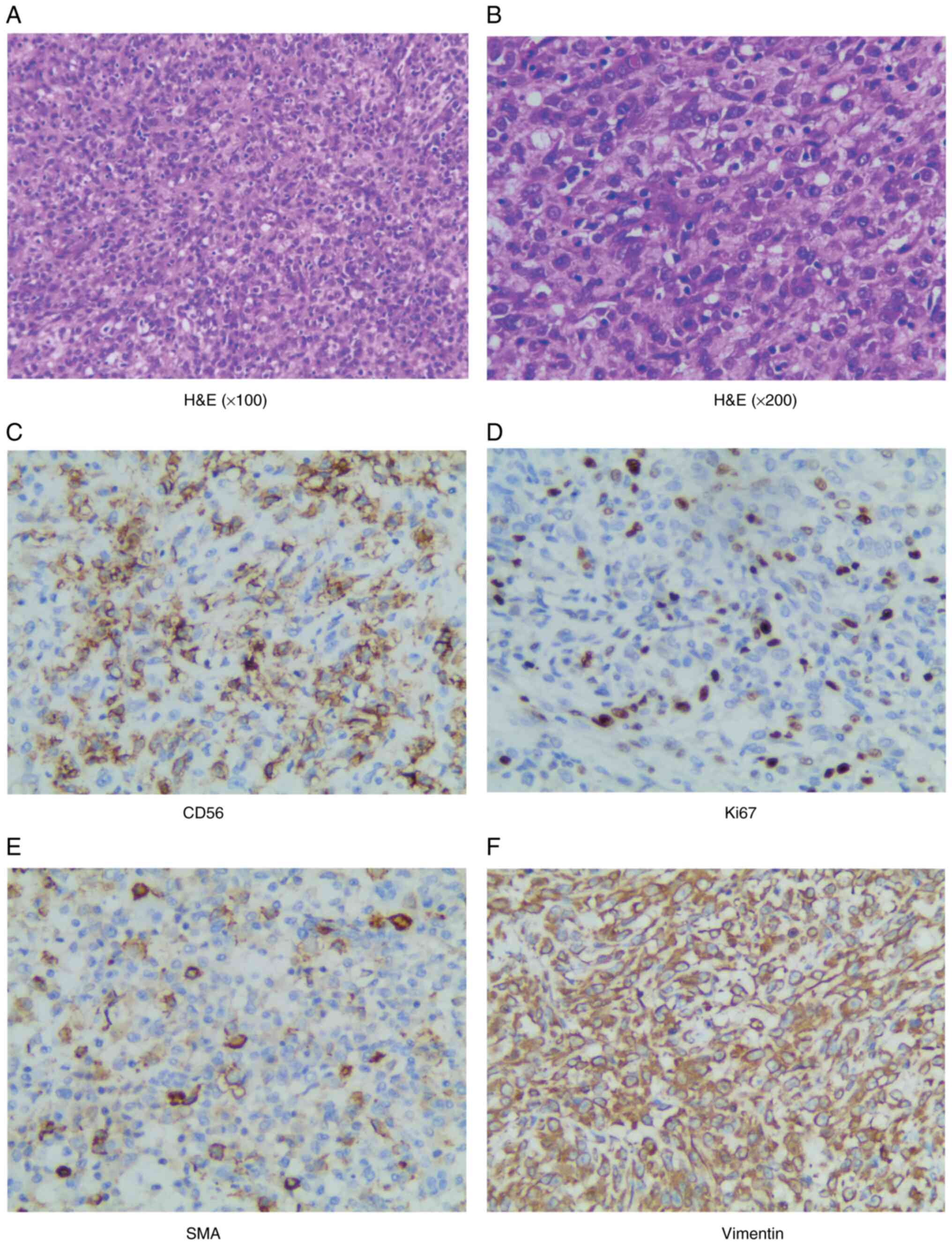 | Figure 2.Histopathological and
immunohistochemistry staining images showing the results of
microscopic examination after H&E staining, which reveal
extensive hyperplasia of short spindle-shaped cells interspersed
with areas of necrosis. (A) H&E (magnification, ×100), (B)
H&E (magnification, ×200), (C) CD56 positive (magnification,
×200), (D) Ki67 positive (magnification, ×200), (E) SMA positive
(magnification, ×200) and (F) Vimentin positive (magnification,
×200). H&E, hematoxylin-eosin; SMA, smooth muscle actin. |
Immunohistochemical analysis demonstrated the
following positive results: Vimentin, CD56, smooth muscle actin
(SMA), special AT-rich sequence-binding protein 2 (SATB-2), P53,
minimal weak positivity for GATA-Binding Factor 3 (GATA-3),
scattered positivity for CK5/6, weak to positive expression of
epithelial membrane antigen (EMA), partial positivity for actin,
weak P504s, partially weak transcription factor E3 (TFE-3), minimal
positivity for S-100. The expression rate of Ki67 was ~60%, and
succinate dehydrogenase complex iron sulfur subunit B (SDHB) was
also expressed in the tumor tissue. (Fig. 2C-F). Conversely, the analysis
revealed negative results for CK8, paired box (Pax)-8, Pax-2, P63,
human melanoma black 45 (HMB-45), CD34, Wilms tumor protein 1
(WT-1), E-cadherin, CK, CK7, Desmin, H-caldesmon, Myogenin,
transcription termination factor 1 (TTF-1), P40, Calponin, CD10,
CD117, Syn and chromogranin A (CgA) (data not shown).
Given the extreme rarity of the condition and the
absence of osseous material in the tissue samples, diagnosis was
highly challenging. Consequently, a pathological consultation was
sought from the Ruijin Hospital Affiliated with Shanghai Jiao Tong
University School of Medicine (Shanghai, China). The expert team at
Ruijin Hospital conducted a comprehensive assessment of the
patient, including microscopic examination, immunohistochemical
analysis, and pathological evaluation, leading to the definitive
diagnosis of primary renal osteosarcoma. The pathological staging
was determined to be pT3aN1M0, in accordance with the 2017 American
Joint Committee on Cancer staging for renal cancer (6). A total of 2 months after the surgical
procedure, the patient returned to Yunnan Cancer Hospital for a
follow-up examination. PET-CT disclosed irregular tissue density
shadows with elevated metabolism in the surgical region (data not
shown) and a maximum standardized uptake value (SUVmax) of 21. This
finding was indicative of tumor recurrence. Additionally, the scan
revealed multiple hypermetabolic nodules in several locations,
including the left posterior renal space, the angle of the left
diaphragm, the left paraspinal muscles, and the left lumbar psoas
major muscles (data not shown). These were suggestive of tumor
metastases. Moreover, PET-CT identified multiple hypermetabolic
lymph nodes in the middle and lower retroperitoneum (data not shown
with an SUVmax of 25.5, which were suggestive of tumor metastases.
Considering the swift progression of the illness, the poor physical
condition of the patient, and after a thorough review of the
therapeutic options, the patient was started on a combination
chemotherapy regimen that included cisplatin, adriamycin and
cyclophosphamide. However, due to the poor physical condition of
the patient, they were lost to follow-up after completing a cycle
of chemotherapy.
Methodology
For H&E staining, the tissue samples were fixed
in 10% formalin for 24 h at room temperature, ensuring the
preservation of tissue structure. The fixed tissues were then
embedded in paraffin to facilitate the subsequent sectioning,
yielding sections with a uniform thickness of 4–6 µm. The staining
sequence involved an initial application of Gill II hematoxylin for
15 min at room temperature, providing a blue color to the cell
nuclei, followed by a brief eosin staining for 30 sec at room
temperature, which imparted a pink hue to the cytoplasm.
Post-staining, the sections underwent a dehydration process to
prepare for mounting. The dehydrated sections were mounted with
neutral balsam, safeguarding the stained layer and enhancing sample
stability. The mounted sections were then scrutinized under a light
microscope to elucidate the cellular and tissue architecture.
For immunohistochemistry, the ready-to-use
UltraSensitive™ SAP immunohistochemistry kit (cat. no.
KIT-9710); Fuzhou Maixin Biotechnology Development Co., Ltd.) was
used, and the procedure was as follows (all conditions are the same
as H&E): Initially, the deparaffinization and hydration step
was performed, where paraffin-embedded tissue sections were treated
with xylene and a descending series of alcohol concentrations,
followed by rinsing with tap water to remove paraffin from the
sections and rehydrate the tissue. Subsequently, the primary
antibodies were incubated overnight at 4°C. All antibodies and
staining reagents used were purchased from Fuzhou Maixin
Biotechnology Development Co., Ltd. and were provided pre-diluted
by the manufacturer: Vimentin (cat. no. MAB-0735), CD56 (cat. no.
MAB-0743), SMA (cat. no. MAB-0890), SATB-2 (cat. no. RMA-0750), P53
(cat. no. MAB-0674), GATA-3 (cat. no. MAB-0695), CK5/6 (cat. no.
MAB-0744), EMA (cat. no. Kit-0011), actin (cat. no. MAB-0871),
P504s (cat. no. RMA-0546), TFE-3 (cat. no. RMA-0663), S-100 (cat.
no. RAB-0150) and Ki67 (cat. no. MAB-0672), SDHB (cat. no.
MAB-0888), CK8 (cat. no. MAB-1002), CK18 (cat. no. MAB-0737), Pax-8
(cat. no. MAB-0837), Pax-2 (cat. no. RMA-0816), P63 (cat. no.
MAB-0694), HMB-45 (cat. no. MAB-0098), CD34 (cat. no. Kit-0004),
WT-1 (cat. no. MAB-0678), E-cadherin (cat. no. MAB-0738), CK (cat.
no. RAB-0050), CK7 (cat. no. MAB-0828), Desmin (cat. no. MAB-0766),
H-caldesmon (cat. no. MAB-0634), Myogenin (cat. no. MAB-0866),
TTF-1 (cat. no. MAB-0677), P40 (cat. no. RMA-0815), Calponin (cat.
no. MAB-0712), CD10 (cat. no. MAB-0668), CD117 (cat. no. Kit-0029),
Syn (cat. no. MAB-0742) and CgA (cat. no. RMA-0548). Following
this, a peroxidase blocking step was performed to prevent
interference from endogenous peroxidase activity. This involved the
removal of PBS, application of the peroxidase blocking reagent, and
a 10-min incubation at room temperature, after which the sections
were rinsed three times with PBS for 3 min each. The non-specific
staining blocking step was then implemented by applying a
non-specific staining blocker, incubation at room temperature for
10 min to reduce background staining, and rinsing again with PBS.
After the removal of the blocking agent, the aforementioned primary
antibodies were applied and incubated at room temperature for 60
min, followed by three rinses with PBS for 3 min each to ensure
specific binding of the antibodies to the target antigen. Once PBS
was removed, biotinylated secondary antibodies were added and
incubated at room temperature for 10 min, then rinsed with PBS.
Streptavidin-anti-biotin peroxidase reagent was introduced to
further amplify the signal, with a subsequent 10-min incubation at
room temperature and three rinses with PBS. The color development
process was terminated using tap water after rinsing with PBS, and
then fresh DAB chromogen reagent was applied to visualize the
specifically bound antibody complex. After color development,
hematoxylin counterstaining for 1–2 min at room temperature was
performed to enhance the contrast of the cell nuclei, followed by
bluing with PBS. Finally, the sections were mounted with synthetic
resin and examined under a light microscope.
Discussion
Among primary renal malignancies, clear cell renal
cell carcinoma is the most common, whilst the renal osteosarcoma
subtype is exceedingly rare and highly aggressive (4). Historical case reports indicate that
>50% of the patients are diagnosed with stage T4 disease
accompanied by lymph node metastasis at the time of initial
presentation, and distant metastases are found in >80% of cases
(3). The patient in the present
report was diagnosed with stage pT3aN1M0.
The most common symptoms of renal osteosarcoma
include lower back pain and a palpable presence of a mass in the
lumbar region (3). In cases of
advanced disease, hematuria often emerges as the primary symptom
(3). CT is a highly valuable
diagnostic tool for renal malignancies. Characteristic CT features
of renal osteosarcoma encompass a large, mixed-density,
cystic-solid mass with areas of calcification, observed in ~50% of
the patients (7). Furthermore, CT
is instrumental in ruling out sarcomas of osseous origin and in
detecting lymph node and systemic metastases. It has been suggested
that renal osteosarcoma may exhibit a distinctive ‘sunburst’
pattern on imaging (8). MRI
findings for extraosseous osteosarcoma are less well characterized.
On T1-weighted images, the signal intensity is similar to that of
skeletal muscle, whereas on T2-weighted images, it appears
isointense or hyperintense (7).
PET-CT typically demonstrates a high metabolic signal, often with a
central necrotic region that may show reduced metabolic activity.
Additionally, extraosseous osteosarcoma tends to exhibit a narrower
range of SUVmax values compared with its osseous counterpart
(7).
A review of the existing literature on primary renal
osteosarcoma indicates that diagnosis is primarily achieved through
a process of exclusion and the use of immunohistochemical
diagnostic methods. Initially, it is crucial to rule out the
metastasis of osteosarcoma tissues from other regions, requiring
whole-body CT or MRI (9). In the
present case, the patient showed no evidence of metastasis from
other sources in the CT examination. Secondly, differentiating
between ossification in renal clear cell carcinoma (RCC) and
primary renal osteosarcoma is essential, as the existing literature
highlights. The probability of RCC ossification is extremely low,
and primary renal osteosarcoma does not contain carcinoma (8–10). The
distinct tissue origins of RCC and primary renal osteosarcoma
result in different expressions of immunohistochemical markers,
which are key for differentiation (for example, Vimentin, CD56, SMA
and SATB-2 positivity) (1). As the
present case was distinctive in that no discernible bone-like
tissue was observed in the resected kidney or metastatic lymph
nodes, a multitude of immunohistochemical markers were used to
substantiate the diagnosis through exclusion, and a comprehensive
array of neoplastic cells was identified, including nephroblasts,
uroepithelial, neurogenic, rhabdomyosarcoma and melanin. However,
the expression of Vimentin, CD56, SMA, and SATB-2 in the tumor
cells ultimately led to the diagnosis of primary osteosarcoma of
the kidney.
In addition, with the popularization of genetic
testing and targeted therapy, there have been reports on the
genetic testing of primary renal osteosarcoma. The genes with
differences reported include: Phosphatidylinositol-4,5-bisphosphate
3-kinase catalytic subunit α (PIK3CA), CCCTC-binding factor (CTCF),
Ras p21 protein activator 1 (RASA1), MutS Homolog 6 (MSH6), Fanconi
anemia complementation group F (FANCF) and excision repair
cross-complementing rodent repair deficiency complementation group
4 (ERCC4) (3,8). Certain studies have suggested that the
aforementioned genes may lead to tumor progression and poor
prognosis in other types of cancer, and they may enhance the tumor
response to chemotherapy (11,12).
However, their role in renal osteosarcoma is currently not well
understood.
In summary, the diagnostic criteria for primary
renal osteosarcoma are encapsulated by the following points: i)
Imaging assessment: Renal space-occupying lesions are identified
via CT or MRI, with meticulous exclusion of extra-renal metastases;
ii) immunohistochemical profiling: Tumor cells are found to express
osteoblast-differentiation markers, such as Vimentin, CD56, SMA and
SATB-2; and iii) differentiation from other renal tumors: A
definitive exclusion of alternative renal neoplasms, notably
ossified renal cell carcinoma, is imperative. Furthermore, the
diagnosis of renal osteosarcoma is bolstered when imaging studies
rule out metastatic renal tumors, and pathological examination
reveals the presence of bone-like components within the tumor.
The primary treatment for renal osteosarcoma is
surgery, with the objective of achieving complete tumor resection
and ensuring negative surgical margins. Whilst there is a limited
body of research on chemotherapeutic regimens specific to renal
osteosarcoma, a study on extraosseous osteosarcoma indicated that
platinum-based adjuvant chemotherapy can markedly extend patient
survival (13). A commonly used
treatment protocol involves a triple drug combination of
doxorubicin, ifosfamide and cisplatin (9). A study also explored the potential of
targeted therapy, particularly in conjunction with anlotinib
(8). Radiotherapy is frequently
used in the treatment of extraosseous osteosarcoma, with evidence
suggesting it is more effective than chemotherapy for improving
recurrence-free survival. Moreover, the concurrent use of
radiotherapy and chemotherapy has been demonstrated to notably
enhance patient survival rates compared with surgery alone
(14,15). However, there is a scarcity of cases
involving the use of radiotherapy for primary renal osteosarcoma.
One documented case involved a patient who experienced local
recurrence shortly after undergoing postoperative radiotherapy at a
dose of 50 Gy and was then switched to adjuvant chemotherapy
involving methotrexate and vincristine (4). After comprehensive consideration, the
patient was given a combination chemotherapy regimen consisting of
cisplatin, adriamycin and cyclophosphamide.
In conclusion, primary renal osteosarcoma is an
exceedingly rare malignancy, with a mere 30 documented cases
reported to date. Clinical symptoms of this condition are often
subtle in the early stages, making them easily overlooked by
patients. It is not uncommon for the disease to progress to later
stages before symptoms such as lower back pain and hematuria become
apparent. The present report describes a case where hematuria was
the initial presenting symptom. Additionally, a comprehensive
review of the imaging characteristics and therapeutic approaches
from previous cases are discussed, with the intent of providing a
reference that may aid in the early diagnosis and treatment of
primary renal osteosarcoma.
Acknowledgements
Not applicable.
Funding
The present research was funded by the National Natural Science
Foundation of China (grant no. 82160511) and the National Cancer
Center Climbing Fund (grant no. NCC201925B01).
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
KZ, LD and HW designed the study and wrote the
manuscript. BZ and YW advised on patient treatment, analyzed
patient data and confirm the authenticity of all the raw data. KZ,
LD and HW gathered medical pictures and assessed the patient
information. EN, FY, JL, CZ and YB contributed to the
conceptualization of the study, general design and quality
assurance. All authors have read and approved the final
manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
The patient provided informed consent for
publication of the present case report and associated images.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Ahomadégbé C, Bennani-Guebessi N and
Karkouri M: Primary renal osteosarcoma: A case report. Afr J Urol.
20:189–192. 2014. View Article : Google Scholar
|
|
2
|
Uhlig J, Uhlig A, Bachanek S, Onur MR,
Kinner S, Geisel D, Köhler M, Preibsch H, Puesken M, Schramm D, et
al: Primary renal sarcomas: Imaging features and discrimination
from non-sarcoma renal tumors. Eur Radiol. 32:981–989. 2022.
View Article : Google Scholar
|
|
3
|
Chen J, Liao H, Zhan R, Zheng Q, Deng J,
Wang G and Zhang J: Case report: Primary osteosarcoma of the
kidney. Front Oncol. 13:11755182023. View Article : Google Scholar
|
|
4
|
Weingärtner K, Gerharz EW, Neumann K,
Pflüger KH, Grüber M and Riedmiller H: Primary osteosarcoma of the
kidney. Case report and review of literature. Eur Urol. 28:81–84.
1995. View Article : Google Scholar
|
|
5
|
Lopez-Beltran A, Montironi R, Carazo JL,
Vidal A and Cheng L: Primary renal osteosarcoma. Am J Clin Pathol.
141:747–752. 2014. View Article : Google Scholar
|
|
6
|
Paner GP, Stadler WM, Hansel DE, Montironi
R, Lin DW and Amin MB: Updates in the eighth edition of the
tumor-node-metastasis staging classification for urologic cancers.
Eur Urol. 73:560–569. 2018. View Article : Google Scholar
|
|
7
|
Hesni S, Lindsay D, O'Donnell P and
Saifuddin A: Extra-skeletal osteosarcoma: A review. Skeletal
Radiol. 52:633–648. 2023. View Article : Google Scholar
|
|
8
|
Huang C, Zhu X, Xiong W, Zhao X and Xu R:
A case report of primary osteosarcoma originating from kidney.
Medicine (Baltimore). 98:e142342019. View Article : Google Scholar
|
|
9
|
Allan CJ and Soule EH: Osteogenic sarcoma
of the somatic soft tissues. Clinicopathologic study of 26 cases
and review of literature. Cancer. 27:1121–1133. 1971. View Article : Google Scholar
|
|
10
|
Pan H, Wu D, Wang H, Pan Y, Zhang T and
Zhou J: Clear cell renal cell carcinoma with extensive osseous
metaplasia: Report of a rare case. Urology. 105:e3–e5. 2017.
View Article : Google Scholar
|
|
11
|
Wang MJ, Zhu Y, Guo XJ and Tian ZZ:
Genetic variability of genes involved in DNA repair influence
treatment outcome in osteosarcoma. Genet Mol Res. 14:11652–11657.
2015. View Article : Google Scholar
|
|
12
|
Zehir A, Benayed R, Shah RH, Syed A,
Middha S, Kim HR, Srinivasan P, Gao J, Chakravarty D, Devlin SM, et
al: Mutational landscape of metastatic cancer revealed from
prospective clinical sequencing of 10,000 patients. Nat Med.
23:703–713. 2017. View Article : Google Scholar
|
|
13
|
Paludo J, Fritchie K, Haddox CL, Rose PS,
Arndt CAS, Marks RS, Galanis E, Okuno SH and Robinson SI:
Extraskeletal osteosarcoma: Outcomes and the role of chemotherapy.
Am J Clin Oncol. 41:832–837. 2018. View Article : Google Scholar
|
|
14
|
Heng M, Gupta A, Chung PW, Healey JH,
Vaynrub M, Rose PS, Houdek MT, Lin PP, Bishop AJ, Hornicek FJ, et
al: The role of chemotherapy and radiotherapy in localized
extraskeletal osteosarcoma. Eur J Cancer. 125:130–141. 2020.
View Article : Google Scholar
|
|
15
|
Campos F, Téres R, Sebio A, Bettim BB and
Martinez-Trufero J: Survival differences of patients with resected
extraskeletal osteosarcoma receiving two different (Neo)adjuvant
chemotherapy regimens: A systematic review and meta-analysis. Clin
Oncol (R Coll Radiol). 35:e720–e727. 2023. View Article : Google Scholar
|