Cholangiocarcinoma (CCA) is a type of malignant
tumor that originates from the epithelial cells of the biliary
system. CCA ranks among the most common malignant tumors affecting
the biliary system and is the second most prevalent primary liver
tumor following hepatocellular carcinoma. In the US, ~23,000
individuals are diagnosed annually, while the condition is more
prevalent in Asia, partly due to parasitic infections common in
that region. In Australia, ~1,300 new cases are reported each year.
The incidence rate of CCA in the US is ~9.4 per 100,000
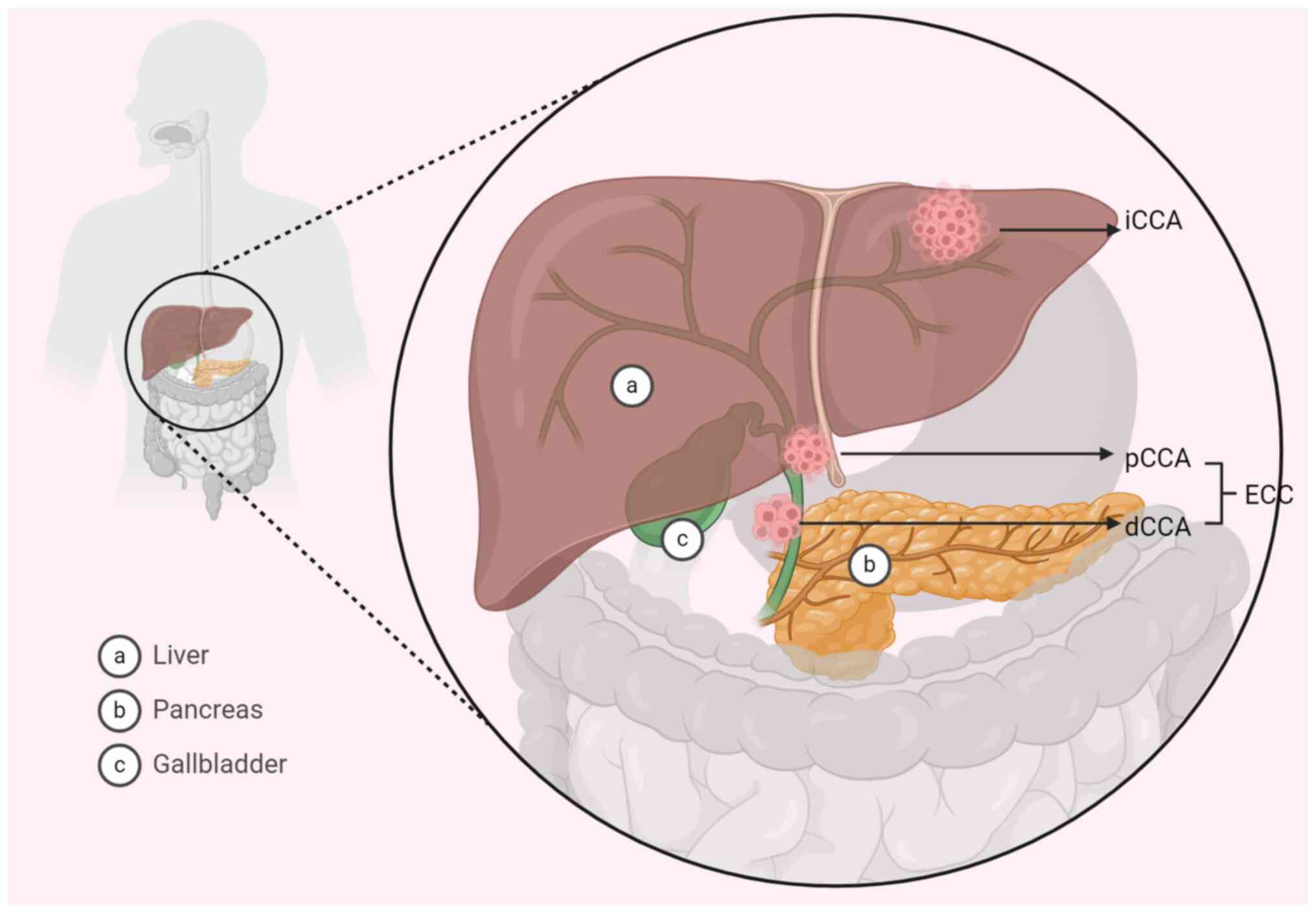
individuals, with a mortality rate of ~6.6 per 100,000 (1). CCA can be categorized based on its
location as intrahepatic CCA (iCCA) or extrahepatic CCA (eCCA;
Fig. 1) (2,3).
Numerous factors influence the onset of CCA. Prolonged inflammation
in the biliary tract, associated with factors such as cholecystitis
and bile duct stones, is deemed a significant risk factor for bile
duct cancer. Parasitic infections, particularly liver fluke
infections prevalent in northeastern Thailand (4), are also associated to the development
of bile duct cancer. Additionally, environmental, genetic and
dietary factors closely intertwine with the occurrence of bile duct
cancer (5).
Ferroptosis, a novel form of programmed cell death
that is associated with intracellular iron ion accumulation and
oxidative stress, manifests key features such as intracellular iron
overload and subsequent lipid peroxidation (6). However, recent studies have shown that
ferroptosis is associated with the development and progression of a
variety of types of cancers, such as liver, triple-negative breast
and non-small cell lung cancers (7–9). An
association between ferroptosis and CCA has been previously
reported (5). Therefore, an
in-depth exploration of the role of ferroptosis in CCA is important
to improve the current understanding of the developmental
mechanisms of CCA, the development of new diagnostic and
therapeutic strategies and the assessment of patient prognosis.
Inflammation represents the natural defense and
repair response of an organism to various stimuli, such as
infection or injury, and typically manifests as redness, swelling,
heat, pain and dysfunction of local tissues (17). Abnormal inflammatory responses are
closely associated with disorders in iron metabolism and imbalances
in the redox system (18).
Pro-inflammatory cytokines, including IL-6 and IL-1β, impact iron
processing by hepatocytes, which influences iron storage and
distribution throughout the body (19). The release of TNF-α and IFN-γ
disrupts the redox system and increases intracellular oxidative
stress (20). This state of
oxidative stress affects the accumulation and utilization of
intracellular iron ions, which potentially results in abnormal iron
accumulation and triggers iron-related cell damage, including
ferroptosis (21). NF-κB, a
transcription factor with specific DNA-binding activity, is
involved in classical pro-inflammatory signaling pathways [TNF-α,
IL-1, IKK (the IKK complex is a key regulator in the NF-κB
signaling pathway and consists of three subunits, IKKα, IKKβ and
NEMO); NF-κB essential modulator, also known as IKKγ] that regulate
inflammation and the immune system. Modulating the NF-κB signaling
pathway can reduce inflammatory responses and injury, while also
regulating iron metabolism pathways during inflammation (22). For instance, dimethyl fumarate (DMF)
serves a role in reducing neuroinflammation and ferroptosis by
regulating the NF-κB signaling pathway (23). It has been reported that DMF
demonstrates efficacy in improving cognitive deficits caused by
vessel occlusion in a rat model of chronic cerebral hypoperfusion
(24). A previous study by Zhao
et al (25) reported that
artemisinin prevents ferroptosis-induced liver injury by inhibiting
reactive oxygen species (ROS) and inflammation through the
activation of the nuclear factor E2-related factor 2 (Nrf2)/heme
oxygenase-1/glutathione peroxidase 4 (GPX4) pathway and
downregulation of NF-κB.
The MAPK family, including ERK, JNK and p38 MAPK
isoforms, serve crucial roles in intracellular signaling. When
activated by external stimuli, such as the role of cytokines,
oxidative stress and mechanical stress. These kinases trigger a
series of enzymatic reactions, which lead to the expression of
inflammation-related genes and inflammatory responses (26). Similar to NF-κB, the activation of
the MAPK pathway is associated with the process of ferroptosis. It
has been suggested that salvianolic acid B, a potent polyphenolic
compound derived from Salvia miltiorrhiza (Danshen) known
for its strong antioxidant, anti-inflammatory and cardioprotective
properties, may attenuate ferroptosis and apoptosis during
myocardial ischemia and reperfusion injury by inhibiting ROS
production or modulating ROS levels, thereby inhibititing
activation of the JNK/MAPK pathway (27). Furthermore, Toll-like receptor 4
(TLR4) is a crucial immune receptor that initiates the p38 MAPK
pathway upon activation, which prompts the production of cytokines
such as IL-1β, IL-6 and IL-18. This process may impact the
expression levels of solute carrier family 7 member 11 (SLC7A11)
and GPX4, both of which regulate redox homeostasis and antioxidant
stress responses (28). A reduction
in SLC7A11 and GPX4 expression levels may lead to neuroinflammation
and ferroptosis (29). The
aforementioned evidence suggests a notable association between
inflammation and ferroptosis.
Mitochondria serve as the energy-producing hubs
within cells, generating adenosine triphosphate (ATP) through
oxidative phosphorylation to supply energy to the cell (30). Beyond their role in energy
production, mitochondria are crucial in regulating intracellular
signaling pathways (31). Moreover,
mitochondria are involved in numerous cell death pathways,
including apoptosis, necrosis and ferroptosis (32). In the context of ferroptosis,
intracellular iron ions catalyze the production of ROS that
initiate lipid peroxidation and lead to cell death. Mitochondria
act as the primary source of ROS (33). The electron transport chain within
mitochondria, a component of oxidative phosphorylation, generates
ROS, with numerous electrons binding to oxygen molecules to form
superoxide anions. Moreover, diverse metabolic processes that occur
within mitochondria, including lipid and amino acid metabolism,
actively participate in the production of ROS (34). The mitochondrial generation of ROS
can potentially contribute to ferroptosis by fostering lipid
peroxidation (35). In situations
of intracellular ATP deficiency, the energy sensor AMP-activated
protein kinase (AMPK) is activated. AMPK activation inhibits the
activity of acetyl-coenzyme A carboxylase, which impacts the rate
of fatty acid synthesis (36).
Furthermore, AMPK activity influences the intracellular iron
metabolism and ferroptosis by decreasing the uptake of iron ions by
intestinal epithelial cells, as well as the storage and release of
iron ions by the liver (37). By
contrast, in conditions of abundant ATP, AMPK activation is less
efficient, which leads to the activation of acetyl-coenzyme A
carboxylase. This activation, in turn, promotes the synthesis of
polyunsaturated fatty acid (PUFA) phospholipids and consequently
supports the occurrence of ferroptosis (38). Nrf2 is a crucial transcription
factor with roles in antioxidant and cytoprotective functions
within cells. By regulating the expression of antioxidant defense
systems, including antioxidant enzymes within mitochondria, Nrf2
serves a crucial role in ferroptosis (39). Nrf2 also influences mitochondrial
energy metabolism and cell signaling, which impacts cell survival
and antioxidant capacity (40).
Given the association of ferroptosis with ROS, Nrf2 regulation of
mitochondrial function has far-reaching effects on ferroptosis.
Specifically, Nrf2 protects cells from damage by maintaining
mitochondrial function and reducing ROS production, thereby
reducing oxidative stress from ferritin deposition (41). In addition, exposure to exogenous
hydrogen peroxide can activate iron response elements involved in
the regulation of iron metabolism by binding to mRNA, which can
inhibit the translation of ferritin and reduce ferritin synthesis,
thus increasing the concentration of free iron in cells; in
addition, it can promote the degradation of mRNA and further
inhibit the expression of ferritin. The increase in free iron may
lead to enhanced Fenton reaction and generation of more ROS, thus
exacerbating intracellular oxidative stress (42).
In healthy cells, iron is an essential trace element
involved in a number of biological processes such as DNA synthesis,
the respiratory chain and oxygen delivery (43). The body must control the uptake,
storage and excretion of iron in order to maintain intracellular
and body iron homeostasis (44).
The human body contains iron in the form of ferric ions. When
intracellular ferric ions are overloaded, harmful ROS and lipid
peroxides, which are catalyzed by Fe2+ via the Fenton
reaction, can be produced, which potentially results in cell death.
Thus, a major factor in cellular iron toxicity is the absorption,
release, storage and transit of intracellular iron ions (45). Several studies have reported that
the demand for iron ions is typically higher in cancer cells
compared with healthy cells, because cancer cells need more iron to
support their rapid proliferation and metabolic activities
(46). Therefore, regulating
intracellular iron storage and release can have a direct impact on
the survival and death of cancer cells (47,48).
Cellular iron uptake occurs primarily through the transferrin (Tf)
receptor (TfR)-mediated pathway. Tf binds Fe3+, which
then binds to TfR and enters the cell via receptor-mediated
endocytosis. The acidic environment within the endocytosed vesicle
dissociates Fe3+ from Tf, which is reduced to
Fe2+ by a metal reductase, such as
hexamethylenetetramine reductase 1 and STEAP family member 1.
Fe2+ is subsequently transported via the divalent metal
transporter 1 into the cytoplasm of the cell (49,50).
Dysregulation of iron metabolism can lead to excessive accumulation
of intracellular Fe2+. Fe2+ participates in
the Fenton reaction, which generates high ROS levels, including
hydroxyl radicals. These high ROS levels can trigger lipid
peroxidation and damage the cellular membranes, which ultimately
leads to ferroptosis (51).
Deletion or dysfunction of the heavy chain of ferritin leads to
aberrant accumulation of iron and increased sensitivity of cells to
ferroptosis. Furthermore, the regulation of ferritin expression is
overseen by the iron-responsive element and iron regulatory
protein. These proteins govern the translation of ferritin and
transferrin receptors and thereby exert finely tuned control over
intracellular iron homeostasis (52).
The mechanisms of ROS generation and lipid
peroxidation are central features of ferroptosis. Examples of ROS
include substances such as superoxide anion, hydrogen peroxide and
hydroxyl radicals (53). These
substances are products of redox reactions and are important cell
signaling molecules. ROS can act as secondary messengers to
regulate a variety of cell signaling pathways, including
inflammation, apoptosis and cell proliferation. Oxidative stress
refers to the excessive production of highly reactive molecules,
such as ROS and reactive nitrogen species, in the body (54). When the body is subjected to various
harmful stimuli (ultraviolet radiation, pollution, smoking,
inflammatory reactions or infections), the oxidizing capacity
exceeds the capacity of its own oxidant scavenging systems and the
oxidative and antioxidant systems are imbalanced, thus leading to
tissue pathology and damage (55).
Iron ions are involved in a number of biological processes within
the cell, but an excess of free iron ions can lead to the
production of large amounts of free radicals, thus causing
oxidative stress (56). Oxidative
stress affects the metabolism and storage of iron ions, which leads
to an abnormal accumulation of iron ions in the cells (57). Excessive intracellular ROS leads to
lipid peroxidation, a key feature of ferroptosis. Phospholipids are
a major component of cell membranes, of which
phosphatidylethanolamines (PEs) are important. Since PE is rich in
polyunsaturated fatty acids (PUFA), these unsaturated bonds are
highly susceptible to attack by ROS, generating lipid peroxides
(e.g., PUFA-PE-OOH). Arachidonic acid (AA) and its derivative
adrenergic acid (ADA) are fatty acids that bind to PEs to form
phospholipids (58). AA and ADA are
key phospholipids in which oxidation occurs and are thought to be
important contributors to iron-related cell death (10). Acyl coenzyme A synthase long-chain
family member 4 (ACSL4) acylates AA to fatty acyl-coenzyme A, and
lysophosphatidylcholine acyltransferase 3 (LPCAT3) catalyzes the
acylation of ADA to membrane phospholipids. This process increases
the oxidative sensitivity of membrane-sensitive fatty acids such as
PUFA, which leads to the development of lipid peroxidation and
further triggers ferroptosis (59).
During ferroptosis, an abnormal accumulation of iron ions may lead
to the production of lethal levels of lipid peroxides that can
damage cell membrane integrity and cause cell death (60).
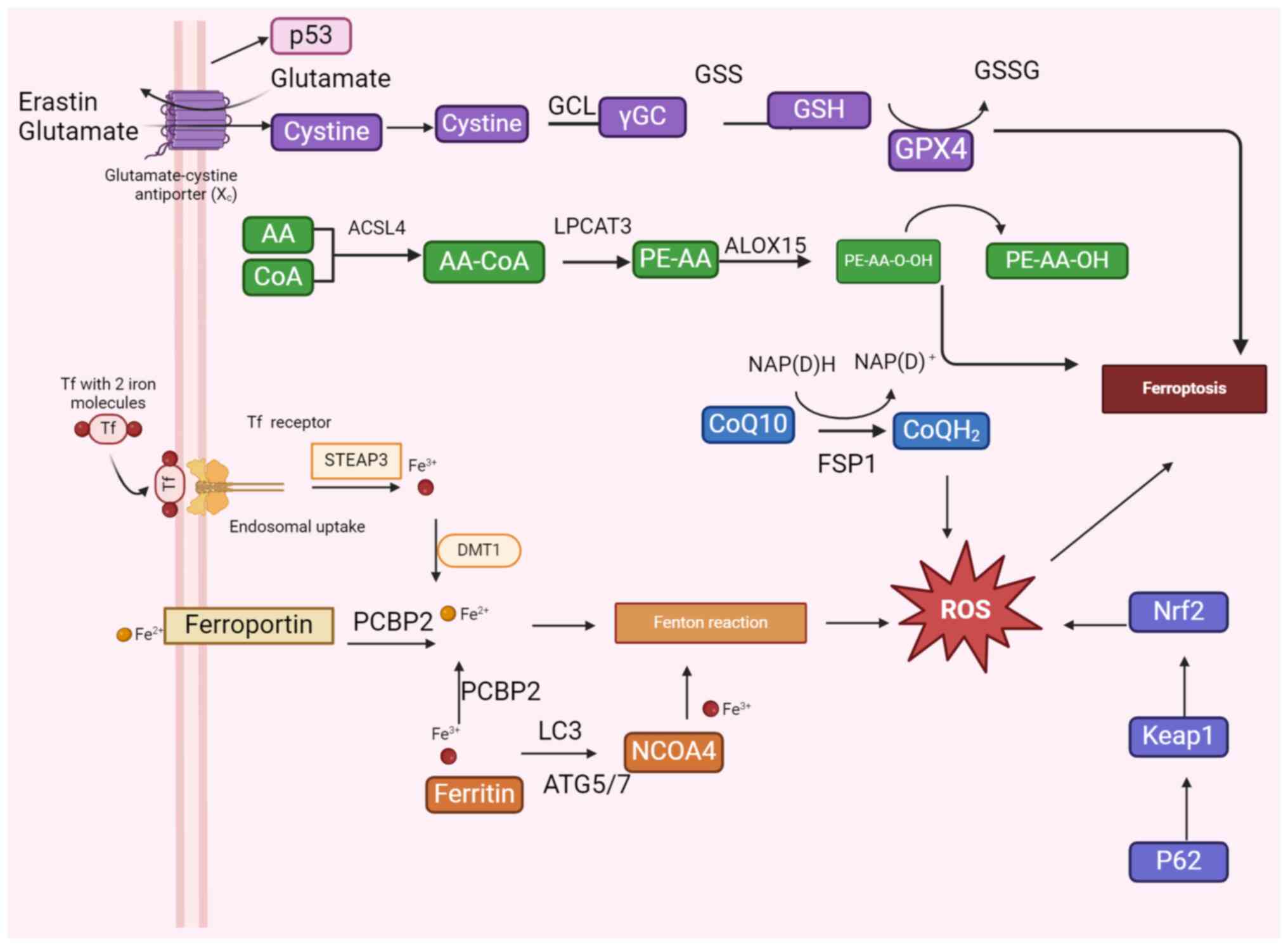
GSH, a tripeptide composed of glutamate, cysteine
and glycine, is an important antioxidant in cells. GSH exists
mainly in the reduced form which protects cells from oxidative
damage by donating electrons to neutralize ROS and other free
radicals (61). The main function
of GPX4 is to catalyze the glutathione GSH-dependent reduction of
membrane lipid peroxides to their corresponding alcohols, thereby
protecting cell membranes from oxidative damage (62). In addition, GPX4 is involved in the
regulation of intracellular iron ion homeostasis, which is
important for maintaining intracellular redox homeostasis and
reducing oxidative stress (63).
GPX4 utilizes GSH as a substrate to reduce lipid peroxides and
prevent the expansion of lipid peroxidation reaction, which
protects the integrity and function of the cell membrane. The
depletion or inhibition of GSH results in inefficient lipid
peroxide scavenging, leading to uncontrolled lipid peroxidation
chain reaction and ultimately triggering the ferroptosis process
(64,65). The Xc− system, also known
as the cysteine-glutamate reverse transporter system, is a
transmembrane transporter protein complex consisting of two
subunits: i) SLC7A11, which is responsible for cysteine uptake; and
ii) SLC3A2, which aids in the function of SLC7A11. The activity of
the Xc−system has a direct effect on intracellular
levels of GSH (50). An adequate
supply of cysteine by the Xc− system ensures continuous
synthesis of GSH and maintenance of intracellular antioxidant
capacity, thus indirectly enhancing the function of GPX4 and
preventing the accumulation of lipid peroxides and ferroptosis
(66). The expression levels and
activity of the Xc−system are regulated by a number of
factors, including oxidative stress, intracellular glutamate levels
and multiple signaling pathways, such as the Nrf2 pathway (53). Nrf2 can regulate the expression of
antioxidant genes, including components of the Xc−system
and SLC7A11 (67).
p53 is an important tumor suppressor gene, and the
p53 protein serves a key role in a number of physiological
processes such as cell cycle regulation, DNA repair and cell death
(68). Recent studies have shown
that p53 also serves an important role in ferroptosis, mainly
affecting the ferroptosis pathway through the regulation of several
factors (SLC7A11, GPX4, FPN1) (69–72).
p53 can reduce the expression levels of SLC7A11 by directly
inhibiting its transcription, which reduces cysteine uptake and GSH
synthesis, thereby impairing GPX4 activity and increasing cellular
sensitivity to ferroptosis (73,74).
Arachidonate 12-lipoxygenase, 12S type (ALOX12) is an enzyme
involved in lipid metabolism, and its full name is
‘12-lipoxygenase’. It is primarily responsible for catalyzing the
production of specific lipid metabolites from polyunsaturated fatty
acids such as arachidonic acid. p53 can promote lipid peroxidation
by regulating ALOX12 expression. It has been shown that p53 can
activate ALOX12 transcription under conditions of DNA damage or
oxidative stress and increase the production of lipid peroxides,
thereby promoting ferroptosis (75). p53 can enhance the sensitivity to
ferroptosis by upregulating the expression levels of SAT1 and
p53-induced nuclear protein 1 (TP53INP1). TP53INP1 is a
stress-responsive protein regulated by the p53 protein and is
involved in the regulation of apoptosis, proliferation, and stress
response. TP53INP1 is considered a downstream effector of the p53
protein. When cells are stressed or injured, TP53INP1 enhances p53
activity, further driving ferroptosis. The upregulation of SAT1 can
trigger an increase in intracellular oxidative stress and the
accumulation of lipid peroxides, which further promote ferroptosis
(76,77).
p62, also known as sequestosome 1, is a
multifunctional junction protein involved in a number of cellular
processes including autophagy, signaling and protein degradation
(78). Keap1 is a cytoplasmic
protein that, under normal conditions, can bind to Nrf2 to inhibit
its activity by promoting Nrf2 ubiquitination and degradation,
thereby regulating the stability and activity of Nrf2 (79). p62 can bind to Keap1 through its
Keap1-interacting region, which causes Nrf2 to be released from the
Keap1 complex, preventing the inhibitory effect of Keap1 on Nrf2.
Nrf2-activated antioxidant genes, such as GPX4, reduce
intracellular lipid accumulation of peroxides and prevent
ferroptosis (80). Nrf2 can also
affect intracellular iron distribution and storage by regulating
the expression of ferredoxin and iron transporter proteins, thus
indirectly affecting the sensitivity to ferroptosis (81).
FSP1 is a ferroptosis inhibitory protein, also known
as apoptosis-inducing factor mitochondria associated 2. FSP1
functions independently of GPX4 and participates in ferroptosis
pathways (82). CoQ10 is an
antioxidant that dissolves in fat and is found in almost all body
cells particularly the inner membrane of the mitochondria. FSP1 can
reduce ferroptosis by decreasing CoQ10 expression levels and
inhibiting the lipid peroxidation chain reaction (83). NAD(P)H is the phosphorylated form of
NADH that is essential for cellular metabolism and antioxidant
responses. By providing the electrons needed for FSP1 to reduce
CoQ10 and by preserving the levels of CoQ10H2, NAD(P)H maintains
antioxidant defense. CoQ10H2 is the reduced form of coenzyme Q10
(CoQ10), also known as ubiquinol. CoQ10H2 is a potent fat-soluble
antioxidant that protects cell membranes from oxidative damage by
directly neutralizing free radicals, especially by trapping and
neutralizing lipid peroxides (84).
The FSP1-CoQ10-NAD(P)H pathway protects the structure and function
of cell membranes, while also directly scavenging free radicals and
lipid peroxides to strengthen cellular antioxidant defenses
(Fig. 2) (85).
Numerous cancer-related signaling pathways have been
shown to control ferroptosis in cancer cells (47). In CCA cells, iron metabolism may be
abnormal, which leads to iron accumulation and increased oxidative
stress triggering ferroptosis (86). The abnormal iron accumulation in CCA
cells can result from various factors, including heightened iron
uptake, irregular expression of transporter proteins and anomalies
in iron storage proteins (87).
Ferroportin (FPN) is a pivotal protein responsible for regulating
iron export. It serves a crucial role in the cell membrane as it
facilitates the export of intracellular iron to maintain the
balance of iron ions both inside and outside the cell (88). It was shown that the expression of
FPN was significantly reduced in CCA (89). Bile duct stones, bile duct stenosis,
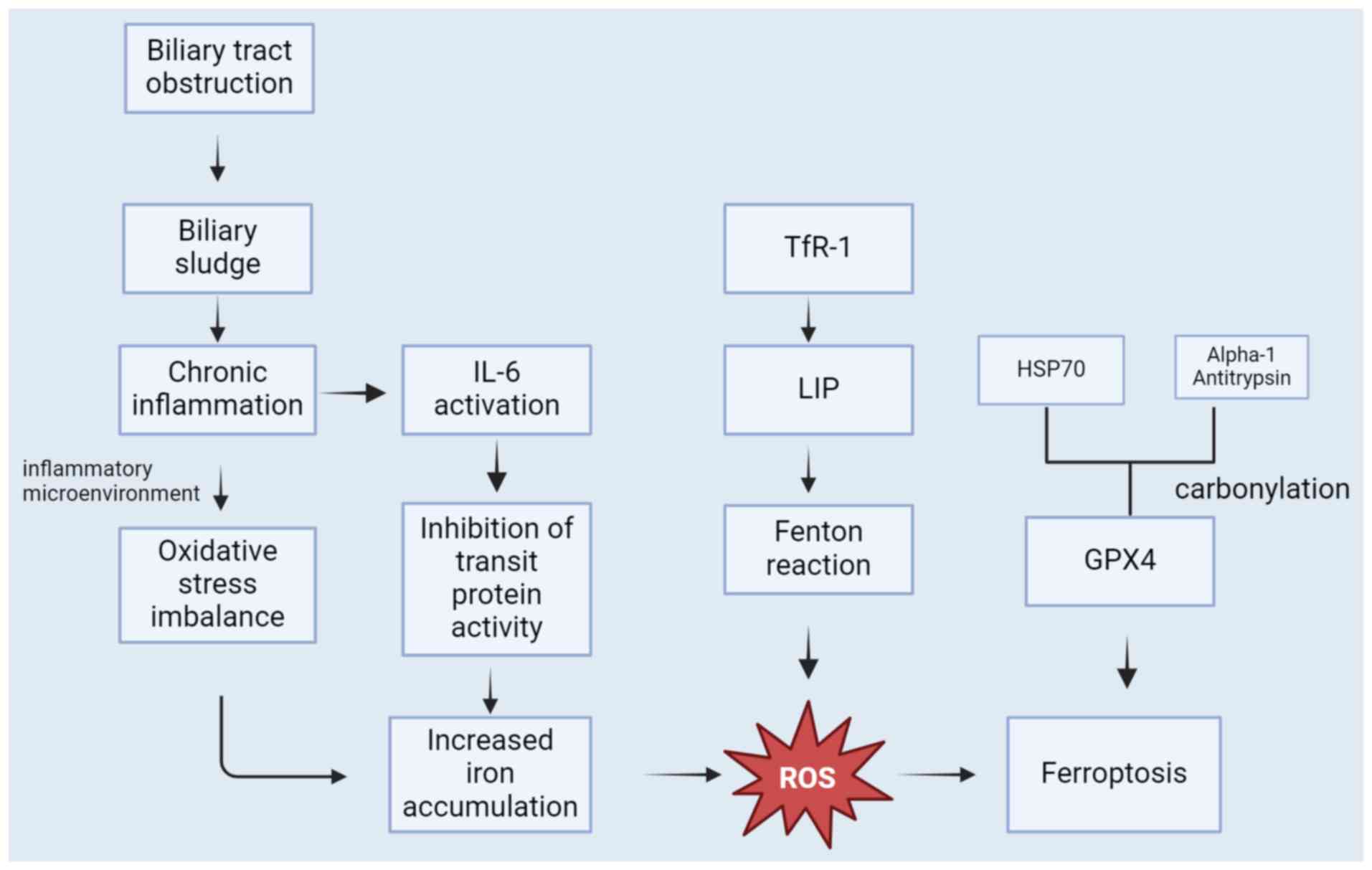
and bile duct parasitic infections can cause biliary obstruction.
Chronic bile duct obstruction can cause cholestasis, which in turn
promotes chronic bile duct inflammation is an important factor that
can cause CCA. ROS and reactive nitrogen species can damage
biomolecules in the inflammatory milieu, such as DNA, proteins and
lipids, and cause malfunction to create a cycle of oxidative stress
imbalance that eventually promotes tumorigenesis and progression,
including cholangiocarcinoma (90).
Oxidative stress from inflammation can result in Fe3+
binding and TfR oxidation, which promotes the release and buildup
of iron. Iron transporter protein function is inhibited by IL-6
activation in response to inflammatory or infectious stimuli
through FPN-dependent pathways or alternative routes (91). Carbonylation of serum transferrin,
heat shock protein 70 (HSP70) and α1-antitrypsin that occurs
through inflammation may be crucial. Carbonylation alters the
function of these key proteins, leading to a worsening of the
inflammatory response, increased oxidative stress and deeper
cellular damage (92).
Carbonylation of serum transferrin improves the Fenton reaction,
which leads to the accumulation of iron in intracellular
accumulations and extracellular release in case of cell damage or
death. When iron is released and free iron increases, the iron
generates large amounts of ROS via the Fenton reaction, leading to
oxidative stress. Carbonylation of a1-antitrypsin, a protease
inhibitor, and HSP70, an antioxidant, causes their malfunction,
GPX4 degradation and ferroptosis promotion. These factors
contribute to the advancement of CCA and are associated with a poor
prognosis (93). Therefore, it is
important to understand the survival mechanism of ferroptosis in
CCA cells to develop new therapeutic strategies that prevent the
growth and spread of CCA cells (Fig.
3).
In its early stages, CCA typically presents with
minimal symptoms, which poses a challenge for early diagnosis. A
substantial proportion of patients with CCA receive a diagnosis
only in the advanced stages and miss the optimal window for
treatment (2). Although commonly
utilized, the prevailing serologic diagnostic tool, CA-199,
exhibits limitations in specificity and sensitivity (52). Notably, doublecortin-like kinase 1
(DCLK1) is a promising candidate for the diagnosis of bile duct
cancers, such as CCA (94). Given
the inconspicuous nature of CCA symptoms, early detection of CCA
often relies on histological testing methods. Immunohistochemical
staining is a frequently employed technique as it allows for the
identification of characteristic alterations in ferroptosis within
patient tissue sections. This is achieved by labeling iron-related
proteins or molecules associated with iron metabolism using
specific antibodies (CK7, CK19, CK20, MUC5AC) (95). In the diagnostic evaluation of CCA,
assessing protein expression levels related to iron metabolism,
such as transferrin, iron carrier proteins and ferritin, is
instrumental in gauging intracellular iron accumulation. To
complement histological testing methods, imaging techniques offer
additional avenues for diagnosis (96). Ultrasonography facilitates the
examination of tumor morphology and hemodynamic characteristics in
patients with CCA, while CT scans provide insights into changes in
tissue density and morphological features of tumors (97). In the context of ferroptosis, CT
scans may identify distinct features that distinguish ferroptosis
from other forms of cell death, such as areas of low tissue density
or uniform enhancement within the tumor (98). However, the direct detection of
ferroptosis remains a challenge through CT imaging. In CCA, the
progression of tumorigenesis correlates with the extent of
ferroptosis-induced oxidative stress (99). Therefore, the degree of
intracellular oxidative stress may be indirectly assessed by
measuring oxidative stress markers, such as ROS levels and the
activity of peroxidases, including superoxide dismutase, GSH
peroxidase and catalase (92).
While histological examination is a pivotal tool for
a definitive diagnosis of CCA, its utility is hampered by limited
access to tissue specimens, as this often necessitates invasive
procedures such as surgery or puncture. The inherent risks
associated with these invasive methods, particularly in patients
with compromised health, underscore the importance of alternative
diagnostic approaches (100).
Liquid biopsy, characterized by the extraction of exosomes from CCA
cells, has potential to improve diagnostic accuracy and highlights
the demand for precise non-invasive biomarkers for CCA (101). However, this technique has yet to
find application in routine clinical practice (102). Therefore, serologic diagnostic
methods could be further explored and improved to enhance the
accurate diagnosis and treatment of CCA in the future.
At present, the primary approach to treat patients
with CCA remains rooted in surgical interventions, and the
prognosis for patients ineligible for surgery is poor (103). Survival outcomes, however, show
promise with the integration of chemotherapeutic modalities. The
combination of gemcitabine and cisplatin is currently suggested as
the most effective first-line treatment option for CCA to improve
patient prognosis (104). Recent
studies have explored the efficacy of combining bevacizumab with
Gemcitabine and cisplatin cytotoxic therapy, which demonstrated
improved survival rates for patients with CCA (105,106). Based on the roles of ferroptotic
mechanisms and pathways in cancer, it has been shown that the
addition of ferroptosis-regulating drugs to the certain treatments
may increase their efficacy (99).
The negative regulator of ferroptosis, p53-induced glycolysis and
apoptosis regulator (TIGAR), inhibits glycolysis by regulating the
activity of phosphofructokinase-2 in the glycolytic pathway,
thereby reducing intracellular ROS production (107). Studies have shown that combination
treatment of cisplatin and low expression of TIGAR significantly
induced ferroptosis (108).
Several ferroptosis inducers, such as Ras selective lethal 3
(RSL3), sulfasalazine and erastin, have shown potential to induce
ferroptosis in CCA cells in vitro and in animal models
(109). The drug reduces
intracellular antioxidant capacity by inhibiting system
Xc− and increases cellular sensitivity to ferroptosis
(110). In the treatment of CCA,
iron chelators may inhibit tumor growth and metastasis by
regulating the balance of intracellular iron metabolism and
reducing the toxic effects of iron ions. The use of chelators may
improve the therapeutic efficacy compared with the use of
platinum-based chemotherapeutic agents (111). In addition, the use of
multi-targeted drugs, including those involving iron chelators and
iron-chelator complexes, is a therapeutic direction to be
considered (112). Application of
ferroptosis antioxidants may help to attenuate tumor cell damage
and death. Antioxidants may inhibit ferroptosis by scavenging
excessive intracellular ROS and attenuating oxidative stress
(113). Glutathione is an
important antioxidant molecule that can help reduce other oxidized
antioxidants and reduce cellular damage from oxidative stress
(66). Moreover, Yang et al
(114) have identified a novel
ferroptosis inhibitor, YL-939, which is distinct from traditional
iron chelators and antioxidants. YL-939, a non-classical
ferroptosis inhibitor, has been reported to target prohibitin 2
(PHB2), which suggests binding of YL-939 to PHB2 promotes
expression of the iron storage protein ferritin, which reduces iron
content and thus susceptibility to ferroptosis. Despite the
association of YL-939 with liver injury, this discovery opens new
avenues for investigating the role of YL-939 in CCA (114).
It has been shown that aberrant p53 expression
levels are typically closely associated with tumor development
(115). p53, a well-established
oncogene, is a critical regulator of apoptosis and cell cycle
processes. In response to DNA damage or stresses such as oxidative
stress, anticancer drugs and inflammatory responses, p53 proteins
are activated and play important protective roles in cells by
orchestrating survival or death pathways (116). p53 may indirectly increase
intracellular oxidative stress and lipid peroxidation through the
activation of SAT1 and regulation of the ALOX-15 pathways. These
processes are implicated in regulating ferroptosis, contributing to
cholangiocarcinogenesis (76).
Through in vitro and in vivo experiments, low
expression levels of FBXO31 have been demonstrated to promote
ferroptosis by facilitating the ubiquitination of GPX4 and leading
to protease degradation. This process increases the sensitivity of
CCA stem cells to cisplatin, which exerts a tumor-suppressive
effect (117).
Mechanistic pathways through which ferroptosis is
regulated can also influence CCA progression. The regulatory
network in CCA involves microRNA (miR)-3202 and GPX4, a protein
pivotal in intracellular oxidative stress and iron metabolism. The
interplay of JUND/linc00976 have been identified as a regulator of
intracellular iron metabolism and modulates the ferroptosis process
through the miR-3202/GPX4 pathway. This regulatory mechanism
promotes the progression and metastasis of CCA (118). Immunohistochemistry was used to
show that hydroxysteroid dehydrogenase-like 2 (HSDL2) expression
levels in tissues was lower compared with those in matched
neighboring non-tumor bile duct tissues. By decreasing
malondialdehyde, ROS levels and inhibiting ferroptosis via the
p53/SLC7A11 axis, HSDL2 knockdown was found to promote CCA
progression. Therefore, HSDL2 may be a useful treatment target and
prognosis indicator for CCA (119). A previous study demonstrated the
potential of downregulating hsa_circ_0050900, identifying its role
as a sponge that inhibits SLC3A2 expression while promoting the
expression of hsa-miR-605-3p. This mechanism induces ferroptosis in
CCA cells, which results in the inhibition of cell proliferation
and migration (120). Notably,
trematode infection stands among the risk factors for CCA (121). Photodynamic therapy (PDT) utilizes
photosensitizers to generate ROS for the targeted eradication of
CCA tumor cells (122,123). Recent research has demonstrated
that by causing iron mortality in in vitro assays and tumor
models, tofacitinib in conjunction with PDT could suppress CCA
(124).
The integration of ferroptosis inducers in
combination with PDT presents a dual-enhancement strategy for tumor
therapy. Firstly, the substantial ROS production induced by PDT
complements the necessary accumulation of lipid ROS for
ferroptosis, which amplifies the therapeutic impact. Secondly, the
ferroptosis inducer further augments the cytotoxic effect on tumor
cells. By employing this combined strategy, a synergistic effect
may emerge, which improves the overall efficacy against tumors
(125). It has been shown that PDT
inhibits cancer progression and induces ferroptosis and apoptosis
by targeting the p53/GPX4/SLC7A11 signaling pathway in CCA
(126). Although PDT has shown
positive results in the treatment of CCA, the study is still in the
early stages of clinical trials in mice (127). This combined therapeutic strategy
may provide novel breakthroughs and research directions for the
application of ferroptosis in tumor therapy (Fig. 4).
CCA, characterized by its high heterogeneity and
aggressive nature within the bile duct, poses a formidable
challenge in clinical management, marked by a lack of precise
prognostic biomarkers and poor overall prognosis. In this
challenging landscape, the emerging field of ferroptosis, a
regulated cell death mechanism linked to cancer progression, offers
a novel perspective and potential avenues for prognostic assessment
in CCA. It has been demonstrated that patients with advanced CCA
can live longer when using a combination of PDT, an efficient
anticancer treatment, and a novel multi-kinase inhibitor sofantinib
(SUR) (128). A previous study
measured the levels of ROS, lipid peroxides, malondialdehyde and
glutathione to demonstrate that SUR combined with PDT can promote
ferroptosis to suppress CCA cell proliferation. Therefore, it could
be suggested that further research on combination therapeutic
approaches that address ferroptosis could enhance patient prognosis
(127,129). Furthermore, it was reported that
circFOXP1-231aa, a recently identified protein, controls the
stability of nuclear receptor coactivator 4; it is involved in iron
homeostasis regulation and regulates intracellular iron release by
mediating ferritin autophagy through de-ubiquitination
modification, increase in ferroptosis in ICC cells (refers to
cancer cells originating from the epithelium of the intrahepatic
bile ducts, which constitute the major cell type of iCCA) and
preventing ICC recurrence (130).
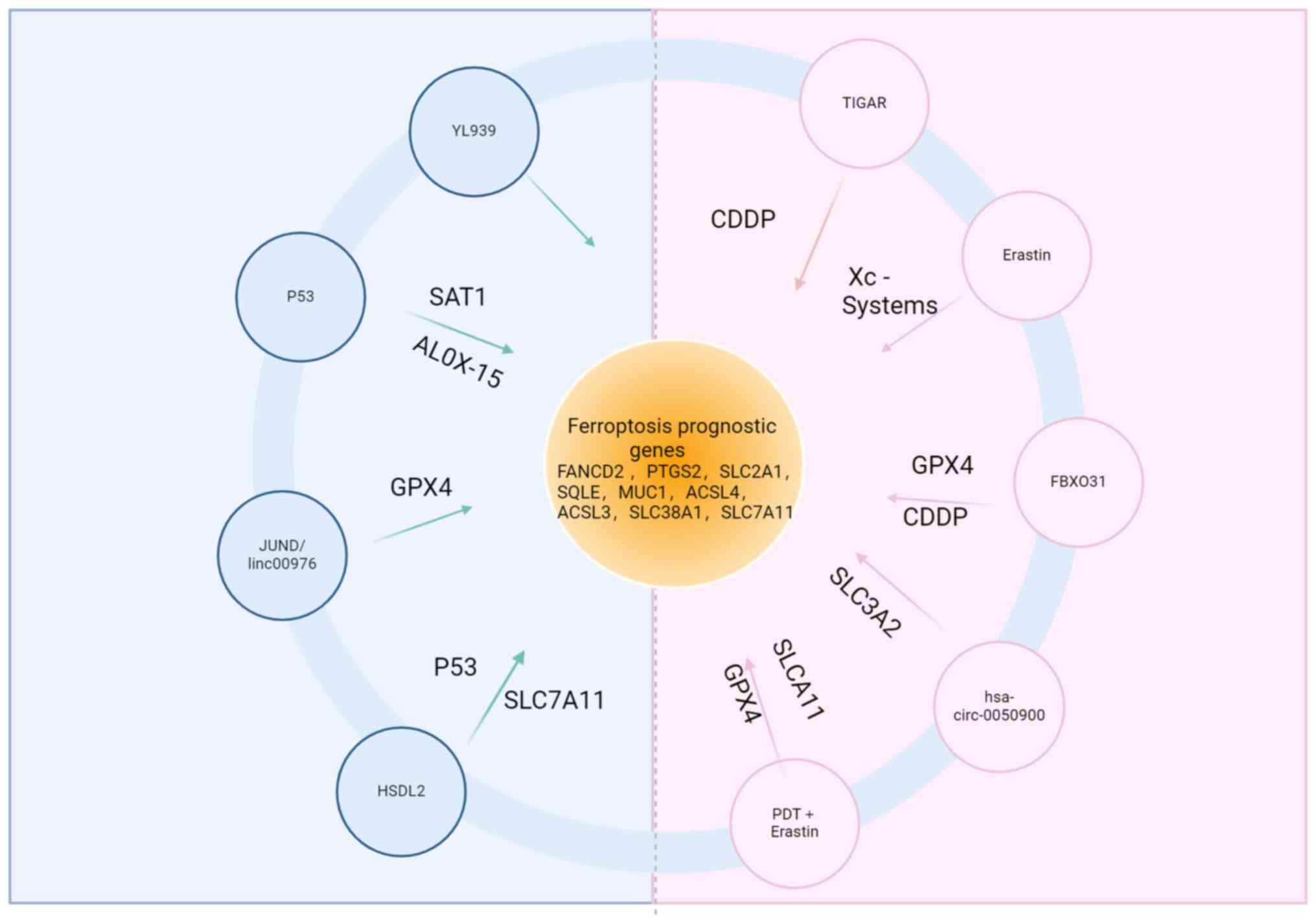
Several ferroptosis-related genes have demonstrated prognostic
implications not only in CCA but also in hepatocellular carcinoma
and pancreatic cancer (131).
Sae-Fung et al (99)
conducted a comprehensive database analysis and identified several
genes (FRG, FANCD2, PTGS2, SLC2A1, SQLE, ACO1 and GOT1) associated
with ferroptosis. Notably, FANCD2, PTGS2, SLC2A1 and SQLE exhibit
increased expression levels in CCA tumor-associated tissues, while
ACO1 and GOT1 demonstrate reduced expression levels. These findings
suggest a potential correlation with poor prognosis in patients
with CCA (132,133). Additionally, five
ferroptosis-related genes (MUC1, ACSL4, ACSL3, SLC38A1 and SLC7A11)
not only hold promise for immunotherapeutic interventions in CCA
but also exhibit prognostic relevance in the context of this
malignancy (134). Among the
highlighted ferroptosis-related genes, ACSL4 stands out as a
crucial regulator of lipid metabolism and intracellular iron ion
homeostasis. A significant correlation between increased ACSL4
expression levels in CCA and adverse patient prognosis has been
reported, which suggests a potential role for ACSL4 as a prognostic
marker for CCA (135). Another
pivotal participant in the ferroptosis process is GPX4, and initial
studies have suggested its candidacy as a prognostic marker for
CCA, further contributing to the expanding understanding of the
intricate molecular landscape associated with CCA (136,137). Although the results of these
clinical studies provide a preliminary indication of the
relationship between ferroptosis and the prognosis of CCA, further
large-scale multicenter clinical studies are needed to validate and
explore this relationship in depth.
Ferroptosis, as a distinct form of cell death
deviating from traditional paradigms, has gained increasing
prominence in clinical practice, particularly with the ongoing
exploration of its mechanisms. Its pivotal role in a number of
diseases, notably cancer, has generated significant interest due to
the potential significance in the diagnosis, treatment and
prognosis of CCA. There are still many unanswered concerns
regarding the mechanism of ferroptosis and treatment approaches in
CCA, which are currently in the exploratory stage. Future studies
may concentrate on combinations of therapeutic strategies in order
to further create and optimize strategies for treating ferroptosis.
The safety, efficacy and prognosis improvement of therapeutic
options related to ferroptosis may be assessed by large-scale
clinical trials and prospective research, thereby offering a
scientific foundation for clinical practice.
Not applicable.
The present study was supported by grants from the Shandong
Provincial Nature Science Foundation (grant no. 2015ZRE27571),
Shandong Provincial Nature Foundation (grant no. ZR2020MH321) and
Shandong Provincial Key Research and Development Program (grant no.
2016G5F201169).
Not applicable.
XiZ, MZ, JH, XL and XuZ contributed to the idea and
design of the article. Data collection and analysis were carried
out by MZ, XL and JH. The first draft of the manuscript was written
by XiZ. XiZ, MZ, JH, XL and XuZ commented on previous versions of
the manuscript. All authors read and approved the final manuscript.
Data authentication is not applicable.
Not applicable.
Not applicable.
The authors declare that they have no competing
interests.
|
1
|
Rizvi S and Gores GJ: Pathogenesis,
diagnosis, and management of cholangiocarcinoma. Gastroenterology.
145:1215–1229. 2013. View Article : Google Scholar
|
|
2
|
Cardinale V: Classifications and
misclassification in cholangiocarcinoma. Liver Int. 39:260–262.
2019. View Article : Google Scholar
|
|
3
|
Brindley PJ, Bachini M, Ilyas SI, Khan SA,
Loukas A, Sirica AE, The BT, Wongkham S and Gores GJ:
Cholangiocarcinoma. Nat Rev Dis Primers. 7:652021. View Article : Google Scholar
|
|
4
|
Sripa B, Suwannatrai AT, Sayasone S, Do
DT, Khieu V and Yang Y: Current status of human liver fluke
infections in the Greater Mekong Subregion. Acta Trop.
224:1061332021. View Article : Google Scholar
|
|
5
|
Qurashi M, Vithayathil M and Khan SA:
Epidemiology of cholangiocarcinoma. Eur J Surg Oncol. 9:1070642023.
View Article : Google Scholar
|
|
6
|
Kim R, Taylor D, Vonderheide RH and
Gabrilovich DI: Ferroptosis of immune cells in the tumor
microenvironment. Trends Pharmacol Sci. 44:542–552. 2023.
View Article : Google Scholar
|
|
7
|
Xu Y, Xing Z, Suliman RAI, Liu Z and Tang
F: Ferroptosis in liver cancer: A key role of post-translational
modifications. Front Immunol. 15:13755892024. View Article : Google Scholar
|
|
8
|
Zhou TJ, Zhang MM, Liu DM, Huang LL, Yu
HQ, Wang Y, Xing L and Jiang HL: Glutathione depletion and
dihydroorotate dehydrogenase inhibition actuated
ferroptosis-augment to surmount triple-negative breast cancer.
Biomaterials. 305:1224472024. View Article : Google Scholar
|
|
9
|
Deng J, Lin X, Qin J, Li Q, Zhang Y, Zhang
Q, Ji C, Shen S, Li Y, Zhang B and Lin N: SPTBN2 suppresses
ferroptosis in NSCLC cells by facilitating SLC7A11 membrane
trafficking and localization. Redox Biol. 70:1030392024. View Article : Google Scholar
|
|
10
|
Dixon SJ, Lemberg KM, Lamprecht MR, Skouta
R, Zaitsev EM, Gleason CE, Patel DN, Bauer AJ, Cantley AM, Yang WS,
et al: Ferroptosis: An iron-dependent form of nonapoptotic cell
death. Cell. 149:1060–1072. 2012. View Article : Google Scholar
|
|
11
|
Nakamura T, Naguro I and Ichijo H: Iron
homeostasis and iron-regulated ROS in cell death, senescence and
human diseases. Biochim Biophys Acta Gen Subj. 1863:1398–1409.
2019. View Article : Google Scholar
|
|
12
|
D'Arcy MS: Cell death: A review of the
major forms of apoptosis, necrosis and autophagy. Cell Biol Int.
43:582–592. 2019. View Article : Google Scholar
|
|
13
|
Fu C, Cao N, Zeng S, Zhu W, Fu X, Liu W
and Fan S: Role of mitochondria in the regulation of ferroptosis
and disease. Front Med (Lausanne). 10:13018222023. View Article : Google Scholar
|
|
14
|
Otasevic V, Vucetic M, Grigorov I,
Martinovic V and Stancic A: Ferroptosis in different pathological
contexts seen through the eyes of mitochondria. Oxid Med Cell
Longev. 2021:55373302021. View Article : Google Scholar
|
|
15
|
Xie LH, Fefelova N, Pamarthi SH and
Gwathmey JK: Molecular mechanisms of ferroptosis and relevance to
cardiovascular disease. Cells. 11:27262022. View Article : Google Scholar
|
|
16
|
Liu J, Kang R and Tang D: Signaling
pathways and defense mechanisms of ferroptosis. FEBS J.
289:7038–7050. 2022. View Article : Google Scholar
|
|
17
|
Liu T, Zhang L, Joo D and Sun SC: NF-κB
signaling in inflammation. Signal Transduct Target Ther.
2:170232017. View Article : Google Scholar
|
|
18
|
Ahmad A and Ahsan H: Biomarkers of
inflammation and oxidative stress in ophthalmic disorders. J
Immunoassay Immunochem. 41:257–271. 2020. View Article : Google Scholar
|
|
19
|
Yang W, Wang Y, Zhang C, Huang Y, Yu J,
Shi L, Zhang P, Yin Y, Li R and Tao K: Maresin1 protect against
ferroptosis-induced liver injury through ROS inhibition and
Nrf2/HO-1/GPX4 activation. Front Pharmacol. 13:8656892022.
View Article : Google Scholar
|
|
20
|
Tanase DM, Gosav EM, Anton MI, Floria M,
Isac PN, Hurjui LL, Tarniceriu CC, Costea CF, Ciocoiu M and Rezus
C: Oxidative stress and NRF2/KEAP1/ARE pathway in diabetic kidney
disease (DKD): New perspectives. Biomolecules. 12:12272022.
View Article : Google Scholar
|
|
21
|
Ueda N and Takasawa K: Impact of
inflammation on ferritin, hepcidin and the management of iron
deficiency anemia in chronic kidney disease. Nutrients.
10:11732018. View Article : Google Scholar
|
|
22
|
Chen Y, Fang ZM, Yi X, Wei X and Jiang DS:
The interaction between ferroptosis and inflammatory signaling
pathways. Cell Death Dis. 14:2052023. View Article : Google Scholar
|
|
23
|
Schmitt A, Xu W, Bucher P, Grimm M,
Konantz M, Horn H, Zapukhlyak M, Berning P, Brändle M, Jarboui MA,
et al: Dimethyl fumarate induces ferroptosis and impairs NF-κ/STAT3
signaling in DLBCL. Blood. 138:871–884. 2021. View Article : Google Scholar
|
|
24
|
Yan N, Xu Z, Qu C and Zhang J: Dimethyl
fumarate improves cognitive deficits in chronic cerebral
hypoperfusion rats by alleviating inflammation, oxidative stress,
and ferroptosis via NRF2/ARE/NF-κB signal pathway. Int
Immunopharmacol. 98:1078442021. View Article : Google Scholar
|
|
25
|
Zhao C, Xiao C, Feng S and Bai J:
Artemisitene alters LPS-induced oxidative stress, inflammation and
ferroptosis in liver through Nrf2/HO-1 and NF-kB pathway. Front
Pharmacol. 14:11775422023. View Article : Google Scholar
|
|
26
|
Yeung YT, Aziz F, Guerrero-Castilla A and
Arguelles S: Signaling pathways in inflammation and
anti-inflammatory therapies. Curr Pharm Des. 24:1449–1484. 2018.
View Article : Google Scholar
|
|
27
|
Xu X, Mao C, Zhang C, Zhang M, Gong J and
Wang X: Salvianolic Acid B inhibits ferroptosis and apoptosis
during myocardial ischemia/reperfusion injury via decreasing the
ubiquitin-proteasome degradation of GPX4 and the ROS-JNK/MAPK
pathways. Molecules. 28:41172023. View Article : Google Scholar
|
|
28
|
Lu JS, Wang JH, Han K and Li N: Nicorandil
regulates ferroptosis and mitigates septic cardiomyopathy via
TLR4/SLC7A11 signaling pathway. Inflammation. 47:975–988. 2024.
View Article : Google Scholar
|
|
29
|
Zhu K, Zhu X, Sun S, Yang W, Liu S, Tang
Z, Zhang R, Li J, Shen T and Hei M: Inhibition of TLR4 prevents
hippocampal hypoxic-ischemic injury by regulating ferroptosis in
neonatal rats. Exp Neurol. 345:1138282021. View Article : Google Scholar
|
|
30
|
Cardoso-Pires C and Vieira HLA: Carbon
monoxide and mitochondria: Cell energy and fate control. Biochim
Biophys Acta Mol Basis Dis. 1870:1674462024. View Article : Google Scholar
|
|
31
|
Du L, Guo C, Zeng S, Yu K, Liu M and Li Y:
Sirt6 overexpression relieves ferroptosis and delays the
progression of diabetic nephropathy via Nrf2/GPX4 pathway. Ren
Fail. 46:23777852024. View Article : Google Scholar
|
|
32
|
Sun K, Zhi Y, Ren W, Li S, Zhou X, Gao L
and Zhi K: The mitochondrial regulation in ferroptosis signaling
pathway and its potential strategies for cancer. Biomed
Pharmacother. 169:1158922023. View Article : Google Scholar
|
|
33
|
Murphy MP: How mitochondria produce
reactive oxygen species. Biochem J. 417:1–13. 2009. View Article : Google Scholar
|
|
34
|
Su D, Ding C, Wang R, Qiu J, Liu Y, Tao J,
Luo W, Weng G, Yang G and Zhang T: E3 ubiquitin ligase RBCK1
confers ferroptosis resistance in pancreatic cancer by facilitating
MFN2 degradation. Free Radic Biol Med. 221:136–154. 2024.
View Article : Google Scholar
|
|
35
|
Zheng J and Conrad M: The metabolic
underpinnings of ferroptosis. Cell Metab. 32:920–937. 2020.
View Article : Google Scholar
|
|
36
|
Xiang Q, Wen J, Zhou Z, Dai Q, Huang Y,
Yang N, Guo J, Zhang J, Ren F, Zhou X, et al: Effect of
hydroxy-α-sanshool on lipid metabolism in liver and hepatocytes
based on AMPK signaling pathway. Phytomedicine. 132:1558492024.
View Article : Google Scholar
|
|
37
|
Copur S, Yildiz AB, Covic A and Kanbay M:
Is there any robust evidence showing that SGLT2 inhibitor
predisposes to cancer? Eur J Clin Invest. 54:e141312024. View Article : Google Scholar
|
|
38
|
Gao M, Yi J, Zhu J, Minikes AM, Monian P,
Thompson CB and Jiang X: Role of mitochondria in ferroptosis. Mol
Cell. 73:354–363. e3532019. View Article : Google Scholar
|
|
39
|
Qi Y, Zheng J, Zi Y, Song W, Chen X, Cao
S, Zhou Q, Fu H and Hu X: Loureirin C improves mitochondrial
function by promoting NRF2 nuclear translocation to attenuate
oxidative damage caused by renal ischemia-reperfusion injury. Int
Immunopharmacol. 138:1125962024. View Article : Google Scholar
|
|
40
|
Zhang T, Jing M, Fei L, Zhang Z, Yi P, Sun
Y and Wang Y: Tetramethylpyrazine nitrone delays the aging process
of C. elegans by improving mitochondrial function through the
AMPK/mTORC1 signaling pathway. Biochem Biophys Res Commun.
723:1502202024. View Article : Google Scholar
|
|
41
|
Lane DJR, Metselaar B, Greenough M, Bush
AI and Ayton SJ: Ferroptosis and NRF2: An emerging battlefield in
the neurodegeneration of Alzheimer's disease. Essays Biochem.
65:925–940. 2021. View Article : Google Scholar
|
|
42
|
Zhang JY, Meng X, Zhu XL, Peng SR, Li HB,
Mo HZ and Hu LB: Thymol induces fenton-reaction-dependent
ferroptosis in vibrio parahemolyticus. J Agric Food Chem.
72:14337–14348. 2024. View Article : Google Scholar
|
|
43
|
Torti SV and Torti FM: Iron and cancer:
More ore to be mined. Nat Rev Cancer. 13:342–355. 2013. View Article : Google Scholar
|
|
44
|
Zhang C: Essential functions of
iron-requiring proteins in DNA replication, repair and cell cycle
control. Protein Cell. 5:750–760. 2014. View Article : Google Scholar
|
|
45
|
Koppenol WH and Hider RH: Iron and redox
cycling. Do's and don'ts. Free Radic Biol Med. 133:3–10. 2019.
View Article : Google Scholar
|
|
46
|
Xu L, Liu Y, Chen X, Zhong H and Wang Y:
Ferroptosis in life: To be or not to be. Biomed Pharmacother.
159:1142412023. View Article : Google Scholar
|
|
47
|
Hassannia B, Vandenabeele P and Berghe TV:
Targeting ferroptosis to iron out cancer. Cancer Cell. 35:830–849.
2019. View Article : Google Scholar
|
|
48
|
Fang X, Ardehali H, Min J and Wang F: The
molecular and metabolic landscape of iron and ferroptosis in
cardiovascular disease. Nat Rev Cardiol. 20:7–23. 2023. View Article : Google Scholar
|
|
49
|
Truman-Rosentsvit M, Berenbaum D, Spektor
L, Cohen LA, Belizowsky-Moshe S, Lifshitz L, Ma J, Li W, Kesselman
E, Abutbul-Ionita I, et al: Ferritin is secreted via 2 distinct
nonclassical vesicular pathways. Blood. 131:342–352. 2018.
View Article : Google Scholar
|
|
50
|
Rochette L, Dogon G, Rigal E, Zeller M,
Cottin Y and Vergely C: Lipid peroxidation and iron metabolism: Two
corner stones in the homeostasis control of ferroptosis. Int J Mol
Sci. 24:4492022. View Article : Google Scholar
|
|
51
|
Chen X, Yu C, Kang R and Tang D: Iron
metabolism in ferroptosis. Front Cell Dev Biol. 8:5902262020.
View Article : Google Scholar
|
|
52
|
Yu X, Guo Q, Zhang H, Wang X, Han Y and
Yang Z: Hypoxia-inducible factor-1alpha can reverse the Adriamycin
resistance of breast cancer adjuvant chemotherapy by upregulating
transferrin receptor and activating ferroptosis. FASEB J.
38:e238762024. View Article : Google Scholar
|
|
53
|
Sies H: Oxidative stress: A concept in
redox biology and medicine. Redox Biol. 4:180–183. 2015. View Article : Google Scholar
|
|
54
|
Chen S, Li Q, Shi H, Li F, Duan Y and Guo
Q: New insights into the role of mitochondrial dynamics in
oxidative stress-induced diseases. Biomed Pharmacother.
178:1170842024. View Article : Google Scholar
|
|
55
|
Jomova K, Raptova R, Alomar SY, Alwasel
SH, Nepovimova E, Kuca K and Valko M: Reactive oxygen species,
toxicity, oxidative stress, and antioxidants: Chronic diseases and
aging. Arch Toxicol. 97:2499–2574. 2023. View Article : Google Scholar
|
|
56
|
Galaris D, Barbouti A and Pantopoulos K:
Iron homeostasis and oxidative stress: An intimate relationship.
Biochim Biophys Acta Mol Cell Res. 1866:1185352019. View Article : Google Scholar
|
|
57
|
Carocci A, Catalano A, Sinicropi MS and
Genchi G: Oxidative stress and neurodegeneration: The involvement
of iron. Biometals. 31:715–735. 2018. View Article : Google Scholar
|
|
58
|
Boldyreva LV, Morozova MV, Saydakova SS
and Kozhevnikova EN: Fat of the gut: Epithelial phospholipids in
inflammatory bowel diseases. Int J Mol Sci. 22:116822021.
View Article : Google Scholar
|
|
59
|
Feng H and Stockwell BR: Unsolved
mysteries: How does lipid peroxidation cause ferroptosis? PLoS
Biol. 16:e20062032018. View Article : Google Scholar
|
|
60
|
Liang D, Minikes AM and Jiang X:
Ferroptosis at the intersection of lipid metabolism and cellular
signaling. Mol Cell. 82:2215–2227. 2022. View Article : Google Scholar
|
|
61
|
Li FJ, Long HZ, Zhou ZW, Luo HY, Xu SG and
Gao LC: System X(c) (−)/GSH/GPX4 axis: An important antioxidant
system for the ferroptosis in drug-resistant solid tumor therapy.
Front Pharmacol. 13:9102922022. View Article : Google Scholar
|
|
62
|
Hussain S, Gupta G, Shahwan M, Bansal P,
Kaur H, Deorari M, Pant K, Ali H, Singh SK, Allam VS, et al:
Non-coding RNA: A key regulator in the Glutathione-GPX4 pathway of
ferroptosis. Noncoding RNA Res. 9:1222–1234. 2024. View Article : Google Scholar
|
|
63
|
Xie Y, Kang R, Klionsky DJ and Tang D:
GPX4 in cell death, autophagy, and disease. Autophagy.
19:2621–2638. 2023. View Article : Google Scholar
|
|
64
|
Liu Y, Wan Y, Jiang Y, Zhang L and Cheng
W: GPX4: The hub of lipid oxidation, ferroptosis, disease and
treatment. Biochim Biophys Acta Rev Cancer. 1878:1888902023.
View Article : Google Scholar
|
|
65
|
Liu Y, Lu S, Wu LL, Yang L, Yang L and
Wang J: The diversified role of mitochondria in ferroptosis in
cancer. Cell Death Dis. 14:5192023. View Article : Google Scholar
|
|
66
|
Jiang X, Stockwell BR and Conrad M:
Ferroptosis: Mechanisms, biology and role in disease. Nat Rev Mol
Cell Biol. 22:266–282. 2021. View Article : Google Scholar
|
|
67
|
Dodson M, Castro-Portuguez R and Zhang DD:
NRF2 plays a critical role in mitigating lipid peroxidation and
ferroptosis. Redox Biol. 23:1011072019. View Article : Google Scholar
|
|
68
|
Liu Y, Su Z, Tavana O and Gu W:
Understanding the complexity of p53 in a new era of tumor
suppression. Cancer Cell. 42:946–967. 2024. View Article : Google Scholar
|
|
69
|
Shin D, Lee J and Roh JL: Pioneering the
future of cancer therapy: Deciphering the p53-ferroptosis nexus for
precision medicine. Cancer Lett. 585:2166452024. View Article : Google Scholar
|
|
70
|
Hu H, Zhang G, Tian M, Yin Y, Bao Y, Guan
X, Ding C and Yu S: Brucella rough RB51 infection activates
P53-Slc7a11-Gpx4/GSH pathway to induce ferroptosis to attenuate the
intracellular survival on macrophages. Vet Microbiol.
298:1102242024. View Article : Google Scholar
|
|
71
|
Liao W, Zhang R, Chen G, Zhu X, Wu W, Chen
Z, Jiang C, Lin Z, Ma L and Yu H: Berberine synergises with
ferroptosis inducer sensitizing NSCLC to ferroptosis in
p53-dependent SLC7A11-GPX4 pathway. Biomed Pharmacother.
176:1168322024. View Article : Google Scholar
|
|
72
|
Fu R, You Y, Wang Y, Wang J, Lu Y, Gao R,
Pang M, Yang P and Wang H: Sanggenol L induces ferroptosis in
non-small cell lung cancer cells via regulating the
miR-26a-1-3p/MDM2/p53 signaling pathway. Biochem Pharmacol.
226:1163452024. View Article : Google Scholar
|
|
73
|
Zeng C, Xiong D, Zhang K and Yao J:
Shank-associated RH domain interactor signaling in tumorigenesis.
Oncol Lett. 20:2579–2586. 2020. View Article : Google Scholar
|
|
74
|
Zeng C, Lin J, Zhang K, Ou H, Shen K, Liu
Q, Wei Z, Dong X, Zeng X, Zeng L, et al: SHARPIN promotes cell
proliferation of cholangiocarcinoma and inhibits ferroptosis via
p53/SLC7A11/GPX4 signaling. Cancer Sci. 113:3766–3775. 2022.
View Article : Google Scholar
|
|
75
|
Chu B, Kon N, Chen D, Li T, Liu T, Jiang
L, Song S, Tavana O and Gu W: ALOX12 is required for p53-mediated
tumour suppression through a distinct ferroptosis pathway. Nat Cell
Biol. 21:579–591. 2019. View Article : Google Scholar
|
|
76
|
Ou Y, Wang SJ, Li D, Chu B and Gu W:
Activation of SAT1 engages polyamine metabolism with p53-mediated
ferroptotic responses. Proc Natl Acad Sci USA. 113:E6806–E6812.
2016. View Article : Google Scholar
|
|
77
|
Lu B, Christensen IT, Yu T, Wang C, Yan Q
and Wang X: SUMOylation evoked by oxidative stress reduced lens
epithelial cell antioxidant functions by increasing the stability
and transcription of TP53INP1 in age-related cataracts. Oxid Med
Cell Longev. 2019:78980692019. View Article : Google Scholar
|
|
78
|
Wang J, Yi H, Li J, Yang Y, Sun G, Xue Y
and He L: P62-autophagic pathway degrades SLC7A11 to regulate
ferroptosis in doxorubicin-induced cardiotoxicity. Life Sci.
356:1229812024. View Article : Google Scholar
|
|
79
|
Lin Z, Wu C, Song D, Zhu C, Wu B, Wang J
and Xue Y: Sarmentosin alleviates doxorubicin-induced
cardiotoxicity and ferroptosis via the p62-Keap1-Nrf2 pathway.
Redox Rep. 29:23923292024. View Article : Google Scholar
|
|
80
|
Zhao Y, Lu J, Mao A, Zhang R and Guan S:
Autophagy inhibition plays a protective role in ferroptosis induced
by alcohol via the p62-Keap1-Nrf2 Pathway. J Agric Food Chem.
69:9671–9683. 2021. View Article : Google Scholar
|
|
81
|
Zhou Y, Yang Y, Yi L, Pan M, Tang W and
Duan H: Propofol mitigates sepsis-induced brain injury by
inhibiting ferroptosis via activation of the Nrf2/HO-1axis.
Neurochem Res. 49:2131–2147. 2024. View Article : Google Scholar
|
|
82
|
Liu N, Wu WL, Wan XR, Wang J, Huang JN,
Jiang YY, Sheng YC, Wu JC, Liang ZQ, Qin ZH and Wang Y: Regulation
of FSP1 myristoylation by NADPH: A novel mechanism for ferroptosis
inhibition. Redox Biol. 73:1031762024. View Article : Google Scholar
|
|
83
|
Dai E, Zhang W, Cong D, Kang R, Wang J and
Tang D: AIFM2 blocks ferroptosis independent of ubiquinol
metabolism. Biochem Biophys Res Commun. 523:966–971. 2020.
View Article : Google Scholar
|
|
84
|
Guo J, Chen L and Ma M: Ginsenoside Rg1
suppresses ferroptosis of renal tubular epithelial cells in
sepsis-induced acute kidney injury via the FSP1-CoQ(10)- NAD(P)H
pathway. Curr Med Chem. 31:2119–2132. 2024. View Article : Google Scholar
|
|
85
|
Li D and Li Y: The interaction between
ferroptosis and lipid metabolism in cancer. Signal Transduct Target
Ther. 5:1082020. View Article : Google Scholar
|
|
86
|
Torti SV and Torti FM: Iron and cancer:
2020 vision. Cancer Res. 80:5435–5448. 2020. View Article : Google Scholar
|
|
87
|
Toshida K, Itoh S, Iseda N, Izumi T, Bekki
Y, Yoshiya S, Toshima T, Iwasaki T, Oda Y and Yoshizumi T: The
association of transferrin receptor with prognosis and biologic
role in intrahepatic cholangiocarcinoma. Ann Surg Oncol.
23:10.1245/s10434–024-16065-3. 2024.
|
|
88
|
Vogt AS, Arsiwala T, Mohsen M, Vogel M,
Manolova V and Bachmann MF: On iron metabolism and its regulation.
Int J Mol Sci. 22:45912021. View Article : Google Scholar
|
|
89
|
Guo W, Zhang S, Chen Y, Zhang D, Yuan L,
Cong H and Liu S: An important role of the hepcidin-ferroportin
signaling in affecting tumor growth and metastasis. Acta Biochim
Biophys Sin (Shanghai). 47:703–715. 2015. View Article : Google Scholar
|
|
90
|
Raggi C, Gammella E, Correnti M, Buratti
P, Forti E, Andersen JB, Alpini G, Glaser S, Alvaro D, Invernizzi
P, et al: Dysregulation of iron metabolism in cholangiocarcinoma
stem-like cells. Sci Rep. 7:176672017. View Article : Google Scholar
|
|
91
|
Kong L, Liu Y, Li J, Wang Y, Ji P, Shi Q,
Han M, Xu H and Li W and Li W: Ginsenoside Rg1 alleviates chronic
inflammation-induced neuronal ferroptosis and cognitive impairments
via regulation of AIM2-Nrf2 signaling pathway. J Ethnopharmacol.
330:1182052024. View Article : Google Scholar
|
|
92
|
Caligiuri A, Becatti M, Porro N, Borghi S,
Marra F, Pastore M, Taddei N, Fiorillo C and Gentilini A: Oxidative
stress and redox-dependent pathways in cholangiocarcinoma.
Antioxidants (Basel). 13:282023. View Article : Google Scholar
|
|
93
|
Thanan R, Oikawa S, Yongvanit P, Hiraku Y,
Ma N, Pinlaor S, Pairojkul C, Wongkham C, Sripa B, Khuntikeo N, et
al: Inflammation-induced protein carbonylation contributes to poor
prognosis for cholangiocarcinoma. Free Radic Biol Med.
52:1465–1472. 2012. View Article : Google Scholar
|
|
94
|
Nevi L, Di Matteo S, Carpino G, Zizzari
IG, Samira S, Ambrosino V, Costantini D, Overi D, Giancotti A,
Monti M, et al: DCLK1, a putative stem cell marker in human
cholangiocarcinoma. Hepatology. 73:144–159. 2021. View Article : Google Scholar
|
|
95
|
Takahashi Y, Dungubat E, Kusano H, Ganbat
D, Tomita Y, Odgerel S and Fukusato T: Application of
immunohistochemistry in the pathological diagnosis of liver tumors.
Int J Mol Sci. 22:57802021. View Article : Google Scholar
|
|
96
|
Voigtländer T, Metzger J, Husi H, Kirstein
MM, Pejchinovski M, Latosinska A, Frantzi M, Mullen W, Book T,
Mischak H and Manns MP: Bile and urine peptide marker profiles:
Access keys to molecular pathways and biological processes in
cholangiocarcinoma. J Biomed Sci. 27:132020. View Article : Google Scholar
|
|
97
|
Xin H, Zhang Y, Lai Q, Liao N, Zhang J,
Liu Y, Chen Z, He P, He J, Liu J, et al: Automatic origin
prediction of liver metastases via hierarchical
artificial-intelligence system trained on multiphasic CT data: A
retrospective, multicentre study. EClinicalMedicine. 69:1024642024.
View Article : Google Scholar
|
|
98
|
Yang Q, Cai Q, Wen H, Mao Y, Ban X, Rong D
and Zhang R: The CT and MRI features of primary intrahepatic
lymphoepithelioma-like cholangiocarcinoma. AJR Am J Roentgenol.
216:393–402. 2021. View Article : Google Scholar
|
|
99
|
Sae-Fung A, Mutirangura A and Jitkaew S:
Identification and validation of a novel ferroptosis -related gene
signature for prognosis and potential therapeutic target prediction
in cholangiocarcinoma. Front Immunol. 13:10512732022. View Article : Google Scholar
|
|
100
|
Bao F, Liu J, Chen H, Miao L, Xu Z and
Zhang G: Diagnosis biomarkers of cholangiocarcinoma in human bile:
An evidence-based study. Cancers (Basel). 14:39212022. View Article : Google Scholar
|
|
101
|
Lapitz A, Azkargorta M, Milkiewicz P,
Olaizola P, Zhuravleva E, Grimsrud MM, Schramm C, Arbelaiz A,
O'Rourke CJ, La Casta A, et al: Liquid biopsy-based protein
biomarkers for risk prediction, early diagnosis, and
prognostication of cholangiocarcinoma. J Hepatol. 79:93–108. 2023.
View Article : Google Scholar
|
|
102
|
El-Deiry WS, Goldberg RM, Lenz HJ, Shields
AF, Gibney GT, Tan AR, Brown J, Eisenberg B, Heath EI, Phuphanich
S, et al: The current state of molecular testing in the treatment
of patients with solid tumors, 2019. CA Cancer J Clin. 69:305–343.
2019. View Article : Google Scholar
|
|
103
|
Scott A, Wong P and Melstrom LG: Surgery
and hepatic artery infusion therapy for intrahepatic
cholangiocarcinoma. Surgery. 174:113–115. 2023. View Article : Google Scholar
|
|
104
|
Benson AB III, D'Angelica MI, Abrams TA,
Are C, Bloomston PM, Chang DT, Clary BM, Covey AM, Ensminger WD,
Iyer R, et al: Hepatobiliary cancers, version 2.2014. J Natl Compr
Canc Netw. 12:1152–1182. 2014. View Article : Google Scholar
|
|
105
|
Moris D, Palta M, Kim C, Allen PJ, Morse
MA and Lidsky ME: Advances in the treatment of intrahepatic
cholangiocarcinoma: An overview of the current and future
therapeutic landscape for clinicians. CA Cancer J Clin. 73:198–222.
2023. View Article : Google Scholar
|
|
106
|
Saito N, Hatanaka T, Nakano S, Hazama Y,
Yoshida S, Hachisu Y, Tanaka Y, Yoshinaga T, Kashiwabara K, Kubo N,
et al: A case of unresectable combined hepatocellular and
cholangiocarcinoma treated with atezolizumab plus bevacizumab. Clin
Case Rep. 10:e61292022. View Article : Google Scholar
|
|
107
|
Simon-Molas H, Sanchez-de-Diego C,
Navarro-Sabate A, Castaño E, Ventura F, Bartrons R and Manzano A:
The expression of TP53-induced glycolysis and apoptosis regulator
(TIGAR) can be controlled by the antioxidant orchestrator NRF2 in
human carcinoma cells. Int J Mol Sci. 23:19052022. View Article : Google Scholar
|
|
108
|
Toshida K, Itoh S, Iseda N, Izumi T,
Yoshiya S, Toshima T, Ninomiya M, Iwasaki T, Oda Y and Yoshizumi T:
Impact of TP53-induced glycolysis and apoptosis regulator on
malignant activity and resistance to ferroptosis in intrahepatic
cholangiocarcinoma. Cancer Sci. 115:170–183. 2024. View Article : Google Scholar
|
|
109
|
Shi Y, Gong M, Deng Z, Liu H, Chang Y,
Yang Z and Cai L: Tirapazamine suppress osteosarcoma cells in part
through SLC7A11 mediated ferroptosis. Biochem Biophys Res Commun.
567:118–124. 2021. View Article : Google Scholar
|
|
110
|
Cao JY and Dixon SJ: Mechanisms of
ferroptosis. Cell Mol Life Sci. 73:2195–2209. 2016. View Article : Google Scholar
|
|
111
|
Johnstone TC, Suntharalingam K and Lippard
SJ: The next generation of platinum drugs: Targeted Pt(II) agents,
nanoparticle delivery, and Pt(IV) prodrugs. Chem Rev.
116:3436–3486. 2016. View Article : Google Scholar
|
|
112
|
Kontoghiorghes GJ: New iron metabolic
pathways and chelation targeting strategies affecting the treatment
of all types and stages of cancer. Int J Mol Sci. 23:139902022.
View Article : Google Scholar
|
|
113
|
Chen X, Li J, Kang R, Klionsky DJ and Tang
D: Ferroptosis: Machinery and regulation. Autophagy. 17:2054–2081.
2021. View Article : Google Scholar
|
|
114
|
Yang W, Mu B, You J, Tian C, Bin H, Xu Z,
Zhang L, Ma R, Wu M, Zhang G, et al: Non-classical ferroptosis
inhibition by a small molecule targeting PHB2. Nat Commun.
13:74732022. View Article : Google Scholar
|
|
115
|
Zhang C, Liu J, Xu D, Zhang T, Hu W and
Feng Z: Gain-of-function mutant p53 in cancer progression and
therapy. J Mol Cell Biol. 12:674–687. 2020. View Article : Google Scholar
|
|
116
|
Hanson RL and Batchelor E: Coordination of
MAPK and p53 dynamics in the cellular responses to DNA damage and
oxidative stress. Mol Syst Biol. 18:e114012022. View Article : Google Scholar
|
|
117
|
Zhang C, Liu X, Jin S, Chen Y and Guo R:
Ferroptosis in cancer therapy: A novel approach to reversing drug
resistance. Mol Cancer. 21:472022. View Article : Google Scholar
|
|
118
|
Lei S, Cao W, Zeng Z, Zhang Z, Jin B, Tian
Q, Wu Y, Zhang T, Li D, Hu C, et al: JUND/linc00976 promotes
cholangiocarcinoma progression and metastasis, inhibits ferroptosis
by regulating the miR-3202/GPX4 axis. Cell Death Dis. 13:9672022.
View Article : Google Scholar
|
|
119
|
Ma S, Ma Y, Qi F, Lei J, Chen F, Sun W,
Wang D, Zhou S, Liu Z, Lu Z and Zhang D: HSDL2 knockdown promotes
the progression of cholangiocarcinoma by inhibiting ferroptosis
through the P53/SLC7A11 axis. World J Surg Oncol. 21:2932023.
View Article : Google Scholar
|
|
120
|
Shi X, Yang J, Wang M, Xia L, Zhang L and
Qiao S: Hsa_circ_0050900 affects ferroptosis in intrahepatic
cholangiocarcinoma cells by targeting hsa-miR-605-3p to regulate
SLC3A2. Oncol Lett. 27:22024. View Article : Google Scholar
|
|
121
|
Kaida T, Beppu T, Hayashi H, Imai K,
Yamamura K, Okabe H, Matsumura K, Yoshii D, Komohara Y, Akahoshi S,
et al: Inflammatory liver tumor caused by fasciola hepatica
mimicking intrahepatic cholangiocarcinoma. Anticancer Res.
40:2795–2800. 2020. View Article : Google Scholar
|
|
122
|
Agostinis P, Berg K, Cengel KA, Foster TH,
Girotti AW, Gollnick SO, Hahn SM, Hamblin MR, Juzeniene A, Kessel
D, et al: Photodynamic therapy of cancer: An update. CA Cancer J
Clin. 61:250–281. 2011. View Article : Google Scholar
|
|
123
|
Zhang ZJ, Huang YP, Li XX, Liu ZT, Liu K,
Deng XF, Xiong L, Zou H and Wen Y: A novel ferroptosis-related
4-gene prognostic signature for cholangiocarcinoma and photodynamic
therapy. Front Oncol. 11:7474452021. View Article : Google Scholar
|
|
124
|
Gonzalez-Carmona MA, Bolch M, Strassburg
CP and Weismuller TJ: Editorial: Shining a light on
cholangiocarcinoma-a new dawn for photodynamic therapy? Authors'
reply. Aliment Pharmacol Ther. 49:953–954. 2019. View Article : Google Scholar
|
|
125
|
Du J, Wan Z, Wang C, Lu F, Wei M, Wang D
and Hao Q: Designer exosomes for targeted and efficient ferroptosis
induction in cancer via chemo-photodynamic therapy. Theranostics.
11:8185–8196. 2021. View Article : Google Scholar
|
|
126
|
Yan X, Li Z, Chen H, Yang F, Tian Q and
Zhang Y: Photodynamic therapy inhibits cancer progression and
induces ferroptosis and apoptosis by targeting P53/GPX4/SLC7A11
signaling pathways in cholangiocarcinoma. Photodiagnosis Photodyn
Ther. 47:1041042024. View Article : Google Scholar
|
|
127
|
Huang YP, Wang YX, Zhou H, Liu ZT, Zhang
ZJ, Xiong L, Zou H and Wen Y: Surufatinib combined with
photodynamic therapy induces ferroptosis to inhibit
cholangiocarcinoma in vitro and in tumor models. Front Pharmacol.
15:12882552024. View Article : Google Scholar
|
|
128
|
Ingle J and Basu S: Mitochondria targeted
AIE probes for cancer phototherapy. ACS Omega. 8:8925–8935. 2023.
View Article : Google Scholar
|
|
129
|
Kojima Y, Tanaka M, Sasaki M, Ozeki K,
Shimura T, Kubota E and Kataoka H: Induction of ferroptosis by
photodynamic therapy and enhancement of antitumor effect with
ferroptosis inducers. J Gastroenterol. 59:81–94. 2024. View Article : Google Scholar
|
|
130
|
Wang P, Hu Z, Yu S, Su S, Wu R, Chen C, Ye
Y, Wang H, Ye X, Zhou Z, et al: A novel protein encoded by
circFOXP1 enhances ferroptosis and inhibits tumor recurrence in
intrahepatic cholangiocarcinoma. Cancer Lett. 598:2170922024.
View Article : Google Scholar
|
|
131
|
Zhang Z, Akuetteh PD, Lin L, Wu Y, Li Y,
Huang W, Ni H, Lv H and Zhang Q: Development and validation of a
ferroptosis-related model for three digestive tract tumors based on
a pan-cancer analysis. Epigenomics. 13:1497–1514. 2021. View Article : Google Scholar
|
|
132
|
Sae-Fung A, Mutirangura A and Jitkaew S:
Identification and validation of a novel ferroptosis-related gene
signature for prognosis and potential therapeutic target prediction
in cholangiocarcinoma. Front Immunol. 13:10512732022. View Article : Google Scholar
|
|
133
|
Yao X, Chen B, Wang M, Zhang S, He B, Shi
Z, Deng T, Bao W, Wang Y, Chen G and Bo Z: Exploration and
validation of a novel ferroptosis-related gene signature predicting
the prognosis of intrahepatic cholangiocarcinoma. Acta Biochim
Biophys Sin (Shanghai). 54:1376–1385. 2022.
|
|
134
|
Wang Z, Zhang Y, Chen Y, Liu S, Li C and
Li X: Identification of a ferroptosis-related gene signature for
predicting the prognosis of cholangiocarcinoma. Expert Rev
Gastroenterol Hepatol. 16:181–191. 2022. View Article : Google Scholar
|
|
135
|
Liu S, Fan S, Wang Y, Chen R, Wang Z,
Zhang Y, Jiang W, Chen Y, Xu X, Yu Y, et al: ACSL4 serves as a
novel prognostic biomarker correlated with immune infiltration in
Cholangiocarcinoma. BMC Cancer. 23:4442023. View Article : Google Scholar
|
|
136
|
Hori Y, Yoh T, Nishino H, Okura K,
Kurimoto M, Takamatsu Y, Satoh M, Nishio T, Koyama Y, Ishii T, et
al: Ferroptosis-related gene glutathione peroxidase 4 promotes
reprogramming of glucose metabolism via Akt-mTOR axis in
intrahepatic cholangiocarcinoma. Carcinogenesis. 45:119–130. 2023.
View Article : Google Scholar
|
|
137
|
Xu L, Gao X, Xing J and Guo Z:
Identification of a necroptosis-related gene signature as a novel
prognostic biomarker of cholangiocarcinoma. Front Immunol.
14:11188162023. View Article : Google Scholar
|