Introduction
Primary squamous cell carcinoma of the liver (PSCCL)
is an exceptionally rare and highly aggressive malignancy, with
only a few cases reported worldwide (1), with <60 articles on primary
hepatocellular carcinoma identified in the present comprehensive
literature search from 1972 to 2024 (1). Unlike more common hepatic tumors,
PSCCL lacks specific clinical and radiologic features, often
leading to delay in diagnosis and treatment (2). Although the pathogenesis of PSCCL
remains unclear, chronic liver disease and inflammatory responses
are considered potential contributing factors (3). Due to its rarity, research into the
therapeutic strategies for PSCCL primarily rely on case reports and
small case series, with surgical resection being the most commonly
employed intervention (4). However,
the prognosis remains poor due to the high recurrence rate and
limited efficacy of adjuvant therapies (5). It has been reported that only a
limited number of patients survive beyond 12 months, even with
treatment (1–11). Zhang et al (8) documented a median survival time of 7.5
months (range, 0.3 to 84 months) among 32 patients with available
prognostic data (8). The present
case report reviews the pathogenesis, clinical presentation,
imaging characteristics and treatment outcomes of a patient with
PSCCL, aiming to enhance the awareness of this rare tumor among
clinicians and radiologists. The present report underscores the
need for heightened suspicion of PSCCL in relevant clinical
settings and imaging findings, with the goal of improving early
diagnostic accuracy and optimizing patient outcomes.
Case report
The patient, a 64-year-old woman, came to the
Department of Hepatobiliary Surgery of the People's Hospital of
Zhuji (Zhuji, China) in October 2023, complaining of a right upper
abdominal pain that had been persisting for two months, with no
nausea or vomiting, and no chills or fever. Physical examination
revealed the patient was alert and oriented, with no jaundice in
the skin or mucous membranes, a soft and protuberant abdomen,
tenderness in the upper abdomen without rebound pain, no palpable
mass, and the liver and spleen not palpable below the ribs. The
patient was negative for Murphy's sign and shifting dullness. The
patient had no history of hepatitis infection, no surgical history,
no family history of hereditary diseases and no other underlying
diseases. Ultrasonography suggested the following: i) Heterogeneous
echo mass near the gallbladder fossa in the right liver, with
further examination recommended; ii) calcifications in the right
liver; iii) rough gallbladder wall with multiple gallstones; and
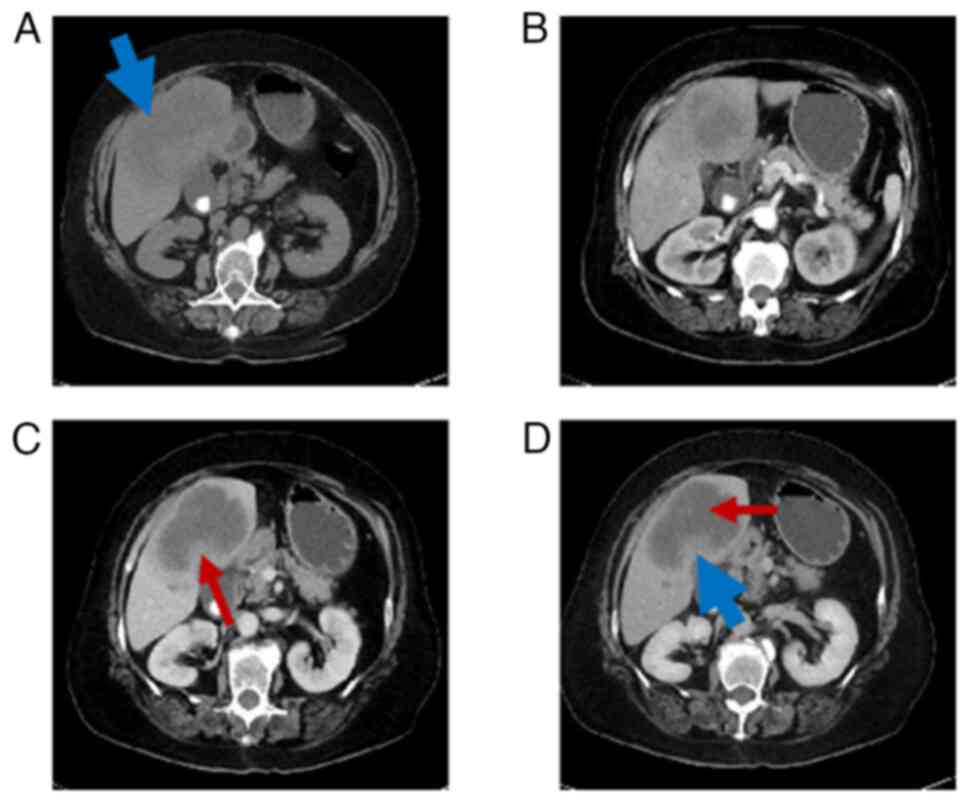
iv) enhanced pancreatic echogenicity. Abdominal CT scan and
enhancement suggested (Fig. 1): i)
Lesion near the gallbladder fossa in the right lobe of the liver,
with magnetic resonance imaging (MRI) recommended for further
examination; ii) multiple enlarged lymph nodes at the hepatic
hilum; and iii) gallstones and cholecystitis. Liver MRI scan and
enhancement suggested (Fig. 2): i)
Occupying lesion in the right lobe of the liver with mild dilation
of the intrahepatic bile ducts, suggesting the possibility of
intrahepatic cholangiocarcinoma; and ii) gallstones and
cholecystitis. Subsequent imaging studies failed to reveal any
primary tumor.
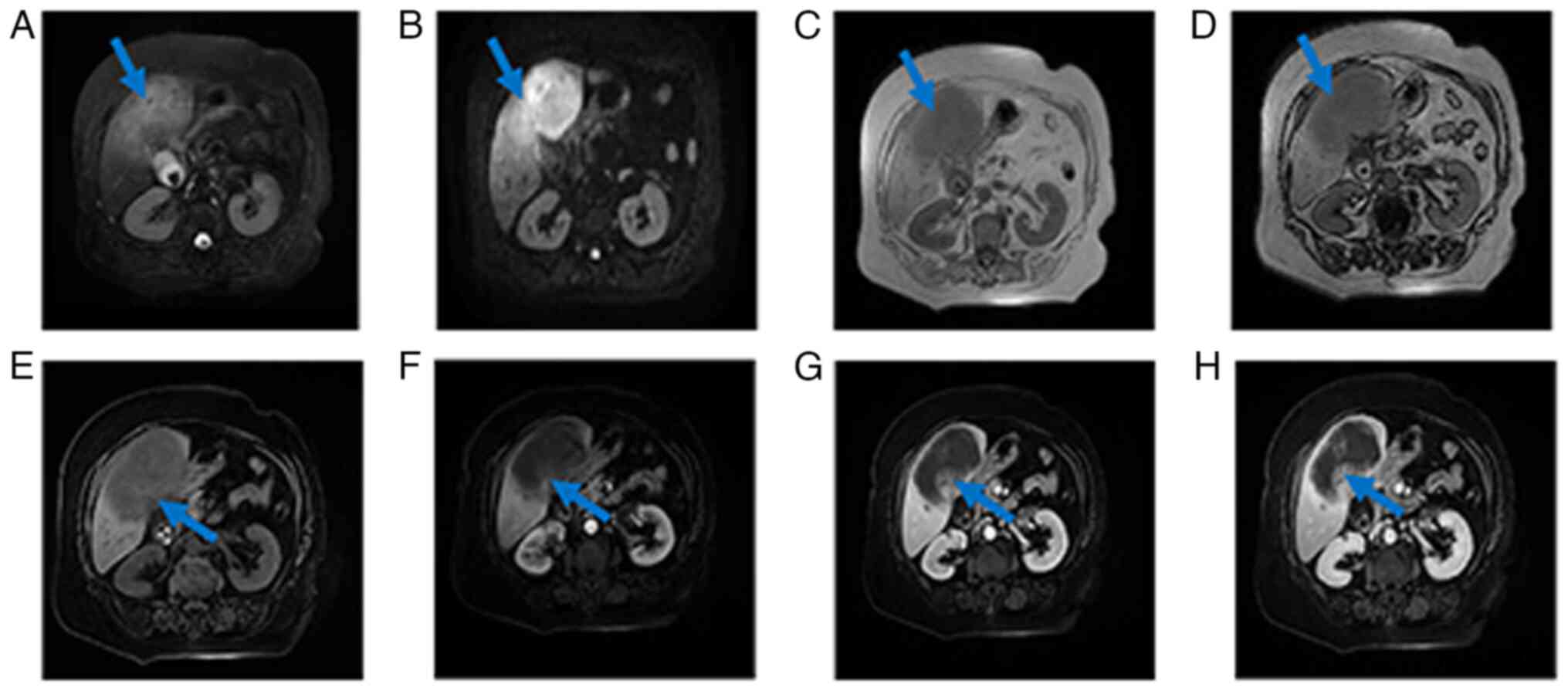 | Figure 2.MRI scan images of the patient. (A)
MRI T2WI sequence, a large mass in the right lobe of the liver can
be seen, which is hyperintense, with uneven intern signals and
unclear borders (blue arrow). (B) DWI sequence showed that the
lesions showed obvious hyperintensity (blue arrow). (C) T1WI
phase-inverse sequence, the lesion was hypointense (blue arrow),
and (D) the reverse position was not reduced compared with the
in-phase signal (blue arrow). (E) The right lobe of the liver is a
large mass, and the LVAV sequence (blue arrow) shows that the
lesion is hypointense and the margins are not clear. (F) Arterial
phase scan showed mild enhancement of the edge of the lesion (blue
arrow), no enhancement inside and the inner wall was not uniform.
(G) Venous phase scan showed continuous enhancement at the edges of
the lesion (blue arrow) and no enhancement internally. (H) The
portal scan showed further enhancement at the edge of the lesion
(blue arrow), and the extent was slightly larger than before, but
the center was still not enhanced and had unclear borders. T1WI,
T1-weighted imaging; T2WI, T2-weighted imaging; MRI, magnetic
resonance imaging; DWI, diffusion weighted imaging; LVAV, liver
vascular anatomy visualization. |
Written informed consent was provided by the patient
to obtain clinical data and information, as well as for
publication. The present study included no personal information
disclosure, and was approved by The Ethics Committee of Zhuji
People's Hospital [Zhuji, China; approval no. (2024); MedEthics no.
(0506)].
Laboratory results
Blood samples were collected from the patient
through venipuncture. Various hematological parameters were
processed and analyzed using ELC electrochemiluminescence and IFCC
(International Federation of Clinical Chemistry and Laboratory
Medicine) methods. Tumor markers were detected using
electrochemiluminescence, employing the fully automated Cobas e801
electrochemiluminescence immunoassay system [Roche Diagnostics
(Shanghai) Ltd.]. Alanine aminotransferase (ALT) and aspartate
aminotransferase (AST) were measured according to the IFCC method
(12), while albumin (ALB) was
assessed using the bromocresol green method (13), utilizing the fully automated
BS-2800M biochemical analyzer (Shenzhen Mindray Bio-Medical
Electronics Co., Ltd.). These data were derived from the patient's
medical records. The laboratory results were as follows:
α-Fetoprotein (AFP), 2.4 µg/l (reference values, 0.0–7.0 µg/l);
carcinoembryonic antigen (CEA), 11.6 µg/l (reference values,
0.0–5.0 µg/l); cancer antigen 125 (CA-125), 32.3 KIU/l (reference
values, 0.0–35.0 KIU/l); CA19-9, 228.0 KIU/l (reference values,
0.0–30.0 KIU/l); CA15-3, 11.2 KIU/l (reference values, 0.0–24.0
KIU/l); albumin, 35.3 g/l (reference values, 40.0–55.0 KIU/l);
globulin, 26.4 g/l (reference values, 20.0–40.0 KIU/l);
albumin/globulin ratio, 1.34 (reference values, 1.20–2.40); alanine
transaminase, 7 U/l (reference values, 7–40 U/l); aspartate
transferase, 8 U/l (reference values, 13–35 U/l); γ-glutamyl
transferase, 43 U/l (reference values, 7–45 U/l); and white blood
cells, 11.9×109/l (reference values,
3.5–9.5×109/l).
The patient presented with upper abdominal pain,
tumor markers were elevated to varying degrees and there was no
history of hepatitis or family history of tumors. Imaging studies
revealed a liver mass, suggesting a malignant liver tumor. Based on
the Barcelona Clinic Liver Cancer (BCLC) classification system
(14), a preoperative assessment
was conducted for the patient, evaluating tumor size, number, liver
function, overall health status and the presence of extrahepatic
metastasis. The patient exhibited normal liver and kidney function,
with no distant metastases, and demonstrated adequate surgical
tolerance. The patient was classified as BCLC A, making surgical
resection the optimal treatment option. To prevent the ongoing
inflammatory stimulation caused by gallstones, which could
potentially lead to tumor recurrence, the patient exhibited normal
liver and renal function, absence of distant metastasis and
sufficient surgical tolerance. These factors enhanced the
likelihood of surgical resection, and thus the patient met the
criteria for surgical intervention.
The patient underwent a hepatic lobectomy and
cholecystectomy at the People's Hospital of Zhuji (Zhuji, China) in
October 2023. During the surgery, extensive adhesions were observed
within the abdominal cavity, with severe adhesions between the
right liver and the diaphragm. The liver texture was hard, with an
uneven surface. Adhesions between the diaphragm and the liver
surface were separated, revealing a tumor measuring ~6.5 cm in
diameter located in the posterior lobe of the right liver. A biopsy
specimen was first fixed using Zhejiang Jinhua Tonghe Biotechnology
Co., Ltd. Biological Tissue Fixative (10% neutral buffered formalin
fixative; ready-to-use) at 25°C for 12 h, after which samples were
cut to 3 µm-thick. The sections were stained with HE stain at 25°C
for 45 min and imaged using a Zeiss Axio-Lab-A1 microscope.
Postoperative pathology revealed moderately
differentiated squamous cell carcinoma of the right liver
(measuring 12×6.5×6 cm), with the tumor infiltrating the full
thickness of the gallbladder to the mucosa (Fig. 3A) and invading the muscular layer of
the colon, while showing no invasion into omental tissue. Tumor
tissue exhibited infiltrative growth, forming nests, pseudoadenoid
structures and visible keratinized beads (Fig. 3B). The tumor cells were enlarged
with significant atypia, abundant red cytoplasm, round nuclei,
coarse chromatin, partial nucleoli and atypical nuclear division
(Fig. 3C). The resection margins of
the cystic duct and both sides of the colon were negative for tumor
involvement. No definite tumor was observed in the stomach wall,
liver or bile duct tissues. For immunohistochemistry, tissue
sections (3-µm thick) were fixed in Zhejiang Jinhua Tonghe
Biotechnology Co., Ltd., Biological Tissue Fixative (10% neutral
buffered formalin) at 25°C for 12 h, and embedded in paraffin.
Staining was performed using the DAKO Autostainer Link 48 system
(Agilent Technologies, Inc.). The following primary antibodies
(prediluted by the manufacturer) from Guangzhou Anbiping Medical
Laboratory Co., Ltd., were used: Ki67 (cat. no. IM098), CD10 (cat.
no. IM025), EMA (cat. no. IR074), PAX-8 (cat. no. IR191), Vimentin
(cat. no. IM142), CK7 (cat. no. IM061), CK (cat. no. IM067), CD34
(cat. no. IM034), P504s (cat. no. IR127), CK20 (cat. no. IR385),
SMA (cat. no. IHC-M005), P53 (cat. no. IM123) and CD30 (cat. no.
IM032). All primary antibodies were incubated with the samples at
25°C for 30 min. The secondary antibodies EnVision FLEX+, Mouse,
High pH (Link; prediluted by the manufacturer; cat. no. K8002;
Agilent Technologies, Inc.; EnVision FLEX+) were used at 25°C for
20 min. Blocking was performed with 3% peroxidase blocking reagent
(cat. no. DAKO SM801; Agilent Technologies, Inc.) at 25°C for 5
min, followed by incubation with EnVision FLEX/HRP at 25°C for 20
min. (cat. no. DAKO SM802; Agilent Technologies, Inc.). DAB
incubation was carried out at 25°C for 5 min (cat. no. DAKO DM827).
The microscope used for examination was an Olympus BX-51 microscope
with a camera adaptor (Olympus U-TV0.5XC-3; Olympus Corporation)
for capturing images. Immunohistochemistry showed the following:
P53 (wild type; data not shown), P63 (+; Fig. 3D), P40 (+; Fig. 3E), Ki-67 40% (+; data not shown),
cytokeratin (CK)7 (+; data not shown), glypican-3 (−; data not
shown), Her-2 (0; data not shown), HepPar1 (−; data not shown), AFP
(−; data not shown), Arginase-1 (−; data not shown), CK19 (+; data
not shown), CK20 (−; data not shown), CD34 (−; data not shown),
SSTR2 (−; data not shown), CD56 (−; data not shown), Syn (−; data
not shown), HSP 70 (+; data not shown) and GS (+; data not shown).
Unfortunately, the patient declined treatment due to financial
constraints and the high cost of chemotherapy and passed away in
April 2024.
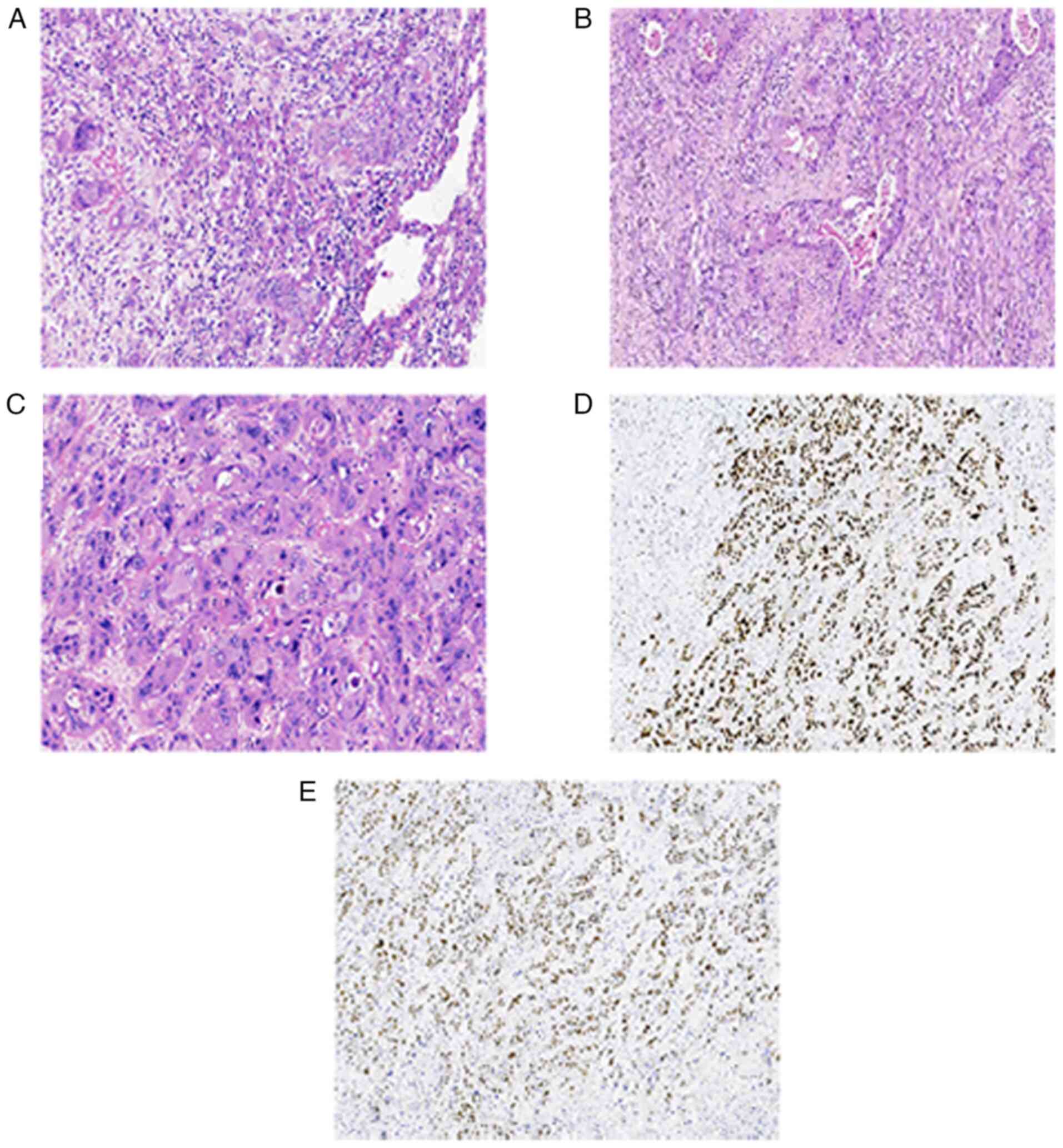 | Figure 3.Postoperative pathological and
immunohistochemical results of the patient. (A) Tumor tissue
exhibited infiltrative growth, forming nests, pseudoadenoid
structures (magnification, ×200) and (B) visible keratinized beads
(magnification, ×100). (C) The tumor cells were enlarged with
significant atypia, abundant red cytoplasm, round nuclei, coarse
chromatin, partial nucleoli and atypical nuclear division
(magnification, ×200). (D) The presence of P63 was detected in the
nucleus (magnification, ×100). (E) The presence of P40 was detected
in the nucleus (magnification, ×100). |
Following the collection and analysis of blood
specimens, postoperative tumor marker reassessments provided the
following results (Table I). A
total of 40 days postoperatively, CEA and CA 19–9 levels returned
to the normal range. However, during the 90-day follow-up, both
markers showed an increase. By contrast, AFP remained within the
normal range both preoperatively and postoperatively. After the
observed increase in tumor marker levels, a comprehensive abdominal
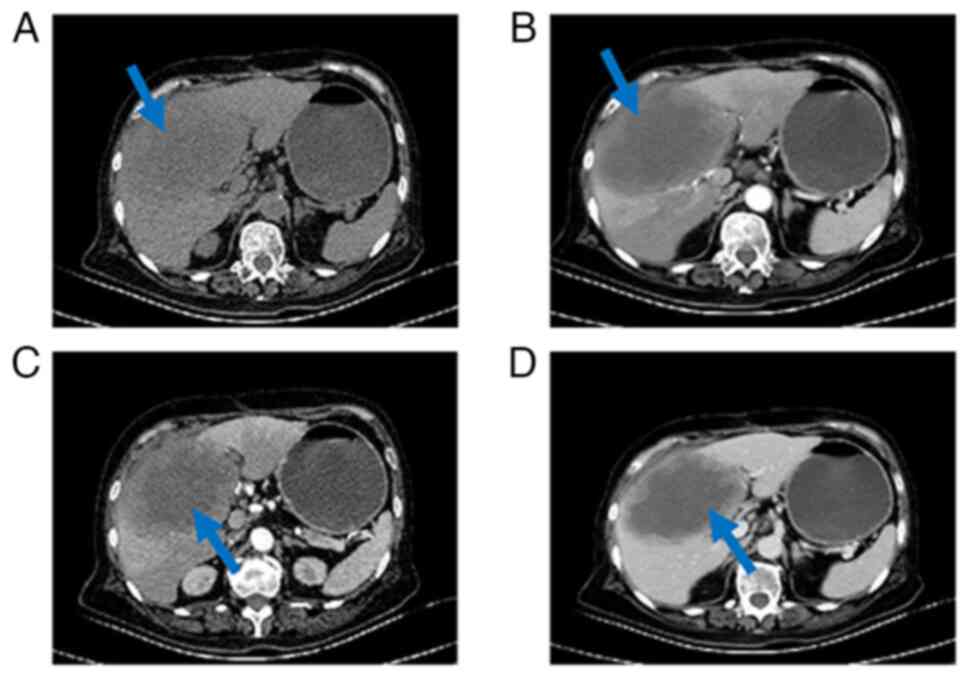
contrast CT scan was performed 100 days post-surgery (Fig. 4). A large occupying lesion in the
surgical area, measuring ~12×8 cm was observed. The enhancement
scan revealed rim and nodular enhancement at the periphery with no
central enhancement.
 | Table I.Temporal changes in tumor marker
levels surrounding surgical intervention. |
Table I.
Temporal changes in tumor marker
levels surrounding surgical intervention.
| Time of
examination | α-fetoprotein,
µg/l | Carcinoembryonic
antigen, µg/l | Carbohydrate antigen
19-9, KIU/l |
|---|
| Preoperative | 2.4 | 11.6 | 228 |
| 7 days
postoperative | 3 | 4.6 | 44.1 |
| 40 days
postoperative | 3.8 | 3.7 | 10.8 |
| 90 days
postoperative | 1.9 | 12.8 | 321 |
| 100 days
postoperative | 2 | 12.6 | 440 |
Discussion
Pathological mechanism
PSCCL is an extremely rare hepatic malignancy, with
only a handful of cases reported worldwide (1,6). The
pathogenesis of PSCCL remains largely unclear. Studies suggest that
PSCCL may be closely associated with chronic liver disease,
inflammatory responses, intrahepatic epithelial metaplasia and
squamous cell hyperplasia (2,4,6,7).
Chronic liver conditions, such as cirrhosis and hepatitis B virus
infection, can lead to prolonged chronic inflammation in hepatic
cells (2,8). This persistent inflammatory state can
activate multiple signaling pathways, triggering gene mutations and
abnormal cell proliferation, thereby laying the groundwork for the
development of PSCCL (1,4,8). As
the chronic inflammation persists, normal intrahepatic glandular
epithelial cells may undergo squamous metaplasia, a precursor for
PSCCL formation (8). Additionally,
PSCCL has been reported in association with hepatic cysts,
intrahepatic stones, and, in rare instances, teratomas (9). In the case described in the present
study, the patient initially presented with suspected
cholecystitis, indicating a history of inflammation. Subsequent CT
imaging revealed a hepatic mass, initially misdiagnosed as a liver
abscess. However, upon further evaluation with imaging studies,
tumor markers and immunohistochemical analysis, the final diagnosis
of PSCCL was established. This case aligns with the proposed
mechanisms of PSCCL pathogenesis.
Clinical presentation
The clinical manifestations of PSCCL are typically
nonspecific and resemble those of other hepatic malignancies,
making early diagnosis challenging (2,7). Most
patients with PSCCL initially present with symptoms such as
abdominal discomfort, fatigue, and weight loss (1,8). As
the disease progresses, more pronounced signs may develop,
including palpable liver masses (3,4,10).
Rapid tumor growth may lead to right upper quadrant tenderness and
abdominal distension (7,10). When the bile ducts are compressed or
liver function is impaired, serum bilirubin and transaminase levels
may be elevated, indicating that the disease has likely advanced to
a late stage (10). Some patients
may develop systemic symptoms, such as fever, particularly when the
tumor undergoes necrosis or secondary infection, occasionally
leading to liver abscess formation (4). At present, no specific serum
biomarkers for PSCCL have been identified, complicating the
diagnostic process (2,4). In the present case, the patient
exhibited significantly elevated levels of CA19-9 and CEA, which
decreased following tumor resection. However, during follow-up,
both markers rose again, and imaging studies suggested tumor
recurrence, highlighting the potential importance of CA19-9 and CEA
in the diagnosis and monitoring of PSCCL. Additionally, AFP levels
in this patient did not show a significant increase, consistent
with the findings of Zhao et al (1), who reported that AFP levels typically
remain within normal ranges in PSCCL cases, whereas elevations in
CEA, CA125 and CA19-9 are more common.
Imaging features
Due to the lack of specific clinical presentations
and a poorly defined etiology, combined with the highly malignant
and rapidly metastatic nature of PSCCL, early diagnosis is
challenging (9). Although PSCCL
lacks distinctive imaging features, a multimodal imaging approach
can enhance diagnostic accuracy (10). Ultrasound is typically used as an
initial screening tool, where PSCCL often appears as an
inhomogeneous hypoechoic mass within the liver, with a potential
for extensive necrosis or cystic degeneration (1,6). CT
scans, one of the primary diagnostic tools for PSCCL, usually
reveal iso- or hypodense, solitary or multiple masses with
indistinct margins and without a complete capsule (7). Larger tumors frequently exhibit
extensive low-density necrotic areas (7,8). In
contrast-enhanced CT scans, there is typically no significant
enhancement during the arterial phase, although the tumor margins
may show high-density rim enhancement; in the venous phase, the
tumor edges usually appear isodense and may be associated with
gallstones and liver cysts (8,10). CT
can occasionally demonstrate tumor invasion into surrounding
tissues or bile ducts, further supporting the diagnosis of PSCCL
(4). In MRI, PSCCL typically
presents as low signal intensity on T1-weighted imaging and high
signal intensity on T2-weighted imaging, with diffusion-weighted
imaging showing restricted diffusion (11). Early enhancement is usually weak,
with peripheral nodular enhancement observed during the delayed
phase (3,11). MRI is particularly valuable in
delineating the extent of tumor infiltration and vascular
involvement, which is crucial for surgical planning (3,11).
PET-CT often reveals significant FDG uptake in PSCCL, indicating
its highly invasive and malignant potential (9). Although literature on the PET-CT
findings of PSCCL is limited, this imaging modality plays an
important role in tumor staging and the assessment of systemic
metastasis (8,9). In summary, while the imaging
characteristics of PSCCL are not specific, the use of multiple
imaging techniques can improve diagnostic accuracy. Ultrasound
serves as an initial screening tool, while CT and MRI are pivotal
in assessing the characteristics of the lesion and the staging of
the tumor, with PET-CT demonstrating advantages in evaluating
systemic metastasis. Further imaging studies may provide deeper
insights into the imaging features of PSCCL and their diagnostic
value.
Differential diagnosis
PSCCL needs to be differentially diagnosed from the
following conditions:
1. Cholangiocarcinoma: Cholangiocarcinoma typically
exhibits an infiltrative growth pattern with indistinct borders
from the surrounding liver parenchyma and is often associated with
bile duct dilatation (9). Enhanced
CT or MRI usually demonstrates delayed enhancement, which is
characteristic of this malignancy (9,15).
Squamous cell carcinoma antigen testing may assist in
differentiation (3).
2. Hepatocellular carcinoma (HCC): In liver
contrast-enhanced imaging, HCC usually shows marked arterial phase
enhancement ~35–45 sec after contrast injection, which is key for
diagnosis (16). The late arterial
phase is preferred for diagnosing and staging HCC, with rapid
‘washout’ observed during the portal venous and delayed phases
(17). In the present case, MRI
arterial phase scanning was performed ~35 sec after contrast
injection, aligning with the traditional late arterial phase
timing.
3. Hepatic cyst: When PSCCL exhibits significant
necrosis, its imaging characteristics may resemble those of hepatic
cysts, although important differences exist (18). Simple hepatic cysts appear as
well-defined, non-enhancing low-density or low-signal areas on
imaging, whereas PSCCL typically presents with thicker, irregular
walls and shows enhancement on contrast studies, along with
heterogeneous signals on MRI and prominent necrotic regions
(19). Malignant transformation of
hepatic cysts may show thickened walls, internal nodules or
septations, typically displaying a more complex internal structure,
while PSCCL tends to appear as a solid mass (8,18,19).
4. Metastatic tumors: Metastatic liver tumors
usually present as single or multiple hypodense nodules with ring
enhancement, often accompanied by a history of malignancy in other
organs (20). Common imaging signs
include the ‘target sign’ and ‘bull's-eye sign’, and PET-CT can be
helpful in identifying the primary tumor (21).
5. Liver abscess: On CT scanning, a liver abscess
appears as a round hypodense lesion with enhanced walls during
contrast scans, while the surrounding edema remains non-enhancing,
forming a ring sign (22). It is
usually associated with fever and elevated white blood cell counts
(9,22).
Treatments and prognosis
PSCCL is an exceedingly rare and highly aggressive
hepatic malignancy (1–11). Due to its rarity, treatment
strategies are primarily based on case reports and small case
series (1–11). Common treatment modalities include
surgical resection, chemotherapy, radiotherapy and transcatheter
arterial chemoembolization (TACE) (2,4–8).
However, the efficacy of these approaches remains uncertain due to
the aggressive nature and high recurrence rate of PSCCL (2,5,7).
Surgical resection is generally considered one of the most
effective treatments for PSCCL (4,8,23).
Studies by Iimuro et al (6)
and Zhao et al (1) indicate
that early surgical intervention before tumor spread into the
surrounding hepatic parenchyma is closely associated with improved
prognosis. Weimann et al (19) reported a case of long-term survival
following surgery alone, with the patient surviving over four years
without adjuvant chemotherapy or radiotherapy. This outcome
suggests that early and complete tumor resection may prolong
survival. The systematic review by Zhao et al (1) also states that chemotherapy and
radiotherapy are commonly employed for patients with PSCCL
post-surgery or those who are inoperable, aiming to control tumor
progression. Boscolo et al (24) reported successful treatment of
advanced primary SCC with systemic therapy using CDDP and 5-FU
along with surgical resection. Although these treatments are
standard in oncology, and their efficacy in PSCCL has not been
well-established (1,4,6).
The prognosis for PSCCL is generally poor. Although
early surgical intervention may extend survival, the majority of
cases eventually recur (5,7,8). Zhang
et al (8) indicated that
patients who undergo curative surgery have significantly longer
overall survival than those receiving palliative treatment
(8). However, the high recurrence
rate remains a major challenge even after curative resection
(5,7,8).
Rezvani et al (25) reported
on a case of recurrence six weeks after radical surgery. No
reoperation or chemotherapy was performed, and after five months of
palliative care, the patient succumbed to the disease. Weimann
et al (19) also found that
despite some patients achieving prolonged survival without adjuvant
therapy, most still face the risk of recurrence post-surgery
(19). Further research and the
accumulation of more cases are essential to explore more effective
treatment options to improve patient quality of life and
prognosis.
In summary, PSCCL is an extremely rare and highly
aggressive hepatic malignancy, characterized by non-specific
clinical and imaging features, making diagnosis and treatment
challenging. The pathogenesis of this tumor may be associated with
factors such as chronic liver disease and inflammatory responses.
Due to the difficulty in early diagnosis, comprehensive evaluation
typically requires multimodal imaging techniques. Although early
surgical resection is considered the primary treatment approach,
the high recurrence rate significantly impacts prognosis. To
improve the diagnostic accuracy and treatment outcomes for PSCCL,
future research should focus on several key areas including deeper
exploration of its pathogenesis, the development of more advanced
multimodal imaging techniques, optimization of personalized
treatment strategies and enhanced sharing of global case data.
These efforts hold the potential to extend patient survival and
improve quality of life. Additionally, tumor markers such as CEA
and CA19-9 may provide valuable insights for diagnosis and
prognostic assessment. Increasing awareness and understanding of
this rare malignancy are crucial for early detection, accurate
diagnosis and effective treatment.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
GS and XM designed the study and participated in the
literature search. GS obtained the medical images, contributed to
the literature review and prepared the draft manuscript. XY, FY and
ZW revised the manuscript, participated in data analysis and
provided treatment recommendations for the patient. XM and GS
confirm the authenticity of all the raw data. All authors read and
approved the final version of the manuscript.
Ethics approval and consent to
participate
The present study included no personal information
disclosure, and was approved by The Ethics Committee of Zhuji
People's Hospital [Zhuji, China; approval no. (2024); MedEthics no.
(0506)].
Patient consent for publication
Written informed consent was provided by the patient
to obtain clinical data and information, as well as for
publication.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Zhao L, Zhou Y, Ding J, Qin Z, Zhou H and
Jing X: Primary hepatic squamous cell carcinoma: Case report and
systematic review of the literature. Front. Oncol.
13:12299362023.
|
|
2
|
Benhamdane A, Adioui T, Berrag S, Nejjari
F, Allaoui M and Tamzaourte M: Primary squamous cell carcinoma of
the liver. Eur J Case Rep Intern Med. 11:0046282024.
|
|
3
|
Fakhreddine O, Fadlallah Y, Turfa J,
Hassan MA, Chamseddine N and Assi HI: Primary squamous cell
carcinoma of the liver: Case report and review of literature. Case
Rep Oncol. 15:480–485. 2022. View Article : Google Scholar
|
|
4
|
Lee HL, Liu YY, Yeh CN, Chiang KC, Chen TC
and Jan YY: Primary squamous cell carcinoma of the liver: A
successful surgically treated case. World J Gastroenterol.
12:5419–5421. 2006. View Article : Google Scholar
|
|
5
|
Lyagoubi M, Mehdaoui C, Haloui A, Karish
N, Ismaili Z and Bennani A: Primary hepatic squamous cell
carcinoma: A case report. Cureus. 16:e638032024.
|
|
6
|
Iimuro Y, Asano Y, Suzumura K, Yada A,
Hirano T, Iijima H, Nishiguchi S, Hirota S and Fujimoto J: Primary
squamous cell carcinoma of the liver: An uncommon finding in
contrast-enhanced ultrasonography imaging. Case Rep Gastroenterol.
5:628–635. 2011. View Article : Google Scholar
|
|
7
|
Xiao J, Ma L, Li J, Yin B, Liang J and
Wang J: Primary squamous cell carcinoma of the liver is rare but
hostile: Case series and comprehensive review of the literature.
Cancer Manag Res. 13:829–837. 2021. View Article : Google Scholar
|
|
8
|
Zhang XF, Du ZQ, Liu XM and Lv Y: Primary
squamous cell carcinoma of liver: Case series and review of
literatures. Medicine (Baltimore). 94:e8682015. View Article : Google Scholar
|
|
9
|
Sun Y and Jin G: Primary squamous cell
carcinoma of the liver: A case report. J Int Med Res.
49:30006052110212752021. View Article : Google Scholar
|
|
10
|
Song Y, Shi J, Zhang X, Qiao M, Sun Z and
Tian S: Diagnostic value of imaging modalities in primary squamous
cell carcinoma of the liver. J Clin Ultrasound. 51:887–897. 2023.
View Article : Google Scholar
|
|
11
|
Atiq M, Ammar AS, Ali RM, Haider S, Ahmed
I and Dar FS: Primary squamous cell carcinoma of liver. First case
report from Pakistan and South Asia. Int J Surg Case Rep.
99:1076552022. View Article : Google Scholar
|
|
12
|
Valenti L, Pelusi S, Bianco C, Ceriotti F,
Berzuini A, Iogna Prat L, Trotti R, Malvestiti F, D'Ambrosio R,
Lampertico P, et al: Definition of healthy ranges for alanine
aminotransferase levels: A 2021 update. Hepatol Commun.
5:1824–1832. 2021. View Article : Google Scholar
|
|
13
|
Zhang Y, Abdollahi A, Andolino C, Tomoo K,
Foster BM, Aryal UK and Henderson GC: Performance evaluation of
different albumin assays for the detection of analbuminemia. PLoS
One. 19:e03001302024. View Article : Google Scholar
|
|
14
|
Llovet JM, Kelley RK, Villanueva A, Singal
AG, Pikarsky E, Roayaie S, Lencioni R, Koike K, Zucman-Rossi J and
Finn RS: Hepatocellular carcinoma. Nat Rev Dis Primers. 7:62021.
View Article : Google Scholar
|
|
15
|
Viganò L and Fiz F: ASO author
reflections: Radiomics for intrahepatic cholangiocarcinoma: A
further step toward precision surgery. Ann Surg Oncol.
31:5647–5648. 2024. View Article : Google Scholar
|
|
16
|
Kim YY, Lee S, Shin J, Son WJ, Roh YH,
Hwang JA and Lee JE: Diagnostic performance of CT versus MRI liver
imaging reporting and data system category 5 for hepatocellular
carcinoma: A systematic review and meta-analysis of comparative
studies. Eur Radiol. 32:6723–6729. 2022. View Article : Google Scholar
|
|
17
|
Zhao C, Dai H, Shao J, He Q, Su W, Wang P,
Tang Q, Zeng J, Xu S, Zhao J and Xiang S: Accuracy of various forms
of contrast-enhanced MRI for diagnosing hepatocellular carcinoma: A
systematic review and meta-analysis. Front Oncol. 11:6806912021.
View Article : Google Scholar
|
|
18
|
Armutlu A, Quigley B, Choi H, Basturk O,
Akkas G, Pehlivanoglu B, Memis B, Jang KT, Erkan M, Erkan B, et al:
Hepatic cysts: Reappraisal of the classification, terminology,
differential diagnosis, and clinicopathologic characteristics in
258 Cases. Am J Surg Pathol. 46:1219–1233. 2022. View Article : Google Scholar
|
|
19
|
Weimann A, Klempnauer J, Gebel M, Maschek
H, Bartels M, Ringe B and Pichlmayr R: Squamous cell carcinoma of
the liver originating from a solitary non-parasitic cyst case
report and review of the literature. HPB Surg. 10:45–49. 1996.
View Article : Google Scholar
|
|
20
|
Berlin JW, Gore RM, Yaghmai V, Pereles FS
and Miller FH: Radiologic imaging and staging of primary and
metastatic liver tumors. Cancer Treat Res. 109:39–58. 2001.
View Article : Google Scholar
|
|
21
|
Fukumoto W, Nakamura Y, Higaki T,
Tatsugami F, Iida M and Awai K: Additional value of
diffusion-weighted MRI to Gd-EOB-DTPA-enhanced Hepatic MRI for the
detection of liver metastasis: The difference depending on the
experience of the radiologists. Hiroshima J Med Sci. 64:15–21.
2015.
|
|
22
|
Sutherland T, Temple F, Lee WK and
Hennessy O: Evaluation of focal hepatic lesions with ultrasound
contrast agents. J Clin Ultrasound. 39:399–407. 2011. View Article : Google Scholar
|
|
23
|
Okuda Y, Abe T, Ikeda M, Kurihara K,
Shimizu A, Oshita A, Yonehara S and Hanada K: Curative surgery for
primary squamous cell carcinoma of the liver: A rare case study.
Clin J Gastroenterol. 16:263–269. 2023. View Article : Google Scholar
|
|
24
|
Boscolo G, Jirillo A and Da Pian P:
Complete remission of poorly differentiated squamous liver
carcinoma after systemic chemotherapy and surgery. A case report.
Tumori. 91:71–72. 2005. View Article : Google Scholar
|
|
25
|
Rezvani H, Azhdari Tehrani H, Salari S,
Feiziazar S and Darnahal M: Primary squamous cell carcinoma of the
liver: A case report. Gastroenterol Hepatol Bed Bench. 15:430–434.
2022.
|