Introduction
According to the most recent predictions from the
International Organization for Research on Cancer, there will be
>20 million new cases of cancer (which includes nonmelanoma skin
cancers) and 9.7 million cancer-associated deaths (including
nonmelanoma skin cancers) worldwide in 2022 (1). According to demographic forecasts, the
annual incidence of new cancer cases globally will exceed 35
million by 2050 (1). Bladder cancer
is the tenth most frequent malignancy worldwide and is ~4 times
more likely to occur in men than in women (2). In China, lung cancer has the highest
incidence and mortality (3).
Bladder cancer is one of the main cancer types in China (4). Bladder cancer is a prevalent
urological malignancy with a poor prognosis in the
advanced/metastatic stage. Bladder cancer patients are typically
treated with a variety of severe treatments, involving radical
cystectomy, chemotherapy, immunological therapy and radiotherapy.
However, these treatments frequently result in problems and
undesirable effects, such as postoperative infection, impaired
urination and weakened immune system (5).
Toxoplasma gondii is an opportunistic
protozoan parasite that can infect warm-blooded animals, birds and
humans (6). When infected, oocysts
break in the gut, releasing sporozoites into the lumen. Sporozoites
infect enterocytes and differentiate into tachyzoites, which are
highly proliferative, mobile and spread quickly throughout the
body, eliciting an immediate immunological response (7). T. gondii primarily infects
humans through the following ways: eating food contaminated with
the parasite's eggs or oocysts; breaking through skin mucous
membranes and coming into contact with soil contaminated with the
parasite's eggs or oocysts; coming into contact with animal
excrement contaminated with the parasite eggs or oocysts; and
vertical transmission from the mother to the fetus via the placenta
(8). Depending on the person, T.
gondii infection might have different health implications
(9). When healthy individuals get
T. gondii, the majority of them have concealed infections,
which frequently resolve on their own and lack clear clinical signs
and symptoms. Some infected people may have mild symptoms such as
low-grade fever, headache, swelling of superficial lymph nodes, and
occasionally pneumonia, pleurisy and myocarditis. However, those
who have hypoplasia or immunodeficiency may experience more severe
infection symptoms, including the possibility of systemic
toxoplasmosis (10). In extreme
situations, toxoplasmic encephalitis may potentially result in
death (11). Toxoplasma infections
in pregnant women can have particularly severe consequences for the
fetus. Infections occurring during the early and middle stages of
pregnancy may result in miscarriage, stillbirths, or congenital
malformations. Conversely, infections that arise during the later
stages of pregnancy can lead to preterm delivery and may be
associated with hydrocephalus, meningoencephalitis, visual
disturbances, or epilepsy in newborns within a few months to
several years post-delivery (12).
African countries have the greatest average incidence rate of
infection of T. gondii (61.4%), followed by Oceania (38.5%),
South America (31.2%), Europe (29.6%), the United States and Canada
(17.5%) and Asia (16.4%) (13);
thus, T. gondii has a high prevalence globally. T.
gondii infection is more prevalent among immunocompromised
patients, pregnant women, blood donors, women of reproductive age
and newborns, compared with the general population (14). Therefore, special attention needs to
be paid to these groups. Some studies have shown that patients with
cancer are more frequently positive for T. gondii than
healthy individuals; however, whether T. gondii causes
cancer is unclear (15–20).
Recent studies have explored the potential
anticancer properties of T. gondii, demonstrating its
ability to invade and destroy various human cancer cell lines, such
as breast cancer cells (21), colon
cancer cells (22) and lung cancer
cells (23). T. gondii has
been formerly categorized into three primary lineages based on its
virulence: Types I, II and III (24). ToxoDB #59 and #2 are the most
prevalent in America, #1 is the most common in Africa, #9 is the
most prevalent genotype in China (17,25,26).
However, the effect of T. gondii on bladder cancer cells is
uncertain. Therefore, the aim of the present study was to examine
the influence of T. gondii on bladder cancer cells and the
outcomes will fill a gap in the investigation of T. gondii
in bladder cancer and provide novel ideas for medication
development in bladder cancer.
Materials and methods
Cell lines and cell culture
Bladder cancer 5637 cells were purchased from Wuhan
Punosai Life Technology Co. Ltd. The cell lines were cultured in
RPMI-1640 medium (Gibco; Thermo Fisher Scientific, Inc.)
supplemented with 10% FBS (Gibco; Thermo Fisher Scientific, Inc.)
and 1% penicillin-streptomycin solution (Beijing Solarbio Science
& Technology Co., Ltd.). Cells were cultured in a humidified
incubator at 37°C with 5% CO2.
Preparation of culture supernatant of
T. gondii tachyzoites
A total of three six-week-old male BALB/c mice
(weight, 20–22 g (Beijing Viton Lihua Lab Animal Technology Co.
Ltd.) were used. These mice were accommodated in a hygienic,
temperature-regulated cage (Temperature: 18~29°C; Humidity: 40~70%)
setting with a 12-h light-dark cycle. They were given unrestricted
access to a laboratory-standard diet and water. Daily monitoring of
animal health and behavior was undertaken. The exclusion criteria
encompassed mortality during the research duration; however, this
study did not witness any such. T. gondii tachyzoites
(Laboratory of Dali University, China) was administered
intraperitoneally to three randomly selected BALB/c mice
(4×106 per mouse) and peritoneal fluid was collected
after 72 h. The peritoneal fluid was centrifuged at 1,400 × g for 8
min at 37°C, the supernatant was discarded and the sample was
washed twice with PBS. The supernatant was filtered through CF-11
cellulose column, the column was loaded into a sterile syringe with
PBS and the hole was sealed with filter paper at the bottom. The
height of the column was 3 cm and the liquid was kept on the
surface of the column. The suspension of T. gondii was
quickly passed through the column and the filtrate was collected
and centrifuged at 3,000 × g for 8 min on 37°C to remove the
supernatant and the precipitate was the T. gondii. These
were collected and counted microscopically before being resuspended
in RPMI-1640 (including 10 ml/l fetal bovine serum, Thermo Fisher
Scientific, Inc.). To determine the optimal concentration of
tachyzoite culture supernatant infecting bladder cancer cells, the
quantity of tachyzoites was adjusted to 2, 4 and
8×107/ml and then tachyzoites were inoculated into
6-well plates for 24 h. The supernatant was collected and filtered
through 0.22-µm pore size filter membranes. Then, two sets of
flasks (each containing three groups) containing bladder cancer
5637 cells were inoculated with T. gondii tachyzoite culture
supernatants at 10:1, 100:1, 1,000:1 and 2,000:1. Thereafter,
culture supernatants were harvested at various days until bladder
cancer 5637 cells were destroyed and tachyzoites were counted.
Finally, the appropriate ratio was bladder cancer: T.
gondii=10:1. At the culmination of the experiment, all mice
were sacrificed via CO2 inhalation (flow rate was
regulated at 30% of the cage volume per min) and mortality was
confirmed by the absence of respiration or cardiac activity. All
animal experiments received approval from the Institutional Animal
Care and Use Committee of the University of Dali, Yunnan, China
(approval no. 2024-0833).
Cell morphology
The changes of cell size, shape and nuclear
morphology of bladder cancer cells before and after infection with
the supernatant of tachyzoite culture of T. gondii were
observed by contrast microscope.
Cell proliferation assay
A Cell Counting Kit-8 (CCK-8; Beijing Solarbio
Science & Technology Co., Ltd.) was used to evaluate the
proliferation of bladder cancer cells. Bladder cancer 5637 cells
were seeded in 96-well plates at a density of 1×106
cells/well and treated with the culture supernatant of T.
gondii tachyzoites for 24, 48 and 72 h at 37°C. Following the
treatment, 10 µl CCK-8 solution was added to each well and cells
were incubated for 1 h, according to the manufacturer's protocol.
The optical density (OD) was measured at 450 nm using a microplate
reader (Multiskan GO; Thermo Fisher Scientific, Inc.).
Proliferation inhibition rate (%)=(average OD value of control
group-average OD value of experimental group)/average OD value of
control group ×100%.
Wound healing assay
A marker was used to draw lines on the backs of the
6-well plates. Bladder cancer cells (1×106, no FBS) were
inoculated into a 6-well plate at 37°C in a 5% CO2
atmosphere. A 10-µl micropipette tip was used to draw lines on the
6-well plates until the cells reached 80–90% confluence. After
washing twice with PBS, the 6-well plates were observed under a
fluorescence microscope and images were captured to record the
scratch width at 0 h. The control group cells were added to 2 ml
serum-free culture medium, while the experimental group cells were
added to 2 ml T. gondii tachyzoite culture supernatant at
37°C in a 5% CO2 atmosphere. After 24 and 36 h, all
plates were removed for washing with PBS and then images were
captured. ImageJ 1.46 (National Institutes of Health) was used to
determine migration rate.
Transwell migration and invasion
assays
Typically, 1×106 bladder cancer 5637
cells and T. gondii tachyzoite culture supernatants +
bladder cancer 5637 cells (10:1) in serum-free media were
separately seeded into the upper chambers with (invasion) or
without (migration) Matrigel coating (diluted in RPMI-1640;
Corning, Inc.; 4°C for 3 h) and the bottom chambers were filled
with 600 µl supplemented RPMI-1640 medium. After 36 h, the cells
that had migrated and invaded through to the bottom of the inserts
were fixed with methanol and stained with 0.1% crystal violet for
30 min at room temperature. In five random fields of view, the
numbers of cells that had migrated/invaded were observed under an
inverted light microscope (magnification, ×200) and images captured
and quantified.
Cell apoptosis assay
A TUNEL Apoptosis Detection Kit was obtained from
Shanghai Biyuntian Biotechnology Co., Ltd. T. gondii
tachyzoite culture supernatants were added when bladder cancer
cells reached 60–70% confluency. After 48 h, the cells were fixed
with 500 µl 4% paraformaldehyde (Beijing Solarbio Science &
Technology Co., Ltd.) and incubated with a highly permeable
immunostaining solution for 5 min at room temperature. The TUNEL
detection solution (50 µl) was added to each well, followed by
incubation for 5 min. The apoptotic rate was determined using
fluorescence microscopy.
Western blotting
The primary antibodies used for western blotting
were as follows: Rabbit anti-Bax (cat. no. ab32503; 1:1,000;
Abcam), rabbit anti-Bcl-2 (cat. no. ab112; 1:1,000; Beyotime
Institute of Biotechnology) and rabbit anti-β-actin (cat. no.
ab209857; 1:1,000; Abcam). The protein concentration was determined
using the BCA method. Equal amounts of protein (40 µg/lane) were
applied to a 10% SDS-PAGE for electrophoresis and transferred to a
PVDF membrane (Beijing Solarbio Science & Technology Co.,
Ltd.). The membrane was transferred to a blocking solution (5% BSA;
Beijing Solarbio Science & Technology Co., Ltd.) for 1 h on a
shaking bed at room temperature and incubated with the primary
antibody at 4°C overnight and secondary antibodies (cat. no.
ab2307391; 1:10,000; Jackson; Horseradish Peroxidase) at 37°C for 1
h. Finally, the membrane was visualized using an ECL Luminescence
Assay Kit (Shanghai Biyuntian Biotechnology Co., Ltd.). Western
blot density was assessed using ImageJ 1.46 (National Institutes of
Health).
Statistical analysis
SPSS Statistics 26.0 (IBM Corp.) was used for
statistical analysis. ImageJ 1.46 (National Institutes of Health)
was used for densitometry analysis. Comparisons between groups were
assessed using one-way ANOVA and LSD as a post hoc test. P<0.05
was considered to indicate a statistically significant
difference.
Results
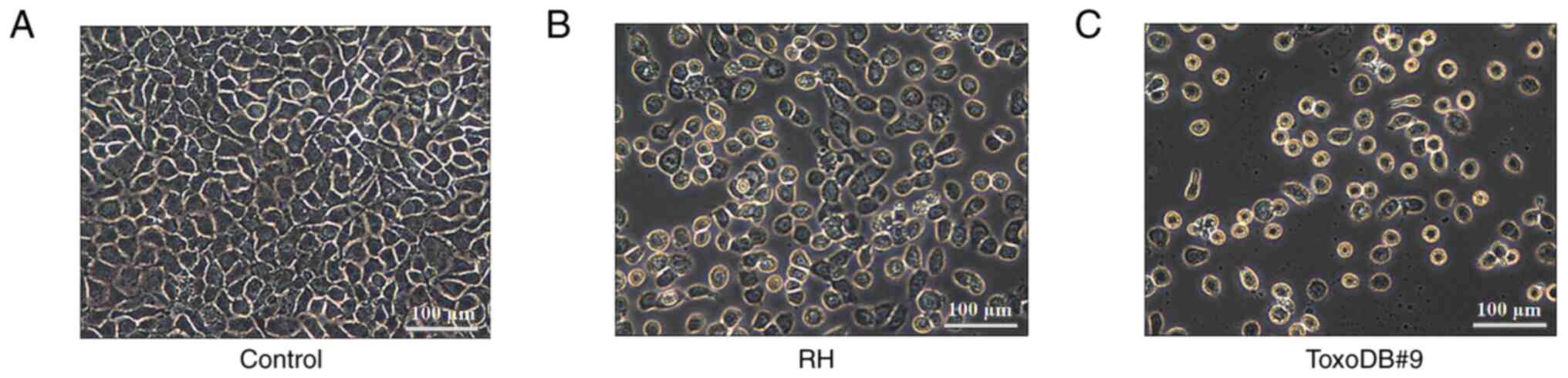
Morphology of bladder cancer 5637
cells altered by culture supernatant of T. gondii tachyzoites
After 24 h, the morphology of bladder cancer 5637
cells was observed (Fig. 1A). The
cells in the control groups were tightly arranged and evenly sized
and exhibited consistent morphology, with a large proportion of
cell nuclei and cytoplasm. The experimental groups exhibited a
decrease in cell quantity, reduced cell volume, wrinkled cells,
decreased nuclear-cytoplasmic ratios and apoptosis, including
nuclear condensation and rupture (Fig.
1B and C). The results indicated that T. gondii
tachyzoite culture supernatant was able to alter the morphology of
bladder cancer 5637 cells.
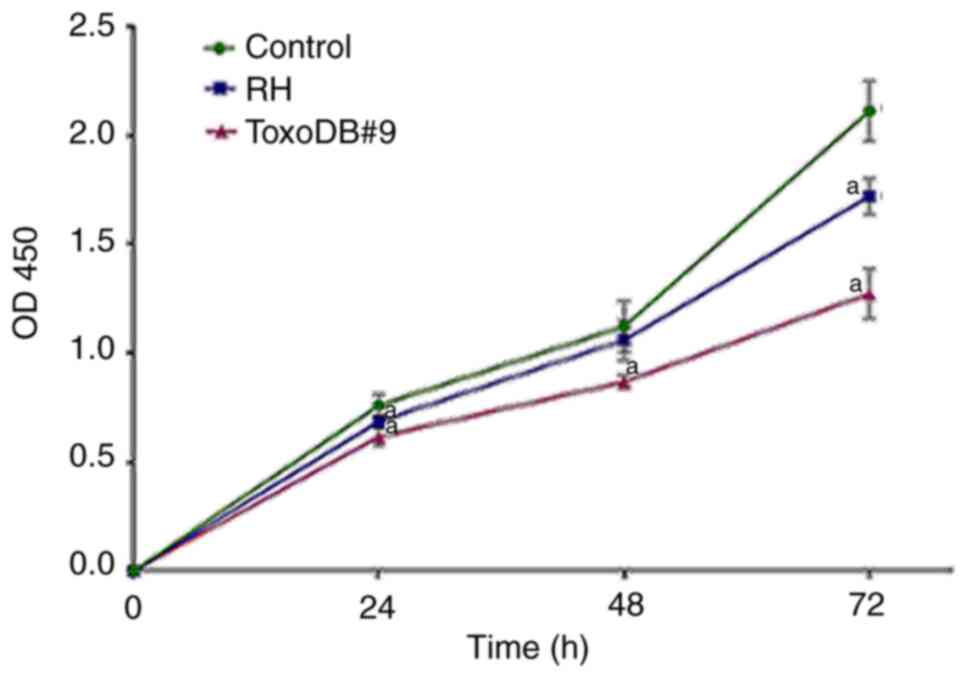
T. gondii tachyzoite culture
supernatant inhibits proliferation of bladder cancer 5637 cells in
vitro
To assess whether T. gondii tachyzoite
culture supernatant can inhibit proliferation of bladder cancer
5637 cells in vitro, CCK-8 assay was used to detect the
effects of T. gondii tachyzoite culture supernatant on the
proliferation of bladder cancer 5637 cells. The OD values were
measured at 24, 48 and 72 h and growth curves were plotted based on
the results (Fig. 2). After T.
gondii culture supernatant acted on bladder cancer 5637 cells,
proliferation was inhibited to different degrees. At 72 h, the
inhibition rates of the RH experimental group and ToxoDB#9
experimental group reached 18.57 and 35.24%, respectively and the
inhibition rate of T. gondii culture supernatant stimulated
at the same time in the ToxoDB#9 experimental group was higher than
that of the RH experimental group (P<0.01; Table I). For the selection of T.
gondii culture supernatant doses in subsequent experiments, the
concentrations were based on the results of the pre-experiment and
the MOI of T. gondii tachyzoite culture supernatant with
bladder cancer 5637 cells was 10:1. These experiments helped to
determine the appropriate range of T. gondii tachyzoite
culture supernatant concentrations that would not cause excessive
cell death, while still eliciting a biological response.
 | Table I.Cell inhibition rate assessed using a
Cell Counting Kit-8 after treatment of bladder cancer cells with
T. gondii culture supernatant at 24, 48 and 72 h (n=6). |
Table I.
Cell inhibition rate assessed using a
Cell Counting Kit-8 after treatment of bladder cancer cells with
T. gondii culture supernatant at 24, 48 and 72 h (n=6).
|
| 24 h | 48 h | 72 h |
|---|
|
|
|
|
|
|---|
| Group | OD450 | Inhibition rate
(%) | OD450value | Inhibition rate
(%) | OD450value | Inhibition rate
(%) |
|---|
| Control | 0.76±0.04 | - | 1.12±0.10 | - | 2.11±0.12 | - |
| RH |
0.69±0.03a | 9.68 | 1.06±0.08 | 5.59 |
1.72±0.07a | 18.57 |
| ToxoDB#9 |
0.62±0.03a | 18.88 |
0.87±0.03a | 22.70 |
1.27±0.10a | 35.24 |
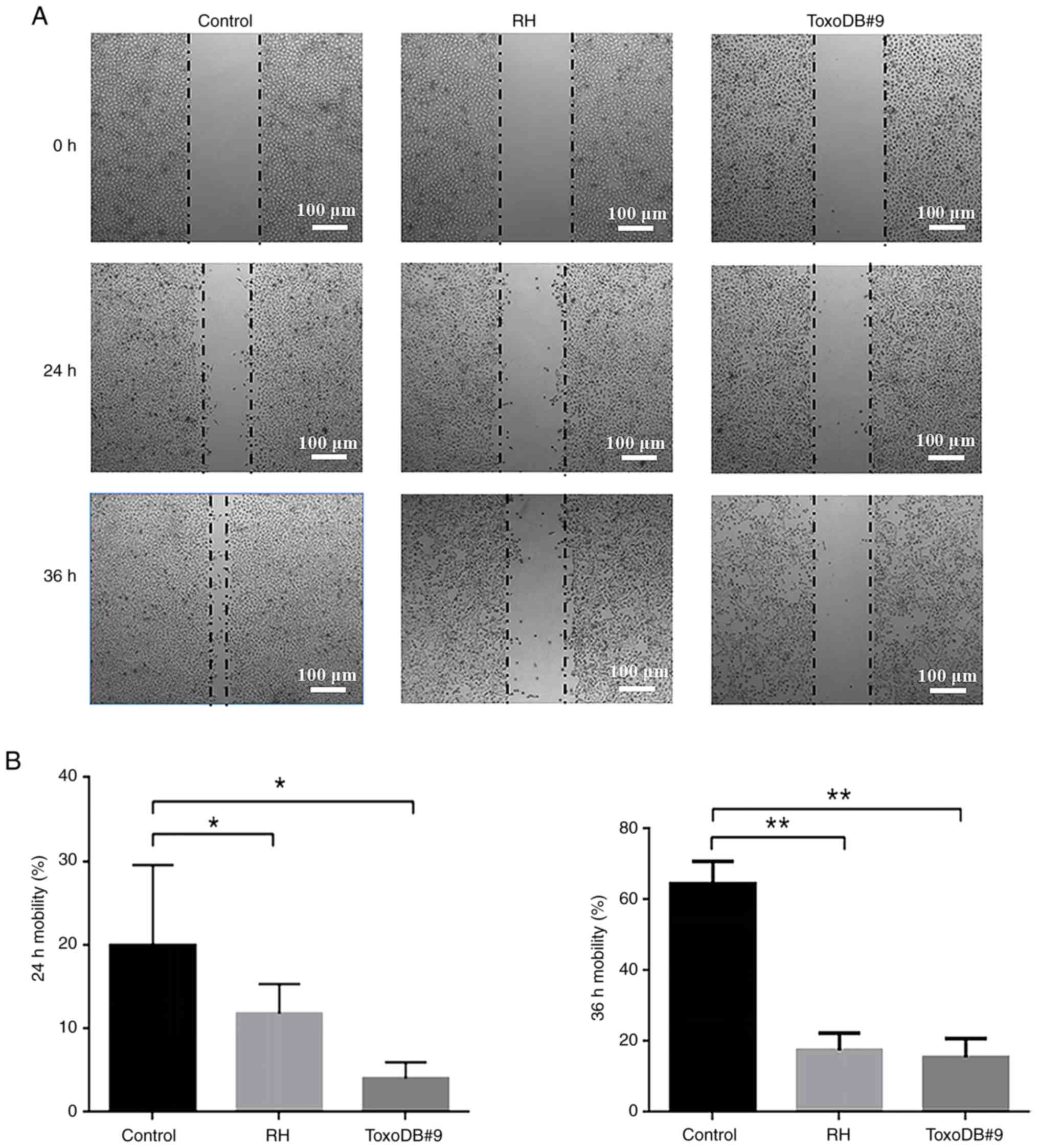
T. gondii tachyzoite culture
supernatant inhibits the migration of bladder cancer 5637 cells in
vitro
After T. gondii tachyzoites culture
supernatant was applied to bladder cancer 5637 cells, the cell
migration rates of the control, RH experimental and ToxoDB#9
experimental groups were 19.92±8.62, 11.80±3.18 and 4.02±1.73% at
24 h and 64.60±5.60, 17.57±4.24 and 15.47±4.82% at 36 h,
respectively (Fig. 3A). At the same
time, the cell migration rates of the RH and ToxoDB#9 experimental
groups were significantly lower than those of the control group
(Fig. 3B). The results indicated
that T. gondii tachyzoite culture supernatant inhibited the
migration of bladder cancer 5637 cells and the inhibition rate of
T. gondii tachyzoite ToxoDB#9-type strain culture
supernatant was higher than that of the RH-type strain.
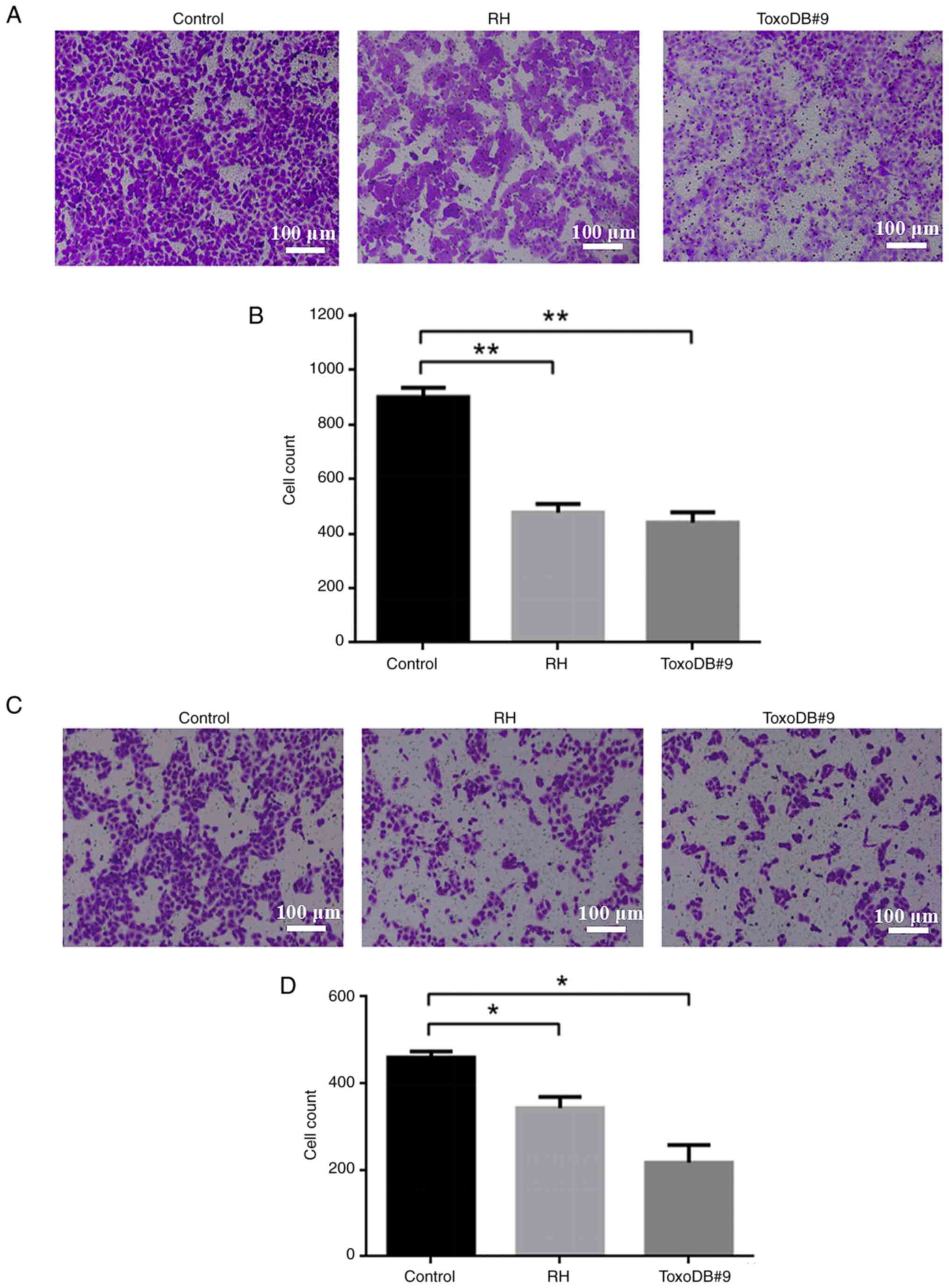
T. gondii tachyzoite culture
supernatant inhibits the migration and invasion of bladder cancer
5637 cells in vitro
Following the action of T. gondii culture
supernatant on bladder cancer 5637 cells, the number of migrating
cells in the control, RH experimental and ToxoDB#9 experimental
groups were 899.33±28.19, 476.00±25.96 and 439.00±31.38 (Fig. 4A and B) and the number of invading
cells were 459.67±11.61, 343.33± 20.74, 217.33±32.42 (Fig. 4C and D). The number of migrating and
invading cells was reduced in both experimental groups compared
with that in the control group. The results indicated that the
culture supernatant of T. gondii tachyzoite inhibited the
migration and invasion of bladder cancer 5637 cells. On the other
hand, ToxoDB#9 experimental group has fewer migrating and invading
cells compared with RH experimental group, which means that the
culture supernatant of T. gondii ToxoDB#9 genotype was more
inhibitory than RH genotype to the migration and invasion of
bladder cancer cells.
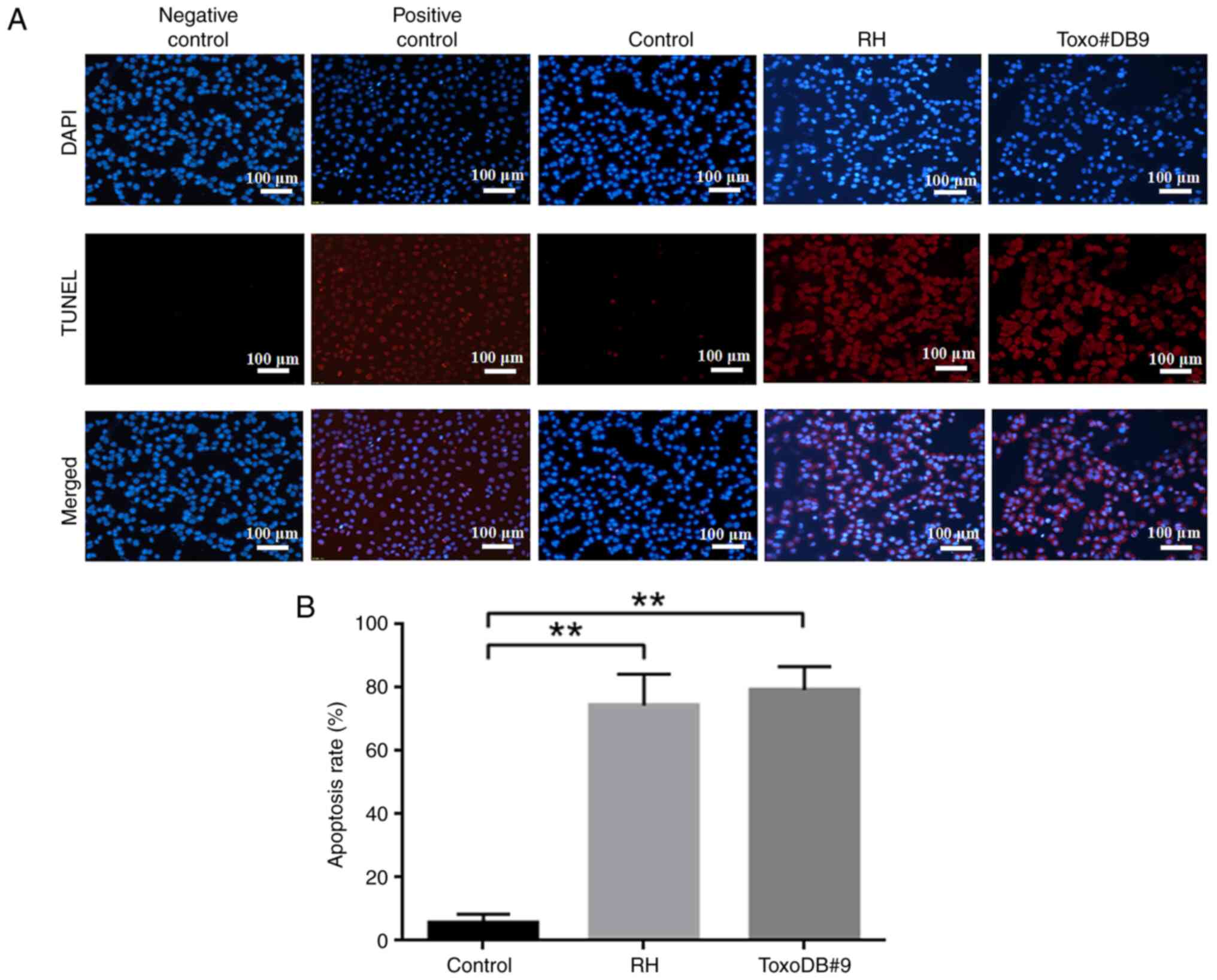
T. gondii tachyzoite culture
supernatant promotes apoptosis in bladder cancer 5637 cells
The present study evaluated the effect of T.
gondii tachyzoite culture supernatant on the induction of
apoptosis in bladder cancer 5637 cells. The apoptosis rates of the
control, RH experimental and ToxoDB#9 experimental groups were
5.71±2.37, 74.35±5.88 and 79.29±6.54%, respectively, after the
action of T. gondii tachyzoite culture supernatant on 5637
bladder cancer cells (Fig. 5A and
B). Compared with that in the control group, the apoptosis rate
was higher in the RH and ToxoDB#9 experimental groups, which
indicated that the culture supernatant of T. gondii
tachyzoite promoted the apoptosis of bladder cancer 5637 cells.
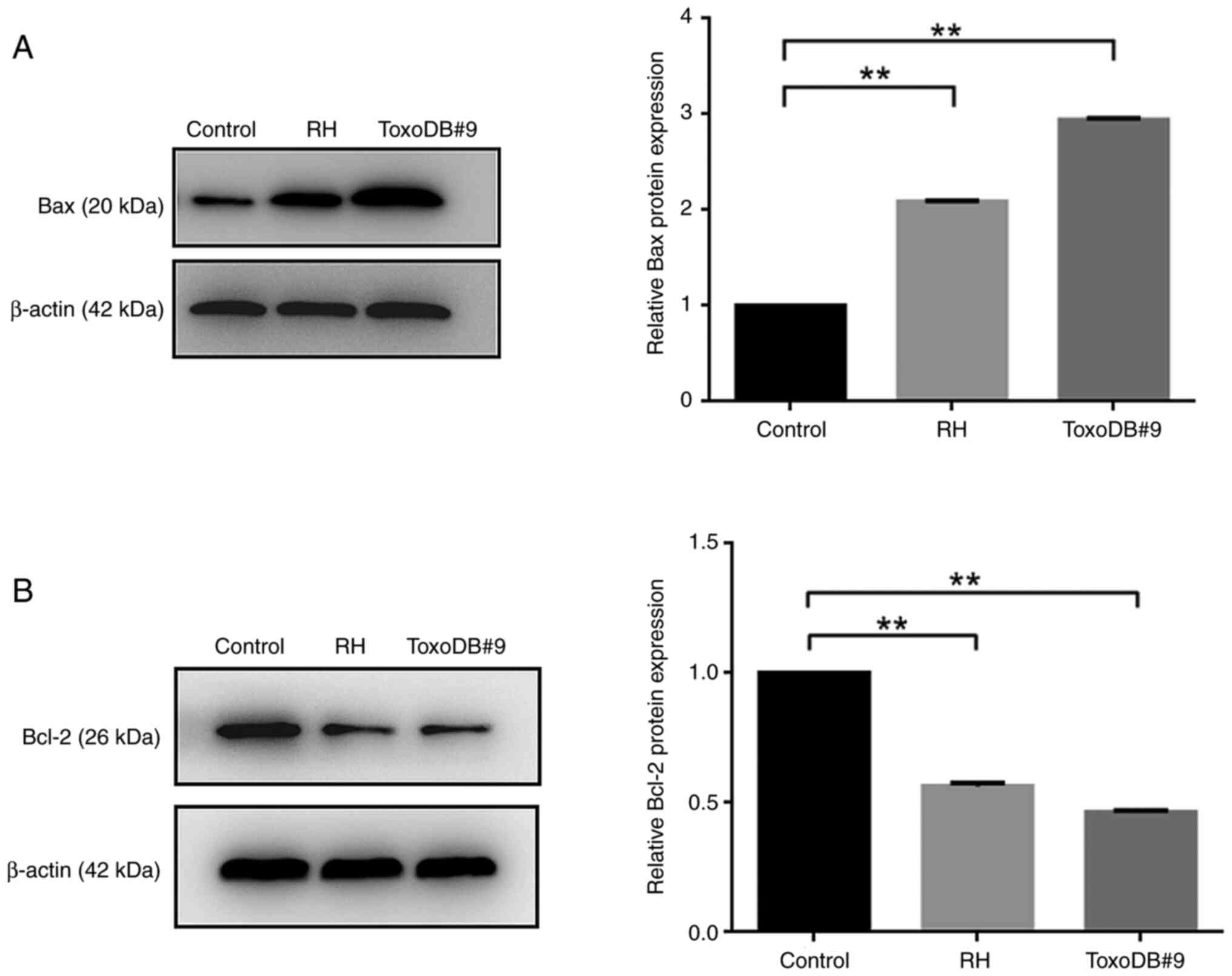
T. gondii tachyzoites culture
supernatants promotes apoptosis in bladder cancer 5637 cells by
modulating apoptotic proteins
Upon the action of T. gondii tachyzoite
culture supernatant on bladder cancer 5637 cells, the expression
levels of Bax in the RH and ToxoDB#9 experimental groups were
significantly increased, the expression levels of Bcl-2 were
significantly decreased and the Bcl-2/Bax ratio were decreased
compared with those in the control group (Fig 6A and B), suggesting T. gondii
tachyzoite culture supernatant may promote the apoptosis of bladder
cancer by regulating apoptotic genes.
Discussion
Bladder cancer is the most prevalent malignant
neoplasm affecting the urinary system and is characterized by its
propensity for frequent recurrence and metastasis, often resulting
in a poor prognosis (27). The
primary treatment modalities for this condition typically involve
surgical intervention in conjunction with radiotherapy or
chemotherapy; however, the efficacy of these approaches remains
limited. Consequently, there is a pressing demand for the
development of novel therapeutic strategies that are both safe and
efficacious for the management of bladder cancer. The landscape of
cancer treatment is continuously evolving, with a growing emphasis
on biological therapy due to its reduced toxicity profile and
minimal adverse effects. Notably, the existing literature from both
domestic and international sources suggests that T. gondii
or its secreted metabolites may exert a dual influence on the
initiation and progression of malignant tumors (28).
Xu et al (29) used CRISPR/CRISPR associated protein
9 technology to create an uracil auxotroph T. gondii RH
strain with orotidine 5′-monophosphate decarboxylase gene deletion
(RH-Δompdc). The authors revealed that inserting RH-Δompdc
immediately into the 4T1 tumor prompts anti-infection and antitumor
immunity in mice, inhibiting tumor growth and metastasis, promoting
tumor-bearing survival and increasing IL-12 and IFN-γ secretion in
serum and the tumor microenvironment. Ye et al (21) reported that T. gondii
transcriptionally regulates various signaling pathways by modifying
hub genes such as BRCA1, MYC and IL-6, which can suppress breast
tumor development and migration (21). Zhu et al (30) used ultracentrifugation to isolate
exosomes that from uninfected dendritic cells (DCs) and T.
gondii Me49-infected DCs and found that the exosomes inhibit
polarization of macrophages to M2 phenotype and regulate suppressor
of cytokine signaling 1 expression by functional microRNA-155-5p to
inhibit colorectal cancer. Ismail et al (31) reported that T. gondii-derived
antigen changes the tumor microenvironment of the Ehrlich solid
carcinoma mouse model and increases the immunotherapeutic efficacy
of cyclophosphamide. Eissa et al (32) demonstrate a prophylactic
antineoplastic activity of autoclaved Toxoplasma vaccine against
Ehrlich solid carcinoma. Zhou et al (33) report that T. gondii rhoptry
protein 18 reduces human glioma cell apoptosis via a mitochondrial
route, targeting host cell P2X1. Mani et al (34) report that T. gondii rGRA6Nt
is a unique and successful protein enhancer when used in
vaccinations with non-replicable cancer cells to effectively
activate immune defenses, particularly against the cancer cells
used in the vaccine. All these studies have shown that T.
gondii and its secretions have antitumor effects. However, it
is not known whether T. gondii and its secretions can
inhibit the growth of bladder cancer. Therefore, the present
experiment was designed to explore the role of T. gondii
tachyzoite culture supernatant in bladder cancer 5637 cells.
In the present study, an in vitro study was
conducted using the human bladder cancer 5637 cell line. The cells
were cultured in the presence of varying concentrations of T.
gondii tachyzoite culture supernatant. The initial observations
revealed that the T. gondii tachyzoite culture supernatant
had an effect on the proliferation, migration, invasion and
apoptosis of 5637 cells. To elucidate the underlying mechanisms, a
series of molecular analyses were performed. Western blotting and
quantitative PCR assays demonstrated that the supernatant induced
apoptosis in the bladder cancer cells, as evidenced by the
increased expression of pro-apoptotic proteins such as Bax and
cleaved caspase-3 and a corresponding decrease in the levels of
anti-apoptotic proteins such as Bcl-2. Furthermore, it was observed
that the T. gondii tachyzoite culture supernatant
upregulated the expression of tumor suppressor genes and
downregulated oncogene expression, indicating a potential
tumor-suppressive role of T. gondii tachyzoite culture
supernatant. Notably, supernatant appeared to impede the migration
and invasion of 5637 cells, as measured by wound healing and
Transwell assays. The present study suggested that T. gondii
tachyzoite culture supernatant may be able to limit the metastatic
spread of bladder cancer, which is an important part of cancer
progression.
In conclusion, the results of the present study
demonstrated that T. gondii tachyzoite culture supernatant
had the ability to inhibit the proliferation, migration and
invasion and promote the apoptosis of bladder cancer 5637 cells.
These findings suggested that T. gondii or its secretions
could be a novel source of therapeutic agents for the treatment of
bladder cancer. However, there are still some limitations to the
present study. Although the culture supernatant of T. gondii
tachyzoites was found to inhibit the proliferation, migration and
invasion and promote the apoptosis of bladder cancer 5637 cells,
the exact mechanism is not known and the present study lacked in
vivo experiments. Therefore, future studies should focus on
identifying the specific components of the supernatant responsible
for these effects and evaluating their efficacy in vivo, as
well as assessing any potential adverse effects that may arise from
the use of T. gondii-derived treatments.
Acknowledgements
Not applicable.
Funding
This work was supported by the Department of Education Science
Research Foundation of Yunnan Province (Research Grant Number
2024J0833).
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
SD designed and performed the experiments, analyzed
the data and wrote the manuscript. YY, TW, LZ and HL performed
experiments and data analyses. SD and YL designed and supervised
the project, acquired the funding and revised the manuscript. LZ
and HL confirm the authenticity of all the raw data. All authors
read and approved the final manuscript.
Ethics approval and consent to
participate
The present study was approved by the Medical Ethics
Committee of Dali University (approval number: 2024-0833).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Bray F, Laversanne M, Sung H, Ferlay J,
Siegel RL, Soerjomataram I and Jemal A: Global cancer statistics
2022: GLOBOCAN estimates of incidence and mortality worldwide for
36 cancers in 185 countries. CA Cancer J Clin. 74:229–263. 2024.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Lobo N, Afferi L, Moschini M, Mostafid H,
Porten S, Psutka SP, Gupta S, Smith AB, Williams SB and Lotan Y:
Epidemiology, screening, and prevention of bladder cancer. Eur Urol
Oncol. 5:628–639. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Zheng RS, Chen R, Han BF, Wang SM, Li L,
Sun KX, Zeng HM, Wei WW and He J: Cancer incidence and mortality in
China, 2022. Zhonghua Zhong Liu Za Zhi. 46:221–231. 2024.(In
Chinese). PubMed/NCBI
|
|
4
|
Li HZ, Zheng RS, Du LB, Zhang SW, Zhu C,
Wei WW and He J: Bladder cancer incidence, mortality and temporal
trends in China. Zhonghua Zhong Liu Za Zhi. 43:293–298. 2021.(In
Chinese). PubMed/NCBI
|
|
5
|
Miyata Y, Matsuo T, Ohba K, Mitsunari K,
Mukae Y, Otsubo A, Harada J, Matsuda T, Kondo T and Sakai H:
Present status, limitations and future directions of treatment
strategies using fucoidan-based therapies in bladder cancer.
Cancers (Basel). 12:37762020. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Abdelbaset AE, Abushahba MFN and Igarashi
M: Toxoplasma gondii in humans and animals in Japan: An
epidemiological overview. Parasitol Int. 87:1025332022. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Hill DE, Chirukandoth S and Dubey JP:
Biology and epidemiology of Toxoplasma gondii in man and animals.
Animal Health Res Rev. 6:41–61. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Uddin A, Hossain D, Ahsan MI, Atikuzzaman
M and Karim MR: Review on diagnosis and molecular characterization
of Toxoplasma gondii in humans and animals. Trop Biomed.
38:511–539. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Milne G, Webster JP and Walker M:
Toxoplasma gondii: AnUnderestimated threat? Trends Parasitol.
36:959–969. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Dubey JP, Murata FHA, Cerqueira-Cézar CK,
Kwok OCH and Villena I: Congenital toxoplasmosis in humans: An
update of worldwide rate of congenital infections. Parasitology.
148:1406–1416. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Halonen SK and Weiss LM: Toxoplasmosis.
Handb Clin Neurol. 114:125–145. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Halici-Ozturk F, Yakut K, Öcal FD, Erol A,
Gökay S, Çağlar AT, Engin-Üstün Y and Ozgu-Erdinc AS:
Seroprevalence of Toxoplasma gondii infections in Syrian pregnant
refugee women in Turkey. Eur J Obstet Gynecol Reprod Biol.
256:91–94. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Molan A, Nosaka K, Hunter M and Wang W:
Global status of Toxoplasma gondii infection: Systematic review and
prevalence snapshots. Trop Biomed. 36:898–925. 2019.PubMed/NCBI
|
|
14
|
Rahmanian V, Rahmanian K, Jahromi AS and
Bokaie S: Seroprevalence of toxoplasma gondii infection: An
umbrella review of updated systematic reviews and meta-analyses. J
Family Med Prim Care. 9:3848–3855. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Abdollahi A, Razavian I, Razavian E,
Ghodsian S, Almukhtar M, Marhoommirzabak E, Sartip B, Parsa H and
Rostami A: Toxoplasma gondii infection/exposure and the risk of
brain tumors: A systematic review and meta-analysis. Cancer
Epidemiol. 77:1021192022. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Anvari D, Sharif M, Sarvi S, Aghayan SA,
Gholami S, Pagheh AS, Hosseini SA, Saberi R, Chegeni TN,
Hosseininejad Z and Daryani A: Seroprevalence of Toxoplasma gondii
infection in cancer patients: A systematic review and
meta-analysis. Microb Pathog. 129:30–42. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Cong W, Liu GH, Meng QF, Dong W, Qin SY,
Zhang FK, Zhang XY, Wang XY, Qian AD and Zhu XQ: Toxoplasma gondii
infection in cancer patients: Prevalence, risk factors, genotypes
and association with clinical diagnosis. Cancer Lett. 359:307–313.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Yu Y, Guo D, Qu T, Zhao S, Xu C, Wang L,
Wang Z, Fu H, Zhang X and Zhou N: Increased risk of Toxoplasma
gondii infection in patients with colorectal cancer in Eastern
China: Seroprevalence, risk factors, and a case-control study.
Biomed Res Int. 2020:25394822020. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhou N, Zhang XY, Li YX, Wang L, Wang LL
and Cong W: Seroprevalence and risk factors of Toxoplasma gondii
infection in oral cancer patients in China: A case-control
prospective study. Epidemiol Infect. 146:1891–1895. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zúñiga-Contreras M, Noemi-Hauck I,
Valenzuela-Cortés R, Barraza-Olivares M and Santolaya-de-Pablo ME:
Toxoplasma gondii IgG seroprevalence in children with cancer from
National Children's Antineoplastic Drug Program network in the
Metropolitan Region, Chile: A multicenter study. Rev Chilena
Infectol. 38:212–217. 2021.(In Spanish). View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ye H, Zhou X, Zhu B, Xiong T, Huang W, He
F, Li H, Chen L, Tang L and Ren Z: Toxoplasma gondii suppresses
proliferation and migration of breast cancer cells by regulating
their transcriptome. Cancer Cell Int. 24:1442024. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ong CY, Abdalkareem EA and Khoo BY:
Functional roles of cytokines in infectious disease associated
colorectal carcinogenesis. Mol Biol Rep. 49:1529–1535. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Lotfalizadeh N, Sadr S, Morovati S,
Lotfalizadeh M, Hajjafari A and Borji H: A potential cure for
tumor-associated immunosuppression by Toxoplasma gondii. Cancer Rep
(Hoboken). 7:e19632024. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Hosseini SA, Sharif M, Sarvi S, Janbabai
G, Keihanian S, Abediankenari S, Gholami S, Amouei A, Javidnia J,
Saberi R, et al: Toxoplasmosis among cancer patients undergoing
chemotherapy: A population study based on the serological,
molecular and epidemiological aspects. Trans R Soc Trop Med Hyg.
115:677–686. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Costa W, Barbosa I, Prado D, Domann N and
Rezende HHA: A systematic review of Toxoplasma gondii genotypes in
Gallus gallus domesticus worldwide: The focus is Brazil. Transbound
Emerg Dis. 69:2440–2450. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Wang L, He LY, Meng DD, Chen ZW, Wen H,
Fang GS, Luo QL, Huang KQ and Shen JL: Seroprevalence and genetic
characterization of Toxoplasma gondii in cancer patients in Anhui
Province, Eastern China. Parasit Vectors. 8:1622015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Chen H, Yang W, Xue X, Li Y, Jin Z and Ji
Z: Integrated analysis revealed an inflammatory cancer-associated
fibroblast-based subtypes with promising implications in predicting
the prognosis and immunotherapeutic response of bladder cancer
patients. Int J Mol Sci. 23:159702022. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zhang Y, Lai BS, Juhas M and Zhang Y:
Toxoplasma gondii secretory proteins and their role in invasion and
pathogenesis. Microbiol Res. 227:1262932019. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Xu LQ, Yao LJ, Jiang D, Zhou LJ, Chen M,
Liao WZ, Zou WH and Peng H: A uracil auxotroph Toxoplasma gondii
exerting immunomodulation to inhibit breast cancer growth and
metastasis. Parasit Vectors. 14:6012021. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Zhu S, Lu J, Lin Z, Abuzeid AMI, Chen X,
Zhuang T, Gong H, Mi R, Huang Y, Chen Z and Li G: Anti-tumoral
effect and action mechanism of exosomes derived from toxoplasma
gondii-infected dendritic cells in mice colorectal cancer. Front
Oncol. 12:8705282022. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Ismail CA, Eissa MM, Gaafar MR, Younis LK
and El Skhawy N: Toxoplasma gondii-derived antigen modifies tumor
microenvironment of Ehrlich solid carcinoma murine model and
enhances immunotherapeutic activity of cyclophosphamide. Med Oncol.
40:1362023. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Eissa MM, Gaafar MR, Younis LK, Ismail CA
and El Skhawy N: Prophylactic antineoplastic activity of Toxoplasma
gondii RH derived antigen against ehrlich solid carcinoma with
evidence of shared antigens by comparative immunoblotting. Infect
Agents Cancer. 18:212023. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Zhou LJ, Chen M, Puthiyakunnon S, He C,
Xia J, He CY, Deng SQ and Peng HJ: Toxoplasma gondii ROP18 inhibits
human glioblastoma cell apoptosis through a mitochondrial pathway
by targeting host cell P2X1. Parasit Vectors. 12:2842019.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Mani R, Martin CG, Balu KE, Wang Q,
Rychahou P, Izumi T, Evers BM and Suzuki Y: A novel protozoa
parasite-derived protein adjuvant is effective in immunization with
cancer cells to activate the cancer-specific protective immunity
and inhibit the cancer growth in a murine model of colorectal
cancer. Cells. 13:1112024. View Article : Google Scholar : PubMed/NCBI
|