Introduction
Epithelioid hemangioendothelioma (EHE) is a rare
soft-tissue vascular neoplasm with a prevalence of one in one
million (1). Clinically, EHE can
involve the liver alone (21%), liver and lungs (18%), lungs alone
(12%) and bones alone (14%), and may occur at various other sites
throughout the body (1,2). The clinical manifestations of EHE
range from bone pain to neurologic symptoms or swelling at the site
of the lesion, and systemic manifestations can include weight loss
and anemia (1,3). However, to the best of our knowledge,
EHE involving the auricle has not been reported.
It is easy to confuse auricular EHE with auricular
pseudocyst in clinical practice since pseudocyst of the auricle
presents as an asymptomatic cystoid swelling (4), as does an EHE. EHE can be diagnosed
based on morphological characteristics, including intranuclear
inclusions, intracytoplasmic vacuoles and stromal changes (5), as well as histological
characteristics, including endothelial cells arranged in nests and
cords, the presence of spindle-shaped tumor cells and various sized
lumens (1). Immunohistochemistry
can also be helpful in the diagnosis of EHEs. Positivity for both
FLI-1 and CD31 can be considered diagnostic of EHE (6). In the present case report, two
patients with clinical symptoms of unilateral soft non inflammatory
auricular swelling are described. The initial diagnosis for these
two cases was pseudocyst of the auricle. During the surgery, it was
found that each cyst had been formed by the accumulation of sterile
fluid between two layers of auricular cartilage, which resembled a
pseudocyst of the auricle. However, postoperative pathological
examination of the cartilage capsule wall suggested a diagnosis of
auricular EHE. Immunohistochemical examination showed that the
specimens were positive for CD31, CD34, friend leukemia integration
1 (FLI-1), coagulation factor 8 and E26
transformation-specific-related gene (ERG), which was consistent
with EHE.
Case reports
Case 1
A 65-year-old man presented with a 5-year history of
swelling on the left ear. The swelling initially manifested as a
2×3-mm lesion with pruritus, which gradually increased in size, but
did not feel tender. The patient visited the outpatient department
of China-Japan Friendship Hospital (Beijing, China). The patient
had no history of previous auricular trauma or frostbite. His
medical history was unremarkable, except that he had undergone
colon cancer surgery in 2009 in a local hospital, 10 years
previously. Physical examination detected swelling in the
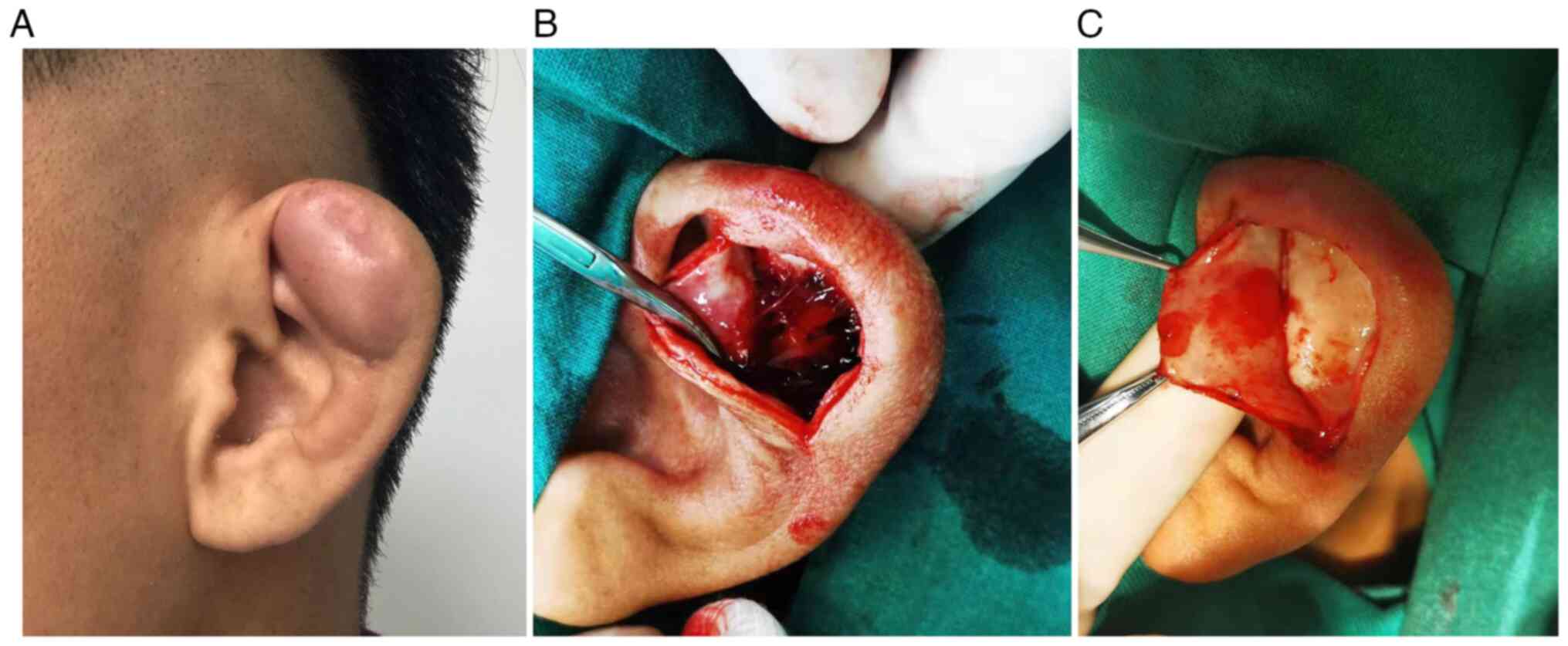
triangular fossa region of the left ear without tenderness
(Fig. 1A), and the patient was
diagnosed with an auricular pseudocyst. Auricular excision surgery
was performed under general anesthesia. During intraoperative
examination, it was found that the auricular cartilage was divided
into two layers, and the space between these layers was filled with
transparent liquid. The effusion was completely aspirated with an
aspirator (Fig. 1B), and the
swollen upper cartilage and cyst wall were removed (Fig. 1C). A compression bandage was placed
on the head of the patient, and broad-spectrum intravenous
antibiotics were administered for 2 days.
The removed upper auricular cartilage and the
swollen cyst wall were sent for pathological examination (7). Postoperative pathological microscopic
examination at low magnification revealed tumor invasion and
destruction of cartilage tissue. At medium magnification, the tumor
cells were seen to be oval, short spindle-shaped, and scattered or
irregularly distributed in sheets. At high magnification, it was
observed that the tumor cells had abundant, light-stained
eosinophilic cytoplasm, mostly small nuclei, and inconspicuous or
small nucleoli. In some areas, vacuoles were visible in the
cytoplasm of the tumor cells, and red blood cells were frequently
present in the vacuoles. Pathological mitotic figures were rare.
Immunohistochemical examination showed that the specimen had a Ki67
index of 20%, as detected using monoclonal antibody Ki67 [MIB-1;
Ki67 index] (8). In addition, the
specimen was positive for FLI-1, ERG, coagulation factor 8 (F8),
CD31, vimentin, the CD68-targeting antibody Ki-61 protein 1 (KP-1;
scattered positive) and CD34, and was negative for desmin, S100, α
smooth muscle actin (α-SMA) and epithelial membrane antigen (EMA)
(Fig. 2).
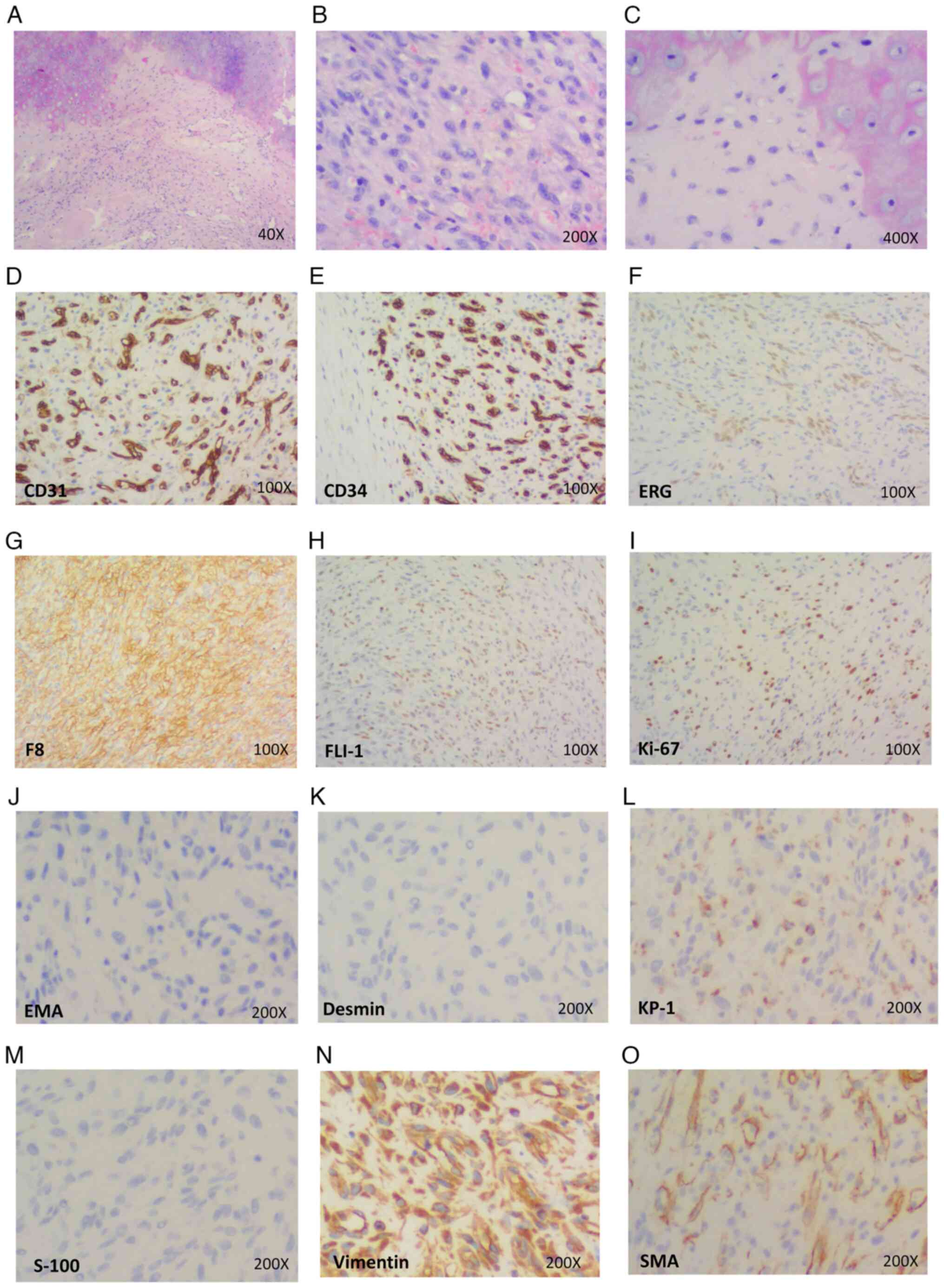 | Figure 2.Pathological features of case 1. (A)
Presence of vacuoles in the cytoplasm of tumor cells in some areas,
with tumor cells invading the cartilage (H&E staining;
magnification, ×40). (B) Scattered or irregular distribution of
oval or short spindle-shaped tumor cells, with red blood cells
frequently visible in the vacuoles (H&E staining;
magnification, ×200). (C) High magnification showed that the tumor
cells had abundant, light-stained eosinophilic cytoplasm, mostly
small nuclei, and inconspicuous or small nucleoli. In some areas,
vacuoles were present in the cytoplasm of the tumor cells, and red
blood cells were commonly found in the vacuoles (H&E staining;
magnification, ×400). (D-N) Immunohistochemical results showed that
tumor cells were positive for the vascular markers (D) CD31, (E)
CD34, (F) ERG, (G) F8, (H) FLI-1, (I) Ki-67, (L) KP-1 and (N)
vimentin, and negative for the markers (J) EMA, (K) desmin and (M)
S100. (O) The tumor cells were negative for SMA; the sites of
positive staining for SMA were vascular smooth muscle
(magnification, ×100 in D-I and ×200 in J-O). ERG, ETS-related
gene; F8, coagulation factor 8; FLI-1, friend leukemia integration
1; KP-1, antibody against CD68; EMA, epithelial membrane antigen;
SMA, smooth muscle actin. |
These examination results led to a pathological
diagnosis of low-grade malignant angiogenic tumor, consistent with
EHE. The 2-year follow-up after surgery showed that no tumor was
present in the auricle. The last follow-up was conducted and the
patient did not revisit in the later stage.
Case 2
A 48-year-old man presented with a 1-month history
of pruritic swelling of his right ear on October 30, 2019. The
swelling increased gradually without redness, purulence or
tenderness. No history of previous trauma or frostbite was
reported. The patient had been repeatedly treated with cyst
puncture and compression in other hospitals, but the swelling was
not relieved after treatment, and gradually became aggravated and
tender. The patient presented at China-Japan Friendship Hospital
(Beijing, China) for further treatment. During physical
examination, swelling in the triangular fossa region of the right
ear was observed, with tenderness on palpation. Based on these
findings, the patient was diagnosed with an auricular pseudocyst.
The auricular lesion was excised under general anesthesia, with
intraoperative examination revealing an accumulation of sterile
fluid between the layers of the auricular cartilage. Following
complete aspiration of the effusion using an aspirator, the swollen
upper cartilage and cyst wall were removed and sent for
pathological examination (8).
Intraoperative frozen pathology revealed that the resection margin
was free of tumor cells. However, no clinical or surgical images of
case 2 were captured at the time of treatment. A compression
bandage was applied to the head of the patient, and broad-spectrum
intravenous antibiotics were administered for 2 days.
The postoperative pathological features of case 2
were consistent with those of case 1. Specifically,
immunohistochemical examination showed that the lesion had a Ki67
(MIB-1) index of 20% and was positive for CD31, F8, CD34, FLI-1 and
ERG (Fig. 3). The pathology report
also disclosed that the lesion was scattered positive for KP-1, and
negative for desmin, α-SMA, S-100 and EMA (data not shown)
(8).
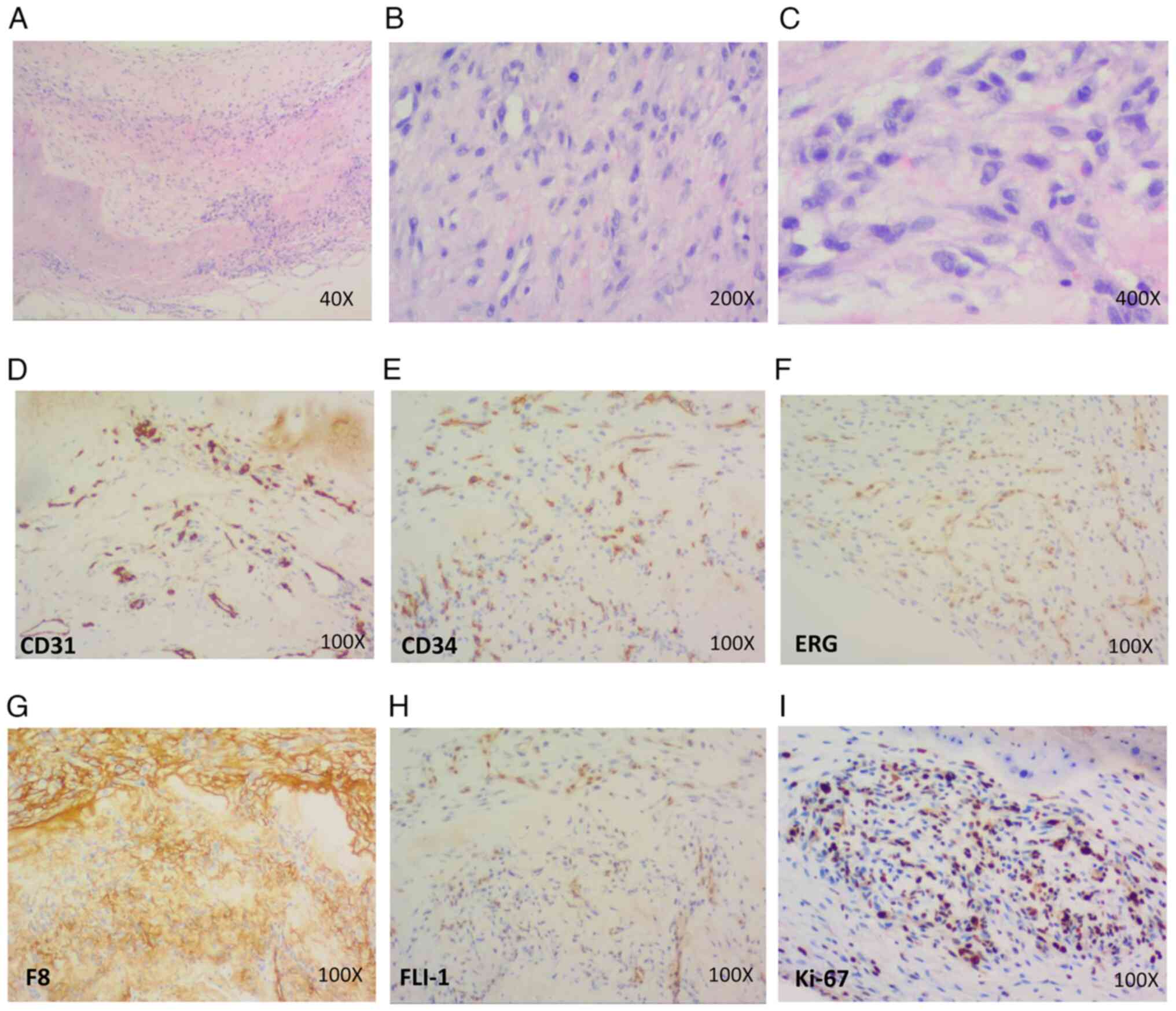 | Figure 3.Pathological features of case 2. (A)
Vacuoles are visible in the cytoplasm of the tumor cells in some
areas, and tumor cells are invading the cartilage (H&E
staining; magnification, ×40). (B) Oval or short spindle-shaped
tumor cells are scattered or irregularly distributed, and red blood
cells are prevalent in the vacuoles (H&E staining;
magnification, ×200). (C) At the highest magnification, it was
evident that the tumor cells had plentiful, lightly stained
eosinophilic cytoplasm, generally small nuclei, and inconspicuous
or small nucleoli. Vacuoles were visible in the cytoplasm of the
tumor cells in some areas and red blood cells were frequently
observed in the vacuoles (H&E staining; magnification, ×400
(D-I) Immunohistochemical results showed that the tumor cells were
positive for the vascular markers (D) CD31, (E) CD34, (F) ERG, (G)
F8 and (H) FLI-1, as well as (I) Ki-67 (magnification, ×100). ERG,
ETS-related gene; F8, coagulation factor 8; FLI-1, friend leukemia
integration 1. |
Pathologic examination of the specimens isolated
from the patient led to the diagnosis of an angiogenic tumor. The
morphology and immunohistochemistry of the lesion were consistent
with those of EHE. A follow-up performed 6 months after surgery
revealed that no new tumor was present in the auricle. The last
follow-up was conducted and the patient did not revisit in the
later stage.
Discussion
EHE is an extremely rare tumor that develops from
vascular endothelial or pre-endothelial cells (1). EHE was initially described in 1975 and
named epithelioid hemangioendothelioma in 1982 (1,9). EHE
tends to present during middle age, with a median age of 36 years,
and is 4-fold more common in women than men (1). Approximately 30% of these tumors
present as pulmonary EHEs, which are typically first diagnosed
incidentally from abnormal chest imaging results (10). Other primary sites of EHE include
subcutaneous fat, bone, retroperitoneum, lymph nodes, ovaries,
prostate glands, eyelids and pleura (1,2). The
clinical manifestations of EHE range from bone pain to neurologic
symptoms or swelling at the site of the lesion, and systemic
manifestations can include weight loss and anemia (1,3). To
the best of our knowledge, only three cases of pseudocysts
associated with malignant tumors have been reported, where the
malignant tumors include lymphoma and hepatocellular carcinoma
(11–13), and EHE has not been previously
reported in the auricles.
EHE in the auricle is easily misdiagnosed as
auricular pseudocyst due to these two conditions having similar
clinical symptoms. Pseudocysts, first described in 1966 (14), manifest as rare benign swellings;
when they affect the auricles, they are characterized by
degeneration and separation of the cartilage, and subsequent cyst
formation (11). Most pseudocysts
of the auricles present as asymptomatic, unilateral soft
skin-colored noninflammatory swellings (11). The two patients described in the
present case report presented with noninflammatory swelling of the
auricle, with intraoperative examinations showing that these cysts
comprised an accumulation of sterile fluid between layers of
auricular cartilage. These clinical manifestations and
intraoperative findings are not able to distinguish auricular
pseudocyst from auricular EHE. The final diagnosis requires
postoperative pathological examination. Auricular pseudocysts are
characterized by the infiltration of chronic inflammatory cells
without the destruction of auricular cartilage (4,11).
However, the postoperative pathology of the two patients in the
present study showed the presence of scattered or irregularly
distributed tumor cells that were oval or short fusiform in
morphology. In some of the tumor cells, vacuoles were present in
the cytoplasm, and numerous red blood cells were visible in the
vacuoles. Pathological mitoses were rare. Also, the cartilage was
invaded by low-grade malignant tumor cells, and immunohistochemical
analyses showed that the tumor cells were positive for the vascular
markers F8, ERG, CD34, CD31 and FLI-1. These pathological
characteristics indicate that these lesions were
hemangioendotheliomas.
EHE can be diagnosed based on morphological
characteristics, including intranuclear inclusions,
intracytoplasmic vacuoles and stromal changes (5), as well as histological
characteristics, including endothelial cells arranged in nests and
cords, the presence of spindle-shaped tumor cells and various sized
lumens (1). Some cells contain
intracytoplasmic inclusions, resulting in a signet-ring appearance
(15). Immunohistochemistry can
also be helpful in the diagnosis of EHEs. CD34 is a vascular tumor
marker expressed in 90% of vascular tumors and is not specific for
EHE (1). By contrast, CD31 is more
specific, and FLI-1, a transcription factor expressed in
endothelial cells, is important for revealing the vascular nature
of EHE (1). Therefore, positivity
for both FLI-1 and CD31 can be considered diagnostic of EHE
(6).
Due to the low incidence of EHE, no optimal
treatment strategy has yet been designed. Localized lesions can be
surgically resected, whereas watchful waiting may be considered as
a reasonable strategy for patients with asymptomatic diffuse
lesions (10). The treatment
options for patients with metastatic EHE include cytotoxic
chemotherapy, immunotherapy and targeted therapy (2). A recently reported case (16) diagnosed with pulmonary endovascular
EHE was treated with tri-weekly paclitaxel (175 mg/m2)
and carboplatin (area under the curve 5) chemotherapy regimen. A
clear response was observed after 5 cycles (21 days per cycle) and
pembrolizumab (200 mg once monthly) as maintenance treatment.
Similarly, Ye et al (17)
reported that three patients with pulmonary EHE who received
combination chemotherapy with carboplatin, paclitaxel and
bevacizumab all achieved partial responses. They survived after
follow-up for 6–25 months. However, the efficacy of chemotherapy is
still uncertain. Bansal et al (18) reported a patient with pleural EHE
who died due to disease progression after 4 months, even after the
use of chemotherapy. In addition to surgery, the efficacy of
postoperative external beam irradiation has also been studied. A
previous study of 5 patients with spinal EHE found that 4 of the
patients received surgery and postoperative external beam
irradiation. One of these patients died 34 months after surgery,
and the others survived for 25–72 months of follow-up (19). Some researchers have shifted their
focus toward targeted molecular therapy. For instance, apatinib
provided some symptomatic improvements and positive imaging changes
in a case of pulmonary EHE (20).
In addition, sorafenib achieved a partial response in a case of
liver EHE (21), and the treatment
of multi-metastatic pulmonary EHE with pazopanib for >2 years
resulted in a stable disease (22).
The two patients in the present study had Ki67 (MIB-1) indices of
20%, suggesting that their tumors were of low malignancy. Both
patients recovered after surgical resection, and showed no evidence
of tumor recurrence on follow-up.
In summary, auricular EHE is rare and lacks typical
clinical features, with clinical manifestations similar to those of
auricular pseudocysts. Comprehensive analysis of clinical, imaging
and pathomorphological results is important, but the final
diagnosis mainly depends on histopathology and
immunohistochemistry. In cases when the course of disease is
prolonged and symptomatic treatment has been ineffective, the
possibility of a tumor should be considered. If the tumor is highly
malignant, radiotherapy and chemotherapy can be administered.
However, the effectiveness of radiotherapy and chemotherapy for the
treatment EHE is poor, and there is no ideal targeted drug therapy
at present. Postoperative follow-up is necessary to prevent
recurrence. Therefore, it is necessary to conduct a thorough
analysis, carefully observe, and accumulate experience by
integrating relevant clinical cases.
Acknowledgements
Not applicable.
Funding
The present study was supported by National High Level Hospital
Clinical Research Funding, Elite Medical Professionals project of
China-Japan Friendship Hospital (grant no. ZRJY2021-QM03) and the
National Natural Science Foundation of China (grant no.
82101235).
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
JL and YW were responsible for conceptualization. YN
and ZM analyzed the pathological sections. JZ and RZ performed the
case review and collected the medical records. YW prepared the
original draft of the manuscript. JZ, YN and JL reviewed and edited
the manuscript. JW and JL checked and confirmed the authenticity of
the raw data. All authors read and approved the final version of
the manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
The patients provided written informed consent for
the publication of their case reports, including case data and
images.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Sardaro A, Bardoscia L, Petruzzelli MF and
Portaluri M: Epithelioid hemangioendothelioma: An overview and
update on a rare vascular tumor. Oncol Rev. 8:2592014.
|
|
2
|
Rosenberg A and Agulnik M: Epithelioid
Hemangioendothelioma: Update on diagnosis and treatment. Curr Treat
Options Oncol. 19:192018. View Article : Google Scholar
|
|
3
|
Bagan P, Hassan M, Barthes FL, Peyrard S,
Souilamas R, Danel C and Riquet M: Prognostic factors and surgical
indications of pulmonary epithelioid hemangioendothelioma: A review
of the literature. Ann Thorac Surg. 82:2010–2013. 2006. View Article : Google Scholar
|
|
4
|
Cohen V, Fortier-Riberdy G, Saliba I and
Davar S: A case of auricular pseudocyst. J Cutan Med Surg.
20:573–574. 2016. View Article : Google Scholar
|
|
5
|
Anderson T, Zhang L, Hameed M, Rusch V,
Travis WD and Antonescu CR: Thoracic epithelioid malignant vascular
tumors: A clinicopathologic study of 52 cases with emphasis on
pathologic grading and molecular studies of WWTR1-CAMTA1 fusions.
Am J Surg Pathol. 39:132–139. 2015. View Article : Google Scholar
|
|
6
|
Gill R, O'Donnell RJ and Horvai A: Utility
of immunohistochemistry for endothelial markers in distinguishing
epithelioid hemangioendothelioma from carcinoma metastatic to bone.
Arch Pathol Lab Med. 133:967–972. 2009. View Article : Google Scholar
|
|
7
|
Tosta TA, de Faria PR, Neves LA and do
Nascimento MZ: Computational normalization of H&E-stained
histological images: Progress, challenges and future potential.
Artif Intell Med. 95:118–132. 2019. View Article : Google Scholar
|
|
8
|
Ribeiro MB and Ibiapina JO:
Immunohistochemical analysis by KI67 and IDH1 in patients with
chondrosarcoma. Acta Ortop Bras. 31:e2672122023. View Article : Google Scholar
|
|
9
|
Weiss SW and Enzinger FM: Epithelioid
hemangioendothelioma: A vascular tumor often mistaken for a
carcinoma. Cancer. 50:970–981. 1982. View Article : Google Scholar
|
|
10
|
Kitaichi M, Nagai S, Nishimura K, Itoh H,
Asamoto H, Izumi T and Dail DH: Pulmonary epithelioid
haemangioendothelioma in 21 patients, including three with partial
spontaneous regression. Eur Resp J. 12:89–96. 1998. View Article : Google Scholar
|
|
11
|
Abbas O, Chedraoui A, Baki JA, Kibbi AG
and Ghosn S: Bilateral auricular pseudocysts as the presenting sign
of hepatocellular carcinoma. Clin Exp Dermatol. 35:e34–e36. 2010.
View Article : Google Scholar
|
|
12
|
Pereira FC, Chinelli PA, Takahashi MD and
Nico MM: Bilateral pseudocyst of the auricle in a man with pruritus
secondary to lymphoma. Int J Dermatol. 42:818–821. 2003. View Article : Google Scholar
|
|
13
|
Hoffmann TJ, Richardson TF, Jacobs RJ and
Torres A: Pseudocyst of the auricle. J Dermatol Surg Oncol.
19:259–262. 1993. View Article : Google Scholar
|
|
14
|
Engel D: Pseudocysts of the auricle in
Chinese. Arch Otolaryngol. 83:197–202. 1966. View Article : Google Scholar
|
|
15
|
Flucke U, Vogels RJ, de Saint Aubain
Somerhausen N, Creytens DH, Riedl RG, van Gorp JM, Milne AN,
Huysentruyt CJ, Verdijk MA, van Asseldonk MM, et al: Epithelioid
hemangioendothelioma: Clinicopathologic, immunhistochemical, and
molecular genetic analysis of 39 cases. Diagn Pathol. 9:1312014.
View Article : Google Scholar
|
|
16
|
Guo W, Zhou D, Huang H, Chen H, Wu X, Yang
X, Ye H and Hong C: Successful chemotherapy with continuous
immunotherapy for primary pulmonary endovascular epithelioid
hemangioendothelioma: A case report. Medicine (Baltimore).
102:e329142023. View Article : Google Scholar
|
|
17
|
Ye B, Li W, Feng J, Shi JX, Chen Y and Han
BH: Treatment of pulmonary epithelioid hemangioendothelioma with
combination chemotherapy: Report of three cases and review of the
literature. Oncol Lett. 5:1491–1496. 2013. View Article : Google Scholar
|
|
18
|
Bansal A, Chawla M, Cohen PJ and Kwon JS:
Pleural epithelioid hemangioendothelioma. Lung. 190:469–470. 2012.
View Article : Google Scholar
|
|
19
|
Ma J, Wang L, Mo W, Yang X and Xiao J:
Epithelioid hemangioendotheliomas of the spine: Clinical characters
with middle and long-term follow-up under surgical treatments. Eur
Spine J. 20:1371–1376. 2011. View Article : Google Scholar
|
|
20
|
Zheng Z, Wang H, Jiang H, Chen E, Zhang J
and Xie X: Apatinib for the treatment of pulmonary epithelioid
hemangioendothelioma: A case report and literature review. Medicine
(Baltimore). 96:e85072017. View Article : Google Scholar
|
|
21
|
Kobayashi N, Shimamura T, Tokuhisa M, Goto
A and Ichikawa Y: Sorafenib Monotherapy in a patient with
unresectable hepatic epithelioid hemangioendothelioma. Case Rep
Oncol. 9:134–137. 2016. View Article : Google Scholar
|
|
22
|
Semenisty V, Naroditsky I, Keidar Z and
Bar-Sela G: Pazopanib for metastatic pulmonary epithelioid
hemangioendothelioma-a suitable treatment option: Case report and
review of anti-angiogenic treatment options. BMC Cancer.
15:4022015. View Article : Google Scholar
|